Abstract
Objective/Background
Adolescents who experience sleep problems are less able to resist impulses. Furthermore, youths who show more impulsive behaviors are, in turn, assumed to have more sleep problems, which sets the stage for a negative cycle over time. Empirical research has shown some evidence that sleep problems affect impulse control, but the bidirectional link has previously not been tested. Therefore, the aim of this study was to test this assumption.
Methods
In this study, we used cross-lagged models to investigate the bidirectional association between sleep problems (ie, insomnia and sleep duration) and impulsive behaviors over two years in a cohort of young adolescents (n = 2767, mean age ∼13.7, 47.6% girls). We also investigated the moderating role of age and gender.
Results
The results showed that the links between sleep duration/insomnia and impulsive behavior are bidirectional. Youths who experienced sleep problems also experienced increased difficulties with impulse control, and problems regulating impulses were also linked with increases in sleep problems, and these effects were systematic over two years. Moreover, age did not moderate these associations but impulsive behaviors had a larger impact on girls’ insomnia as compared to boys.
Conclusions
By confirming the bi-directionality of this association, this study supports the importance of developing interventions to promote sleep health in adolescents but also the need to tailor such programs to adolescents’ development because adolescents might not be able to prioritize sleep if they cannot control their impulses.
Keywords: Sleep duration, Insomnia, Urgency, Impulsive behaviors, Adolescents, Bidirectional
Highlights
-
•
Poor sleep and impulsive behavior were bidirectionally linked over three years.
-
•
Girls might be at higher risk of insomnia when displaying impulsive behavior.
-
•
Sleep interventions should take into account adolescents' lack of impulse control.
1. Introduction
Adolescents go through many biological and psychosocial changes, which deeply affect both their sleep and their ability to regulate emotions and behaviors [1]. There is growing evidence that poor sleep, both quality and quantity, is associated with adolescents' risk behaviors (eg, delinquency, unprotected sex, suicide behaviors, and alcohol intoxication) [2], [3]. Some research suggests that the ability to regulate emotions and behaviors – impulse control – may be impaired by poor sleep [4]. Conversely, it has been suggested that poor impulse control is related to irregular sleep routines and heightened arousal, which is antithetical to sleep [1]. This assumption has not been tested previously, as most studies examined concurrent associations or the effects of sleep on impulsive behavior but not the reverse [2], [3]. Understanding the interplay between sleep and impulse control over time is important to inform interventions to promote sleep health and prevent adolescents’ risk behaviors.
A growing body of research shows that adolescents do not get enough sleep as sleep duration tends to decrease throughout adolescence [5], [6]. Moreover, adolescents report increases in sleep complaints, especially problems falling asleep [7]. Specifically, girls report more sleep disturbances than boys and these differences are maintained through adulthood [8]. Gender differences in sleep duration, conversely, are not as clear and have been mixed or non-significant [9]. The negative consequences of poor sleep in adolescents have gained much attention, ranging from emotional problems such as depression [10] to daytime functioning such as attention problems [11] and long-term consequences as poor school performance [12] and worse physical health [13]. In particular, new attention has been given to the association between poor sleep and adolescents’ involvement in risky behaviors because, in parallel to an increase in sleep problems, adolescence is characterized by an increase in impulsive behaviors and sensation seeking [14].
Research shows a peak in impulsive behavior around middle adolescence (before age 15 years), and this trend seems somewhat more pronounced in boys as compared to girls [15], [16]. Increases in impulsive behavior have been explained by the faster maturation of the socioemotional system, which is sensitive to rewards, in contrast to the later maturation of the cognitive control system, which is responsible for self-regulation [17]. Neuroimaging studies support this dual-systems model [14] and provide evidence that impulse control improves linearly from early adolescence into adulthood whereas reward-seeking follows a curvilinear shape, peaking in mid-adolescence [14]. Experimental studies show that adolescents are capable of performing as well as adults (and better than children) in tasks requiring impulse control, such as in a classic Stroop color–word task [18]. Yet, they act more impulsively in situations that elicit emotional arousal [19], suggesting that the emotional system interferes when adolescents try to control impulses but that this interference is better controlled in adulthood. Therefore, it seems that an imbalance between the control and reward-seeking systems and an increase in sleep problems co-occur in adolescents, yet an understanding of how and why they are related is less developed.
The majority of experimental studies have focused on determining the effects of sleep on impulse control. The ability to control impulses has been described as a limited resource that may be running low when the individual is tired [20]. Moreover, several studies show that poor sleep intensifies emotional reactivity to both positive and negative stimuli [21]. Thus, one explanation is that poor sleep increases emotional reactivity which in turn worsens impulse control [22]. Several studies have found that one night of sleep deprivation was associated with more impulsive responses to negative stimuli in Go–NoGo tasks in healthy adults [23], [24]. Similarly, 1-h sleep restriction (vs 1 h of extra sleep) in children (age 7–11 years) showed clear increases in teacher-rated impulsive behaviors [25]. Yet, other studies have found no effects of sleep restriction on adolescents’ daytime impulsivity (including self- and parent reports) (eg Refs. [26], [27]). Finally, brain activity in adolescents who reported poor sleep quality showed decreased connectivity between cognitive control and reward-seeking areas during a Go–NoGo task [4]. Thus, there is some evidence pointing at sleep disturbances as an additional factor contributing to impulsive behavior.
Conversely, increased impulsivity might explain why adolescents report worse sleep routines [1]. For example, adolescents scoring higher on impulsivity might have a harder time maintaining a regular sleep routine and get easily caught in stimulating activities, such as social networking late in the evening [28]. Fewer studies have examined this direction; that is, whether impulsive behavior is a risk for sleep disturbances. To support this idea, an experimental study found that adolescents who perceived the negative consequences of their risk-taking behaviors as mild also kept playing video games longer and thus delayed their bedtimes more [29]. This suggests, according to the dual-system model [14], that a lack of impulse control and higher risk-taking may lead to shorter sleep duration. Another study found that college students who were more impulsive also reported more dysfunctional thought control strategies (eg, worry), which in turn were related to more severe symptoms of insomnia [30]. Although this study gives important information about potential mechanisms explaining why lack of impulse control might exacerbate sleep problems, the cross-sectional design limits the ability to ascertain directionality. To conclude, there is some evidence supporting the idea that impulsive behavior might be a risk factor for the development of sleep problems, however, no study to our knowledge has explicitly investigated whether impulsive behavior predicts later sleep disturbances – and vice versa – over a longer period of time.
The aim of this study was to examine the bidirectional associations between sleep and impulsive behavior over three years in a large cohort of adolescents. We considered two indicators of sleep: sleep duration and symptoms of insomnia. In addition to testing the bidirectional associations between sleep and impulsive behavior, we also considered the potential moderating role of gender and age. Research on gender and age differences in the links between sleep and impulsivity is scarce. Thus, we included moderation by gender and age as an exploratory question.
2. Method
2.1. Design
We used a cross-lagged panel design with three measurement points over two years. The data are from the first three waves (2014–2016) of a five-year longitudinal study (2014–2018) of Swedish adolescents, the Three cities’ study [31]. Annual measurements followed students from the upper levels of secondary school through to high school.
2.2. Participants
Participants were 2767 adolescents attending one of 18 public schools across three towns of central Sweden. Retention rate was 91% from wave 1 to wave 2 and 72% from wave 1 to wave 3, N = 1982 participated at all three waves. Common reasons for drop-out were that students were not present at data collection or that they changed school. The adolescents included in this study had to be present at waves 1 and 2 or 3.
The mean age at wave 1 was 13.7 years (SD = 0.65, range 12–15 years); 43.3% (N = 1197) adolescents were ∼13 years old at the first year of data collection and 56.7% (N = 1566) were ∼14 years old. Distribution across gender was even: 47.6% (N = 1315) were girls, and 52.4% (N = 1449) were boys. Most adolescents were born in Sweden (88.5%) and lived with both parents (71.7%).
2.3. Procedures
Data collection took place once per year, during the spring term. Adolescents completed the paper and pen questionnaire in the classroom, during school hours. Trained test leaders administered the surveys allowing students 90 min to complete the questionnaires and a snack for each participant was distributed during data collection. In addition, each class received 300 Swedish crowns in recognition of participation. Before participation we received active consent from students and passive consent from parents. That is, parents received information about the study via mail and were invited to fill-out and send in a form if they did not want their child to participate in the study. We then used parents’ passive consent to increase participation rate and limit sampling bias [32], [33]. Moreover, students were informed onsite about confidentiality, that participation was voluntary and that they could choose to withdraw from the study at any time, and they actively filled out a consent form.
The retention rate in the first three waves of data used in this paper was 68%. Adolescents who did not speak Swedish, or reported other difficulties with understanding written language at the time of data collection were excluded from the study. The project was approved by the Regional Ethical Board in Uppsala, Sweden.
2.4. Measures
The survey contained a range of standardized measures as well as questions developed specifically for the study (see Ref. [31]); not all will be reported in this paper.
2.4.1. Sleep duration
Weekday sleep duration was estimated by calculating the interval between students' self-reported bed time (‘What time do you usually go to bed on school days?‘) and wake time (‘What time do you usually wake up on school days?‘), subtracting self-reported sleep onset latency (‘On school nights, after you go to bed, about how long does it take for you to fall asleep?‘). These items were drawn from the School Sleep Habits Survey [34], which has shown good validity when compared with actigraphic measures [35]. Cronbach's alpha was 0.83 for this study.
2.4.2. Symptoms of insomnia
Symptoms of insomnia were assessed through the Insomnia Severity Index (ISI) [36]. The scale includes seven items assessing the symptoms of insomnia (ie falling asleep, waking up during the night, or too early in the morning), overall satisfaction with sleep, interference with daily activities, and worry about the current sleep [36]. Answers range from 0 to 4 on a Likert scale with higher scores indicating more severe sleep problems. The time frame was changed from two weeks (in the original) to the last six months to match the rest of the survey. The scale has good psychometric properties, also when used in adolescent populations [37], [38]. Cronbach's alpha was 0.88 for this study.
2.4.3. Impulsive behavior (urgency)
The urgency subscale of the UPPS (Urgency, Premeditation, Perseverance, Sensation seeking) impulsive behavioral scale [39] was used to assess strong impulses, especially when experiencing negative feelings (eg, ‘When I am upset I often act without thinking’). The original subscale included 12 items but the first item was mistakenly excluded. Answers ranged from 0 to 3, with higher scores indicating more impulsivity (except for one reverse item: ‘I am always able to keep my feelings under control’). The UPPS impulsivity behavior scale has good psychometric properties [39]; Cronbach's alpha was 0.97.
2.5. Analyses
First, we looked at descriptive statistics and correlations among variables of interest (ie, sleep duration and quality and impulse control). Second, we tested a longitudinal cross-path analysis using the full imputation maximum likelihood (FIML) estimator in Mplus version 8 [40]. The model tested bidirectional association between sleep (ie insomnia and sleep duration) and impulsive behavior across three waves of data, and included both stability paths and concurrent correlations (not controlling for age and gender) (see Fig. 1, Fig. 2). As a second step, we tested equivalence across groups to control for potential gender and age differences; that is, testing the model separately for boys vs girls and age 13 vs 14 years. We used global fit indices to assess the fit of the model and for group comparisons. In particular, we used Chi-squared, Comparative Fit Index (CFI), Standardized Root Means Square Residual (SRMSR), Root Mean Square Error of Approximation (RMSEA), and Chi-squared difference for group comparisons [40].
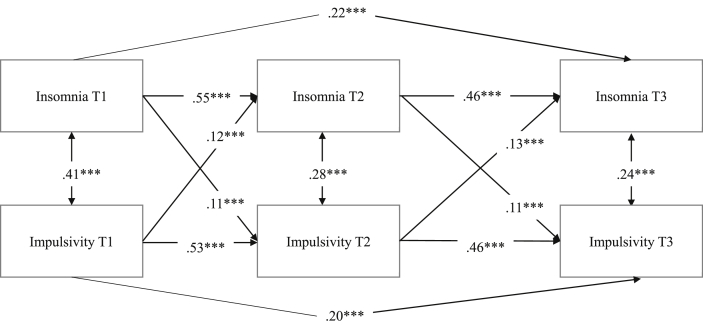
Fig. 1.
Cross-lagged panel model of insomnia and impulsive behavior over two years displaying regression weights and significance level (*p < 0.05, **p < 0.01, ***p < 0.001).
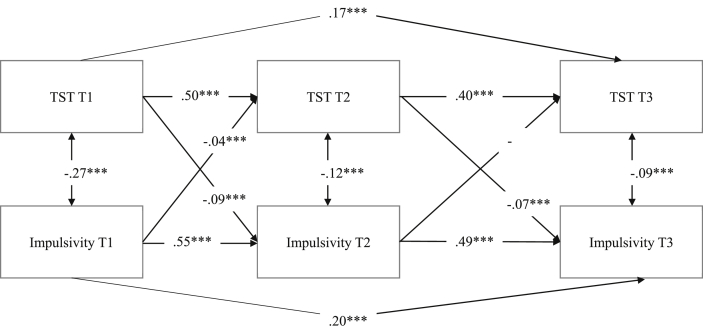
Fig. 2.
Cross-lagged panel model of total sleep time (TST) and impulsive behavior over two years displaying regression weights and significance level (*p < 0.05, **p < 0.01, ***p < 0.001).
2.6. Missing data
To handle missing data, we used the FIML estimator in Mplus [40]. Cases with complete missing data patterns was low (<5%). At Time 1 there were no significant differences between the adolescents who participated in all three waves and those who dropped out of the study regarding insomnia symptoms, sleep duration, gender, age, and country of birth. However, adolescents retained in all three waves were less likely to report impulsive behavior (OR = 0.69, p = 0.02). Yet, this model only explained 3.4% of the variance, thus we do not expect a significant impact on the results.
3. Results
3.1. Descriptive statistics
Table 1 shows the descriptive statistics of the total sample, boys and girls, and age ∼13 years and age ∼14 years, including means for total sleep time (TST), insomnia, and impulsive behavior at Times 1 through 3.
Table 1.
Descriptives of total sleep time (TST), insomnia & impulsivity in the sample.
| Main variables | All (N = 2767) | Boys (N = 1449) | Girls (N = 1315) | Age 13 (N = 1197) | Age 14 (N = 1566) |
|---|---|---|---|---|---|
| Sleep variables (Mean, SD) | |||||
| TST Time 1 | 7:59 (1:09) | 8:12 (1:03) | 7:44 (1:11) | 8:09 (1:02) | 7:51 (1:12) |
| TST Time 2 | 7:49 (1:11) | 7:59 (1:13) | 7:37 (1:06) | 7:56 (1:10) | 7:43 (1:11) |
| TST Time 3 | 7:38 (1:12) | 7:43 (1:12) | 7:32 (1:11) | 7:42 (1:14) | 7:35 (1:10) |
| Insomnia Time 1 | 5.52 (4.9) | 4.75 (4.43) | 6.38 (5.25) | 5.08 (4.67) | 5.85 (5.05) |
| Insomnia Time 2 | 5.87 (5.11) | 4.84 (4.47) | 6.99 (5.51) | 5.37 (4.75) | 6.26 (5.35) |
| Insomnia Time 3 | 6.41 (5.46) | 5.38 (5.96) | 7.60 (5.77) | 6.08 (5.39) | 6.74 (5.52) |
| Impulsivity (Mean, SD) | |||||
| Impulsive behavior Time 1 | 15.87 (5.77) | 15.26 (5.49) | 16.52 (5.99) | 15.37 (5.44) | 16.24 (5.98) |
| Impulsive behavior Time 2 | 15.99 (5.78) | 15.16 (5.29) | 16.91 (6.15) | 15.64 (5.68) | 16.27 (5.85) |
| Impulsive behavior Time 3 | 15.69 (5.96) | 14.81 (5.46) | 16.73 (6.34) | 15.63 (5.99) | 15.75 (5.93) |
3.2. Links between youth insomnia, TST and impulsive behavior
To answer how youth insomnia and impulsive behavior are related, we tested the cross-lagged panel design model presented in Fig. 1. Standardized estimates of all cross-paths that we tested in the model are presented in Table 1. The fit of this model was good, χ2 = 1.75, degrees of freedom (df) = 2, p = 0.42, CFI = 1.00, RMSEA = 0.01 (0.00–0.04), SRMR = 0.004. Youth insomnia and impulsive behavior had moderate stability over time (standardized autoregressive coefficients ranged from 0.46 to 0.55). All cross-paths in the model were significant. This suggests that youths who report sleep problems at Time 1 display more impulsive behaviors at Time 2 (b = 11, p = 0.000). It seems also that youths who report higher levels of impulsive behavior at Time 1 report more sleep problems at Time 2 (b = 0.12, p = 0.000). These effects were significant over two years which suggests that the links between insomnia and impulsive behavior are bidirectional in early through middle adolescence. The model explained 37% and 46% of the variance in insomnia symptoms at Time 2 and Time 3, respectively; and 34% and 43% of the variance in impulsive behavior at Time 2 and Time 3, respectively.
Next, we tested relations between youths' TST and impulsive behavior. The model fit was good, χ2 = 2.80, df = 2, p = 0.25, CFI = 1.00, RMSEA = 0.02 (0.00–0.04), SRMR = 0.005. For all cross-path estimates tested in the model, see Fig. 2. Youths’ TST and impulsive behavior shows moderate stability over time (standardized autoregressive coefficients ranged from 0.40 to 0.55). Results from this model show a significant negative bidirectional relationship between total hours of sleep and impulsive behavior over two years. Youths who reported fewer hours of sleep displayed more impulsive behavior at Time 2 (b = −0.09, p = 0.000), and youths who reported higher levels of impulsive behavior at Time 1 reported sleeping fewer hours per night at Time 2 (b = −0.04, p = 0.000). These effects were also significant between Time 2 and Time 3. The model explained 26% and 28% of the variance in sleep duration at Time 2 and Time 3, respectively; and 34% and 43% of the variance in impulsive behavior at Time 2 and Time 3, respectively. Thus, our findings suggest that youths who report sleeping fewer hours during the night report more impulsive behaviors and vice versa – reporting higher levels of impulsive behavior is linked with sleeping less.
Overall, our results show a consistent pattern where sleep problems, measured as insomnia and total hours of sleep per night, predict changes in impulsive behavior and, in turn, that impulsive behavior predicts changes in both indicators of sleep problems in adolescents.
3.3. Adolescent gender as a moderator
To examine whether adolescent gender moderated the links between sleep problems and impulsive behavior, we repeated the same set of analyses with gender-based group comparisons on all cross-lagged paths, in all directions. We compared the constrained model with the unconstrained models in which the structural paths were set free across adolescent gender. Equality constraints in multiple group analyses were compared using χ2 difference tests. The only significant difference we found was that impulsive behavior at Time 2 was a stronger predictor of insomnia problems at Time 3 in girls (b = −0.17, p < 0.001) as compared to boys (b = −0.08, p = 0.004), χ2(1) = 6.18, p = 0.014.
3.4. Adolescent age as a moderator
To examine whether age moderated the links between sleep problems and impulsive behavior, we carried out the same procedure as for gender. No significant differences were found.
4. Discussion
This study tested the theoretical assumption that youths who experience sleep problems are less able to resist impulses, and youths who show more impulsive behavior, in turn, report more sleep problems. While empirical research has shown some evidence that sleep problems affect impulse control, the bidirectional link has previously not been tested. The results showed that the links between sleep quantity and quality and impulsive behavior are bidirectional. Youths who experience sleep problems also experience increased difficulties with impulse control, and problems regulating impulses are also linked with increases in sleep problems, and these effects were systematic over three years. Moreover, there seem to be some gender differences. That is, impulsive behavior seems to have a larger impact on girls' insomnia as compared to boys’.
The bidirectional associations between sleep and impulsive behavior found in this study are in line with several experimental, neuroimaging studies and the theoretical assumption of a negative cycle of sleep and impulsive behaviors [1], [2], [3]. Yet, this is the first study to our knowledge to test this assumption with a rigorous cross-lagged design. By confirming the bidirectionality of this association, this study supports the importance of developing interventions to promote sleep health in adolescents but also the importance of tailoring such programs to adolescents' development. In fact, adolescents might not be able to change sleep behaviors if they can not control their impulses (eg, of using their phone before bed). Therefore, working to create an environment that supports good sleep habits is strongly recommended to be able to improve sleep behaviors. For example, adolescents whose parents set clear rules about bedtime sleep longer than peers who do not have set bedtimes [41]. Moreover, current sleep interventions solely targeting adolescents have shown limited success, whereas a recent intervention targeting smartphone use at bedtime showed promising results [42]. Another example is that postponing school start times have shown significant benefits [43]. These examples, together with knowledge of adolescents’ development and the current findings, suggest that structural changes that support heathy sleep might be a more effective way of promoting good sleep habits.
It is worth mentioning that although the bidirectional model was clear for both symptoms of insomnia and sleep duration, the bidirectional associations with impulsive behavior were stronger for insomnia. This might be explained by the more precise measure of sleep disturbance, which includes daytime impairment [36]. That is, aspects such as daytime sleepiness and fatigue rather than sleep length might be more directly linked to the inability to control impulses. In line with this idea, one study has suggested that sleepiness and chronotype rather than sleep duration are more closely associated with adolescents’ self-regulation and risk-health behaviors [44]. Nevertheless, interventions targeting both sleep duration and quality in at-risk adolescents have yielded positive effects on problem behaviors [45], [46]. Therefore, both sleep quality and quantity seem to be viable targets for interventions aimed at reducing risk behaviors.
Furthermore, the predictive association between impulsive behavior and insomnia was stronger for girls as compared to boys. This difference might be due to the measure of impulsive behaviors (ie, urgency) which refers to reactions to negative affect [39]. Negative affect in turn is generally more elevated in teenage girls than boys, and girls might also be more likely to respond with maladaptive strategies, such as repetitive negative thinking, when experiencing a negative mood state [47]. Repetitive negative thinking in turn has been found to be associated with sleep disturbances, and insomnia in particular [48]. Nevertheless, possible mechanisms behind this difference should be investigated further.
This study has both strengths and limitations. One limitation is the reliance on one subscale (urgency) to tap into impulsive behavior. However, previous research shows that urgency, in particular, is related to insomnia severity and other sleep disturbances [30]. Nevertheless, even though our results are in line with previous studies using broader measures of impulsive behavior, future studies should replicate our findings using a more comprehensive measure of impulsive behavior. Another limitation is the reliance on youths' self-reports of sleep quality and quantity. Given that there are objective means of attaining data on sleep (eg, actigraphs and electroencephalograms), our measures provide individual perceptions of sleeping behavior. Conversely, adolescents’ self-reports have been found to be reliable when compared to objective measures [34], [35]. Despite these limitations, this study has a number of strengths. First, we used data from a large, community-based sample. We included longitudinal data, which allowed us to systematically test for direction of effects between sleep disturbances and impulsive behavior. Hence, our design provides unique insight into the developmental processes at play in a large group of young people.
5. Conclusions
Sleep and impulsive behaviors influence one another bidirectionally during adolescence. Sleep interventions are warranted but need to take into account that it might be challenging for adolescents to control their impulses and they might therefore need additional support and strategies to allow themselves to go to sleep at bedtime. Therefore, programs to promote sleep health in adolescents should target contextual factors (eg, parents, peers, technological devices) to support changes in sleep behaviors.
Funding
This work was supported by the Swedish research agencies FORMAS, FORTE, Vinnova, and Vetenskapsrådet [grant number 2012-65].
Acknowledgements
This study was made possible by access to data from the Three City Study, a longitudinal research program at the department of Law, Psychology and Social work at Örebro University, Sweden and financed by the Swedish research agencies FORMAS, FORTE, Vinnova, and Vetenskapsrådet [grant number 2012-65].
Footnotes
Conflicts of interest
None.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleepx.2019.100009.
Conflicts of interest
The following is the supplementary data to this article:
References
- 1.Dahl R.E., Lewin D.S. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31(6):175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 2.Shochat T., Cohen-Zion M., Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014;18(1):75–87. doi: 10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Short M.A., Weber N. Sleep duration and risk-taking in adolescents: a systematic review and meta-analysis. Sleep Med Rev. 2018;41:185–196. doi: 10.1016/j.smrv.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Telzer E.H., Fuligni A.J., Lieberman M.D. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colrain I.M., Baker F.C. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21(1):5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maslowsky J., Ozer E.J. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014;54(6):691–697. doi: 10.1016/j.jadohealth.2013.10.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivertsen B., Harvey A.G., Pallesen S. Trajectories of sleep problems from childhood to adolescence: a population-based longitudinal study from Norway. J Sleep Res. 2017;26(1):55–63. doi: 10.1111/jsr.12443. [DOI] [PubMed] [Google Scholar]
- 8.Johnson E.O., Roth T., Schultz L. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–e256. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- 9.Gradisar M., Gardner G., Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12(2):110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Lovato N., Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev. 2014;18(6):521–529. doi: 10.1016/j.smrv.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Beebe D.W., Rose D., Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adolesc Health. 2010;47(5):523–525. doi: 10.1016/j.jadohealth.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewald J.F., Meijer A.M., Oort F.J. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14(3):179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Paiva T., Gaspar T., Matos M.G. Sleep deprivation in adolescents: correlations with health complaints and health-related quality of life. Sleep Med. 2015;16(4):521–527. doi: 10.1016/j.sleep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Shulman E.P., Smith A.R., Silva K. The dual systems model: review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross C.P., Copping L.T., Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137(1):97. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- 16.Cyders M.A. Impulsivity and the sexes: measurement and structural invariance of the UPPS-P impulsive behavior scale. Assessment. 2013;20:86–97. doi: 10.1177/1073191111428762. [DOI] [PubMed] [Google Scholar]
- 17.Casey B., Jones R.M., Somerville L.H. Braking and accelerating of the adolescent brain. J Res Adolesc. 2011;21(1):21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews-Hanna J.R., Seghete K.L.M., Claus E.D. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vink M., Zandbelt B.B., Gladwin T. Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum Brain Map. 2014;35(9):4415–4427. doi: 10.1002/hbm.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraven M., Baumeister R.F. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol Bull. 2000;126(2):247. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- 21.Palmer C.A., Alfano C.A. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. doi: 10.1016/j.smrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Figner B., Mackinlay R.J., Wilkening F. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J Exp Psychol Learn Mem Cogn. 2009;35(3):709. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- 23.Anderson C., Platten C.R. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217(2):463–466. doi: 10.1016/j.bbr.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Drummond S.P., Paulus M.P., Tapert S.F. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15(3):261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 25.Gruber R., Cassoff J., Frenette S. Impact of sleep extension and restriction on children's emotional lability and impulsivity. Pediatrics. 2012;130(5):e1155–e1161. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- 26.Baum K.T., Desai A., Field J. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiat. 2014;55(2):180–190. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker S.P., Epstein J.N., Tamm L. Shortened sleep duration causes sleepiness, inattention, and oppositionality in adolescents with attention-deficit/hyperactivity disorder: findings from a crossover sleep restriction/extension study. J Am Acad Child Adolesc Psychiatry. 2019;58(4):433–442. doi: 10.1016/j.jaac.2018.09.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troxel W.M., Hunter G., Scharf D. Say “GDNT”: frequency of adolescent texting at night. Sleep Health. 2015;1(4):300. doi: 10.1016/j.sleh.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds C.M., Gradisar M., Kar K. Adolescents who perceive fewer consequences of risk-taking choose to switch off games later at night. Acta Paediatr. 2015;104(5):e222–e227. doi: 10.1111/apa.12935. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt R.E., Gay P., Ghisletta P. Linking impulsivity to dysfunctional thought control and insomnia: a structural equation model. J Sleep Res. 2010;19:3–11. doi: 10.1111/j.1365-2869.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 31.Boersma K. 2019. Processes that buffer against youth mental health problems: a longitudinal-experimental approach.https://www.oru.se/trestadsstudienhttps://www.oru.se/trestadsstudien Retrieved from. [Google Scholar]
- 32.Pokorny S.B., Jason L.A., Schoeny M.E. Do participation rates change when active consent procedures replace passive consent. Eval Rev. 2001;25(5):567–580. doi: 10.1177/0193841X0102500504. [DOI] [PubMed] [Google Scholar]
- 33.Shaw T., Cross D., Thomas L.T. Bias in student survey findings from active parental consent procedures. Br Educ Res J. 2015;41(2):229–243. [Google Scholar]
- 34.Wolfson A.R., Carskadon M.A. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 35.Short M.A., Gradisar M., Lack L.C. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13(4):378–384. doi: 10.1016/j.sleep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Morin C.M. Guilford Press; 1993. Insomnia: psychological assessment and management. [Google Scholar]
- 37.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 38.Chung K.F., Kan K.K.-K., Yeung W.-F. Assessing insomnia in adolescents: comparison of insomnia severity index, Athens insomnia scale and sleep quality index. Sleep Med. 2011;12(5):463–470. doi: 10.1016/j.sleep.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside S.P., Lynam D.R. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Ind Diff. 2001;30(4):669–689. [Google Scholar]
- 40.Muthén L.K., Muthén B.O. Muthén & Muthén; Los Angeles, CA: 1998–2017. Mplus user's guide. [Google Scholar]
- 41.Bartel K., Gradisar M., Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015;21:72–85. doi: 10.1016/j.smrv.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Bartel K., Scheeren R., Gradisar M. Altering adolescents' pre-bedtime phone use to achieve better sleep health. Health Commun. 2019;34(4):456–462. doi: 10.1080/10410236.2017.1422099. [DOI] [PubMed] [Google Scholar]
- 43.Minges K.E., Redeker N.S. Delayed school start times and adolescent sleep: a systematic review of the experimental evidence. Sleep Med Rev. 2016;28:86–95. doi: 10.1016/j.smrv.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owens J.A., Dearth-Wesley T., Lewin D. Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics. 2016;138(6) doi: 10.1542/peds.2016-1406. [DOI] [PubMed] [Google Scholar]
- 45.Blake M.J., Snoep L., Raniti M. A cognitive-behavioral and mindfulness-based group sleep intervention improves behavior problems in at-risk adolescents by improving perceived sleep quality. Behav Res Ther. 2017;99:147–156. doi: 10.1016/j.brat.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Haynes P.L., Bootzin R.R., Smith L. Sleep and aggression in substance-abusing adolescents: results from an integrative behavioral sleep-treatment pilot program. Sleep. 2006;29(4):512–520. [PubMed] [Google Scholar]
- 47.Nolen-Hoeksema S. An interactive model for the emergence of gender differences in depression in adolescence. J Res Adolesc. 1994;4(4):519–534. [Google Scholar]
- 48.Hiller R.M., Johnston A., Dohnt H. Assessing cognitive processes related to insomnia: a review and measurement guide for Harvey's cognitive model for the maintenance of insomnia. Sleep Ned Rev. 2015;23:46–53. doi: 10.1016/j.smrv.2014.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.