Abstract
Delayed sleep–wake phase disorder (DSPD) is the most frequently occurring intrinsic circadian rhythm sleep–wake disorder, with the highest prevalence in adolescence. Melatonin is the first-choice drug treatment. However, to date melatonin (in a controlled-release formulation) is only authorised for the treatment of insomnia in children with autism or Smiths-Magenis syndrome. Concerns have been raised with respect to the safety and efficacy of melatonin for more general use in children, as melatonin has not undergone the formal safety testing required for a new drug, especially long-term safety in children. Melatonin is known to have profound effects on the reproductive systems of rodents, sheep and primates, as well as effects on the cardiovascular, immune and metabolic systems.
The objective of the present article was therefore to establish the efficacy and safety of exogenous melatonin for use in children with DSPD, based on in vitro, animal model and clinical studies by reviewing the relevant literature in the Medline database using PubMed.
Acute toxicity studies in rats and mice showed toxic effects only at extremely high melatonin doses (>400 mg/kg), some tens of thousands of times more than the recommended dose of 3–6 mg in a person weighing 70 kg. Longer-term administration of melatonin improved the general health and survival of ageing rats or mice. A full range of in vitro/in vivo genotoxicity tests consistently found no evidence that melatonin is genotoxic. Similarly long term administration of melatonin in rats or mice did not have carcinogenic effects, or negative effects on cardiovascular, endocrine and reproductive systems.
With regard to clinical studies, in 19 randomised controlled trials comprising 841 children and adolescents with DSPD, melatonin treatment (usually of 4 weeks duration) consistently improved sleep latency by 22–60 min, without any serious adverse effects. Similarly, 17 randomised controlled trials, comprising 1374 children and adolescents, supplementing melatonin for indications other than DSPD, reported no relevant adverse effects. In addition, 4 long-term safety studies (1.0–10.8 yr) supplementing exogenous melatonin found no substantial deviation of the development of children with respect to sleep quality, puberty development and mental health scores. Finally, post-marketing data for an immediate-release melatonin formulation (Bio-melatonin), used in the UK since 2008 as an unlicensed medicine for sleep disturbance in children, recorded no adverse events to date on sales of approximately 600,000 packs, equivalent to some 35 million individual 3 mg tablet doses (MHRA yellow card adverse event recording scheme).
In conclusion, evidence has been provided that melatonin is an efficacious and safe chronobiotic drug for the treatment of DSPD in children, provided that it is administered at the correct time (3–5 h before endogenous melatonin starts to rise in dim light (DLMO)), and in the correct (minimal effective) dose. As the status of circadian rhythmicity may change during long-time treatment, it is recommended to stop melatonin treatment at least once a year (preferably during the summer holidays).
Keywords: Melatonin, Children, Adolescents, Delayed sleep–wake phase disorder, DSPD, Safety
Highlights
-
•
Melatonin improves sleep onset without serious adverse effects in youths with DSPD. Change th text after the fourth bullet into: Melatonin is an efficacious and safe chronobiotic drug for the treatment of DSPD in youths.
-
•
Melatonin for indications other than DSPD, dose not cause relevant adverse effects.
-
•
Long term melatonin treatment does not impair sleep, puberty, and mental health.
-
•
Melatonin is an efficacious and safe chronobiotic drug for the treatment of DSPD in youths.
-
•
Melatonin should be administered at the correct time and in the minimal effective dose.
1. Introduction
Delayed sleep–wake phase disorder (DSPD) is the most frequently occurring intrinsic circadian rhythm sleep–wake disorder [1], with the highest prevalence in adolescence [2]. DSPD is characterized by difficulty in falling asleep and waking in the morning, while sleep duration and quality are usually normal DSPD can affect children both with and without associated mental or neurological problems. In DSPD the endogenous melatonin rhythm is delayed, and is no longer in alignment with the desired sleep time. This misalignment explains the difficulty falling asleep and waking in the morning, and may result in daytime sleepiness, poor school performance, anxiety, and behavioural problems [3].
There is convincing evidence for the short-term efficacy of non-medical chronobiological treatments for DSPD, including behavioural and light therapy. However, long-term treatment outcomes can be improved [4,5]. For those subjects for whom non-pharmacological treatments have been unsuccessful, melatonin is considered as first-choice drug treatment for DSPD [6].
Exogenous melatonin is a chronobiotic drug with some soporific and hypnotic properties [6,7]. It may shift circadian rhythms, including the sleep–wake cycle and is increasingly used to improve sleep in children and adolescents [6]. However, melatonin is not authorised for general use in children [8]; melatonin (in controlled-release form) is currently authorised for use in children with autism or Smiths-Magenis syndrome in European Community countries, but not for more common sleep disorders such as DSPD. Supplemental (exogenous) melatonin is available in both controlled-release and immediate-release formulations. For sleep induction, as is required for DSPD, immediate-release melatonin is considered to be the more effective formulation [9], and this is the melatonin form used in most clinical studies on DSPD. Food grade melatonin is available over-the-counter (OTC) in some countries. The manufacturing quality and bio-availability of melatonin differs in these unlicensed melatonin preparations [10]. Consequently there is a clinical need for an authorised immediate-release melatonin medicinal product.
Although there is a clear rationale for the use of supplementary melatonin in DSPD in children, concerns have been raised with respect to safety and efficacy. Melatonin has not undergone the formal safety testing expected for a new drug, especially long-term safety in children; it is known to have profound effects on the reproductive systems of rodents, sheep, and primates, as well as effects on the cardiovascular, immune, and metabolic systems. In addition, there is the potential for important interactions with drugs sometimes prescribed for children [[11], [12], [13]]. Furthermore, the efficacy of melatonin for insomnia has been questioned, although in part this can be ascribed to the heterogeneity of the sleep disorders studied, varying from largely unspecified insomnia to the better defined DSPD [14],as well as co-morbidities associated with the described sleep disorder [15,16].
The purpose of this paper is therefore to review evidence from the literature relating to safety and efficacy of exogenous melatonin for the treatment DSPD in children.
2. Methods
The published literature in the Medline database was searched via PubMed for relevant articles on the efficacy and safety of exogenous melatonin using in vitro, animal model and clinical studies, the latter specifically via randomised controlled trials in children.
3. Delayed sleep–wake phase disorder
DSPD is a circadian rhythm sleep disorder, first described by Weizmann et al., in 1981 [17], and characterized by habitual sleep–wake times that are delayed in comparison to conventional or socially acceptable sleep times [18]. When allowed to choose their preferred sleep–wake schedule, individuals with DSPD will exhibit normal sleep quality and duration for age [19], although with deviated timing.
DSPD is the most frequent circadian rhythm sleep disorder [20] and is particularly common among adolescents, with a prevalence of 7–16% [21,22], compared to a prevalence in adults of 0.13–0.17% [23,24].
Patients with DSPD complain of difficulty in falling sleep, and not obtaining sufficient sleep on school or work nights. Such individuals often have difficulty rising at a socially acceptable wake time. DSPD is associated with poor school adherence, lower school grades, smoking, alcohol usage, anxiety, and depression [[25], [26], [27], [28]].
Several co-morbidities are associated with DSPD, including attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) [6,[29], [30], [31], [32], [33]]. Co-morbidity and social features may mask characteristic symptoms of DSPD. Thus patients, especially children with DSPD and ADHD or ASD, may have less trouble waking in the morning than DSPD patients without these co-morbidities. Consequently chronic sleep onset insomnia can be the single mean characteristic. In children, sleep onset insomnia is sometimes considered as an issue for the caregiver and not necessarily for the child. However, in DSPD the sleep disturbance is associated with impairment of social, occupational or other areas of functioning [2] and consequently is an issue also for the child.
In DSPD endogenous circadian rhythms are not in accordance with socially conventional and desired times for sleep/wake. Several genetic, physiological and behavioural mechanisms have been suggested but there is sparse evidence for most of these [34]. An abnormally long intrinsic circadian period τ is crucial in the pathogenesis of DSPD [35]. A length polymorphism in the PER3 gene was reported to be linked to DSPD and extreme diurnal preference [36]. Up to 40% of those affected by DSPD may have a family history of this disorder, possibly associated with this polymorphism [37].
In mammals, the temporal organization of metabolism, physiology, and behaviour around 24 h is controlled by a network of multiple cellular clocks, synchronized via neuronal and hormonal signals by a master clock located in the suprachiasmatic nuclei (SCN), and peripheral clocks located throughout the body. The SCN clock is set to solar time by photic input pathways originating in the retina, while secondary circadian clocks in other brain areas and peripheral clocks can be reset by meal timing [38,39]. This explains why irregular meals are adversely associated with metabolic risk [40].
Alterations in entrainment of the circadian clock to synchronizing agents such as light and physical activity contribute to the delayed timing of sleep [25]. Individuals with DSPD may have an altered responsiveness to light [41], and are more sensitive to evening light [42]. Furthermore, alterations in the homeostatic regulation of sleep and inappropriate meal timing (chrononutrition) may also play a crucial role in the development and maintenance of DSPD [43,44].
Current treatments for DSPD include light therapy, behavioural, and environmental approaches [4,[45], [46], [47]]. There is convincing evidence for the short-term efficacy of these chronobiological treatments for DSPD. However, relapse of symptomology is common [4], and long-term treatment outcomes can be improved [4,5]. Therefore supplemental melatonin is often recommended [6].
3.1. Rationale for the use of exogenous (supplemental) melatonin to enhance sleep
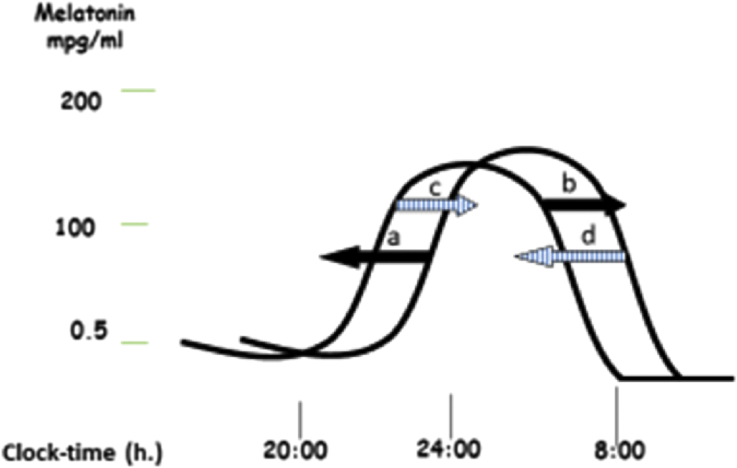
Exogenous (supplemental) melatonin influences sleep mainly by its interaction with endogenous melatonin. Therefore the influence of endogenous melatonin on sleep is summarized, followed by a discussion of the most powerful influencers on endogenous melatonin ie exogenous melatonin and bright light (summarised in Fig. 1).
Fig. 1.
Exogenous-melatonin-induced shifts and bright-light-induced shifts of the endogenous melatonin rhythm. The endogenous melatonin rhythm is advanced the most when exogenous melatonin is administered 5 h before the dim light melatonin onset (DLMO) (a); when administered 10 h after DLMO it is delayed the most (b). Bright light during the increasing phase of the melatonin curve delays the endogenous melatonin rhythm (c). Bright light during the decreasing phase of the melatonin curve advances the endogenous melatonin rhythm (d). As the natural sleep–wake rhythm is associated with the endogenous melatonin rhythm, exogenous melatonin- and bright light-induced shifts of the endogenous melatonin rhythm parallel sleep–wake rhythm shifts.
3.1.1. Relationship between endogenous melatonin and sleep
To achieve consolidated sleep (and wakefulness), two processes should interact and balance each other. The first includes a homeostatic sleep drive (process S), determined by recent sleep–wake history such that the longer you are awake, the more the homeostatic pressure to sleep. The second is the circadian rhythm (process C), which is largely independent of recent sleep and waking activity. It influences the timing, duration, and internal structure of sleep, and is regulated by a central endogenous circadian pacemaker (ECP) located in the suprachiasmatic nuclei (SCN) of the hypothalamus. The ECP regulates many biological functions, such as sleep, metabolism, and temperature. The ECP has a slightly-more-than-24-h period which is synchronized to geophysical time by regular exposure to light and darkness [48].
The ECP has a higher frequency of firing during the day compared to the night. In reaction to a decrease in firing of the ECP during the night, melatonin is secreted by the pineal gland. The timing of its secretion is influenced by several clock-genes (eg PER3, PER2, Clock) [49]. Furthermore, melatonin secretion is suppressed by light [50], particularly blue light [51]. Physiological melatonin levels begin to rise in the evening, peak during night-time and decrease early in the morning: they are low or absent during the daytime (Fig. 1).
Melatonin exerts its physiological action via MT1 and MT2 receptors, located particularly in the SCN, but also in other areas of the brain. Melatonin inhibits SCN neuronal firing via the MT1 receptors, and activation of MT2 receptors mediates melatonin's phase shifting effects, although a role of the MT1 receptor on phase shifting cannot be excluded [51,52]. Melatonin may also alter the functions of the GABA-A-benzodiazepine receptor complex. These effects, linked to the activation of GABAergic mechanisms in the SCN, are putative mechanisms by which melatonin mediates the circadian timing of the sleep–wake cycle [[53], [54], [55]].
The time at which melatonin starts to rise in dim light, the so-called Dim Light Melatonin Onset (DLMO) is the characteristic of the 24-h melatonin curve which is most relevant for clinical practice [56]. When endogenous melatonin starts to rise (usually late in the evening), the possibility to fall asleep increases [57]. When other circumstances are also favourable (eg enough rest, lying in bed, absence of mental activities, etc.), one may fall asleep, and wake up rested some 8–9 h later. When endogenous melatonin starts to rise later than at a conventional time, sleep-onset and sleep offset are delayed. Patients then suffer from sleep-onset insomnia and have difficulty waking up in the morning at a conventional time. Thus, the discrepancy between biological and social clocks, so-called “social jetlag”, occurs [58,59]. When melatonin starts to rise earlier than at a conventional time, the sleep–wake rhythm is advanced. Thus patients fall asleep too early in the evening and wake up too early in the morning.
The 24-h endogenous melatonin rhythm is associated with the 24-h temperature rhythm. This association is in an opposing direction: when melatonin increases, temperature decreases and vice versa. Circadian variations in temperature may act as an input signal to sleep-regulating systems [60].
The slightly-more-than-24-h circadian rhythm is corrected to a 24-h rhythm by bright light in the morning. Consequently the sleep–wake rhythm in most people follows a 24-h rhythm. Failure of bright light exposure in the morning results in an automatically occurring delay of the sleep–wake rhythm. This natural synchroniser of the endogenous melatonin rhythm also explains why people adapt more easily to changing time zones after westward travel rather than after eastward travel. Thus eastward travel is paralleled by automatically occurring delayed sleep–wake rhythm, which needs to be advanced. Bright light in the morning, especially during the phase when endogenous melatonin is decreasing, or exogenous melatonin, administered 5 h before DLMO (as mentioned in Fig. 1, to be explained later in this review) will help to advance the endogenous melatonin rhythm and the sleep–wake rhythm that is associated with it [61].
The natural tendency to delayed sleep–wake rhythms seems to be stronger during adolescence than in other phases of life [58], mostly due to an age related physiological process. Additional to this natural tendency, adolescents tend to use electronic devices more frequently than adults for social media during the rising phase of the melatonin curve, which might enhance the delay of the sleep–wake rhythm (Fig. 1). Due to these two factors, advancing the delayed sleep–wake rhythms of adolescents requires much more effort than in adults. This might be a major reason for the increased prevalence of DSPD in adolescents when compared to adults, although new technologies in cell phones and other electronic devices reduce this exposure in blue light, and eventually only the evolutionary difference between adolescents and adults will remain.
3.2. Influence of exogenous melatonin and bright light on endogenous melatonin and sleep
Exogenous melatonin may shift the endogenous melatonin rhythm, and with it the associated circadian rhythms, including the sleep–wake rhythm. The direction of the shift depends upon the time at which exogenous melatonin is administered. The melatonin (and the sleep–wake) rhythm is advanced most when in adults exogenous melatonin is administered 5 h before DLMO. Consequently it is an effective treatment for circadian rhythm sleep disorders, especially DSPD [62]. In clinical practice, melatonin is administered for (adolescent) DSPD patients not earlier than 19:00 h, otherwise the hypnotic effects of melatonin induce sleepiness too early in the evening. Exogenous melatonin delays endogenous melatonin rhythm (and the sleep–wake rhythm) most when administered 10 h after DLMO [63]. Consequently it can be used to delay sleep–wake rhythm in advanced sleep phase disorder (ASPD). Melatonin should then be taken somewhere in the middle of the night. However, in clinical practice ASPD is preferably treated with bright light early in the evening, during the rising phase of melatonin production.
Several pharmacopeias, and also the European Food Safety Authority, as well as all summaries of product characteristics (SPCs) of authorized medicinal products advise to take melatonin at a time (eg half an hour) before (desired) bed time [64]. However, as discussed above this is incorrect; the correct time to administer melatonin is related to the DLMO and not the desired bedtime. This was confirmed in children with chronic sleep-onset insomnia and late DLMO, showing a significant correlation between DLMO-determined time of administration and treatment effect on DLMO and sleep-onset time [65]. As the DLMO cannot be estimated in patients with sleep disorders [66], DLMO has to be measured. Fortunately, this can easily be done by collecting saliva at the patients home [48]. When melatonin administration does not have the desired effect within 1–2 weeks, administration time and/or dose should than be modified, as described below.
Not only the administration time, but also the dosage, is important for correct melatonin treatment. When the dose of melatonin administered in the evening is too high, the next day not all exogenous melatonin is metabolised, and melatonin remains present in the body. When the following evening melatonin is administered again in a too high dose, the melatonin in the body will increase again (accumulation). This spill-over will cover up the endogenous melatonin rhythm and the exogenous melatonin loses its efficacy. Accumulation may occur in “normal” doses when melatonin is metabolised slowly [67]. This slow melatonin metabolisation is associated with polymorphisms of the CYP1A2 gene, which occurs in 5–10% of the population, and possibly more in patients with autism spectrum disorder [68]. In that case the melatonin dose should be lowered, eg to 0.1–0.3 mg. For optimal treatment effects melatonin should be timed and dosed individually. Consequently melatonin treatment is an example of personalized/precision sleep medicine [66].
As noted above, light also may shift endogenous melatonin rhythm. Therefore light therapy is also recommended for the treatment of circadian rhythm sleep disorders [62], possibly in combination with melatonin treatment [69]. However, there is not enough evidence for the efficacy of the former [70]. A study comparing bright light therapy with melatonin treatment in children with DSPD showed only slight effects of bright light, while the effects of melatonin were much stronger [71].
4. Pharmacokinetics of melatonin
Melatonin is present in multiple tissues within the body. Due to its small molecular size and lipophilic nature, melatonin moves rapidly across cell membranes. Most circulatory melatonin is reversibly bound to albumin, is distributed to all tissues, and can cross the blood–brain barrier. Melatonin is principally metabolised in the liver; the elimination half-life is 35–45 min in extensive metabolizers [72], but might increase to 6.6 h in poor metabolizers [68]. The level of endogenous melatonin production is fixed after age 1 yr, probably because the pineal gland does not appear to grow, in contrast to the pituitary gland that doubles in size. This explains why endogenous melatonin is high in early childhood, and decreases in relation to body size [73]. Exogenous melatonin is efficiently absorbed from the digestive tract, but the bioavailability is low (ie <15%) due to first pass elimination [74]. This first pass effect can be by-passed by oro-mucosal (buccal) administration. The rate of clearance in children is greater than in adults, because in children enzymatic activity of CYP1A2 (the principal metabolizer of melatonin) in the liver is increased [75]. Children require a relative-to-bodyweight higher dose to induce sleep than adults. Most clinical studies in children with DSPD have been performed with immediate-release melatonin preparations.
In order to mimic the endogenous melatonin profile, a controlled-release melatonin preparation was developed. A paper by Chua et al. published in 2016 in the journal Pharmaceutics recommended the use of divided or crushed Circadin tablets (as a licensed product) where an immediate release melatonin was required, in preference to a manufactured immediate release tablet (unlicensed in the UK) [76]. However what the authors had not stated was that this represents off-license use of a licensed medicine, promotion of which is illegal [77]. Clinical studies to compare the efficacy of immediate- and controlled-release melatonin preparations have not yet been published [78].
5. Melatonin supplement formulation
Supplemental (exogenous) melatonin is available in three different formulations, immediate-release orally and oro-mucosally, and oral controlled-release. Immediate-release oral melatonin is worldwide the most frequently used formulation, often as an over-the-counter (OTC) product. There are no melatonin-based medicinal products authorised for use in DSPD, or for generalised use in children, but medicinal products authorised for adults with jetlag are available in some EU countries. The active content of unlicensed OTC melatonin products, and potential contaminants, can vary widely [10,79]. For sleep induction (as is required for DSPD), immediate-release melatonin is considered more effective, whilst controlled-release melatonin is considered to be more effective for sleep maintenance. Controlled-release melatonin was developed to mimic the nocturnal release of endogenous melatonin, for example for the treatment of certain types of insomnia in the elderly with pineal gland deficiency [79,80]. However, since there is no strong evidence that the amount of melatonin produced at night directly influences sleep quality [45], there seems to be no reason to prefer controlled-release above immediate-release formulations in children. Immediate-release formulations typically consist of the active melatonin dose dispersed in an inactive (usually cellulose-based) matrix. Taking Bio-Melatonin as an example, complete tablet disintegration occurs within 15 min in the pH range 1.2–6 [77], well within the ICH (International Conference on Harmonisation) guideline requirements for immediate-release tablets (80% tablet dissolution at 30 min). In the case of controlled-release formulations, the matrix serves to control the release of the melatonin activity over a more sustained time period. It is of note that controlled-release formulations may release up to 25% of the total dosage on an immediate basis [81], so there is a degree of overlap between the formulations.
In addition to controlled-release formulations of melatonin, melatoninergic drugs with a longer half-life have been developed, such as ramelteon, tasimelteon, and agomelatine, for (seasonal) depression. With all these medicinal products, improvements of sleep are statistically demonstrable, but clinically relevant effects remain limited [82].
6. Clinical efficacy studies with melatonin in children
6.1. Delayed sleep phase disorder
A total of 19 randomised, double blind, placebo-controlled trials assessed the efficacy of melatonin in more than 800 children and adolescents with DSPD phenotypes [77]. The most frequently used dosage was 3–6 mg/day. Eighteen studies used an immediate-release melatonin formulation, and one study used a mixed immediate-release/controlled-release formulation. Time of administration varied between approximately 30 min prior to bedtime and 2–3 h before DLMO. Most studies reported an improvement in sleep latency of at least 30 min. A meta-analysis of 7 randomized controlled trials assessing efficacy and safety of melatonin in 387 children and adolescents concluded that melatonin advanced mean sleep-onset time by 37 min and DLMO by 49 min [83]. Sleep was measured using sleep-dairies in all studies, and actigraphy in 5 studies. In four of the latter studies [14,[84], [85], [86]], sleep-times before and after melatonin treatment were described, making comparison with published normal values possible [87]. Mean sleep latency decreased significantly from 52–63 min to 27–36 min (normal value: 19 min), and mean sleep-onset times in from 21:40–22:45 h to 21:00–21:42 h.
6.1.1. Melatonin dose
Melatonin does not show a dose–response relationship within a dose range of 0.05–0.15 mg/kg with regard to sleep-onset time, sleep-onset latency and DLMO [65]. This suggests that melatonin activates a “switch” in the brain, resulting in a shift of circadian rhythmicity. For clinical use this means that it is pointless to increase the dose further, when melatonin has been effective. Probably most important for efficacy is the time of administration (TOA) in relation to the DLMO, and in extensive metabolizers the highest dose might seem more effective when this interval of TOA and DLMO is increased (ie the dose is taken earlier, or the DLMO is severely delayed), while a lower dose might be as efficacious when the interval is shorter [65].
6.1.2. Duration of melatonin treatment
In most placebo-controlled studies the duration of melatonin treatment was 4 weeks. Some studies reported results of treatment continuous up to 10.8 years, showing that after 3.1 years 20% of the children successfully stopped melatonin treatment [88,89], and after 10.8 years this figure increased to 75%. The sleep disturbance might simply be better tolerated by the individual or by the carers as the person becomes older, enabling the melatonin to be “successfully stopped”.
The differences in characteristics of the children who were able to stop, and who were unable to stop melatonin supplementation are unknown. To establish if treatment is still necessary it is advisable to stop melatonin treatment every year, preferably for a few weeks during the summer holidays; stopping treatment then usually influences quality of life as little as possible.
6.2. Other disorders
The efficacy of melatonin has been studied in randomised controlled trials of children in situations other than DSPD. These include studies on migraine [90], Dravet syndrome [91], atopic dermatitis [92], medication induced weight gain in bipolar disorder [93], and as sedative premedication for surgical treatments [[94], [95], [96], [97]],anaesthesia induction [98], neonatal analgesia [99], postoperative anxiety [100,101], blood withdrawal anxiety [100], EEG premedication [[102], [103], [104]] and MRI premedication [105]. The results from these studies suggest that melatonin can be beneficial in the management of these disorders, or during these interventions.
6.3. Studies with prolonged-release melatonin in children
Three randomised controlled studies of controlled-release melatonin have been performed, comprising 220 children and adolescents age 2–17.5 years, all of whom had autism spectrum disorder or neurogenetic disorders [[106], [107], [108]]. Controlled-release melatonin improved sleep and caregivers' quality of life. Except for somnolence relevant adverse effects did not occur [108]. As studies comparing controlled-release melatonin with immediate-release melatonin in children have not yet been performed [78], the question remains unresolved as to which of these treatments is the best for children with circadian rhythm sleep disorders.
7. Safety of melatonin administration
To assess the safety of melatonin we reviewed the results of in vitro, animal, and human studies.
7.1. Animal and in vitro studies
Toxicity studies are summarized in Table 1. Single and repeated dose toxicity studies in rats and mice with melatonin given orally, intraperitoneally (i.p.), subcutaneously (s.c.) and intravenously (i.v.) at different doses only showed toxic effects at extremely high melatonin doses (>400 mg/kg). The LD50 values of i.p., s.c. and i.v. administration of melatonin were similar for mice and rats, but the LD50 for oral administration was lower in mice (1250 mg/kg) than rats (3200 mg/kg) [109]. For comparison, the daily recommended dose of 3–6 mg in a person weighing 70 kg equates to a value of 0.04–0.08 mg/kg, some tens of thousands of times less than the melatonin dose causing death in 50% of rats or mice.
Table 1.
Summary of toxicity studies with melatonin. SD: single Dose. LT: long-term administration. GT: genotoxicity. CG: carcinogenicity. O: other toxicity studies. i.p.: intraperitoneally, s.c. subcutaneously, i.v. intravenously. dw: drinking water.
| Study type | Animal | Route | Duration | Results | Reference |
|---|---|---|---|---|---|
| SD | Rats in vivo | Orally, i.p.,s.c., i.v. | Once-only | At doses >400 mg/kg: vasodilatation, piloerection, ptosis, impairment of righting reflex, lack of motor activity, decrease in body temperature and respiratory problems preceding death | [109] |
| LT | Aged rats and mice in vivo | 10 mg/L in dw | 16 months | Improved health and survival of aged rats and mice | [111,112] |
| LT | Diabetic mice and hypercholestaemia-susceptible rats in vivo | s.c or in dw | Enhanced survival | [114,115] | |
| GT | In vitro non-mammalian cell system | No mutagenicity in bacterial strains. | [113,[116], [117], [118]] | ||
| GT | In vitro mammalian cell system | No chromosome aberrations and no clastogenic activity. Protective anti-clastogenic activity. Nop DNA strand breaks. | [113,117,119] | ||
| GT | In vivo cell system | 5 mg/kg s.c. | Not mutagenic in mouse bone marrow cells; reduced chromosome aberration rates. Not mutagenic in mice sperm head anomaly test; reduced sperm head anomaly rates. |
[120] | |
| GT | In vivo cell system | 10 mg/kg i.p. | No adverse effects on rat peripheral blood micronucleus test. | [121] | |
| GT | In vivo study in rats or mice | 4–10 mg/kg i.p. | Protective effects against genotoxic action of potassium dichromate, cobalt, ethanol, paraquat | [[122], [123], [124], [125]] | |
| GT | In vitro study using human lymphocytes | 0.2 mM | Anti-genotoxic effect on mercuric chloride and gossypol | [110,126] | |
| CG | Transgenic mice at increased risk for prostate adenocarcinoma | 10–20 mcg/L in dw | 18 weeks | Strong prostate cancer inhibitory effect | [127] |
| CG | Transgenic mice susceptible for mammary tumours | 50–200 mcg/kg per day via gavage | 30 weeks | Reduced incidence and growth rate of mammary tumours | [128,144] |
| CG | Rats, mice susceptible for breast cancer | 10–20 mcg/L in dw | >1 yr | No induction of uterine tumours, lower incidence of mammary tumours. | [129,130] |
Long-term administration of melatonin [110] in drinking water (10 mg/L, resulting in a dose of approximately 1 mg/kg/day) improved the general health and survival of aged rats [111] or aged mice [112,113], diabetic mice [114] and hypercholesterolaemia-susceptible rats [115].
A full range of genotoxicity tests, including in vitro non-mammalian [113,[116], [117], [118]] and in vitro mammalian cell systems [113,117,119], and in vivo mammalian system [120,121] tests consistently found no evidence that melatonin is genotoxic (neither mutagenic or clastogenic). Furthermore, several studies in various in vitro/in vivo systems have reported the anti-genotoxic action of melatonin [[122], [123], [124], [125], [126]], usually ascribed to its antioxidant activity.
Medium term (18–30 week) and long term (>one year) studies showed that melatonin had a strong prostate cancer inhibitory effect [127], and decreased breast cancer tumour incidence and growth rate [[128], [129], [130]],in transgenic mice lines susceptible to breast cancer. Furthermore, melatonin did not induce uterine tumours in rats [131], and resulted in a lower incidence of mammary tumours in mice. The anti-cancer action of melatonin is thought to involve inhibition of cancer initiation, progression and metastasis [132], and results via a number of mechanisms, including antioxidant action, regulation of oestrogen metabolism, inhibition of telomerase activity, inhibition of metastasis, anti-angiogenesis, and activation of the immune system [133].
In diurnal primates, an oral melatonin dose of 0.005 mg/kg administered 2 h before lights-off time promoted significantly earlier sleep-onset. Long-term melatonin administration did not result in development of tolerance or sensitisation to melatonin effects on sleep [134].
Intravenous administration of melatonin (10 mg/kg) had no effect on blood pressure in the cat, and no effect on heart function in the dog [135]. Intraperitoneal administration of melatonin (8 mg/kg) had no effect on neurological behaviour in mice [136].
Rodent studies indicate that melatonin has a cardioprotective action in myocardial infarction. In a mouse model, melatonin pre-treatment (0.150 mg/kg i.p.) significantly reduced the infarct size [137]. Similar results were obtained in rats, with the cardioprotective effect thought to result from the free radical scavenging capacity of melatonin [138].
No treatment-related effects were found for the endocrine system, including serum concentrations of 17 beta-oestradiol, progesterone, prolactin, luteinising hormone, and the glandular area of mammary tissue as measured on day 20 of gestation, when doses of melatonin in the range 1–200 mg/kg/d by gavage were administered to groups of female rats on days 6–19 of gestation, while aversion to treatment (at doses> 100 mg/kg/d) and reduced maternal weight gain (at doses >150 mg/kg/d) were observed. There was no maternal morbidity/mortality, and foetuses showed normal development [139]. Oral administration of melatonin (4 mg/L in drinking water, ∼0.4 mg/kg/day) for 3 months in middle aged rats resulted in decreases in body weight, intra-abdominal adiposity, plasma leptin, and plasma insulin levels, whilst locomotor activity was restored to more youthful levels. Intraperitoneal administration of melatonin (4 mg/day for 4 months; the authors did not specify the weight of rats used) resulted in beneficial effects on blood cholesterol levels in rats genetically predisposed to development of hypercholesterolaemia [115].
Melatonin receptors are located on a wide range of cells throughout the body, not just the SCN [140]. Some of these may play a role on glucose metabolism [141]. Acute melatonin administration in humans impairs glucose tolerance in the morning and evening [142].
Since both melatonin and benzodiazepines bind to GABA receptors, there is potential for their interaction. Murine data obtained in vitro [143] and in vivo [136] support this concept, although it should be noted that very high doses (260 mg/kg) of melatonin were used in these studies.
7.1.1. Animal studies on reproduction
The results of animal and in vitro studies as to the influence of melatonin on reproduction are summarized in Table 2. No adverse effects on fertilisation and early embryonic development were identified. Interpretation of such non-clinical data in relation to clinical use is complicated by the fact that species for which most data are available (rats, mice) are seasonal breeders ie a shorter photoperiod during winter suppresses sexual development/reproduction, whilst a longer photoperiod in spring suppresses melatonin synthesis and sexual development/reproduction begins. Since sexual maturation and the reproductive cycle in humans are not dependent on seasonal photoperiod, it is unlikely that melatonin plays a significant role in decreasing the development of sexual maturity [144].
Table 2.
Summary of reproductive and developmental toxicity studies with melatonin. s.c.: subcutaneous. dw: drinking water.
| Dose | Period of administration | Results | Reference | |
|---|---|---|---|---|
| Male Wistar rats | 110 mcg s.c. Daily | Age 20–45 days | Reduced pituitary GnRH receptor content at 70 days of age | [150] |
| Pre-pubertal female Sprague–Dawley rats | 110 mcg s.c. Daily | Age 20–70 days | Normal sexual maturation | [151] |
| Female Holzman rats | 10 mcg/L in dw | Age 10–380 days | Delayed vaginal opening, no effect on oestrus cycle | [131] |
| Adult rats | 4 mg/l in dw | 12 weeks | No adverse effect on sexual behaviour | [152] |
| Sexually active male Wistar rats | 10–100 mcg/kg intraperitoneally | Once before mating | No adverse effect on sexual behaviour | [153] |
| Embryonic in vitro studies in rat, mouse, and pig | 10(-5) M to 10(-13) M for 48 or 72 h | No adverse effect on in vitro fertilisation and early embryonic development | [139,154,155] | |
| Pregnant Sprague–Dawley rats | 200 mcg/kg gavage | Gestational days 6–19 | No toxic effect on embryo-foetal development | [139] |
| Pregnant rats | 300 mcg/rat s.c. | Gestational days 8–21 | No effect on litter size, live young birth weights and incidence of stillbirths | [155] |
Several studies suggest that melatonin might improve progesterone function in human granulosa-lutein cells [145],follicular cells [146] and corpus luteum [147].Therefore melatonin has been suggested to be a relevant medication for improving ovarian and luteal function and in the early stages of pregnancy, opening new opportunities for the management of several ovarian-luteal and pregnancy diseases [148].
Melatonin doses are 10–100 fold higher than the level provided by a 3–6 mg dose in man. It should be noted that adults produce 20–40 mcg melatonin per night [149]. When a 15 kg child is administered 6 mg of melatonin, this is 150 times more than an adult produces each night.
7.2. Clinical studies in children
In the 19 randomised trials of exogenous melatonin for children with DSPD (n = 841) [77] no serious adverse effects were reported, irrespective of patient category or dose up to 10 mg/day; studies were typically of 4 weeks' duration, although some studies extended to 3 months. The safety of melatonin for children with DSPD-like features was confirmed in 4 meta-analyses [83,[156], [157], [158]]; in two of these analyses patients with neurodevelopmental disorders were included.
The long-term safety of melatonin supplementation in children was determined in 3 studies. One study included 44 children with neurodevelopmental disorders and treatment-resistant circadian rhythm sleep disorders [159]; the second comprised 94 children with ADHD and DSPD [160], and third study 51 children with DSPD [88]. The mean follow up intervals were 3.8, 3.7 and 3.1 years respectively. Participants of the latter study were additionally followed up to 10.8 years [89] without any substantial deviation of the development of children with respect to sleep quality, puberty development and mental health scores. In addition, the fourth study, including 95 children and adolescents with autism spectrum disorder (ASD) and neurogenetic disorders (NGD) with/without attention-deficit/hyperactivity disorder comorbidity, long-term treatment with 5 or 10 mg controlled-release melatonin over 39–52 weeks was both safe and efficacious [107].
Supplemental melatonin has been widely used in children for surgical premedication, and prior to EEG or magnetic resonance procedures. Furthermore melatonin is used for migraine prophylaxis, Dravet syndrome, atopic dermatitis, and to treat olanzapine-related weight gain in bipolar disorder. Safety outcomes of these 17 randomised trials including 1374 children are summarised in Table 3. Since an immediate-release melatonin formulation is required for such applications, and no commercially available immediate-release melatonin products licensed for use in children are available, the use of unlicensed products is necessitated; these can vary in quality from food grade supplements to off-license use of products licensed within the EC such as Bio-Melatonin.
Table 3.
Summary of safety outcomes from randomised controlled studies of melatonin for indications other than DSPD in children.
| Study | Indication | Number of subjects (n)/age range | Melatonin dose/formulation (as single dose unless otherwise indicated) | Reported adverse effects (vs control) |
|---|---|---|---|---|
| Fallah et al. [90] | Migraine prophylaxis | n = 80/5–15 yrs | 0.3 mg/kg/day for 3 months | Daytime somnolence in 3 children |
| Myers et al. [91] | Dravet syndrome | n = 13/2–50 yrs | 6 mg/day for 2 weeks | No adverse effects |
| Ardakani et al. [92] | Atopic dermatitis | n = 70/6–12 yrs | 6 mg/day for 6 weeks | No adverse effects |
| Impellizzeri et al. [162] | Surgical premedication | n = 80/9–11 yrs | 0.5 mg/kg to 20 mg max | No adverse effects |
| Mostafavi et al. [93] | Weight gain in bipolar disorder | n = 48/11–17 yrs | 3 mg/day for 12 weeks | No adverse effects |
| Gitto et al. [94] | Surgical premedication | n = 92/5–14 yrs | 0.5 mg/kg/day | No adverse effects |
| Marseglia et al. [100] | Blood withdrawal anxiety | n = 60/1–14 yrs | 0.5 mg/kg/day to 5 mg max | No adverse effects |
| Fallah et al. [102] | EEG premedication | n = 60/1–8 yrs | 0.3 mg/kg/day | No adverse effects |
| Almenrader et al. [98] | Anaesthesia induction | n = 87/12–71 yrs | 0.3 mg/kg/day | No adverse effects |
| Gitto et al. [99] | Neonatal analgesia | n = 60/5–14 yrs | 0.5 mg/kg/day | No adverse effects |
| Sander et al. [103] | EEG premedication | n = 50/1–18 yrs | 3 mg < 15 kg 6 mg > 15 kg |
No adverse effects |
| Ozcengiz et al. [101] | Post-operative anxiety | n = 100/3–9 yrs | 0.1 mg/kg/day | No adverse effects |
| Kain et al. [95] | Surgical premedication | 148/2–8 yrs | 0.05–0.4 mg/kg/day | No adverse effects |
| Isik et al. [96] | Surgical premedication | n = 60/508 yrs | 3 mg/day | No adverse effects |
| Sury [105] | Sedation for magnetic resonance imaging | n = 98/0.3–4 yrs | 3–6 mg/day | No adverse effects |
| Samarkandi et al. [97] | Surgical premedication | n = 105/2–5 yrs | 0.1–0.5 mg/kg/day | No adverse effects |
| Wassmer et al. [104] | EEG sleep study | n = 163/1–16 yrs | 2–10 mg/day | No adverse effects |
Melatonin influences the human gonadotrophin-releasing hormone pulse generator, suggesting that melatonin could be used as contraceptive [161]. However, we could find no published studies of contraceptive effects in humans.
7.3. Adverse events
Severe adverse events associated with oral melatonin are scarce [[163], [164], [165]]. Mild generalized epilepsy was mentioned in two studies [84,166]. However, melatonin also has an anticonvulsant action as shown by animal and human studies [167]. Nevertheless, a Cochrane review could not draw any conclusion about the role of melatonin in reducing seizure frequency or improving quality of life in people with epilepsy [168,169]. Hypothermia was reported in a child with autism after melatonin ingestion, suggested to be an extreme variant of the normal physiologic action of melatonin on decreasing body temperature [169]. In one case, melatonin was suspected to be involved in the sudden death of a twin infant [170]. A critical systematic review of clinical evidence for adverse events associated with oral administration concluded that melatonin supplementation in humans has a generally favourable safety profile with some exceptions, relating to fatigue, mood or psychomotor and neurocognitive performance. These events were generally minor, short-lived and easily managed, eg by dosing in accordance with natural circadian rhythms [163]. Also in the authors' clinical experience, sometimes mentioned vivid dreams and frequent awakenings at night are usually short lived (clinical experience, MS). Sleep maintenance problems may point at a too high dose of melatonin (clinical experience, MS).
Finally, post-marketing data for an immediate-release melatonin formulation (Bio-Melatonin), used in the UK since 2008 as an unlicensed medicine for sleep disturbance in children, recorded no adverse events to date on sales of approximately 600,000 packs, equivalent to some 35 million individual 3 mg tablet doses (MHRA yellow card adverse event reporting scheme, and personal communication, DM).
7.4. Overall conclusion on safety
Melatonin has low acute toxicity, with LD50 values some 4 orders of magnitude greater than melatonin doses recommended in humans. Long-term administration of melatonin in rodents resulted in improved general health, and survival of aged animals. Melatonin is not genotoxic or carcinogenic. Sexual maturation in young rats was somewhat delayed, but not prevented following melatonin administration in doses 100-fold greater than the proposed dose in humans. Interpreting non-clinical data in relation to clinical use is complicated by the fact that species for which most data are available (rats, mice) are seasonal breeders ie a shorter photoperiod during winter suppresses sexual development/reproduction, whilst a longer photoperiod in spring suppresses melatonin synthesis and sexual development/reproduction begins. In animals with a reproduction cycle of once per year, this phenomenon is even more pronounced. For sheep, melatonin implant injections are authorized in Europe to stimulate twice yearly breeding, overruling their seasonality. As humans are non-seasonal breeders, this is not applicable in man. Thus caution should be exercised in the extrapolation of non-clinical or veterinary data to man.
No adverse effects of melatonin on early embryonic development in rodents were detected in vitro or in vivo. Similarly administration of melatonin to pregnant rats had no toxic effect on embryo-foetal development, and the No Observed Adverse Effect Level value established of 200 mg/kg/day is approximately one thousand fold greater the equivalent recommended dose in man. Limited indications of mild maternal toxicity were obtained in pregnant mice or rats treated with very high doses of melatonin-the maternal Lowest Observed Adverse Effect value was 200 mg/kg/day.
Melatonin administration in the morning decreased glucose tolerance primarily by decreasing insulin release, and in the evening, by decreasing insulin sensitivity. The clinical significance for patients with impaired glucose tolerance of this feature of chrononutrition [171] found in a study with healthy humans [142], has not yet been studied. Nevertheless, when patients with impaired glucose tolerance start melatonin treatment at night it could be useful to ask them for signs and symptoms of increased glucose levels. Eventually mealtimes could then be advanced [142].
8. Discussion
Clinical efficacy studies have demonstrated that melatonin is remarkably effective for children and adolescents with sleep problems, provided that these sleep problems are due to DSPD, and that melatonin is administered at the correct time and in the correct dose. Furthermore, animal, in vitro and human safety studies did not reveal any evidence of potentially harmful effects of melatonin treatment in children. We did not find indications for clinical relevant effects on cardiovascular, immune, and metabolic systems.
Sleep problems due to DSPD may resemble those of insomnia. For example, delayed sleep-onset is one of the main characteristics of DSPD, but it also one of the features of insomnia, since insomnia is defined as difficulty in initiating/maintaining sleep or nonrestorative sleep. Delayed sleep-onset in DSPD is a consequence of delayed circadian rhythmicity, which consequently usually responds to melatonin treatment. However, sleep-onset insomnia can also be due to insufficient sleep hygiene, or insomnia. The first choice of treatment for insomnia is cognitive behaviour therapy (CBT-I) [172]. Usually melatonin treatment is not helpful then. Furthermore, when the sleep–wake rhythm is delayed for a long time due to DSPD, and lifestyle factors are not in accordance with the biological clock, sleep maintenance problems may arise in addition to sleep-onset and sleep offset issues according to our clinical experience. These sleep problems can also be diagnosed as insomnia; however, not CBT-I, but melatonin treatment will then be helpful. Consequently, for optimal treatment with melatonin, a correct diagnosis is crucial. When the sleep–wake rhythm alone is insufficient to diagnose DSPD, assessment of the DLMO can help considerably [48]. However, clinicians should be aware of a possible pitfall: according to our experience DLMO fluctuates for the first few weeks after stopping melatonin [160]. Therefore we advise measurement of DLMO no earlier than 4–6 weeks after stopping melatonin treatment (clinical experience, MS).
Ideally the DLMO should be known, not only for aiding diagnosis, but also for optimal melatonin treatment. The melatonin concentration is readily determined in saliva collected at the children's home, and several laboratories are able to establish the DLMO, including saliva samples sent from abroad (eg www.melatoninecheck.nl). Nevertheless, practical drawbacks may make it unfeasible to determine DLMO. In that case, we would advise starting melatonin treatment 3–5 h before bedtime. Try to find the lowest effective melatonin dose, starting with 1 mg in children between 6 and 18 years. If after one week no change occurs, increase the dose by 1 mg weekly until an effect occurs. When a 1 mg melatonin dose is already effective, try to lower the dose until a minimal effective dose is reached. If there is no effect using a 3–6 mg dose, stop melatonin treatment and try to measure DLMO, or reconsider the diagnosis [48] (clinical experience, MS).
The present review did not reveal evidence of potentially harmful effects of melatonin treatment in children. Concerns for possible adverse effects of melatonin on reproductive function are not justified on the basis of evidence reviewed in the present article. In vitro and animal studies indicate that melatonin can be used safely in the recommended doses. This is supported by the critical systematic review of adverse events associated with oral administration of melatonin [168], and a search of Medline for adverse effects of melatonin, identifying 789 papers limited to ‘human’ and 543 to ‘animal’ [6]. Nevertheless melatonin has the potential to cause problems if it is used incorrectly. Thus when melatonin is administered at the wrong time (usually shortly before bedtime without knowing DLMO), or in the wrong dose (usually too high), successful treatment of the sleep disorder may be postponed unnecessarily. During our 25-years' experience we saw some medically important side effects including diarrhoea, headache, and enuresis, which have not received much attention in the literature. When diarrhoea is caused by melatonin, we suggest treatment should be stopped, as we have found all conventional methods to stop this intestinal problem to be unsuccessful. The same scenario applies to melatonin induced persistent headache or enuresis (clinical experience, MS). Other treatments should then be considered ie bright light, behavioural interventions or adaptation of lifestyle to the delayed biological clock [58].
A rapidly increasing number of children are using melatonin, with the numbers doubling from 2004 to 2011 [173]. The Dutch Generation R study reported in 2019 that 6% of school-aged children used melatonin regularly. Caregiver- and child-reported shorter total sleep time appeared to be the indication for melatonin use, without taking into account the cause of the poor sleep [8].
To conclude, melatonin is a safe chronobiotic drug for the treatment of delayed sleep–wake phase disorder in children, at least in the short term, provided that it is administered at the right time and in the right dose. Treatment should be tailored to the specific patient in relation to the particular status of the biological clock. As this status may change through the years we recommend stopping melatonin treatment at least once a year (preferably during the summer holidays).
9. Keypoints
-
•
Clinical efficacy studies demonstrated that melatonin is remarkably effective for children and adolescents with sleep problems, provided that these sleep problems are due to DSPD, and that melatonin is administered at the correct time and in the correct dose.
-
•
Animal, in vitro and human safety studies did not reveal any evidence of potentially harmful effects of melatonin treatment in children.
CRediT author statement
D. Mantle: conceptualization, methodology, investigation, review and editing, visualization. M. Smits: writing original draft, investigation, review and editing, project administration. M. Boss: methodology, visualization, review and editing. I. Miedema: validation, review and editing. I. van Geijlswijk: supervision, validation, review and editing.
Footnotes
David Mantle is medical adviser at Pharma Nord (UK) Ltd. Marcel Smits is chronobiological adviser of Pharma Nord ApS (Denmark). Myrthe Boss, Irene Miedema, and Inge van Geijlswijk have no competing interests to declare.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleepx.2020.100022.
Conflict of interest
The following is the supplementary data related to this article:
References
- 1.Nesbitt A.D. Delayed sleep-wake phase disorder. J Thorac Dis. 2018 January;10(Suppl. 1):S103–S111. doi: 10.21037/jtd.2018.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine . 3rd ed. American Academy of Sleep Medicine; Chicago, Illinois: Darien, IL: 2014. International classification of sleep disorders. [Google Scholar]
- 3.Gradisar M., Crowley S.J. Delayed sleep phase disorder in youth. Curr Opin Psychiatry. 2013 November;26(6):580–585. doi: 10.1097/YCO.0b013e328365a1d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson C., Cain N., Bartel K. A randomised controlled trial of bright light therapy and morning activity for adolescents and young adults with delayed sleep-wake phase disorder. Sleep Med. 2018 May;45:114–123. doi: 10.1016/j.sleep.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Danielsson K., Jansson-Frojmark M., Broman J.E. Cognitive behavioral therapy as an adjunct treatment to light therapy for delayed sleep phase disorder in young adults: a randomized controlled feasibility study. Behav Sleep Med. 2016;14(2):212–232. doi: 10.1080/15402002.2014.981817. [DOI] [PubMed] [Google Scholar]
- 6.Bruni O., Alonso-Alconada D., Besag F. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015 March;19(2):122–133. doi: 10.1016/j.ejpn.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Wirz-Justice A., Armstrong S.M. Melatonin: nature's soporific? J Sleep Res. 1996 June;5(2):137–141. [PubMed] [Google Scholar]
- 8.Koopman-Verhoeff M.E., van den Dries M.A., Van Seters J.J. Association of sleep problems and melatonin use in school-aged children. JAMA Pediatr. 2019 July 22;173(9):883–885. doi: 10.1001/jamapediatrics.2019.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jan J.E., Freeman R.D., Fast D.K. Melatonin treatment of sleep-wake cycle disorders in children and adolescents. Dev Med Child Neurol. 1999 July;41(7):491–500. [PubMed] [Google Scholar]
- 10.Erland L.A., Saxena P.K. Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J Clin Sleep Med. 2017 February 15;13(2):275–281. doi: 10.5664/jcsm.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan V., Spence W.D., Pandi-Perumal S.R. Melatonin and human reproduction: shedding light on the darkness hormone. Gynecol Endocrinol. 2009 December;25(12):779–785. doi: 10.3109/09513590903159649. [DOI] [PubMed] [Google Scholar]
- 12.Janjua I., Goldman R.D. Sleep-related melatonin use in healthy children. Can Fam Physician. 2016 April;62(4):315–317. [PMC free article] [PubMed] [Google Scholar]
- 13.Kennaway D.J. Potential safety issues in the use of the hormone melatonin in paediatrics. J Paediatr Child Health. 2015 June;51(6):584–589. doi: 10.1111/jpc.12840. [DOI] [PubMed] [Google Scholar]
- 14.Smits M.G., van Stel H.F., van der H.K. Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: a randomized placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2003 November;42(11):1286–1293. doi: 10.1097/01.chi.0000085756.71002.86. [DOI] [PubMed] [Google Scholar]
- 15.Anand S., Tong H., Besag F.M.C. Safety, tolerability and efficacy of drugs for treating behavioural insomnia in children with attention-deficit/hyperactivity disorder: a systematic review with methodological quality assessment. Paediatr Drugs. 2017 June;19(3):235–250. doi: 10.1007/s40272-017-0224-6. [DOI] [PubMed] [Google Scholar]
- 16.Hollway J.A., Aman M.G. Pharmacological treatment of sleep disturbance in developmental disabilities: a review of the literature. Res Dev Disabil. 2011 May;32(3):939–962. doi: 10.1016/j.ridd.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Weitzman E.D., Czeisler C.A., Coleman R.M. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry. 1981 July;38(7):737–746. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- 18.Sack R.L., Auckley D., Auger R.R. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007 November 1;30(11):1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxvig I.W., Wilhelmsen-Langeland A., Pallesen S. Objective measures of sleep and dim light melatonin onset in adolescents and young adults with delayed sleep phase disorder compared to healthy controls. J Sleep Res. 2013 August;22(4):365–372. doi: 10.1111/jsr.12030. [DOI] [PubMed] [Google Scholar]
- 20.Dagan Y., Eisenstein M. Circadian rhythm sleep disorders: toward a more precise definition and diagnosis. Chronobiol Int. 1999 March;16(2):213–222. doi: 10.3109/07420529909019087. [DOI] [PubMed] [Google Scholar]
- 21.Barion A., Zee P.C. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007 September;8(6):566–577. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelayo R.P., Thorpy M.J., Glovinsky P. Prevalence of delayed sleep phase syndrome among adolescents. J Sleep Res. 1988;17:392. [Google Scholar]
- 23.Schrader H., Bovim G., Sand T. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res. 1993;2:51–55. doi: 10.1111/j.1365-2869.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 24.Yazaki M., Shirakawa S., Okawa M. Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci. 1999 April;53(2):267–268. doi: 10.1046/j.1440-1819.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 25.Regestein Q.R., Monk T.H. Delayed sleep phase syndrome: a review of its clinical aspects. Am J Psychiatry. 1995 April;152(4):602–608. doi: 10.1176/ajp.152.4.602. [DOI] [PubMed] [Google Scholar]
- 26.Crowley S.J., Acebo C., Carskadon M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007 September;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Saxvig I.W., Pallesen S., Wilhelmsen-Langeland A. Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. 2012 February;13(2):193–199. doi: 10.1016/j.sleep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Thorpy M.J., Korman E., Spielman A.J. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988 January;9(1):22–27. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 29.Kotagal S., Broomall E. Sleep in children with autism spectrum disorder. Pediatr Neurol. 2012 October;47(4):242–251. doi: 10.1016/j.pediatrneurol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Van Veen M.M., Kooij J.J., Boonstra A.M. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010 June 1;67(11):1091–1096. doi: 10.1016/j.biopsych.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Van der Heijden K.B., Smits M.G., Someren E.J. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22(3):559–570. doi: 10.1081/CBI-200062410. [DOI] [PubMed] [Google Scholar]
- 32.Rossignol D.A., Frye R.E. Melatonin in autism spectrum disorders. Curr Clin Pharmacol. 2014;9(4):326–334. doi: 10.2174/15748847113086660072. [DOI] [PubMed] [Google Scholar]
- 33.Bijlenga D., Vollebregt M.A., Jjs Kooij. The role of the circadian system in the etiology and pathophysiology of ADHD: time to redefine ADHD? Atten Defic Hyperact Disord. 2019 March;11(1):5–19. doi: 10.1007/s12402-018-0271-z. [DOI] [PubMed] [Google Scholar]
- 34.Bromundt V., Taddio M., Cajochen C. Circadian rhythm sleep disorders. Pathophysiology. In: Bassetti Claudio, Dogas Zoran, Peigneux Philippe., editors. Sleep medine textbook. European Sleep Research Society; 2014. pp. 327–335. [Google Scholar]
- 35.Jones C.R., Huang A.L., Ptacek L.J. Genetic basis of human circadian rhythm disorders. Exp Neurol. 2013 May;243:28–33. doi: 10.1016/j.expneurol.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archer S.N., Robilliard D.L., Skene D.J. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003 June 15;26(4):413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 37.Archer S.N., Carpen J.D., Gibson M. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. 2010 May;33(5):695–701. doi: 10.1093/sleep/33.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehrens S.M.T., Christou S., Isherwood C. Meal timing regulates the human circadian system. Curr Biol. 2017 June 19;27(12):1768–1775. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Challet E., Kalsbeek A. Editorial: circadian rhythms and metabolism. Front Endocrinol. 2017;8:201. doi: 10.3389/fendo.2017.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pot G.K., Hardy R., Stephen A.M. Irregularity of energy intake at meals: prospective associations with the metabolic syndrome in adults of the 1946 British birth cohort. Br J Nutr. 2016 January 28;115(2):315–323. doi: 10.1017/S0007114515004407. [DOI] [PubMed] [Google Scholar]
- 41.Rufiange M., Dumont M., Lachapelle P. Correlating retinal function with melatonin secretion in subjects with an early or late circadian phase. Investig Ophthalmol Vis Sci. 2002 July;43(7):2491–2499. [PubMed] [Google Scholar]
- 42.Aoki H., Ozeki Y., Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001 March;18(2):263–271. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- 43.Klerman E.B., Gershengorn H.B., Duffy J.F. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002 April;17(2):181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 44.Berendsen M., Boss M., Smits M. Chrono-nutrition and diet quality in adolescents with delayed sleep-wake phase disorder. Nutrients. 2020 February 19;12(2) doi: 10.3390/nu12020539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxvig I.W., Wilhelmsen-Langeland A., Pallesen S. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: effects on subjective and objective sleep. Chronobiol Int. 2014 February;31(1):72–86. doi: 10.3109/07420528.2013.823200. [DOI] [PubMed] [Google Scholar]
- 46.Jaspers-Fayer F., Lin S.Y., Belschner L. A case-control study of sleep disturbances in pediatric obsessive-compulsive disorder. J Anxiety Disord. 2018 April;55:1–7. doi: 10.1016/j.janxdis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Danielsson K., Jansson-Frojmark M., Broman J.E. Light therapy with scheduled rise times in young adults with delayed sleep phase disorder: therapeutic outcomes and possible predictors. Behav Sleep Med. 2018 July;16(4):325–336. doi: 10.1080/15402002.2016.1210150. [DOI] [PubMed] [Google Scholar]
- 48.Keijzer H., Smits M.G., Duffy J.F. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014 August;18(4):333–339. doi: 10.1016/j.smrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Fukada Y., Okano T. Circadian clock system in the pineal gland. Mol Neurobiol. 2002 February;25(1):19–30. doi: 10.1385/MN:25:1:019. [DOI] [PubMed] [Google Scholar]
- 50.Lewy A.J., Wehr T.A., Goodwin F.K. Light suppresses melatonin secretion in humans. Science. 1980 December 12;210(4475):1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 51.Nagare R., Plitnick B., Figueiro M.G. Effect of exposure duration and light spectra on nighttime melatonin suppression in adolescents and adults. Light Res Technol. 2019 June;51(4):530–543. doi: 10.1177/1477153518763003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubocovich M.L. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007 December;8(Suppl. 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Macchi M.M., Bruce J.N. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004 September;25(3–4):177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Zhdanova I.V., Wurtman R.J., Morabito C. Effects of low oral doses of melatonin, given 2-4 hours before habitual bedtime, on sleep in normal young humans. Sleep. 1996 June;19(5):423–431. doi: 10.1093/sleep/19.5.423. [DOI] [PubMed] [Google Scholar]
- 55.Carpentieri A., Diaz de B.G., Areco V. New perspectives in melatonin uses. Pharmacol Res. 2012 April;65(4):437–444. doi: 10.1016/j.phrs.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Lewy A.J., Cutler N.L., Sack R.L. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999 June;14(3):227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 57.Lewy A. Clinical implications of the melatonin phase response curve. J Clin Endocrinol Metab. 2010 July;95(7):3158–3160. doi: 10.1210/jc.2010-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin J.S., Gaudreault M.M., Perron M. Chronotype, light exposure, sleep, and daytime functioning in high school students attending morning or afternoon school shifts: an actigraphic study. J Biol Rhythms. 2016 April;31(2):205–217. doi: 10.1177/0748730415625510. [DOI] [PubMed] [Google Scholar]
- 59.Doi Y., Ishihara K., Uchiyama M. Associations of chronotype with social jetlag and behavioral problems in preschool children. Chronobiol Int. 2015;32(8):1101–1108. doi: 10.3109/07420528.2015.1063503. [DOI] [PubMed] [Google Scholar]
- 60.Raymann R.J., Swaab D.F., van Someren E.J. Cutaneous warming promotes sleep onset. Am J Physiol Regul Integr Comp Physiol. 2005 June;288(6):R1589–R1597. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 61.Zee P.C., Goldstein C.A. Treatment of shift work disorder and jet lag. Curr Treat Options Neurol. 2010 September;12(5):396–411. doi: 10.1007/s11940-010-0090-9. [DOI] [PubMed] [Google Scholar]
- 62.Auger R.R., Burgess H.J., Emens J.S. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2015 October 15;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewy A.J., Ahmed S., Jackson J.M. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992 October;9(5):380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 64.EFSA PoDPNaAN Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698,1780,4080) pursuant to article 13(1) of regulation (EC) No 1924/2006. EFSA J. 2011;9(2241) [Google Scholar]
- 65.van Geijlswijk I.M., Van der Heijden K.B., Egberts A.C. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl) 2010 October;212(3):379–391. doi: 10.1007/s00213-010-1962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keijzer H., Snitselaar M.A., Smits M.G. Precision medicine in circadian rhythm sleep-wake disorders: current state and future perspectives. Per Med. 2017 March;14(2):171–182. doi: 10.2217/pme-2016-0079. [DOI] [PubMed] [Google Scholar]
- 67.Braam W., van G I., Keijzer H. Loss of response to melatonin treatment is associated with slow melatonin metabolism. J Intellect Disabil Res. 2010 June;54(6):547–555. doi: 10.1111/j.1365-2788.2010.01283.x. [DOI] [PubMed] [Google Scholar]
- 68.Braam W., Keijzer H., Struijker B.H. CYP1A2 polymorphisms in slow melatonin metabolisers: a possible relationship with autism spectrum disorder? J Intellect Disabil Res. 2013 November;57(11):993–1000. doi: 10.1111/j.1365-2788.2012.01595.x. [DOI] [PubMed] [Google Scholar]
- 69.Wilhelmsen-Langeland A., Saxvig I.W., Pallesen S. A randomized controlled trial with bright light and melatonin for the treatment of delayed sleep phase disorder: effects on subjective and objective sleepiness and cognitive function. J Biol Rhythms. 2013 October;28(5):306–321. doi: 10.1177/0748730413500126. [DOI] [PubMed] [Google Scholar]
- 70.Auger R.R., Burgess H.J., Emens J.S. Do evidence-based treatments for circadian rhythm sleep-wake disorders make the GRADE? Updated guidelines point to need for more clinical research. J Clin Sleep Med. 2015 October 15;11(10):1079–1080. doi: 10.5664/jcsm.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van M.A., Meijer A.M., Smits M.G. Effects of melatonin and bright light treatment in childhood chronic sleep onset insomnia with late melatonin onset: a randomised controlled study. Sleep. 2017 Feb 1;40(2) doi: 10.1093/sleep/zsw038. [DOI] [PubMed] [Google Scholar]
- 72.Fourtillan J.B., Brisson A.M., Gobin P. Bioavailability of melatonin in humans after day-time administration of D(7) melatonin. Biopharm Drug Dispos. 2000 January;21(1):15–22. doi: 10.1002/1099-081x(200001)21:1<15::aid-bdd215>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 73.Zeitzer J.M., Daniels J.E., Duffy J.F. Do plasma melatonin concentrations decline with age? Am J Med. 1999 November;107(5):432–436. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- 74.Harpsoe N.G., Andersen L.P., Gogenur I. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015 August;71(8):901–909. doi: 10.1007/s00228-015-1873-4. [DOI] [PubMed] [Google Scholar]
- 75.Anderson G.D. Developmental pharmacokinetics. Semin Pediatr Neurol. 2010;17:208–213. doi: 10.1016/j.spen.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Chua H.M., Hauet R.N., Swedrowska M. Dissolution of intact, divided and crushed Circadin tablets: prolonged vs. immediate release of melatonin. Pharmaceutics. 2016 January 7;8(1) doi: 10.3390/pharmaceutics8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mantle D. Immediate-release supplemental melatonin for delayed sleep phase disorder in children: an overview. Br J Neurosci Nurs. 2019;15(1) [Google Scholar]
- 78.Bruni O., Alonso-Alconada D., Besag F. Immediate and prolonged-release melatonin in children with neurodevelopmental disabilities. Author reply to Prof. Zisapel. Eur J Paediatr Neurol. 2017 March;21(2):420–421. doi: 10.1016/j.ejpn.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Grigg-Damberger M.M., Ianakieva D. Poor quality control of over-the-counter melatonin: what they say is often not what you get. J Clin Sleep Med. 2017 February 15;13(2):163–165. doi: 10.5664/jcsm.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quera-Salva M.A., Claustrat B. Melatonin: physiological and pharmacological aspects related to sleep: the interest of a prolonged-release formulation (Circadin((R))) in insomnia. Encephale. 2018 December;44(6):548–557. doi: 10.1016/j.encep.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Bunn R. Melatonin and its use in children. Pharm J. 2013;290:147–149. [Google Scholar]
- 82.Hardeland R. New approaches in the management of insomnia: weighing the advantages of prolonged-release melatonin and synthetic melatoninergic agonists. Neuropsychiatr Dis Treat. 2009;5:341–354. doi: 10.2147/ndt.s4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei S., Smits M.G., Tang X. Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: a meta-analysis of randomized controlled trials. Sleep Med. 2020 Apr;68:1–8. doi: 10.1016/j.sleep.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 84.Smits M.G., Nagtegaal J.E., van der Heijden J. Melatonin for chronic sleep onset insomnia in children: a randomized placebo-controlled trial. J Child Neurol. 2001 February;16(2):86–92. doi: 10.1177/088307380101600204. [DOI] [PubMed] [Google Scholar]
- 85.van M.A., Meijer A.M., Smits M.G. Effects of melatonin and bright light treatment in childhood chronic sleep onset insomnia with late melatonin onset: a randomized controlled study. Sleep. 2017 February 1;40(2) doi: 10.1093/sleep/zsw038. [DOI] [PubMed] [Google Scholar]
- 86.Van der Heijden K.B., Smits M.G., van Someren E.J. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007 February;46(2):233–241. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 87.Galland B.C., Short M.A., Terrill P. Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta-analysis. Sleep. 2018 April 1;41(4) doi: 10.1093/sleep/zsy017. [DOI] [PubMed] [Google Scholar]
- 88.van Geijlswijk I.M., Mol R.H., Egberts T.C. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology. 2011 July;216(1):111–120. doi: 10.1007/s00213-011-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zwart T.C., Smits M.G., Egberts T.C.G. Long-term melatonin therapy for adolescents and young adults with chronic sleep onset insomnia and late melatonin onset: evaluation of sleep quality, chronotype, and lifestyle factors compared to age-related randomly selected population cohorts. Healthcare (Basel) 2018 March 2;6(1) doi: 10.3390/healthcare6010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fallah R., Fazelishoroki F., Sekhavat L. A randomized clinical trial comparing the efficacy of melatonin and amitriptyline in migraine prophylaxis of children. Iran J Child Neurol. 2018;12(1):47–54. [PMC free article] [PubMed] [Google Scholar]
- 91.Myers K.A., Davey M.J., Ching M. Randomized controlled trial of melatonin for sleep disturbance in Dravet syndrome: the DREAMS study. J Clin Sleep Med. 2018 October 15;14(10):1697–1704. doi: 10.5664/jcsm.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ardakani A., Farrehi M., Sharif M. The effect of melatonin administration on disease severity ans sleep quality in children with atopic dermatitis: a randomized, double blind, placebo-controlled trial. Pediatr Allergy Immunol. 2018;29(8):834–840. doi: 10.1111/pai.12978. [DOI] [PubMed] [Google Scholar]
- 93.Mostafavi S.A., Solhi M., Mohammadi M.R. Melatonin for reducing weight gain following administration of atypical antipsychotic olanzapine for adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2017 June;27(5):440–444. doi: 10.1089/cap.2016.0046. [DOI] [PubMed] [Google Scholar]
- 94.Gitto E., Marseglia L., D'Angelo G. Melatonin versus midazolam premedication in children undergoing surgery: a pilot study. J Paediatr Child Health. 2016 March;52(3):291–295. doi: 10.1111/jpc.13007. [DOI] [PubMed] [Google Scholar]
- 95.Kain Z.N., MacLaren J.E., Herrmann L. Preoperative melatonin and its effects on induction and emergence in children undergoing anesthesia and surgery. Anesthesiology. 2009 July;111(1):44–49. doi: 10.1097/ALN.0b013e3181a91870. [DOI] [PubMed] [Google Scholar]
- 96.Isik B., Baygin O., Bodur H. Premedication with melatonin vs midazolam in anxious children. Paediatr Anaesth. 2008 July;18(7):635–641. doi: 10.1111/j.1460-9592.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- 97.Samarkandi A., Naguib M., Riad W. Melatonin vs. midazolam premedication in children: a double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005 March;22(3):189–196. doi: 10.1017/s0265021505000335. [DOI] [PubMed] [Google Scholar]
- 98.Almenrader N., Haiberger R., Passariello M. Steal induction in preschool children: is melatonin as good as clonidine? A prospective, randomized study. Paediatr Anaesth. 2013 April;23(4):328–333. doi: 10.1111/pan.12105. [DOI] [PubMed] [Google Scholar]
- 99.Gitto E., Aversa S., Salpietro C.D. Pain in neonatal intensive care: role of melatonin as an analgesic antioxidant. J Pineal Res. 2012 April;52(3):291–295. doi: 10.1111/j.1600-079X.2011.00941.x. [DOI] [PubMed] [Google Scholar]
- 100.Marseglia L., D'Angelo G., Manti S. Analgesic, anxiolytic and anaesthetic effects of melatonin: new potential uses in pediatrics. Int J Mol Sci. 2015 January 6;16(1):1209–1220. doi: 10.3390/ijms16011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozcengiz D., Gunes Y., Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesth. 2011 April;25(2):184–188. doi: 10.1007/s00540-011-1099-2. [DOI] [PubMed] [Google Scholar]
- 102.Fallah R., Yadegari Y., Behdad S. Melatonin and intravenous midazolam administered orally in drug induced sleep electroencephalography of children: randomized clinical trial of efficacy. Arch Iran Med. 2014 November;17(11):741–745. [PubMed] [Google Scholar]
- 103.Sander J., Shamdeen M.G., Gottschling S. Melatonin does not influence sleep deprivation electroencephalogram recordings in children. Eur J Pediatr. 2012 April;171(4):675–679. doi: 10.1007/s00431-011-1640-1. [DOI] [PubMed] [Google Scholar]
- 104.Wassmer E., Quinn E., Whitehouse W. Melatonin as a sleep inductor for electroencephalogram recordings in children. Clin Neurophysiol. 2001 April;112(4):683–685. doi: 10.1016/s1388-2457(00)00554-x. [DOI] [PubMed] [Google Scholar]
- 105.Sury M.R., Fairweather K. The effect of melatonin on sedation of children undergoing magnetic resonance imaging. Br J Anaesth. 2006 August;97(2):220–225. doi: 10.1093/bja/ael144. [DOI] [PubMed] [Google Scholar]
- 106.Schroder C.M., Malow B.A., Maras A. Pediatric prolonged-release melatonin for sleep in children with autism spectrum disorder: impact on child behavior and caregiver's quality of life. J Autism Dev Disord. 2019 August;49(8):3218–3230. doi: 10.1007/s10803-019-04046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maras A., Schroder C.M., Malow B.A. Long-term efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2018 December;28(10):699–710. doi: 10.1089/cap.2018.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gringras P., Nir T., Breddy J. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017 November;56(11):948–957. doi: 10.1016/j.jaac.2017.09.414. [DOI] [PubMed] [Google Scholar]
- 109.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983 December;227(3):587–591. [PubMed] [Google Scholar]
- 110.Patel T.A., Rao M.V. Antigenotoxic effect of melatonin against mercuric chloride in human peripheral blood lymphocytes. Toxicol Ind Health. 2018 November;34(11):778–786. doi: 10.1177/0748233718795747. [DOI] [PubMed] [Google Scholar]
- 111.Oaknin-Bendahan S., Anis Y., Nir I. Effects of long-term administration of melatonin and a putative antagonist on the ageing rat. Neuroreport. 1995 March 27;6(5):785–788. doi: 10.1097/00001756-199503270-00020. [DOI] [PubMed] [Google Scholar]
- 112.Pierpaoli W., Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci U S A. 1994 January 18;91(2):787–791. doi: 10.1073/pnas.91.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Musatov S.A., Anisimov V.N., Andre V. Effects of melatonin on N-nitroso-N-methylurea-induced carcinogenesis in rats and mutagenesis in vitro (Ames test and COMET assay) Cancer Lett. 1999 April 26;138(1–2):37–44. doi: 10.1016/s0304-3835(98)00365-6. [DOI] [PubMed] [Google Scholar]
- 114.Conti A., Maestroni G.J. Role of the pineal gland and melatonin in the development of autoimmune diabetes in non-obese diabetic mice. J Pineal Res. 1996 April;20(3):164–172. doi: 10.1111/j.1600-079x.1996.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 115.Aoyama H., Mori N., Mori W. Effects of melatonin on genetic hypercholesterolemia in rats. Atherosclerosis. 1988 February;69(2–3):269–272. doi: 10.1016/0021-9150(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 116.Neville S., Arendt J., Ioannides C. A study of the mutagenicity of melatonin and 6-hydroxymelatonin. J Pineal Res. 1989;6(1):73–76. doi: 10.1111/j.1600-079x.1989.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 117.Reddy M.V., Storer R.D., Laws G.M. Genotoxicity of naturally occurring indole compounds: correlation between covalent DNA binding and other genotoxicity tests. Environ Mol Mutagen. 2002;40(1):1–17. doi: 10.1002/em.10088. [DOI] [PubMed] [Google Scholar]
- 118.Martinez A., Urios A., Blanco M. Mutagenicity of 80 chemicals in Escherichia coli tester strains IC203, deficient in OxyR, and its oxyR(+) parent WP2 uvrA/pKM101: detection of 31 oxidative mutagens. Mutat Res. 2000 April 13;467(1):41–53. doi: 10.1016/s1383-5718(00)00020-6. [DOI] [PubMed] [Google Scholar]
- 119.De S.R., Fiore M., Aglitti T. Inhibitory action of melatonin on. Mutagenesis. 1999 January;14(1):107–112. doi: 10.1093/mutage/14.1.107. [DOI] [PubMed] [Google Scholar]
- 120.Musatov S.A., Rosenfeld S.V., Togo E.F. The influence of melatonin on mutagenicity and antitumor action of cytostatic drugs in mice. Vopr Onkol. 1997;43(6):623–627. [PubMed] [Google Scholar]
- 121.Ortega-Gutierrez S., Lopez-Vicente M., Lostale F. Protective effect of melatonin against mitomycin C-induced genotoxic damage in peripheral blood of rats. J Biomed Biotechnol. 2009;2009:791432. doi: 10.1155/2009/791432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cengiz M., Alansal N.O., Tuncdemir M. Evaluation of effects of melatonin and caffeic acid phenethyl ester on acute potassium dichromate toxicity and genotoxicity in rats. Indian J Pharmacol. 2016 July;48(4):407–411. doi: 10.4103/0253-7613.186213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y., Yang X., Wang W. Melatonin counteracts cobalt nanoparticleinduced cytotoxicity and genotoxicity by deactivating reactive oxygen speciesdependent mechanisms in the NRK cell line. Mol Med Rep. 2017 October;16(4):4413–4420. doi: 10.3892/mmr.2017.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sousa Coelho I.D.D., Lapa Neto C.J.C., Souza T.G.D.S. Protective effect of exogenous melatonin in rats and their offspring on the genotoxic response induced by the chronic consumption of alcohol during pregnancy. Mutat Res Genet Toxicol Environ Mutagen. 2018 August;832–833:52–60. doi: 10.1016/j.mrgentox.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 125.Ortiz G.G., Reiter R.J., Zuniga G. Genotoxicity of paraquat: micronuclei induced in bone marrow and peripheral blood are inhibited by melatonin. Mutat Res. 2000 January 24;464(2):239–245. doi: 10.1016/s1383-5718(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 126.Rao M.V., Narechania M.B. The genotoxic effects of anti-cancer drug gossypol on human lymphocytes and its mitigation by melatonin. Drug Chem Toxicol. 2016 October;39(4):357–361. doi: 10.3109/01480545.2015.1039646. [DOI] [PubMed] [Google Scholar]
- 127.Jung-Hynes B., Schmit T.L., Reagan-Shaw S.R. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res. 2011 March;50(2):140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao G.N., Ney E., Herbert R.A. Effect of melatonin and linolenic acid on mammary cancer in transgenic mice with c-neu breast cancer oncogene. Breast Cancer Res Treat. 2000 December;64(3):287–296. doi: 10.1023/a:1026552405042. [DOI] [PubMed] [Google Scholar]
- 129.Anisimov V.N., Alimova I.N., Baturin D.A. Dose-dependent effect of melatonin on life span and spontaneous tumor incidence in female SHR mice. Exp Gerontol. 2003 April;38(4):449–461. doi: 10.1016/s0531-5565(02)00240-1. [DOI] [PubMed] [Google Scholar]
- 130.Subramanian A., Kothari L. Melatonin, a suppressor of spontaneous murine mammary tumors. J Pineal Res. 1991 April;10(3):136–140. doi: 10.1111/j.1600-079x.1991.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 131.Meredith S., Jackson K., Dudenhoeffer G. Long-term supplementation with melatonin delays reproductive senescence in rats, without an effect on number of primordial follicles. Exp Gerontol. 2000 May;35(3):343–352. doi: 10.1016/s0531-5565(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 132.Reiter R.J., Rosales-Corral S.A., Tan D.X. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017 April 17;18(4) doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mediavilla M.D., Sanchez-Barcelo E.J., Tan D.X. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem. 2010;17(36):4462–4481. doi: 10.2174/092986710794183015. [DOI] [PubMed] [Google Scholar]
- 134.Zhdanova I.V., Geiger D.A., Schwagerl A.L. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002 April 1;75(4):523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- 135.Barchas J., DaCosta F., Spector S. Acute pharmacology of melatonin. Nature. 1967 May 27;214(5091):919–920. doi: 10.1038/214919a0. [DOI] [PubMed] [Google Scholar]
- 136.Guardiola-Lemaitre B., Lenegre A., Porsolt R.D. Combined effects of diazepam and melatonin in two tests for anxiolytic activity in the mouse. Pharmacol Biochem Behav. 1992 February;41(2):405–408. doi: 10.1016/0091-3057(92)90118-y. [DOI] [PubMed] [Google Scholar]
- 137.Chen Z., Chua C.C., Gao J. Protective effect of melatonin on myocardial infarction. Am J Physiol Heart Circ Physiol. 2003 May;284(5):H1618–H1624. doi: 10.1152/ajpheart.00874.2002. [DOI] [PubMed] [Google Scholar]
- 138.Reiter R.J., Melchiorri D., Sewerynek E. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995 January;18(1):1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 139.Jahnke G., Marr M., Myers C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci. 1999 August;50(2):271–279. doi: 10.1093/toxsci/50.2.271. [DOI] [PubMed] [Google Scholar]
- 140.Dubocovich M.L., Delagrange P., Krause D.N. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010 September;62(3):343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Karamitri A., Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019 February;15(2):105–125. doi: 10.1038/s41574-018-0130-1. [DOI] [PubMed] [Google Scholar]
- 142.Rubio-Sastre P., Scheer F.A., Gomez-Abellan P. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014 October 1;37(10):1715–1719. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marangos P.J., Patel J., Hirata F. Inhibition of diazepam binding by tryptophan derivatives including melatonin and its brain metabolite N-acetyl-5-methoxy kynurenamine. Life Sci. 1981 July 20;29(3):259–267. doi: 10.1016/0024-3205(81)90242-3. [DOI] [PubMed] [Google Scholar]
- 144.Boafo A., Greenham S., Alenezi S. Could long-term administration of melatonin to prepubertal children affect timing of puberty? A clinician's perspective. Nat Sci Sleep. 2019;11:1–10. doi: 10.2147/NSS.S181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fang L., Li Y., Wang S. Melatonin induces progesterone production in human granulosa-lutein cells through upregulation of StAR expression. Aging. 2019 October 16;11(20):9013–9024. doi: 10.18632/aging.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Minguini I.P., Luquetti C.M., Baracat M.C.P. Melatonin effects on ovarian follicular cells: a systematic review. Rev Assoc Med Bras. 2019 September 12;65(8):1122–1127. doi: 10.1590/1806-9282.65.8.1122. [DOI] [PubMed] [Google Scholar]
- 147.Scarinci E., Tropea A., Notaristefano G. “Hormone of darkness” and human reproductive process: direct regulatory role of melatonin in human corpus luteum. J Endocrinol Investig. 2019 October;42(10):1191–1197. doi: 10.1007/s40618-019-01036-3. [DOI] [PubMed] [Google Scholar]
- 148.Tagliaferri V., Romualdi D., Scarinci E. Melatonin treatment may be able to restore menstrual cyclicity in women with PCOS: a pilot study. Reprod Sci. 2018 February;25(2):269–275. doi: 10.1177/1933719117711262. [DOI] [PubMed] [Google Scholar]
- 149.Fourtillan J.B., Brisson A.M., Fourtillan M. Melatonin secretion occurs at a constant rate in both young and older men and women. Am J Physiol Endocrinol Metab. 2001 January;280(1):E11–E22. doi: 10.1152/ajpendo.2001.280.1.E11. [DOI] [PubMed] [Google Scholar]
- 150.Lang U., Aubert M.L., Rivest R.W. Daily afternoon administration of melatonin does not irreversibly inhibit sexual maturation in the male rat. Endocrinology. 1984 December;115(6):2303–2310. doi: 10.1210/endo-115-6-2303. [DOI] [PubMed] [Google Scholar]
- 151.Nordio M., Vaughan M.K., Sabry I. Undernutrition potentiates melatonin effects in maturing female rats. J Endocrinol Investig. 1989 February;12(2):103–110. doi: 10.1007/BF03349933. [DOI] [PubMed] [Google Scholar]
- 152.Brotto L.A., Gorzalka B.B. Melatonin enhances sexual behavior in the male rat. Physiol Behav. 2000 February;68(4):483–486. doi: 10.1016/s0031-9384(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 153.Drago F., Busa L., Benelli A. Acute low doses of melatonin stimulate rat sex behavior: the role of serotonin neurotransmission. Eur J Pharmacol. 1999 November 26;385(1):1–6. doi: 10.1016/s0014-2999(99)00701-3. [DOI] [PubMed] [Google Scholar]
- 154.Do L.T., Shibata Y., Taniguchi M. Melatonin supplementation during in vitro maturation and development supports the development of porcine embryos. Reprod Domest Anim. 2015 December;50(6):1054–1058. doi: 10.1111/rda.12607. [DOI] [PubMed] [Google Scholar]
- 155.Bosc M.J. Time of parturition in rats after melatonin administration or change of photoperiod. J Reprod Fertil. 1987 July;80(2):563–568. doi: 10.1530/jrf.0.0800563. [DOI] [PubMed] [Google Scholar]
- 156.van Geijlswijk I.M., Korzilius H.P., Smits M.G. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010 December 1;33(12):1605–1614. doi: 10.1093/sleep/33.12.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Braam W., Smits M.G., Didden R. Exogenous melatonin for sleep problems in individuals with intellectual disability: a meta-analysis. Dev Med Child Neurol. 2009 May;51(5):340–349. doi: 10.1111/j.1469-8749.2008.03244.x. [DOI] [PubMed] [Google Scholar]
- 158.Abdelgadir I.S., Gordon M.A., Akobeng A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018 December;103(12):1155–1162. doi: 10.1136/archdischild-2017-314181. [DOI] [PubMed] [Google Scholar]
- 159.Carr R., Wasdell M.B., Hamilton D. Long-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disorders. J Pineal Res. 2007 November;43(4):351–359. doi: 10.1111/j.1600-079X.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 160.Hoebert M., Van der Heijden K.B., van Geijlswijk I.M. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res. 2009 August;47(1):1–7. doi: 10.1111/j.1600-079X.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 161.Voordouw B.C., Euser R., Verdonk R.E. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992 January;74(1):108–117. doi: 10.1210/jcem.74.1.1727807. [DOI] [PubMed] [Google Scholar]
- 162.Impellizzeri P., Vinci E., Gugliandolo M.C. Premedication with melatonin vs midazolam: efficacy on anxiety and compliance in paediatric surgical patients. Eur J Pediatr. 2017 July;176(7):947–953. doi: 10.1007/s00431-017-2933-9. [DOI] [PubMed] [Google Scholar]
- 163.Foley H.M., Steel A.E. Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther Med. 2019 February;42:65–81. doi: 10.1016/j.ctim.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 164.Williams B.A., Hirtz D., Oskoui M. Practice guideline: treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2020 March 3;94(9):392–404. doi: 10.1212/WNL.0000000000009033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parker A., Beresford B., Dawson V. Oral melatonin for non-respiratory sleep disturbance in children with neurodisabilities: systematic review and meta-analyses. Dev Med Child Neurol. 2019 August;61(8):880–890. doi: 10.1111/dmcn.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sheldon S.H. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet. 1998 April 25;351(9111):1254. doi: 10.1016/S0140-6736(05)79321-1. [DOI] [PubMed] [Google Scholar]
- 167.Vishnoi S., Raisuddin S., Parvez S. Glutamate excitotoxicity and oxidative stress in epilepsy: modulatory role of melatonin. J Environ Pathol Toxicol Oncol. 2016;35(4):365–374. doi: 10.1615/JEnvironPatholToxicolOncol.2016016399. [DOI] [PubMed] [Google Scholar]
- 168.Brigo F., Igwe S.C. Melatonin as add-on treatment for epilepsy. Cochrane Database Syst Rev. 2016 March 17;3:CD006967. doi: 10.1002/14651858.CD006967.pub3. [DOI] [PubMed] [Google Scholar]
- 169.Deacon S., Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995 August 7;688(1–2):77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- 170.Shimomura E.T., Briones A.J., Gordon C.J. Case report of sudden death in a twin infant given melatonin supplementation: a challenging interpretation of postmortem toxicology. Forensic Sci Int. 2019 November;304:109962. doi: 10.1016/j.forsciint.2019.109962. [DOI] [PubMed] [Google Scholar]
- 171.Panda S. Circadian physiology of metabolism. Science. 2016 November 25;354(6315):1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Riemann D., Baglioni C., Bassetti C. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017 December;26(6):675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 173.Hartz I., Furu K., Bratlid T. Hypnotic drug use among 0-17 year olds during 2004-2011: a nationwide prescription database study. Scand J Public Health. 2012 December;40(8):704–711. doi: 10.1177/1403494812464446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.