Abstract
Hypercoagulability and virally-mediated vascular inflammation have become well-recognized features of the SARS-CoV-2 virus infection, COVID-19. Of growing concern is the apparent ineffectiveness of therapeutic anticoagulation in preventing thromboembolic events among some at-risk patient subtypes with COVID-19. We present a 43-year-old female with a history of seropositive-antiphospholipid syndrome and systemic lupus erythematosus who developed an acute ischemic stroke in the setting of mild COVID-19 infection despite adherence to chronic systemic anticoagulation. The clinical significance of SARS-CoV-2-mediated endothelial cell dysfunction and its potential to cause macrovascular events in spite of full anticoagulation warrants further investigation and likely represents another disease-defining pathology of COVID-19.
Key Words: ischemic stroke, Anti-phospholipid, Hypercoagulable state, COVID-19, Anticoagulation
Case Description
A 43-year-old female with co-morbidities of localization-related epilepsy, systemic lupus erythematosus (SLE) managed with mycophenolate and hydroxychloroquine, a remote history of deep vein thrombosis and left hemispheric stroke, and lupus-anticoagulant-positive antiphospholipid syndrome (APS) on chronic warfarin presented to an emergency department with low-grade fever and loss of taste and smell. The patient tested positive for SARS-CoV-2. The National Institute of Health guidelines classify patients positive for the SARS-CoV-2 infection without shortness of breath or radiographic evidence of lung involvement as mild disease.1 Since this patient's only symptoms included low grade fever and loss of taste and smell, she was diagnosed with mild COVID-19 per NIH guidelines.1 She was discharged home with prednisone and instructions to self-quarantine.
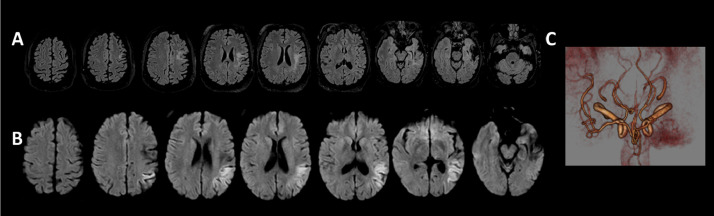
Two weeks later, the patient returned to a hospital after a seizure. Her exam was notable for fluent aphasia and right upper and lower extremity weakness. She was not a candidate for acute intervention as the last-known-well time was unknown, and the patient's INR was 2.49 at the time of presentation. She was normotensive, afebrile, and tachycardic with normal sinus rhythm. Cerebral imaging revealed an area of diffusion restriction of the left parietal and superior temporal lobes on diffusion-weighted magnetic resonance imaging (MRI) consistent with acute ischemic infarction (Fig. 1 a & 1b) and left middle cerebral artery occlusion (Fig. 1c). An echocardiographic evaluation, stroke labs, and telemetry were unremarkable. After an uneventful hospitalization, the patient was discharged home with outpatient rehabilitative services and transitioned to apixaban for anticoagulation by her hematologist. Of note, prior to this stroke, the patient had not had any recent thrombotic events related to her lupus and anti-phospholipid syndrome since being initiated on immunosuppressants and coumadin greater than 10 years prior.
Fig. 1.
A) & B) Diffusion restriction of the left parietal and superior temporal lobes as demonstrated on diffusion-weighted magnetic resonance imaging and hyperintensity on the fluid attenuated inversion recovery sequence consistent with acute ischemic infarction. C) Proximal left middle cerebral artery occlusion demonstrated on magnetic resonance angiography.
Discussion
Acute thrombotic events have become an important feature of COVID-19, attributable to both a proinflammatory hypercoagulable state and virally-mediated vascular inflammation.2 , 3 Numerous studies have substantiated that COVID-19-associated coagulopathy (characterized by mild thrombocytopenia, elevated D-dimer, elevated serum cytokines, elevated serum inflammatory markers, and prolonged prothrombin time)4 appears to worsen with increasing disease severity, evidenced by associations between mortality rate and D-dimer level. As such, prophylactic anticoagulation has become a guideline recommendation for all patients hospitalized with COVID-19, regardless of severity.4, 5, 6 For patients with pre-existing hypercoagulable states, such as APS, recommendations speculate that a higher target of anticoagulation may be necessary to attenuate the risk of thrombotic events.7 However, reports of acute thrombotic events among individuals with COVID-19 despite anticoagulation are concerning, in particular among those with severe disease who are at increased risk.8 Acute thromboses have also been reported among those with only mild disease (without viral pneumonia or hypoxia) and in whom the features of COVID-19-associated coagulopathy are absent.2 , 9 , 10 Animal models have demonstrated that SARS-CoV-2 can invade the brain through axonal transport via infection of olfactory neurons, but data demonstrating direct causality of stroke by neuroinvasion of SARS-CoV-2 in humans remains limited.11 Altogether, these findings suggest that SARS-CoV-2 endotheliitis may play a more significant role than expected in the formation of thromboses in COVID-19.
Endothelial cell dysfunction is emerging as the disease-defining pathology underlying COVID-19.12 , 13 It is now well established that SARS-CoV-2 infects the endothelial cells of multiple organ systems via the angiotensin converting enzyme 2 (ACE2) receptor, ultimately resulting in apoptosis and necrosis of endothelial cells.14 As inflammation builds, further organ systems deteriorate and serve as inflammatory furnaces of cytokine production through well understood physiological positive-feedback loops.12 Quickly, the elements of Virchow's Triad (vascular inflammation, hemostasis, and hypercoagulability) develop with a widespread capacity to precipitate formation of micro- and macro- vascular thromboses. To substantiate this, pathological studies have overwhelmingly demonstrated evidence of multimodal microvascular injury consistent with both vascular inflammation and endothelial cell dysfunction.15 This may explain why thrombosis occurs despite activation of the ACE2/angiotensin-(1-7)/Mas receptor axis, a pathway shown to attenuate thrombosis formation in mouse models.16
In conclusion, SARS-CoV-2 endotheliitis may represent an unfamiliar mechanism of stroke which both meets clinical significance and warrants further investigation. Of great concern is the possibility that risk of major thrombotic phenomena may not be reassuringly attenuated by means of anticoagulation in all patients. Studies of mild COVID-19 examining the association between disease symptoms and incidence of cerebrovascular sequelae are likely to be worthwhile. Additionally, clinical trials addressing alternative therapeutic treatment goals of anticoagulation would be informative, as well as trials examining anti-inflammatory agents and their effect on the incidence of cerebrovascular events in patients with COVID-19.
Declaration of Competing Interest
None of the authors have any conflicts of interest to disclose.
Footnotes
Grant Support: Not applicable.
Institution: All work was performed in the Department of Neurology at the University of Florida.
References
- 1.NIH. National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) treatment guidelines. 2021: Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed 13/31/2021. [PubMed]
- 2.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Tecson KM, McCullough PA. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020;21(3):315–319. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miesbach W, Makris M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBane RD, Torres Roldan VD, Niven AS, et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clin Proc. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason A, Rose E, Edwards CJ. Clinical management of Lupus patients during the COVID-19 pandemic. Lupus. 2020;29(13):1661–1672. doi: 10.1177/0961203320961848. [DOI] [PubMed] [Google Scholar]
- 8.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crippa S, Kägi G, Graf L, Meyer Sauteur PM, Kohler P. Stroke in a young adult with mild COVID-19 suggesting endotheliitis. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 11.Kumari P, Rothan HA, Natekar JP, et al. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses. 2021;13(1) doi: 10.3390/v13010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladka MM, Maack C. The endothelium as Achilles' heel in COVID-19 patients. Cardiovasc Res. 2020;116(14):e195–e197. doi: 10.1093/cvr/cvaa327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraga-Silva RA, Sorg BS, Wankhede M, et al. ACE2 activation promotes antithrombotic activity. Mol Med. 2010;16(5-6):210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]