Abstract
Introduction
The potential for Bacille Calmette-Guerin vaccination to mitigate COVID-19 severity and perhaps infection susceptibility has been hypothesized, attracting global attention given its off-target benefits shown in several respiratory viral infections.
Methods
In this retrospective study, patients with laboratory-confirmed COVID-19 from Wuhan Pulmonary Hospital, China were categorized into Bacille Calmette-Guerin‒vaccinated and nonvaccinated groups. Clinical records, demography, laboratory results, and chest computed tomography scans were extracted from electronic medical records and compared between the 2 groups.
Results
No adverse events were observed, except for an increased frequency of chills in the Bacille Calmette-Guerin‒vaccinated group compared with that in the unvaccinated group (p=0.014). There were no significant differences in oxygen demand for breathing, computed tomography scans, treatments, or outcomes between the 2 groups. However, Bacille Calmette-Guerin‒vaccinated group had significantly less severe pneumonia (p=0.028) and milder deficiency in liver function, consistent with a lower death rate than in the unvaccinated group.
Conclusions
Bacille Calmette-Guerin vaccination received in childhood is associated with less severe COVID-19 pneumonia and milder liver function deficiency in addition to a lower death rate in Bacille Calmette-Guerin‒vaccinated patients than in nonvaccinated individuals.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global public health and economic crisis since its initial appearance in Wuhan, China in December 2019.1 , 2 Various resources worldwide have been allocated to the development of COVID-19 vaccines and to searching for effective treatments to manage this devastating disease.3 , 4 Although COVID-19 vaccines and effective therapeutics are emerging, repurposing the available medical regimens against this disease remains a vital strategy for the current and future pandemics. Among these, the potential for Bacille Calmette-Guerin (BCG) vaccination to mitigate COVID-19 severity and perhaps susceptibility has attracted attention worldwide for the following reasons. First, BCG, a live attenuated vaccine, was developed to prevent tuberculosis 100 years ago and has been since administered to infants worldwide with a proven safety record over the last century. It is the most used vaccine in the world, and 130 million children receive the vaccine each year owing to its effectiveness and low cost. Second, epidemiologic, preclinical, and clinical studies have found a wide range of off-target benefits of the vaccine, especially with regard to respiratory tract viral infections—including those from respiratory syncytial virus, influenza A virus, and herpes simplex virus type 2.5 , 6 Finally, the number of confirmed COVID-19 cases appear to be fewer and the death toll seems to be lower in countries with universal BCG vaccine coverage than in countries without it.7 , 8 These observations boost hopes for use of BCG vaccination as a bridging strategy to protect against a wide range of emerging pathogens, including SARS-CoV-2, their mutations, or future new severe acute respiratory syndrome viruses before specific vaccines are developed.
However, definitive proof of its benefit is required to validate this broad approach, which remains lacking. Most current ecologic studies fail to consider some major confounding factors in the linkage between COVID-19 heterogenicity and the prevalence of BCG childhood vaccination program in different countries. These confounding factors include differences in demography, ethnicity, time of infections, age, sex, BMI, chronic underlying medical conditions, nonpharmaceutical interventions, and diagnosis and reporting of COVID-19 cases. As a consequence, conflicting results are reported with either positive or no real correlation between BCG vaccination and reduced COVID-19 incidence and fatality rates.9 , 10 When COVID-19 infection rates were investigated in young adults aged 35–41 years differing only in BCG status in Israel, BCG vaccination in childhood was found to have no effect on COVID-19 infection rates.11 Effects of BCG vaccination on the severity of COVID-19 was not assessed in that study because those young adults exhibit mostly mild or moderate disease.
The BCG vaccination program was initiated in China in 1950 but did not become a routine vaccination until 1982, which provides a unique opportunity to investigate the influence of BCG vaccination on COVID-19 severity and infection rates in the same demographic, ethnic, epidemiologic infection curve, social, cultural, and climate environment. Accordingly, this study analyzed a cohort of patients with COVID-19 from the same hospital in the same period of the pandemic curve to eliminate any of the aforementioned inherent biases and determine the beneficial effects of BCG vaccination on COVID-19 severity between 2 similar groups of patients with only 1 variable: BCG immunization history. The investigation provides the first direct evidence that BCG vaccination in childhood may attenuate COVID-19 pneumonia severity and improve liver function in association with a lower death rate.
METHODS
Study Sample
A total of 428 patients with COVID-19 were admitted to the Wuhan Pulmonary Hospital in China from February 1, 2020 to March 30, 2020. All patients were confirmed positive for SARS-CoV-2 with ≥2 real-time polymerase chain reaction tests. To avoid any influence of age, the medical records of patients at age 39–62 years were analyzed because patients at these ages receiving BCG vaccination in childhood were relatively sufficient in number. Patients born before 1958 or after the BCG vaccination program became mandated in China in 1982 were excluded from the investigation. Patients with or without BCG vaccination were verified on the basis of the presence of a BCG scar on their arm and were compared across a number of clinical characteristics. The study was approved by the Research Ethics Commission of Wuhan Pulmonary Hospital, China (2020–15).
Measures
Basic information, medical history, clinical symptoms, clinical laboratory tests, treatments, and outcome data were extracted from electronic medical records. All information was obtained and curated with a customized data collection form. Pneumonia severity was determined according to the Chinese COVID-19 diagnosis and treatment protocol (seventh edition). Briefly, mild disease is defined as no signs of pneumonia on computed tomography (CT) imaging; moderate disease refers to symptoms such as fever, respiratory tract lesion, and manifestations of pneumonia on CT imaging; severe disease must have 1 of the following: onset of shortness of breath, respiration rate ≥30 times/minute, finger oxygen saturation ≤93% when at rest, partial arterial oxygen pressure/fraction of inspiration oxygen ≤300 mm Hg, or pulmonary imaging showing a significant progression of lesions within 24–48 hours (>50%); and critical disease is defined by any of the following: respiratory failure and mechanical ventilation required or shock alone or combined with other organ failures, requiring intensive-care-unit admission. Two investigators independently reviewed the data collection forms to ensure that the data collection was unbiased.
Statistical Analysis
Continuous variables are presented as median (IQR), and categorical variables are presented as n (%). Mann–Whitney U-test, Wilcoxon signed-rank test, independent-samples t-test, Fisher's exact test, or chi-square test was initially used to compare the differences between BCG vaccination and nonvaccination groups as appropriate. Variables that were significant in the univariate analysis were further examined with the multivariate logistic regression model to ascertain independent factors after adjusting age, sex, and BMI. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS, version 22.
RESULTS
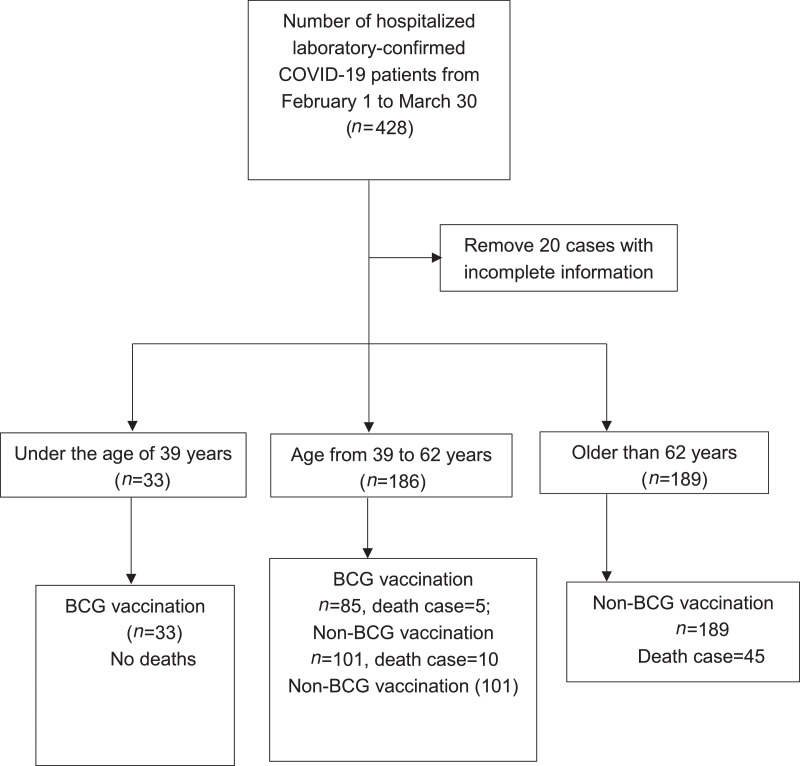
From February 1, 2020 to March 30, 2020, a total of 428 patients with COVID-19 were admitted to the Wuhan Pulmonary Hospital in China. SARS-CoV-2 viral infection was confirmed with at least 2 real-time polymerase chain reaction tests. Patients (n=20) with incomplete medical records were removed from the study. Overall, the death rate was significantly lower in the BCG-vaccinated group (4.2%, 5 of 118) than in the unvaccinated group (19.0%, 55 of 290) (p=0.00014, chi-square test). However, young patients aged <39 years were all BCG vaccinated because BCG vaccination became mandatory in China in 1982, and they are less likely to die of COVID-19. By contrast, none of the older patients (n=189) aged >62 years received the BCG vaccine because the BCG vaccine was seldom administered in China before 1958. It has been well documented that elderly patients died of the disease or had the disease at a relatively high mortality rate. Therefore, patients in these 2 groups were excluded from the investigation to circumvent any influence of age on COVID-19 (Figure 1 ). The remaining 186 patients were divided into 2 groups on the basis of BCG vaccination history (Figure 1). The median age of the BCG-vaccinated group was 51.5 (SD=6.469) years, consisting of 43 male (50.588%) and 42 female (49.412%) patients. The unvaccinated group had a median age of 53 (SD=7.090) years, among whom 48 (47.525%) were male and 53 (52.475%) were female (Table 1 ). There was no significant difference in age or sex between the 2 groups. Clinical symptoms such as fever, cough, sore throat, stuffy nose, fatigue, muscle aches, chest tightness, difficult breathing, breath-holding spells, digestion-related symptoms, dizziness, or heart palpitations also did not differ significantly between the 2 groups (Table 1). BMI and chronic underlying conditions, including pulmonary disease, cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic liver disease, chronic gastropathy, hyperlipidemia, diabetes, cancer, surgery history, and other diseases were all similar irrespective of BCG vaccination history (Table 1). However, a significantly higher percentage of patients in the BCG-vaccinated group experienced chills than in the unvaccinated group (p=0.014) (Table 1).
Figure 1.
A depiction of patient with COVID-19 enrollment in the study.
BCG, Bacille Calmette-Guerin.
Table 1.
Clinical Characteristics of the Patients
| Characteristics | Total(n=186) | BCG vaccination(n=85) | Non-BCG vaccination(n=101) | p-value |
|---|---|---|---|---|
| Age, years, 62≥ age ≥39, median (IQR) | 52±6.813 | 51.5±6.469 | 53±7.090 | 0.370 |
| Sex, n (%) | 0.977 | |||
| Male | 91 (48.925) | 43 (50.588) | 48 (47.525) | |
| Female | 95 (51.075) | 42 (49.412) | 53 (52.475) | |
| BMI (18.5‒23), n (%) | 0.523 | |||
| BMI >23 | 101 (54.301) | 44 (51.766) | 57 (56.436) | |
| 23≥BMI≥18.5 | 81 (43.548) | 39 (45.882) | 42 (41.584) | |
| BMI<18.5 | 4 (2.151) | 2 (2.353) | 2 (1.980) | |
| Coexisting conditions, n (%) | ||||
| Pulmonary diseasea | 15 (8.065) | 9 (10.588) | 6 (5.941) | 0.228 |
| Cardiovascular diseaseb | 11 (5.914) | 5 (5.882) | 6 (5.941) | 0.984 |
| Cerebrovascular disease | 8 (4.301) | 5 (5.882) | 3 (2.970) | 0.471 |
| Chronic kidney disease | 4 (2.151) | 3 (3.529) | 1 (0.990) | 0.329 |
| Chronic liver disease | 9 (4.839) | 4 (4.706) | 5 (4.950) | 0.965 |
| Chronic gastropathy | 9 (4.839) | 5 (5.882) | 4 (3.960) | 0.734 |
| Hypertension | 53 (28.495) | 26 (30.588) | 27 (26.733) | 0.500 |
| Hyperlipidemia | 43 (23.118) | 20 (23.529) | 23 (22.772) | 0.839 |
| Diabetes | 25 (13.441) | 12 (14.118) | 13 (12.871) | 0.759 |
| Cancer | 6 (3.226) | 2 (2.353) | 3 (2.970) | 1 |
| Had surgery | 2 (1.075) | 1 (1.176) | 1 (0.990) | 1 |
| Othersc | 38 (20.430) | 19 (22.353) | 19 (18.812) | 0.502 |
| Clinical symptoms, n (%) | ||||
| Fever >37.3°C | 133 (71.505) | 61 (71.765) | 72 (71.287) | 0.760 |
| High fever >39°C | 36 (19.355) | 15 (17.647) | 21 (20.792) | 0.639 |
| Coughd | 91 (48.925) | 39 (45.882) | 52 (51.485) | 0.537 |
| Chill | 47 (25.269) | 29 (34.118) | 19 (18.812) | 0.014 |
| Sore throat | 10 (5.376) | 4 (4.706) | 6 (5.941) | 0.736 |
| Stuffy nose | 5 (2.688) | 1 (1.176) | 4 (3.960) | 0.252 |
| Fatigue | 82 (44.086) | 34 (40) | 48 (47.525) | 0.368 |
| Muscle ache | 48 (25.806) | 19 (22.353) | 29 (28.713) | 0.367 |
| Chest tightness | 58 (31.183) | 28 (32.941) | 30 (29.703) | 0.566 |
| Difficulty breathing | 41 (22.043) | 22 (25.882) | 19 (18.812) | 0.216 |
| Breathing spell | 30 (16.129) | 15 (17.647) | 15 (14.851) | 0.561 |
| Digestion relatedf | 62 (33.333) | 29 (34.118) | 33 (32.673) | 0.755 |
| Dizziness | 7 (3.763) | 3 (3.529) | 4 (3.960) | 1 |
| Heart palpitations | 5 (2.688) | 1 (1.176) | 4 (3.960) | 0.380 |
| Othersg | 41 (22.043) | 25 (29.412) | 27 (26.733) | 0.619 |
Note: Boldface indicates statistical significance (p<0.05). The values shown are based on available data. Plus minus values are means±SD.
Pulmonary diseases: chronic obstructive pulmonary disease, asthma, branch expansion, pulmonary fibrosis, pulmonary emphysema, and pulmonary tuberculosis.
Cerebrovascular diseases: coronary heart disease and arrhythmia.
Others in this study include rheumatoid arthritis, gout, uterine fibroids, psoriasis, and others.
Cough in this study includes cough with or without sputum or hemoptysis.
Digestion-related symptoms: anorexia, nausea, vomiting, and diarrhea.
Others: conjunctival hyperemia, poor sleep, and weight loss.
BCG, Bacille Calmette-Guerin.
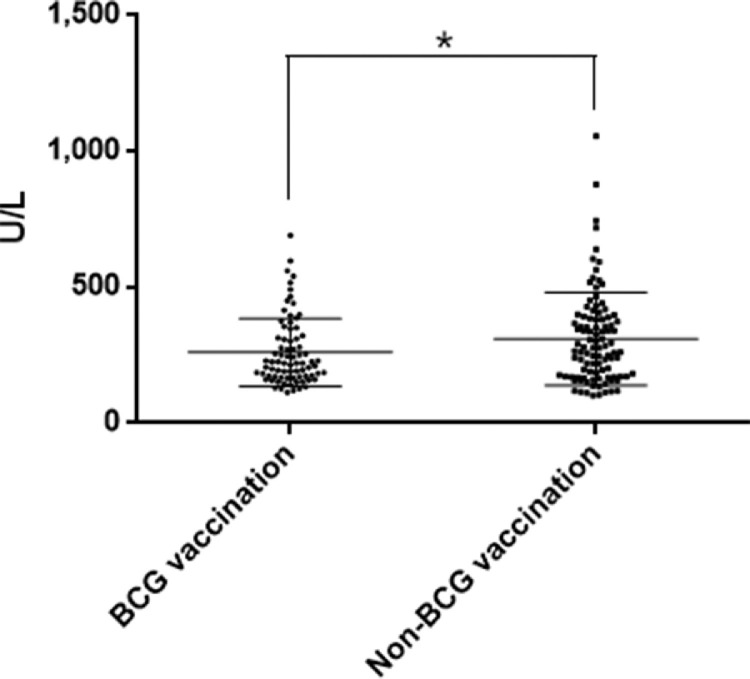
There were 10 deaths (9.9%) in the unvaccinated group and 5 deaths (5.9%) among BCG-vaccinated patients, a 40% reduction (Figure 1). In continuation of this positive trend, the percentages of patients suffering from severe and critical pneumonia were significantly lower in the BCG-vaccinated group than in the nonvaccinated group. Only 1 patient (1.18%) was in critical condition in the BCG-vaccinated group, whereas 6 patients (5.94%) were critically ill in the unvaccinated group (Table 2 ). Concomitantly, more patients or a higher proportion of patients with mild or moderate pneumonia were identified in the BCG-vaccinated group than in the unvaccinated group (Table 2). The higher percentage of patients with mild and moderate pneumonia, along with a lower proportion of patients with severe and critical COVID-19, suggested a significant reduction of COVID-19 pneumonia severity in BCG-vaccinated compared with that in the unvaccinated groups (p=0.028) (Table 2). Moreover, a significant decrease in the mean concentration of serum lactate dehydrogenase (p=0.031) (Figure 2 ) and a declining trend in lactate dehydrogenase levels were observed in the BCG-vaccinated group compared with those in the unvaccinated patients (Table 2). Lactate dehydrogenase has been reported to be an independent risk factor for lung injury and severe COVID-19 because it is released from injured cells.12 Finally and unexpectedly, investigators found significantly lower percentages of patients producing abnormal levels of alanine aminotransferase (p=0.005) or aspartate transaminase (p=0.029) in the BCG-vaccinated group than in the unvaccinated group (Table 2).
Table 2.
Patients’ Status and the Treatments They Received in the Entire Hospitalization Period
| Characteristics | Total(n=186) | BCG vaccination(n=85) | Non-BCG vaccination(n=101) | p-value |
|---|---|---|---|---|
| Severity level of pneumonia, n (%) | 0.028* | |||
| 1=Mild | 12 (6.452) | 8 (9.412) | 4 (3.960) | |
| 2=Moderate | 96 (51.613) | 47 (55.294) | 49 (48.515) | |
| 3=Severe | 71 (38.172) | 29 (34.118) | 42 (41.584) | |
| 4=Critical | 7 (3.763) | 1 (1.176) | 6 (5.941) | |
| Oxygen demand for breathing, n (%) | 0.265 | |||
| Not requiring supplemental oxygen | 68 (36.559) | 33 (38.824) | 35 (34.653) | |
| Requiring LFNC supplemental oxygen | 55 (29.570) | 28 (32.941) | 27 (26.733) | |
| Requiring HFNC or NIMV | 51 (27.419) | 19 (22.353) | 32 (31.683) | |
| Requiring ECMO, IMV, or both | 12 (6.452) | 5 (5.882) | 7 (6.931) | |
| CT results | ||||
| Ground-glass image in the lung, n (%) | 174 (93.548) | 79 (92.941) | 95 (94.059) | 0.774 |
| Number of affected lung lobes, median (IQR), n | 3.44 (2,5) | 3.38 (2.5,5) | 3.51 (3,5) | 0.317 |
| Patch, stripe shadow, n (%) | 86 (46.237) | 40 (47.059) | 46 (45.545) | 0.836 |
| Pleural adhesion, thickening, or effusion, n (%) | 24 (12.903) | 11 (12.941) | 13 (12.871) | 1 |
| Others,an (%) | 17 (9.140) | 6 (7.059) | 11 (10.891) | 0.391 |
| Laboratory test results, n (%) | ||||
| White-cell count <4 × 10−9/L | 38 (20.430) | 19 (22.353) | 19 (18.812) | 0.502 |
| Lymphocyte count <1.0 × 10−9/L | 88 (47.312) | 39 (45.882) | 49 (48.515) | 0.827 |
| Hypersensitive −CRP>5 mg/L | 105 (56.452) | 47 (55.294) | 58 (57.426) | 0.901 |
| ALT (male >50 U/L, female >40 U/L) | 74 (39.785) | 24 (28.235) | 50 (49.505) | 0.005⁎⁎ |
| AST (male >40 U/L, female >35 U/L) | 62 (33.333) | 21 (24.706) | 41 (40.594) | 0.029* |
| LDH >245 U/L | 85 (45.699) | 32 (37.647) | 53 (52.475) | 0.059 |
| Mass creatine kinase MB isoenzyme >5.86 ng/mL | 10 (5.376) | 3 (3.529) | 7 (6.931) | 0.516 |
| Myoglobin increase >100 ng/mL | 14 (7.527) | 4 (4.706) | 10 (9.901) | 0.267 |
| Troponin I >0.1 ng/mL | 14 (7.527) | 5 (5.882) | 9 (8.911) | 0.460 |
| ESR (male >15 mm/h, female >20 mm/h) | 143 (76.882) | 63 (74.118) | 80 (79.208) | 0.581 |
| Procalcitonin >0.25 ng/mL | 21 (11.90) | 7 (8.235%) | 14 (13.861) | 0.248 |
| D-dimer >0.5 mg/L | 75 (40.323) | 33 (38.824) | 42 (41.584) | 0.794 |
| Treatments, n (%) | ||||
| Using interferon | 126 (67.742) | 60 (70.588) | 66 (65.347) | 0.329 |
| Noninvasive mechanical ventilation | 28 (15.054) | 10 (11.765) | 18 (17.822) | 0.276 |
| Invasive mechanical ventilation | 12 (6.452) | 5 (5.882) | 7 (6.931) | 0.801 |
| ECMO | 4 (2.151) | 2 (2.353) | 2 (1.980) | 1 |
| Antibiotic agent | 103 (55.376) | 46 (54.118) | 57 (56.436) | 0.878 |
| Glucocorticoid therapy | 81 (43.548) | 32 (37.647) | 49 (48.515) | 0.173 |
| Outcome after treatments | ||||
| Days of recovered,b median (IQR) | 16.2 (13‒22) | 15.5 (12‒21) | 0.317 | |
| Recovered rate, n (%) | 94 | 90 | 0.297 |
Note: Boldface indicates statistical significance (*p<0.05; ⁎⁎p<0.01).
Others: bronchial inflation sign, enlarged mediastinal lymph nodes, and pericardial effusion.
Recovered means no pathologic symptoms and also that viral nucleic acid test, conducted twice, was negative.
ALT, alanine aminotransferase; AST, aspartate transaminase; BCG, Bacille Calmette-Guerin; CRP, C-reactive protein; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; ESR, erythrocyte sedimentation rate; HFNC, high-flow nasal cannula oxygen therapy; IMV, invasive mechanical ventilation; LDH, lactate dehydrogenase; LFNC, low-flow nasal cannula oxygen therapy; MB, muscle and brain; NIMV, noninvasive mechanical ventilation.
Figure 2.
The mean value of lactate dehydrogenase in BCG-vaccinated and unvaccinated groups.
Note: Each symbol represents data from 1 patient; *p<0.05.
BCG, Bacille Calmette-Guerin.
The beneficial effects of BCG vaccination were independent of age or chronic underlying health conditions (Table 1). Moreover, the oxygen demand for breathing was assessed by any need for supplemental oxygen or requiring low-flow nasal cannula oxygen therapy, high-flow nasal cannula oxygen therapy, noninvasive mechanical ventilation, extracorporeal membrane oxygenation, or invasive mechanical ventilation, none of which were discriminable statistically in the presence or absence of BCG vaccination (Table 2). CT scans also showed no difference between the 2 groups while comparing ground-glass opacities in the lung, number of affected lung lobes, patches, stripe shadows, pleural adhesion, thickening, or effusion (Table 2). In accordance with the CT scans, there were no significant differences between these 2 groups when examining white-cell counts; lymphocyte counts; or the levels of hypersensitive C-reactive protein, creatine kinase muscle and brain isoenzyme, myoglobin, troponin I, erythrocyte sedimentation rate, procalcitonin, or D-dimer (Table 2). The investigation also failed to identify any significant differences in treatment options patients received during hospitalization or in the subsidiary cabin medical facility irrespective of BCG vaccination history.
DISCUSSION
This study provides the first direct clinical evidence that BCG vaccination received in childhood is associated with less severe COVID-19 pneumonia and milder deficiency in liver function in connection with a lower death rate in BCG-vaccinated patients than in unvaccinated individuals. This finding agrees with recent ecologic studies and the well-recognized benefits of BCG vaccination leading to a decreased number of infections of all causes, especially of respiratory tract infections, in people of all ages.5 , 6 , 13 , 14 Similar to the BCG vaccine, measles, mumps, and rubella vaccines and the oral polio vaccine have exhibited off-target benefits.14 , 15 The broad protective and lifelong benefit of BCG vaccination is intriguing and could serve a desperately urgent need for protecting people from a wide range of emerging pathogens. For instance, if a more lethal severe acute respiratory syndrome virus or mutated SARS-CoV-2 transmit among humans in the future, the world could experience another emerging pathogen with potentially devastating consequences. This possibility cannot be ruled out because SARS-CoV-2 is mutating and the SARS-CoV-2 D614G variant is reported to be 10 times more contagious than the original virus.16 Conceivably, BCG vaccination could protect people to some degree at the beginning of a pandemic, bridging the 1- to 2-year period until a specific vaccine is developed in case this occurs.
Apart from COVID-19 severity, a previous investigation suggests that BCG vaccination in childhood provides little prophylaxis against SARS-CoV-2 infection.11 The authors could not determine any prophylactic effect of BCG vaccination on SARS-CoV-2 viral infection in this study because the BCG vaccination rate among people aged 39–62 years is unknown. Yet, in the authors’ view, any conclusion on the impact of childhood BCG vaccination on infection should not serve as a reference to predict whether single or multiple doses of BCG vaccination in adults, as proposed in ongoing clinical trials, may prevent SARS-CoV-2 infection.17 In fact, BCG revaccination may decrease susceptibility to SARS-CoV-2, as recently shown (I Amirlak, MD, FACS, unpublished data, August 2020). BCG vaccination could induce trained immunity by activation and reprogramming of innate immune cells through histone modifications and epigenetic reprogramming at the regulatory sites of genes encoding inflammatory cytokines in cells.18 , 19 Hematopoietic stem cells and myeloid progenitors in the bone marrow or microbiota in the gut may be also imprinted by BCG-mediated metabolic and epigenetic processes either directly or indirectly and may be involved in trained immunity, especially when the BCG vaccine is administered at birth.20 , 21 Epigenetically trained monocytes and natural killer cells can respond to bacterial or viral pathogens more strongly through pathogen-associated molecular patterns or other mechanisms and promote host defense.19 Although trained immunity is thought to be long lasting, whether it can be fully or only partially sustained throughout life is unknown. Therefore, recent BCG vaccination, especially multiple doses, may confer much stronger trained immunity than childhood BCG vaccination does, an assumption that remains to be tested, however.22
One concern has been raised with respect to whether nonspecific augmentation of the innate immune response by BCG can deteriorate the exaggerated cytokine responses associated with complications in patients with COVID-19. This concern can be eased, at least in part, by the current investigation showing that BCG's imprint exerts no significant adverse events except for an increased chill response, which may be due to an enhanced innate immune response,23 although the mechanism underlying the chill response remains to be determined. Nevertheless, this response is minor and greatly outweighed by the benefits of BCG vaccination. Another unexpected finding is less liver dysfunction in patients with COVID-19 with BCG immunization history, which is likely associated with metabolic reprogramming that alters certain metabolites that can function as cofactors in some liver enzymes.24
Limitations
Despite the extensive adjustment, it is still possible that some unmeasured factors may play a role and that some data may be missing or inaccurate in some patients’ electronic health records. For instance, patients with tuberculosis infection history, irrespective of whether it was latent or active, may be categorized into the unvaccinated group, and the significance of BCG vaccination might be underestimated. Moreover, the effect may be more predominant in patients with mild or no symptoms who were not admitted to the hospital or included in this study, as suggested by a recent study showing a significant decrease in hospital admission rate (3.7% vs 15.8%) in people with BCG vaccination history versus those without BCG vaccination history.25
CONCLUSIONS
This investigation suggests that BCG vaccination in children is associated with less severe COVID-19 pneumonia, milder liver function deficiency, and a lower death rate than no BCG vaccination. This conclusion is supported after controlling for major confounding factors, including age, sex, ethnicity, BMI, the pandemic curve, demography, diabetes, and other underlying medical conditions.
ACKNOWLEDGMENTS
The authors acknowledge the sacrifice, devotion, and commitment of all healthcare workers. This study is dedicated to the memory of those who have given their lives in the care of patients with COVID-19. FC, GC, and JZ contributed equally to this article.
The study is supported in part by Wellman Center Discretionary Fund, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. https://doi.org/10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F, Zhao S, Yu B. A new coronavirus associated with human respiratory disease in China [published correction appears in Nature. 2020;580(7803):E7] Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. https://doi.org/10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn DG, Shin HJ, Kim MH. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. https://doi.org/10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. https://doi.org/10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodridge HS, Ahmed SS, Curtis N. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16(6):392–400. doi: 10.1038/nri.2016.43. https://doi.org/10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. https://doi.org/10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat Rev Urol. 2020;17(6):316–317. doi: 10.1038/s41585-020-0325-9. https://doi.org/10.1038/s41585-020-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyasaka M. Is BCG vaccination causally related to reduced COVID-19 mortality? EMBO Mol Med. 2020;12(6):e12661. doi: 10.15252/emmm.202012661. https://doi.org/10.15252/emmm.202012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–337. doi: 10.1038/s41577-020-0337-y. https://doi.org/10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor E, Teh J, Kamat AM, Lawrentschuk N. Bacillus Calmette Guérin (BCG) vaccination use in the fight against COVID-19 – what's old is new again? Future Oncol. 2020;16(19):1323–1325. doi: 10.2217/fon-2020-0381. https://doi.org/10.2217/fon-2020-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323(22):2340–2341. doi: 10.1001/jama.2020.8189. https://doi.org/10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y, Zhang H, Mu S. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) 2020;12(12):11245–11258. doi: 10.18632/aging.103372. https://doi.org/10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2) doi: 10.1016/j.cell.2020.08.051. 315–323.e9https://doi.org/10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA. 2014;311(8):826–835. doi: 10.1001/jama.2014.470. https://doi.org/10.1001/jama.2014.470. [DOI] [PubMed] [Google Scholar]
- 15.Sørup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis. 2015;3(1):ofv204. doi: 10.1093/ofid/ofv204. https://doi.org/10.1093/ofid/ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korber B, Fischer WM, Gnanakaran S. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4) doi: 10.1016/j.cell.2020.06.043. 812–827.e19https://doi.org/10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ten Doesschate T, Moorlag SJCFM, van der Vaart TW. Two randomized controlled trials of bacillus Calmette-Guérin vaccination to reduce absenteeism among health care workers and hospital admission by elderly persons during the COVID-19 pandemic: a structured summary of the study protocols for two randomised controlled trials. Trials. 2020;21(1):481. doi: 10.1186/s13063-020-04389-w. https://doi.org/10.1186/s13063-020-04389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netea MG, Joosten LA, Latz E. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. https://doi.org/10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed S, Quintin J, Kerstens HH. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345(6204) doi: 10.1126/science.1251086. https://doi.org/10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke TB. Microbial programming of systemic innate immunity and resistance to infection. PLoS Pathog. 2014;10(12) doi: 10.1371/journal.ppat.1004506. https://doi.org/10.1371/journal.ppat.1004506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann E, Sanz J, Dunn JL. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1–2) doi: 10.1016/j.cell.2017.12.031. 176–190.e19https://doi.org/10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Gursel M, Gursel I. Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic? Allergy. 2020;75(7):1815–1819. doi: 10.1111/all.14345. https://doi.org/10.1111/all.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arts RJW, Moorlag SJCFM, Novakovic B. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1) doi: 10.1016/j.chom.2017.12.010. 89–100.e5https://doi.org/10.1016/j.chom.2017.`12.010. [DOI] [PubMed] [Google Scholar]
- 24.Ryan DG, O'Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38:289–313. doi: 10.1146/annurev-immunol-081619-104850. https://doi.org/10.1146/annurev-immunol-081619-104850. [DOI] [PubMed] [Google Scholar]
- 25.Weng CH, Saal A, Butt WW. Bacillus Calmette-Guérin vaccination and clinical characteristics and outcomes of COVID-19 in Rhode Island, United States: a cohort study. Epidemiol Infect. 2020;148:e140. doi: 10.1017/S0950268820001569. https://doi.org/10.1017/S0950268820001569. [DOI] [PMC free article] [PubMed] [Google Scholar]