Abstract
Neuropilin 1 (Nrp1) is a type I transmembrane protein that plays important roles in axonal guidance, neuronal development, and angiogenesis. Nrp1 helps migrate thymus-derived regulatory T cells to vascular endothelial growth factor (VEGF)-producing tumors. Little is known about the role of Nrp1 on CD4 T cells during atherosclerosis. In ApoE−/− mice fed a western diet (WD) for 15 weeks, we found a two-fold increase in Nrp1+Foxp3− CD4 T cells in the spleens, peri-aortic lymph nodes (PaLN) and aortas of those mice compared to chow-fed mice. Nrp1+Foxp3− CD4 T cells had higher proliferation potential, expressed higher levels of the memory marker CD44, and produced more IFNγ when compared to Nrp1− CD4 T cells. Treatment of CD4 T cells with oxLDL increased Nrp1 expression. Using atherosclerosis-susceptible mice that were selectively deficient for Nrp1 expression on T cells, we found that mice lacking Nrp1 developed less atherosclerosis than their Nrp1-sufficient counterparts. Mechanistically, we found that CD4 T cells that express Nrp1 have higher capacity to migrate to the aorta and PaLN than Nrp1− T cells, suggesting that the expression of Nrp1 facilitates the recruitment of CD4 T cells into the aorta where they can be pathogenic. Thus, we have identified a novel role of Nrp1 on CD4 T cells in atherosclerosis. These results suggest that manipulation of Nrp1 expression on T cells can affect the outcome of atherosclerosis and lower disease incidence.
Introduction:
Neuropilin 1 (Nrp1) is a type I transmembrane protein that acts as a receptor for the semaphorin 3 family of proteins (1–3) and a co-receptor for vascular endothelial growth factor isoforms (4). It is expressed on a wide range of tissues and has a variety of functions including migration, proliferation, and adhesion (5). Its expression is essential for axonal guidance and neuronal development as well as cardiac formation and angiogenesis. Global deficiency of Nrp1 in mice is embryonically lethal due to a heart, blood vessels and neuronal development defect (6, 7). In the immune system, Nrp1 is expressed primarily on natural occurring regulatory T cells (Treg cells) (8), recent thymic immigrants of IL-17 producing invariant NKT cells (9), and some myeloid cells (10, 11). Expression of Nrp1 is thought to be able to differentiate between natural occurring Treg cells and peripherally induced cells (12, 13), although there is some controversy in the matter (14). Nrp1 expression on Treg cells may play a role in increasing the interaction between dendritic cells and Treg cells, preventing other T cells from interacting with dendritic cells and therefore reducing priming of effector T cells as blocking antibodies against Nrp1 limits such interactions (15, 16). Nrp1 is also thought to be a receptor for inactive TGFβ promoting Treg cell activity (17).
In recent years, neuropilins have gained a spotlight as immunological modulators both in physiological and pathological conditions (reviewed in (18)). In disease, much of Nrp1 role in the immune system has been studied in the context of tumor immunity. Studies have shown that the expression of Nrp1 on Treg cells allow for these suppressive cells to infiltrate tumor microenvironment rich in VEGF expression, where they can suppress effector T cell activation at the local environment, and therefore, support tumor growth (19). In addition, a study by Delgoffe et al. showed that the lack of Nrp1 on Treg cells lessens Treg capacity to suppress anti-tumor responses without impairing suppression of auto-immunity (20). This occurs through the Nrp1 and Semaphorin-4A axis rather than Nrp1 and VEGF interaction, suggesting the dual suppressive action of Nrp1 against anti-tumor responses.
Due to its suppressive role on Treg cells, Nrp1 signaling is thought to reduce the incidence of inflammatory diseases. A study examining the role of Nrp1 in EAE model of multiple sclerosis showed that the lack of Nrp1 resulted in an increase in inflammation, and EAE disease severity due to the increase of IL-17 production by CD4 T cells (21). Another study showed that showed that the administration of a plasmid encoding Semaphorin 3A reduced the severity of experimental rheumatoid arthritis by increasing the development of Nrp1+ suppressive CD4 T cells (22). Transfer of Nrp1+ Treg cells allows for skin graft survival and reduction of effector CD4 T cells (23).
Yet, little is known about the role of Nrp1 in cardiovascular disease and atherosclerosis. Since effector CD4 T cells and Treg cells play important, yet opposing roles in the pathogenesis of atherosclerosis (reviewed in (24–27), it is important to understand the role Nrp1 plays during disease progression. Is Nrp1 protective against the development of atherosclerosis? In this study, we sought to answer the question regarding the role of Nrp1 on atherosclerosis development focusing on the T cell arm of the immune system.
Material and Methods:
Mice
Original breeding pairs of B6, ApoE−/−, Ldlr−/−, Nrp1fl/fl, and LCKCre mice were purchased from The Jackson Laboratories (Bar Harbor, Maine; Stock numbers 000664, 002055, 002207, 005247, and 003802, respectively). Foxp3-YFP-IRES-Cre-Rosa26-dt-RFP-ApoE−/− (LT-ApoE−/−) and Foxp3-YFP-IRES-Cre-Rosa26-dt-RFP-Ldlr−/− mice (LT-Ldlr−/−) were developed in house at the La Jolla Institute for Immunology, as previously described (28, 29). Mice were used for experiments at 6-10 weeks of age. All mice were fed a standard chow diet containing 0% cholesterol and 5% calories from fat (Pico lab, #5053, Saint Louis, MO), a Western Diet (42% from fat, 0.2% from cholesterol) (Harlan, #TD 88137, Placentia, CA), or a high cholesterol diet (High Fat Rodent Diet with Regular Casein and 1.25% added cholesterol (Research Diets Inc, #D12108C, New Brunswick, NJ). Mice were housed in a pathogen free animal facility of LJI. Experiments followed guidelines of the LJI Animal Care and Use Committee and the use of rodents was approved according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health.
Flow Cytometry
Peri-aortic LNs (PaLN), inguinal lymph nodes (ILN), or spleens were passed through a 40 μm cell strainers. Spleen RBCs were lysed with 1X RBCs lysis buffer (Biolegend, San Diego, CA). Aortas were perfused with ice cold PBS, explanted, and digested with DNAse I, Collagenase type I, Collagenase type XI and Hyaluronidase type I. All antibodies were purchased from Ebioscience or Biolegend (San Diego, CA) unless otherwise indicated. Samples were stained with Live Dead fixable dye (ThermoFisher, Carlsbad, CA). Cells were surface stained with antibodies against CD4 (clone RM4-4), TCRβ (clone H57-597), Nrp1 (clone N43-7; MBL International, Woburn, MA), CD44 (clone IM7), CD25 (clone PC61), and CCR5 (clone HM-CCR5), in cold FACS buffer (2% BSA, 0.01% sodium azide in PBS). Cells were fixed and permeabilized with Foxp3 staining buffer kit (Ebioscience), then stained with antibody against Foxp3 (clone FJK-16S). All staining was done on ice for 30 minutes. In case of LT-ApoE−/− mice, YFP and RFP were detected without further staining. For IFNγ, IL-17, and TNFɑ, cells were stimulated with PMA and ionomycin (50 ng/ml and 1 μg/ml, respectively) for 4-5 hours with the addition of Golgiplug (BD Biosciences; 1 μl/ml), and cells were stained intracellularly with antibodies against IFNγ (clone XMG-1.2), IL-17 (clone ebio1787), and TNFɑ (MP6-XT22). For analysis of PD-1 expression, splenocytes were stimulated with ɑ-CD3/28 dynabeads (Gibco) for 48 hrs. Samples were acquired using LSRII (BD, Bioscience, San Diego, CA) and data were analyzed using Flowjo software (Tree Star, Ashland, OR).
In vivo Proliferation Assay
Mice were injected i.p with 1 mg of BrdU and tissue were harvested 36 hours after injection. Cell suspensions were stained as above, then treated with DNase and stained for BrdU as recommended by the BrdU staining kit (BD Biosciences).
Quantitative Real Time PCR
Nrp1+YFP− or Nrp1−YFP− from LT-ApoE−/− mice were stained as above and sorted using BD Aria. Total cellular RNA from sorted cells were extracted by Trizol (Life Technologies) followed by RNA purification using Direct-zol™ RNA miniPrep (Zymo Research, Irvine, CA) per manufacturer’s instructions. RNA purity and quantity were determined by a NanoDrop spectrophotometer (Thermo Scientific) and equal amounts of RNA was used to synthesize cDNA using iscript cDNA synthesis kit (Bio-Rad). mRNA expression was measured in real time quantitative PCR using TaqMan Gene Expression system and predesigned TaqMan primers for Nrp1 (Mm00435379_m1), Tbx21 (Mm00450960_m1), IFNγ (Mm01168134_m1), IL-2 (Mm00434256_m1), Abca1(Mm00442646_m1), Abcg1 (Mm00437390_m1) and β-actin (Applied Biosystems). Data were analyzed and presented on the basis of relative expression method. The ΔΔCT method was used with β-actin as a housekeeping gene.
OxLDL stimulation
Equal numbers of lymphocytes from ApoE−/− mice or sorted naive (CD44lowCD62Lhi), central memory (CD44hiCD62Lhi), or effector memory (CD44hiCD62Llow) CD4 T cells from B6 mice were cultured in serum free CST OpTimizer™ T cell expansion media (ThermoFisher), supplemented with L-glutamine, penicillin and streptomycin and β-mercaptoethanol, and incubated with or without human oxLDL (unlabeled), or Dil-oxLDL (KalenBiomed, Montgomery Village, MD) for 3 days.
Atherosclerosis Quantification
Mouse aortae were perfused, collected and immersed in paraformaldehyde and stained with Oil Red O, then opened longitudinally and pinned as previously (30). Images were scanned and the percentage of lesion surface area was determined with Photoshop software.
In vitro migration assay
Equal numbers of sorted Nrp1hi and Nrp1low were placed in the upper chamber of a 5 μm transwell plate. In the lower chamber, either media, VEGF-165 (R&D systems), or oxLDL (Kalen Biomedical) was placed. Cells were incubated at 37°C for 3 hours and cells that migrated to the lower chamber were counted. Migration index was calculated by dividing the number of cells that migrated in a particular well by the number of cells that migrated in the control wells (media only).
In vivo migration assay
Total lymphocytes from spleens and peripheral lymph nodes from WD-fed ApoE−/− mice were harvested and stained with Cell trace violet (CTV; ThermoFisher) according to manufacturer’s instructions. Equal numbers of cells were transferred to age matched chow or WD-fed ApoE−/− mice. Cells were allowed to engraft for 18-24 hours before the mice were sacrificed. Aorta, PaLN and ILN were collected and donor cells (CTV+) were determined using flow cytometry as described above.
Statistical Analyses:
All results are reported as mean ± s.e.m. Results were analyzed by unpaired two-tailed Student’s t-test for two groups and one-way analysis of variance (one-way ANOVA), followed by Tukey’s multiple comparisons test for three groups. A P value of < 0.05 is considered to be significant. Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software, Inc.).
Results:
Nrp1+Foxp3− CD4 T cells are increased in atherosclerosis:
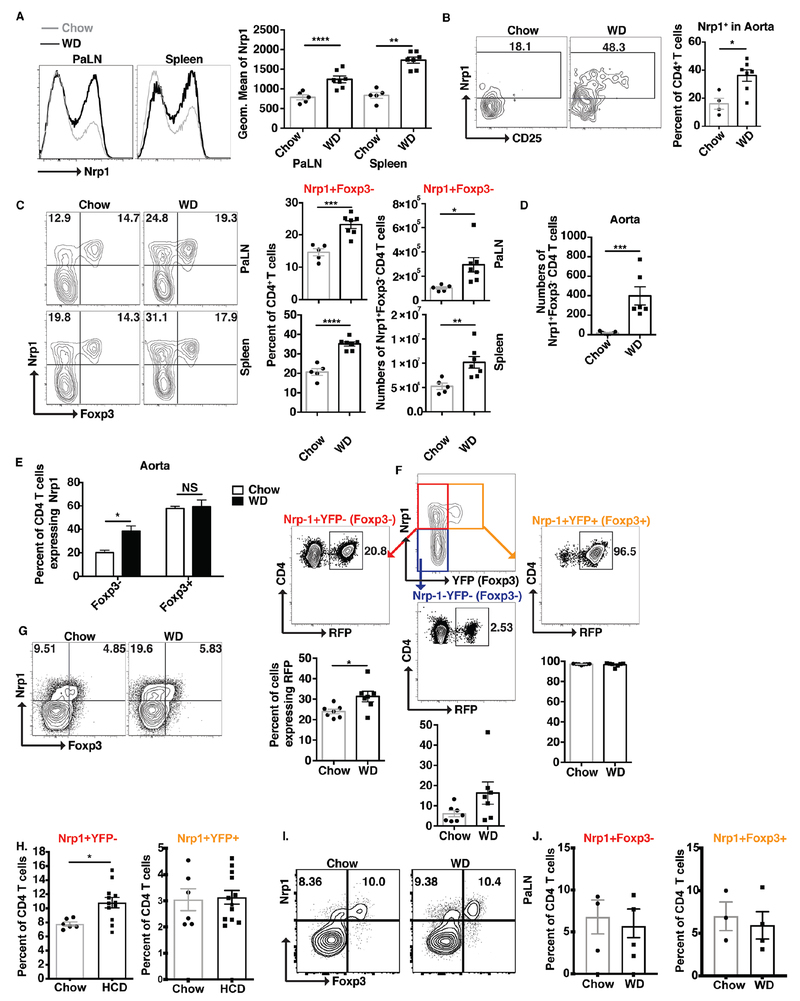
To determine the role of Nrp1 in atherosclerosis, we first examined the expression levels of Nrp1 on CD4 T cells during atherosclerosis development in chow versus western diet (WD)-fed LT-ApoE−/−. We found a 2-fold increase in Nrp1 expression on CD4 T cells in the peri-aortic lymph nodes (PaLNs) and spleens of WD-fed mice (Fig. 1A). We also saw a similar increase in the aortas of these animals (Fig. 1B) compared to chow controls. Upon detailed examination, we found that most of the increase in Nrp1 expression occurred in non Treg cells (CD4+TCRβ+Foxp3−) (Fig. 1C; PaLN and spleen, Fig 1D and 1E; aorta). Since we have previously reported that Treg cells lose their phenotype during atherosclerosis (28), we wanted to ensure that this was not merely a shift of Treg cells that have lost expression of Foxp3. By looking at the expression of RFP, that marks previous expression of Foxp3, our results show that there was only 7% increase in RFP expression in the Nrp1+Foxp3− CD4 T cell population, between chow and WD-fed mice (Fig. 1F). This suggested that conversion of Treg cells is not merely the explanation for the 2 fold increase in Nrp1+Foxp3− CD4 T cell population that we observe in WD-fed mice compared to their chow counterparts, rather that this increase may be driven by atherosclerosis development. Furthermore, to validate that this was not a phenotype of the LT-ApoE−/− mice, we observed the same 2-fold increase in Nrp1+Foxp3− CD4 T cells in ApoE−/− mice (Fig. 1G). Moreover, increased Nrp1+Foxp3− CD4 T cells were observed in the peripheral blood of LT-LDL receptor deficient, Ldlr−/−, a distinct atherosclerosis-prone mouse strain at 6 weeks post high cholesterol diet (HCD), while frequencies of Nrp1+YFP+ cells remained the same (Fig. 1H). Finally, three weeks on an atherogenic diet failed to elicit an increase in Nrp1+Foxp3− CD4 T cells within PaLNs from B6 mice (Fig. 1I, J) or spleen (data not shown), indicating that this increase in Nrp1+Foxp3− cells is governed by atherosclerosis development and a phenomenon of atherosclerosis-prone, not B6 mouse strains.
Figure 1: Nrp1+Foxp3− CD4 T cells are increased in atherosclerosis.
LT-ApoE−/− (A-F) or ApoE−/− (G) mice were fed a WD for 15 weeks, and CD4 T cells were examined for the expression of Nrp1. (A-B) Histograms, flow cytometry plots and graphs showing the expression of Nrp1 on CD4 T cells from PaLN, spleens (A), and aorta (B) from chow and WD-fed mice. (C-D) Flow cytometry plots and graphs showing the expression of Nrp1 and Foxp3 on CD4 T cells in the PaLN, spleens (C) and aorta (D) of the above mice. (E) Graphs showing the percentage of expression of Nrp1 on Foxp3− versus Foxp3+ CD4 T cells in the aorta of the above mice. (F) Distribution of Treg cell signature (RFP+) in the different CD4 T cell population based on the expression of Nrp1 and Foxp3. (G) Flow cytometry plots showing the expression of Nrp1 on CD4 T cells from PaLN from chow and WD-fed ApoE−/− mice. (H) Pooled frequencies of Nrp1+YFP− or Nrp1+YFP+ CD4 T cells in LT-Ldlr−/− mice at 6 weeks post high cholesterol diet (HCD) (n=6 (Chow); n=12 (HCD)). (I) Representative contour plots of Foxp3 by Nrp1 for B6 mice fed chow or WD for 3 weeks. (J) Pooled percentages of Nrp1+Foxp3− and Nrp1+Foxp3+ CD4 T cells from B6 mice fed a western diet for 3 weeks (n=3 mice/group). Results are expressed as the mean ± s.e.m from one of three independent experiments (n=5-7) (A-G), n=6 mice/group (chow), n=12 mice/group (HCD) (H) (one independent experiment), and 3 mice/group (Chow), n=4 mice/group (WD) (J, I) (one independent experiment). Statistically significant differences were at * P<0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001.
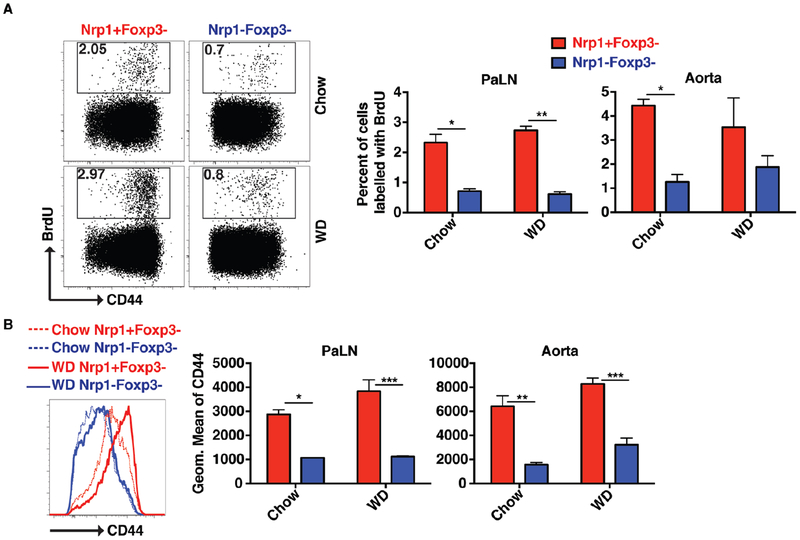
Nrp1+Foxp3− CD4 T cells are more proliferative and highly activated cells:
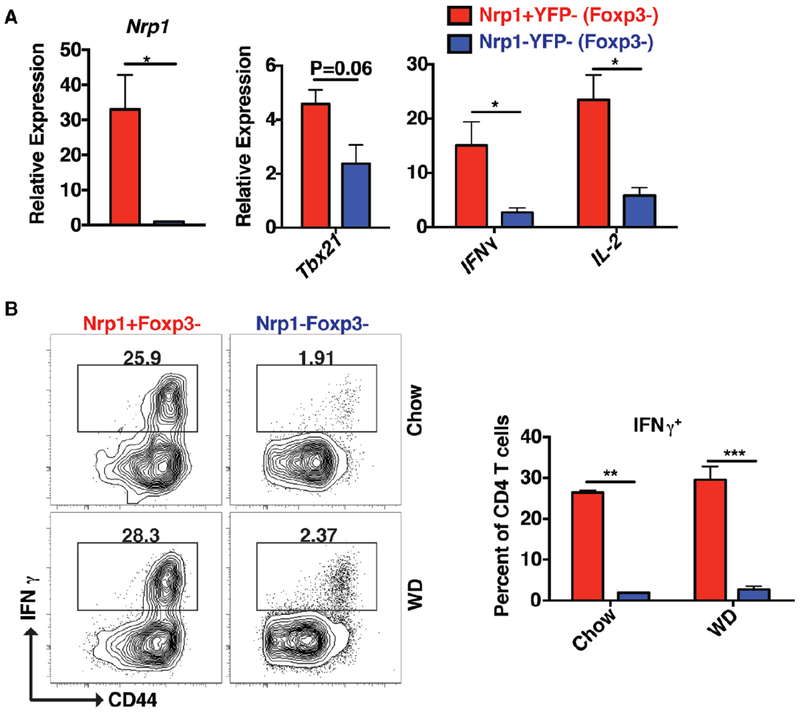
We next sought to characterize the behavior of these Nrp1+Foxp3− CD4 T cells in the aorta and PaLN of chow- and WD-fed ApoE−/− mice. Using BrdU injection to label dividing cells, our results showed that Nrp1+Foxp3− CD4 T cells had a 2 to 3-fold higher proliferative capacity compared to their Nrp1− counterparts (Fig. 2A). This change was accompanied by a 2 to 3-fold increase in the expression of the T cell memory marker CD44 (Fig. 2B). When both cell populations were sorted and assessed for mRNA expression levels, Nrp1+YFP− (Foxp3−) CD4 T cells had higher mRNA expression of Tbx21, IFNγ and IL-2 (Fig. 3A). Nrp1+YFP− (Foxp3−) CD4 T cells also showed ~ 20-fold higher capacity to make IFNγ cytokine compared to Nrp1− CD4 T cells (Fig. 3B).
Figure 2: Nrp1+Foxp3− CD4 T cells are more proliferative and highly activated cells.
Chow and WD-fed ApoE−/− mice were injected with BrdU and CD4 T cells from the PaLN and aorta were harvested after 36 hours to detect the level of BrdU incorporation. (A) Flow cytometry plots and bar graphs showing the percentage of BrdU incorporated in Nrp1+Foxp3− versus Nrp1−Foxp3− CD4 T cells. (B) Histograms and bar graphs showing the expression of CD44 on Nrp1+Foxp3− versus Nrp1−Foxp3− CD4 T cells. Results are expressed as the mean ± s.e.m from one of two independent experiments (n=3). Statistically significant differences were at * P<0.05, ** P < 0.01, and *** P < 0.001.
Figure 3: Nrp1+Foxp3− CD4 T cells are high IFNγ producers.
(A) Nrp1+YFP− and Nrp1−YFP− CD4 T cells were sorted and the mRNA message level for Nrp1, Tbx21, IFNγ and IL-2 was detected via qPCR. (B) Flow cytometry plots and graphs showing the production of IFNγ by intracellular staining on Nrp1+Foxp3− versus Nrp1−Foxp3− CD4 T cells. Results are expressed as the mean ± s.e.m from one of two independent experiments (n=3). Statistically significant differences were at * P < 0.05, ** P < 0.01, and *** P < 0.001.
To determine functional differences between Nrp1+ and Nrp1− cells that were Foxp3−, splenocytes from ApoE−/− mice fed a WD were stimulated with PMA/Ionomycin for 4-5hrs, and synthesis of IFNɣ and TNFɑ by Nrp1+Foxp3− and Nrp1−Foxp3− CD4 T cells was determined by flow cytometry (Supplemental Fig. 1). Nrp1+Foxp3− CD4 T cells selectively increased secretion of IFNɣ rather than TNFɑ (Supplemental Fig. 1A), with a 1.3-fold diminution in IFNɣ−TNFɑ+ CD4 T cells, a 2-fold reduction in IFNɣ+TNFɑ+ CD4 T cells, and a 2.5-fold increase in IFNɣ+TNFɑ− CD4 T cells (Supplemental Fig. 1B). Concomitant with an impaired ability to secrete the pro-inflammatory cytokine TNFɑ, Nrp1+Foxp3− CD4 T cells displayed increased functional exhaustion (Supplemental Fig. 1C), with a 1.6-fold enhancement in CD44+PD-1+ T cells (Supplemental Fig. 1D) and a 2.8-fold increase in PD-1 gMFI (Supplemental Fig. 1E) with ɑCD3, CD28 stimulation. Together, these results suggest that Nrp1 expression on non-Treg cells mark an inflammatory, exhausted subset of CD4 T cells that expands during atherosclerosis.
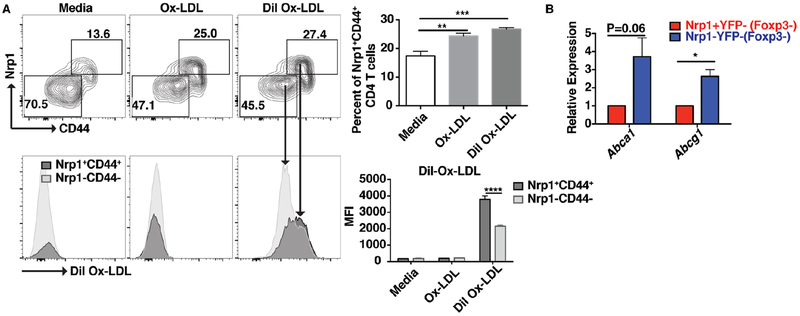
Nrp1 expression is induced by oxLDL:
The increased Nrp1 expression on non-Treg CD4 T cells suggests that an increase in cholesterol or lipids level may contribute to the increase in Nrp1 level on non Treg CD4 T cells. To determine the role of cholesterol on Nrp1 expression, lymphocytes from ApoE−/− mice were incubated with oxLDL (unlabeled or Dil-labeled) for 3 days and the expression of Nrp1 and CD44 was determined on Foxp3− CD4 T cells. Our results show that incubation of CD4 T cells with oxLDL increased the expression of Nrp1 and CD44 (Fig. 4A). In addition, oxLDL treated Nrp1+CD44+ CD4 T cells showed a 2-fold increase in labeling with Dil-oxLDL compared to the CD4 T cell population that did not upregulate Nrp1 (Fig. 4A). These results suggested that Nrp1+ CD4 T cells may either uptake oxLDL more readily or have a defect in cholesterol transport. When we examined the expression of mRNA levels of cholesterol transporters Abca1 and Abcg1, we found that Nrp1+Foxp3− CD4 T cells expressed 3-fold lower levels of mRNA of both Abca1 and Abcg1 (Fig. 4B), suggesting that these cells have a decreased capacity to efflux cholesterol compared to Nrp1− CD4 T cells.
Figure 4: Nrp1 expression is induced by oxLDL.
(A) Splenocytes from ApoE−/− mice were cultured with or without oxLDL or Dil-oxLDL (10 μg/ml) for 3 days. The expression of Nrp1 and CD44, and the uptake of oxLDL were determined using flow cytometry. The top contour plots are gated on live CD4+TCRβ+ cells and show the expression of Nrp1 and CD44. The bottom histograms are gated on Nrp1+CD44+ or Nrp1−CD44− cells and show the uptake of labeled Dil-oxLDL. (B) Bar graphs showing the relative expression of mRNA of Abca1 and Abcg1 in sorted Nrp1+YFP+ or Nrp1−YFP− CD4 T cells. Results are expressed as the mean ± s.e.m from one of two independent experiments (n=6 (A) and n=3 (B)). Statistically significant differences were at * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
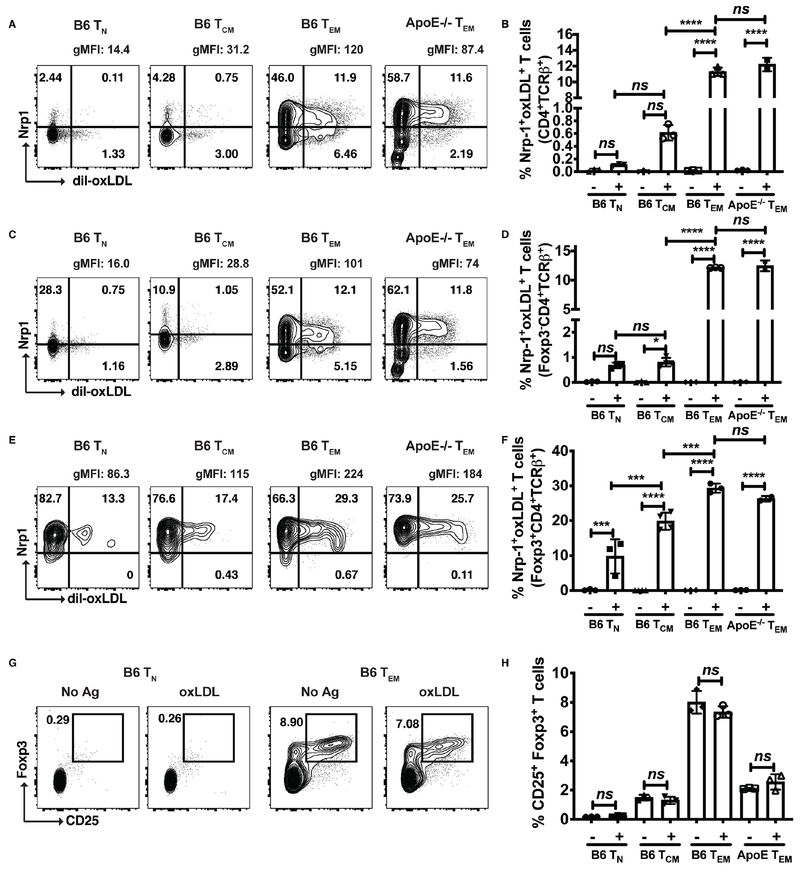
Effector memory CD4 T cells exhibit increased Nrp1+oxLDL+ uptake compared to naive and central memory T cells
To determine the mechanisms responsible for oxLDL uptake by Nrp1+ cells, naive (TN) (CD44loCD62Lhi), central memory (TCM) (CD44hiCD62Lhi), and effector memory (TEM) (CD44hiCD62Llo) CD4 T cells were sorted from both B6 and ApoE−/− mice and incubated with dil-oxLDL for 48 hrs. As TN cells encounter antigen, they expand and differentiate into effector T cells (Teff), which home to peripheral tissues and perform their effector functions. Following expansion, a majority of these TEff cells die by apoptosis, but a fraction of them mature into long-lived memory cells, which are broadly divided into central memory (TCM) and effector memory (TEM) T cells, classified by expression of CD62L and CCR7 (31). In contrast to TCM, TEM cells, characterized by the loss of CD62L and CCR7, possess a phenotype most similar to TEff cells, keen ability to differentiate into IFNɣ-secreting TEff cells, and rapid acquisition of effector functions. We reasoned that because Nrp1+Foxp3− cells are highly activated, secreting IFN-ɣ, that pro-inflammatory uptake of oxLDL would be highest on CD4+ TEM cells compared to TN. Analysis of Dil-oxLDL uptake by CD4+ TN, TCM, and TEM subsets revealed the highest frequencies of Dil-oxLDL+Nrp1+ T cells by TEM in comparison to TN and TCM subsets. This was true when gating on bulk CD4+ T cells (Fig. 5A, B), Foxp3− CD4+ T cells (Fig. 5C, D) and Foxp3+ CD4+ T cells (Fig. 5E, F). Interestingly, expression of Dil-oxLDL+Nrp1+ T cells was highest on CD4+Foxp3+ T cells (Fig. 5E, F) compared to the CD4+Foxp3− fraction. This expansion of Nrp1+oxLDL+ T cells was not due to an expansion of Tregs with oxLDL, as there were no differences in frequencies of CD25+Foxp3+ T cells in the absence or presence of oxLDL (Fig. 5G, H).
Figure 5: Effector memory CD4+ T cells displayed the highest frequency of Nrp1+oxLDL+ T cells.
Representative contour plots of dil-oxLDL by Nrp1 for B6 TN, TCM, and TEM subsets, in addition to ApoE−/− TEM cells (A, C, E) and pooled frequencies of Nrp1+oxLDL+ T cells, gated on CD4+TCRβ+ T cells (B), Foxp3−CD4+TCRβ+ T cells (D), and Foxp3+CD4+TCRβ+ T cells (F). Representative contour plots of CD25 by Foxp3, gated on CD4+TCRβ+ T cells (G) and pooled frequencies of CD25+Foxp3+ Tregs (H). Results are expressed as the mean + s.e.m. from one independent experiment. Statistically significant differences were at *P < 0.05, ***P < 0.001, and ****P < 0.0001.
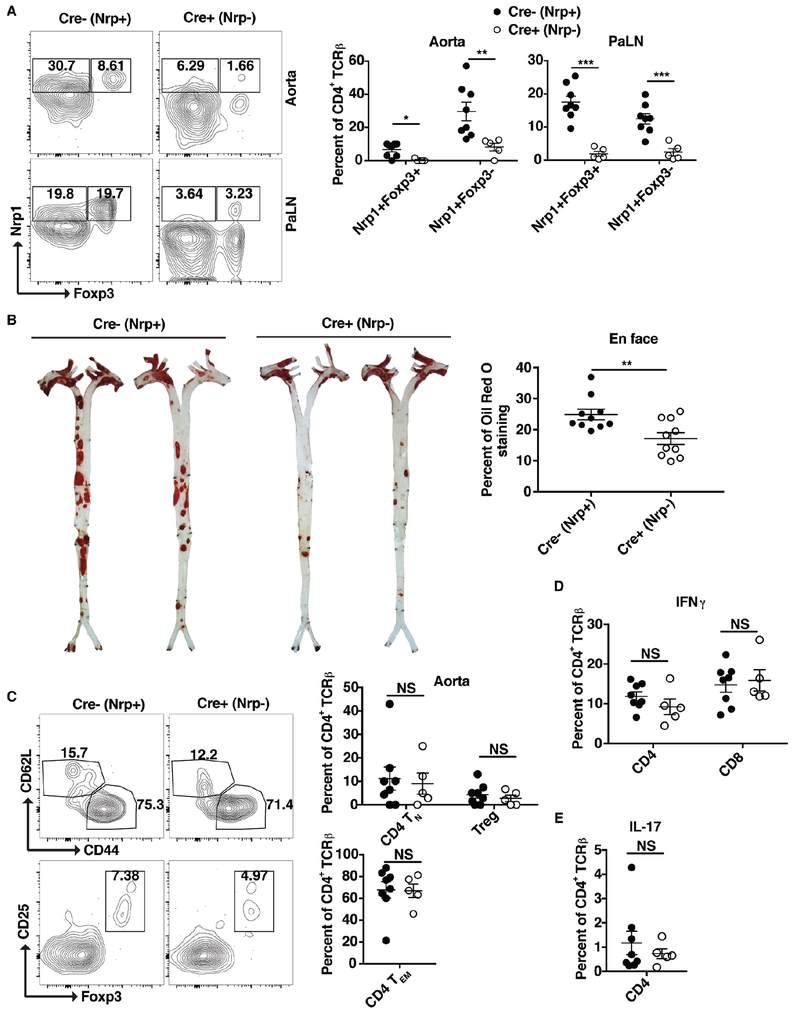
Nrp1 deficiency in CD4 T cells reduces atherosclerosis development:
Afterwards, we moved to determine the role of Nrp1 on atherosclerosis progression. Thus, we generated Nrp1fl/fl/LCKCre+/ApoE−/− mice (designated here as Cre+, Nrp1− mice). These mice lack Nrp1 expression specifically in T cells and are atherosclerosis susceptible as a result of the ApoE−/− background. These mice were fed a WD for 15 weeks and then assessed for atherosclerosis development using enface aorta Oil Red O staining. First, we validated that CD4 T cells in these mice were Nrp1-depleted. Our results show that the levels of Nrp1 expression on both Foxp3− and Foxp3+ CD4 T cells were reduced to background levels in the aorta and PaLN of Cre+ mice compared to the Cre− (Nrp1-sufficient) mice (Fig. 6A). Subsequently, Nrp1fl/fl/LCKCre+/ApoE−/− (Cre+, Nrp1−) exhibited less atherosclerosis development compared to their Nrp1 T cell-sufficient counterparts (Fig. 6B). Upon further examination of the aorta and PaLN of Nrp1 deficient mice, there was no difference in the percentage of naïve, effector memory or Treg cells (Fig. 6C), IFNγ production (Fig. 6D) or IL-17 production (Fig. 6E). These results suggest that the expression of Nrp1 on CD4 T cells is atherogenic and contributes to disease progression.
Figure 6: Mice with Nrp1 deficient CD4 T cells have reduced atherosclerosis.
Nrpfl/fl/LCkCre−/ApoE−/− (Cre−; Nrp+) and Nrpfl/fl/LCkCre+/ApoE−/− (Cre+; Nrp−) were fed a WD for 15 weeks. (A) Flow cytometry plots and bar graphs showing the percentages of Nrp1+Foxp3+ and Nrp1+Foxp3− CD4 T cells in the above mice in the PaLN and Aorta. (B) Images and bar graphs of enface Oil red O staining showing the level of atherosclerosis in the above mice. (C-E) Flow cytometry plots and/or bar graphs showing the percentages of naïve, effector memory CD4 T cells and Treg cells (C), IFNγ (D), and IL-17 production (E) in Aorta (C) and PaLN (D-E) of the above mice. Results are expressed as the mean ± s.e.m from two independent experiments (n=5-8 (A, C-E) and n=10 (B)). Statistically significant differences were at * P < 0.05, ** P < 0.01, and *** P < 0.001. NS = no significant differences.
Nrp1 facilitates the migration of Foxp3− CD4 T cells to the aorta:
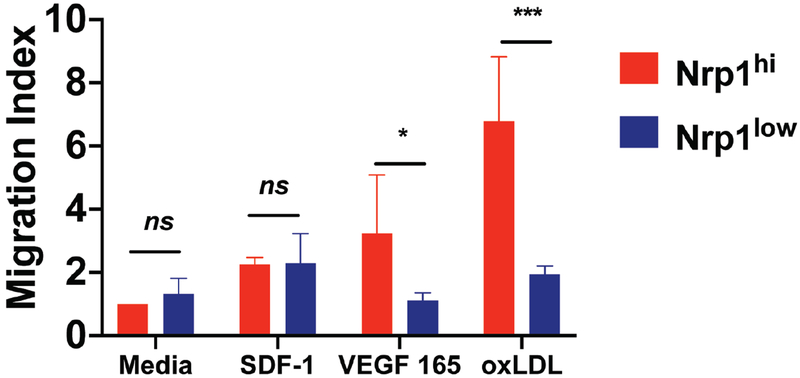
To determine the mechanism behind the protective role of Nrp1 deficient mice, we examined the ability of cells expressing Nrp1 to migrate toward VEGF165 and oxLDL, which are highly expressed in atherosclerotic tissues in an in vitro migration assay (Fig. 7) (32, 33). Sorted Nrp1hi cells displayed a 2.9-fold increased ability to migrate toward VEGF compared to Nrp1low cells in a trans-well assay. Migration toward oxLDL was even higher for Nrp1hi cells than VEGF and excitingly, there was a 3.5-fold increase compared to Nrp1low (Fig. 7).
Figure 7: Nrp1 expression is required for migration towards VEGF 165 and oxLDL.
Sorted Nrp1hi/Nrp1lo CD4 T cells were tested for the ability to migrate towards VEGF 165 and oxLDL using an in vitro migration assay with transwell plates. Graphs show the migration index of each cell population under a specific condition in comparison to media only wells. In certain wells, SDF1 was used as a control. Results are expressed as the mean ± s.e.m of one from two independent experiments (n=3).
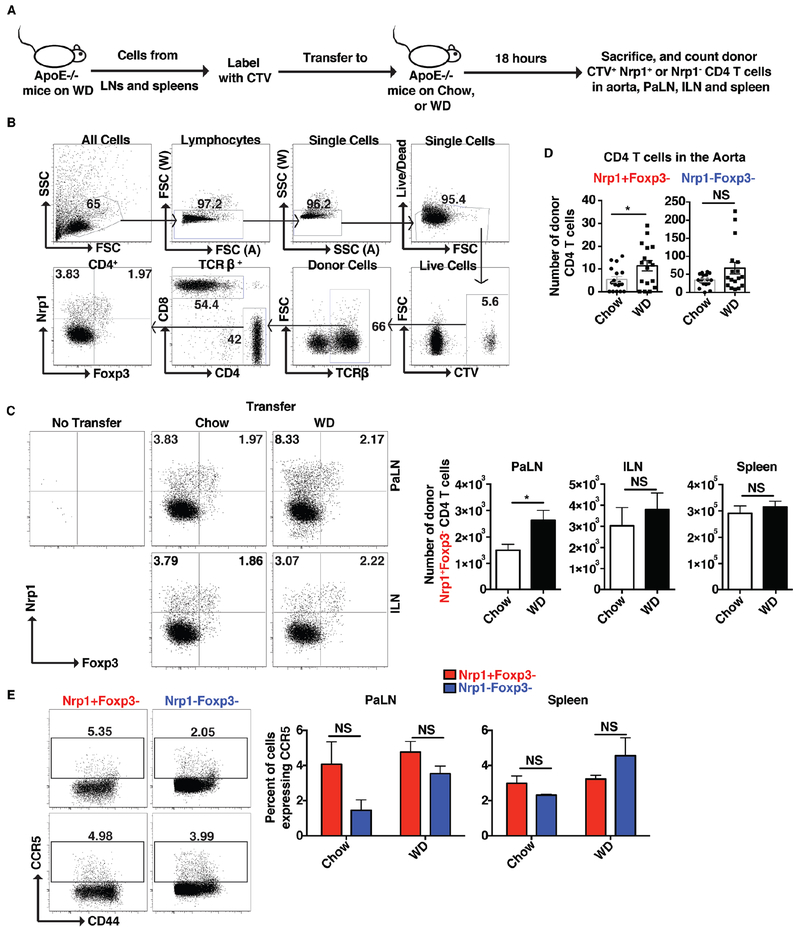
Finally, we sought to examine the ability of cells expressing Nrp1+ to migrate to the aorta. Lymphocytes were isolated from WD-fed ApoE−/− mice, labeled with CTV and adoptively transferred into age-matched chow- or WD-fed mice. Transferred cells were tracked 18 hours later (See Fig. 8A for experimental design). Donor cells were detected using the gating strategy outlined in Fig. 8B. Our results show a 2-fold increase in the total number of donor Nrp1+Foxp3− CD4 T cells that migrated to the PaLN (Fig. 8C), or the aorta (Fig. 8D) of WD-fed mice compared to chow-fed mice. This was not the case for Nrp1− CD4 T cells (Fig. 8D). In addition, Nrp1+Foxp3− CD4 T cells showed no difference in their migration patterns to the ILN or spleens of chow versus WD-fed mice (Fig. 8C). Since the expression of CCR5 is essential for the recruitment of T cells into the aorta during atherosclerosis (34), we examined the expression of CCR5 on the Nrp1+Foxp3− CD4 T cells in WD-fed mice and found no difference in the expression of CCR5 on these cells versus their Nrp1− counterparts (Fig. 8E). Together, our results suggest that the expression of Nrp1 elicits the recruitment of CD4 T cells to the atherosclerosis-involved organs expressing oxLDL and VEGF. Indeed, because Nrp1+Foxp3− CD4 T cells were more inflammatory in nature, the reduction of these cells in Nrp1fl/fl/LCKCre+/ApoE−/− would result in less atherosclerosis development.
Figure 8: Nrp1+ facilitates the migration of CD4 T cells to the aorta.
(A) Experimental design: Lymphocytes from peripheral lymph nodes and spleens of ApoE−/− mice fed a WD for 15 weeks were labeled with CTV and equal numbers were transferred to age matched ApoE−/− mice fed either a chow or WD (for 15 weeks). Mice were sacrificed 18 hours later and donor cells were assessed. (B) Flow cytometry plots showing the gating strategy used to find the donor cells in the recipient mice. (C-D) Flow cytometry plots and/or graphs showing the percentages and numbers of donor CD4 T cells that migrated to the PaLN, ILN, spleen (C) or aorta (D). (E) Flow cytometry plots and graphs showing the expression of CCR5 on the Nrp1+Foxp3− versus Nrp1−Foxp3− CD4 T cells populations in PaLN and spleen. Results are expressed as the mean ± s.e.m from three independent experiments (n=11 (A-C), n=17 (D)), or one experiment (n=3 (E)). Statistically significant differences were at * P<0.05. NS = no significant differences.
Discussion:
In this study, we examined the role of Nrp1 on CD4 T cells in the development of atherosclerosis. We show that Nrp1 expression is increased on non Treg cells during atherosclerosis and that Nrp1+Foxp3− CD4 T cells display an upregulated activated/inflammatory phenotype. Interestingly, the ablation of this population of cells is protective against atherosclerosis development. We thus hypothesize that expression of Nrp1 on CD4 T cells aids in the migration of these cells to the aortic tissue and draining lymph nodes where Nrp1-expressing cells will produce inflammatory IFNγ and thus worsen the outcome of atherosclerosis.
The role of neuronal guidance cues in the immune system has become increasingly appreciated in recent years. Work from Kathryn’s Moore laboratory has shown the importance of such molecules in retention of the immune cells in the inflammation sites. Specifically, this group has shown that Netrin-1, an axonal growth secreted protein, is increased during obesity in adipose tissue (35). The increase in Netrin-1 levels reduced tissue macrophage migratory capacity and increased their ability to remain within adipose tissue where they produced inflammatory cytokines. Thus, blocking of Netrin-1 expression results in macrophage emigration and reduced inflammation. Similarly, Netrin-1 is produced by macrophages in atherosclerotic plaque tissue and prevents their egress from tissue (36). Deletion of Netrin-1 in macrophages during atherosclerosis reduces plaque burden in LDLR−/− mice and promotes the egress of macrophages from plaques. Here, we show that Nrp1, also an axonal guidance molecule, is increased on CD4 T cells during atherosclerosis and that these cells exhibit an inflammatory phenotype and have higher capacity to be retained in the aorta and PaLN. Specific deletion of Nrp1 in CD4 T cells reduced the numbers of these cells in the aorta and PaLN and also reduced plaque burden. Nrp1 expression on Tregs directs these cells to migrate to VEGF-producing tumors (19). Furthermore, VEGF and oxLDL expression increases within atherosclerotic tissues (32, 33). Subsequently, we hypothesize that Nrp1+ CD4 T cells migrate towards VEGF and oxLDL in the aortic tissue.
The role of Nrp1 in inflammatory disease is controversial. On one hand, mouse models of EAE and rheumatoid arthritis (22) have shown that Nrp1 loss can worsen disease and increase inflammation. Meanwhile, in lupus patients with glomerulonephritis, expression of Nrp1 was increased compared to controls (37). In addition, this study found that Nrp1 expression was only in damaged glomeruli and correlated with pathology of renal disease. Similarly, the depletion of Nrp1 in macrophages using Nrp1-myeloid specific knockout mice, showed opposing results in different inflammatory models. Nrp1-myeloid specific knockout mice were more prone to LPS- induced sepsis, as well as cecal ligation (38). This correlated with decreased survival of the mice and increased production of pro-inflammatory cytokines. Meanwhile, in the same mouse model, high fat feeding resulted in decreased insulin resistance and reduced systemic inflammation (39). Furthermore, this effect was found to be due to the deletion of Nrp1 in macrophages, which activated the NF-kB pathway, and decreased the Nlrp3-inflammasome complex, resulting in attenuated macrophage activation. Whether similar effects occur in atherosclerosis is yet to be determined. Overall, this study is concordant with these previous findings and suggests that Nrp1 may present both beneficial and harmful effects in different inflammatory diseases.
The high expression of Nrp1 on Treg cells begs the question of why there is no increased Treg cell recruitment into the aortic tissue during atherosclerosis. Our results show that during atherosclerosis, increased Nrp1 is observed only in Foxp3− CD4 T cells and not Foxp3+ CD4 T cells, suggesting that Nrp1 is only induced on non-Treg cells (Fig. 1). In addition, our results show that Nrp1 is induced on Foxp3− CD4 T cells by oxLDL (Fig. 4). These data indicate that the dynamics of expression of Nrp1 on Tregs may differ from other CD4 T cells. The stability and function of Treg cells seems to be dependent on the ligation of Sema3A to Nrp1 (20). Little is known concerning whether this effect is also exhibited by the Nrp1-VEGF axis. If this axis elicited no effects on Treg stability, increasing concentrations of VEGF in plaque tissue would not increase Treg stability. Another factor could be the state of Treg cells in atherosclerosis. There is evidence that Treg cell function is disrupted during atherosclerosis either due to their conversion into other pathogenic T cell subsets (28) or due to their apoptosis (40). This finding suggests that in atherosclerosis, Treg cells may not be able to perform their suppressive functions even following recruitment into an inflammatory environment, in the context of atherosclerosis.
The protection against atherosclerosis that we observed with depletion of Nrp1+CD4+ T cells indicates that Nrp1+Foxp3− CD4 T cells exacerbate atherosclerosis progression. Concordant with this notion is our observation that Nrp1+Foxp3− cells were highly activated cells and potent producers of the pro-atherogenic cytokine IFNɣ. Nevertheless, examination of paLNs revealed no significant reduction in IFN-ɣ synthesis or attenuation in memory CD4+ TEM cell frequencies, with depletion of Nrp1+CD4+ T cells in ApoE−/− mice fed a western diet. We believe that this is due, in part, to the fact that Nrp1 expression was depleted on total CD4 T cells, not only the Foxp3− subset. While our in vitro studies demonstrated that Nrp1+Foxp3− CD4 T cells secreted IFNɣ, Nrp1−Foxp3− T cells were marked by a robust attenuation in TNFɑ and in CD4+ T cells expressing both TNFɑ and IFNɣ. TNFɑ deficiency attenuated atherosclerosis plaque severity in ApoE−/− mice, accompanied by attenuated expression of I-CAM1 and V-CAM1 adhesion molecules and monocyte chemotactic protein (MCP-1) (41). In addition to attenuated dual IFNɣ and TNFɑ production, Nrp1+Foxp3− cells displayed increased expression of PD-1, a marker of T cell exhaustion. Previous studies have demonstrated that Nrp1 is induced on self-reactive CD8+ T cells denoted by markers of T cell exhaustion and inhibitory receptors, including PD-1, Lag-3, and CTLA-4 (42). Ultimately, Nrp1+Foxp3− CD4 T cells may represent an exhausted, IFNɣ-producing subset that is increased in atherosclerosis.
Our results show that Nrp1 on CD4+ T cells is induced by WD feeding and that this effect is phenocopied with oxLDL. These findings indicate that expression of Nrp1 is regulated by cholesterol levels or by the lack of ability to excrete cholesterol, as seen by the decreased expression of Abca1 and Abcg1 (Fig. 4). Increased cholesterol or lipid cellular intake can affect the capacity of the cells to function and can push them toward an inflammatory state. For example, studies have shown that during obesity, CD4 T cells accumulate more lipids due to an increase in the fatty acid synthesis (27), and this results in an increase in inflammatory cytokine production by T cells. Work from our own group has shown that increased cholesterol content in Treg cells results in a Treg to T follicular helper cell switch (28). These T follicular helper cells are pro-atherogenic, and result in increased plaque formation. The accumulation of oxLDL in Nrp1+ CD4 T cells furthers supports that cholesterol and/or oxLDL biases CD4 T cells toward an inflammatory and pathogenic phenotype. While it is still unknown how oxLDL accumulates in CD4 T cells, our results suggest that manipulation of the cholesterol content in CD4 T cells could affect the phenotype of these cells, tipping the balance toward more inflammatory subsets.
In summary, our results show that the loss of Nrp1 on T cells can be beneficial against atherosclerosis development. Design of therapies that target either the expression of Nrp1 or its downstream signaling targets could reduce atherosclerosis burden and lead to better outcomes for patients with coronary vascular diseases.
Supplementary Material
Key Points:
Western-diet feeding increases Nrp1 on Foxp3− CD4 T cells in ApoE−/− mice.
Nrp1+Foxp3− CD4 T cells are highly proliferative and atherogenic.
Nrp1+Foxp3− CD4 T cells preferentially migrate to the aorta and PaLN.
Acknowledgements:
We would like to thank Deborah Yoakum for managing the mice colony, and the La Jolla Institute Flow Cytometry Core Facility for sorting. We also thank Daniel Araujo for critical reading of the manuscript.
This work was supported by the American Heart Association (13POST16990031 to D.E.G. and 19POST34450020 to L.E.P.), American Diabetes Association (#7-12-MN-13 to D.E.G. and C.C.H.), and the National Institutes of Health (P01 HL055798 and P01 HL136275 to C.C.H, R01 HL112276 to C.C.H., T32 AI125279-01 Training in Immunological Mechanisms Training Grant to L.E.P., and S10 RR027366-01A1 to LJI Flow Cytometry Core Facility).
Footnotes
Abbreviations used in this article: Nrp1, Neuropilin 1; ApoE−/−, apolipoprotein E knockout mice; PaLN, peri-aortic lymph node; ILN, inguinal lymph node
References:
- 1.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, and Ginty DD. 1997. Neuropilin is a semaphorin III receptor. Cell 90: 753–762. [DOI] [PubMed] [Google Scholar]
- 2.He Z, and Tessier-Lavigne M. 1997. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90: 739–751. [DOI] [PubMed] [Google Scholar]
- 3.Zachary I 2014. Neuropilins: role in signalling, angiogenesis and disease. Chem. Immunol. Allergy 99: 37–70. [DOI] [PubMed] [Google Scholar]
- 4.Soker S, Takashima S, Miao HQ, Neufeld G, and Klagsbrun M. 1998. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92: 735–745. [DOI] [PubMed] [Google Scholar]
- 5.Pellet-Many C, Frankel P, Jia H, and Zachary I. 2008. Neuropilins: structure, function and role in disease. Biochem. J 411: 211–226. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, and Fujisawa H. 1999. A requirement for neuropilin-1 in embryonic vessel formation. Development 126: 4895–4902. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz Q 2004. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes & Development 18: 2822–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, and Hansen W. 2004. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol 34: 623–630. [DOI] [PubMed] [Google Scholar]
- 9.Milpied P, Massot B, Renand A, Diem S, Herbelin A, Leite-de-Moraes M, Rubio M-T, and Hermine O. 2011. IL-17-producing invariant NKT cells in lymphoid organs are recent thymic emigrants identified by neuropilin-1 expression. Blood 118: 2993–3002. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi K, Suzuki E, Nishie M, Kii I, Kataoka TR, Hirata M, Inoue M, Pu F, Iwaisako K, Tsuda M, Yamaguchi A, Haga H, Hagiwara M, and Toi M. 2017. Downregulation of neuropilin-1 on macrophages modulates antibody-mediated tumoricidal activity. Cancer Immunology, Immunotherapy 66: 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrer A, Moimas S, Zacchigna S, Pattarini L, Zentilin L, Ruozi G, Mano M, Sinigaglia M, Maione F, Serini G, Giraudo E, Bussolino F, and Giacca M. 2012. Neuropilin-1 identifies a subset of bone marrow Gr1- monocytes that can induce tumor vessel normalization and inhibit tumor growth. Cancer Res. 72: 6371–6381. [DOI] [PubMed] [Google Scholar]
- 12.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, and Lafaille JJ. 2012. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med 209: 1723–42, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, and Bluestone JA. 2012. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. The Journal of Experimental Medicine 209: 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P, and Ignatowicz L. 2015. Differences in Expression Level of Helios and Neuropilin-1 Do Not Distinguish Thymus-Derived from Extrathymically-Induced CD4+Foxp3+ Regulatory T Cells. PLoS One 10: e0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarris M, Andersen KG, Randow F, Mayr L, and Betz AG. 2008. Neuropilin-1 Expression on Regulatory T Cells Enhances Their Interactions with Dendritic Cells during Antigen Recognition. Immunity 28: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizui M, and Kikutani H. 2008. Neuropilin-1: the glue between regulatory T cells and dendritic cells? Immunity 28: 302–303. [DOI] [PubMed] [Google Scholar]
- 17.Glinka Y, and Prud’homme GJ. 2008. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol 84: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumanogoh A, and Kikutani H. 2013. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol 13: 802–814. [DOI] [PubMed] [Google Scholar]
- 19.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, Schadendorf D, Buer J, and Helfrich I. 2012. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med 209: 2001–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgoffe GM, Woo S-R, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, and Vignali DAA. 2013. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon BD, Mueller C, Chae W-J, Alabanza LM, and Bynoe MS. 2011. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A 108: 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catalano A 2010. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J. Immunol 185: 6373–6383. [DOI] [PubMed] [Google Scholar]
- 23.Campos-Mora M, Morales RA, Pérez F, Gajardo T, Campos J, Catalan D, Aguillón JC, and Pino-Lagos K. 2015. Neuropilin-1+ regulatory T cells promote skin allograft survival and modulate effector CD4+ T cells phenotypic signature. Immunol. Cell Biol 93: 113–119. [DOI] [PubMed] [Google Scholar]
- 24.Mallat Z, Taleb S, Ait-Oufella H, and Tedgui A. 2009. The role of adaptive T cell immunity in atherosclerosis: Fig. 1. Journal of Lipid Research 50: S364–S369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Profumo E, Buttari B, Tosti ME, Tagliani A, Capoano R, D’Amati G, Businaro R, Salvati B, and Riganò R. 2013. Plaque-infiltrating T lymphocytes in patients with carotid atherosclerosis: an insight into the cellular mechanisms associated to plaque destabilization. J. Cardiovasc. Surg 54: 349–357. [PubMed] [Google Scholar]
- 26.Businaro R, Tagliani A, Buttari B, Profumo E, Ippoliti F, Di Cristofano C, Capoano R, Salvati B, and Riganò R. 2012. Cellular and molecular players in the atherosclerotic plaque progression. Ann. N. Y. Acad. Sci 1262: 134–141. [DOI] [PubMed] [Google Scholar]
- 27.Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ, Tokuyama H, Yokote K, and Nakayama T. 2015. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 12: 1042–1055. [DOI] [PubMed] [Google Scholar]
- 28.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, Sorci-Thomas MG, and Hedrick CC. 2018. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun 9: 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr, Muller W, and Rudensky AY. 2008. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H-Y, Gaddis DE, Wu R, McSkimming C, Haynes LD, Taylor AM, McNamara CA, Sorci-Thomas M, and Hedrick CC. 2016. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Invest 126: 3236–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Geginat J, and Lanzavecchia A. 2004. CentralMemory andEffectorMemoryT CellSubsets: Function, Generation, and Maintenance. Annual Review of Immunology 22: 745–763. [DOI] [PubMed] [Google Scholar]
- 32.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Chun T-H, Masatsugu K, Becker AE, and Nakao K. 1998. Vascular Endothelial Growth Factor (VEGF) Expression in Human Coronary Atherosclerotic Lesions. Circulation 98: 2108–2116. [DOI] [PubMed] [Google Scholar]
- 33.Wolanska M, Bankowska-Guszczyn E, Sobolewski K, and Kowalewski R. 2015. Expression of VEGFs and its receptors in abdominal aortic aneurysm. Int. Angiol 34: 520–528. [PubMed] [Google Scholar]
- 34.Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, Miller J, Weber C, and Ley K. 2016. CCR5 T-bet FoxP3 Effector CD4 T Cells Drive Atherosclerosis. Circulation Research 118: 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramkhelawon B, Hennessy EJ, Ménager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner KJ, and Moore KJ. 2014. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med 20: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Gils JM, Ramkhelawon B, Fernandes L, Stewart MC, Guo L, Seibert T, Menezes GB, Cara DC, Chow C, Kinane TB, Fisher EA, Balcells M, Alvarez-Leite J, Lacy-Hulbert A, and Moore KJ. 2013. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler. Thromb. Vasc. Biol 33: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vadasz Z, Ben-Izhak O, Bejar J, Sabo E, Kessel A, Storch S, and Toubi E. 2011. The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus 20: 1466–1473. [DOI] [PubMed] [Google Scholar]
- 38.Dai X, Okon I, and Zou M-H. 2017. Myeloid cell neuropilin 1 ameliorates high-fat diet-induced insulin resistance via suppression of Nlrp3 inflammasome. Macrophage (Houst) 4. [PMC free article] [PubMed] [Google Scholar]
- 39.Dai X, Okon I, Liu Z, Bedarida T, Wang Q, Ramprasath T, Zhang M, Song P, and Zou M-H. 2017. Ablation of Neuropilin 1 in Myeloid Cells Exacerbates High-Fat Diet-Induced Insulin Resistance Through Nlrp3 Inflammasome In Vivo. Diabetes 66: 2424–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maganto-García E, Tarrio ML, Grabie N, Bu D-X, and Lichtman AH. 2011. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 124: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, and Jovinge S. 2004. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol 24: 2137–2142. [DOI] [PubMed] [Google Scholar]
- 42.Jackson SR, Berrien-Elliott M, Yuan J, Hsueh EC, and Teague RM. 2014. Neuropilin-1 expression is induced on tolerant self-reactive CD8+ T cells but is dispensable for the tolerant phenotype. PLoS One 9: e110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.