Abstract
Since the emergence of lead halide perovskites for photovoltaic research, there has been mounting effort in the search for alternative compounds with improved or complementary physical, chemical, or optoelectronic properties. Here, we report the discovery of Cu2AgBiI6: a stable, inorganic, lead-free wide-band-gap semiconductor, well suited for use in lead-free tandem photovoltaics. We measure a very high absorption coefficient of 1.0 × 105 cm–1 near the absorption onset, several times that of CH3NH3PbI3. Solution-processed Cu2AgBiI6 thin films show a direct band gap of 2.06(1) eV, an exciton binding energy of 25 meV, a substantial charge-carrier mobility (1.7 cm2 V–1 s–1), a long photoluminescence lifetime (33 ns), and a relatively small Stokes shift between absorption and emission. Crucially, we solve the structure of the first quaternary compound in the phase space among CuI, AgI and BiI3. The structure includes both tetrahedral and octahedral species which are open to compositional tuning and chemical substitution to further enhance properties. Since the proposed double-perovskite Cs2AgBiI6 thin films have not been synthesized to date, Cu2AgBiI6 is a valuable example of a stable Ag+/Bi3+ octahedral motif in a close-packed iodide sublattice that is accessed via the enhanced chemical diversity of the quaternary phase space.
1. Introduction
Hybrid lead perovskites APb2+X3 (A = FA+, MA+, Cs+; X = Br–, I–) continue to be intensely studied as solar absorbers for photovoltaic (PV) applications due to their high absorption coefficients suitable for thin-film technology,1−3 long charge-carrier diffusion lengths,4−7 and high radiative efficiencies. In single-junction devices the current certified record power conversion efficiency (PCE) stands at 25.5%.8 This is close to matching the very highest efficiencies delivered by silicon PV cells, and high PCEs are a way to minimize the cost of energy from PVs. However, this becomes increasingly difficult, as heavily optimized systems approach their maximum theoretical efficiency limits. A crucial strategy to overcome this limitation is to combine suitably wide band gap (Eg) materials (Eg from 1.6 to 2.0 eV) with well-established c-Si (Eg ≈ 1.1 eV) technology to construct tandem cells, which can achieve much higher PCEs in comparison to single-junction cells.9,10
The tunability of the band gap in mixed iodide–bromide lead halide perovskites has opened up the possibility of multijunction solar cells with c-Si, currently delivering a record PCE of 29.5%, with efficiency improvements to over 32% being feasible.9,11 There remain a number of compromises which could be improved upon with the discovery of new wide-band-gap, stable, lead-free, inorganic solar absorber materials. These include the yet unresolved challenge of obtaining band-gap-stable, low-defect I–Br mixed halide perovskites,12 the reliance upon organic ammonium cations to deliver a crystallographically phase stable lead halide perovskite compound, which leads to lower thermal stability in comparison to conventional inorganic semiconductors, and finally the fact that these materials contain lead, which requires careful management due to the known toxicological issues. One strategy for replacing Pb2+ is with isoelectronic Bi3+. Bismuth bromide and chloride networks have been synthesized as the double perovskites (MA)2KBiCl6 (3.04 eV),13 (MA)2AgBiBr6 (2.02 eV),14 Cs2AgBiCl6 (2.77 eV), and Cs2AgBiBr6 (2.19 eV);15,16 however, their absorption profiles remain unsuitable for use in tandem cells. Bismuth iodides A3Bi23+I9 (A = K+, Rb+, Cs+, MA+, NH4+)17−22 have been reported as 2D perovskites (A = NH4+, K+, Rb+) or as 0D isolated [Bi2I9]3+ units (A = Cs+, MA+), which are not ideal for isotropic charge transport and carrier mobility. Hypothetical bismuth iodide double perovskites, such as Cs2AgBiI6, would possess a lower, more ideal band gap but so far have not been stable enough to be synthesized,23 apart from in nanocrystal form,24 which identifies a clear opportunity for materials discovery. Searching for other possible bismuth iodide networks with suitably lower band gaps leads to BiI3 and the ternary compounds Ag1–3xBi1+xI4 and CuBiI4. BiI3 has been reported with an indirect band gap of 1.67(1) eV,25 and PV devices have reached PCEs of 1.0%.26−28 Ag1–3xBi1+xI4 and CuBiI4 have been reported with suitable band gaps of 1.64–1.93 eV; the variation arises from composition, sample type, and assumption of direct or indirect band gaps.25,29,30 Devices based on a x = −0.33 Ag1–3xBi1+xI4(Ag3BiI6) solar absorber have reached PCEs of 4.3%,31 and introducing small amounts of sulfur to the layer has recently been shown to increase the Jsc values of devices, increasing the maximum PCE to 5.44(7)%.32 Cu-containing CuBiI4 films have also recently been processed into devices reaching PCEs of 1.1%.30,33 However, we show here that CuBiI4 is not a stable phase and decomposes on standing at room temperature. As with the initial reports of MAPbI3 devices, the low initial PCEs of devices using these recent materials are a result of limited investigations into optimal device architectures, charge carrier layers, control of crystallinity and passivation, and further materials chemistry. Here, we synthesize the new compound Cu2AgBiI6 as crystals, powders, and solution-processed thin films, solve its crystal structure, and present its properties. By expanding this family of materials to the quaternary Cu–Ag–Bi–I system, we gain an extra degree of chemical tunability, which can be further optimized to increase the performance and stability of this lead-free absorber material. Cu2AgBiI6 represents the use of Ag+ to stabilize CuBiI4 and the use of Cu+ to reduce the content of Ag+ in comparison to Ag1–3xBi1+xI4 compounds.
2. Results
2.1. Cu2AgBiI6 Crystal Structure
We
synthesized Cu2AgBiI6 powders and crystals
by a solid-state synthesis in evacuated fused-silica ampules as described
in the Supporting Information. We found
that it is important to quench the material from the synthesis temperature
of 350 °C rather than cool it down slowly through a range of
temperatures, which induces compositional heterogeneity as measured
by the TEM EDX (Figure S1). Crystals were
picked from the powder reaction and were found to be of suitable quality
for single-crystal X-ray diffraction (SCXRD) (Figure S2). Larger crystals grown by chemical vapor transport
and cooling of the melt were found to contain large amounts of heterogeneity
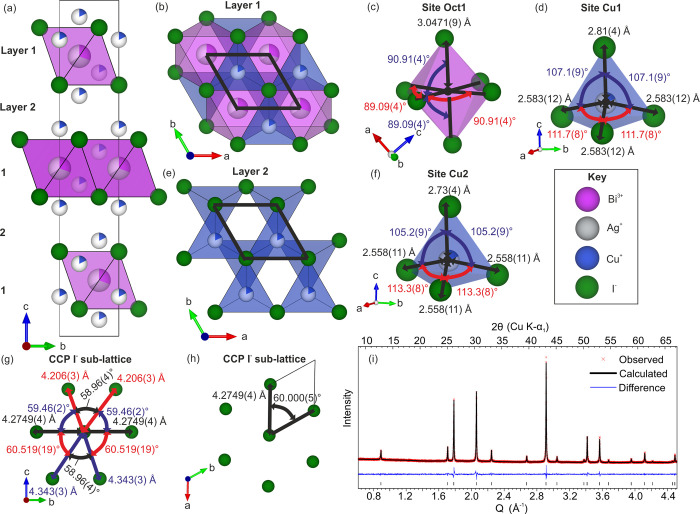
and twinning. We solved the structure of Cu2AgBiI6 using SCXRD data collected at 100 K (Figure 1, Table 1 and Tables S2 and S3).
The observed reflections could be fitted with a twinning of four trigonal
unit cells with space group R3m and lattice parameters a = 4.2749(3)
Å and c = 20.9395(16) Å, which is metrically
cubic within 2σ error (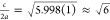 ). The trigonal unit cell and definition
of rhombohedral strain are shown in Figure S3a,b. Table S1 gives the contribution of each
twin and the twinning matrices. The twinning is complex and has been
reported in more detail for AgBiI4,25 which, due to the twinning, has two indistinguishable structural
solutions—a defect spinel and/or twinning of a CdCl2 structure. Here, we find that Cu2AgBiI6 consists
of a cubic close-packed (CCP) iodide sublattice (Figure S3a), as reported for the Ag1–3xBi1+xI4 and
CuBiI4 materials. The octahedral cations Ag+ and Bi3+ then adopt a CdCl2 octahedral motif
in a disordered fashion (Figure S3c). This
consists of layers of 2D edge-sharing octahedra separated by a layer
of vacant octahedral sites. The atomic occupancies of Ag+ and Bi3+ are 34.7% and 30.6%, respectively. Rather than
a direct refinement of the composition, the Ag+ and Bi3+ occupancies were constrained to the average composition
Cu2.15(16)Ag1.04(5)Bi0.92(7)I6.00(11)—the composition of the powder measured by TEM
EDX (Figure 2). This
compositional constraint was required due to the high number of correlated
parameters in the refinement derived from cation disorder and the
four twin components. A comparison to AgBiI4 shows that
adding tetrahedral Cu+ in to the structure has reduced
the octahedral occupancy of the Ag+ and Bi3+ and introduced octahedral vacancies to maintain charge balance.
The formula Cu4x(AgBi)1–xI4 expresses this case, where equal amounts
of Ag+ and Bi3+ are substituted for Cu+, with x = 0.33 corresponding to Cu2AgBiI6. The electron density in the difference Fourier map shows
two Cu+ sites (Cu1 and Cu2) with equal occupancy (Figure S3d). They occupy every possible tetrahedral
site in the CCP iodide sublattice, as in the reported CuBiI4 structure.35 The Cu+ atomic
occupancies were fixed to occupancies of 17.9%, in line with the measured
composition. Cu2AgBiI6 provides the initial
report and understanding of a quaternary phase in the CuI–AgI–BiI3 phase space (Figure 2). The Cu2AgBiI6 structure is analogous
with oxides, where occupancy of this pattern of tetrahedral sites
within the R3m space group and CdCl2-type octahedral site occupancy
motif have been observed: for example, in nonstoichiometric lithium
vanadium oxides such as Li0.22VO2, which have
cationic disorder due to delithiation.39 We performed a Pawley fit on room-temperature laboratory powder
X-ray diffraction (PXRD) data of Cu2AgBiI6,
yielding lattice parameters of a = 4.3151(2) Å
and c = 21.141(1) Å (Figure 1i), which is metrically cubic within error
(
). The trigonal unit cell and definition
of rhombohedral strain are shown in Figure S3a,b. Table S1 gives the contribution of each
twin and the twinning matrices. The twinning is complex and has been
reported in more detail for AgBiI4,25 which, due to the twinning, has two indistinguishable structural
solutions—a defect spinel and/or twinning of a CdCl2 structure. Here, we find that Cu2AgBiI6 consists
of a cubic close-packed (CCP) iodide sublattice (Figure S3a), as reported for the Ag1–3xBi1+xI4 and
CuBiI4 materials. The octahedral cations Ag+ and Bi3+ then adopt a CdCl2 octahedral motif
in a disordered fashion (Figure S3c). This
consists of layers of 2D edge-sharing octahedra separated by a layer
of vacant octahedral sites. The atomic occupancies of Ag+ and Bi3+ are 34.7% and 30.6%, respectively. Rather than
a direct refinement of the composition, the Ag+ and Bi3+ occupancies were constrained to the average composition
Cu2.15(16)Ag1.04(5)Bi0.92(7)I6.00(11)—the composition of the powder measured by TEM
EDX (Figure 2). This
compositional constraint was required due to the high number of correlated
parameters in the refinement derived from cation disorder and the
four twin components. A comparison to AgBiI4 shows that
adding tetrahedral Cu+ in to the structure has reduced
the octahedral occupancy of the Ag+ and Bi3+ and introduced octahedral vacancies to maintain charge balance.
The formula Cu4x(AgBi)1–xI4 expresses this case, where equal amounts
of Ag+ and Bi3+ are substituted for Cu+, with x = 0.33 corresponding to Cu2AgBiI6. The electron density in the difference Fourier map shows
two Cu+ sites (Cu1 and Cu2) with equal occupancy (Figure S3d). They occupy every possible tetrahedral
site in the CCP iodide sublattice, as in the reported CuBiI4 structure.35 The Cu+ atomic
occupancies were fixed to occupancies of 17.9%, in line with the measured
composition. Cu2AgBiI6 provides the initial
report and understanding of a quaternary phase in the CuI–AgI–BiI3 phase space (Figure 2). The Cu2AgBiI6 structure is analogous
with oxides, where occupancy of this pattern of tetrahedral sites
within the R3m space group and CdCl2-type octahedral site occupancy
motif have been observed: for example, in nonstoichiometric lithium
vanadium oxides such as Li0.22VO2, which have
cationic disorder due to delithiation.39 We performed a Pawley fit on room-temperature laboratory powder
X-ray diffraction (PXRD) data of Cu2AgBiI6,
yielding lattice parameters of a = 4.3151(2) Å
and c = 21.141(1) Å (Figure 1i), which is metrically cubic within error
( ).
).
Figure 1.
(a) Structure of Cu2AgBiI6 solved from 100 K SCXRD data with the composition constrained in line with the average composition Cu2.15(16)Ag1.04(5)Bi0.92(7)I6.00(11) from TEM EDX. (b) Layer 1 contains the sites Oct1 (occupied by 34.6% Ag+ and 30.6% Bi3+) and Cu1 with coordination environments shown in (c) and (d), respectively. (e) Layer 2 contains the site Cu2 with the coordination environment shown in (f). Sites Cu1 and Cu2 are both occupied by 17.9% Cu+. The I–I distances and I–I–I angles of the cubic close-packed (CCP) iodide sublattice in the bc and ab planes are shown in (g) and (h), respectively. The green, blue, gray, and pink spheres/polyhedra represent I–, Cu+, Ag+ and Bi3+ ions, respectively. (i) Pawley fit performed on room-temperature laboratory PXRD data.
Table 1. Refined Structural Data for the Cu2AgBiI6 Structure Solved from 100 K SCXRD Data with the Composition Constrained to Match the Average Composition Cu2.15(16)Ag1.04(5)Bi0.92(7)I6.00(11) from TEM EDXa.
| site | atom | x | y | z | occ | U (103 Å2) | Wyckoff position | point group (Hermann– Mauguin) |
|---|---|---|---|---|---|---|---|---|
| I1 | I | 2/3 | 1/3 | 0.08133(7) | 1 | 16.6(7) | 6c | 3m |
| Oct1 | Bi | 1/3 | 2/3 | 1/6 | 0.306 | 24.0(9) | 3b | 3̅m |
| 24.0(9) | ||||||||
| Ag | 1/3 | 2/3 | 1/6 | 0.347 | 3b | 3̅m | ||
| Cu1 | Cu | 0 | 0 | 0 1177(19) | 0.179 | 22 | 6c | 3m |
| Cu2 | Cu | 2/3 | 1/3 | –0.0492(19) | 0.179 | 22 | 6c | 3m |
Crystal data: Cu2AgBilI6, space group R3̅m (No. 166), 100 K, formula sum Cu2.15Ag1.04Bi0.92I6, Z = 1, formula mass 1202.46 g/mol, cell parameters a = 4.2749(3) Å and c = 20.9395(16) Å, trigonal crystal system, cell volume 331.40(5) Å3, calculated density 6.025 g/cm3.
Figure 2.
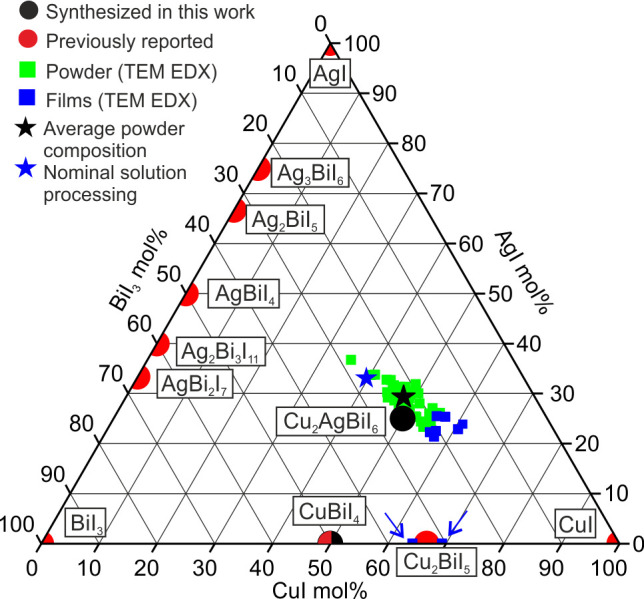
Compounds of the CuI–AgI–BiI3 phase space. The previously reported compounds are shown in red, including Ag1–3xBi1+xI4, CuBiI4, and Cu2BiI5.34−38 Shown in black are the phases synthesized here, including Cu2AgBiI6, the first report of a quaternary phase in the CuI–AgI–BiI3 phase space. The TEM EDX measurements of powder samples shown in green give an average composition of Cu2.15(16)Ag1.04(5)Bi0.92(7)I6.00(11) (black star). The composition dissolved in solution for processing thin films is shown by a blue star. The TEM EDX measurements of films are shown in blue, with a Cu2AgBiI6 main phase, and an impurity phase of Cu2BiI5, indicated by the blue arrows.
2.2. Bulk Stability
Powders of the previously reported CuBiI4 and AgBiI4 were obtained by a solid-state synthesis in evacuated fused-silica ampules as described in the Supporting Information. The PXRD pattern of CuBiI4 was fitted to a cubic unit cell with the lattice parameter a = 12.1580(2) Å, larger than the a = 12.134(6) Å reported by Fourcroy et al. (Figure S4).35 SEM EDX confirmed a composition of Cu1.21(5)Bi1.11(7)I4.00(9), within 3σ error of CuBiI4 (Figure S5). We find that CuBiI4 is a metastable material that decomposes back to the starting materials BiI3 and CuI at room temperature, even in the dark (Figure S6). We could slow the rate of decomposition of CuBiI4 by storing the powder at −20 °C. In contrast, we find that Cu2AgBiI6 powder is stable when it is kept in the dark, in air, at room temperature. We exposed synthesized Cu2AgBiI6 and AgBiI4 powders to a simulated AM1.5 solar spectrum for 1 week, sealed in capillaries with synthetic (dry) air, laboratory air, and He atmospheres. AgBiI4 and Cu2AgBiI6 showed no color change after 1 week in the solar spectrum and showed no signs of decomposition by PXRD (Figure S7) or Raman spectroscopy (Figure S8). The Cu2AgBiI6 composition therefore represents the stabilization of a Cu-containing bismuth iodide solar absorber and is as stable as AgBiI4 under the investigated conditions. This is promising, since unencapsulated devices using AgBiI4 absorber layers have been shown to retain 96% of their initial PCE after 1000 h of storage in air at 26% relative humidity.43
2.3. Optical Properties
We solution-processed Cu2AgBiI6 into thin films for optical property measurements and device fabrication, as we describe in the Supporting Information. The films were found to be consistently Cu rich in comparison to the nominal composition in solution, showing loss of Ag and Bi during the film processing. Therefore, the nominal Cu-poor composition in solution reported here (Cu1.53Ag1.26Bi1.07I6.00) was to compensate for this, bringing the compositions of films close to the composition of the powders (Figure 2). Two phases were detected in the films. The most abundant phase had a measured composition of Cu2.52(9)Ag1.02(7)Bi0.82(11)I6.00(20). The minor phase was identified as Cu2BiI5, containing no silver (Figure 2). We performed a Pawley fit of PXRD data collected on the film (Figure S9), which shows the major phase to have a trigonal unit cell (R3m) with lattice parameters of a = 4.3476(8) Å and c = 20.868(9) Å and the impurity phase to have a trigonal unit cell (R3m), with lattice parameters a = 4.322(1) Å and c = 20.80(1) Å. This is consistent with the two phases identified in the TEM EDX. We found that the film deposition was very sensitive to the annealing temperature, which we optimized to a two-step anneal to improve the film morphology from large rough dendritic crystallites (Figure S10) to a more uniform smooth film (Figure S11).
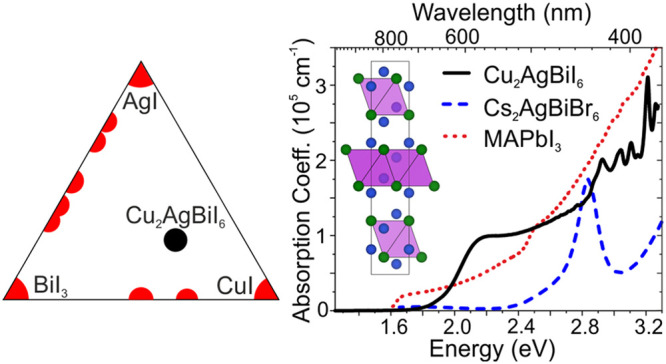
We determined the absorption coefficient spectra of Cu2AgBiI6 thin films by using a combination of a Fourier transform infrared (FTIR) spectrometer to accurately determine the band gap absorption spectra and photothermal deflection spectroscopy (PDS) to accurately measure the low-energy parts of the spectrum. The raw PDS data (Figure S12a) were scaled to match the FTIR data, and then the two data sets were combined as shown in Figure S12b. Unusually, for this broad family of compounds, Cu2AgBiI6 presents a strong absorption coefficient profile with a steep increase typical of a direct band gap semiconductor (Figure 3a). The absorption strength that we measure for Cu2AgBiI6 at the first peak just above the band edge (1.0 × 105 cm–1) is considerably stronger than that for MAPbI3 films measured here (0.3 × 105 cm–1) and reported in the literature,44 which are already very strongly absorbing semiconductors near the band edge. Crucially, Cu2AgBiI6 has a much more suitable absorption profile in comparison to that of the alternative wide-band-gap, lead-free double perovskite Cs2AgBiBr6, which consists of an initial peak in the absorption spectrum centered at 2.8 eV, followed by a minimum. In the literature, it is unresolved whether this absorption peak in Cs2AgBiBr6 can be attributed to excitonic contributions or to the nature of the density of states near the band edge.15,45−47 Although a Tauc analysis is often used to approximate the band gap of lead halide perovskites, this is unphysical since a Tauc analysis assumes that the absorption at the band edge is directly into the continuum of states and neglects the exciton contribution that can dominate features near the band edge. The more accurate approach is a fit according to Elliott theory,41,48 which accounts for contributions from the excitonic contribution and continuum of states. In Figure 3b, we show a fit to the absorption coefficient based on the Elliott model, which reveals a band gap of 2.06(1) eV and an exciton binding energy (EB) of 25(2) meV. This value of the exciton binding energy is higher than that determined for MAPbI3 but very similar to that determined for CsPbBr3 and notably comparable to the thermal energy at room temperature.49,50 This indicates that under light absorption at room temperature free carriers, as opposed to bound excitons, will be generated. Therefore, we have stabilized a close-packed iodide framework with three metal species that together give high absorption, and both the band gap and exciton binding energies are lower than those in a comparable bromide (Cs2AgBiBr6, Tauc plot Eg ≈ 2.2 eV, EB ≈ 220 meV).40,51 Both the strong absorption properties and low exciton binding energy are very encouraging for the potential use of Cu2AgBiI6 as a solar absorber, in comparison to the previously reported double perovskites. We note that, although the band gap of the continuum of states at 2.06 eV appears to be quite large for PV applications, there exists considerable absorption at lower energies due to the excitonic states. This indicates that the optical, or PV, band gap will be at lower energy.52 We will return to this point later on.
Figure 3.
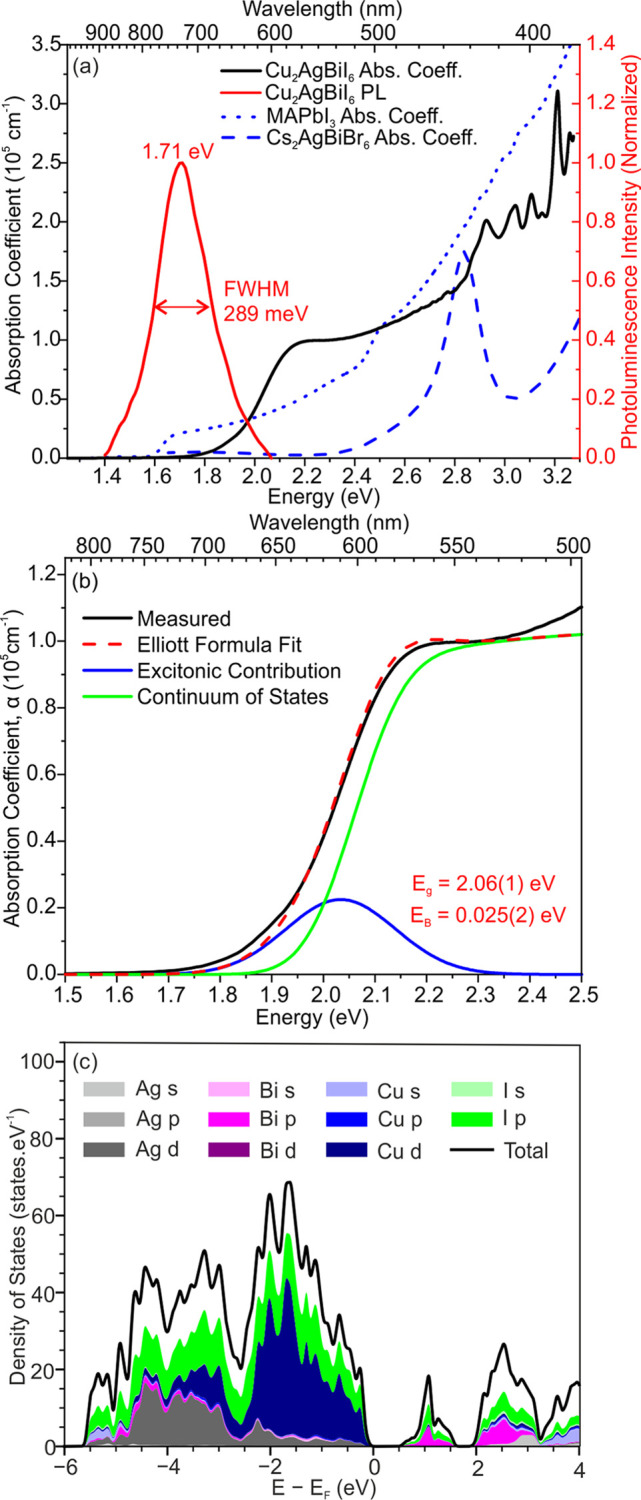
(a) Absorption coefficient of Cu2AgBiI6 thin films (black) measured by a combination of Fourier transform infrared (FTIR) spectroscopy and photothermal deflection spectroscopy (PDS). This is compared to the reported absorption coefficients of MAPbI3 (blue dotted line) and Cs2AgBiBr6 (blue dashed line), reproduced from Davies et al. and Longo et al., respectively.40−42 Also shown is the photoluminescence (PL) spectrum of Cu2AgBiI6. (b) Elliott model fitting of the absorption coefficient spectrum, giving a band gap of 2.06(1) eV and an exciton binding energy of 25(2) meV. (c) Partial density of states of Cu2AgBiI6 computed with density functional theory for configurations of cations with the lowest computed energy. The cumulative contributions from each species are shown along with the total density of states for energies relative to the computed Fermi energy.
To gain some insight into the nature of the electronic transitions underlying optical absorption, we have performed density functional theory calculations on ordered structural models of Cu2AgBiI6 based on the refined experimental disordered structures. Partial density of states plots (Figure 3c and Figure S13) show that the bottom of the conduction band is dominated by Bi 6p and I 5p states, similar to the case for AgBiI4 and BiI3.25 In contrast, Cu 3d states dominate at the top of the valence band in Cu2AgBiI6, mixed with the I 5p states which dominate when Cu is absent. Optical transitions near the band gap energy of Cu2AgBiI6 will involve considerable Cu 3d to Bi 6p/I 5p character, in contrast to the I 5p to Bi 6p/I 5p transitions present in Ag1–3xBi1+xI4 and BiI3. This suggests that the Cu+, which is well dispersed throughout the structure, is a functional part of the electronic network. Band structure plots for the lowest energy computed structure of Cu2AgBiI6 (Figure S21) are shown in Figure S14 and naturally reflect the precise ordering selected in the supercell used for the calculations. The in-plane effective masses of the holes and electrons are relatively low at 1.0 and 0.6 me, respectively, and are similar to those calculated for AgBiI4.25 We find that the layered nature of the structure leads to flat bands in the kz direction (c direction) in the ordered supercell studied.
In Figure 3a we also show the photoluminescence (PL) of the Cu2AgBiI6 thin film, which we fit to a pseudo-Voigt function (convolution of a Gaussian and Lorentzian function) with a full width half-maximum (fwhm) of 289 meV. The PL peak of Cu2AgBiI6 is centered at 1.71 eV, corresponding to a Stokes shift of 350 meV in comparison to the estimated direct band gap. For comparison, we show the absorption and emission profiles of MAPbI3 in Figure S15. We can fit the PL of MAPbI3 to a Gaussian function with a fwhm of 96 meV and a Stokes shift of 10 meV. Although the Stokes shift for Cu2AgBiI6 is larger than in MAPbI3, it is still substantially less than the 1 eV separation between the direct gap energy and PL peak in the indirect band gap material Cs2AgBiBr6.45 Due to the disordered nature of the Cu2AgBiI6 crystal structure, the sub-band-gap states of the thin film were investigated using PDS. PDS is a scatter-free absorption measurement capable of assessing the presence of sub-band-gap states and/or a broad distribution of states near the band edge. Interestingly, the PDS measurement reveals absorption at lower energies down to 1.25 eV due to sub-band-gap states (Figure S16a). We recorded time-resolved PL transients for the Cu2AgBiI6 thin films (Figure S16b) and fitted the decays to a stretched exponential function, yielding an average lifetime of 33 ns. This function phenomenologically accounts for a superposition of monoexponential decays,53 which may be a result of inhomogeneous trap distributions.54 Longer charge-carrier lifetimes are more favorable for photovoltaic applications, since they allow more time for the charge carriers to reach the contacts and be extracted but are sensitive to the trap density in the films and hence their processing conditions: for MAPbI3, monomolecular charge-carrier lifetimes ranging from 4 ns to over 1 μs have been reported.54 PL lifetime measurements of Cs2AgBiBr6 have also been made and are reproduced from Longo et al. in Figure S16b,40 and a stretched exponential was found to describe the long-term decays in this material as well, highlighting the heterogeneity of recombination processes in both Cs2AgBiBr6 and Cu2AgBiI6. Both materials have an initial fast PL decay, but Cu2AgBiI6 shows a higher proportion of signals from long-lived (>200 ns) PL in comparison to Cs2AgBiBr6, which is reflected in the lower average PL lifetime (10 ns) of the latter decay.
To gain an insight into charge-carrier
mobilities in Cu2AgBiI6, we performed transient
THz photoconductivity measurements
using optical-pump, terahertz-probe spectroscopy, which gave a value
for the electron–hole sum mobility of 1.7(5) cm2 V–1 s–1. This value is higher
than that measured for the double perovskite Cs2AgBiBr6 (0.8 cm2 V–1 s–1),55 though not as high as that found
in MAPbBr3 or in current best-in-class hybrid perovskites
(8–70 cm2 V–1 s–1).54,56 Charge-carrier mobilities can be limited
by intrinsic factors such as the effective mass of charge carriers
and couplings of charge carriers to phonons but can also be influenced
significantly by extrinsic factors such as crystallinity, energetic
disorder, and carrier–carrier scattering.54 For instance, the first reports of room-temperature THz
mobilities in CH3NH3SnI3 were only
1.6 cm2 V–1 s–1.57 However, through compositional tuning and more
optimized processing, which resulted in reducing the crystalline disorder
and background charge carrier density, this has been raised to over
80 cm2 V–1 s–1 for
tin iodide perovskites.58 Mixed-cation,
mixed-halide lead halide perovskites are similarly sensitive to extrinsic
factors.56 Given the already-promising
value for Cu2AgBiI6 measured here, an improved
understanding of both the limits due to intrinsic factors and the
influences of extrinsic factors could lead to further enhancements
in charge-carrier mobilities in Cu2AgBiI6. Good
charge-carrier diffusion lengths are critical to efficient solar cell
operation, and a simplified calculation of 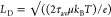 , using the values measured here and neglecting
higher-order recombination, yields an estimated value of 530 nm, which
is greater than the first estimates for MAPbI3,59,60 showing a charge-carrier diffusion length suitable for charge extraction,
despite the high cation disorder in the structure.
, using the values measured here and neglecting
higher-order recombination, yields an estimated value of 530 nm, which
is greater than the first estimates for MAPbI3,59,60 showing a charge-carrier diffusion length suitable for charge extraction,
despite the high cation disorder in the structure.
2.4. Cu2AgBiI6 Single-Junction Photovoltaic Devices
In order to assess if this material does function as an absorber layer in a photovoltaic cell, we fabricated “n-i-p” planar heterojunction devices incorporating a compact SnO2 n-type charge extraction layer and a spiro-OMeTAD hole-extraction layer. We fully describe the cell preparation and measurements in the Supporting Information, with the device architecture being shown in the inset in Figure 4b. The cell did function and delivered a PCE of 0.43%, a Jsc of 1.54 mA/cm2, a Voc of 0.47 V, and a fill factor of 59.6% (Figure 4a). The device shows hysteresis between the forward bias (FB)-to-short circuit (SC) and the SC-to-FB scan, with the first showing higher performance. However, it is interesting to note that the steady-state performances, measured at the maximum power point, present good short-term stability, with both the current density and the PCE increasing over time (Figure 4b). The results show that this device architecture can deliver photocurrent and photovoltage, but it is clear that significant effort is required to further optimize the devices. Here we have chosen the archetypical charge extraction materials and device configuration for lead halide perovskite cells, and it is likely that a new selection of charge extraction layers and/or different device architectures will be required in order to reach the full potential for this material. In addition, we expect that an improved understanding of the optoelectronic properties, and passivating defects, will also be important for device development.
Figure 4.
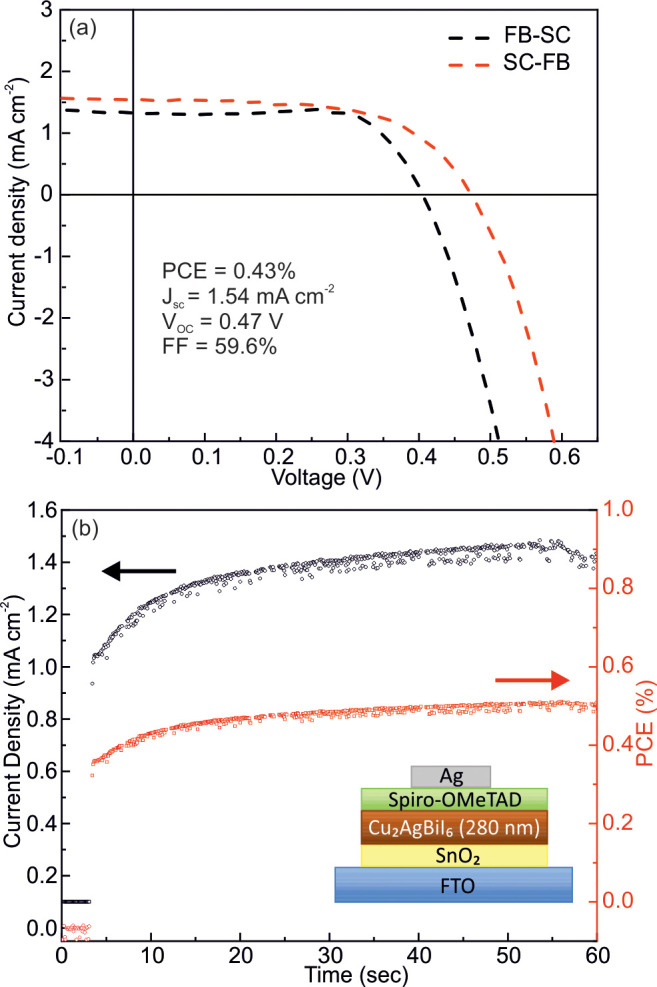
(a) J–V curves of the forward (FB) to short circuit (SC) and SC-FB scans for the device using Cu2AgBiI6 as the solar absorber. (b) Steady state performance, measured at the maximum power point, presenting good stability with both the current density and the PCE increasing over time. The inset shows the device architecture used.
We have demonstrated a certain degree of PV operation from this new material, yet the fundamental optical and electronic properties appear to promise significantly higher performance. Prior to expending significant effort upon materials and device optimization, however, it is important to estimate the ultimate potential efficiency achievable for this material. With knowledge of the above- and below-band-gap optical properties of a solar absorber material, it is possible to model its performance in a PV cell, following a detailed balanced thermodynamic approach,52 as we describe in the Supporting Information. In the thermodynamic assessment of a solar cell, Shockley and Queisser introduced an idealized step-function absorption profile, where the band gap is clearly defined.61 For a real material, the absorption onset is never infinitely steep, and the “PV band gap” is defined as the steepest point of the absorptance curve, which is easily deduced by taking the maximum of the differential of the external quantum efficiency spectrum. This PV band gap is therefore not an intrinsic property of the material but a property of the PV cell, which is influenced by the absorber layer thickness, its optical absorption properties, and the overall optical structure of the solar cell. In Figure S17b, we show the PV band gap of the Cu2AgBiI6 junction, as a function of the thickness of this layer. Very encouragingly, although the Elliott model band gap is 2.06(1) eV, the PV band gap drops from 2.0 eV all the way down to 1.7 eV for film thicknesses from 100 to 1200 nm. A band gap of 1.7 eV is close to optimal for combining with Si in a tandem cell, which will allow current matching of the two junctions.11 We therefore constructed an optical and electronic model for Cu2AgBiI6-on-Si tandem cells of the following structure: LiF/ITO/SnO2/ C60/Cu2AgBiI6/PolyTPD/ITO/nc-SiOx:H/(i)a-Si:H/c-Si/(i)a-Si:H/(p)a-Si:H/AZO/Ag (Figure S17a). We simulated the performance of a Cu2AgBiI6-on-Si tandem solar cell, using a transfer matrix optical model, coupled with a detailed balanced approach for simulating the current voltage curves. We determined the diode parameters for simulating the current–voltage curves via fitting lead halide perovskite and Si J–V curves reported in the literature (see the Supporting Information for more details and assumptions made during the modeling). Our main assumption is that, electronically, we can optimize the lead-free Cu2AgBiI6 to work as well as a lead halide perovskite cell, where radiative recombination accounts for 1% of the total recombination events (1% external radiative efficiency (ERE)), which is well below the world record lead halide perovskite cell that approaches 10% ERE but has not yet been reached in the related Ag1–3xBi1+xI4 materials. Our model does account for the optical properties, including sub-band-gap absorption onset, of our experimentally measured Cu2AgBiI6 thin films. Our results suggest that, with a thickness of 1710 nm, the Cu2AgBiI6 thin film is capable of being the top cell in a Si tandem, yielding a matched current density of 19.0 mA/cm2 (Figure S17c), a Voc of 1.92 V, an FF of 83%, and a corresponding PCE of 30.2% (Figure S17d). We show the influence of the decreasing Cu2AgBiI6 absorber layer thickness upon the tandem cell photovoltaic performance in Figure S18 in the Supporting Information. For a Cu2AgBiI6 layer thickness of 530 nm, the estimated carrier diffusion length leads to a modeled tandem efficiency of 28.1%. Thus, provided that the defects responsible for nonradiative recombination can be reduced in density or passivated to such an extent that a PV cell with a 1% external radiative efficiency can be created, then this material would compete on efficiency with lead halide perovskites integrated into multijunction PV cells. For comparison, we show in Figure S19 that Cs2AgBiBr6 cannot be current-matched with Si due to very low absorption in the red end of the visible spectrum.
3. Conclusion
In summary, we have synthesized stable compound Cu2AgBiI6 as powders, crystals, and solution-processed thin films. Cu2AgBiI6 provides the initial report and understanding of a quaternary phase in the CuI–AgI–BiI3 phase space. The structure is based on a 2D edge-sharing octahedral network. Octahedral sites are occupied by Ag+ and Bi3+ in a disordered fashion, and Cu+ occupies all possible tetrahedral sites located in the cubic close-packed iodide sublattice. Fitting the absorption profile using the Elliott model shows a band gap of the continuum of states of 2.06(1) eV and an exciton binding energy of only 25(2) meV. The steep rise in absorption from the band edge to a high absorption coefficient of 1.0 × 105 cm–1 just above the onset, several times higher than for MAPbI3 (0.3 × 105 cm–1), indicates great promise for the use of Cu2AgBiI6 as a thin-film absorber material in PV devices. In contrast, the highly studied lead-free double perovskite Cs2AgBiBr6 has a much less suitable absorption profile and exciton binding energy in comparison to Cu2AgBiI6 and a band gap too wide to be combined with c-Si in a tandem cell. The properties of this cation-decorated cubic close-packed iodide array containing Bi3+ emphasizes the scope for this chemistry to control optoelectronic properties without lead and beyond the perovskite structural family. Cu2AgBiI6 may also prove fruitful for other applications such as light emission and radiation detection.
Acknowledgments
We thank Prof. L. Hardwick and Dr. A. Cowan at the Stephenson Institute for Renewable Energy (SIRE), University of Liverpool, U.K., for helpful discussions and use of equipment and K. Dawson and M. Bilton at ICaL, Liverpool, U.K., for guidance and maintenance of TEM microscopes. We thank Dr. C. Robertson and Dr. E. Carrington (University of Liverpool, U.K.), and Dr. G. F. S. Whitehead (University of Manchester, U.K.) for helpful discussions regarding the single-crystal X-ray diffraction. L.M.H. is a TUM-IAS Hans Fischer Senior Fellow. M.J.R. thanks the Royal Society for the award of a Research Professorship. The data as presented in this paper is freely available at 10.17638/datacat.liverpool.ac.uk/1110.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c00495.
Experimental and calculation details and characterization data (PDF)
Accession Codes
CCDC 2013668 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Present Address
⊥ G.L.: Northumbria University, Department of Mathematics, Physics and Electrical Engineering, Ellison Place, Newcastle upon Tyne NE18ST, U.K.
Author Present Address
# M.J.P.: CEMHTI CNRS UPR3079, 45071 Orléans, France.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
H.C.S. is thankful to the EPSRC for support under EP/N004884/1 and for a Ph.D. studentship at the University of Liverpool, and the EPSRC Prosperity Partnership EP/S004947/1 for current funding at the University of Oxford. G.L. acknowledges the EPSRC EP/M015254/2. A.D.W. acknowledges support from the EPSRC EP/P033229/1. L.R.V.B. acknowledges funding from the Oxford-Radcliffe Scholarship and the EPSRC Centre for Doctoral Training in New and Sustainable Photovoltaics. S.M. acknowledges funding from the Rhodes Trust (India & Worcester 2016). M.A.-J. acknowledges the Royal Society (RGS\R1\211068), Cambridge Materials Limited, and Wolfson College, University of Cambridge for their funding and technical support. This work used the Cirrus UK National Tier-2 HPC Service at EPCC (http://www.cirrus.ac.uk) funded by the University of Edinburgh and EPSRC (EP/P020267/1).
The authors declare the following competing financial interest(s): We declare that we have filed a patent protecting quaternary Cu-Ag-Bi-I phases and their use in optoelectronic devices.
Supplementary Material
References
- Leguy A. M. A.; Azarhoosh P.; Alonso M. I.; Campoy-Quiles M.; Weber O. J.; Yao J.; Bryant D.; Weller M. T.; Nelson J.; Walsh A.; van Schilfgaarde M.; Barnes P. R. F. Experimental and Theoretical Optical Properties of Methylammonium Lead Halide Perovskites. Nanoscale 2016, 8 (12), 6317–6327. 10.1039/C5NR05435D. [DOI] [PubMed] [Google Scholar]
- Stoumpos C. C.; Malliakas C. D.; Kanatzidis M. G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52 (15), 9019–9038. 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- Sun S.; Salim T.; Mathews N.; Duchamp M.; Boothroyd C.; Xing G.; Sum T. C.; Lam Y. M. The Origin of High Efficiency in Low-Temperature Solution-Processable Bilayer Organometal Halide Hybrid Solar Cells. Energy Environ. Sci. 2014, 7 (1), 399–407. 10.1039/C3EE43161D. [DOI] [Google Scholar]
- Johnston M. B.; Herz L. M. Hybrid Perovskites for Photovoltaics: Charge-Carrier Recombination, Diffusion, and Radiative Efficiencies. Acc. Chem. Res. 2016, 49 (1), 146–154. 10.1021/acs.accounts.5b00411. [DOI] [PubMed] [Google Scholar]
- Dong Q.; Fang Y.; Shao Y.; Mulligan P.; Qiu J.; Cao L.; Huang J. Electron-Hole Diffusion Lengths > 175 μm in Solution-Grown CH3NH3PbI3 Single Crystals. Science 2015, 347 (6225), 967–970. 10.1126/science.aaa5760. [DOI] [PubMed] [Google Scholar]
- Ponseca C. S.; Savenije T. J.; Abdellah M.; Zheng K.; Yartsev A.; Pascher T.; Harlang T.; Chabera P.; Pullerits T.; Stepanov A.; Wolf J.-P.; Sundström V. Organometal Halide Perovskite Solar Cell Materials Rationalized: Ultrafast Charge Generation, High and Microsecond-Long Balanced Mobilities, and Slow Recombination. J. Am. Chem. Soc. 2014, 136 (14), 5189–5192. 10.1021/ja412583t. [DOI] [PubMed] [Google Scholar]
- Shi D.; Adinolfi V.; Comin R.; Yuan M.; Alarousu E.; Buin A.; Chen Y.; Hoogland S.; Rothenberger A.; Katsiev K.; Losovyj Y.; Zhang X.; Dowben P. A.; Mohammed O. F.; Sargent E. H.; Bakr O. M. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347 (6221), 519–522. 10.1126/science.aaa2725. [DOI] [PubMed] [Google Scholar]
- National Renewable Energy Laboratory (NREL) , Best Research-Cell Efficiency Chart; https://www.nrel.gov/pv/cell-efficiency.html (accessed December 2020).
- Eperon G. E.; Hörantner M. T.; Snaith H. J. Metal Halide Perovskite Tandem and Multiple-Junction Photovoltaics. Nat. Rev. Chem. 2017, 1 (12), 0095. 10.1038/s41570-017-0095. [DOI] [Google Scholar]
- Leijtens T.; Bush K. A.; Prasanna R.; McGehee M. D. Opportunities and Challenges for Tandem Solar Cells Using Metal Halide Perovskite Semiconductors. Nat. Energy 2018, 3 (10), 828–838. 10.1038/s41560-018-0190-4. [DOI] [Google Scholar]
- Hörantner M. T.; Leijtens T.; Ziffer M. E.; Eperon G. E.; Christoforo M. G.; McGehee M. D.; Snaith H. J. The Potential of Multijunction Perovskite Solar Cells. ACS Energy Lett. 2017, 2 (10), 2506–2513. 10.1021/acsenergylett.7b00647. [DOI] [Google Scholar]
- Mahesh S.; Ball J. M.; Oliver R. D. J.; McMeekin D. P.; Nayak P. K.; Johnston M. B.; Snaith H. J. Revealing the Origin of Voltage Loss in Mixed-Halide Perovskite Solar Cells. Energy Environ. Sci. 2020, 13 (1), 258–267. 10.1039/C9EE02162K. [DOI] [Google Scholar]
- Wei F.; Deng Z.; Sun S.; Xie F.; Kieslich G.; Evans D. M.; Carpenter M. A.; Bristowe P. D.; Cheetham A. K. The Synthesis, Structure and Electronic Properties of a Lead-Free Hybrid Inorganic-Organic Double Perovskite (MA)2KBiCl6 (MA = Methylammonium). Mater. Horiz. 2016, 3 (4), 328–332. 10.1039/C6MH00053C. [DOI] [Google Scholar]
- Wei F.; Deng Z.; Sun S.; Zhang F.; Evans D. M.; Kieslich G.; Tominaka S.; Carpenter M. A.; Zhang J.; Bristowe P. D.; Cheetham A. K. Synthesis and Properties of a Lead-Free Hybrid Double Perovskite: (CH3NH3)2AgBiBr6. Chem. Mater. 2017, 29 (3), 1089–1094. 10.1021/acs.chemmater.6b03944. [DOI] [Google Scholar]
- McClure E. T.; Ball M. R.; Windl W.; Woodward P. M. Cs2AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28 (5), 1348–1354. 10.1021/acs.chemmater.5b04231. [DOI] [Google Scholar]
- Slavney A. H.; Hu T.; Lindenberg A. M.; Karunadasa H. I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138 (7), 2138–2141. 10.1021/jacs.5b13294. [DOI] [PubMed] [Google Scholar]
- Saparov B.; Hong F.; Sun J.-P.; Duan H.-S.; Meng W.; Cameron S.; Hill I. G.; Yan Y.; Mitzi D. B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27 (16), 5622–5632. 10.1021/acs.chemmater.5b01989. [DOI] [Google Scholar]
- Harikesh P. C.; Mulmudi H. K.; Ghosh B.; Goh T. W.; Teng Y. T.; Thirumal K.; Lockrey M.; Weber K.; Koh T. M.; Li S.; Mhaisalkar S.; Mathews N. Rb as an Alternative Cation for Templating Inorganic Lead-Free Perovskites for Solution Processed Photovoltaics. Chem. Mater. 2016, 28 (20), 7496–7504. 10.1021/acs.chemmater.6b03310. [DOI] [Google Scholar]
- Lehner A. J.; Fabini D. H.; Evans H. A.; Hébert C.-A.; Smock S. R.; Hu J.; Wang H.; Zwanziger J. W.; Chabinyc M. L.; Seshadri R. Crystal and Electronic Structures of Complex Bismuth Iodides A3Bi2I9 (A = K, Rb, Cs) Related to Perovskite: Aiding the Rational Design of Photovoltaics. Chem. Mater. 2015, 27 (20), 7137–7148. 10.1021/acs.chemmater.5b03147. [DOI] [Google Scholar]
- Hoye R. L. Z.; Brandt R. E.; Osherov A.; Stevanović V.; Stranks S. D.; Wilson M. W. B.; Kim H.; Akey A. J.; Perkins J. D.; Kurchin R. C.; Poindexter J. R.; Wang E. N.; Bawendi M. G.; Bulović V.; Buonassisi T. Methylammonium Bismuth Iodide as a Lead-Free, Stable Hybrid Organic-Inorganic Solar Absorber. Chem. Eur. J. 2016, 22 (8), 2605–2610. 10.1002/chem.201505055. [DOI] [PubMed] [Google Scholar]
- Sun S.; Tominaka S.; Lee J.-H.; Xie F.; Bristowe P. D.; Cheetham A. K. Synthesis, Crystal Structure, and Properties of a Perovskite-Related Bismuth Phase, (NH4)3Bi2I9. APL Mater. 2016, 4 (3), 031101. 10.1063/1.4943680. [DOI] [Google Scholar]
- Hebig J.-C.; Kühn I.; Flohre J.; Kirchartz T. Optoelectronic Properties of (CH3NH3)3Sb2I9 Thin Films for Photovoltaic Applications. ACS Energy Lett. 2016, 1 (1), 309–314. 10.1021/acsenergylett.6b00170. [DOI] [Google Scholar]
- Savory C. N.; Walsh A.; Scanlon D. O. Can Pb-Free Halide Double Perovskites Support High-Efficiency Solar Cells?. ACS Energy Lett. 2016, 1 (5), 949–955. 10.1021/acsenergylett.6b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz S. E.; Crites E. N.; De Siena M. C.; Gamelin D. R. Colloidal Nanocrystals of Lead-Free Double-Perovskite (Elpasolite) Semiconductors: Synthesis and Anion Exchange to Access New Materials. Nano Lett. 2018, 18 (2), 1118–1123. 10.1021/acs.nanolett.7b04659. [DOI] [PubMed] [Google Scholar]
- Sansom H. C.; Whitehead G. F. S.; Dyer M. S.; Zanella M.; Manning T. D.; Pitcher M. J.; Whittles T. J.; Dhanak V. R.; Alaria J.; Claridge J. B.; Rosseinsky M. J. AgBiI4 as a Lead-Free Solar Absorber with Potential Application in Photovoltaics. Chem. Mater. 2017, 29 (4), 1538–1549. 10.1021/acs.chemmater.6b04135. [DOI] [Google Scholar]
- Hamdeh U. H.; Nelson R. D.; Ryan B. J.; Bhattacharjee U.; Petrich J. W.; Panthani M. G. Solution-Processed BiI3 Thin Films for Photovoltaic Applications: Improved Carrier Collection via Solvent Annealing. Chem. Mater. 2016, 28 (18), 6567–6574. 10.1021/acs.chemmater.6b02347. [DOI] [Google Scholar]
- Lehner A. J.; Wang H.; Fabini D. H.; Liman C. D.; Hébert C.-A.; Perry E. E.; Wang M.; Bazan G. C.; Chabinyc M. L.; Seshadri R. Electronic Structure and Photovoltaic Application of BiI3. Appl. Phys. Lett. 2015, 107 (13), 131109. 10.1063/1.4932129. [DOI] [Google Scholar]
- Brandt R. E.; Kurchin R. C.; Hoye R. L. Z.; Poindexter J. R.; Wilson M. W. B.; Sulekar S.; Lenahan F.; Yen P. X. T.; Stevanović V.; Nino J. C.; Bawendi M. G.; Buonassisi T. Investigation of Bismuth Triiodide (BiI3) for Photovoltaic Applications. J. Phys. Chem. Lett. 2015, 6 (21), 4297–4302. 10.1021/acs.jpclett.5b02022. [DOI] [PubMed] [Google Scholar]
- Shao Z.; Le Mercier T.; Madec M. B.; Pauporté T. AgBi2I7 Layers with Controlled Surface Morphology for Solar Cells with Improved Charge Collection. Mater. Lett. 2018, 221, 135–138. 10.1016/j.matlet.2018.03.085. [DOI] [Google Scholar]
- Zhang B.; Lei Y.; Qi R.; Yu H.; Yang X.; Cai T.; Zheng Z. An In-Situ Room Temperature Route to CuBiI4 Based Bulk-Heterojunction Perovskite-Like Solar Cells. Sci. China Mater. 2019, 62 (4), 519–526. 10.1007/s40843-018-9355-0. [DOI] [Google Scholar]
- Turkevych I.; Kazaoui S.; Ito E.; Urano T.; Yamada K.; Tomiyasu H.; Yamagishi H.; Kondo M.; Aramaki S. Photovoltaic Rudorffites: Lead-Free Silver Bismuth Halides Alternative to Hybrid Lead Halide Perovskites. ChemSusChem 2017, 10 (19), 3754–3759. 10.1002/cssc.201700980. [DOI] [PubMed] [Google Scholar]
- Pai N.; Lu J.; Gengenbach T. R.; Seeber A.; Chesman A. S. R.; Jiang L.; Senevirathna D. C.; Andrews P. C.; Bach U.; Cheng Y.-B.; Simonov A. N. Silver Bismuth Sulfoiodide Solar Cells: Tuning Optoelectronic Properties by Sulfide Modification for Enhanced Photovoltaic Performance. Adv. Energy Mater. 2018, 9 (5), 1803396. 10.1002/aenm.201803396. [DOI] [Google Scholar]
- Hu Z.; Wang Z.; Kapil G.; Ma T.; Iikubo S.; Minemoto T.; Yoshino K.; Toyoda T.; Shen Q.; Hayase S. Solution-Processed Air-Stable Copper Bismuth Iodide for Photovoltaics. ChemSusChem 2018, 11 (17), 2930–2935. 10.1002/cssc.201800815. [DOI] [PubMed] [Google Scholar]
- Oldag T.; Aussieker T.; Keller H.-L.; Preitschaft C.; Pfitzner A. Solvothermale Synthese und Bestimmung der Kristallstrukturen von AgBiI4 und Ag3BiI6. Z. Anorg. Allg. Chem. 2005, 631 (4), 677–682. 10.1002/zaac.200400508. [DOI] [Google Scholar]
- Fourcroy P. H.; Carre D.; Thevet F.; Rivet J. Structure du Tetraiodure de Cuivre(I) et de Bismuth(III), CuBiI4. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1991, 47 (10), 2023–2025. 10.1107/S0108270191005309. [DOI] [Google Scholar]
- Fourcroy P. H.; Palazzi M.; Rivet J.; Flahaut J.; Céolin R. Etude du Systeme AgIBiI3. Mater. Res. Bull. 1979, 14 (3), 325–328. 10.1016/0025-5408(79)90096-5. [DOI] [Google Scholar]
- Mashadieva L. F.; Aliev Z. S.; Shevelkov A. V.; Babanly M. B. Experimental Investigation of the Ag-Bi-I Ternary System and Thermodynamic Properties of the Ternary Phases. J. Alloys Compd. 2013, 551, 512–520. 10.1016/j.jallcom.2012.11.033. [DOI] [Google Scholar]
- Dzeranova K. B.; Kaloev N. I.; Bukhalova G. A. The BiI3 - AgI System. Russ. J. Inorg. Chem. 1985, 30, 1700–1701. [Google Scholar]
- de Picciotto L. A.; Thackeray M. M.; David W. I. F.; Bruce P. G.; Goodenough J. B. Structural Characterization of Delithiated LiVO2. Mater. Res. Bull. 1984, 19 (11), 1497–1506. 10.1016/0025-5408(84)90264-2. [DOI] [Google Scholar]
- Longo G.; Mahesh S.; Buizza L. R. V.; Wright A. D.; Ramadan A. J.; Abdi-Jalebi M.; Nayak P. K.; Herz L. M.; Snaith H. J. Understanding the Performance-Limiting Factors of Cs2AgBiBr6 Double-Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2200–2207. 10.1021/acsenergylett.0c01020. [DOI] [Google Scholar]
- Davies C. L.; Filip M. R.; Patel J. B.; Crothers T. W.; Verdi C.; Wright A. D.; Milot R. L.; Giustino F.; Johnston M. B.; Herz L. M. Bimolecular Recombination in Methylammonium Lead Triiodide Perovskite is an Inverse Absorption Process. Nat. Commun. 2018, 9 (1), 293. 10.1038/s41467-017-02670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. D.; Verdi C.; Milot R. L.; Eperon G. E.; Pérez-Osorio M. A.; Snaith H. J.; Giustino F.; Johnston M. B.; Herz L. M. Electron-Phonon Coupling in Hybrid Lead Halide Perovskites. Nat. Commun. 2016, 7 (1), 11755. 10.1038/ncomms11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.; Zhang J.; Sun H.; Hou D.; Gan X.; Shang M.-h.; Li Y.; Hu Z.; Zhu Y.; Han L. Inorganic and Lead-Free AgBiI4 Rudorffite for Stable Solar Cell Applications. ACS Appl. Energy Mater. 2018, 1 (9), 4485–4492. 10.1021/acsaem.8b01202. [DOI] [Google Scholar]
- De Wolf S.; Holovsky J.; Moon S.-J.; Löper P.; Niesen B.; Ledinsky M.; Haug F.-J.; Yum J.-H.; Ballif C. Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 5 (6), 1035–1039. 10.1021/jz500279b. [DOI] [PubMed] [Google Scholar]
- Schade L.; Wright A. D.; Johnson R. D.; Dollmann M.; Wenger B.; Nayak P. K.; Prabhakaran D.; Herz L. M.; Nicholas R.; Snaith H. J.; Radaelli P. G. Structural and Optical Properties of Cs2AgBiBr6 Double Perovskite. ACS Energy Lett. 2019, 4 (1), 299–305. 10.1021/acsenergylett.8b02090. [DOI] [Google Scholar]
- Bartesaghi D.; Slavney A. H.; Gélvez-Rueda M. C.; Connor B. A.; Grozema F. C.; Karunadasa H. I.; Savenije T. J. Charge Carrier Dynamics in Cs2AgBiBr6 Double Perovskite. J. Phys. Chem. C 2018, 122 (9), 4809–4816. 10.1021/acs.jpcc.8b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M. R.; Hillman S.; Haghighirad A. A.; Snaith H. J.; Giustino F. Band Gaps of the Lead-Free Halide Double Perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from Theory and Experiment. J. Phys. Chem. Lett. 2016, 7 (13), 2579–2585. 10.1021/acs.jpclett.6b01041. [DOI] [PubMed] [Google Scholar]
- Elliott R. J. Intensity of Optical Absorption by Excitons. Phys. Rev. 1957, 108 (6), 1384–1389. 10.1103/PhysRev.108.1384. [DOI] [Google Scholar]
- Galkowski K.; Mitioglu A.; Miyata A.; Plochocka P.; Portugall O.; Eperon G. E.; Wang J. T.-W.; Stergiopoulos T.; Stranks S. D.; Snaith H. J.; Nicholas R. J. Determination of the Exciton Binding Energy and Effective Masses for Methylammonium and Formamidinium Lead Tri-Halide Perovskite Semiconductors. Energy Environ. Sci. 2016, 9 (3), 962–970. 10.1039/C5EE03435C. [DOI] [Google Scholar]
- Yang Z.; Surrente A.; Galkowski K.; Miyata A.; Portugall O.; Sutton R. J.; Haghighirad A. A.; Snaith H. J.; Maude D. K.; Plochocka P.; Nicholas R. J. Impact of the Halide Cage on the Electronic Properties of Fully Inorganic Cesium Lead Halide Perovskites. ACS Energy Lett. 2017, 2 (7), 1621–1627. 10.1021/acsenergylett.7b00416. [DOI] [Google Scholar]
- Wu C.; Zhang Q.; Liu Y.; Luo W.; Guo X.; Huang Z.; Ting H.; Sun W.; Zhong X.; Wei S.; Wang S.; Chen Z.; Xiao L. The Dawn of Lead-Free Perovskite Solar Cell: Highly Stable Double Perovskite Cs2AgBiBr6 Film. Adv. Sci. 2018, 5 (3), 1700759. 10.1002/advs.201700759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak P. K.; Mahesh S.; Snaith H. J.; Cahen D. Photovoltaic Solar Cell Technologies: Analysing the State of the Art. Nat. Rev. Mater. 2019, 4 (4), 269–285. 10.1038/s41578-019-0097-0. [DOI] [Google Scholar]
- Patel J. B.; Milot R. L.; Wright A. D.; Herz L. M.; Johnston M. B. Formation Dynamics of CH3NH3PbI3 Perovskite Following Two-Step Layer Deposition. J. Phys. Chem. Lett. 2016, 7 (1), 96–102. 10.1021/acs.jpclett.5b02495. [DOI] [PubMed] [Google Scholar]
- Herz L. M. Charge-Carrier Dynamics in Organic-Inorganic Metal Halide Perovskites. Annu. Rev. Phys. Chem. 2016, 67 (1), 65–89. 10.1146/annurev-physchem-040215-112222. [DOI] [PubMed] [Google Scholar]
- Hutter E. M.; Gélvez-Rueda M. C.; Bartesaghi D.; Grozema F. C.; Savenije T. J. Band-Like Charge Transport in Cs2AgBiBr6 and Mixed Antimony-Bismuth Cs2AgBi1-xSbxBr6 Halide Double Perovskites. ACS Omega 2018, 3 (9), 11655–11662. 10.1021/acsomega.8b01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman W.; McMeekin D. P.; Patel J. B.; Milot R. L.; Johnston M. B.; Snaith H. J.; Herz L. M. Photovoltaic Mixed-Cation Lead Mixed-Halide Perovskites: Links Between Crystallinity, Photo-Stability and Electronic Properties. Energy Environ. Sci. 2017, 10 (1), 361–369. 10.1039/C6EE03014A. [DOI] [Google Scholar]
- Noel N. K.; Stranks S. D.; Abate A.; Wehrenfennig C.; Guarnera S.; Haghighirad A.-A.; Sadhanala A.; Eperon G. E.; Pathak S. K.; Johnston M. B.; Petrozza A.; Herz L. M.; Snaith H. J. Lead-Free Organic-Inorganic Tin Halide Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7 (9), 3061–3068. 10.1039/C4EE01076K. [DOI] [Google Scholar]
- Savill K. J.; Ulatowski A. M.; Farrar M. D.; Johnston M. B.; Snaith H. J.; Herz L. M. Impact of Tin Fluoride Additive on the Properties of Mixed Tin-Lead Iodide Perovskite Semiconductors. Adv. Funct. Mater. 2020, 30 (52), 2005594. 10.1002/adfm.202005594. [DOI] [Google Scholar]
- Stranks S. D.; Eperon G. E.; Grancini G.; Menelaou C.; Alcocer M. J. P.; Leijtens T.; Herz L. M.; Petrozza A.; Snaith H. J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342 (6156), 341–344. 10.1126/science.1243982. [DOI] [PubMed] [Google Scholar]
- Xing G.; Mathews N.; Sun S.; Lim S. S.; Lam Y. M.; Grätzel M.; Mhaisalkar S.; Sum T. C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342 (6156), 344–347. 10.1126/science.1243167. [DOI] [PubMed] [Google Scholar]
- Shockley W.; Queisser H. J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32 (3), 510–519. 10.1063/1.1736034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.