Abstract
Although psychosis is a defining feature of Lewy body disease, psychotic symptoms occur in a subset of patients with every major neurodegenerative disease. Few studies, however, have compared disease-related rates of psychosis prevalence in a large autopsy-based cohort, and it remains unclear how diseases differ with respect to the nature or content of the psychosis. We conducted a retrospective chart review of 372 patients with autopsy-confirmed neurodegenerative pathology: 111 with Alzheimer’s disease, 59 with Lewy body disease and concomitant Alzheimer’s disease, 133 with frontotemporal lobar degeneration (FTLD) with tau inclusions (including progressive supranuclear palsy, corticobasal degeneration or Pick’s disease), and 69 with FTLD and TDP inclusions (FTLD-TDP, including types A–C). Psychosis content was classified by subtype, and the frequency of each subtype was compared among pathological diagnoses using logistic regression. A total of 111 of 372 patients had psychosis. Compared to other groups, patients with Lewy body disease/Alzheimer’s disease pathology were significantly more likely to have hallucinations and were more likely to have more than one subtype of hallucination. Patients with Braak Parkinson stage 5–6 Lewy body disease were significantly more likely than those with no Lewy body disease to have visual hallucinations of misperception, peripheral hallucinations, hallucinations that moved, hallucinations of people/animals/objects, as well as delusions regarding a place and delusions of misidentification. The feeling of a presence occurred significantly more frequently in patients with Lewy body disease/Alzheimer’s disease than all other pathologies. Patients with FTLD-TDP were significantly more likely to have delusions, and for the delusions to occur in the first 3 years of the disease, when compared to patients with Alzheimer’s disease and FTLD-tau, though rates were not significantly greater than patients with Lewy body disease/Alzheimer’s disease. Paranoia occurred more frequently in the FTLD-TDP and Lewy body disease/Alzheimer’s disease categories compared to patients with Alzheimer’s disease or FTLD-tau. Patients with FTLD-TDP pathology had delusions of misidentification as frequently as patients with Lewy body disease/Alzheimer’s disease, and were significantly more likely to have self-elevating delusions such as grandiosity and erotomania compared to patients with other pathologies including FTLD-tau. These data show that the nature and content of psychosis can provide meaningful information about the underlying neurodegenerative pathology, emphasizing the importance of characterizing patients’ psychoses for prediction of the neuropathological diagnosis, regardless of a patient’s clinical syndrome.
Keywords: psychosis, pathology, TDP-43, frontotemporal dementia, Lewy body disease
Naasan et al. report that the nature and content of psychosis in patients with dementia correlate with the underlying neurodegenerative pathology. Characterizing patients’ psychoses can thus provide valuable information on the neuropathological diagnosis, regardless of a patient’s clinical syndrome.
Introduction
Neuropsychiatric symptoms, including psychosis, are common manifestations of neurodegenerative disorders (Lyketsos et al., 2001, 2012) and can often be the first sign of disease (Haddad and Benbow, 1992; Auning et al., 2011). Advancing our understanding of psychotic symptoms in patients with neurodegenerative disease is critical for clinical care, because studies have shown that people who have delusions may act on their false beliefs in a manner that can be harmful to themselves and their surroundings (Gilleen and David, 2005). Furthermore, psychosis is a major source of stress, anxiety and depression, placing a significant burden both on people suffering from the disorder and on their caregivers (Lyketsos et al., 2012). Psychosis is associated with earlier institutionalization and a higher cost of care (Herrmann et al., 2006). The low prevalence of psychosis in typically ageing cohorts suggests that most late-life psychotic symptoms are likely indicative of underlying neurodegeneration (Geda et al., 2008; Ehrenberg et al., 2018). However, studies clearly linking specific neuropathologies with psychotic symptoms are lacking, and prevalence rates remain unclear.
Moreover, the phenomenology of psychosis in neurodegenerative disease has implications for understanding both disease mechanisms and the neural basis of psychosis. Patients with psychiatric, neurodegenerative, and eye disease show contrasting hallucination patterns and content, suggesting diverse underlying biological and physiological substrates (Dudley et al., 2019). Current medical management of psychosis in neurodegenerative disease tends to treat all different subtypes of delusions and hallucinations as a general problem in which cognition deviates from reality. However, this approach is based on the assumption that psychotic symptoms arise from a common underlying brain dysfunction, and thus are unlikely to show predictable content variability across diseases—an assumption that is not supported by existing clinical studies in patients with neurodegenerative disorders.
Hallucinations are a form of psychosis defined as a lived sensory experience in the absence of a sensory stimulus. Hallucinations are one of the core clinical diagnostic criteria of dementia with Lewy bodies syndrome (McKeith et al., 2017). According to a systematic meta-analysis, hallucinations occur in 13–16% of patients with Alzheimer-type dementia syndrome (Alzheimer’s disease dementia) (Zhao et al., 2016). Another study found that visual, auditory and tactile hallucinations occurred in 36% of patients with behavioural variant frontotemporal dementia syndrome (bvFTD) who carried an expansion in one of the most common FTD-causing genes, C9orf72, compared to 17% of bvFTD non-carriers (Devenney et al., 2017). In dementia with Lewy bodies, visual hallucinations of people and those of non-human objects may arise from distinct neural origins (Nagahama et al., 2007, 2010). One theory of hallucinations is that all hallucinations share a common neural network that includes the right superior temporal sulcus, while the specific sensory modality and content of the hallucination relates to connections between that network and other sensory networks (Kim et al., 2019).
Delusions are another form of psychosis and are defined as ‘fixed beliefs that are not amenable to change in light of conflicting evidence’ (American Psychiatric Association, 2013). Up to 35% of patients with Alzheimer’s disease dementia have delusions (Zhao et al., 2016). Delusions were the presenting neuropsychiatric symptom in 21% of patients with bvFTD and 18% of patients with FTD and motor neuron disease (FTD-MND) with an expansion in C9orf72 (Sha et al., 2012). One neuropsychological theory of delusions proposes a two-factor hypothesis whereby a delusion requires (i) a neuropsychological impairment that leads to the formation of the false belief, in addition to (ii) an impairment in processes that would normally lead to the rejection of the belief in question (Coltheart, 2010). This hypothesis suggests that the first impairment, which dictates the nature or content of the delusion, could be disease-specific, unlike the second impairment, which leads to the general fixation and persistence of the false belief, and is a process likely common to all conditions in which delusions manifest clinically. As an example of the disease specificity of delusion content, the delusions associated with the C9orf72 expansion can be grandiose (Shinagawa et al., 2015). In contrast, the delusions associated with Alzheimer’s disease dementia are often of a paranoid nature, such as delusions of theft or persecution, and may be associated with volume loss in the right hippocampus (Geroldi et al., 2000). One factor analysis of psychotic symptoms in clinically defined dementia with Lewy bodies yielded four clusters: (i) misidentification of people and reduplication of people and places; (ii) belief that absent or deceased relatives are in the house; (iii) visual hallucinations of non-human objects; and (iv) visual hallucinations of people (Nagahama et al., 2007, 2010).
If delusions and hallucinations differ based on a patient’s underlying neuropathology, then the content of the psychosis could have a predictive role, providing clinicians with valuable assistance in differential diagnosis. Such clinical tools are increasingly important in an era when pathology-specific pharmacological interventions are being designed and tested. The objective of this study was to assess the rates of specific types of psychosis content found in each major disease category in a large autopsied cohort of patients with neurodegenerative disease. Specifically, we hypothesized that distinct types and content of delusions and hallucinations would appear in patients with different neurodegenerative conditions.
Materials and methods
Participants
We screened 830 patients that came to autopsy at the University of California San Francisco Memory and Aging Center between 1999 and 2017 who had autopsy-confirmed primary neurodegenerative disease. Full pathology reports of all participants were reviewed. Patients were screened into the psychosis group if delusions or hallucinations were reported on the Neuropsychiatric Inventory (NPI) (Cummings et al., 1994) or the Alzheimer’s Disease Research Center (ADRC) UDS Symptom Checklist at any time during their illness. The medical records of this subgroup were reviewed, and participants were excluded if details regarding their psychosis were absent or if their pathology reports were incomplete. Patients with no reported psychosis on the NPI or the ADRC symptom checklist were included in the control group if they had available pathology reports. If pathology reports mentioned psychosis as part of the clinical syndrome for patients who were originally assigned to the control group, their full medical charts were reviewed and they were switched to the case group if psychosis was verified. All patients or their surrogates consented to participate in the study, and the protocol was approved through the institution’s internal review board, in accordance with the Declaration of Helsinki.
Chart review
Medical records were systematically reviewed for information about patients’ psychosis by trained coders who were blind to neuropathological diagnosis. To maximize reliability among coders, 20% of the charts of patients with psychosis were rated by two coders and discrepancies were discussed, resolved, and incorporated into an enhanced coding manual. Hallucinations were classified by sensory modality (visual, auditory, tactile, and olfactory) and by content of the hallucinated stimulus (visual misperceptions, simple visual hallucinations of shapes and colours, well-formed visual hallucinations including of people or animals, and non-visual hallucinations). Other characteristics of visual hallucinations, such as peripheral/extracampine hallucinations (Wood et al., 2015) and hallucinations of images that move were also coded separately. Delusion subtypes were selected according to the DSM-5 criteria. First, all delusions were subclassified into either bizarre or non-bizarre based on the definition that non-bizarre delusions are conceivably possible whereas bizarre fall outside of the realm of possibility. Then, delusions were divided into five major groups including: (i) erotomanic; (ii) grandiose; (iii) jealous; (iv) persecutory; and (v) somatic (American Psychiatric Association, 2013). In addition, we added a category to capture delusions of misidentification of people, including the Capgras delusion, and for delusions regarding a particular place, including a person’s own home and reduplicative paramnesia. We further subclassified persecutory delusions into delusions of theft, intention to hurt, intrusion into home, and suspiciousness, for paranoid delusions that did not meet any of the other classifications. Finally, we included the feeling of presence, an overwhelming feeling that another person is present in the same room when no one is there, as its own category as its classification is unclear (Nagahama et al., 2007). Operational definitions for all psychosis classifications are presented in the Supplementary material. The duration and frequency of psychosis were also recorded.
Neuropathological assessment
The neuropathological procedure is detailed in the Supplementary material. We classified patients into major categories according to four primary pathologies: (i) Alzheimer’s disease without Lewy body disease (LBD) defined as a primary pathology of Alzheimer’s disease meeting the ADNC criteria (Montine et al., 2012) without concomitant LBD or with LBD showing a Parkinson’s disease Braak stage < 3; (ii) mixed LBD and Alzheimer’s disease (LBD/AD), defined as concomitant Alzheimer’s disease and LBD pathology where the Parkinson’s disease Braak stage was ≥ 3; (iii) frontotemporal lobar degeneration with TDP inclusions (FTLD-TDP), including types A–C; and (iv) FTLD-tau, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Pick’s disease. Patients with FTLD were diagnosed according to international consensus criteria (Mackenzie et al., 2011). All patients with other primary pathologies were excluded from the analysis to constrain scope and to avoid creating many small subgroups with limited statistical power. In addition to classifying patients according to primary pathological diagnoses, we also coded all contributing pathologies as either present/absent. For example, if a patient had a primary pathology of FTLD-TDP-B, along with MND pathology, Alzheimer’s disease Braak 3 and LBD Braak 2, that patient would receive a primary pathology classification of FTLD-TDP B, along with secondary pathology codes for MND, Alzheimer’s disease, and LBD (present), and tau (absent).
Genetic analysis
A subgroup of 49 participants received genetic testing as part of their research protocol. For these patients, we coded available information regarding APOE and tau haplotype status as well as the presence of an expansion in the C9orf72 gene and of any mutation in Alzheimer’s disease autosomal dominant gene (APP, PSEN1, and PSEN2), and in any of the other most common FTLD autosomal dominant genes [FUS, GRN, MAPT, and TARDBP (encoding TDP)].
Statistical analysis
All analyses were conducted using SPSS version 25. First, we determined the frequencies of hallucinations and delusions and all symptom subtypes across primary pathological diagnoses. Then, we conducted Fisher’s exact chi-squared tests of independence to compare the frequencies of each symptom, both among the four major pathological categories detailed above and then among specific pathological diagnoses. In a secondary analysis, we used chi-square tests to determine if the presence of a co-pathology influenced whether patients with a specific primary neuropathological diagnosis were more likely to have a particular psychosis subtype (e.g. in all patients with FTLD-TDP-A who had LBD co-pathology, we compared the frequency of patients who had persecutory delusions to those who did not). We performed logistic regressions with the four primary pathology categories to assess their effect on specific psychotic symptoms. For each psychosis subtype, we used a single model which included: (i) Alzheimer’s disease severity, as measured by the Alzheimer’s disease NIA-AA ADNC likelihood (low, intermediate, or high); (ii) Parkinson’s disease Braak staging [grouped into Braak 1–2 (brainstem-predominant), Braak 3–4 (transitional limbic) and Braak 5–6 (diffuse neocortical)]; and (iii) TDP presence (none, limbic only, or diffuse), controlling for gender, level of education, and age at death. We corrected for multiple pairwise testing analyses according to the B-Y method (Benjamini and Yekutieli, 2001).
Data availability
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants, but are available on request from the corresponding author.
Results
A total of 372 patients met inclusion criteria, of which 111 patients had psychosis. The primary neurodegenerative diagnoses were: 111 Alzheimer’s disease, 59 LBD/AD, 63 PSP, 44 CBD, 26 Pick’s disease, 17 FTLD-TDP-A, 33 FTLD-TDP-B, and 19 FTLD-TDP-C. The demographic characteristics of patients across the major primary pathology diagnoses are presented in Table 1. There were no statistical differences in gender distribution or level of education across diagnostic groups, although patients with LBD/AD were more likely to be males. Patients with FTLD-TDP B were significantly younger at disease onset than patients with LBD/AD or PSP.
Table 1.
Demographics and psychosis breakdown
| AD | LBD/AD | FTLD-Tau |
FTLD-TDP |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pick’s | PSP | CBD | A | B | C | |||||
| Total subjects with and without psychosis, n | 111 | 59 | 26 | 63 | 44 | 17 | 32 | 19 | NA | |
| Female, % | 41.4 | 27.1 | 38.5 | 49.2 | 54.5 | 41.2 | 42.4 | 46.8 | 0.193 | |
| Years of education, mean | 16.1 | 17.2 | 15.9 | 16.0 | 15.9 | 15.7 | 16 | 16.5 | 0.371 | |
| Age at disease onset, mean | 61 | 64 | 57 | 63 | 60 | 57 | 56 | 56 | 0.001 | |
| Clinical diagnosis, % | ||||||||||
| MCI | 3.6 | – | – | – | – | – | 3 | 5.3 | NA | |
| Clinical AD | 70.3 | 54.2 | 3.8 | 3.2 | 6.8 | 11.8 | – | – | ||
| DLB | 3.6 | 35.6 | – | 1.6 | 2.3 | – | 3 | – | ||
| bvFTD | 2.7 | – | 53.8 | 4.8 | 27.3 | 47.1 | 48.5 | 26.3 | ||
| FTD/MND | - | – | – | – | 2.3 | – | 24.2 | – | ||
| CBS | 8.1 | 1.7 | 15.4 | 17.5 | 27.3 | 23.5 | 3 | – | ||
| PSP | - | – | – | 66.7 | 6.8 | – | – | – | ||
| nfvPPA | 0.9 | – | 15.4 | 3.2 | 15.9 | – | 3 | – | ||
| svPPA | - | 1.7 | 3.8 | – | – | 5.9 | 9.1 | 68.4 | ||
| Any psychosis, % | 22.5a,b | 55.9c | 23.1a,b,c | 22.2a,b | 9.1b | 52.9a,c | 42.4a,c | 31.6a,b,c | ||
| Hallucinations, % | 13.5a | 48.3b | 11.5a | 22.2a,b | 9.1a | 23.5a,b | 30.3a,b | 5.3a | <0.0001 | |
| Hallucinations in first 3 years, % | 5a | 26.3b | 4.3a,b | 9.5a,b | 2.3a | 23.3b | 18.2a,b | 0a,b | <0.0001 | |
| Delusions, % | 16.2a,b | 25.9a,b,c | 15.4a,b,c | 12.7b,d | 2.3d | 35.3a,c | 36.4c | 31.6a,b,c | <0.0001 | |
| Delusions in first 3 years, % | 1.8a | 10.2b,c,d,e,f | 7.7a,b,c,d,e,f | 4.8a,e,f | 2.3a,d,f | 23.5c | 18.2c | 0a,b,d,e,f | 0.001 | |
| Genetics, n | 83 | 34 | 25 | 51 | 35 | 15 | 33 | 18 | 0.001 | |
| PSEN1 | 1 | – | – | – | – | – | – | – | NA | |
| PSEN2 | - | – | – | – | – | – | – | – | ||
| APP | 2 | 1 | – | – | – | – | – | – | ||
| C9orf72 | - | – | – | – | – | 3 | 12 | – | ||
| GRN | 1 | – | – | – | – | 7 | – | – | ||
| MAPT | - | – | – | – | 1 | – | – | – | ||
Statistical significance set at P = 0.01273 per B-Y method for k = 28. AD = primary Alzheimer’s disease pathology; LBD = primary Lewy body disease; LBD/AD = Lewy body disease and primary Alzheimer’s disease of equal pathological burden; MND = motor neuron disease; Tau = primary Tau pathology (including CBD, PSP, AGD, and unspecified); TDP = primary TDP pathology (including TDP types A, B, C and unspecified). One patient with AGD diagnosis was not included in the table. NA = not applicable.
Each superscript letter denotes diagnostic categories whose proportions do not significantly differ from each other for that respective row (e.g. in hallucinations, the frequency of hallucinations in patients with LBD/AD was significantly different than that in patients with FTLD-tau CBD).
Frequency of psychosis
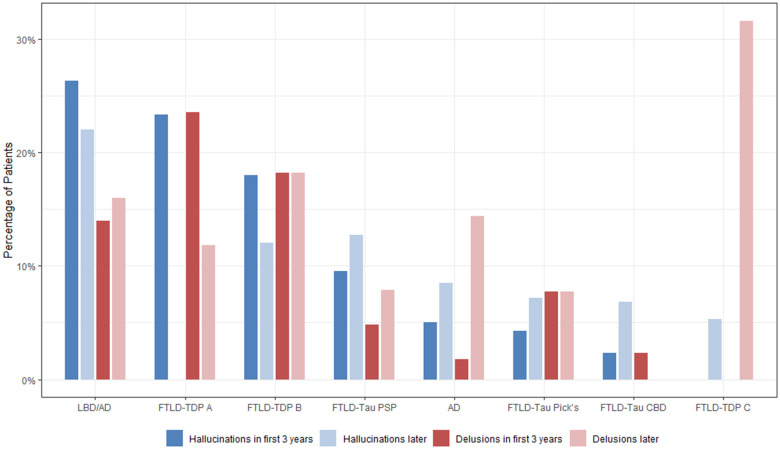
Compared to the other diagnostic groups, patients with LBD/AD or with FTLD-TDP-A or -B were more likely to have psychosis consistently throughout their disease course rather than in isolated incidents (Table 1). Patients with FTLD-TDP-A and patients with LBD/AD were more likely to have hallucinations present in the first 3 years of the disease as compared to patients with Alzheimer’s disease and patients with CBD (P < 0.0001) (Fig. 1 and Table 1). The median number of distinct hallucination types co-occurring in a single patient was two for both patients with Alzheimer’s disease and LBD/AD as compared to one type for patients with FTLD. The duration and frequency of hallucinations did not significantly differ across disease category. After removing patients with infrequent hallucinations (i.e. patients who experienced hallucinations once a year or less) and rerunning the analysis, none of these results changed meaningfully. Patients with LBD/AD, TDP-A and TDP-B were more likely to have delusions in the first 3 years of disease as compared to patients with Alzheimer’s disease and CBD (P = 0.001) (Figs 1 and 2, Table 1 and Supplementary Table 1).
Figure 1.
Frequency of psychosis symptoms. Graph shows the frequency of psychosis symptoms across all major classes of neuropathology, including nature of psychosis (hallucinations or delusions) and whether the symptom appeared early (in the first 3 years after disease onset) or later in the disease course. AD = Alzheimer’s disease.
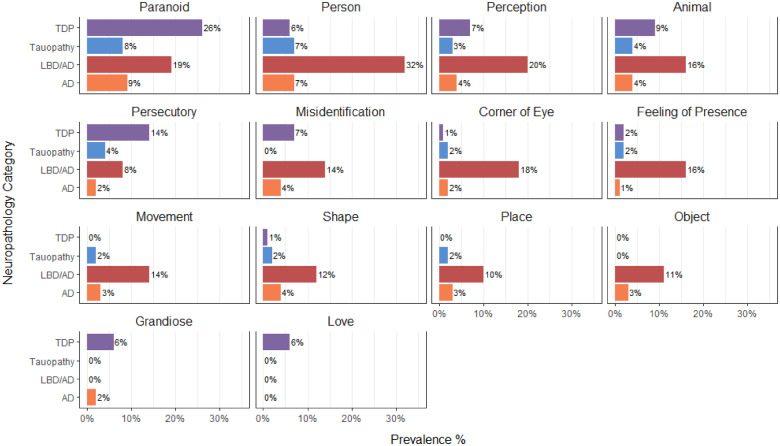
Figure 2.
Psychosis subtypes by major pathology groups. Bars represent percentage of the patients in the sample with that class of primary pathology who exhibited the particular type of psychosis at some point during the course of their neurodegenerative disease. AD = Alzheimer’s disease.
Non-formed visual hallucinations: shapes, shadows and visual misperceptions
Hallucinations described as shapes and colours occurred in 12.5% of patients with LBD/AD, significantly more than in patients with Alzheimer’s disease (3.7%), FTLD-tau (1.5%) or FTLD-TDP (1.4%) (P = 0.002). In the two patients with FTLD-tau and non-formed hallucinations, one had Pick’s disease with LBD pathology in the substantia nigra and the other had CBD with brainstem-only LBD pathology. Four patients with Alzheimer’s disease (3.7%) had hallucinations of shapes and colours and none of them had LBD co-pathology, although one had a vascular infarct in the putamen.
Peripheral hallucinations described as ‘shadows off the corner of the eye’ occurred almost exclusively in patients with LBD/AD (17.5%) as compared to patients with Alzheimer’s disease, FTLD-tau, and FTLD-TDP pathology (1.8%, 1.5%, and 1.4%, respectively; P < 0.0001). The only two patients with FTLD-tau who had peripheral hallucinations both had CBD and limbic TDP pathology. Moreover, two patients with Alzheimer’s disease had hallucinations of peripheral shadows; one had LBD pathology confined to the amygdala and the other had limbic argyrophilic grain disease (AGD).
Visual misperceptions, wherein an existing visual stimulus appeared distorted or modified, also occurred significantly more frequently in patients with LBD/AD (19.6%) as compared to patients with Alzheimer’s disease, FTLD-tau, and FTLD-TDP (3.7%, 3%, and 7.2%, respectively; P < 0.0001). One of the four patients with FTLD-tau and visual misperceptions had CBD pathology with LBD confined to the brainstem. In six patients with FTLD-TDP and visual misperceptions, one had brainstem only LBD, one had an infarct in the globus pallidus, and one had argyrophilic thorny astrocyte clusters (ATAC) in the amygdala consistent with ageing-related tau astrogliopathy (ARTAG) (Kovacs et al., 2016). In all groups, visual misperceptions were more likely to be new images as opposed to distortions of existing visual stimuli.
A logistic regression model (see ‘Materials and methods’ section) correctly classifying 96.4% of cases showed that patients with a Parkinson’s disease Braak stage of 5–6 were 52 times more likely to have hallucinations of peripheral shadows than patients with no LBD pathology (P < 0.0001, Nagelkerke R2 = 34.4%). No other pathology significantly predicted this type of hallucination. Moreover, patients with Parkinson’s disease Braak stage 5–6 were eight times more likely to have misperceptions than those with no LBD (P < 0.0001, R2 = 18.4%, correctly classifying 93.1% of cases).
Formed visual hallucinations: people, animals, and inanimate objects
One-third of patients with LBD/AD had hallucinations of people significantly more frequently than patients with Alzheimer’s disease (7.3%), FTLD-tau (6.8%), or any FTLD-TDP (6.1%, P < 0.0001). The majority of hallucinations of people were of strangers, while only 20% were of familiar people. Of the eight patients with Alzheimer’s disease and hallucinations of people, one had an infarct in the putamen, and none had LBD co-pathology. Of the nine patients with FTLD-tau pathology who had people hallucinations, two had LBD confined to the brainstem, one had Alzheimer’s disease Braak 6 and one had Alzheimer’s disease Braak 2. Of four patients with FTLD-TDP, one had brainstem-predominant LBD and one had Alzheimer’s disease Braak 6.
Hallucinations of animals occurred in 16.1% of patients with LBD/AD, more frequently than in patients with Alzheimer’s disease (3.7%), FTLD-tau (4.1%), or FTLD-TDP (8.7%), though the differences were not statistically significant. The one patient with FTLD-TDP-C who had hallucinations of animals was the only patient with TDP-C in the entire cohort to have any hallucinations and had concomitant focal traumatic tauopathy in frontal, temporal and limbic structures. Of the three patients with FTLD-TDP-B who had animal hallucinations had LBD Braak 3, and one of two patients with FTLD-TDP-A who had animal hallucinations had ATAC confined to the amygdala. Of six patients with FTLD-tau, one had LBD Braak 4 co-pathology and another had limbic AGD.
Hallucination of insects was seen only in patients with LBD/AD (7.3%), Alzheimer’s disease (5.6%), PSP (1.6%) and FTLD-TDP-B (3%) with no statistically significant difference in prevalence across groups. Nevertheless, the one patient with TDP-B who had insect hallucinations had a vascular infarct in the globus pallidus and limbic AGD, whereas the one patient with PSP who had insect hallucinations had concomitant Alzheimer’s disease Braak 4.
Ten per cent of patients with LBD/AD hallucinated inanimate objects as compared to none from the FTLD-tau and FTLD-TDP groups, and only 2.8% of patients with Alzheimer’s disease (P < 0.0001).
A logistic regression model correctly classifying 92.8% of cases showed that patients with a Parkinson’s disease Braak stage 5–6 were seven times more likely to have hallucinations of animals than patients with no LBD pathology (P = 0.01, R2 = 14.3%). Moreover, patients with a Parkinson’s disease Braak stage 5–6 were 11 times more likely to have hallucinations of people than those with no LBD (P < 0.0001, R2 = 23.8%, classifying 89.2% of cases). Finally, patients with Parkinson’s disease Braak Stage 5–6 were seven times more likely to have hallucination of objects than those with no LBD pathology (P = 0.021, R2 = 34.5%, classifying 97.3% of cases).
Non-visual hallucinations: auditory, tactile and olfactory
Auditory hallucinations were reported in 4.3% of all patients and across all four main pathology categories without statistical differences across groups (Table 2). Only one patient with LBD/AD and one patient with Pick’s had olfactory hallucinations. Only two patients with LBD/AD and one patient with TDP-B had tactile hallucinations. None of the patients in our cohort reported gustatory hallucinations.
Table 2.
Frequency of hallucination and delusion subtypes across neurodegenerative categories
| AD (n = 111) | LBD/AD (n = 59) | FTLD-tau (n = 133) | FTLD-TDP (n = 69) | P-value | |
|---|---|---|---|---|---|
| Female, % | 41.4 | 27.1 | 48.9 | 40.6 | 0.046 |
| Right handedness | 92.2 | 80.4 | 92.1 | 95.6 | 0.133 |
| Any psychosis | 22.5a | 55.9b | 18a | 42b | <0.0001 |
| Had hallucinations and delusions | 7.2 | 19 | 7.5 | 14.5 | 0.044 |
| Hallucinations | 13.5a | 48.3b | 15.8a | 21.7a | <0.0001 |
| Onset in first 3 years disease | 5a | 26.3b | 6.2a | 18.5b | <0.0001 |
| More than one subtyped | 10a | 35.2b | 6.2a | 9.1a | <0.0001 |
| Misperception | 3.7a | 19.6b | 3a | 7.2a | <0.0001 |
| Shapes and colours | 3.7a | 12.5b | 1.5a | 1.4a | 0.002 |
| Corner of eye | 1.8a | 17.5b | 1.5a | 1.4a | <0.0001 |
| Moving image | 2.8a | 14.3b | 1.5a | 0a | <0.0001 |
| Object | 2.8a | 10.7b | 0a | 0a | <0.0001 |
| Animal | 3.7a | 16.1b | 4.5a | 8.7a,b | 0.014 |
| Insect | 5.6 | 7.3 | 0.8 | 1.4 | 0.049 |
| Person | 7.3a | 32.1b | 6.8a | 6.1a | <0.0001 |
| Sound | 1.8 | 3.5 | 3 | 1.5 | 0.827 |
| Voice | 1.8 | 3.6 | 1.5 | 2.9 | 0.791 |
| Feeling of presence | 0.9a | 15.5b | 1.5a | 1.5a | <0.0001 |
| Delusions | 16.2a | 32.8b | 10.5a | 34.8b | <0.0001 |
| Onset in first 3 years disease | 1.8a | 10.2b,c | 4.5a,b | 14.5c | 0.004 |
| More than one subtyped | 6.8a | 20b | 2.5a | 18.8b | <0.0001 |
| Non-bizarre | 12.7a,b | 22.4b,c | 9.8a | 35.3c | <0.0001 |
| Bizarre | 3.6 | 6.9 | 0.8 | 7.4 | 0.064 |
| Paranoid | 9.1a,b | 19b,c | 8.3a | 26.5c | 0.001 |
| Persecutory | 1.8a | 8.5b,c | 3.8a,c | 14.5b | 0.003 |
| Intrusion | 0.9a,b | 5.2b,c | 0a | 7.4c | 0.004 |
| Theft | 0 | 3.4 | 2.3 | 4.4 | 0.212 |
| Hurt | 0.9 | 5.2 | 1.5 | 5.9 | 0.111 |
| Jealousy | 0.9 | 5.2 | 1.5 | 5.9 | 0.111 |
| Place | 2.7a | 10.3b | 2.3a | 0a | 0.007 |
| Misidentification of people | 4.5a | 13.8b | 0c | 7.2a,b | 0.001 |
| Grandeur | 1.8a,b | 8.5a,b | 3.8b | 14.5a | 0.003 |
| Erotomania | 0a | 0a,b | 0a | 5.8b | 0.001 |
Values represent percentage of patients within diagnostic category. Values in bold indicate statistical significance set at P = 0.02041 per B-Y method for k = 6 comparisons. AD = Alzheimer’s disease.
Each superscript letter denotes diagnostic categories whose proportions do not significantly differ from each other for that respective row (e.g. in ‘shapes and colours’, the frequency of that hallucinations in patients with LBD/AD was significantly different than all other groups, who were not different from each other).
Defined as number of subtypes occurring at the same time in the same patients at any point in time of the disease.
Hallucinations: other characteristics
Hallucinations were reported to move in 14.3% of patients with LBD/AD as compared to only 2.8% of patients Alzheimer’s disease and 1.5% of patients with FTLD-tau, while none of the patients with TDP reported moving hallucinations (P < 0.0001). Moreover, both patients with FTLD-tau and moving hallucinations had brainstem-predominant LBD co-pathology. A logistic regression model showed that patients with a Parkinson’s disease Braak staging of 5–6 were 11 times more likely to have hallucinations that moved than patients with no LBD pathology (P = 0.001, R2 = 27.8%, classifying 96.1% of cases).
In a subgroup analysis of all patients with hallucinations (n = 79), there was a trend for patients with LBD/AD to have insight during and after their hallucinatory episode as compared to patients with FTLD-tau or FTLD-TDP (P = 0.026 and 0.044, respectively, threshold for significance set at P = 0.02041 per B-Y method for k = 6; Table 3). None of the patients with Pick’s (n = 1), CBD (n = 3), or TDP-B (n = 8) who had hallucinations had insight, either during the episode or in retrospect. Hallucinations were reported to occur in association with sleep (waking up from sleep or falling asleep) in 61.5% of patients with Alzheimer’s disease who hallucinated, significantly more frequently than in all other patients (P = 0.002). Hallucinations were reported around sleep in a quarter of patients with LBD, but this was not statistically different from other groups. There was a non-significant trend in which patients with LBD were more likely to interact with their hallucinations as compared to patients with Alzheimer’s disease, FTLD-tau, or FTLD-TDP (32.1% versus 15.4%, 0%, and 13.3%, respectively, P = 0.033). Of the two patients with TDP who interacted with their hallucinations, both had TDP-B and one had concomitant LBD Braak 1. Of the two patients with Alzheimer’s disease who interacted with their hallucinations, one had LBD only in the amygdala. Hallucinations were reported as ‘disturbing’ in equal proportions across diagnostic groups, ranging from 11% to 17% of patients.
Table 3.
Other characteristics of hallucinations in patients with hallucinations
| AD (n = 15) | LBD/AD (n = 28) | FTLD-tau (n = 21) | FTLD-TDP (n = 15) | |
|---|---|---|---|---|
| Disturbing (n = 73), % | 16.7 | 11.1 | 15.8 | 13.3 |
| Interact with hallucination (n = 76), % | 15.4 | 32.1 | 0 | 13.3 |
| Insight during halluc. (n = 55), % | 40 | 52.6 | 14.3 | 8.3 |
| Insight in retrospect (n = 54), % | 40 | 61.1 | 21.4 | 16.7 |
| Associated with sleep (n = 71), % | 61.5 | 26.1 | 9.5 | 7.1 |
| More than 1 halluc. (n = 78), % | 71.4 | 67.9 | 38.1 | 33.3 |
| Also had delusions (n = 79), % | 53.3 | 53.6 | 52.4 | 66.7 |
| Number of hallucination subtypes, median | 2 | 2 | 1 | 1 |
Values represent percentage of patients within diagnostic category except where otherwise noted. AD = Alzheimer’s disease.
The feeling of presence
The feeling of presence, defined as a reported feeling of someone present in the room when no one is actually there, occurred in 15.5% of patients with LBD/AD, significantly more than in patients with any other pathology (0.9% in Alzheimer’s disease, 1.5% in FTLD-tau, and 1.5% in FTLD-TDP, P < 0.0001). Of the four patients who reported the feeling of presence and did not have primary LBD/AD, one had LBD Braak 3 co-pathology. A logistic regression model showed that patients with a Parkinson’s disease Braak staging of 5–6 were 16 times more likely to have the feeling of presence than patients with no LBD pathology (P < 0.0001, R2 = 26.2%, classifying 96.2% of cases).
Bizarre and non-bizarre delusions
Non-bizarre delusions were the most common type of delusions across all diagnoses and occurred at a frequency of 17.3% in the entire cohort. They occurred in a third of patients with TDP pathology and in 22% of patients LBD/AD pathology, significantly more frequently than in other diagnostic groups (P < 0.0001; Tables 2 and 4). Conversely, bizarre delusions were not common and were found in only 3.8% of all patients. They appeared to occur more frequently in patients with any FTLD-TDP and in patients with LBD/AD. As an example of a bizarre delusion, a patient with a pathological diagnosis of PSP and Alzheimer’s disease Braak 4 believed that his arm and other body parts were replaced by robotic implants. This was the only patient in our cohort who had a delusion of misidentification of body parts. A patient with FTLD-TDP-A and AGD was convinced that his wife had been replaced by a demon. Ideas of reference only occurred in four patients who had a pathology of Alzheimer’s disease, LBD/AD or TDP-B.
Table 4.
Other characteristics of delusions in patients with delusions
| AD (n = 18) | LBD/AD (n = 15) | FTLD-tau (n = 13) | FTLD-TDP (n = 24) | |
|---|---|---|---|---|
| Harmful (n = 63), % | 50 | 50 | 58 | 74 |
| Positive (n = 63), % | 0 | 0 | 0 | 9 |
| Also had hallucinations (n = 70) | 44.4 | 73.3 | 76.9 | 41.7 |
| Number of delusion subtypes, median | 1 | 2 | 1 | 2 |
Values represent percentage of patients within diagnostic category except where otherwise noted. AD = Alzheimer’s disease.
Paranoia, persecutory delusions and delusions of jealousy
Paranoid non-bizarre delusions were the most common subtype of delusions and occurred significantly more frequently in patients with any FTLD-TDP (26.5%) as compared to patients with Alzheimer’s disease (9.1%) and FTLD-tau (8.3%). In particular, paranoid delusions were most frequent in patients with TDP-A (29.4%), TDP-B (28.1%), and LBD/AD (19%).
Within the category of paranoid delusions, persecutory delusions encompassing delusions of theft, hurt, and intrusion to the patient’s own home occurred more frequently in patients with FTLD-TDP-A–C (11.8, 15.2 and 15.8%, respectively) as compared to patients with Alzheimer’s disease (1.8%). In particular, delusions of intrusion to home drove these findings (12.5% in TDP-B versus 0.9% in Alzheimer’s disease). Delusions of jealousy were reported in only a small number of patients distributed evenly across most neuropathological diagnoses. Logistic regression modelling did not significantly discriminate any of these diagnostic groups.
Delusions regarding a place
Delusions regarding a place (e.g. ‘home’) were significantly more frequent in patients with LBD/AD pathology (10.3%) than in any other group, and did not appear at all in patients with FTLD-TDP. As an example, one patient with LBD/AD reported that when he was not paying attention, someone was moving his house to a different location. He found it odd that the interior of the house remained the same despite the fact that the house had moved many miles away. None of the patients with CBD or Pick’s disease had delusions regarding a place. A logistic regression model showed that patients with a Parkinson’s disease Braak staging of 5–6 were five times more likely to have delusions regarding a place than patients with no LBD pathology (P = 0.017, R2 = 27%, classifying 96.4% of cases).
Delusions of misidentification of people
Delusions of misidentification of people were significantly more frequent in patients with LBD/AD (13.8%) and patients with FTLD-TDP (7.2%) when compared to patients with other diagnoses. None of the patients with FTLD-TDP who had this delusion had an LBD co-pathology, except for one who had LBD at a very early Parkinson’s disease Braak 1 stage. A logistic regression model showed that patients with a Parkinson’s disease Braak staging of 5–6 were 10 times more likely to misidentify people than patients with no LBD pathology (P = 0.001, R2 = 34.9%, classifying 95% of cases) and that patients with FTLD-TDP pathology were also 10 times more likely to misidentify people than patients with no FTLD-TDP pathology (P = 0.011).
Only one patient in our cohort had a true Capgras delusion, and believed that his physician was an imposter. This patient had a diagnosis of FTLD-TDP-B along with MND pathology, no contributing Alzheimer’s disease or LBD pathology, and an expansion in the C9orf72 gene.
Delusions of grandeur and erotomania
Grandiose delusions were primarily seen in patients with FTLD-TDP-B pathology at a frequency of 9.4% (supplementary Table 1) and with a non-significant trend to be more frequent than in patients with Alzheimer’s disease, LBD/AD or FTLD-tau-Pick’s pathology (P = 0.022). Delusions of erotomania were only reported in patients with TDP pathologies, suggesting that this delusion subtype is most predictive of TDP pathology. Even though these two delusions are classified separately in the DSM-V, and because (i) they have comparable neurological mechanism involving inflation of self perception; and (ii) they seem to occur in a similar pattern across diagnoses, we combined them together under one category of self-elevating delusions and performed a logistic regression model, which showed that patients with FTLD-TDP were 22 times more likely to have self-elevating delusions than patients with no FTLD-TDP pathology (P = 0.009, R2 = 31.1%, classifying 97.6% of cases).
Direct comparison of the FTLD pathologies
For clarity, a direct comparison of the psychosis profile of the two main FTLD molecular classes is summarized in Table 5. Whereas none of the hallucination subtypes differentiated between FTLD pathologies, the presence of delusions in general, as well as delusions that are bizarre, paranoid, persecutory, intrusionary, erotomanic, or grandiose, and delusions of misidentification, all were significantly more likely to be present in FTLD-TDP than in FTLD-tau.
Table 5.
Direct comparison of frequency of specific psychoses in patients with FTLD-TDP versus those with FTLD-tau
| FTLD-tau (n = 133) | FTLD-TDP (n = 69) | P-value | |
|---|---|---|---|
| Hallucinations, % | 15.8 | 21.7 | 0.295 |
| Feeling of presence, % | 1.5 | 1.5 | 0.985 |
| Delusions, % | 9.8 | 34.8 | <0.0001 |
| Non-bizarre, % | 9.8 | 35.3 | <0.0001 |
| Bizarre, % | 0.8 | 7.4 | 0.009 |
| Paranoid, % | 8.3 | 26.5 | 0.001 |
| Persecutory, % | 3.8 | 14.5 | 0.006 |
| Intrusion, % | 0 | 7.4 | 0.002 |
| Theft, % | 2.3 | 4.4 | 0.395 |
| Hurt, % | 1.5 | 5.9 | 0.084 |
| Jealousy, % | 1.5 | 5.9 | 0.084 |
| Place, % | 2.3 | 0 | 0.212 |
| Misidentification of people | 0 | 7.2 | 0.002 |
| Grandeur, % | 0 | 5.9 | 0.005 |
| Erotomania, % | 0 | 5.8 | 0.005 |
Values in bold indicate P significant at 0.05. Numbers represent percentage within diagnostic category.
Genetic analysis
Genetic analysis is summarized in Tables 1 and 6. There was an equal proportion of APOE ε4 carriers with and without psychosis across all diagnoses, except in patients with Pick’s disease, in whom 2/4 with psychosis had an APOE ε4 allele versus 4/22 without psychosis. In patients with FTLD-TDP-A, there was a trend towards a higher proportion of C9orf72 expansions in patients with delusions (n = 2 mutation positive/5 mutation negative) compared to those without (n = 1/10), but there was equal proportion of GRN mutations in both groups. Thirty-three patients with FTLD-TDP-B received genetic testing and 12 (36.4%) had an expansion in the C9orf72, in equal proportion between patients with and without delusions (Table 6).
Table 6.
Psychosis characteristics across genetic mutations
| Sporadic (n = 326) | PSEN1 (n = 1) | APP (n = 3) | C9orf72 (n = 30) | GRN (n = 9) | MAPT (n = 6) | |
|---|---|---|---|---|---|---|
| Any psychosis, % | 28.8 | 0 | 0 | 26.7 | 55.6 | 33.3 |
| Hallucinations, % | 19.7 | 0 | 0 | 10 | 22.2 | 0 |
| Perception | 5.6 | 0 | 0 | 0 | 11.1 | 0 |
| Shapes and colours | 3.8 | 0 | 0 | 0 | 0 | 0 |
| Corner of eye | 2.8 | 0 | 0 | 0 | 0 | 0 |
| Moving image | 3.1 | 0 | 0 | 0 | 0 | 0 |
| Animal | 6 | 0 | 0 | 3.3 | 0 | 0 |
| Insect | 3.2 | 0 | 0 | 0 | 0 | 0 |
| Person | 9.8 | 0 | 0 | 0 | 11.1 | 0 |
| Feeling of presence, % | 2.2 | 0 | 0 | 0 | 0 | 0 |
| Delusions, % | 19.7 | 0 | 0 | 23.3 | 33.3 | 33.3 |
| Non-bizarre | 17.3 | 0 | 0 | 23.3 | 33.3 | 16.7 |
| Bizarre | 4 | 0 | 0 | 6.7 | 0 | 16.7 |
| Paranoid | 11.8 | 0 | 0 | 20 | 33.3 | 16.7 |
| Persecutory | 4.9 | 0 | 0 | 6.9 | 11.1 | 16.7 |
| Intrusion | 1.9 | 0 | 0 | 3.3 | 0 | 0 |
| Theft | 2.2 | 0 | 0 | 0 | 11.1 | 0 |
| Hurt | 1.9 | 0 | 0 | 3.3 | 0 | 16.7 |
| Jealousy | 1.6 | 0 | 0 | 10 | 0 | 0 |
| Place | 2.5 | 0 | 0 | 0 | 0 | 0 |
| Misidentification | 4 | 0 | 0 | 6.7 | 11.1 | 0 |
| Grandeur | 1.5 | 0 | 0 | 6.7 | 0 | 16.7 |
| Erotomania | 0.9 | 0 | 0 | 0 | 11.1 | 0 |
Statistical analysis not performed due to small group sizes. Numbers represent percentage within gene category.
Discussion
Our findings reveal that specific contents of hallucinations and delusions are preferentially associated with specific pathological diagnoses, and thus may reflect the anatomical and physiological differences among these conditions. Our analysis showed that patients with LBD/AD were more likely to have visual misperceptions, and hallucinations that are shapeless, peripheral and with images that moved, in addition to well-formed hallucinations, and also were more likely to experience the feeling of presence. Delusions of misidentification occured more frequently in patients with LBD/AD and patients with FTLD-TDP. Furthermore, patients with FTLD-TDP were more likely than those with any other pathology to report paranoid delusions, as well as delusions that were self-elevating, including grandiosity and erotomania. These results suggest that knowledge of delusion subtype may help differentiate patients with clinical FTD who have underlying FTLD-TDP from those who have FTLD-tau. Additionally, our results suggest that the neurobiology of psychosis in different neurodegenerative diseases may differ, which may have implications for future pharmacological treatments of dementia related psychosis. While there may be a core neural aetiology for all symptoms involving deviation from reality, if differences in psychosis content are reflective of divergent neurobiology, then future precision medicine treatments may also need to target the additional neural networks or neurotransmitters corresponding to that content.
Prior clinical studies have suggested that patients with different clinical syndromes display different subtypes of psychosis (Geroldi et al., 2000; Nagahama et al., 2007, 2010; Shinagawa et al., 2015). Our results confirm this relationship in a pathology-proven cohort. One hypothesis to explain this relationship posits that all patients with psychosis share a common anatomical network that makes them prone to deviate from reality, but it interacts with dysfunctions in other anatomical networks that are specific to each disease, thereby leading to disease-specific psychosis content (Coltheart, 2010; Kim et al., 2019). For example, Sellami and colleagues demonstrated that in a cohort of patients with genetic mutations known to cause FTLD, different mutations were associated with different patterns of cortical atrophy in those who displayed psychotic and other neuropsychiatric symptoms (Sellami et al., 2018). Interestingly, patients with the C9orf72 expansion who had delusions also had cortical atrophy in left frontal circuitry (Sellami et al., 2018), which may explain why we found a correlation between FTLD-TDP-B pathology, the presence of a C9orf72 expansion, and grandiose delusions. A previous investigation found grandiose delusions in 2 of 14 and 2 of 42 patients with bvFTD with and without the C9orf72 expansion, respectively (Devenney et al., 2017). Our findings also suggested that erotomania occurs more frequently in patients with GRN mutations, while Sellami et al. (2018) found that patients with GRN mutations who have delusions also have atrophy in anterior insular structures, which play a role in self-awareness. To our knowledge, this is the first report of erotomania in a patient with a GRN mutation. The literature for erotomania consists mostly of case reports in clinical Alzheimer’s disease (Cipriani et al., 2012), though we did not find this in our autopsy-confirmed cohort. Not only does our data underline the association between FTLD-TDP pathology and self-elevating delusions, both grandiose and erotomanic, but also highlights the usefulness of this symptom in differentiating TDP from tau pathology in patients with an FTLD syndrome.
In addition to self-elevating delusions, we found a high occurrence of paranoia, and specifically delusions of intrusion, in patients with FTLD-TDP and in patients with mixed LBD/AD pathologies. Previous clinical studies have suggested that the frequency of persecutory delusions was up to 29% and 14% in patients with clinical bvFTD, with and without the C9orf72 expansion, respectively (Takada and Sha, 2012; Devenney et al., 2017). We confirm this finding, and highlight that paranoia is not exclusively a characteristic of Alzheimer’s disease, as the literature in clinical studies seems to suggest (Geroldi et al., 2000; Nakatsuka et al., 2013, 2014), but should prompt clinicians to consider FTLD-TDP or LBD pathologies, especially when it occurs early after disease onset. The neurobiological source of paranoia remains unclear, although it may stem from dysfunction in the default mode network (DMN) (Huang et al., 2018), which can be affected in patients with LBD and was found to correlate with anxiety in patients with GRN mutations (Sellami et al., 2018). In the literature, delusional jealousy was found to be more prevalent in patients with clinically defined dementia with Lewy bodies (26.3%) as compared to patients with clinical Alzheimer’s disease (5.5%) (Hashimoto et al., 2015). Patients with FTD were not included in that study. The association between the delusion of infidelity and bvFTD was reported in the literature in clinical series where genetic testing for the C9orf72 expansion was conducted (Takada and Sha, 2012; Shinagawa et al., 2015; Devenney et al., 2017). In our cohort, we found low occurrence of jealousy delusions in any diagnostic group. We acknowledge that the delusion of theft may have been underrepresented in our Alzheimer’s disease sample because physicians may be less likely to document it in the chart as an important phenomenon, since, at least anecdotally, a large number of patients with Alzheimer’s disease will believe at some point that someone is stealing from them, especially as they forget where they have placed their belongings.
Our findings show that both Parkinson’s disease Braak 5–6 and the presence of an FTLD-TDP pathology were both independently predictive of misidentification delusions. In clinical studies without pathology confirmation, 15.8% of patients with clinical Alzheimer’s disease, 16.6% of patients with dementia with Lewy bodies, and 8.3% of patients with semantic variant primary progressive aphasia were found to have some form of misidentification delusions (Harciarek and Kertesz, 2008). In a cohort of 41 patients with autopsy-confirmed LBD and 70 patients with autopsy-confirmed Alzheimer’s disease, misidentification was not found to differ between the two groups (Ferman et al., 2013). However, that study did not look at the effect of contributing co-pathology, and the misidentification delusion was captured through a structured questionnaire that only asked whether the patient had difficulties recognizing family members, which could overlap with prosopagnosia and visual symptoms that are not necessarily fixed beliefs like delusions are. The neurobiological aetiology of misidentification delusions remains unclear; however, they may stem from the interplay between sensory circuitry and memory circuitry, as suggested by Hirstein and Ramachandran in the case of Capgras delusions (Hirstein and Ramachandran, 1997). We highlight the importance of considering both underlying FTLD-TDP and LBD pathologies on the diagnostic differential when evaluating patients who report misidentification delusions.
Patients with mixed LBD/AD pathology had a unique pattern of hallucinations, composed of hallucinations that are shapeless, occurring in the periphery of their visual field, and that were described as moving, in addition to visual misperceptions and the feeling of presence that occurred almost exclusively in patients with LBD/AD pathology. In a study examining the frequency of extracampine hallucinations, defined as a ‘sense of a presence or fleeting movement in the absence of an associated visual percept’ in patients with clinical Parkinson’s disease, half of them reported this hallucination (Wood et al., 2015). However, it is unclear if some of the patients in that study would have described their hallucination as ‘shadows’. In addition, our findings that non-formed hallucinations experienced in the periphery of the visual field and hallucinations of images that moved were significantly more likely to occur in patients with a Parkinson’s disease Braak stage of 5–6 are consistent with other studies in clinical cohorts of patients with Parkinson’s disease. These studies show evidence that minor hallucinations and illusions, including peripheral shadows and passage hallucinations, correlate with posterior cortical atrophy and hypometabolism on brain MRI and FDG-PET, respectively (Nishio et al., 2017). We also found that Parkinson’s disease Braak 5–6 predicted hallucinations of people, animals and objects, which also occurred in other pathologies, albeit less frequently. The feeling of presence [leibhaftige Bewusstheit (Iseki et al., 2002)] was previously reported to occur at a 23% frequency in both patients with clinical dementia with Lewy bodies and patients with clinical Parkinson’s disease. In a cohort of patients clinically diagnosed with dementia with Lewy bodies, the feeling of presence belonged to a cluster of symptoms that also included visual hallucinations, and was associated with hypoperfusion to the left ventral occipital gyrus and bilateral parietal lobes (Nagahama et al., 2010). In a cohort of patients clinically diagnosed with Parkinson’s disease, the feeling of presence belonged to a cluster of symptoms including peripheral, minor and non-formed hallucinations as well as misperceptions, associating with posterior cortical atrophy and/or hypoperfusion (Nishio et al., 2017). To our knowledge, ours is the first clinicopathological analysis that establishes a statistically significant relationship between the feeling of presence and LBD pathology, especially in direct association with high Parkinson’s disease Braak stages of 5–6. This finding underlines the importance of asking about this particular symptom when evaluating patients with cognitive decline in a clinical setting, because this may increase the chance of accurately detecting LBD pathology early on in the disease, when other defining symptoms of the pathology have not yet occurred.
Limitations and conclusions
Our study has several limitations, including its retrospective nature. The descriptions of the delusions were extracted from physician notes, which may not have been complete or documented systematically. Moreover, there were small sample sizes for some of the delusion types, while a larger sample might have represented a broader selection of contributory primary or co-pathologies. Because the Memory and Aging Center at UCSF evaluates a large number of patients with FTD, the overall number of patients with FTLD pathologies may be over-represented in our sample; however, this would not have impacted the accuracy of the rates of psychosis we observed in these patients. Measures of disease severity such as a Mini-Mental State examination (MMSE) score were not captured by our chart review, in part because we did not consistently have an MMSE time locked with onset of psychosis, especially when the onset was many years prior to the first presentation of the patient to the centre for evaluation. Furthermore, our sample lacked a group of patients with LBD pathology without Alzheimer’s disease, which may affect hallucination frequency as shown in clinically series without pathology confirmation (Lemstra et al., 2017). However, the differences in psychosis content remain valid. Our data also lacked information about concomitant neuropsychiatric symptoms such as anxiety and depression, therefore preventing additional analysis of how these other symptoms may affect the manifestation of psychosis. Finally, our study did not examine how regional distribution of pathology in the brain may mediate this relationship between pathology and psychotic symptom, thus this factor will be an important component of future investigations.
Collectively, our findings highlight the role of the nature and content of psychosis in predicting the underlying neuropathology of different neurodegenerative illnesses. It is important for clinicians who are evaluating patients with cognitive and behavioural changes to systematically ask about the content of their patients’ fixed beliefs, as this may help with predicting their neuropathology ante-mortem, which is becoming increasingly more important as new pathology-specific therapies are emerging. Our findings also lay the groundwork for future research in which prospective systematic recording of the timing of psychotic symptoms over the disease course can be correlated with regional imaging markers of advancing neuropathology, in order to further refine the neuroanatomy and mechanistic neurophysiology of psychosis, which could lead to research into pharmacological and non-pharmacological interventions that more precisely target specific subtypes of psychosis by content.
Funding
This work was supported by an National Institutes of Health funded grants R03AG045491 and R01AG029577 (K.P.R.), K08AG052648 (S.S.), P01AG019724 and P50AG023501 (B.L.M.), The Rainwater Charitable Foundation, The Bluefield Project to Cure FTD.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
- CBD
corticobasal degeneration
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- LBD
Lewy body disease
- LBD/AD
concomitant Lewy body disease and Alzheimer’s disease
- PSP
progressive supranuclear palsy
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Auning E, Rongve A, Fladby T, Booij J, Hortobagyi T, Siepel FJ, et al. Early and presenting symptoms of dementia with Lewy bodies. Dement Geriatr Cogn Disord 2011; 32: 202–8. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statist 2001; 29: 1165–88. [Google Scholar]
- Cipriani G, Logi C, Di Fiorino AA.. Romantic delusion: de Clerambault's syndrome in dementia. Geriatr Gerontol Int 2012; 12: 383–7. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The neuropsychology of delusions. Ann N Y Acad Sci 2010; 1191: 16–26. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J.. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–14. [DOI] [PubMed] [Google Scholar]
- Devenney EM, Landin-Romero R, Irish M, Hornberger M, Mioshi E, Halliday GM, et al. The neural correlates and clinical characteristics of psychosis in the frontotemporal dementia continuum and the C9orf72 expansion. Neuroimage Clin 2017; 13: 439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley R, Aynsworth C, Mosimann U, Taylor JP, Smailes D, Collerton D, et al. A comparison of visual hallucinations across disorders. Psychiatry Res 2019; 272: 86–92. [DOI] [PubMed] [Google Scholar]
- Ehrenberg AJ, Suemoto CK, Franca Resende EP, Petersen C, Leite REP, Rodriguez RD, et al. Neuropathologic correlates of psychiatric symptoms in Alzheimer's disease. J Alzheimers Dis 2018; 66: 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Arvanitakis Z, Fujishiro H, Duara R, Parfitt F, Purdy M, et al. Pathology and temporal onset of visual hallucinations, misperceptions and family misidentification distinguishes dementia with Lewy bodies from Alzheimer's disease. Parkinsonism Relat Disord 2013; 19: 227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65: 1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geroldi C, Akkawi NM, Galluzzi S, Ubezio M, Binetti G, Zanetti O, et al. Temporal lobe asymmetry in patients with Alzheimer's disease with delusions. J Neurol Neurosurg Psychiatry 2000; 69: 187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleen J, David AS.. The cognitive neuropsychiatry of delusions: from psychopathology to neuropsychology and back again. Psychol Med 2005; 35: 5–12. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Benbow SM.. Visual hallucinations as the presenting symptom of senile dementia. Br J Psychiatry 1992; 161: 263–5. [DOI] [PubMed] [Google Scholar]
- Harciarek M, Kertesz A.. The prevalence of misidentification syndromes in neurodegenerative diseases. Alzheimer Dis Assoc Disord 2008; 22: 163–9. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sakamoto S, Ikeda M.. Clinical features of delusional jealousy in elderly patients with dementia. J Clin Psychiatry 2015; 76: 691–5. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Lanctot KL, Sambrook R, Lesnikova N, Hebert R, McCracken P, et al. ; The COSID Investigators. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriat Psychiatry 2006; 21: 972–6. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Ramachandran VS.. Capgras syndrome: a novel probe for understanding the neural representation of the identity and familiarity of persons. Proc R Soc Lond B 1997; 264: 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu C, Chen J, Zou J, Chen C, Wu S, et al. Altered corticostriatal pathway in first-episode paranoid schizophrenia: resting-state functional and causal connectivity analyses. Psychiatry Res Neuroiaging 2018; 272: 38–45. [DOI] [PubMed] [Google Scholar]
- Iseki E, Marui W, Nihashi N, Kosaka K.. Psychiatric symptoms typical of patients with dementia with Lewy bodies - similarity to those of levodopa-induced psychosis. Acta Neuropsychiatr 2002; 14: 237–41. [DOI] [PubMed] [Google Scholar]
- Kim NY, Hsu J, Talmasov D, Joutsa J, Soussand L, Wu O, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry 2019. doi: 10.1038/s41380-019-0565-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 2016; 131: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemstra AW, de Beer MH, Teunissen CE, Schreuder C, Scheltens P, van der Flier WM, et al. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2017; 88: 113–8. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Miller DS; Neuropsychiatric Syndromes Professional Interest Area of the International Society to Advance Alzheimer's Research and Treatment. Addressing the Alzheimer's disease crisis through better understanding, treatment, and eventual prevention of associated neuropsychiatric syndromes. Alzheimers Dement 2012; 8: 60–4. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard JM, Steinberg M, Tschanz JA, Norton MC, Steffens DC, et al. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the cache county study. Int J Geriat Psychiatry 2001; 16: 1043–53. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011; 122: 111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okina T, Suzuki N, Matsuda M.. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain 2010; 133: 557–67. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okina T, Suzuki N, Matsuda M, Fukao K, Murai T.. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry 2007; 15: 961–7. [DOI] [PubMed] [Google Scholar]
- Nakatsuka M, Meguro K, Nakamura K, Akanuma K, Yamaguchi S.. ‘Residence is not home’ is a particular type of delusion associated with cognitive decline of Alzheimer's disease. Dement Geriatr Cogn Disord 2014; 38: 46–54. [DOI] [PubMed] [Google Scholar]
- Nakatsuka M, Meguro K, Tsuboi H, Nakamura K, Akanuma K, Yamaguchi S.. Content of delusional thoughts in Alzheimer's disease and assessment of content-specific brain dysfunctions with BEHAVE-AD-FW and SPECT. Int Psychogeriatr 2013; 25: 939–48. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Yokoi K, Uchiyama M, Mamiya Y, Watanabe H, Gang M, et al. Deconstructing psychosis and misperception symptoms in Parkinson's disease. J Neurol Neurosurg Psychiatry 2017; 88: 722–9. [DOI] [PubMed] [Google Scholar]
- Sellami L, Bocchetta M, Masellis M, Cash DM, Dick KM, van Swieten J, et al. ; on behalf of the Genetic FTD Initiative. Distinct neuroanatomical correlates of neuropsychiatric symptoms in the three main forms of genetic frontotemporal dementia in the GENFI cohort. J Alzheimers Dis 2018; 65: 1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology 2012; 79: 1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa S, Naasan G, Karydas AM, Coppola G, Pribadi M, Seeley WW, et al. Clinicopathological study of patients with C9ORF72-associated frontotemporal dementia presenting with delusions. J Geriatr Psychiatry Neurol 2015; 28: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada LT, Sha SJ.. Neuropsychiatric features of C9orf72-associated behavioral variant frontotemporal dementia and frontotemporal dementia with motor neuron disease. Alzheimers Res Ther 2012; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RA, Hopkins SA, Moodley KK, Chan D.. Fifty percent prevalence of extracampine hallucinations in Parkinson's disease patients. Front Neurol 2015; 6: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta-analysis. J Affect Disord 2016; 190: 264–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants, but are available on request from the corresponding author.