Abstract
Deep brain stimulation (DBS) of the subthalamic nucleus, pallidum, and thalamus is an established therapy for various movement disorders. Limbic targets have also been increasingly explored for their application to neuropsychiatric and cognitive disorders. The brainstem constitutes another DBS substrate, although the existing literature on the indications for and the effects of brainstem stimulation remains comparatively sparse. The objective of this review was to provide a comprehensive overview of the pertinent anatomy, indications, and reported stimulation-induced acute and long-term effects of existing white and grey matter brainstem DBS targets. We systematically searched the published literature, reviewing clinical trial articles pertaining to DBS brainstem targets. Overall, 164 studies describing brainstem DBS were identified. These studies encompassed 10 discrete structures: periaqueductal/periventricular grey (n = 63), pedunculopontine nucleus (n = 48), ventral tegmental area (n = 22), substantia nigra (n = 9), mesencephalic reticular formation (n = 7), medial forebrain bundle (n = 8), superior cerebellar peduncles (n = 3), red nucleus (n = 3), parabrachial complex (n = 2), and locus coeruleus (n = 1). Indications for brainstem DBS varied widely and included central neuropathic pain, axial symptoms of movement disorders, headache, depression, and vegetative state. The most promising results for brainstem DBS have come from targeting the pedunculopontine nucleus for relief of axial motor deficits, periaqueductal/periventricular grey for the management of central neuropathic pain, and ventral tegmental area for treatment of cluster headaches. Brainstem DBS has also acutely elicited numerous motor, limbic, and autonomic effects. Further work involving larger, controlled trials is necessary to better establish the therapeutic potential of DBS in this complex area.
Keywords: neuroanatomy, deep brain stimulation, brainstem, clinical neurophysiology
Elias et al. review 164 studies of brainstem DBS, encompassing the stimulation of 10 distinct grey or white matter structures. By cataloguing the acute and long-term effects of stimulating these structures, they glean new insights into the therapeutic potential of this complex region
Introduction
Deep brain stimulation (DBS) is a neuromodulatory therapy in which intracranial electrodes are used to deliver electrical impulses to targeted brain structures. Well-established as a safe and effective treatment for movement disorders such as Parkinson’s disease, essential tremor, and dystonia (Anderson and Lenz, 2006; DeLong and Wichmann, 2012), DBS has also seen longstanding use for chronic pain (Hosobuchi, 1986; Levy et al., 1987) and has been increasingly explored as a treatment for psychiatric and cognitive disorders such as obsessive-compulsive disorder (Greenberg et al., 2006; Roh et al., 2012), major depressive disorder (Mayberg et al., 2005; Kennedy et al., 2011), and Alzheimer’s disease (Hamani et al., 2008; Laxton et al., 2010). However, the variable benefit observed in stimulating exploratory targets (Holtzheimer et al., 2017), the evocation of undesirable side-effects (e.g. postural instability in Parkinson’s disease) with traditional targets (Fasano et al., 2015), and a continued desire to expand the therapeutic indications of DBS has prompted the exploration for suitable target alternatives outside the established cortico-basal ganglia-thalamo-cortical loop [e.g. subthalamic nucleus (STN), pallidum, and thalamus] (Lozano and Lipsman, 2013).
The brainstem is an intricate neuroanatomical structure that may hold therapeutic potential as a target for DBS, owing to its manifold fibre connections and functionally diverse nuclei. It is composed of grey matter, formed primarily by the cranial nerve nuclei, mesencephalic nuclei, reticular formation, and pontine nuclei, as well as ascending and descending white matter fibre tracts (Ángeles Fernández-Gil et al., 2010; Benarroch, 2018). In addition to its role as a crucial conduit for numerous motor and sensory pathways, the brainstem performs vital vegetative functions—including cardiorespiratory and cardiovascular control (Benarroch, 2018) and maintenance/regulation of consciousness and sleep—and a pivotal role in coordination, posture, and locomotion (Drew et al., 1986; Prentice and Drew, 2001). While the brainstem’s importance, complexity, and diverse functionality present an attractive rationale for its targeting with DBS, these same factors mean that the consequences of operative complications in this area are particularly grave. Moreover, the brainstem’s caudal positioning and susceptibility to physiological noise on imaging (e.g. movement artefact during breathing) impose significant technical challenges that have likely obstructed exploration of this structure (Jagannathan and Krovvidi, 2014; Sclocco et al., 2018).
The brainstem DBS literature is spread over several decades and encompasses a wide range of substructures and indications. The present review was devised with the intent to provide an accessible and comprehensive summary of this literature, shedding light on how brainstem DBS has evolved, where it stands today with respect to both acute effects and long-term clinical consequences of stimulation, and what avenues for potential growth might be.
Literature search
We conducted a literature search of all published original research pertaining to DBS of brainstem targets in humans (Supplementary material). This review, performed in November 2019, consisted of an initial search of the NCBI PubMed database using the terms ‘electric stimulation therapy’[MAJR], ‘brain’[mesh], ‘humans’[mesh], and ‘english’[lang], which were combined with the boolean operator ‘AND’. This generated 7632 papers. Two raters (G.J.B.E., A.N.) subsequently scanned each abstract to filter the results for relevance; a third (A.P.) resolved any disagreements. Reviews, animal studies, surgical technique papers lacking in clinical outcome information, and papers dealing with non-DBS stimulation modalities were excluded at this stage. From this selection, studies in which at least one patient received DBS of a brainstem structure were included for review. Information regarding sample size, patient clinical indication and demographics, stimulation target and stimulation parameters, clinical outcome, and any reported acute/peri-operative stimulation effects were extracted from each paper.
The STN and structures within its immediate vicinity (e.g. zona incerta, fields of Forel, and dentato-rubro-thalamic tract) were considered to be beyond the scope of this review and papers involving these targets were excluded from analysis. The effects of stimulating these targets have been extensively catalogued in prior studies (Fytagoridis and Blomstedt, 2010; Welter et al., 2014; Fenoy and Schiess, 2017).
DBS of brainstem structures
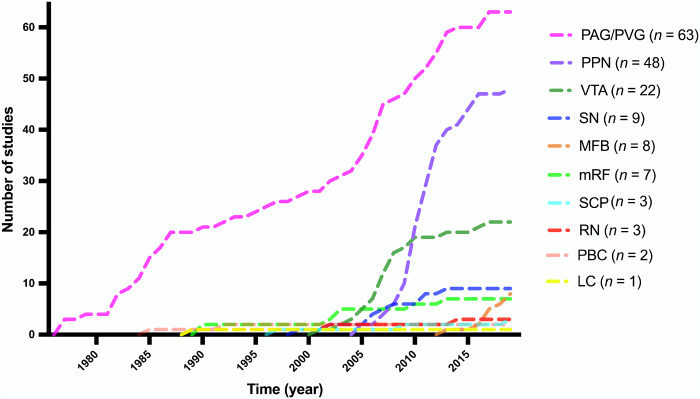
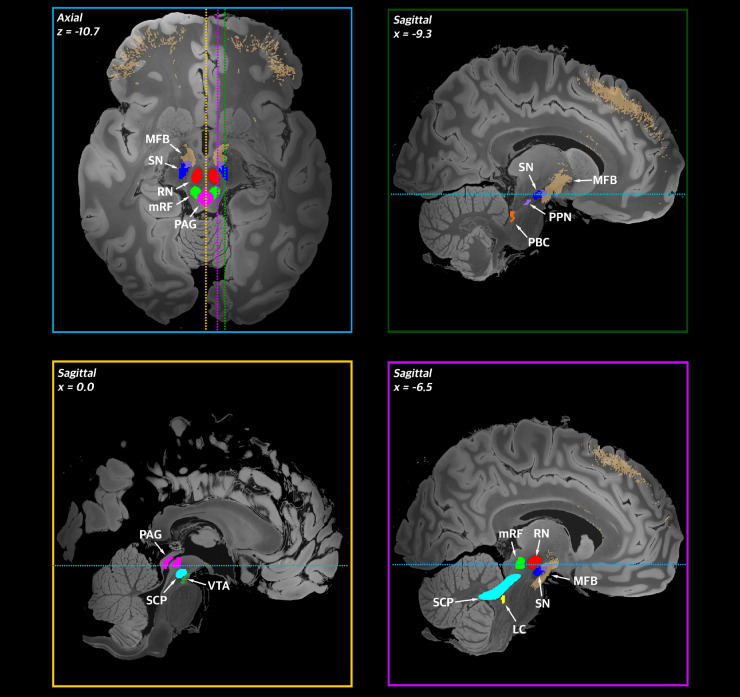
In total, we identified 164 clinical studies that reported on DBS of brainstem structures. Ten brainstem structures have been targeted using DBS: periaqueductal grey-periventricular grey (63 studies), the pedunculopontine nucleus (48 studies), ventral tegmental area (22 studies), substantia nigra (nine studies), medial forebrain bundle (eight studies), mesencephalic reticular formation (seven studies), red nucleus (three studies), superior cerebellar peduncle (three studies), parabrachial complex (two studies), and locus coeruleus (one study). Two studies involved two different brainstem targets; these were double counted for the purpose of tallying papers per target. A graph showing the cumulative number of studies involving each target over time can be seen in Fig. 1. The targets themselves are depicted in Fig. 2 (Edlow et al., 2012; Glasser et al., 2013).
Figure 1.
Cumulative number of brainstem DBS studies over time. Graph demonstrating the cumulative number of published studies for each brainstem target over time. LC = locus coeruleus; PBC = parabrachial complex; RN = red nucleus; SCP = superior cerebellar peduncle.
Figure 2.
Brainstem regions and nuclei targeted by DBS. Brainstem regions and nuclei that have been targeted by DBS are shown on a high resolution brain template [7 T, 100-µm resolution brain in MNI152 (nonlin asym) space]. Brainstem targets (taken from the Harvard Ascending Arousal Network atlas or constructed from Human Connectome Project imaging data (http://www.humanconnectomeproject.org/) are shown in two planes (top left: axial; top right and bottom: sagittal). Dotted lines denote the anatomical level displayed in panels bordered by the corresponding colour (e.g. the blue dotted line denotes the level of the axial slice displayed in the top left panel). LC = locus coeruleus; PBC = parabrachial complex; RN = red nucleus; SC = superior colliculus; SCP = superior cerebellar peduncle.
Supplementary Table 1 details the clinical outcomes of these studies, organized by target, while Supplementary Table 2 presents acute effects that were observed during intra- and post-operative stimulation. Below, we summarize the salient patterns of outcomes and acute effects that have been reported for each target. This summary is ordered according to rostrocaudal convention (Fig. 2).
Substantia nigra
Situated in the ventral midbrain tegmentum just ventral to STN, the substantia nigra (SN) is a critical component of the basal ganglia circuit. This nucleus can be subdivided into the dopaminergic, neuromelanin-rich pars compacta (SNc) and the GABAergic pars reticulata (SNr). SNc projects via the nigrostriatal pathway to the striatum, which then relays signals to SNr via the direct and indirect pathways. In turn, SNr projects nigrothalamic axons that disperse and terminate in the ventral (motor) and mediodorsal thalamus, exerting inhibitory GABAergic control over the thalamocortical network (Halliday, 2004). The degeneration of SNc neurons and subsequent diminution of nigrostriatal dopamine is a crucial element of Parkinson’s disease pathophysiology (Ulla et al., 2011), resulting in the cardinal Parkinson’s disease symptoms related to dysfunction of motor control. More generally, dopamine is also implicated in widespread modulation of cognition, motivation, punishment and reward, prolactin inhibition, sleep, mood, attention, working memory, and learning (Calabresi et al., 2007). Finally, animal studies have implicated the nigral system in epileptic neuronal transmission (Löscher et al., 1998).
Nine studies to date have described SN-DBS. One study deliberately targeted both SNr and STN to treat resistant axial motor impairment in Parkinson’s disease (n = 12); here, combined STN-SNr stimulation did not confer any significant improvement over STN stimulation alone. More encouraging results were observed across three studies (n = 7 in total) in which SNr—because of its putative connections with a dorsal midbrain anticonvulsant zone (Benabid et al., 2001; Dinner et al., 2002)—was stimulated alongside STN in epilepsy patients, leading to reports of reduced seizures in all patients. These papers primarily reported on progressive myoclonic epilepsy syndromes and tentatively support a role for STN-SNr stimulation in treating these indications. Paralleling STN-DBS for motor symptoms in Parkinson’s disease, these findings suggest greatest improvement might be attained in patients with less severe disease. The utility of STN-SNr-DBS for other forms of epilepsy is largely unknown. Finally, six case reports/series described the acute effects of SN stimulation in Parkinson’s disease patients undergoing STN-DBS. These primarily highlighted the ability of SN (possibly in combination with ventral STN given their proximity) stimulation to rapidly and reversibly induce pronounced limbic side-effects, including mania and depressive symptoms. Functional imaging studies conducted during these episodes have suggested that subcortically driven mania is accompanied by activation of areas such as dorsal anterior cingulate cortex (Ulla et al., 2011), while acute depression during left SN-DBS on (contrasted with SN-DBS off) has been linked to increased regional cerebral blood flow in areas such as ipsilateral orbitofrontal cortex, amygdala, and anterior thalamus (Bejjani et al., 1999).
Red nucleus
Lying dorsomedial to SN in the midbrain tegmentum, the oval-shaped red nucleus comprises caudal magnocellular and rostral parvocellular components. These provide efferents that contribute to the rubrospinal tract and central tegmental tract, respectively. Both cell populations also receive cerebellar and cerebral cortical inputs (Miller and Gibson, 2009). Additionally, at the level of the oculomotor nucleus, red nucleus is crossed by, but does not interact with, fibres of the oculomotor nerve (Paxinos et al., 2012). Functionally, red nucleus is believed to supplement the dominant corticospinal tract in controlling gross trunk and limb movements. In clinical practice, this is reflected in the decerebrate posturing—a loss of flexion in the upper extremities—observed following red nucleus destruction (Mihailoff and Haines, 2018).
Only one case report has described prospective DBS targeting of red nucleus (n = 1); this was in a patient with cerebellar tremor, which is typically refractory to stimulation at conventional tremor targets. This study did not evince any benefits of red nucleus-DBS (Lefranc et al., 2014). Another study reported on intra-operative effects of high frequency (130 Hz) stimulation at contacts that were localized to the white matter between red nucleus and SN in Parkinson’s disease-STN patients (n = 4), while a third briefly described the effect of stimulating red nucleus alongside other targets (Feinstein et al., 1989). Patients stimulated in this region experienced diplopia—likely due to stimulation of the nearby oculomotor nerve—as well as contralateral upper limb signs (dystonic posturing and tremor) that were consistent with the sequelae of red nucleus lesions (Bejjani et al., 2002). Overall, red nucleus-DBS appears to be of limited therapeutic value.
Ventral tegmental area
The ventral tegmental area (VTA) is a dopaminergic midbrain structure that projects to the nucleus accumbens, amygdala, hippocampus, and prefrontal cortex (Corner and Swaab, 1976) via the mesolimbic and mesocortical pathways, thereby playing a key role in reward, executive function, and motivation (Alcaro et al., 2007). May et al. (1998) first implicated VTA in cluster headache, observing increased PET activation in this area during cluster headache attacks. Since then, numerous groups have performed DBS of the region, variously referring to it as the VTA or the posterior hypothalamic region (Leone et al., 2001, 2013; Franzini et al., 2003; Akram et al., 2016). A detailed discussion of this anatomical debate is beyond this review’s purview; for the sake of inclusiveness, we have gathered all studies targeting this general region under the umbrella of VTA-DBS.
Overall, 22 studies describing VTA-DBS were identified. Most patients received VTA-DBS for cluster headache, while smaller contingents underwent treatment for aggressiveness or atypical facial pain. Overall, cluster headache patients experienced improvements in headache symptoms, with many benefiting from sustained pain reduction at long-term follow-up (>5 years), and even years after the cessation of stimulation. In studies that provided clearly defined data on individual patients, we identified 20% of patients as being pain free or nearly pain free, 47% of patients as being responders (>30% reduction in headache severity or frequency), and 31% of patients as non-responders (no improvement, or transient improvement with subsequent relapse). While a randomized controlled trial did not report any benefit of VTA-DBS over placebo, methodological limitations of the study (Nowacki et al., 2019), and the positive findings reported across multiple open-label studies suggest VTA-DBS is a potentially effective treatment for cluster headache, warranting further investigation. A handful of patients receiving VTA-DBS for aggressiveness (n = 2) experienced behavioural improvement, while treatment for atypical facial pain (n = 1) was not beneficial. Across all studies, acute side-effects of VTA stimulation included seizures (n = 1) and diplopia (n = 3), the latter of which could be avoided by adjusting stimulation parameters.
Medial forebrain bundle
The medial forebrain bundle (MFB) is a large white matter tract extending through the brainstem as a single main trunk, before bifurcating at the level of the VTA along divergent paths. The infero-medial branch (imMFB) traces the wall of the third ventricle anteriorly before terminating at the lateral hypothalamus. Conversely, the supero-lateral branch (slMFB) passes laterally beneath the thalamus, ascending to the anterior limb of the internal capsule and projecting to nucleus accumbens, orbitofrontal cortex, dorsolateral prefrontal cortex, and other regions via the mesolimbic and mesocortical pathways (Coenen et al., 2012). Through these extensive dopaminergic connections, MFB is believed to be integral in mediating reward and motivation. Indeed, affective disorders such as melancholic depression have been associated with microstructural changes in these pathways (Bracht et al., 2014). In the absence of histological descriptions of MFB in human brain specimens, possibly as a consequence of its complicated course and lack of myelin, the most comprehensive mapping of human MFB has come from diffusion tensor imaging studies (Coenen et al., 2018b).
Eight clinical studies have targeted MFB—specifically slMFB given its connections with mood-implicated prefrontal cortex—for psychiatric indications. The anatomical locus of stimulation along the slMFB for these trials was a triangle bound by the STN, SNc, and red nucleus (Coenen et al., 2018a). Overall, most treated patients had treatment-resistant depression, while a far smaller number had obsessive-compulsive disorder, co-morbid treatment-resistant depression and anorexia nervosa, or bipolar depression. Open-label MFB-DBS trials for treatment-resistant depression, all of which used 130 Hz frequency, 60 µs stimulation with minor variation in voltage/current (3–4.3 V; 2.3–4.9 mA), have demonstrated reproducible improvements in depression symptoms. Antidepressant responses in these studies were typically notably rapid (within 1 week) and sustained, with multiple groups reporting a ≥ 50% reduction in depressive symptoms at 12-month follow-up among most patients. It should be noted, however, that one randomized controlled trial comparing MFB-DBS stimulation against sham stimulation in a treatment-resistant depression cohort found no statistically significant differences between treatment arms (Coenen et al., 2019). This pattern is similar to that seen in trials of another major target for treatment-resistant depression DBS, the subcallosal/subgenual anterior cingulate area; these demonstrated robust antidepressant responses (≥50% symptom reduction) in open-label studies but less convincing results in controlled trials (Kringelbach et al., 2007; Lozano et al., 2008; Holtzheimer et al., 2012). Mitigating factors for the negative results of the MFB-DBS randomized controlled trial include its small size, relative short follow-up duration (8 weeks), potential microlesioning ‘insertional’ effects, and the difficulty of interpreting placebo responses in depression cohorts (Schatzberg and Kraemer, 2000). Moreover, acute relapse occurred in some patients after double-blinded battery failure (Bewernick et al., 2017; Coenen et al., 2019). Thus, the balance of evidence supports a promising role for MFB-DBS in treating treatment-resistant depression. MFB-DBS for the management of obsessive-compulsive disorder has also been reported to result in moderate improvements in overall symptoms, although confident conclusions are precluded by the small sample size to date. Reports of acute stimulation-induced strabismus and diplopia were common to all trials; these can be attributed to slMFB’s proximity to the origin of the oculomotor nerve.
Mesencephalic reticular formation
The reticular formation is a loose collection of interconnected nuclei located in the central tegmentum that extends from the midbrain to the medulla oblongata (Horn and Adamczyk, 2012). Within the pons and medulla, the reticular formation can be divided into three columns: (i) the medial tegmental field with descending afferents involved in postural control; (ii) the raphe nuclei, which modulate firing rates in the sensory or motor spinal circuits; and (iii) the lateral tegmental field, containing interneurons to various cranial nerve nuclei and to motor neurons in the spinal cord implicated in respiration, micturition, and blood pressure (Holstege, 1991). Pertinent to DBS studies of this region, ascending fibres from the mesencephalic reticular formation (mRF) contribute to the ascending reticular activating system, which serves to regulate states of consciousness (Edlow et al., 2012).
While seven papers have described mRF-DBS, all were reported follow-ups of the same trial at varying time points. In part, this trial described a cohort of 21 persistent vegetative state patients, 19 of whom received centromedian/parafascicular (CMPf) thalamic DBS and two of whom received mRF-DBS. Low frequency stimulation (25–50 Hz) was used in all cases. At 24 months post-operation, eight DBS patients had progressed from persistent vegetative state and were able to obey simple verbal commands, while none of the untreated patients had progressed. Unfortunately, no distinction of outcomes between patients receiving mRF-DBS and CMPf-DBS was made, prohibiting any meaningful conclusions about the therapeutic value of mRF-DBS. Acutely, ‘arousal responses’ (e.g. eye opening) was observed following mRF and thalamic DBS. PET showed acute mRF was associated with increased regional cerebral blood flow while EEG demonstrated increased pain-related P250 (a measure of late positive component of cerebral evoked potential in response to painful stimuli) in response to stimulation, which could not be elicited in response to painful external stimuli (Tsubokawa et al., 1990). While this points to possible mRF-mediated arousal and strengthens the case for further research in this field, it also highlights the potential ethical issues of performing DBS in a population unable to communicate distress or grant informed consent.
Periaqueductal and periventricular grey
The periaqueductal grey (PAG) is an area of grey matter within the midbrain that circumscribes the cerebral aqueduct until it reaches the third ventricle anteriorly, at which point it becomes known as the periventricular grey (PVG). Functionally, this continuous PAG/PVG region is involved in the coordination of autonomic and behavioural responses—especially to pain and other aversive stimuli—by integrating inputs from the prefrontal cortex, amygdala, reticular formation, hypothalamus, and nociceptive and sympathetic afferents (Green et al., 2005). These responses, which include cardiovascular, respiratory, motor responses as well as central modulation of nociceptive signalling, are driven by the PAG/PVG’s connections to other brainstem and hypothalamic nuclei (Benarroch, 2018). PAG/PVG DBS has a long and extensive history as a treatment for neuropathic pain under the premise that stimulation of this target engages endogenous, opioid-releasing neurons that are capable of inhibiting or altering nociceptive signals (Basbaum and Fields, 1978; Young et al., 1993; Green et al., 2009; Sims-Williams et al., 2017). However, the PAG/PVG’s more general role in the orchestration of autonomic responses has also prompted investigation of its relevance for purposes beyond analgesia.
PAG/PVG-DBS was identified as the most studied brainstem target. Sixty-three studies have reported on patients who received PAG/PVG-DBS for the management of medication-refractory pain, most commonly neuropathic pain secondary to stroke, trauma, or amputation. When all studies that reported individual patient outcome data were pooled, ∼52% of patients experienced good-to-excellent pain relief (≥50% improvement), 23% experienced mild relief (20–50% improvement), and 26% had poor or no benefit from stimulation. While 50% improvement has been argued by some to represent a benchmark for clinically useful pain relief (Owen et al., 2006), the fact that treated patients are typically refractory to all other forms of therapy and that even moderate pain improvements in this context can lead to noticeable gains in quality of life (Farrell et al., 2018), should be kept in mind. Overall, the available data—derived from a legacy of studies dating back to the 1970s—support a role for PAG/PVG-DBS in managing refractory pain conditions. PAG/PVG alone may represent a particularly useful target for treating refractory nociceptive pain; by contrast, best outcomes in the context of neuropathic pain were more often achieved using combined PAG/PVG and sensory thalamus stimulation.
In patients already receiving PAG/PVG-DBS for the treatment of chronic pain, more recent studies have also demonstrated associated changes in various autonomic functions. These include modulation of the cardiovascular system, with one group demonstrating clinical improvement in patients with hypertension or orthostatic hypotension. PAG/PVG-DBS has also been associated with increases in lung function (Hyam et al., 2012a), and bladder capacity (Green et al., 2012). These studies point to the potential of PAG/PVG-DBS as a tool for managing a broad range of autonomic dysfunctions (Hyam et al., 2012b).
When reported, the frequency of PAG/PVG-DBS stimulation was typically low (<50 Hz), while the voltage and pulse width of stimulation varied between 0.6–7 V and 60–120 µs, respectively. The propensity for lower stimulation frequencies may relate to the acute adverse effects often observed with higher frequencies of stimulation (>100 Hz). These include eye bobbing and eye deviation—attributable to current spread to the nearby superior colliculus and oculomotor nerve—and anxiety. Autonomic side-effects including nausea and diaphoresis have been reported at relatively higher voltages (>3 V). By contrast, therapeutic stimulation parameters were often documented as producing warmth/cold sensations or paraesthesias acutely.
Pedunculopontine nucleus
Situated in the dorsolateral portion of the ponto-mesencephalic tegmentum at the level of the trochlear nucleus, the pedunculopontine nucleus (PPN) comprises part of the upper brainstem’s mesencephalic locomotor region (together with the cuneiform and subcuneiform nuclei). The two subnuclei of PPN, the pars compacta and pars dissipata, have widespread cholinergic, glutamatergic, and GABAergic bidirectional projections with the basal ganglia, cerebellum, cortex, thalamus, and spinal cord (Pienaar et al., 2017). To date, studies have implicated PPN in the maintenance of posture, modulation of attention, arousal, sleep (as part of the reticular formation), and initiation of gait (Semba et al., 1990; Fuller et al., 2007; Boutin et al., 2017). These processes are often impaired in Parkinson’s disease patients, in whom the PPN is known to degenerate (Ricciardi et al., 2015). Importantly, PPN has been studied for its relevance to freezing of gait (Nutt et al., 2011)—characterized by sudden transient episodes of inability to move the feet forward—and balance. Progressive freezing of gait affects 40–50% of patients with Parkinson’s disease and is frequently refractory to both pharmacotherapy and subthalamic or pallidal DBS (Perez-Lloret et al., 2014; Amboni et al., 2015; Forsaa et al., 2015). As such, PPN has become an attractive DBS target for the improvement of freezing of gait in the subset of Parkinson’s disease patients in whom it arises or persists following treatment via more conventional targets [i.e. STN or globus pallidus interna (GPi)] (Ferraye et al., 2010).
Consistent with this premise, we identified 48 studies that described PPN-DBS—either alone or combined with STN-, GPi- or zona incerta-DBS—for improvement of postural stability and axial symptoms in patients with Parkinson’s disease, as well as a small number of progressive supranuclear palsy, and primary progressive freezing of gait patients. Overall, Parkinson’s disease studies tended to demonstrate statistically significant, although sometimes modest, post-operative improvements in Unified Parkinson’s Disease Rating Scale motor component (UPDRS-III) score, particularly in axial metrics (items 27–30). These scores typically improved between 20% and 50% from baseline. The most robust axial benefit of PPN-DBS appeared to be improved freezing of gait, which has been documented across several case series and double-blind randomised assessments. The reported impact on balance as assessed by UPDRS-III item 30 (i.e. the ‘pull’ test, which may not accurately capture all aspects of balance) was more variable; however, reduced falls, which can be a function of many factors including balance and freezing of gait, was a common finding amongst studies. Conversely, improvements in bradykinesia, rigidity, and tremor rarely occurred with PPN-DBS alone. These findings suggest that PPN-DBS may be a useful therapy in Parkinson’s disease patients for whom falls and freezing of gait are a particular concern, particularly if modest improvements in these areas might substantially benefit quality of life. Across identified studies, the stimulation frequency used was typically low (25–35 Hz), with a larger variation in pulse width and voltage (60–120 µs, 1.0–4.9 V). In line with these overall improvements in axial signs, improvements in verbal fluency were reported in several studies (Stefani et al., 2010a, b; Mazzone et al., 2012). PPN-DBS has also been linked to improvements in sleep quality and architecture (Lim et al., 2009; Stefani et al., 2010a, b), reaction time (Costa et al., 2010), recall and executive function (Stefani et al., 2010a), and visual perception (Strumpf et al., 2016), which may be attributable to PPN’s involvement in the ascending reticular activating system. These clinical findings have been supported by functional imaging, in which PPN-DBS has been associated with altered activation (both metabolic and regional blood flow) of brain areas implicated in movement (Strafella et al., 2008; Ballanger et al., 2009; Wilcox et al., 2011)—specifically the cerebellum and mesencephalic locomotor region—as well as cortical areas implicated in executive function (Ceravolo et al., 2011). The voltage used by PPN-DBS was often limited by unpleasant acute effects, particularly contralateral hemibody paresthesias—likely attributable to current spread to nearby medial lemniscus fibres—and oculomotor effects related to medial longitudinal fasciculus stimulation. Additionally, PPN-DBS induced involuntary urinary voiding in one patient, likely due to inadvertent stimulation of the pontine micturition centre.
Locus coeruleus
The locus coeruleus is a discrete cluster of noradrenergic neurons in the dorsal pontine tegmentum, lying close to the lateral floor of the fourth ventricle. The major source of noradrenaline in the brain, locus coeruleus projects extensively throughout the brainstem, cerebrum, cerebellum, and spinal cord (Counts and Mufson, 2012) and plays an important role in modulating memory function, arousal, attention, and ‘fight or flight’ responses (Foote et al., 1980, 1983).
A single 1989 study used low frequency (50–60 Hz) locus coeruleus-DBS for the treatment of cerebral palsy (n = 1) and generalized tonic-clonic seizures (n = 2) with the intent of suppressing generalized neuronal hyperexcitability mediated through noradrenaline innervation (Feinstein et al., 1989). An acute improvement in hypertonicity during stimulation was reported in the cerebral palsy patient (along with symptom worsening following double-blind battery failure), while decreased seizure frequency and severity was described in the epileptic patients. Continuous stimulation at night was associated with sleep disruption, possibly related to the role of the locus coeruleus in arousal. Locus coeruleus-DBS has not been described since in human trials, perhaps owing to the establishment of the more readily accessible GPi and anterior nucleus of the thalamus for the management of dystonia and epilepsy, respectively (Krauss et al., 2002; Fisher et al., 2010). Overall, the lack of reproducible findings or quantitative outcomes limit confidence in this region as a therapeutic target.
Parabrachial complex
The parabrachial complex is a cluster of neurons surrounding the superior cerebellar peduncles in the dorsolateral pons. Often divided into medial, lateral, and subparabrachial (or Kölliker-Fuse nucleus) subdivisions, the parabrachial complex is known to play a role in cardiorespiratory homeostasis, pain, and aversion/avoidance, integrating ascending sensory signals with input from higher brain areas (Chiang et al., 2019).
Two studies in the 1980s/90s (collective n = 8) described low frequency (10–60 Hz) parabrachial complex-DBS—in several cases in combination with PAG/PVG or thalamic DBS. All patients underwent treatment for chronic intractable pain of varied aetiologies, including post-herpetic neuralgia, spinal cord injury, and malignancy. Overall, parabrachial complex-DBS was reported to produce ‘good’ to ‘excellent pain relief’ in five patients without notable side-effects, although no quantitative measures of pain relief were provided (Katayama et al., 1985; Young et al., 1992). However, it should be noted that some of these patients received concurrent stimulation with other targets, such as PVG or sensory thalamus, making it difficult to ascribe benefit solely to parabrachial complex stimulation. Despite these seemingly promising outcomes and suggestions from animal studies that parabrachial complex-dependent analgesia, unlike PAG/PVG-dependent analgesia, may be partially opioid-independent (Katayama et al., 1984), parabrachial complex-DBS has not been since pursued. This may reflect the structure’s anatomical complexity as well as the fact that both nearby PAG-PVG and sensory thalamus were already well-established as ‘classic’ targets for treating refractory pain (Hosobuchi, 1983; Young et al., 1992).
Superior cerebellar peduncle
One of the three white matter structures that connects the cerebellum to the brainstem, the superior cerebellar peduncle (SCP) attaches to the midbrain immediately below the trochlear nerve, conveying efferent cerebellar fibres—integral for ipsilateral arm and leg coordination—to the brainstem and diencephalon via the dentato-rubro-thalamic tract (DRTT) (Haines and Mihailoff, 2018).
Three studies describing high frequency (200 Hz) SCP-DBS were identified. In both studies, investigators sought to exploit SCPs connection to DRTT in the treatment of hypertonic conditions, namely cerebral palsy (n = 31) and dystonia refractory to previous pallidal intervention (n = 1). Decreased post-operative hypertonicity was reported in the cerebral palsy cohort, albeit in the absence of quantitative measures (Galanda and Hovath, 1997; Harat et al., 2009). By contrast, notable quantitative improvements in muscle tone, pain, and quality of life were noted in the dystonia patient at 6 months follow-up (Horisawa et al., 2020), suggesting that further work on the role of SCP-DBS as a treatment for refractory dystonia may be warranted. Acutely, high voltage stimulation (8.5–10 V) was associated with both oculomotor effects as well as forced laughter (Horisawa et al., 2020), and subjective pleasure and fear (Galanda and Hovath, 1997). These phenomena may reflect infringement on the trochlear nerve and white matter pathways relevant to the cerebellum’s role in emotional regulation and expression (Damasio et al., 2000; Parvizi, 2001), respectively.
Limitations
Several limitations of the literature reviewed here should be acknowledged given their potential to inform future study design. First, many of the studies described in this paper were open-label and uncontrolled, making them susceptible to performance and detection biases. More generally, the well-recognized bias towards reporting and publishing positive results should be kept in mind when reviewing any case studies or case series, as it can lead to an overrepresentation of efficacious DBS cases in the literature (Schlaepfer and Fins, 2010). Another limitation is that comparison across the reviewed studies is challenging because outcome measures were often non-standardized. Additionally, some of the case series reported clinical outcomes as aggregate group measures, which can be heavily skewed by individual outliers when sample size is small and can also obscure specific target-outcome relationships if patients with different stimulation targets are grouped together. Finally, technological limitations in older studies reduce the accuracy and reliability with which implanted electrodes can even be said to be stimulating the purported targets.
Summary and future directions
DBS of the brainstem remains a relatively unexplored field. We identified 164 unique clinical studies on this topic that have been published to date; for context, prior work has shown there were over 500 DBS-related publications per year by the latter part of the 2000s (Lozano and Lipsman, 2013). Indeed, in a recent survey of past and present DBS clinical trials that were registered on clinicaltrials.gov, only 21 (4%) of the total 485 trials identified involved brainstem targets (Harmsen et al., 2020). Data on several targets—namely locus coeruleus, parabrachial complex, reticular formation, and superior cerebellar peduncle—are fairly sparse, primarily deriving from decades-old case series that lack information on follow-up, stimulation parameters, and standardized outcome measures. Charitable interpretations of these areas could tentatively suggest potential avenues of further investigation in disorders such as chronic pain, hypertonic conditions, and disorders of consciousness; however, the paucity of high-quality evidence necessitates a conservative interpretation. SN-DBS may have a role to play in controlling progressive myoclonic epilepsy, although reports on this indication are outnumbered by studies describing acute limbic consequences (mania or depression) of stimulation in this area. This side-effect profile could prove problematic for any future applications of SN-DBS to epilepsy treatment. The most promising targets for therapeutic stimulation within the brainstem appear to be the PPN, PAG/PVG, VTA, and slMFB, in which studies have respectively demonstrated reproducible improvements in axial stability, pain/autonomic dysfunction, cluster headache severity, and depressive symptoms. Of these targets, PAG/PVG has been utilized since the late 1970s, while VTA and PPN have seen increased interest since the early 2000s. However, the number of new DBS studies involving these structures has plateaued in recent years. Conversely, slMFB has emerged as a target of interest for psychiatric indications in the last half decade and continues to be explored in contemporary work (Fig. 1). In addition to positive results, studies investigating these targets have also been more consistent in reporting recognized outcome measures and long-term follow up, increasing their reliability and reproducibility. These factors have likely contributed to the relatively large quantity of studies in these areas when compared to other brainstem substructures. Despite this, there is a clear absence of double-blinded randomized control trials, suggesting that further work is needed in order to validate these areas as reliable and efficacious treatment targets.
The technical challenges of performing DBS in a region as constrained and anatomically complex as the brainstem may be mitigated by advances in stimulation delivery. The arrival of directional leads has allowed for the possibility of greater stimulation precision (Schüpbach et al., 2017), while endoscopic ‘stentrode’ devices may render certain brainstem targets, such as the slMFB, more accessible to stimulation (Neudorfer et al., 2020). Increased focus on the development of closed-loop systems, which offer the prospect of recording neuronal or peripheral activity and adapting stimulation accordingly (Habets et al., 2018), may also be beneficial for brainstem DBS. By allowing stimulation to be fine-tuned in real time based on physiological dynamics, these systems may improve the utility of DBS for brainstem functions such as arousal and autonomic function. In concert with advances in stimulation technology, improved imaging—such as that delivered by ultrahigh-field (e.g. 7 T) MRI—has potential to enhance the visualization of brainstem structures and relevant circuitry (Sclocco et al., 2018).
In summary, we comprehensively reviewed the existing human literature on brainstem DBS, cataloguing the long-term clinical outcomes and acute effects of stimulation as they have been reported. It is hoped this work serves as a concise account of what is known about this field to date in addition to providing a helpful launch-point for future research.
Funding
This study was supported by the RR Tasker Chair in Functional Neurosurgery at University Health Network (AML), the Canadian Institutes of Health Research (reference # 164235: G.J.B.E.), and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG NE 2276/1-1: C.N.).
Competing interests
A.M.L. is the co-founder of Functional Neuromodulation, is a consultant for Boston Scientific, Medtronic, and Abbott (all of which produce DBS devices), and holds intellectual property in the field of DBS. The other authors report no conflicts of interest.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
- DBS
deep brain stimulation
- MFB
medial forebrain bundle
- mRF
mesencephalic reticular formation
- PAG
periaqueductal grey
- PPN
pedunculopontine nucleus
- PVG
periventricular grey
- SNc/r
substantia nigra pars compacta/pars reticulata
- STN
subthalamic nucleus
- VTA
ventral tegmental area
References
- Akram H, Miller S, Lagrata S, Hyam J, Jahanshahi M, Hariz M, et al. Ventral tegmental area deep brain stimulation for refractory chronic cluster headache. Neurology 2016; 86: 1676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaro A, Huber R, Panksepp J.. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev 2007; 56: 283–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amboni M, Stocchi F, Abbruzzese G, Morgante L, Onofrj M, Ruggieri S, et al. Prevalence and associated features of self-reported freezing of gait in Parkinson disease: the DEEP FOG study. Parkinsonism Relat Disord 2015; 21: 644–9. [DOI] [PubMed] [Google Scholar]
- Anderson WS, Lenz FA.. Surgery Insight: deep brain stimulation for movement disorders. Nat Rev Neurol 2006; 2: 310–20. [DOI] [PubMed] [Google Scholar]
- Ángeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP.. Anatomy of the brainstem: a gaze into the stem of life. Semin Ultrasound CT MR 2010; 31: 196–219. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum Brain Mapp 2009; 30: 3901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL.. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 1978; 4: 451–62. [DOI] [PubMed] [Google Scholar]
- Bejjani B-P, Arnulf I, Houeto J-L, Milea D, Demeret S, Pidoux B, et al. Concurrent excitatory and inhibitory effects of high frequency stimulation: an oculomotor study. J Neurol Neurosurg Psychiatry 2002; 72: 517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani B-P, Damier P, Arnulf I, Thivard L, Bonnet A-M, Dormont D, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med 1999; 340: 1476–80. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Koudsié A, Benazzouz A, Vercueil L, Fraix V, Chabardes S, et al. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson’s disease. Extension to new indications such as dystonia and epilepsy. J Neurol 2001; 248: 37–47. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Brainstem integration of arousal, sleep, cardiovascular, and respiratory control. Neurology 2018; 91: 958–66. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Gippert SM, Switala C, Coenen VA, Schlaepfer TE.. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimul 2017; 10: 664–71. [DOI] [PubMed] [Google Scholar]
- Boutin RCT, Alsahafi Z, Pagliardini S.. Cholinergic modulation of the parafacial respiratory group: cholinergic modulation of active expiration. J Physiol 2017; 595: 1377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T, Horn H, Strik W, Federspiel A, Schnell S, Höfle O, et al. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord 2014; 155: 186–93. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M.. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 2007; 30: 211–9. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Brusa L, Galati S, Volterrani D, Peppe A, Siciliano G, et al. Low frequency stimulation of the nucleus tegmenti pedunculopontini increases cortical metabolism in parkinsonian patients. Eur J Neurol 2011; 18: 842–9. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM.. Parabrachial complex: a hub for pain and a version. J Neurosci 2019; 39: 8225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen VA, Bewernick BH, Kayser S, Kilian H, Boström J, Greschus S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology 2019; 44: 1224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Mädler B.. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci 2012; 24: 223–36. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Sajonz B, Reisert M, Bostroem J, Bewernick B, Urbach H, et al. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. Neuroimage Clin 2018. a; 20: 580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen VA, Schumacher LV, Kaller C, Schlaepfer TE, Reinacher PC, Egger K, et al. The anatomy of the human medial forebrain bundle: ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. Neuroimage Clin 2018. b; 18: 770–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner MA, Swaab DF, Progress in brain research. In: Corner MA, Swaab DF, editor(s). Progress in brain research. Amsterdam, New York: Elsevier; 1976. p. ii–x. [Google Scholar]
- Costa A, Carlesimo GA, Caltagirone C, Mazzone P, Pierantozzi M, Stefani A, et al. Effects of deep brain stimulation of the peduncolopontine area on working memory tasks in patients with Parkinson’s disease. Parkinsonism Relat Disord 2010; 16: 64–7. [DOI] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ, Locus coeruleus. In: The human nervous system. London: Elsevier; 2012. p. 425–38. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci 2000; 3: 1049–56. [DOI] [PubMed] [Google Scholar]
- DeLong M, Wichmann T.. Deep brain stimulation for movement and other neurologic disorders. Ann N Y Acad Sci 2012; 1265: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinner DS, Neme S, Nair D, Montgomery EB, Baker KB, Rezai A, et al. EEG and evoked potential recording from the subthalamic nucleus for deep brain stimulation of intractable epilepsy. Clin Neurophysiol 2002; 113: 1391–402. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S.. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol 1986; 55: 375–401. [DOI] [PubMed] [Google Scholar]
- Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 2012; 71: 531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell S, Green A, Aziz T.. The current state of deep brain stimulation for chronic pain and its context in other forms of neuromodulation. Brain Sci 2018; 8: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR.. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol 2015; 11: 98–110. [DOI] [PubMed] [Google Scholar]
- Feinstein B, Gleason CA, Libet B.. Stimulation of locus coeruleus in man. Preliminary trials for spasticity and epilepsy. Stereotact Funct Neurosurg 1989; 52: 26–41. [DOI] [PubMed] [Google Scholar]
- Fenoy AJ, Schiess MC.. Deep brain stimulation of the dentato-rubro-thalamic tract: outcomes of direct targeting for tremor. Neuromodulation 2017; 20: 429–36. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 2010; 133: 205–14. [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R; the SANTE Study Group, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010; 51: 899–908. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE.. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 1980; 77: 3033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G.. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 1983; 63: 844–914. [DOI] [PubMed] [Google Scholar]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G.. A 12-year population-based study of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 254–8. [DOI] [PubMed] [Google Scholar]
- Franzini A, Ferroli P, Leone M, Broggi G.. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery 2003; 52: 1095–9. discussion 1099–1101. [PubMed] [Google Scholar]
- Fuller PM, Saper CB, Lu J.. The pontine REM switch: past and present: the pontine REM switch. J Physiol 2007; 584: 735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fytagoridis A, Blomstedt P.. Complications and side effects of deep brain stimulation in the posterior subthalamic area. Stereotact Funct Neurosurg 2010; 88: 88–93. [DOI] [PubMed] [Google Scholar]
- Galanda M, Hovath S.. Different effect of chronic electrical stimulation of the region of the superior cerebellar peduncle and the nucleus ventralis intermedius of the thalamus in the treatment of movement disorders. Stereotact Funct Neurosurg 1997; 69: 116–20. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage 2013; 80: 105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Stone E, Sitsapesan H, Turney BW, Coote JH, Aziz TZ, et al. Switching off micturition using deep brain stimulation at midbrain sites. Ann Neurol 2012; 72: 144–7. [DOI] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SLF, Xie K, Liu X, Paterson DJ, et al. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport 2005; 16: 1741–5. [DOI] [PubMed] [Google Scholar]
- Green AL, Wang S, Stein JF, Pereira EAC, Kringelbach ML, Liu X, et al. Neural signatures in patients with neuropathic pain. Neurology 2009; 72: 569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 2006; 31: 2384–93. [DOI] [PubMed] [Google Scholar]
- Habets JGV, Heijmans M, Kuijf ML, Janssen MLF, Temel Y, Kubben PL.. An update on adaptive deep brain stimulation in Parkinson’s disease: update on adaptive DBS in Parkinson’s disease. Mov Disord 2018; 33: 1834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DE, Mihailoff GA, An overview of the brainstem. In: Haines DE, Mihailoff GA, editor(s). Fundamental neuroscience for basic and clinical applications. 5th edn. Philadelphia: Elsevier; 2018. p. 152–9. [Google Scholar]
- Halliday G, Substantia nigra and locus coeruleus. In: Paxinos G, Mai JK, editor(s). The human nervous system. San Diego, London: Elsevier; 2004. p. 449–63. [Google Scholar]
- Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 2008; 63: 119–23. [DOI] [PubMed] [Google Scholar]
- Harat M, Radziszewski K, Rudas M, Okon M, Galanda M.. Clinical evaluation of deep cerebellar stimulation for spasticity in patients with cerebral palsy. Neurol Neurochir Pol 2009; 43: 36–44. [PubMed] [Google Scholar]
- Harmsen IE, Elias GJB, Beyn ME, Boutet A, Pancholi A, Germann J, et al. Clinical trials for deep brain stimulation: current state of affairs. Brain Stimul 2020; 13: 378–85. [DOI] [PubMed] [Google Scholar]
- Holstege G, Descending pathways from the periaqueductal gray and adjacent areas. In: Depaulis A, Bandler R, editor (s). The midbrain periaqueductal gray matter. Boston, MA: Springer US; 1991. p. 239–65. [Google Scholar]
- Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017; 4: 839–49. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 2012; 69: 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisawa S, Arai T, Suzuki N, Kawamata T, Taira T.. The striking effects of deep cerebellar stimulation on generalized fixed dystonia: case report. J Neurosurg 2020; 132: 712–6. [DOI] [PubMed] [Google Scholar]
- Horn AKE, Adamczyk C, Reticular formation: eye Movements, Gaze and Blinks. In: Mai JK, Paxinos G, editor(s). The human nervous system. 3rd edn.San Diego: Academic Press; 2012. p. 328–66. [Google Scholar]
- Hosobuchi Y. Combined electrical stimulation of the periaqueductal gray matter and sensory thalamus. Appl Neurophysiol 1983; 46: 112–5. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970-1984). J Neurosurg 1986; 64: 543–53. [DOI] [PubMed] [Google Scholar]
- Hyam JA, Brittain J-S, Paterson DJ, Davies RJO, Aziz TZ, Green AL.. Controlling the lungs via the brain: a novel neurosurgical method to improve lung function in humans. Neurosurgery 2012. a; 70: 469–77. discussion 477–478. [DOI] [PubMed] [Google Scholar]
- Hyam JA, Kringelbach ML, Silburn PA, Aziz TZ, Green AL.. The autonomic effects of deep brain stimulation—a therapeutic opportunity. Nat Rev Neurol 2012. b; 8: 391–400. [DOI] [PubMed] [Google Scholar]
- Jagannathan S, Krovvidi H.. Anaesthetic considerations for posterior fossa surgery. Anaesthesia Crit Care Pa 2014; 14: 202–6. [Google Scholar]
- Katayama Y, Tsubokawa T, Hirayama T, Yamamoto T.. Pain relief following stimulation of the pontomesencephalic parabrachial region in humans: brain sites for nonopiate-mediated pain control. Stereotact Funct Neurosurg 1985; 48: 195–200. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Watkins LR, Becker DP, Hayes RL.. Non-opiate analgesia induced by carbachol microinjection into the pontine parabrachial region of the cat. Brain Res 1984; 296: 263–83. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 2011; 168: 502–10. [DOI] [PubMed] [Google Scholar]
- Krauss JK, Loher TJ, Pohle T, Weber S, Taub E, Barlocher CB, et al. Pallidal deep brain stimulation in patients with cervical dystonia and severe cervical dyskinesias with cervical myelopathy. J Neurol Neurosurg Psychiatry 2002; 72: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Green AL, Owen SLF, Hansen PC, Cornelissen PL, et al. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport 2007; 18: 223–8. [DOI] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 2010; 68: 521–34. [DOI] [PubMed] [Google Scholar]
- Lefranc M, Manto M, Merle P, Tir M, Montpellier D, Constant J-M, et al. Targeting the red nucleus for cerebellar tremor. Cerebellum 2014; 13: 372–7. [DOI] [PubMed] [Google Scholar]
- Leone M, Franzini A, Bussone G.. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med 2001; 345: 1428–9. [DOI] [PubMed] [Google Scholar]
- Leone M, Franzini A, Proietti Cecchini A, Bussone G.. Success, failure, and putative mechanisms in hypothalamic stimulation for drug-resistant chronic cluster headache. Pain 2013; 154: 89–94. [DOI] [PubMed] [Google Scholar]
- Levy RM, Lamb S, Adams JE.. Treatment of chronic pain by deep brain stimulation: long term follow‐up and review of the literature. Neurosurgery 1987; 21: 885–93. [DOI] [PubMed] [Google Scholar]
- Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, Hutchison WD, et al. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol 2009; 66: 110–4. [DOI] [PubMed] [Google Scholar]
- Löscher W, Ebert U, Lehmann H, Rosenthal C, Nikkhah G.. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res 1998; 51: 196–209. [DOI] [PubMed] [Google Scholar]
- Lozano A, Lipsman N.. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013; 77: 406–24. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH.. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 2008; 64: 461–7. [DOI] [PubMed] [Google Scholar]
- May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ.. Hypothalamic activation in cluster headache attacks. Lancet 1998; 352: 275–8. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45: 651–60. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Padua L, Falisi G, Insola A, Florio TM, Scarnati E.. Unilateral deep brain stimulation of the pedunculopontine tegmental nucleus improves oromotor movements in Parkinson’s disease. Brain Stimul 2012; 5: 634–41. [DOI] [PubMed] [Google Scholar]
- Mihailoff GA, Haines DE, Motor System II: corticofugal Systems and the Control of Movement. In: Haines DE, Mihailoff GA, editor(s). Fundamental neuroscience for basic and clinical applications . 5th edn.Elsevier; 2018. p. 360–376.e1. [Google Scholar]
- Miller LE, Gibson AR, Red nucleus. In: Squire LR, editor(s). Encyclopedia of neuroscience. Oxford: Academic Press; 2009. p. 55–62. [Google Scholar]
- Neudorfer C, Bhatia K, Boutet A, Germann J, Elias GJ, Loh A, et al. Endovascular deep brain stimulation: investigating the relationship between vascular structures and deep brain stimulation targets. J Neurosurg 2020; 13: 1668–77. [DOI] [PubMed] [Google Scholar]
- Nowacki A, Moir L, Owen SL, Fitzgerald JJ, Green AL, Aziz TZ.. Deep brain stimulation of chronic cluster headaches: posterior hypothalamus, ventral tegmentum and beyond. Cephalalgia 2019; 39: 1111–20. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A.. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011; 10: 734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SLF, Green AL, Stein JF, Aziz TZ.. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain 2006; 120: 202–6. [DOI] [PubMed] [Google Scholar]
- Parvizi J. Pathological laughter and crying: a link to the cerebellum. Brain 2001; 124: 1708–19. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Xu-Feng H, Sengul G, Watson C, Organization of Brainstem Nuclei. In: Mai JK, Paxinos G, editor(s). The human nervous system. 3rd edn.San Diego: Academic Press; 2012. p. 260–327. [Google Scholar]
- Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, et al. prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson Disease. JAMA Neurol 2014; 71: 884. [DOI] [PubMed] [Google Scholar]
- Pienaar IS, Vernon A, Winn P.. The cellular diversity of the Pedunculopontine nucleus: relevance to behavior in health and aspects of Parkinson’s disease. Neuroscientist 2017; 23: 415–31. [DOI] [PubMed] [Google Scholar]
- Prentice SD, Drew T.. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol 2001; 85: 679–98. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Piano C, Rita Bentivoglio A, Fasano A.. Pedunculopontine nucleus stimulation in Parkinson’s disease dementia. Biol Psychiatry 2015; 77: e35. [DOI] [PubMed] [Google Scholar]
- Roh D, Chang WS, Chang JW, Kim C-H.. Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder. Psychiatry Res 2012; 200: 1067–70. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Kraemer HC.. Use of placebo control groups in evaluating efficacy of treatment of unipolar major depression. Biol Psychiatry 2000; 47: 736–44. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Fins JJ.. Deep brain stimulation and the neuroethics of responsible publishing: when one is not enough. JAMA 2010; 303: 775. [DOI] [PubMed] [Google Scholar]
- Schüpbach WMM, Chabardes S, Matthies C, Pollo C, Steigerwald F, Timmermann L, et al. Directional leads for deep brain stimulation: opportunities and challenges: directional leads for DBS. Mov Disord 2017; 32: 1371–5. [DOI] [PubMed] [Google Scholar]
- Sclocco R, Beissner F, Bianciardi M, Polimeni JR, Napadow V.. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage 2018; 168: 412–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Reiner PB, Fibiger HC.. Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 1990; 38: 643–54. [DOI] [PubMed] [Google Scholar]
- Sims-Williams H, Matthews JC, Talbot PS, Love-Jones S, Brooks JC, Patel NK, et al. Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans. Neuroimage 2017; 146: 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci 2010. a; 289: 44–8. [DOI] [PubMed] [Google Scholar]
- Stefani A, Pierantozzi M, Ceravolo R, Brusa L, Galati S, Stanzione P.. Deep brain stimulation of pedunculopontine tegmental nucleus (PPTg) promotes cognitive and metabolic changes: a target-specific effect or response to a low-frequency pattern of stimulation? Clin EEG Neurosci 2010. b; 41: 82–6. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Lozano AM, Ballanger B, Poon Y-Y, Lang AE, Moro E.. rCBF changes associated with PPN stimulation in a patient with Parkinson’s disease: a PET study. Mov Disord 2008; 23: 1051–4. [DOI] [PubMed] [Google Scholar]
- Strumpf H, Noesselt T, Schoenfeld MA, Voges J, Panther P, Kaufmann J, et al. Deep brain stimulation of the pedunculopontine tegmental nucleus (PPN) influences visual contrast sensitivity in human observers. Plos One 2016; 11: e0155206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa T, Yamamoto T, Katayama Y, Hirayama T, Maejima S, Moriya T.. Deep-brain stimulation in a persistent vegetative state: follow-up results and criteria for selection of candidates. Brain Inj 1990; 4: 315–27. [DOI] [PubMed] [Google Scholar]
- Ulla M, Thobois S, Llorca P-M, Derost P, Lemaire J-J, Chereau-Boudet I, et al. Contact dependent reproducible hypomania induced by deep brain stimulation in Parkinson’s disease: clinical, anatomical and functional imaging study. J Neurol Neurosurg Psychiatry 2011; 82: 607–14. [DOI] [PubMed] [Google Scholar]
- Welter M-L, Schupbach M, Czernecki V, Karachi C, Fernandez-Vidal S, Golmard J-L, et al. Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology 2014; 82: 1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RA, Cole MH, Wong D, Coyne T, Silburn P, Kerr G.. Pedunculopontine nucleus deep brain stimulation produces sustained improvement in primary progressive freezing of gait. J Neurol Neurosurg Psychiatry 2011; 82: 1256–9. [DOI] [PubMed] [Google Scholar]
- Young RF, Bach FW, Van Norman AS, Yaksh TL.. Release of beta-endorphin and methionine-enkephalin into cerebrospinal fluid during deep brain stimulation for chronic pain. Effects of stimulation locus and site of sampling. J Neurosurg 1993; 79: 816–25. [DOI] [PubMed] [Google Scholar]
- Young RF, Tronnier V, Rinaldi PC.. Chronic stimulation of the Kolliker-Fuse nucleus region for relief of intractable pain in humans. J Neurosurg 1992; 76: 979–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.