See the article by Lara-Velazquez et al in this issue pp. 599–610.
The process of stem cell self-renewal is tightly regulated and determines the balance between the generation of differentiated progeny and the maintenance of a population of stem cells. This basic biological process is foundational for tissue development and homeostasis throughout the body. However, alterations in self-renewal also underlie pathogenic processes, including cancer, in which self-renewal is co-opted by a population of therapeutically resistant cancer stem cells. Therefore, understanding how self-renewal is regulated has broad implications, including for the treatment of aggressive cancers such as glioblastoma that are driven by a cancer stem cell population.1 The regulation of self-renewal is complex and occurs through the integration of cell-intrinsic molecular mechanisms (which include core pluripotency transcription factors and their epigenetic accessibility) and cell-extrinsic interactions with the surrounding microenvironment, namely the stem cell niche, via soluble factors and direct cell-cell interactions. While many of the previously reported regulatory mechanisms are conserved between somatic and cancer stem cells across tissue types,2 the brain contains unique anatomical aspects, including cerebrospinal fluid, compared to other organs.
Cerebrospinal fluid is present throughout the brain and spinal cord and has a variety of key functions during development, including ensuring the proper distribution of growth factors for region-specific morphological gradients.3 The cerebrospinal fluid adjacent to the lateral ventricles is also essential for normal neural stem cell maintenance and is considered to be a key part of the neural stem cell niche.4 Given (1) the overlap between neural stem cell and cancer stem cell regulatory mechanisms in glioblastoma2; (2) the hypothesis that more aggressive glioblastoma growth is associated with locations adjacent to the cerebrospinal fluid-filled lateral ventricles, an idea that is supported by observations that glioblastomas closer to the lateral ventricles have a poorer prognosis5,6; and (3) the interest in accessing cerebrospinal fluid to identify biomarkers of disease aggressiveness and progression,7 there is a clear need to identify signaling pathways through which cerebrospinal fluid drives self-renewal. Several observations support an interaction between cerebrospinal fluid and cancer stem cells, including that glioblastomas close to lateral ventricles are enriched in cancer stem cell gene signatures6 and that cerebrospinal fluid from glioblastoma patients stimulates neural stem cell proliferation.3 However, the specific signaling networks underlying these interactions are not clear.
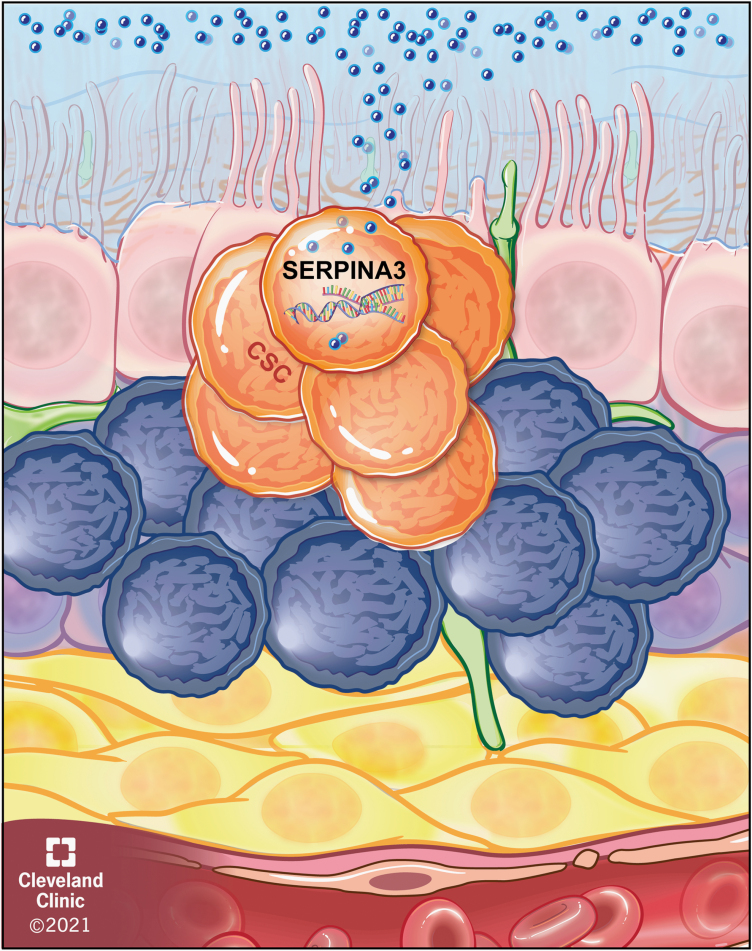
In this issue, Lara-Velazquez et al. reveal a novel signaling network by which cerebrospinal fluid drives cancer stem cell phenotypes via the SERPINA3 gene that encodes the antiprotease alpha 1-antichymotrypsin protein, a member of the serine protease inhibitor family.8 Using patient-derived cancer stem cell models, the authors first observed that cerebrospinal fluid from glioblastoma patients potently induced migration compared to cerebrospinal fluid from donors without cancer. A comparative transcriptional profiling study revealed that SERPINA3 was the top differentially expressed gene in cancer stem cells exposed to cerebrospinal fluid from glioblastoma patients compared to non-cancer control patients. Genetic loss-of-function studies confirmed that the cancer stem cell migration induced by glioblastoma patient cerebrospinal fluid was attenuated by SERPINA3 knockdown. In parallel, genetic gain-of-function studies demonstrated that SERPINA3 drove invasion, which could also be achieved through treatment with recombinant alpha 1-antichymotrypsin protein. Bioinformatics assessments revealed that SERPINA3 was elevated in glioblastoma patients compared to non-cancer patients. Furthermore, SERPINA3 levels increased over glioma grade, and high expression portended poor prognosis. Additional genetic loss-of-function studies revealed a reduction in proliferation, colony formation, cancer stem cell marker expression, and tumor initiation, with a marked decrease in proliferation of SERPINA3 knockdown tumor cells. Mechanistically, SERPINA3 knockdown potently reduced ERK and MEK phosphorylation. Taken together, these findings provide compelling evidence for a signaling network initiated by glioblastoma cerebrospinal fluid exposure that drives cancer stem cell invasion and other phenotypes (Figure 1).
Fig. 1.
SerpinA3 activation in cancer stem cells adjacent to the lateral ventricle. Schematic depicting the activation of SERPINA3 in cancer stem cells adjacent to the lateral ventricle by the cerebrospinal fluid.
These initial findings also raise a series of interesting questions for future studies that have broad implications, ranging from the basic understanding of tumorigenesis to translational applications including the development of next-generation therapeutic approaches to reduce invasion. From these findings, the upstream regulator of SERPINA3 activation and whether there are canonical signaling networks that can be neutralized remain unknown. Comparative proteomics analysis of cerebrospinal fluid and activated receptors may provide insight into this open question. Second, as SERPINA3 is a member of a large family of serine protease inhibitors, the specific downstream proteins altered are also not clear. As some SERPIN family members have been shown to inhibit apoptosis, SERPINA3 could also be a cancer stem cell-specific inhibitor of cell death. This could have potential implications for resistance to radiation and/or temozolomide. Third, while the authors mainly focused on SERPINA3, this family of genes is large (36 human genes), and it is unclear whether SERPINA3 is the only family member that is increased upon cerebrospinal fluid exposure and/or is important for cancer stem cells. Finally, while challenging in terms of specificity, efforts to develop inhibitors to attenuate SERPINA3, either directly or via upstream or downstream mediators, could be a potential glioblastoma therapeutic approach.
More broadly, these findings have multiple implications. This study provides an additional cancer stem cell regulatory mechanism that requires additional experimental interrogation, which could reveal novel molecular mechanisms. Given the localization of cancer stem cells to the cerebrospinal fluid, there may be additional therapeutic delivery opportunities, including CAR-T cells, which have been validated in other brain tumors for cerebrospinal fluid infusion.9 This approach is relevant to recent findings focused on optimizing CAR-T cell efficacy against cancer stem cells in glioblastoma models.10 Taken together, Lara-Velazquez et al. present a timely and interesting set of findings that provides the starting point for additional investigations with the potential to impart much needed insight into glioblastoma and uncover additional therapeutic opportunities.
Acknowledgments
The author thanks Amanda Mendelsohn and the Center for Medical Art and Photography at the Cleveland Clinic for providing the illustration.
Conflict of interest statement. The author declares no conflicts of interest.
Authorship statement. The text is the sole product of the author, and no third party had input or gave support to its writing.
References
- 1. Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 3. Lehtinen MK, Zappaterra MW, Chen X, et al. . The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69(5):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70(4):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berendsen S, van Bodegraven E, Seute T, et al. . Adverse prognosis of glioblastoma contacting the subventricular zone: biological correlates. PLoS One. 2019;14(10):e0222717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steed TC, Treiber JM, Taha B, et al. . Glioblastomas located in proximity to the subventricular zone (SVZ) exhibited enrichment of gene expression profiles associated with the cancer stem cell state. J Neurooncol. 2020;148(3):455–462. [DOI] [PubMed] [Google Scholar]
- 7. Miller AM, Shah RH, Pentsova EI, et al. . Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lara-Velazquez M, Zarco N, Carrano A, et al. . Alpha 1-antichymotrypsin contributes to stem cell characteristics and enhances tumorigenicity of glioblastoma. Neuro Oncol. 2021;23(4):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donovan LK, Delaidelli A, Joseph SK, et al. . Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med. 2020;26(5):720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Prager BC, Gimple RC, et al. . CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies[published online ahead of print December 16,2020]. Cancer Discov. doi: 10.1158/2159-8290.CD-20-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]