Abstract
Background
Radiotherapy may synergize with programmed cell death 1 (PD1)/PD1 ligand (PD-L1) blockade. The purpose of this study was to determine the recommended phase II dose, safety/tolerability, and preliminary efficacy of combining pembrolizumab, an anti-PD1 monoclonal antibody, with hypofractionated stereotactic irradiation (HFSRT) and bevacizumab in patients with recurrent high-grade gliomas (HGGs).
Methods
Eligible subjects with recurrent glioblastoma or anaplastic astrocytoma were treated with pembrolizumab (100 or 200 mg based on dose level Q3W) concurrently with HFSRT (30 Gy in 5 fractions) and bevacizumab 10 mg/kg Q2W.
Results
Thirty-two patients were enrolled (bevacizumab-naïve, n = 24; bevacizumab-resistant, n = 8). The most common treatment-related adverse events (TRAEs) were proteinuria (40.6%), fatigue (25%), increased alanine aminotransferase (25%), and hypertension (25%). TRAEs leading to discontinuation occurred in 1 patient who experienced a grade 3 elevation of aspartate aminotransferase. In the bevacizumab-naïve cohort, 20 patients (83%) had a complete response or partial response. The median overall survival (OS) and progression-free survival (PFS) were 13.45 months (95% CI: 9.46–18.46) and 7.92 months (95% CI: 6.31–12.45), respectively. In the bevacizumab-resistant cohort, PR was achieved in 5 patients (62%). Median OS was 9.3 months (95% CI: 8.97–18.86) with a median PFS of 6.54 months (95% CI: 5.95–18.86). The majority of patients (n = 20/26; 77%) had tumor-cell/tumor-microenvironment PD-L1 expression <1%.
Conclusions
The combination of HFSRT with pembrolizumab and bevacizumab in patients with recurrent HGG is generally safe and well tolerated. These findings merit further investigation of HFSRT with immunotherapy in HGGs.
Keywords: bevacizumab, hypofractionated stereotactic re-irradiation, PD-L1, pembrolizumab, recurrent high-grade glioma
Key Points.
Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade glioma is generally safe and well tolerated.
The median overall survival was 13.45 months in patients with bevacizumab-naïve tumors.
Importance of the Study
Preclinical models suggest that the combination of anti-PD1 or anti–PD-L1 blockade with radiotherapy, especially with moderate hypofractionated radiotherapy dose, may amplify tumor-specific immune responses to cell death and tumor antigen release leading to improved survival in HGGs. In this first reported trial of anti-PD1 blockade combination with HFSRT and vascular endothelial growth factor in recurrent HGGs, pembrolizumab with HFSRT and bevacizumab were very well tolerated with no unexpected treatment related toxicities. Exploratory antitumor activity is encouraging, though our sample size is small and population somewhat heterogeneous. In bevacizumab-naïve patients, median OS was 13.45 months and ~58% of subjects were alive 12 months after starting study treatment. Further research into the strategies to enhance this effect by identifying optimal combination regimens and radiation dose/fractionation/treatment volume may be warranted.
Glioblastoma (GBM) remains one of the most fatal tumors, with poor prognosis and a 5- year survival rate of ~5‒10%.1 Treatment options for patients with recurrent high-grade gliomas (HGGs) such as GBM are limited, with no regimen demonstrating significant improvement in survival. New treatment strategies are therefore needed.
Antibodies targeting immune checkpoints have shown limited activity in patients with recurrent GBM. The phase III CheckMate 143 trial (NCT02017717) compared the efficacy of the anti–programmed cell death 1 (PD1) monoclonal antibody nivolumab versus bevacizumab in patients with GBM at first recurrence after temozolomide chemoradiotherapy. This study failed to show a survival benefit for nivolumab in the overall patient population with a first recurrence of GBM.2
Preclinical investigation has demonstrated that moderate hypofractionated radiotherapy acts synergistically with immunotherapy to enhance the immune response against tumor cells.3–5 In a study by Zeng et al, combination of anti-PD1 antibody with stereotactic radiosurgery (10 Gy) in a mouse orthotopic GBM model generated robust and durable responses and doubled the survival compared with either modality alone.5 In this study, an analysis of the brain and spinal cord of animals treated with combination therapy showed an increase in the ratio of cytotoxic to regulatory T cells (Tregs), with an increased tumor infiltration by cytotoxic T cells (CD8+/interferon-γ+/tumor necrosis factor-α+). However, the optimal human dose fractionation of radiotherapy in combination with immunotherapy in humans is not yet determined. Preclinical experiments have demonstrated the highest tumor-specific T-cell response, lowest Tregs, and best tumor control with the use of a higher dose per fraction.6–8
Moreover, preclinical and clinical evidence indicate that abnormal tumor vasculature, partly caused by pro-angiogenic factors such as vascular endothelial growth factor (VEGF), creates an immunosuppressive tumor microenvironment by increasing immunosuppressive cells (tumor associated macrophages and Tregs) and decreasing antitumor lymphocytes.9,10 Circulating VEGF exerts a systemic influence on the host immune system by affecting proliferation, differentiation, and function of immune cells.9
Elevated levels of VEGF have been associated with inhibition of T-cell immune response by suppressing the maturation of dendritic cell precursors and by enhancing the proliferation and peripheral blood proportion of Tregs.11 The benefit of combined anti-VEGF agents with immunotherapy has been studied in several cancer models and has been approved for treatment of renal cell carcinoma.12–17 In murine models, combination therapy has resulted in increased tumoral infiltration of CD4+ and CD8+ T cells, decreased Tregs, significant decrease in negative co-stimulatory molecules PD1 and cytotoxic T-lymphocyte antigen 4, and increased tumor growth delay.12–16
Safety and efficacy of concomitant administration of bevacizumab with hypofractionated stereotactic radiotherapy (HFSRT) in previously irradiated gliomas has been investigated in a prospective study carried out by Gutin et al, where 25 patients with recurrent HGGs were treated with HFSRT (30 Gy; 6 Gy delivered in 5 fractions) combined with bevacizumab (10 mg/kg) every 2 weeks of 28-day cycles. The combination therapy was safe and well tolerated without any cases of radiation necrosis. The reported clinical outcome was more favorable than other trials using bevacizumab in recurrent HGGs. For the GBM cohort, overall response rate was 50% with median survival of 12.5 months.18
Here we report the results of a phase I clinical trial of an immune checkpoint inhibitor combined with HFSRT and anti-VEGF blockade in patients with recurrent HGGs. This study evaluated the safety and tolerability of pembrolizumab, a fully human immunoglobulin G subclass 4 monoclonal antibody inhibitor of PD1, in combination with HFSRT and bevacizumab in patients with recurrent GBM or anaplastic astrocytoma. Selected efficacy outcomes were also assessed in an exploratory analysis.
Patients and Methods
This single-arm, open-label phase I study was conducted at the Moffitt Cancer Center between 2015 and 2019. It was registered in ClinicalTrials.gov (NCT02313272). The protocol and its amendments were reviewed and approved by the institutional review board. Written informed consent was provided by all patients.
Eligibility Criteria
Patients were eligible if they were ≥18 years of age and had a recurrent World Health Organization (WHO) grade III (except anaplastic oligodendroglioma) or grade IV gliomas; maximum diameter of enhancing tumor (target lesion) ≤3.5 cm; previous first-line treatment with at least standard dose of radiotherapy (total dose ≥54 Gy) and temozolomide; an interval of at least 6 months after the end of prior radiation therapy unless there was a new recurrence outside of the previous radiotherapy treatment field; Karnofsky performance status (KPS) score ≥70%; and adequate pulmonary, liver, kidney, and bone marrow function. Patients were excluded if they had more than 3 recurrences of HGG; received re-irradiation to recurrent disease (in addition to standard frontline definitive radiation therapy); had tumor recurrence within 5 mm of the brainstem and/or the optic chiasm; had evidence of infratentorial or leptomeningeal disease; active, known, or suspected autoimmune disease; had history of gastrointestinal bleeding or any other hemorrhage/bleeding adverse event of grade ≥3 (Common Terminology Criteria for Adverse Events [CTCAE] v4) within 30 days prior to trial enrollment; or required chronic supraphysiologic doses of corticosteroids.
Treatment
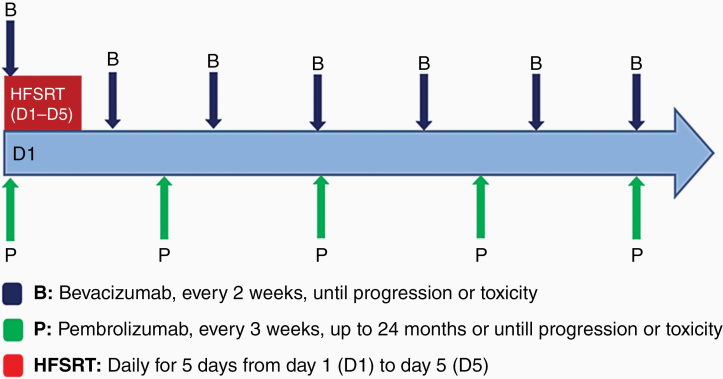
Study treatment was started with 5 days of HFSRT at the beginning of cycle 1 (Fig. 1). The first doses of bevacizumab (10 mg/kg i.v.) and pembrolizumab were administered on cycle 1 day 1 (C1 D1). Bevacizumab 10 mg/kg i.v. was continued every 2 weeks. Two dose levels of pembrolizumab (100 mg and 200 mg i.v. Q3W) were explored. The first 3 patients were treated with pembrolizumab at 100 mg i.v. Q3W dose. The subsequent patients were treated with pembrolizumab at 200 mg i.v. Q3W dose. Patients continued pembrolizumab and bevacizumab until confirmed disease progression, intolerable toxicity, or withdrawal of consent. Dose reductions were not permitted. Both pembrolizumab and bevacizumab could be held for toxicity and restarted when toxicity resolved.
Fig. 1.
Study treatment.
Radiation Technique
Patients underwent CT simulation with noncontrast 1.5 mm CT slice thickness after being immobilized with a BrainLAB non-invasive thermoplastic mask. Volumetric MRI of the brain with 1 mm slices was performed within 1 week of CT simulation for treatment planning. CT images were fused with T1 postcontrast brain MRI. Gross tumor volume (GTV) was defined as the enhancing tumor on T1 postcontrast imaging. For the patients who underwent repeat resection for recurrence, the new resection cavity was also included in the GTV. The GTV was expanded by 3–5 mm margin to create the planning target volume (PTV). All patients had a PTV prescription of 30 Gy in 5 daily fractions. Simultaneous integrated boost technique was used so that the GTV received 35 Gy in 5 fractions while allowing up to 1 cm3 volume of GTV receiving 40 Gy in 5 fractions. Treatment planning was performed with either the BrainLAB system or Pinnacle treatment planning system. A single isocenter plan was used for each patient, and an intensity modulated radiation therapy technique was utilized. The plan was normalized so that 100% of the prescription dose covers 95% of the PTV. Daily image guidance prior to each fraction was performed with cone-beam CT scans. HFSRT started on C1 D1 and was delivered over 5 days. The median GTV was 9.9 cm3 (range, 0.51–39.2 cm3). The mean PTV was 55.07 cm3 (range, 6.87–123.53 cm3).
Safety and Efficacy Assessments
Treatment-related adverse events (TRAEs) were assessed continuously and graded according to the National Cancer Institute CTCAE v 4.0.
Tumor assessments were performed at baseline, and then every 6 weeks (±7 days) thereafter or as clinically indicated. Tumor progression was assessed using contrast-enhanced MRI and according to Response Assessment in Neuro-Oncology (RANO) criteria.19 RANO criteria was chosen as the Immunotherapy RANO (iRANO) criteria do not provide clear guidance with combination of immunotherapy and anti-angiogenic agents where fluid attenuated inversion recovery (FLAIR) changes are of more significance.20 However, to decrease the likelihood of prematurely discontinuing potentially effective therapy, subjects with suspected radiologic disease progression were permitted to receive study treatment until progression was confirmed by MRI performed approximately 8 weeks after the initial radiological assessment of progression. If the follow-up MRI confirmed the progression, the date of initial determination was recorded as the date of tumor progression.
Tumor Sample Analyses
For patients with available tumor samples, PD-L1 tumor expression was determined retrospectively by using immunohistochemistry. Immunohistochemistry for PD-L1 was conducted using Dako clone 22C3 following 20 minutes of antigen retrieval at low pH, and peroxidase development of diaminobenzidine chromogen. Slides were counterstained with hematoxylin, and tumor regions were verified against hematoxylin and eosin (H&E)–stained sections. Immunopositive cells were morphologically assigned to either neoplastic or microenvironment compartments. PD-L1 positivity was defined as membranous staining in 1% or more of tumor cells. In patients who underwent surgical resection for recurrent disease, tumor PD-L1 expression was evaluated on both archival tissue from initial diagnosis and samples at the time of progression.
Moreover, data on tumor O6-methylguanine DNA methyltransferase (MGMT) promoter methylation and isocitrate dehydrogenase (IDH) mutation status were collected in all patients. Molecular testing for IDH1/2 mutations was performed either by polymerase chain reaction or pyrosequencing assay.
Tumor mutation burden (TMB) and microsatellite status were determined through commercial FoundationOne or FoundationOne CDx tumor testing when possible.
Outcomes and Statistical Analyses
The primary objective was to determine the safety/tolerability and the recommended phase II dose of pembrolizumab given in combination with HFSRT and bevacizumab in patients with recurrent HGGs. This objective was analyzed based on reported TRAEs which were graded according to CTCAE v4.0. A secondary objective was to assess the objective response rate (ORR) per RANO criteria. PFS and OS were analyzed as ad hoc exploratory objectives. PFS was defined as the time from the date of treatment initiation to the date of documented progression or death, whichever occurred first. OS was defined as the time from the date of treatment initiation to date of death from any cause. Survival follow-ups were performed every 3 months. PFS and OS were analyzed by Kaplan–Meier estimates and reported with 2-sided 95% CIs.
Results
Patient Characteristics and Treatment
Between 2015 and 2019, a total of 32 patients received study treatment. The first 3 patients received pembrolizumab at 100 mg and the following 29 patients were treated with pembrolizumab at 200 mg. Twenty-four patients had no prior exposure to anti-VEGF treatment. Eight patients had prior tumor progression on bevacizumab. One patient in the bevacizumab-naïve cohort had prior disease progression on nivolumab. Patient baseline demographic and disease characteristics are listed in Table 1.
Table 1.
Patient demographics and clinical characteristics
| Characteristics | BEV Naïve (n = 24) | BEV Resistant (n = 8) |
|---|---|---|
| Age, y | ||
| Median (range) | 55.5 (22–68) | 49.5 (27–61) |
| Sex, n (%) | ||
| Male | 14 (58) | 7 (88) |
| Female | 10 (42) | 1 (12) |
| Histopathologic Diagnosis, n (%) | ||
| Glioblastoma | 22 (92) | 7 (88) |
| Anaplastic astrocytoma | 2 (8) | 1 (12) |
| KPS, n (%) | ||
| 90 | 6 (25) | 0 |
| 80 | 13 (54) | 2 (25) |
| 70 | 5 (21) | 6 (75) |
| MGMT promoter methylation status, n (%) | ||
| Methylated | 12 (50) | 2 (25) |
| Unmethylated | 11 (46) | 2 (25) |
| Unknown | 1 (4) | 4 (50) |
| IDH mutation status, n (%) | ||
| Mutant | 5 (21) | 2 (25) |
| Wildtype | 19 (79) | 3 (37.5) |
| Unknown | 0 | 3 (37.5) |
| Resection prior to study treatment, n (%) | ||
| Yes | 12 (50) | 2 (25) |
| No | 12 (50) | 6 (75) |
| Tumor (enhancing) volume (cm3) | ||
| Median (range) | 5.51 (0.51–39.2) | 15.1 (6.81–27.3) |
| Recurrence(s), n (%) | ||
| 1st | 18 (75) | 0 |
| 2nd | 6 (25) | 7 (88) |
| 3rd | 0 | 1 (12) |
| Steroid use at day 1 of treatment, n (%) | ||
| Yes | 5 (21) | 2 (25) |
| No | 19 (79) | 6 (75) |
| PD-L1 expression levels | ||
| <1% | 15 | 5 |
| ≥1% | 6 | 0 |
| ≥10% | 1 | 0 |
| Unknown | 3 | 3 |
All patients have discontinued the study treatment. The reasons for discontinuation of study treatment included disease progression (n = 21, 65.6%), patient preference (n = 5, 15.6%), a medical condition unrelated to study treatment (n = 5, 15.6%), and treatment-related toxicity (n = 1, 3.2%). The median durations of study treatment for bevacizumab-naïve patients (n = 24) and bevacizumab-resistant patients (n = 8) were 5.7 months (range, 1.4–17.5) and 4.9 months (range, 1.8–7.8), respectively.
At the time of analyses, the median durations of follow-up for bevacizumab-naïve patients (n = 24) and bevacizumab-resistant patients (n = 8) were 3.7 months (range, 1.0–12.9) and 3.1 months (range, 1.2–31.3), respectively.
Safety
Of the 6 patients treated during the dose-finding phase (3 at pembrolizumab 100 mg and 3 at pembrolizumab 200 mg), no dose-limiting toxicities were observed during the 6-week observation period. Therefore, 26 additional patients were treated as a dose expansion cohort with pembrolizumab at 200 mg. The most common TRAEs of all grade were proteinuria, increased alanine aminotransferase, fatigue, and hypertension (Table 2). Grade 3 events were reported in 12 (34.4%) patients, with the most common being hypertension and thromboembolic events. No grade 4/5 TRAEs were reported. No unexpected toxicity or treatment-related neurologic adverse event of clinical significance was observed. One patient discontinued study treatment due to an asymptomatic grade 3 elevation of aspartate aminotransferase.
Table 2.
Treatment-related adverse events
| Treatment-Related Adverse Events | Any Grade, n (%) | Grades 3/4, n (%) |
|---|---|---|
| Proteinuria | 13 (40.6) | 0 |
| Alanine aminotransferase increased | 8 (25) | 0 |
| Fatigue | 8 (25) | 0 |
| Hypertension | 8 (25) | 5 (15.6) |
| Aspartate aminotransferase increased | 6 (18.8) | 1 (3.1) |
| Hypothyroidism | 6 (18.8) | 0 |
| Thromboembolic event | 4 (12.5) | 3 (9.4) |
| Anorexia | 3 (9.4) | 0 |
| Arthralgia | 3 (9.4) | 0 |
| Chills | 2 (6.3) | 0 |
| Confusion | 2 (6.3) | 1 (3.1) |
| Hyperthyroidism | 2 (6.3) | 0 |
| Lymphocyte count decreased | 2 (6.3) | 0 |
| Blood bilirubin increased | 1 (3.1) | 0 |
| Dysgeusia | 1 (3.1) | 0 |
| Dysphagia | 1 (3.1) | 0 |
| Dyspnea | 1 (3.1) | 0 |
| Edema limbs | 1 (3.1) | 0 |
| Blurry vision | 1 (3.1) | 0 |
| Gait disturbance | 1 (3.1) | 0 |
| Headache | 1 (3.1) | 0 |
| Hyperkalemia | 1 (3.1) | 0 |
| 4th cranial nerve palsy | 1 (3.1) | 0 |
| Myalgia | 1 (3.1) | 0 |
| Nausea | 1 (3.1) | 0 |
| Pancreatitis | 1 (3.1) | 1 (3.1) |
| Papulopustular rash | 1 (3.1) | 0 |
| Psychiatric disorders | 1 (3.1) | 1 (3.1) |
| Rash acneiform | 1 (3.1) | 0 |
| Rash maculopapular | 1 (3.1) | 0 |
| Cough | 1 (3.1) | 0 |
| Seizure | 1 (3.1) | 0 |
| Wound complication | 1 (3.1) | 0 |
| Total | 87 | 12 |
There was no evidence of symptomatic radiation necrosis following re-irradiation.
In the bevacizumab-naïve cohort, 8 patients were receiving corticosteroids at the time of screening. After start of study treatment, 6 patients were able to discontinue corticosteroids with no neurologic adverse event. In the bevacizumab failure cohort, 2 out of 8 patients were receiving corticosteroid at the time of trial entry, which was discontinued after start of study treatment. Two patients required systemic corticosteroids due to management of immune toxicity. Due to the small number of patients requiring corticosteroids during therapy, assessing the impact of corticosteroid use on response to therapy was not feasible.
Exploratory Efficacy
The objective response rates in the bevacizumab-naïve and bevacizumab-resistant cohorts were 83% (95% CI: 63‒95) and 62.5% (95% CI: 24.5‒91.5), respectively. Disease control rates, defined by complete response + partial response + stable disease, were 100% (95% CI: 85.8–100) and 75% (95% CI: 34.9–96.8), in the bevacizumab-naïve and -resistant groups, respectively (Table 3). The median durations of response (range) were 8.44 (1.4–21.6) and 5.83 (2.9–18.3) months in the bevacizumab-naïve and bevacizumab-resistant cohorts, respectively.
Table 3.
Best overall response and disease control rate
| Response | BEV Naïve, n = 24 | BEV Resistant, n = 8 |
|---|---|---|
| Best Overall Response, n (%) | ||
| Complete response | 2 (8.3) | 0 |
| Partial response | 18 (75) | 5 (62.5) |
| Stable disease | 4 (16.7) | 1 (12.5) |
| ≥12 wk | 4 | 1 |
| ≥24 wk | 3 | 0 |
| Progressive disease | 0 | 2 (25) |
| Disease Control Rate | ||
| Disease control rate, n (%) | 24 (100) | 6 (75) |
| 95% CI | 85.8–100.0 | 34.9–96.8 |
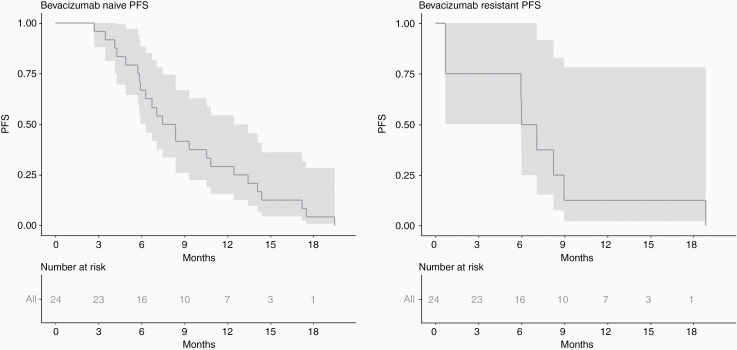
The median PFS among bevacizumab-naïve and bevacizumab-resistant patients was 7.92 months (95% CI: 6.31–12.45) and 6.54 months (95% CI: 5.95–18.86), respectively (Fig. 2).
Fig. 2.
Kaplan–Meier estimates of PFS for patients with bevacizumab-naïve (A) and bevacizumab-resistant (B) tumors.
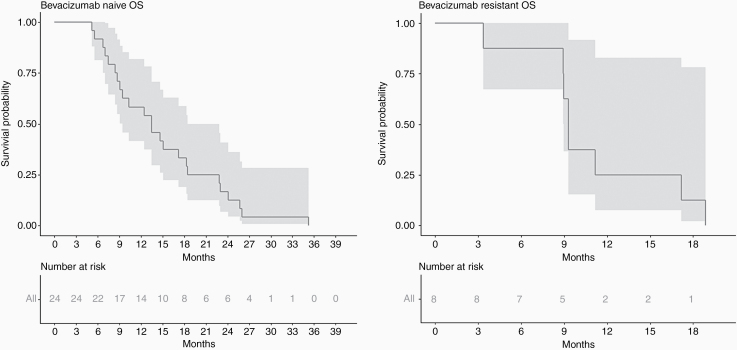
Median OS was 13.45 months (95% CI: 9.46–18.46), and 9.3 months (95% CI: 8.97‒18.86) in bevacizumab-naïve and bevacizumab-resistant patients, respectively (Fig. 3). In bevacizumab-naïve cohort, 6 months and 12 months PFS were 66.7% (95% CI: 44.3–81.7%) and 29.2% (95% CI: 13.0–47.6%). Six months OS was 91.7% (95% CI: 70.6–97.8%), 12 months OS was 58.3% (95% CI: 36.4–75.0%), and 24 months OS was 16.7% (95% CI: 5.2–33.7%).
Fig. 3.
Kaplan–Meier estimates of OS for patients with bevacizumab-naïve (A) and bevacizumab-resistant (B) tumors.
In bevacizumab-resistant cohort, 6 and 12 months OS were 87.5% (95% CI: 38.7– 98.1%) and 25.0% (95% CI: 3.7–55.8%).
Analysis of Tumor Biospecimens
PD-L1 expression level of ≥1% (either by the tumor or tumor microenvironment) was detected in samples of 6 out of 26 patients (23%) with available tissue for analysis. PD-L1 expression level of ≥10% was observed in the recurrent tumor sample of only 1 patient who had PD-L1 expression of 1% at initial diagnosis. This low frequency prohibited any meaningful assessment of response by PD-L1 expression in this study. TMB data were available in 16 patients. Only 1 patient’s tumor had high TMB with 118 mutations per megabase. This analysis was performed on tumor resected after progression on temozolomide. It is not known if the tumor was hypermutated at the time of diagnosis or increased TMB was secondary to treatment with temozolomide and radiotherapy. This patient who was treated in bevacizumab-resistant cohort experienced early disease progression at first imaging obtained after 3 weeks of treatment.
Discussion
Treatment for recurrent GBM and HGGs remains very challenging and the addition of novel therapies to traditional approaches (eg, re-resection, re-irradiation, and chemotherapy) has been disappointing so far. For example, the OS of nivolumab monotherapy at recurrence was only 9.8 months in patients with recurrent GBM. Here we report a small phase I trial in recurrent HGG patients where the survivals (PFS and OS) and toxicity profiles are encouraging using triple therapy with HFSRT, pembrolizumab, and bevacizumab in this setting.
Overall this approach was surprisingly well tolerated. With respect to safety, the primary endpoint, the combination of pembrolizumab (200 mg i.v. Q3W) with HFSRT (30 Gy in 5 fractions) and bevacizumab (10 mg/kg i.v. Q2W) proved safe in the treatment of patients with recurrent HGG. The overall TRAE profiles are similar to those previously reported with pembrolizumab and bevacizumab. No unexpected toxicity or treatment-related neurologic adverse event of clinical significance was observed. We wondered if perhaps bevacizumab may have blunted some of the side effects of re-irradiation, though we cannot be sure in this small number of patients.
The antitumor activity of this combination treatment is encouraging, though our sample size is small and population somewhat heterogeneous. In our patients with bevacizumab-naïve HGG, the overall response rate was 83% with median OS of 13.45 months (95% CI: 9.46–18.46). Response rate and median OS in patients with bevacizumab-naïve GBM receiving PD1 pathway inhibitor monotherapy, nivolumab, has been around 7.8% and 9.8 months (95% CI, 8.2–11.8), respectively.2 A phase II trial investigating 2 cohorts of bevacizumab-naïve recurrent GBM patients receiving either pembrolizumab + bevacizumab (49 patients) or pembrolizumab monotherapy (30 patients) resulted in median OS of 8.78 month (95% CI: 7.69–14.17) and 10.26 (95% CI: 8.45–12.46), respectively.21 Another study, by Gutin et al, which investigated HFSRT given in 30 Gy in 5 factions with bevacizumab in 20 patients with recurrent GBMs and 5 patients with anaplastic gliomas, resulted in an ORR of 52% (95% CI: 28–89%). The median OS was 12.5 months (95% CI: 6.9–22.8 mo) for patients with recurrent GBM.18 Radiation Therapy Oncology Group (RTOG) 1205 is a phase II trial evaluating HFSRT with concurrent bevacizumab versus bevacizumab alone in patients with bevacizumab-naïve recurrent GBM. The final result has not been published, but the authors have reported median OS of 10.1 months for patients who received HFSRT in addition to bevacizumab, which was not significantly different than patients who received bevacizumab monotherapy. It is worth mentioning that the hypofractionated regimen used in RTOG 1205 is 35 Gy given in 10 fractions.22
Preclinical data supporting combining hypofractionated radiation and immunotherapy for malignant glioma are promising. In a murine melanoma model, moderate hypofractionated dose of 7.5 Gy per fraction lead to best tumor control and immunity and lower Tregs.7 Formenti et al have shown that moderate ablative radiation dose such as 8 Gy per fraction × 3 fractions elicits abscopal effects better than high-dose single fraction of 20 Gy when combining with checkpoint inhibitors.23 Several completed and ongoing phase I and II studies for various tumor histologies are investigating efficacy and treatment sequence of this therapeutic approach.24,25 In our trial, we employed moderate ablative regimen of 6 Gy × 5 fractions that is in the range of moderate hypofractionated regimen thought to be immunogenic and synergistic with checkpoint inhibitors. This regimen has also been shown to be safe and tolerable for re-irradiation in patients with recurrent glioblastoma.18
Whether the improved efficacy in our trial is due to patient selection, small numbers of patients, small tumor volume, or improved therapeutic ratio remains to be determined. Our second trial which combines HFSRT (30 Gy in 5 fractions) with bevacizumab, ipilimumab, and nivolumab in bevacizumab-naïve patients with HGG (NCT02829931) is ongoing and will provide more information on efficacy of immunotherapy regimens administered with HFSRT.
Although initial reports suggested high expression of PD-L1 in glioblastoma samples, PD-L1 expression level of ≥1% (either by the tumor or tumor microenvironment) was detected in only 23% of our patients. This is consistent with data from the CheckMate-143 trial.2
Recently, a small trial of 35 patients with recurrent GBM who were randomized to receive pembrolizumab prior to surgical resection versus postsurgical resection suggested that neoadjuvant blockade of PD1 enhances both systemic and local antitumor immune response, which may result in improved OS.26 While these results are provocative and need to be validated in a larger study, the most efficacious combination and sequence of surgical resection, HFSRT, anti-PD1/PD-L1 blockade, and anti-VEGF therapy is not yet fully determined.
There are several limitations in our study. First, while we proposed the use of bevacizumab to enhance immunological responses on the basis of preclinical models in gliomas, we unfortunately were unable to obtain tissue from tumors or serum biomarkers in order to test this hypothesis. Importantly, we did not find any immune-mediated effects mimicking radiographic progression, which have been observed in other studies of checkpoint inhibitors in GBM,20,27 suggesting that clinically apparent inflammatory reactions did not occur but does not address cellular immunologic responses. Interestingly, most patients did not require corticosteroid treatment during study treatment, and patients who were receiving steroids prior to study treatment were able to taper and remain off steroid during treatment. We attribute these findings to use of bevacizumab in this regimen.
Other limitations in our study are related to its phase I nature, which necessarily treats a small number of patients and the correspondingly large confidence intervals of trial endpoints, making definitive conclusions regarding efficacy premature. Our trial included patients with enhancing tumors up to 3.5 cm in maximal diameter, resulting in the median enhancing tumor size of 9.9 cm3. A number of patients underwent surgical resection for cytoreduction prior to study entry. The role of resection prior to combination radioimmunotherapy and bevacizumab could not be evaluated in this small trial. Finally, the patient population is heterogeneous, some were heavily pretreated; the biology of the heavily treated tumors may differ from that of the patients who had first recurrence following initial chemoradiotherapy.
In conclusion, combination of HFSRT (30 Gy; 6 Gy delivered in 5 fractions) with pembrolizumab (200 mg i.v. Q3W) and bevacizumab (10 mg/kg i.v. Q2W) is safe and well tolerated in patients with recurrent HGGs (including GBMs). The result of this phase I trial suggests that efficacy of HFSRT with bevacizumab might be enhanced by the addition of pembrolizumab. Further research into the strategies to enhance this effect by identifying optimal combination regimens, treatment sequence, and radiation dose/fractionation may be warranted.
Acknowledgments
We thank all patients and their families, as well Merck for supporting this investigator initiated trial. The authors would like to acknowledge funding from Moffitt Foundation which made the tissue analyses possible.
Funding
This investigator initiated trial was supported by funding from Merck and Moffitt Foundation.
Conflict of interest statement.
Solmaz Sahebjam: Merck, Bristol Myers Squibb, Eli Lilly, Brooklyn ImmunoTherapeutics, Boehringer-Ingelheim, AbbVie, Cortice Biosciences.
Peter A. Forsyth: AbbVie, Boehringer-Ingelheim, Novellus, Tocagen, Ziopharm, Pfizer, Celgene.
Christine M. Walko: Intermountain Healthcare, Jackson Genetic Labs.
Michael Jaglal: Novartis.
Sepideh Mokhtari: KITE, Novocure.
Sungjune Kim: Bristol-Myers Squibb, AstraZeneca.
Hsiang-Hsuan Michael Yu: BrainLab, UpToDate, Novocure.
Nam D. Tran, John Arrington, Robert Macaulay, Arnold B. Etame, Theresa Boyle, Edwin N. Peguero, Heiko Enderling, Natarajan Raghunand, Tyra Gatewood, Wendy Long, Jennifer L. Dzierzeski, Brittany Evernden, Timothy Robinson, Melissa C. Wicklund, Zachary J. Thompson, Dung-Tsa Chen, Prakash Chinnaiyan: No conflict to disclose.
Authorship statement.
Design: S.S., S.K., P.C.
Implementation: S.S., P.A.F., N.D.T., J.A.A., R.M., A.B.E., C.M.W., E.N.P., M.J, S.M.,
T.G., W.L., J.L.D., B.E., T.R., M.C.W, H.H.M.Y.
Analysis of the data: S.S., C.M.W., T.B., H.E., N.R., Z.J.T., D-T.C.
Interpretation of the data: S.S., R.M., C.M.W., P.C., H-H.M.Y.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kearl TJ, Jing W, Gershan JA, Johnson BD. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013;190(11):5620–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poleszczuk J, Enderling H. The optimal radiation dose to induce robust systemic anti-tumor immunity. Int J Mol Sci. 2018;19(11):3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tartour E, Pere H, Maillere B, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30(1):83–95. [DOI] [PubMed] [Google Scholar]
- 11. Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. [DOI] [PubMed] [Google Scholar]
- 12. Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5(10):2963–2970. [PubMed] [Google Scholar]
- 13. Manning EA, Ullman JG, Leatherman JM, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13(13):3951–3959. [DOI] [PubMed] [Google Scholar]
- 14. Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12(22):6808–6816. [DOI] [PubMed] [Google Scholar]
- 16. Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators . Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. [DOI] [PubMed] [Google Scholar]
- 18. Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 20. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reardon DA, Nayak L, Peters KB, et al. Phase II study of pembrolizumab or pembrolizumab plus bevacizumab for recurrent glioblastoma (rGBM) patients. J Clin Oncol. 2018;36(15_suppl):2006–2006.29763342 [Google Scholar]
- 22. Tsien C, Pugh S, Dicker AP, et al. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRGOncology/RTOG 1205): initial outcomes and RT plan quality report. Int J Radiat Oncol Biol Phys. 2019;105(1):S78. [Google Scholar]
- 23. Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]