Abstract
The family of B-cell lymphoma-2 (Bcl-2) proteins exerts key functions in cellular health. Bcl-2 primarily acts in mitochondria where it controls the initiation of apoptosis. However, during the last decades, it has become clear that this family of proteins is also involved in controlling Ca2+ signaling in cells, a critical process for the function of most cell types, including neurons. Several anti- and pro-apoptotic Bcl-2 family members are expressed in neurons and impact neuronal function. Importantly, expression levels of neuronal Bcl-2 proteins are affected by age. In this review, we focus on the emerging roles of Bcl-2 proteins in neuronal cells. Specifically, we discuss how their dysregulation contributes to the onset, development, and progression of neurodegeneration in the context of Alzheimer’s disease (AD). Aberrant Ca2+ signaling plays an important role in the pathogenesis of AD, and we propose that dysregulation of the Bcl-2-Ca2+ signaling axis may contribute to the progression of AD and that herein, Bcl-2 may constitute a potential therapeutic target for the treatment of AD.
Keywords: Bcl-2, calcium, neurons, mitochondria, apoptosis, Alzheimer disease
1. Neuronal Ca2+ signaling: an overview
In this section we provide a brief overview of neuronal Ca2+ signaling systems relevant for this review; for a more extensive description a recent review by Bootman and Bultynck is highly recommended [1]. Ca2+ is the most important second messenger in neurons and converts incoming signals into activation of effector enzymes that regulate key aspects of neuronal function. The main conductors of Ca2+ in neurons include voltage-gated Ca2+ channels (VGCC), ligand-operated ion channels and store-operated calcium entry channels (SOCE) (Fig 1). SOCE channels are activated by depletion of the endoplasmic reticulum (ER), the main intracellular Ca2+ store, which is detected by STIM1/2, both intraluminal Ca2+ sensor proteins (Fig 1). Metabotropic glutamate receptors (mGluR) mobilize Ca2+ from the ER into the cytoplasm by activating inositol 1,4,5-trisphosphate receptors (IP3Rs), the major ER-localized Ca2+ release channels (Fig 1). Ca2+ can also be released from the ER via ryanodine receptors (RyRs), which are activated by Ca2+ itself via Ca2+-induced Ca2+ release, allowing to amplify cytosolic Ca2+ signals. Besides the ER, other intracellular Ca2+ stores include the Golgi apparatus, the nuclear envelope and lysosomes. A low concentration of Ca2+ in the cytoplasm of neurons is maintained due to the presence of these intracellular stores in which Ca2+ is sequestered via activity of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) [2]. Mitochondria and peroxisomes are involved in Ca2+ signaling as well but are not considered to be constitutive Ca2+ stores. To control Ca2+ levels, neurons utilize different mechanisms including Na+/Ca2+ exchanger (NCE), plasma membrane Ca2+ pumps and Ca2+- buffering proteins like calbindin–D28, calretinin and parvalbumin in the cytosol and calreticulin and calnexin in the ER [3] (Fig 1).
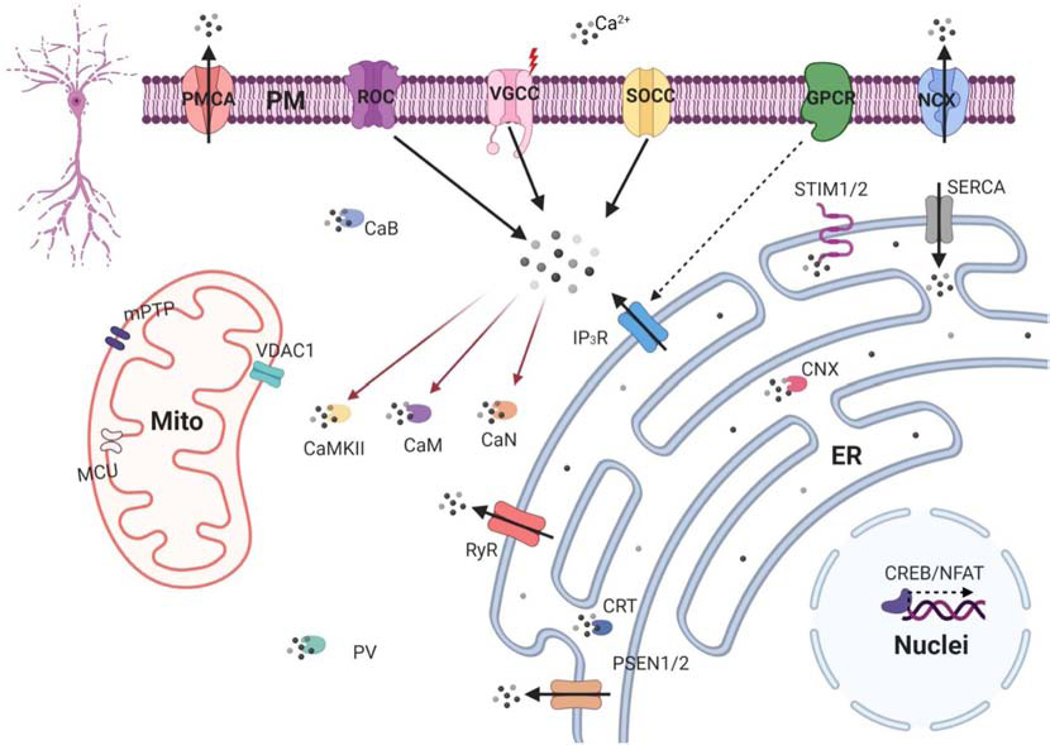
Figure 1. Neuronal calcium signaling.
Schematic representation of neuronal Ca2+ signaling. Main Ca2+ influx sources in plasma membrane (PM) are voltage-gated (VGCC), the ligand–operated (ROC) and the store-operated (SOCE) Ca2+ channels. Plasma membrane Ca2+ ATPase (PMCA) and the Na+/Ca2+ exchanger (NCX) extrude Ca2+ from cytosol into the extracellular space. Activation of metabotropic glutamate receptors (mGluR) stimulate Ca2+ mobilization from endoplasmic reticulum (ER) via inositol 1,4,5 - trisphosphate receptor (IP3R). Ca2+ also can be mobilized from ER via ryanodine receptors (RyR) amplifying cytoplasmic Ca2+ signals. Reuptake Ca2+ into the ER is operated by the ATPase SERCA. ER Ca2+ depletion is detected by Ca2+ sensors STIM1/2. To maintain the balance, Ca2+ is released from ER through passive leakage channels presenilins (PSEN1/2). The transmission of Ca2+-dependent signals to the nucleus occurs with the help of transcription factors cAMP response element-binding protein (CREB) and nuclear factor of activated T-cells (NFAT). The mitochondrial (mito) Ca2+ handling systems include mitochondrial Ca2+ uniporter (MCU), voltage-dependent anion channel type 1 (VDAC1), mitochondrial permeability transition pore (mPTP). Ca2+ concentration in cytosolic maintain with Ca2+-binding proteins: calbindin-28 (CaB), parvalbumin (PV) and calretinin (CaR), and inside ER with calreticulin (CRT) and calnexin (CNX). Ca2+-activated proteins include calmodulin (CaM), Ca2+/ calmodulin-dependent protein kinase type II (CAMKII) and Ca2+/calmodulin-dependent protein phosphatase calcineurin (CaN). Figure created with BioRender.com.
Mitochondria have a special role in neurons acting not only as the main source of ATP necessary to maintain the electrochemical gradients and membrane excitability, but also to provide additional Ca2+ buffering capacity and to participate in many Ca2+-mediated signaling processes [4]. The close proximity of organelles and Ca2+ stores allows for direct communication via membrane contact sites (Loncke J et al, Trends Cell Biol, 2021, accepted for publication [5]). In general, membrane contact sites are enriched with chaperones that stabilize the close apposition of the two lipid bilayers [6]. The membrane contact sites between ER and mitochondria are termed mitochondria-associated ER membranes (MAMs). MAMs are dynamic structures that provide crosstalk between the ER and mitochondria and are necessary to maintain the bioenergetic balance in cells. It was demonstrated that the voltage-dependent anion channel (VDAC) 1 (VDAC1) on the outer mitochondrial membrane is physically linked with the IP3R through glucose-regulated protein 75 (GRP75), thus facilitating the transfer of IP3R-mediated Ca2+ signals towards the mitochondria and allowing “quasi-synaptic” Ca2+ flux from ER to mitochondria [7]. IP3R presence and function at the MAMs is sustained by accessory proteins such as IRE1α [8] and translocase of the outer membrane 70 (TOM70) [9], thereby supporting proper mitochondrial metabolism. A recent review of Loncke et al. further illustrates the impact and function of IP3Rs at MAMs [10]. Impairing IP3R function limits mitochondrial bioenergetics, thereby augmenting the AMP/ATP ratio. This activates AMP-activated protein kinase and subsequently initiates autophagy [11].
For Ca2+ to enter the mitochondrial matrix, it has to pass two membranes. First, the mitochondrial outer membrane where VDAC1 is responsible for Ca2+ transport from the cytosol to the intermitochondrial membrane space (Fig 1). Second, the mitochondrial inner membrane where the mitochondrial calcium uniporter (MCU) complex is responsible for Ca2+ transport from the intermitochondrial membrane space to the mitochondrial matrix [12] (Fig 1). The intimate connection between Ca2+ signaling and mitochondrial metabolism is due to the close apposition of ER and the mitochondria. Additionally, mitochondria have a high driving force for Ca2+ accumulation due to the negative mitochondrial potential (−180 mv).
A key aspect of Ca2+ signaling in neurons is its involvement in the mechanisms of synaptic transmission and synaptic plasticity. Long-term potentiation (LTP) and long-term depression (LTD) are a facilitation or attenuation in synaptic transmission between two neurons which persists for a long time after the termination of the stimulus, and these are considered to be the cellular mechanisms of learning and memory formation. Both LTP and LTD trigger complex post-synaptic Ca2+ signaling pathways enabling continual changes in synaptic strength. Typically, LTP requires initial phosphorylation and subsequent autophosphorylation of CaMKII, while calcineurin initiates dephosphorylation events which often lead to LTD. [13]. Long-lasting changes in the synaptic activity require transcriptional responses, which are also driven by Ca2+ signals and specifically propagate to the nucleus in order to maintain the synthesis of the proteins involved in neuroplasticity. The most studied Ca2+-dependent transcription factor is a cAMP-responsive element-binding protein (CREB) that mediates the conversion of short-term memory to long-term memory (Fig 1). Massive Ca2+ influx through N-methyl-D-aspartate receptor (NMDAR) during LTP induces activation of Ca2+-dependent kinases like CaMKII and subsequently induces phosphorylation of CREB, which in turn is required for activity-induced Ca2+-dependent gene transcription [14]. Another transcription factor called nuclear factor of activated T cells (NFAT) is also regulated by Ca2+ and calcineurin in neurons (Fig 2). NFAT proteins are phosphorylated and reside in the cytoplasm of resting cells. Upon stimulation, they are dephosphorylated by calcineurin, translocate to the nucleus, and become transcriptionally active, thus providing a direct link between intracellular Ca2+ signaling and gene expression in neurons [15].
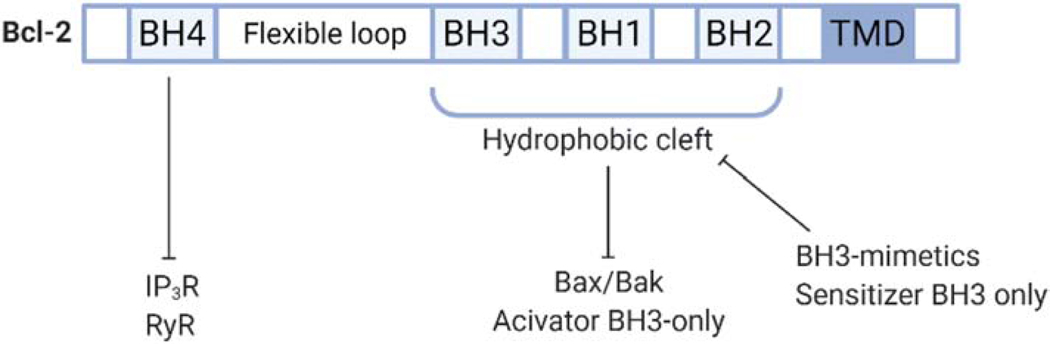
Figure 2. Domain structure of Bcl-2 protein family structure.
Schematic overview of the linear representation of Bcl-2 protein family. Bcl-2 contains four Bcl-2-homology (BH) domains. The BH4 domain is known to bind and inhibit inositol 1,4,5-trisphosphate receptor (IP3R), as well as ryanodine receptor (RyR). The hydrophobic cleft is formed by the BH3, BH1 and BH2 domains and scaffolds pro-apoptotic Bax/Bak and activator BH3-only proteins, thereby neutralizing their pro-apoptotic activities. BH3 mimetics and sensitizer BH3-only proteins can bind to the hydrophobic cleft thereby antagonizing Bcl-2’s ability to bind Bax/Bak and activator BH3-only proteins and unleashing their pro-activity activities. Figure created with BioRender.com.
The mechanisms of Ca2+ regulation are especially important in synapses where the processes of synaptic transmission and synaptic plasticity take place. The postsynaptic structures of the excitatory synapses, called dendritic spines, provide compartmentalization of Ca2+ signals to local microdomains. Morphology of the spines is tightly coupled with synaptic transmission and strongly correlates with ongoing neuronal activity. The structure and function of spines are regulated by Ca2+ activated proteins such as CAMKII and calcineurin (Fig 1). Recent studies also highlight the importance of intracellular Ca2+ stores in formation and maintenance of dendritic spines. Although dendritic spines contain internal Ca2+ stores and several plasma membrane located Ca2+ channels including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), NMDAR and VGCC, they utilize less potent Ca2+-extrusion mechanisms and have lower endogenous Ca2+-buffer capacity when compared to soma and dendrites [16]. Mitochondria play an important role in synaptic function, providing synapses with ATP for neurotransmitter synthesis and release, as well as buffering Ca2+ levels [17]. Synaptic mitochondria are considered to be more vulnerable. It has been shown that nonsynaptic mitochondria are capable of accumulating higher amounts of exogenously added Ca2+ compared to synaptic mitochondria before undergoing mitochondrial permeability transition pore (mPTP) opening [18].
The role of Ca2+ as a second messenger in neurons is difficult to overestimate since the most important functions of neurons, such as control of excitability, synaptic transmission, synaptic plasticity, changes in gene expression and the activation of survival and programmed cell death pathways are regulated by Ca2+. Not surprisingly, Ca2+ levels in neurons are tightly controlled. Even small imbalances in Ca2+ handling in neurons can disrupt the delicate mechanisms of Ca2+ regulation and ultimately lead to cell death [19]. Importantly, many of these pathways become impaired with age and the relation between brain aging and changes in cellular Ca2+ homeostasis is well known [20]. This connection may provide a link with age-related neurodegeneration since similar processes can occur in neuronal cells with the development of neurodegenerative diseases [21–23], as discussed below in more detail for Alzheimer’s disease (AD).
2. Structure and function of the Bcl-2 family
Members of the B-cell lymphoma-2 (Bcl-2) protein family critically control cell death and survival processes by regulating mitochondrial outer membrane permeabilization (MOMP) [24–26]. Bcl-2-family members can be classified into three main groups based on their structure and the presence of Bcl-2-homology (BH) domains. The latter being highly conserved α-helical motifs. The group of anti-apoptotic Bcl-2-family members (i.e. Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl-1, and Bcl-10) is characterized by the presence of four BH domains arranged, from N- to C-terminus, BH4, BH3, BH1, and BH2 (Fig 2). On the other hand, the pro-apoptotic multidomain Bcl-2-family members (i.e. Bax, Bak, and Bok) possess at least three BH domains. Lastly, the BH3-only proteins only contain a single BH3 domain, which is necessary for their pro-apoptotic function [27,28]. BH3-only proteins can be further subdivided into direct activators of Bax and Bak (i.e. Bid and Bim), and sensitizers (i.e. Bad, Bik, Noxa, Puma, Hrk, Bmf). These sensitizers are unable to activate Bax and Bak, but bind to anti-apoptotic Bcl-2 proteins and consequently neutralize them [24,29].
Most forms of apoptosis in vertebrates occur via the intrinsic mitochondrial pathway rather than through receptors of cell death (TNF and Fas receptors) [30]. In this pathway, apoptosis is initiated after the release of apoptogenic proteins from the intermembrane space of mitochondria into the cell cytoplasm. The key event in the mitochondrial apoptosis pathway is an increase in the permeability of the outer mitochondrial membrane [31,32]. The apoptotic Bcl-2 proteins, Bax and Bak, play a significant role in increasing MOMP. The activation and oligomerization of these proteins trigger the onset of apoptosis by forming proteinaceous pores in the mitochondrial outer membrane, resulting in MOMP [33]. This process is responsible for the release of cytochrome c and Smac/Diablo into the cytosol, triggering the formation of the apoptosome and subsequent activation of caspases. While in general MOMP results in apoptotic cell death, low levels of MOMP can actually promote cell transformation and tumorigenesis through cell damage elicited by the sublethal activity of caspases (so-called ‘minority MOMP’) [25,34]. Under normal circumstances, Bak is associated with the mitochondrial outer membrane, whereas Bax resides mostly in the cytosol and is inserted into the mitochondrial outer membrane upon apoptosis induction [27,35–37]. The relevant interaction between the activator BH3-only proteins and Bax/Bak in cells occurs at and within the intracellular membranes [33]. The anti-apoptotic Bcl-2-family members contain a hydrophobic cleft, formed by their BH3, BH1, and BH2 domains, that allows the scaffolding of the BH3 domain of pro-apoptotic members. The formation of such complexes neutralizes Bax/Bak and BH3-only proteins, thereby preventing their pro-apoptotic functions [27]. Recently, Bax/Bak-inhibiting molecules have been developed such as MSN-125 and MSN-150, which can effectively protect cells against pro-apoptotic stimuli [38]. On the other hand, BH3 mimetics drugs, a promising class of precision anti-cancer medicines, have been developed to occupy the hydrophobic groove of the anti-apoptotic Bcl-2 proteins, thereby releasing pro-apoptotic Bcl-2-family members and thus stimulating apoptosis induction [28,39] (Fig. 2). More recently, several BH3 mimetic antagonists that selectively antagonize distinct Bcl-2-family members, including Bcl-2, Bcl-XL and Mcl-1, have emerged [40].
Finally, besides the hydrophobic cleft, Bcl-2’s BH4 domain is also critical for its anti-apoptotic function. At the mechanistic level, the BH4 domain of Bcl-2 can directly target Bax, preventing its conformational activation and its pore-formation properties through non-canonical interaction sites [41]. Additionally, Bcl-2 deletion mutants lacking the BH4 domain (Bcl-2ΔBH4) fail to scaffold Bax and thereby even become pro-apoptotic [42,43]. Besides a potential role of Bcl-2’s BH4 domain in controlling Bax activity, the BH4 domain of anti-apoptotic Bcl-2 proteins has mainly emerged as a key determinant to control intracellular Ca2+ signaling [44,45]. More recently, this BH4 domain also appears an attractive target in anti-cancer strategies, yet on-target small molecule BH4-domain antagonists ought to emerge [46–48].
3. Role of Bcl-2 family members in Ca2+ signaling
The first association between Bcl-2 and intracellular Ca2+ was described in 1993 by Baffy et al. [49]. Later on, multiple studies revealed a profound impact of Bcl-2-family proteins on intracellular Ca2+ signaling [50–52]. We here focus on the importance of Bcl-2 in mediating mitochondrial and ER Ca2+ signaling (Fig 3A).
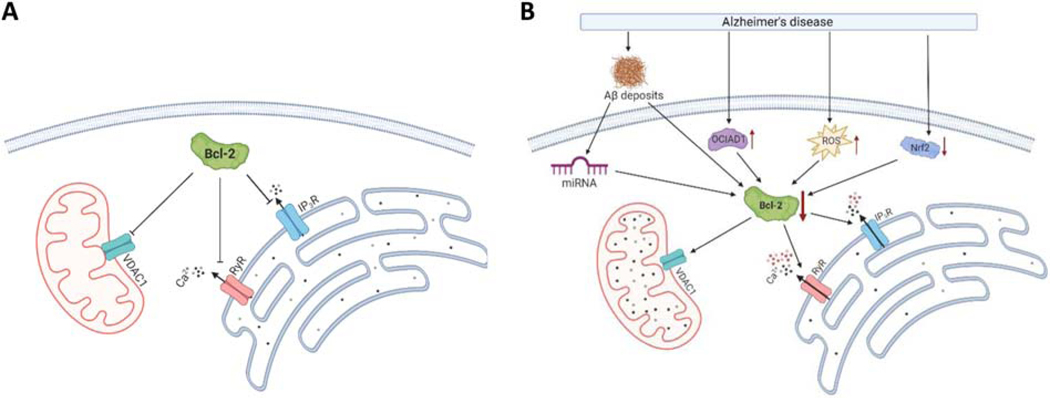
Figure 3. Bcl-2 function in healthy and AD neurons.
(A) In healthy neurons, Bcl-2 diminishes Ca2+ release from the ER by inhibiting ryanodine receptor (RyR) and 1,4,5-trisphosphate receptor (IP3R), and reduces activity of voltage-dependent anion channel 1 (VDAC1). (B) In AD neurons Bcl-2 expression is reduced via various mechanisms including the formation of amyloid-β deposits, the upregulation of Ovarian-Carcinoma-Immunoreactive-Antigen-Domain-Containing-1 (OCIAD1), the upregulation of reactive oxygen species (ROS) and the downregulation of nuclear factor erythroid 2-related factor (Nrf2). The Bcl-2 downregulation reduces the inhibition of ER Ca2+ release and VDAC1 activity, leading to enhanced intracellular Ca2+ release and apoptosis. Figure created with BioRender.com.
3.1. Bcl-2-family members and mitochondrial Ca2+ handling
VDAC is located in the mitochondrial outer membrane and is permeable to mitochondrial metabolites and ions, including Ca2+ [53]. Up until now, three isoforms of VDAC have been identified in higher eukaryotes: VDAC1, VDAC2, and VDAC3, with VDAC1 being the most abundant isoform [54]. Several studies have highlighted the pivotal role of VDAC1 in mitochondria-mediated apoptosis, as silencing of VDAC1 prevented apoptosis [55,56], whereas overexpression induced apoptotic cell death [53,57–60]. VDAC1 is responsible for the transfer of pro-apoptotic Ca2+ signals towards the mitochondria, in part due to its ability to form complexes with IP3Rs located at the MAMs [61]. Moreover, VDAC1 serves as a mitochondrial permeation path that mediates the cross-talk between mitochondria and the rest of the cell, e.g. as an exit pathway for ATP from the intermitochondrial membrane space and the cytosol [62]. VDAC1 channels interact with several pro-survival proteins including hexokinase-I and Bcl-2-family members, thereby promoting cell survival and preventing apoptosis [63]. Particularly, the N-terminal region of VDAC1 is an interaction hub for these proteins [64,65]. These insights have been exploited to elicit cell death in different cancer types using a variety of VDAC1-derived peptides [66]. Also, different anti-apoptotic Bcl-2-family members, Bcl-2, Bcl-XL, and Mcl-1, interact with VDAC1, impacting its channel conductance [53,67]. Bcl-2 and Bcl-XL have been implicated in inhibiting VDAC1 by targeting its N-terminal region, thereby rendering cells more resistant to Ca2+-driven apoptosis. The inhibition of VDAC1 by Bcl-XL involved its BH4 domain, which by itself is sufficient to inhibit VDAC1 single-channel activity and VDAC1-mediated mitochondrial Ca2+ uptake [64]. However, besides an inhibitory effect, Bcl-XL (and also Mcl-1) have been reported to augment VDAC1 activity, thereby boosting mitochondrial metabolism and cancer cell proliferation [68,69]. The binding of Bax to VDAC1 on the other hand evokes cytochrome c release [67,70]. In addition to this, VDAC2 has emerged as an important partner for the mitochondrial import of Bak [71,72] and Bax during apoptosis induction [73]. Besides its interaction with VDAC, the Bcl-2-family proteins display other effects at the mitochondrial level. For example, overexpression of Bcl-2 in mice revealed a significant reduction in the activity of the mitochondrial Na+/Ca2+ exchanger [74]. Additionally, different Bcl-2-family members (i.e. Bak, Bax, Bcl-XL, and Bok) are able to modulate mitochondrial morphology and dynamics by targeting different proteins responsible for mitochondrial fission and fusion [75–78]. Bcl-XL is able to control the mitochondrial respiratory capacity and ATP production, and Bcl-XL knockout (KO) results in increased oxidative stress [79]. Finally, Bcl-XL has been shown to interact directly with the β-subunit of the F1F0 ATP synthase causing an increased transport of H+ during F1F0 ATPase activity [80].
3.2. Bcl-2-family members and ER Ca2+ handling
Bcl-2-family members do not only affect the mitochondrial Ca2+ handling but also control intracellular Ca2+ dynamics at the level of the ER. Bcl-2 directly binds to and inhibits the IP3R, the major Ca2+ release channel and an important player in the crosstalk between the ER and mitochondria. This Bcl-2-IP3R interaction seems crucial for the inhibition of the IP3R-mediated Ca2+ release from the ER by Bcl-2, and thereby Ca2+-mediated apoptosis is prevented [46,81]. The domain responsible for this interaction is the BH4 domain of Bcl-2, which binds to two regions in the IP3R: i. a sequence of 20 amino acids in the central modulatory domain [81]; and ii. the ligand-binding region near the N-terminal part of the IP3R channel [82]. Moreover, the BH4-domain by itself is sufficient to bind to and inhibit IP3Rs [26,44,83]. However, relatively high concentrations of BH4-domain peptides are needed to impact the IP3R function [83]. In cellulo, the relatively low affinity of the BH4 domain for IP3Rs is alleviated by recruiting Bcl-2 in close proximity of IP3Rs through Bcl-2’s transmembrane domain that targets the C-terminal region of the channel [84]. Interestingly, Bcl-2’s binding to IP3Rs appears to occur independently of its hydrophobic cleft that is responsible for scaffolding pro-apoptotic Bcl-2-family members [84].
Other Bcl-2-family members containing a BH4 domain motif too, may interact with these IP3Rs. This has been shown for Nrz/NrH, another anti-apoptotic Bcl-2-family member, as well as for Bcl-w [85–87]. However, the BH4 domain of Bcl-XL, which closely resembles Bcl-2, appears not to be able to target and control IP3R function. At the molecular level, Lys17 in Bcl-2’s BH4 domain was identified as a residue that was critical for its binding to IP3Rs and inhibition of the channel. In the BH4-domain of Bcl-XL, this Lys residue was not present and was replaced by an Asp residue. This may underlie the reported differences between Bcl-2 and Bcl-XL concerning the ability of the BH4 domain of Bcl-2 versus the BH4 domain of Bcl-XL to inhibit IP3Rs [88]. Besides the IP3R, there is another major Ca2+ release channel located in the ER, namely the RyR [89]. The central domain of the RyR contains a stretch of amino acids that displays a strong similarity with the binding site of Bcl-2 in the central domain of the IP3R. Bcl-2 via its BH4 domain can bind to this central domain of RyR channels, thereby enabling RyR/Bcl-2-complex formation and suppressing RyR-mediated Ca2+ release. However, the Lys17 residue of the BH4-domain was not critical for RyR binding and modulation by Bcl-2. Consistent with this, both Bcl-2 and Bcl-XL proteins bind to RyRs and inhibit RyR-mediated Ca2+ release [90].
3.3. Subcellular localization of Bcl-2-family members
It is clear that many of the Bcl-2-family members are localized at the mitochondria where these proteins have several functions such as regulating MOMP [24], mitochondrial Ca2+ uptake [63] and energy production [80]. In addition, several anti-apoptotic Bcl-2 family members also localize to the ER where they modulate ER Ca2+ release (Bcl-2, Bcl-XL and Mcl-1) [91–93] and the structural organization of the ER (Mcl-1) [94]. Pro-apoptotic Bcl-2-family members have also been shown to localize to the ER, thereby regulating ER Ca2+ levels (Bax/Bak) [52] or the stability of the IP3R (Bok) [95]. Besides the ER and mitochondria, Bcl-2-protein family members can also localize in the cytosol or at other compartments such as the nuclear outer membrane, the nucleus, peroxisomes and the Golgi apparatus. As an in-dept discussion of this is beyond the scope of this review, we would like to direct the reader to a recent review of Popgeorgiev et al. dedicated to this topic [96].
4. Functions of Bcl-2-family members in neurons
A large number of Bcl-2-family members, both pro-and anti-apoptotic, are expressed in the central nervous system (CNS) [97]. The pro-apoptotic protein Bok is expressed in the cerebral cortex and hippocampus, whereas Bax is more widely expressed in the brain [98,99]. The anti-apoptotic Bcl-2-family members, Bcl-XL and Bcl-w are present in mature neurons, whereas Bcl-2 is mostly expressed in the developing brain [100–102]. In the following part, we will briefly discuss the known functions of pro-and anti-apoptotic Bcl-2 family members in neurons and refer the reader to Pemberton et al. for an extensive discussion [103].
4.1. Functions of pro-apoptotic Bcl-2-family members in neurons
Bax is one of the major proteins promoting cell death in the developing CNS. More than half of the neurons in the developing CNS die via the apoptotic pathway regulated by Bax [104]. The main proteins responsible for Bax activation in neurons are the BH3-only activators Bid, Bim, and Puma. For instance, single deletion of Puma prevents both neuronal apoptosis [103,105,106] and axon degeneration [107] in vitro and in a variety of neuronal cell types from both the central and peripheral nervous system. This suggests that BH3-only activators play a significant role in regulating neuronal MOMP [103]. The central role of Bax in neuronal apoptosis is illustrated by the observation that Bax KO mice do not exhibit developmental programmed cell death of dorsal root ganglion sensory neurons, superior cervical ganglion sympathetic neurons, or motoneurons (MNs) [108–110]. Multiple studies have indicated that the deletion of the Bax gene protects neurons against excitotoxic apoptosis [97,111,112]. Moreover, the Ca2+ transients during NMDA excitation were reduced in Bax-deficient neurons, and this effect seemed independent of the role of Bax in apoptosis [113]. Besides regulating neuronal cell death, Bax suppresses neurogenesis in the hippocampus and the cerebellum of adult brains [104,114].
Neuronal death in Bak KO mice was found to be more complex as it was either inhibited or enhanced depending on the developmental stage, death stimulus or neuronal subtype [115]. Only Bax/Bak double KO mice demonstrated an increase in neuroprogenitor cells in the periventricular zone of the brain, whereas single KO of Bax (or Bak), did not contribute to an increase in the survival of neuroprogenitor cells in the brain of mice. This suggests a redundancy for Bax and Bak in these progenitor cells, which is not the case in postmitotic differentiating neurons which only require Bax for apoptosis induction [116,117].
Thus, Bak and Bax can contribute to both survival and death of neurons. How Bak and Bax participate in these processes strongly depends on the stage of development, stress stimuli, and the neuronal type. However, it should be noted that despite the almost canonical belief that apoptosis is necessary to ensure proper development, mice lacking both Bax and Bak can successfully develop [116]. Moreover, early stages of embryogenesis occur without any defects despite the loss of both key apoptotic molecules [116]. These data suggest that the induction of Bax/Bak-dependent apoptosis may not be critical for successful embryogenesis to occur.
4.2. Functions of anti-apoptotic Bcl-2-family members in neurons
Similar data were obtained when studying the effects of some anti-apoptotic members of the Bcl-2 family on the process of embryogenesis. Bcl-2-deficient mice embryos did not exhibit any significant neuronal development disturbances or abnormal neuronal apoptosis [118,119]. Nevertheless, severe defects of Bcl-2 loss are observed in postnatal animals, which may indicate a more important role in post-natal development than during early neurogenesis [119,120]. High levels of Bcl-2 expression were detected during early neurulation, which emphasizes its role in preventing apoptosis at this stage. Bcl-2 expression decreases in the neurons of CNS after neural tube formation, while it remains highly expressed in the peripheral nervous cells [102,121].
In the mature brain, Bcl-2 is principally retained in the granule cells of the cerebellum and dentate gyrus of the hippocampus, as well as in sensory and sympathetic adult neurons [102]. Chen et al. reported that Bcl-2 plays a major role in promoting growth and axon regeneration in retinal neurons. Bcl-2 seemed essential but not sufficient for the regeneration of retinal axons in the CNS [122]. Altered Bcl-2 expression is deemed to impair cellular plasticity and resilience in neuropsychiatric patients [123,124]. The mechanisms by which Bcl-2 promotes axonal growth are suggested to be by enhancing the intracellular Ca2+ signaling and by activating CREB and extracellular-regulated kinase (ERK). These latter two proteins are known to induce gene expression essential for neurite growth and plasticity. Bcl-2 reduces the ER Ca2+ uptake in neurons, which leads to increased Ca2+ influx over the plasma membrane. This consequently causes the activation of CREB and ERK transcriptional programs that regulate neurite extension [125]. A recent review of Pemberton et al. further focuses on the regulation of axon degeneration and neuronal cell death by Bcl-2 proteins [103]. Additionally, overexpression of Bcl-2 protects motor neuron cell bodies from apoptosis in a progressive motor neuropathy mouse model. However, the life span of these mice was not affected as overexpression of Bcl-2 did not prevent the degeneration of myelinated motor fibers [126]. Bcl-2 can also influence neuronal Ca2+ signaling through its interaction with RyRs [90]. Co-immunoprecipitation assays on lysates of rat hippocampi proved the presence of RyR-Bcl-2 complexes in rat neurons. The BH4 domain of Bcl-2 was sufficient to inhibit the RyR-mediated Ca2+ release in these neurons. This further underpins an important function of Bcl-2 in the brain.
Bcl-2 expression appears to protect neuroepithelial and hippocampal cells against glutamate-mediated excitotoxicity [127]. It was shown that overexpression of Bcl-2 may improve cortical neuron survival by blocking the translocation of apoptosis-inducing factor (AIF) from mitochondria to the nucleus following focal cerebral ischemia [128]. Instead, mice with Bcl-2 deficiency demonstrate enhanced oxidative stress and alterations in antioxidants levels in the brain [129]. Transgenic mice overexpressing Bcl-2 in neurons display nervous system hypertrophy caused by decreased neuronal cell death, but they also show a 50% reduction in cerebral infarction volume compared to wild-type mice after permanent middle cerebral artery occlusion (MCAO)-induced ischemia [130]. Moreover, transplantation of embryonic stem cells overexpressing Bcl-2 into the brain cavity of adult rats after MCAO led to neuronal differentiation and improved functional recovery [131]. Up-regulation of Bcl-2 may also aid DNA repair following oxidative stress damage [132]. In the model of MCAO, Bcl-2 expression inhibits apoptosis of neonatal neurons in the brains of adult rats [133]. It should be noted that changes in Ca2+ homeostasis of the ER have been shown to induce apoptosis in neurons [134].
As Bcl-2 expression decreases after neurulation, Bcl-XL expression increases and remains elevated throughout neuronal ontogeny with the highest levels in differentiating cells [102,121]. It was demonstrated that Bcl-XL may regulate programmed cell death via supporting the viability of immature cells during the development of the nervous and hematopoietic systems. Strikingly, Bcl-XL-deficient mice die around 13th embryonic day showing extensive apoptotic cell death in postmitotic immature neurons of the developing brain, spinal cord, and dorsal root ganglia [100]. However, the mechanisms and signals regulating Bcl-XL expression in the brain are still not clear. Postnatally, in mature neuronal cells Bcl-XL has been identified as regulator of synaptic plasticity and neurite growth [135]. Injection of Bcl-XL in the presynaptic terminal of squids led to potentiation of the synaptic neurotransmitter release, both in healthy synapses as in synapses in which the transmission had run down. Additionally, Bcl-XL reportedly improved recovery after synaptic depression [135].
Bcl-w, another anti-apoptotic Bcl-2-family member is considered to play an important role in neurons and may be potentially therapeutically relevant target in neurodegenerative diseases [87]. Indeed, Bcl-w contributes to the maintenance of axons [136] and is involved in promoting cell survival after cerebral ischemia. Cell death, and more specifically apoptosis, is implicated in brain injury following cerebral ischemia. Bcl-w is deemed to have a neuroprotective effect given its increased expression in the brain, and mainly in the surviving cells, after the ischemic insult [137,138]. The role of Bcl-w as an endogenous neuroprotector is not limited to cerebral ischemia but is also observed in the β-amyloid-induced cell death in AD [139]. Bcl-w is also implicated in the maintenance of axon integrity in sensory neurons, and a lack of this anti-apoptotic protein can lead to small-fiber sensory neuropathy [140]. Bcl-w can protect against axon degeneration via interaction of its BH4 domain with the IP3R1, the isoform predominantly expressed in the brain [141].
5. Bcl-2 in Alzheimer’s disease
5.1. Deranged Ca2+ signaling in Alzheimer’s disease
AD is the most frequently occurring form of dementia worldwide with an enormous impact on the quality of life of patients and their family. The disease leads to an irreversible loss of neurons and is clinically represented by impaired memory formation, disorientation, troubled judgment, and behavioral changes [142,143]. The main histopathological features of AD are an accumulation of amyloid-beta (Aβ) that aggregates in senile plaques and hyperphosphorylated tau protein which is a major constituent neurofibrillary tangles typically observed in AD patients. Rare familial forms of Alzheimer’s disease (FAD) are linked to mutations in the genes encoding the amyloid precursor protein (APP), presenilin1 (PSEN1), and presenilin 2 (PSEN2). The latter constitute the catalytic subunit of γ-secretase [142,144] that liberates Aβ peptides from their precursor, the APP C-terminal fragment. Based on the genetic nature of these familial forms of AD, an amyloid cascade hypothesis was formulated that linked the Aβ accumulation with degenerative changes in the brain leading to the death of neuronal cells and the development of cognitive impairment [145]. However, the cause of sporadic forms of AD (SAD), still constituting over 95% of all cases, remains elusive. Besides, some controversy exists concerning the Aβ- hypothesis, given that Aβ-deposits are also found in healthy, cognitively well-functioning individuals [146]. Whereas anti-amyloid therapy strategies have so far not been successful in patients [147], the more recently developed Aducanumab bears more promise [148]. Memantine, an NMDAR antagonist, is currently the only disease-modifying drug approved for AD therapy.
The Ca2+ hypothesis of AD was formulated by Zaven S. Khachaturian and is based on the similarity of the processes occurring in neurons during aging and AD [149]. Indeed, many pathological processes observed in neurons during AD progressions such as oxidative and metabolic stress, a decrease in ATP production, and Ca2+ dysregulation are also observed with senescence. Increasing evidence points to an important role for altered Ca2+ signaling in AD, as changes in intracellular Ca2+ signaling occur before the major neuronal loss in AD [150–152]. Among these changes, synaptic dysfunction is especially important since synapses are units of high energy consumption and extremely vulnerable to changes in Ca2+ balance. The elimination of synapses is observed early in the AD development and better correlates with cognitive impairments than other histopathological signs [153,154].
Many physiological processes mediated by Ca2+ can turn into a pathological cascade with aging and AD. An example of such a process is the excitotoxic effect of glutamate. Glutamate is the main excitatory neurotransmitter in the brain, and one of its key effects is the induction of LTP necessary for short-term memory formation. However, the constant massive Ca2+ influx in case of NMDAR overstimulation can become detrimental if neurons are unable to rapidly remove of Ca2+ from the cytosol. The decrease in Ca2+ buffer proteins observed in AD [155] may contribute to the development of excitotoxicity since the accumulation of Ca2+ in the cytoplasm leads to the activation of Ca2+-dependent calpain proteases, which induce neuronal apoptosis. Considering the physiological/pathophysiological effects of glutamate, it is necessary to carefully consider the strategy for correcting Ca2+ imbalance in neurons. Perhaps this is a key feature of memantine which at therapeutic concentrations blocks the excitotoxicity mediated by extrasynaptic NMDARs but does not affect the physiological processes of neuroplasticity in synapses [156].
Evidence that supports the Ca2+ hypothesis comes from studies indicating dysfunctional ER-mediated Ca2+ signaling in FAD [157]. A specific interest in the ER Ca2+ homeostasis related to the AD pathology arises as certain mutations that cause FAD also interfere with ER Ca2+ signaling. An important finding indicating the close relationship between FAD and impaired Ca2+ signaling was the discovery of the role of PSEN1 as potential Ca2+ leak channels [158]. Mutations in PSEN1 that affect this function, disrupt steady-state resting ER Ca2+ levels and promote excessive Ca2+ accumulation in the ER [159]. Alternatively, other groups showed an enhancement of IP3R-mediated Ca2+ signaling in fibroblasts [160] and B lymphoblast’s [161] from AD patients carrying mutations in PSEN1. These changes in Ca2+ signaling were already present before the patients became clinically symptomatic [162]. Similar results have been shown in Xenopus oocyte expression system experiments with AD-linked mutation in PSEN [163] and in mouse cortical neurons of the mutant PSEN1, PS1-M146V knock-in (PS1-M146V-KI), model of AD [164].
Several groups also indicated the role of RyRs in dysregulated Ca2+ signaling as neuronal RyR expression was increased in different AD mouse models and cell lines [165–170]. Dantrolene treatment, a RyRs inhibitor, has been reported to normalize the dysregulated ER Ca2+ signaling and to reduce the Aβ deposition [167]. Despite some uncertainty remaining in proposed mechanisms, the main concept of excessive Ca2+ release from the ER via IP3R1 and RyR caused by FAD-associated mutations in PSEN is gaining in popularity [171].
Another pathological aspect of overfilling of the ER Ca2+ content is decreased activity of neuronal SOCE (nSOCE). As a compensatory response to elevated ER Ca2+ level, a reduction in STIM2 protein has been observed in PSEN1-M146V-KI [172] and APP-KI mouse models [173]. TRPC6-mediated nSOCE activity is necessary for maintaining the persistent activity of CAMKII and stabilization of mature mushroom spines [174]. Downregulation of STIM2 expressions reduces the constant activity of nSOCE and the expression of the active (phosphorylated) form of CAMKII leading to destabilization of mature spines. This provides an important connection between deranged Ca2+ signaling in the ER and the earliest signs of neurodegenerative processes observed in AD. It was also demonstrated that application of Aβ oligomers (Aβo) promotes the loss of nSOCE in aged rat hippocampal cultures. Aβo also exacerbate the increased resting cytosolic Ca2+ concentration and Ca2+ store content. In young neuronal cultures Aβo promoted ER to mitochondrial Ca2+ transfer without detrimental effects whereas, in aged cultured neurons, Aβo suppressed Ca2+ transfer from ER to mitochondria. In these aged cultures Aβo also decreased mitochondrial potential, enhanced reactive oxygen species (ROS) generation and promoted apoptosis [175].
While deranged Ca2+ signaling is mainly considered as a downstream effect of AD-linked mutations, some studies indicate that nSOCE downregulation selectively elevates Aβ42 generation suggesting that reduced Ca2+ entry might be an early cellular event associated with PSEN mutations [176]. It was also shown that exposure of cultured neurons to Ca2+ ionophores increases their production of Aβ [177]. Moreover, a physiological Ca2+ stimulus increases α-secretase cleavage of APP and may thereby decrease Aβ production [178,179].
Impaired Ca2+ balance in the ER leads to dysfunction of mitochondria since its additional buffering capacity is needed to reduce high cytosolic Ca2+ concentration in neurons. Mitochondria’s dysfunction in AD is well described and includes mitochondrial oxidative stress, impaired bioenergetics and biogenesis and formation of mPTP, inducing neuronal apoptosis [180,181]. Recent studies also point to the pivotal role of MAMs in AD pathogenesis. It was shown that PSENs may localize in MAMs [182] where also APP cleavage has been suggested to take place [183]. A significant increase in the contact sites between ER and mitochondria was also demonstrated in the case of FAD and SAD, indicating an imbalance in the functioning of MAMs [184]. Taking into account that Ca2+ transfer from ER into mitochondria occurs predominantly through MAMs [185], it cannot be excluded that the increase in contact sites between the two organelles may result in an increased Ca2+ uptake into mitochondria, eventually leading to mPTP opening and neuronal apoptosis. More recently, in vivo evidence based on an APP/PS1 mouse model emerged that mitochondrial Ca2+ overload occurs prior to neuronal death [186,187]. This further underpins the key role of Ca2+ -signaling dysregulation as an early event in AD [181].
5.2. Bcl-2-family members in AD
Much work has been performed on the potential roles of Bcl-2 in AD as we will describe in the next parts. However, also other Bcl-2-family members have been associated with the disease progression. In general, the balance between pro-and anti-apoptotic Bcl-2 family members shifts towards the pro-apoptotic side during the disease thereby increasing the potential occurrence of neuronal cell death. It is suggested that Aβ-deposits alter the balance of the Bcl-2-family proteins favoring the expression of pro-apoptotic family members. This is illustrated by the observation that Bim is upregulated while Bcl-2 is downregulated after injection of mice brain with oligomeric Aβ [188]. Additionally, increased activation of Bax was observed and bax−/− neurons seemed resistant against Aβ-induced cell death [188]. A similar increase in Bax and downregulation of Bcl-2 in human neuronal cultures derived from fetal brain following Aβ treatment was also observed by other researchers [189]. Finally, by studying senile plaques and neurons from AD patients with neurofibrillary degeneration, a strong immunoreactivity has been observed for Bax as well [190,191]. However, this could not be reproduced by others (Tortosa et al.) [192].
Concerning the anti-apoptotic Bcl-2-family members, Bcl-XL was shown to be expressed in microglia of patients with AD that co-localized with Aβ-deposits and activated astrocytes which may increase the cell survival of these microglia in disease hot spots [193]. Mcl-1 was shown to interact with cyclin dependent kinase 5 (Cdk5) [194], which is involved in neuronal cell death in AD [195]. The interaction between Mcl-1 and Cdk5 induced phosphorylation of Mcl-1 at T91 thereby triggering Mcl-1 ubiquitylation and its subsequent degradation. This renders the neurons more sensitive to cell death. Besides this, selective Mcl-1 antagonism using UMI-77, a BH3 mimetic, induces mitophagy, a mitochondrial quality control process that is inhibited in AD patients. Through this mechanism, UMI-77 could significantly improve the cognitive impairment in mice lacking APP and PSEN1 [196].
5.3. Bcl-2 is downregulated in AD
The expression of members of the Bcl-2-protein family, such as Bcl-XL, Bak, and Bad, is altered in AD [197] (Fig 3B). The alterations in Bcl-2 in healthy brain aging are dissimilar from those observed in brain of AD models [198]. Multiple studies using post-mortem samples of AD patients have indicated a striking immunoreactivity of Bcl-2 in astrocytes surrounding senile Aβ-plaques. This suggests that Bcl-2 is involved in astroglial survival [199–201]. Moreover, it was shown that the immunoreactivity for Bcl-2 in neurons of patients with AD was increased relative to controls in most neurons of the entorhinal cortex, subiculum, CA1, CA2, CA3, hilus, and dentate gyrus [201]. Relative Bcl-2 staining increased in parallel with increasing disease severity. In contrast, Bcl-2 immunoreactivity in neurons from patients with AD with confirmed neurofibrillary degeneration is decreased, indicating the downregulation of Bcl-2 in these degenerating neurons [200–202].
Several mechanisms may contribute to the Bcl-2 downregulation observed in AD. For instance, Aβ-deposits are considered to be able to regulate the expression of certain micro RNAs (miRNAs) that can impact neuronal cell death observed in animal models of AD [203–206]. Interestingly, miR-16–5p, a microRNA that targets Bcl-2 mRNA and thus reduces Bcl-2-protein levels, was found to be upregulated in neurons from a FAD mouse model surrounding Aβ-deposits, thereby promoting apoptosis in the neurons affected by Aβ-plaques [206]. Bhatnagar et al. showed increased levels of miR-34a and miR-34c in blood samples of AD patients [207,208]. Four key target genes are silenced by these miRNAs of which Bcl-2 is one of them, leading to a significantly reduced abundance of Bcl-2 in AD plasma samples [209]. Thus, this implicates Bcl-2 as a potential biomarker for neurodegeneration.
Another possible contributing mechanism was unveiled by bio-informatic approaches identifying Ovarian-Carcinoma-Immunoreactive-Antigen-Domain-Containing-1 (OCIAD1) as a neurodegeneration-associated factor for AD in a FAD mouse model [210]. OCIAD1 is upregulated in neurons of both AD mice and sporadic AD patients, and higher OCIAD1 levels are correlated with disease severity. Different pathways are affected by this protein including mitochondrial functionality through interaction with Bcl-2. The OCIAD1-Bcl-2 interaction was suggested to interfere with Bcl-2/Bax-complex formation [210].
A third mechanism that may play a role is a single-nucleotide polymorphism (SNP). The Bcl-2 gene is subject to SNP rs956572, which significantly alters protein and mRNA expression levels as detected in AD patients and patients suffering from bipolar disorder [211–213]. The AA-genotype is associated with reduced expression of Bcl-2, whereas the G-allele is associated with higher Bcl-2 levels. The Bcl-2 rs956572 polymorphism influences age-related volume reductions of the grey matter, mainly in the cerebellum. More specifically, the Bcl-2 G homozygosity has been found to protect against this age-related grey matter volume reduction [123]. In addition, Chang et al. examined the association between the Bcl-2 rs956572 polymorphism and the structural covariance network in AD patient samples and reported a greater covariance strength in the A homozygotes [211]. Therefore, this polymorphism might also be of importance when examining the role of Bcl-2 in AD.
Another mechanism that has been proposed is the interference of ROS. In AD patients, ROS is increased which subsequently causes a decrease in Bcl-2 levels [214,215]. This may then be associated with the Ca2+ dysregulation in AD. Finally, nuclear factor erythroid 2-related factor (Nrf2), which maintains the level of redox buffer glutathione, is suggested to be an important player in the prevention of AD [216]. Interestingly, Nrf2 Is known to regulate Bcl-2 levels and besides the downregulation of Bcl-2, Nrf2 levels are also significantly reduced in the brains of AD patients [214,217].
5.4. Bcl-2 as therapeutic target for AD
In this section, we focus on the potential of Bcl-2 as a therapeutic target for AD. As discussed above, Ca2+ signaling is dysregulated in AD. Bcl-2 plays a role in the control of intracellular Ca2+ signaling and may potentially be used to normalize Ca2+ signals in AD neurons (Fig 3B).
A transgenic AD mouse model with neuronal overexpression of Bcl-2 showed a reduction in caspase 9 and caspase 3 activation, suppression in the formation of plaques and NTFs, and improved memory retention [218]. More specifically, the prevention of caspase activation led to a limited caspase-mediated cleavage of tau and an intracellular accumulation of APP without the formation of Aβ-plaques. These findings indicate a neuroprotective role of Bcl-2 overexpression [218]. This is further supported by other research groups as Karlnoski et al. demonstrated an association between increased Bcl-2 expression in brain regions containing Aβ deposits and neuroprotection in APP transgenic mice [219]. Besides Bcl-2, Bcl-XL too appears to protect against early-stage and late-stage apoptosis/necrosis following Aβ treatment [220]. Moreover, Bcl-XL was shown to interact with PSENs, which in turn are known to significantly increase Bax expression. However, overexpression of Bcl-XL abolished the enhanced Bax-induced apoptosis mediated by PSENs [221]. Besides these direct overexpression approaches, several therapeutic regimens that ameliorate AD outcomes, also enhance Bcl-2-expression levels. For instance, ibudilast, montelukast, and pranlukast, all currently used for the treatment of inflammatory diseases such as asthma, improved Aβ-induced memory impairment [222–224]. Moreover, these drugs all prevented Bcl-2 downregulation. Other researchers found similar results for prosaposin-derived 18-mer peptide, an amelioration of both Aβ-induced neurotoxicity and Bcl-2 downregulation was observed in mice [225].
6. Conclusion
In this review, we have highlighted the emerging link between Bcl-2 family proteins, neuronal Ca2+ signaling in health and AD. Ca2+ is one of the most versatile secondary messengers required for memory formation and other neuronal-specific processes. Multiple evidence points to an important role of dysregulated Ca2+ signaling in AD. Proteins of the Bcl-2 family are key regulators of cell death and survival, but also regulate intracellular Ca2+ signaling at both mitochondrial and ER levels. Moreover, several Bcl-2 proteins are involved in the integrity of axons and neurons. Since expression of Bcl-2 is suppressed in AD and Bcl-2 proteins act as inhibitors of Ca2+ channels such as IP3R and RyR, de-inhibition of these channels might therefore contribute to aberrant neuronal Ca2+ signaling in AD. Multiple mechanisms have emerged that contribute to the decreased Bcl-2-protein levels, including the expression of miRNAs regulated by Aβ deposition; interaction with OCIAD1, and single nucleotide polymorphism in the Bcl-2 gene. Overexpression of Bcl-2 has multiple neuroprotective effects in models of AD that may go well beyond its canonical anti-apoptotic effects. Indeed, by targeting intracellular Ca2+-release channels, Bcl-2 via its BH4 domain could inhibit excessive IP3R/RyR-mediated release of Ca2+ from the ER. These results suggest that anti-apoptotic Bcl-2 and derived protein domains such as the BH4 domain may have therapeutic potential to prevent the onset of AD and delay neurodegeneration. Therefore, further work is needed to elucidate whether Bcl-2 deregulation contributes to aberrant Ca2+ signaling in AD and whether Bcl-2 or its BH4 domain can help to prevent or delay memory loss and neurodegeneration in AD and possibly other neurodegenerative disorders.
Highlights.
The function of Bcl-2 protein family in the nervous system is discussed
The emerging link between Bcl-2 family proteins, neuronal calcium signaling and Alzheimer’s disease is discussed.
The role of Bcl-2 as therapeutic target for Alzheimer’s disease is discussed
Acknowledgements
Research in the authors’ laboratories was supported by research grants of the Research Foundation - Flanders (FWO) (grants G.0634.13N, G.0C91.14N, G.0A34.16N and G090118N to GB and grant G0E7520N to GB and IB; S006617N, G078117N, G056017N to WA), the Research Council - KU Leuven (OT14/101, C14/19/101 and AKUL/19/34 to GB; C16/15/073 to WA), VIB (to WA), Central European Leuven Strategic Alliance (CELSA/18/040 to GB), Stichting Alzheimer Onderzoek (SAO IP3 RECEPTOR to GB; SAO-FRA #2020-0030 to WA), Eye Hope Foundation/Koning Boudewijnstichting (2020-J1160630-214966 to GB), National Institutes of Health grant R01AG055577 (IB) and Russian Science Foundation grant 20-45-01004 (IB). IB is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer’s Disease Research.
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bootman MD, Bultynck G, Fundamentals of cellular calcium signaling: A primer, Cold Spring Harb. Perspect. Biol 12 (2020). 10.1101/cshperspect.a038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen J, Sitsel A, Benoy V, Sepúlveda MR, Vangheluwe P, Primary active Ca2+ transport systems in health and disease, Cold Spring Harb. Perspect. Biol. 12 (2020). 10.1101/cshperspect.a035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bezprozvanny I, Mattson MP, Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease, Trends Neurosci. 31 (2008) 454–463. 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pivovarova NB, Andrews SB, Calcium-dependent mitochondrial function and dysfunction in neurons: Minireview, FEBS J. 277 (2010) 3622–3636. 10.1111/j.1742-4658.2010.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Loncke J, Kaasik A, Bezprozvanny I, Parys JB, Kerkhofs M, Bultynck G, Balancing ER-mitochondrial Ca2+ fluxes in health and disease, Trends Cell Biol. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hayashi T, Su TP, Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival, Cell. 131 (2007) 596–610. 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- [7].Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R, Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels, J. Cell Biol. 175 (2006) 901–911. 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carreras-Sureda A, Jaña F, Urra H, Durand S, Mortenson DE, Sagredo A, Bustos G, Hazari Y, Ramos-Fernández E, Sassano ML, Pihán P, van Vliet AR, González-Quiroz M, Torres AK, Tapia-Rojas C, Kerkhofs M, Vicente R, Kaufman RJ, Inestrosa NC, Gonzalez-Billault C, Wiseman RL, Agostinis P, Bultynck G, Court FA, Kroemer G, Cárdenas JC, Hetz C, Non-canonical function of IRE1α determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics, Nat. Cell Biol 21 (2019) 755–767. 10.1038/s41556-019-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Filadi R, Leal NS, Schreiner B, Rossi A, Dentoni G, Pinho CM, Wiehager B, Cieri D, Calì T, Pizzo P, Ankarcrona M, TOM70 Sustains Cell Bioenergetics by Promoting IP3R3-Mediated ER to Mitochondria Ca2+ Transfer, Curr. Biol 28 (2018) 369–382.e6. 10.1016/j.cub.2017.12.047. [DOI] [PubMed] [Google Scholar]
- [10].Loncke J, Kerkhofs M, Kaasik A, Bezprozvanny I, Bultynck G, Recent advances in understanding IP3R function with focus on ER-mitochondrial Ca2+ transfers, Curr. Opin. Physiol17 (2020) 80–88. 10.1016/j.cophys.2020.07.011. [DOI] [Google Scholar]
- [11].Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK, Essential Regulation of Cell Bioenergetics by Constitutive InsP3 Receptor Ca2+ Transfer to Mitochondria, Cell. 142 (2010) 270–283. 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rizzuto R, De Stefani D, Raffaello A, Mammucari C, Mitochondria as sensors and regulators of calcium signalling, Nat. Rev. Mol. Cell Biol 13 (2012) 566–578. 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- [13].Evans RC, Blackwell KT, Calcium: Amplitude, duration, or location?, Biol. Bull 228 (2015) 75–83. 10.1086/BBLv228n1p75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kornhauser JM, Cowan CW, Shaywitz AJ, Dolmetsch RE, Griffith EC, Hu LS, Haddad C, Xia Z, Greenberg ME, CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events, Neuron. 34 (2002) 221–233. 10.1016/S0896-6273(02)00655-4. [DOI] [PubMed] [Google Scholar]
- [15].Hogan PG, Chen L, Nardone J, Rao A, Transcriptional regulation by calcium, calcineurin, and NFAT, Genes Dev. 17 (2003) 2205–2232. 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- [16].Sabatini BL, Oertner TG, Svoboda K, The life cycle of Ca2+ ions in dendritic spines, Neuron. 33 (2002) 439–452. 10.1016/S0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- [17].Ly CV, Verstreken P, Mitochondria at the synapse, Neuroscientist. 12 (2006) 291–299. 10.1177/1073858406287661. [DOI] [PubMed] [Google Scholar]
- [18].Brown MR, Sullivan PG, Geddes JW, Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria, J. Biol. Chem 281 (2006) 11658–11668. 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- [19].Toescu EC, Role of calcium in normal aging and neurodegeneration, Aging Cell. 6 (2007) 265. 10.1111/j.1474-9726.2007.00299.x. [DOI] [PubMed] [Google Scholar]
- [20].Bezprozvanny I, Calcium signaling and neurodegenerative diseases, Trends Mol. Med 15 (2009) 89–100. 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khachaturian ZS, Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis, Alzheimer’s Dement. 13 (2017) 178–182.e17. 10.1016/j.jalz.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [22].Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T, Mohan PS, Shea TB, Beermann M, Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease, in: Ann. N. Y. Acad. Sci, Blackwell Publishing Inc., 1994: pp. 77–91. 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- [23].Disterhoft JF, Moyer JR, Thompson LT, The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging, in: Ann. N. Y. Acad. Sci, Blackwell Publishing Inc., 1994: pp. 382–406. 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- [24].Brunelle JK, Letai A, Control of mitochondrial apoptosis by the Bcl-2 family, J. Cell Sci 122 (2009) 437–441. 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tait SWG, Green DR, Mitochondria and cell death: Outer membrane permeabilization and beyond, Nat. Rev. Mol. Cell Biol 11 (2010) 621–632. 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- [26].Ivanova H, Wagner LE, Akihiko II, Elien T, Luyten T, Welkenhuyzen K, Alzayady KJ, Wang L, Hamada K, Mikoshiba K, Bcl-2 and - IP3 compete for the ligand-binding domain of - IP3 Rs modulating - Ca2+ signaling output, (2019) 3843–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vervliet T, Parys JB, Bultynck G, Bcl-2 proteins and calcium signaling: complexity beneath the surface, Oncogene. 35 (2016) 5079–5092. 10.1038/onc.2016.31. [DOI] [PubMed] [Google Scholar]
- [28].Montero J, Letai A, Why do BCL-2 inhibitorswork and where should we use them in the clinic?, Cell Death Differ. 25 (2018) 56–64. 10.1038/cdd.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Del Gaizo Moore V, Letai A, BH3 profiling - Measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions, Cancer Lett. 332 (2013) 202–205. 10.1016/j.canlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elmore S, Apoptosis: A Review of Programmed Cell Death, Toxicol. Pathol 35 (2007) 495–516. 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lin QS, Mitochondria and apoptosis, Acta Biochim. Biophys. Sin. (Shanghai) 31 (1999) 118. 10.1126/science.281.5381.1309. [DOI] [Google Scholar]
- [32].Bock FJ, Tait SWG, Mitochondria as multifaceted regulators of cell death, Nat. Rev. Mol. Cell Biol 21 (2020) 85–100. 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- [33].Kale J, Osterlund EJ, Andrews DW, BCL-2 family proteins: Changing partners in the dance towards death, Cell Death Differ. 25 (2018) 65–80. 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D, Parsons MJ, vandeKooij B, Bouchier-Hayes L, Chalmers AJ, Rooswinkel RW, Oberst A, Blyth K, Rehm M, Murphy DJ, Tait SWG, Limited Mitochondrial Permeabilization Causes DNA Damage and Genomic Instability in the Absence of Cell Death, Mol. Cell 57 (2015) 860–872. 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chipuk JE, Green DR, How do BCL-2 proteins induce mitochondrial outer membrane permeabilization?, (2008). 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kroemer G, Galluzzi L, Brenner C, Mitochondrial Membrane Permeabilization in Cell Death, (2007). 10.1152/physrev.00013.2006.-Irrespective. [DOI] [PubMed] [Google Scholar]
- [37].Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH, Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c, 2000. [DOI] [PubMed] [Google Scholar]
- [38].Niu X, Brahmbhatt H, Mergenthaler P, Zhang Z, Sang J, Daude M, Ehlert FGR, Diederich WE, Wong E, Zhu W, Pogmore J, Nandy JP, Satyanarayana M, Jimmidi RK, Arya P, Leber B, Lin J, Culmsee C, Yi J, Andrews DW, A Small-Molecule Inhibitor of Bax and Bak Oligomerization Prevents Genotoxic Cell Death and Promotes Neuroprotection, Cell Chem. Biol 24 (2017) 493–506.e5. 10.1016/j.chembiol.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Warren CFA, Wong-Brown MW, Bowden NA, BCL-2 family isoforms in apoptosis and cancer, Cell Death Dis. 10 (2019). 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Villalobos-Ortiz M, Ryan J, Mashaka TN, Opferman JT, Letai A, BH3 profiling discriminates on-target small molecule BH3 mimetics from putative mimetics, Cell Death Differ. 27 (2020) 999–1007. 10.1038/s41418-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Barclay LA, Wales TE, Garner TP, Wachter F, Lee S, Guerra RM, Stewart ML, Braun CR, Bird GH, Gavathiotis E, Engen JR, Walensky LD, Inhibition of Pro-Apoptotic BAX by a Noncanonical Interaction Mechanism, Mol. Cell 57 (2015) 873–886. 10.1016/j.molcel.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huang DCS, Adams JM, Cory S, The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hirotani M, Zhang Y, Fujita N, Naito M, Tsuruo T, NH2-terminal BH4 domain of Bcl-2 is functional for heterodimerization with Bax and inhibition of apoptosis, J. Biol. Chem 274 (1999) 20415–20420. 10.1074/jbc.274.29.20415. [DOI] [PubMed] [Google Scholar]
- [44].Monaco G, Vervliet T, Akl H, Bultynck G, The selective BH4-domain biology of Bcl-2-family members: IP3Rs and beyond, Cell. Mol. Life Sci 70 (2013) 1171–1183. 10.1007/s00018-012-1118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Distelhorst CW, Bootman MD, Creating a new cancer therapeutic agent by targeting the interaction between Bcl-2 and IP3 receptors, Cold Spring Harb. Perspect. Biol 11 (2019). 10.1101/cshperspect.a035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].de Ridder I, Kerkhofs M, Veettil SP, Dehaen W, Bultynck G, Cancer cell death strategies by targeting Bcl-2’s BH4 domain, Biochim. Biophys. Acta - Mol. Cell Res 1868 (2021) 118983. 10.1016/j.bbamcr.2021.118983. [DOI] [PubMed] [Google Scholar]
- [47].Vervloessem T, Sasi BK, Xerxa E, Karamanou S, Kale J, La Rovere RM, Chakraborty S, Sneyers F, Vogler M, Economou A, Laurenti L, Andrews DW, Efremov DG, Bultynck G, BDA-366, a putative Bcl-2 BH4 domain antagonist, induces apoptosis independently of Bcl-2 in a variety of cancer cell models, Cell Death Dis. 11 (2020). 10.1038/s41419-020-02944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Birkinshaw RW, Challenges in small-molecule target identification: a commentary on “BDA-366, a putative Bcl-2 BH4 domain antagonist, induces apoptosis independently of Bcl-2 in a variety of cancer cell models,” Cell Death Differ. (2021). 10.1038/s41418-020-00717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baffy G, Miyashita T, Williamson JR, Reed JC, Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production., J. Biol. Chem 268 (1993) 6511–9. [PubMed] [Google Scholar]
- [50].He H, Lam M, McCormick TS, Distelhorst CW, Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl- 2, J. Cell Biol 138 (1997) 1219–1228. 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Magnelli L, Cinelli M, Turchetti A, Chiarugi VP, Bcl-2 Overexpression Abolishes Early Calcium Waving Preceding Apoptosis in NIH-3T3 Murine Fibroblasts, Biochem. Biophys. Res. Commun 204 (1994) 84–90. 10.1006/bbrc.1994.2429. [DOI] [PubMed] [Google Scholar]
- [52].Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ, BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis, Science (80-. ). 300 (2003) 135–139. 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- [53].Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N, VDAC, a multi-functional mitochondrial protein regulating cell life and death, Mol. Aspects Med 31 (2010) 227–285. 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [54].Shoshan-Barmatz V, Ben-Hail D, VDAC, a multi-functional mitochondrial protein as a pharmacological target, Mitochondrion. 12 (2012) 24–34. 10.1016/j.mito.2011.04.001. [DOI] [PubMed] [Google Scholar]
- [55].Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L, Araujo N, Pinna G, Larochette N, Zamzami N, Modjtahedi N, Harel-Bellan A, Kroemer G, Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death, Oncogene. 27 (2008) 4221–4232. 10.1038/onc.2008.63. [DOI] [PubMed] [Google Scholar]
- [56].Yuan S, Fu Y, Wang X, Shi H, Huang Y, Song X, Li L, Song N, Luo Y, Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis, FASEB J. 22 (2008) 2809–2820. 10.1096/fj.08-107417. [DOI] [PubMed] [Google Scholar]
- [57].Ghosh T, Pandey N, Maitra A, Brahmachari SK, Pillai B, A Role for Voltage-Dependent Anion Channel Vdac1 in Polyglutamine-Mediated Neuronal Cell Death, PLoS One. 2 (2007) e1170. 10.1371/journal.pone.0001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Godbole A, Varghese J, Sarin A, Mathew MK, VDAC is a conserved element of death pathways in plant and animal systems, Biochim. Biophys. Acta - Mol. Cell Res 1642 (2003) 87–96. 10.1016/S0167-4889(03)00102-2. [DOI] [PubMed] [Google Scholar]
- [59].Lü AJ, Dong CW, Du CS, Zhang QY, Characterization and expression analysis of Paralichthys olivaceus voltage-dependent anion channel (VDAC) gene in response to virus infection, Fish Shellfish Immunol. 23 (2007) 601–613. 10.1016/j.fsi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- [60].Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V, The voltage-dependent anion channel-1 modulates apoptotic cell death, Cell Death Differ. 12 (2005) 751–760. 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- [61].De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R, VDAC1 selectively transfers apoptotic Ca 2 signals to mitochondria, Cell Death Differ. 19 (2012) 267–273. 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shoshan-Barmatz V, Nahon-Crystal E, Shteinfer-Kuzmine A, Gupta R, VDAC1, mitochondrial dysfunction, and Alzheimer’s disease, Pharmacol. Res. 131 (2018) 87–101. 10.1016/j.phrs.2018.03.010. [DOI] [PubMed] [Google Scholar]
- [63].Shoshan-Barmatz V, Keinan N, Zaid H, Uncovering the role of VDAC in the regulation of cell life and death, J. Bioenerg. Biomembr 40 (2008) 183–191. 10.1007/s10863-008-9147-9. [DOI] [PubMed] [Google Scholar]
- [64].Monaco G, Decrock E, Arbel N, Van Vliet AR, La Rovere RM, De Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, Bultynck G, The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria, J. Biol. Chem 290 (2015) 9150–9161. 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V, The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins, J. Cell Sci 122 (2009) 1906–1916. 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- [66].Shteinfer-Kuzmine A, Amsalem Z, Arif T, Zooravlov A, Shoshan-Barmatz V, Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy, Mol. Oncol 12 (2018) 1077–1103. 10.1002/1878-0261.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Arbel N, Ben-Hail D, Shoshan-Barmatz V, Mediation of the Antiapoptotic Activity of Bcl-xL Protein upon Interaction with VDAC1 Protein *, (2012). 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huang H, Hu X, Eno CO, Zhao G, Li C, White C, An interaction between Bcl-xL and the Voltage-dependent Anion Channel (VDAC) promotes mitochondrial Ca2+ uptake, J. Biol. Chem 288 (2013) 19870–19881. 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Huang H, Shah K, Bradbury NA, Li C, White C, Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+uptake and reactive oxygen species generation, Cell Death Dis. 5 (2014). 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shimizu S, Narita M, Tsujimoto Y, Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC, Nature. 399 (1999) 483–487. 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- [71].Roy SS, Ehrlich AM, Craigen WJ, Hajnóczky G, VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria, EMBO Rep. 10 (2009) 1341–1347. 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Naghdi S, Várnai P, Hajnóczky G, Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis, Proc. Natl. Acad. Sci. U. S. A 112 (2015) E5590–E5599. 10.1073/pnas.1510574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chin HS, Li MX, Tan IKL, Ninnis RL, Reljic B, Scicluna K, Dagley LF, Sandow JJ, Kelly GL, Samson AL, Chappaz S, Khaw SL, Chang C, Morokoff A, Brinkmann K, Webb A, Hockings C, Hall CM, Kueh AJ, Ryan MT, Kluck RM, Bouillet P, Herold MJ, Gray DHD, Huang DCS, van Delft MF, Dewson G, VDAC2 enables BAX to mediate apoptosis and limit tumor development, Nat. Commun 9 (2018). 10.1038/s41467-018-07309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhu L, Yu Y, Chua BHL, Ho YS, Kuo TH, Regulation of sodium - Calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice, J. Mol. Cell. Cardiol 33 (2001) 2135–2144. 10.1006/jmcc.2001.1476. [DOI] [PubMed] [Google Scholar]
- [75].Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ, Bcl-2 family interaction with the mitochondrial morphogenesis machinery, Cell Death Differ. 18 (2011) 235–247. 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Autret A, Martin SJ, Emerging Role for Members of the Bcl-2 Family in Mitochondrial Morphogenesis, Mol. Cell 36 (2009) 355–363. 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- [77].Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z, Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 11649–11654. 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ, Role of Bax and Bak in mitochondrial morphogenesis, Nature. 443 (2006) 658–662. 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- [79].Pfeiffer A, Schneider J, Bueno D, Dolga A, Voss TD, Lewerenz J, Wüllner V, Methner A, Bcl-xL knockout attenuates mitochondrial respiration and causes oxidative stress that is compensated by pentose phosphate pathway activity, Free Radic. Biol. Med 112 (2017) 350–359. 10.1016/j.freeradbiomed.2017.08.007. [DOI] [PubMed] [Google Scholar]
- [80].Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, Chen Y, Messerli SM, Mariggio MA, Rahner C, McNay E, Shore GC, Smith PJS, Hardwick JM, Jonas EA, Bcl-x L regulates metabolic efficiency of neurons through interaction with the mitochondrial F1 FO ATP synthase, Nat. Cell Biol 13 (2011) 1224–1233. 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, Mignery GA, Parys JB, De Smedt H, Distelhorst CW, Targeting Bcl-2-IP3 Receptor Interaction to Reverse Bcl-2’s Inhibition of Apoptotic Calcium Signals, Mol. Cell 31 (2008) 255–265. 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].H I, LE W, A T, E V, T L, K W, KJ A, L W, K H, K M, H DS, L M, DI Y, JB P, G B, Bcl-2 and IP3 compete for the ligand-binding domain of IP3Rs modulating Ca2+ signaling output., Cell. Mol. Life Sci 76 (2019) 3843–3859. 10.1007/S00018-019-03091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW, The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 14397–14402. 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ivanova H, Ritane A, Wagner L, Luyten T, Shapovalov G, Welkenhuyzen K, Seitaj B, Monaco G, De Smedt H, Prevarskaya N, Yule DI, Parys JB, Bultynck G, The trans-membrane domain of Bcl-2β, but not its hydrophobic cleft, is a critical determinant for efficient IP3 receptor inhibition, Oncotarget. 7 (2016) 55704–55720. 10.18632/oncotarget.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bonneau B, Nougarède A, Prudent J, Popgeorgiev N, Peyriéras N, Rimokh R, Gillet G, The Bcl-2 homolog Nrz inhibits binding of IP3to its receptor to control calcium signaling during zebrafish epiboly, Sci. Signal 7 (2014) ra14–ra14. 10.1126/scisignal.2004480. [DOI] [PubMed] [Google Scholar]
- [86].Nougarede A, Popgeorgiev N, Kassem L, Omarjee S, Borel S, Mikaelian I, Lopez J, Gadet R, Marcillat O, Treilleux I, Villoutreix BO, Rimokh R, Gillet G, Breast cancer targeting through inhibition of the endoplasmic reticulum-based apoptosis regulator Nrh/BCL2L10, Cancer Res. 78 (2018) 1404–1417. 10.1158/0008-5472.CAN-17-0846. [DOI] [PubMed] [Google Scholar]
- [87].Hartman ML, Czyz M, BCL-w: apoptotic and non-apoptotic role in health and disease, Cell Death Dis. 11 (2020) 260. 10.1038/s41419-020-2417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, Bultynck G, Selective regulation of IP 3-receptor-mediated Ca 2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl, Cell Death Differ. 19 (2012) 295–309. 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lanner JT, Georgiou DK, Joshi AD, Hamilton SL, Ryanodine receptors: structure, expression, molecular details, and function in calcium release., Cold Spring Harb. Perspect. Biol 2 (2010). 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Vervliet T, Decrock E, Molgó J, Sorrentino V, Missiaen L, Leybaert L, De Smedt H, Kasri NN, Parys JB, Bultynck G, Bcl-2 binds to and inhibits ryanodine receptors, J. Cell Sci 127 (2014) 2782–2792. 10.1242/jcs.150011. [DOI] [PubMed] [Google Scholar]
- [91].Eckenrode EF, Yang J, Velmurugan GV, Kevin Foskett J, White C, Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling, J. Biol. Chem 285 (2010) 13678–13684. 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK, The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R, Nat. Cell Biol 7 (2005) 1021–1028. 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW, Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate, J. Cell Biol 166 (2004) 193–203. 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Varadarajan S, Bampton ETW, Smalley JL, Tanaka K, Caves RE, Butterworth M, Wei J, Pellecchia M, Mitcheson J, Gant TW, Dinsdale D, Cohen GM, A novel cellular stress response characterised by a rapid reorganisation of membranes of the endoplasmic reticulum, Cell Death Differ. 19 (2012) 1896–1907. 10.1038/cdd.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJH, The Bcl-2 protein family member bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage, J. Biol. Chem 288 (2013) 25340–25349. 10.1074/jbc.M113.496570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Popgeorgiev N, Jabbour L, Gillet G, Subcellular localization and dynamics of the Bcl-2 family of proteins, Front. Cell Dev. Biol 6 (2018). 10.3389/fcell.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].D’Orsi B, Mateyka J, Prehn JHM, Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok, Neurochem. Int 109 (2017) 162–170. 10.1016/j.neuint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- [98].D’Orsi B, Engel T, Pfeiffer S, Nandi S, Kaufmann T, Henshall DC, Prehn JHM, Bok is not pro-apoptotic but suppresses poly ADP-ribose polymerase-dependent cell death pathways and protects against excitotoxic and seizure-induced neuronal injury, J. Neurosci 36 (2016) 4564–4578. 10.1523/JNEUROSCI.3780-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Krajewski S, Mai JK, Krajewska M, Sikorska M, Mossakowski MJ, J.C. Reed4, Upregulation of Bax Protein Levels in Neurons following Cerebral lschemia, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Motoyama N, Wang F, Roth KA, Sawa H, Nakayama KI, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh DY, Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice, Science (80-. ). 267 (1995) 1506–1510. 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- [101].O’Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DCS, Strasser A, Tissue expression and subcellular localization of the pro-survival molecule Bcl-w, Cell Death Differ. 8 (2001) 486–494. 10.1038/sj.cdd.4400835. [DOI] [PubMed] [Google Scholar]
- [102].Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ, Bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS, Development. 120 (1994) 301–311. [DOI] [PubMed] [Google Scholar]
- [103].Pemberton JM, Pogmore JP, Andrews DW, Neuronal cell life, death, and axonal degeneration as regulated by the BCL-2 family proteins, Cell Death Differ. (2020). 10.1038/s41418-020-00654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW, Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene bax, J. Neurosci 24 (2004) 11205–11213. 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, Poulter MO, Ferguson SSG, Strasser A, Cregan SP, Puma is a dominant regulator of oxidative stress induced bax activation and neuronal apoptosis, J. Neurosci 27 (2007) 12989–12999. 10.1523/JNEUROSCI.3400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP, Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA, J. Neurosci 30 (2010) 16938–16948. 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, Tešić Mark M, Molina H, Tessier-Lavigne M, Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling, Cell. 164 (2016) 1031–1045. 10.1016/j.cell.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sun W, Gould TW, Vinsant S, Prevette D, Oppenheim RW, Neuromuscular development after the prevention of naturally occurring neuronal death by Bax deletion, J. Neurosci 23 (2003) 7298–7310. 10.1523/jneurosci.23-19-07298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Snider WD, Korsmeyer SJ, BAX is required for neuronal death after trophic factor deprivation and during development, Neuron. 17 (1996) 401–411. 10.1016/S0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- [110].White FA, Keller-Peck CR, Michael Knudson C, Korsmeyer SJ, Snider WD, Widespread elimination of naturally occurring neuronal death in Bax- deficient mice, J. Neurosci 18 (1998) 1428–1439. 10.1523/jneurosci.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sánchez-Gómez MV, Alberdi E, Pérez-Navarro E, Alberch J, Matute C, Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors, J. Neurosci 31 (2011) 2996–3006. 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].D’Orsi B, Bonner H, Tuffy LP, Düssmann H, Woods I, Courtney MJ, Ward MW, Prehn JHM, Calpains are downstream effectors of bax-dependent excitotoxic apoptosis, J. Neurosci 32 (2012) 1847–1858. 10.1523/JNEUROSCI.2345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].D’Orsi B, Kilbride SM, Chen G, Alvarez SP, Bonner HP, Pfeiffer S, Plesnila N, Engel T, Henshall DC, Düssmann H, Prehn JHM, Bax regulates neuronal Ca2+Homeostasis, J. Neurosci 35 (2015) 1706–1722. 10.1523/JNEUROSCI.2453-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Garcia I, Crowther AJ, Gama V, Ryan Miller C, Deshmukh M, Gershon TR, Bax deficiency prolongs cerebellar neurogenesis, accelerates medulloblastoma formation and paradoxically increases both malignancy and differentiation, Oncogene. 32 (2013) 2304–2314. 10.1038/onc.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fannjiang Y, Kim CH, Huganir RL, Zou S, Lindsten T, Thompson CB, Mito T, Traystman RJ, Larsen T, Griffin DE, Mandir AS, Dawson TM, Dike S, Sappington AL, Kerr DA, Jonas EA, Kaczmarek LK, Hardwick JM, BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development, Dev. Cell 4 (2003) 575–585. 10.1016/S1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- [116].Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB, The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues, Mol. Cell 6 (2000) 1389–1399. 10.1016/S1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Marie Hardwick J, Soane L, Multiple functions of BCL-2 family proteins, Cold Spring Harb. Perspect. Biol 5 (2013). 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ, Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair, Cell. 75 (1993) 229–240. 10.1016/0092-8674(93)80065-M. [DOI] [PubMed] [Google Scholar]
- [119].Nakayama K, Nakayama KI, Negishi I, Kuida K, Sawa H, Loh DY, Targeted disruption of Bcl-2αβ in mice: Occurrence of gray hair, polycystic kidney disease, and lymphocytopenia, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 3700–3704. 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M, Thoenen H, Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development, Neuron. 17 (1996) 75–89. 10.1016/S0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- [121].Abe-Dohmae S, Harada N, Yamada K, Tanaka R, BCL-2 Gene is highly expressed during neurogenesis in the central nervous system, Biochem. Biophys. Res. Commun 191 (1993) 915–921. 10.1006/bbrc.1993.1304. [DOI] [PubMed] [Google Scholar]
- [122].Chen DF, Schneider GE, Martinou JC, Tonegawa S, Bcl-2 promotes regeneration of severed axons in mammalian CNS, Nature. 385 (1997) 434–439. 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- [123].Liu ME, Huang CC, Yang AC, Tu PC, Yeh HL, Hong CJ, Chen JF, Liou YJ, Lin CP, Tsai SJ, Effect of Bcl-2 rs956572 Polymorphism on Age-Related Gray Matter Volume Changes, PLoS One. 8 (2013) 56663. 10.1371/journal.pone.0056663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chen G, Manji HK, The extracellular signal-regulated kinase pathway: An emerging promising target for mood stabilizers, Curr. Opin. Psychiatry 19 (2006) 313–323. 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- [125].Jiao J, Huang X, Feit-Leithman RA, Neve RL, Snider W, Dartt DA, Chen DF, Bcl-2 enhances Ca2+ signaling to support the intrinsic regenerative capacity of CNS axons, EMBO J. 24 (2005) 1068–1078. 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sagot Y, Dubois-Dauphin M, Tan SA, De Bilbao F, Aebischer P, Martinou J-C, Katol AC, Bcl-2 Overexpression Prevents Motoneuron Cell Body Loss but Not Axonal Degeneration in a Mouse Model of a Neurodegenerative Disease, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT, Role of NMDA Receptor Subtypes in Governing the Direction of Hippocampal Synaptic Plasticity, Science (80-. ). 304 (2004) 1021–1024. 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- [128].Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK, Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat, J. Cereb. Blood Flow Metab 24 (2004) 681–692. 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]
- [129].Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D, Enhanced oxidative stress and altered antioxidants in brains of Bcl-2- deficient mice, J. Neurochem 71 (1998) 741–748. 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- [130].Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J, Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia, Neuron. 13 (1994) 1017–1030. 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- [131].Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM, Yu SP, Choi DW, Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia, Neurobiol. Dis 19 (2005) 183–193. 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- [132].Deng G, Su JH, Ivins KJ, Van Houten B, Cotman CW, Bcl-2 facilitates recovery from DNA damage after oxidative stress, Exp. Neurol 159 (1999) 309–318. 10.1006/exnr.1999.7145. [DOI] [PubMed] [Google Scholar]
- [133].Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, Signore AP, Chen J, Sun FY, Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion, Neurobiol. Dis 24 (2006) 345–356. 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [134].Mattson MP, Apoptosis in neurodegenerative disorders, Nat. Rev. Mol. Cell Biol 1 (2000) 120–129. 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- [135].Jonas E, BCL-xL regulates synaptic plasticity, Mol. Interv 6 (2006) 208–222. 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- [136].Cosker KE, Pazyra-Murphy MF, Fenstermacher SJ, Segal RA, Target-derived neurotrophins coordinate transcription and transport of Bclw to prevent axonal degeneration, Ann. Intern. Med 158 (2013) 5195–5207. 10.1523/JNEUROSCI.3862-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yan C, Chen J, Chen D, Minami M, Pei W, Yin XM, Simon RP, Overexpression of the cell death suppressor Bcl-w in ischemic brain: Implications for a neuroprotective role via the mitochondrial pathway, J. Cereb. Blood Flow Metab 20 (2000) 620–630. 10.1097/00004647-200003000-00020. [DOI] [PubMed] [Google Scholar]
- [138].Minami M, Lin Jin K, Li W, Nagayama T, Henshall DC, Simon RP, Bcl-w expression is increased in brain regions affected by focal cerebral ischemia in the rat, Neurosci. Lett 279 (2000) 193–195. 10.1016/S0304-3940(99)00987-8. [DOI] [PubMed] [Google Scholar]
- [139].Zhu X, Wang Y, Ogawa O, Lee H, Raina AK, Siedlak SL, Harris PLR, Fujioka H, Shimohama S, Tabaton M, Atwood CS, Petersen RB, Perry G, Smith MA, Neuroprotective properties of Bcl-w in Alzheimer disease, J. Neurochem 89 (2004) 1233–1240. 10.1111/j.1471-4159.2004.02416.x. [DOI] [PubMed] [Google Scholar]
- [140].Courchesne SL, Karch C, Pazyra-Murphy MF, Segal RA, Sensory neuropathy attributable to loss of Bcl-w, J. Neurosci 31 (2011) 1624–1634. 10.1523/JNEUROSCI.3347-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Pease-Raissi SE, Pazyra-Murphy MF, Li Y, Wachter F, Fukuda Y, Fenstermacher SJ, Barclay LA, Bird GH, Walensky LD, Segal RA, Paclitaxel Reduces Axonal Bclw to Initiate IP3R1-Dependent Axon Degeneration, Neuron. 96 (2017) 373–386.e6. 10.1016/j.neuron.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nussbaum RL, Ellis CE, Alzheimer’s Disease and Parkinson’s Disease, N. Engl. J. Med. 348 (2003) 1356–1364. 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- [143].Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, Cedarbaum J, Brashear R, Miller DS, Neuropsychiatric symptoms in Alzheimer’s disease, Alzheimer’s Dement. 7 (2011) 532–539. 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Selkoe DJ, Alzheimer’s disease: Genes, proteins, and therapy, Physiol. Rev 81 (2001) 741–766. 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- [145].Hardy JA, Higgins GA, Alzheimer’s disease: The amyloid cascade hypothesis, Science (80-. ). 256 (1992) 184–185. 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- [146].Morris GP, Clark IA, Vissel B, Inconsistencies and Controversies Surrounding the Amyloid Hypothesis of Alzheimer’s Disease, Acta Neuropathol. Commun 2 (2014) 135. 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Karran E, Mercken M, De Strooper B, The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics, Nat. Rev. Drug Discov 10 (2011) 698–712. 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- [148].Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A, The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease, Nature. 537 (2016) 50–56. 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- [149].Khachaturian ZS, Calcium hypothesis of Alzheimer’s disease and brain aging, in: Ann. N. Y. Acad. Sci, Blackwell Publishing Inc., 1994: pp. 1–11. 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- [150].Mattson MP, Chan SL, Neuronal and glial calcium signaling in Alzheimer’s disease, Cell Calcium. 34 (2003) 385–397. 10.1016/S0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- [151].Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VMY, Foskett JK, Mechanism of Ca2+ Disruption in Alzheimer’s Disease by Presenilin Regulation of InsP3 Receptor Channel Gating, Neuron. 58 (2008) 871–883. 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Stutzmann GE, Calcium dysregulation, IP3 signaling, and Alzheimer’s disease, Neuroscientist. 11 (2005) 110–115. 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- [153].Koffie RM, Hyman BT, Spires-Jones TL, Alzheimer’s disease: Synapses gone cold, Mol. Neurodegener 6 (2011). 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Sheng M, Sabatini BL, Südhof TC, Synapses and Alzheimer’s disease, Cold Spring Harb. Perspect. Biol 4 (2012) 10. 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Riascos D, De Leon D, Baker-Nigh A, Nicholas A, Yukhananov R, Bu J, Wu CK, Geula C, Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer’s disease, Acta Neuropathol. 122 (2011) 565–576. 10.1007/s00401-011-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Xia P, Chen HSV, Zhang D, Lipton SA, Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses, J. Neurosci 30 (2010) 11246–11250. 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Bezprozvanny I, Mattson MP, Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease, Trends Neurosci. 31 (2008) 454–463. 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I, Presenilins Form ER Ca2+ Leak Channels, a Function Disrupted by Familial Alzheimer’s Disease-Linked Mutations, Cell. 126 (2006) 981–993. 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Nelson O, Tu H, Lei T, Bentahir M, De Strooper B, Bezprozvanny I, Familial Alzheimer disease-linked mutations specifically disrupt Ca 2+ leak function of presenilin 1, J. Clin. Invest 117 (2007) 1230–1239. 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL, Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 534–538. 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cheung KH, Mei L, Mak DOD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK, Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons, Sci. Signal 3 (2010). 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL, Calcium responses in fibroblasts from asymptomatic members of Alzheimer’s disease families, Neurobiol. Dis 5 (1998) 37–45. 10.1006/nbdi.1998.0176. [DOI] [PubMed] [Google Scholar]
- [163].Leissring MA, Paul BA, Parker I, Cotman CW, Laferla FM, Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5- trisphosphate-mediated calcium signaling in Xenopus oocytes, J. Neurochem 72 (1999) 1061–1068. 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- [164].Stutzmann GE, Caccamo A, LaFerla FM, Parker I, Dysregulated IP3 Signaling in Cortical Neurons of Knock-In Mice Expressing an Alzheimer’s-Linked Mutation in Presenilin1 Results in Exaggerated Ca2+ Signals and Altered Membrane Excitability, J. Neurosci 24 (2004) 508–513. 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE, Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease, Neurobiol. Aging 33 (2012) 1001.e1–1001.e6. 10.1016/j.neurobiolaging.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Bussiere R, Lacampagne A, Reiken S, Liu X, Scheuerman V, Zalk R, Martin C, Checler F, Marks AR, Chami M, Amyloid β production is regulated by β2-adrenergic signaling-mediated post-translational modifications of the ryanodine receptor, J. Biol. Chem 292 (2017) 10153–10168. 10.1074/jbc.M116.743070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, Stutzmann GE, Stabilizing ER Ca2+ Channel Function as an Early Preventative Strategy for Alzheimer’s Disease, PLoS One. 7 (2012) e52056. 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP, Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons, J. Biol. Chem 275 (2000) 18195–18200. 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- [169].Lacampagne A, Liu X, Reiken S, Bussiere R, Meli AC, Lauritzen I, Teich AF, Zalk R, Saint N, Arancio O, Bauer C, Duprat F, Briggs CA, Chakroborty S, Stutzmann GE, Shelanski ML, Checler F, Chami M, Marks AR, Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits, Acta Neuropathol. 134 (2017) 749–767. 10.1007/s00401-017-1733-7. [DOI] [PubMed] [Google Scholar]
- [170].Stutzmann GE, Smith I, Caccamo A, Oddo S, LaFerla FM, Parker I, Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice, J. Neurosci 26 (2006) 5180–5189. 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Popugaeva E, Bezprozvanny I, Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease, Front. Mol. Neurosci 6 (2013). 10.3389/fnmol.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sun S, Zhang H, Liu J, Popugaeva E, Xu NJ, Feske S, White CL, Bezprozvanny I, Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice, Neuron. 82 (2014) 79–93. 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, Bezprozvanny I, Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s disease, J. Neurosci 35 (2015) 13275–13286. 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhang H, Sun S, Wu L, Pchitskaya E, Zakharova O, Tacer KF, Bezprozvanny I, Store-operated calcium channel complex in postsynaptic spines: A new therapeutic target for Alzheimer’s disease treatment, J. Neurosci 36 (2016) 11837–11850. 10.1523/JNEUROSCI.1188-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Calvo-Rodriguez M, Hernando-Perez E, Nuñez L, Villalobos C, Amyloid β oligomers increase ER-mitochondria Ca2+ cross talk in young hippocampal neurons and exacerbate aging-induced intracellular Ca2+ remodeling, Front. Cell. Neurosci 13 (2019). 10.3389/fncel.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW, Presenilin-mediated modulation of capacitative calcium entry, Neuron. 27 (2000) 561–572. 10.1016/S0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- [177].Querfurth HW, Selkoe DJ, Calcium Ionophore Increases Amyloid β Peptide Production by Cultured Cells, Biochemistry. 33 (1994) 4550–4561. 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- [178].Petryniak MA, Wurtman RJ, Slack BE, Elevated intracellular calcium concentration increases secretory processing of the amyloid precursor protein by a tyrosine phosphorylation-dependent mechanism, Biochem. J 320 (1996) 957–963. 10.1042/bj3200957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Buxbaum JD, Ruefli AA, Parker CA, Cypess AM, Greengard P, Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 4489–4493. 10.1073/pnas.91.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Supnet C, Bezprozvanny I, Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease, J. Alzheimer’s Dis 20 (2010). 10.3233/JAD-2010-100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Calvo-Rodriguez M, Bacskai BJ, Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death, Trends Neurosci. 44 (2021) 136–151. 10.1016/j.tins.2020.10.004. [DOI] [PubMed] [Google Scholar]
- [182].Area-Gomez E, De Groof AJC, Boldogh I, Bird TD, Gibson GE, Koehler CM, Yu WH, Duff KE, Yaffe MP, Pon LA, Schon EA, Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria, Am. J. Pathol 175 (2009) 1810–1816. 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Del Prete D, Suski JM, Oulès B, Debayle D, Gay AS, Lacas-Gervais S, Bussiere R, Bauer C, Pinton P, Paterlini-Bréchot P, Wieckowski MR, Checler F, Chami M, Localization and Processing of the Amyloid-β Protein Precursor in Mitochondria-Associated Membranes, J. Alzheimer’s Dis 55 (2017) 1549–1570. 10.3233/JAD-160953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, De Groof AJC, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA, Upregulated function of mitochondria-associated ER membranes in Alzheimer disease, EMBO J. 31 (2012) 4106–4123. 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G, Imaging Interorganelle Contacts and Local Calcium Dynamics at the ER-Mitochondrial Interface, Mol. Cell 39 (2010) 121–132. 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Calvo-Rodriguez M, Hou SS, Snyder AC, Kharitonova EK, Russ AN, Das S, Fan Z, Muzikansky A, Garcia-Alloza M, Serrano-Pozo A, Hudry E, Bacskai BJ, Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease, Nat. Commun 11 (2020). 10.1038/s41467-020-16074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Calvo-Rodriguez M, Bacskai BJ, High mitochondrial calcium levels precede neuronal death in vivo in Alzheimer’s disease, Cell Stress. 4 (2020) 187–190. 10.15698/cst2020.07.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Kudo W, Lee HP, Smith MA, Zhu X, Matsuyama S, Lee HG, Inhibition of Bax protects neuronal cells from oligomeric Aβ neurotoxicity, Cell Death Dis. 3 (2012). 10.1038/cddis.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Paradis E, Douillard H, Koutroumanis M, Goodyer C, LeBlanc A, Amyloid β peptide of Alzheimer’s disease downregulates bcl-2 and upregulates bax expression in human neurons, J. Neurosci 16 (1996) 7533–7539. 10.1523/jneurosci.16-23-07533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].MacGibbon GA, Lawlor PA, Sirimanne ES, Walton MR, Connor B, Young D, Williams C, Gluckman P, Faull RLM, Hughes P, Dragunow M, Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus, Brain Res. 750 (1997) 223–234. 10.1016/S0006-8993(96)01351-0. [DOI] [PubMed] [Google Scholar]
- [191].SU JH, DENG G, COTMAN CW, Bax Protein Expression Is Increased in Alzheimerʼs Brain, J. Neuropathol. Exp. Neurol 56 (1997) 86–93. 10.1097/00005072-199701000-00009. [DOI] [PubMed] [Google Scholar]
- [192].Tortosa A, Löpez E, Ferrer I, Bcl-2 and Bax protein expression in Alzheimer’s disease, Acta Neuropathol. 95 (1998) 407–412. 10.1007/s004010050817. [DOI] [PubMed] [Google Scholar]
- [193].Drache B, Diehl GE, Beyreuther K, Perlmutter LS, Knig G, Bcl-xl-Specific antibody labels activated microglia associated with Alzheimer’s disease and other pathological states, J. Neurosci. Res 47 (1997) 98–108. . [DOI] [PubMed] [Google Scholar]
- [194].Nikhi K, Shah K, The Cdk5-Mcl-1 axis promotes mitochondrial dysfunction and neurodegeneration in a model of Alzheimer’s disease, J. Cell Sci 130 (2017) 3023–3039. 10.1242/jcs.205666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sun KH, Lee HG, Smith MA, Shah K, Direct and indirect roles of cyclin-dependent kinase 5 as an upstream regulator in the c-Jun NH2-terminal kinase cascade: Relevance to neurotoxic insults in Alzheimer’s disease, Mol. Biol. Cell 20 (2009) 4611–4619. 10.1091/mbc.E09-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Cen X, Xu X, Xia H, Targeting MCL1 to induce mitophagy is a potential therapeutic strategy for Alzheimer disease, Autophagy. (2020). 10.1080/15548627.2020.1860542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, Smith MA, Perry G, Whitehouse PJ, Taniguchi T, Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease, Brain Res. 780 (1998) 260–269. 10.1016/S0006-8993(97)01202-X. [DOI] [PubMed] [Google Scholar]
- [198].Shimohama S, Fujimoto S, Sumida Y, Tanino H, Differential expression of rat brain Bcl-2 family proteins in development and aging, Biochem. Biophys. Res. Commun 252 (1998) 92–96. 10.1006/bbrc.1998.9577. [DOI] [PubMed] [Google Scholar]
- [199].Migheli A, Cavalla P, Piva R, Giordana MT, Bcl–2 protein expression in aged brain and neurodegenerative diseases, Neuroreport. 5 (1994) 1906–1908. 10.1097/00001756-199410000-00016. [DOI] [PubMed] [Google Scholar]
- [200].O’Barr S, Schultz J, Rogers J, Expression of the protooncogene bcl-2 in Alzheimer’s disease brain, Neurobiol. Aging 17 (1996) 131–136. 10.1016/0197-4580(95)02024-1. [DOI] [PubMed] [Google Scholar]
- [201].Satou T, Cummings BJ, Cotman CW, Immunoreactivity for Bcl-2 protein within neurons in the Alzheimer’s disease brain increases with disease severity, Brain Res. 697 (1995) 35–43. 10.1016/0006-8993(95)00748-F. [DOI] [PubMed] [Google Scholar]
- [202].Su JH, Satou T, Anderson AJ, Cotman CW, Up-regulation of Bcl-2 is associated with neuronal DNA damage in Alzheimer’s disease, Neuroreport. 7 (1996) 437–440. 10.1097/00001756-199601310-00015. [DOI] [PubMed] [Google Scholar]
- [203].Schaefer A, O’Carroll D, Chan LT, Hillman D, Sugimori M, Llinas R, Greengard P, Cerebellar neurodegeneration in the absence of microRNAs, J. Exp. Med 204 (2007) 1553–1558. 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Chang F, Zhang LH, Xu WUP, Jing P, Zhan PY, microRNA-9 attenuates amyloidβ-induced synaptotoxicity by targeting calcium/calmodulin-dependent protein kinase kinase 2, Mol. Med. Rep 9 (2014) 1917–1922. 10.3892/mmr.2014.2013. [DOI] [PubMed] [Google Scholar]
- [205].Wu B-W, Wu M-S, Guo J-D, Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer’s disease, J. Cell. Physiol 233 (2018) 5281–5292. 10.1002/jcp.26328. [DOI] [PubMed] [Google Scholar]
- [206].Kim YJ, Kim SH, Park Y, Park J, Lee JH, Kim BC, Song WK, miR-16–5p is upregulated by amyloid β deposition in Alzheimer’s disease models and induces neuronal cell apoptosis through direct targeting and suppression of BCL-2, Exp. Gerontol 136 (2020) 110954. 10.1016/j.exger.2020.110954. [DOI] [PubMed] [Google Scholar]
- [207].Schipper HM, Maes OC, Chertkow HM, Wang E, MicroRNA Expression in Alzheimer Blood Mononuclear Cells, Gene Regul. Syst. Bio 1 (2007) GRSB.S361. 10.4137/grsb.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Bhatnagar S, Chertkow H, Schipper HM, Yuan Z, Shetty V, Jenkins S, Jones T, Wang E, Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma, Front. Mol. Neurosci 7 (2014). 10.3389/fnmol.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zirnheld AL, Regalado EL, Shetty V, Chertkow H, Schipper HM, Wang E, Target Genes of Circulating miR-34c as Plasma Protein Biomarkers of Alzheimer’s Disease and Mild Cognitive Impairment, (2015). 10.4172/2329-8847.1000140. [DOI] [Google Scholar]
- [210].Li X, Wang L, Cykowski M, He T, Liu T, Chakranarayan J, Rivera A, Zhao H, Powell S, Xia W, Wong STC, OCIAD1 contributes to neurodegeneration in Alzheimer’s disease by inducing mitochondria dysfunction, neuronal vulnerability and synaptic damages, EBioMedicine. 51 (2020). 10.1016/j.ebiom.2019.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Chang C-C, Chang Y-T, Huang C-W, Tsai S-J, Hsu S-W, Huang S-H, Lee C-C, Chang W-N, Lui C-C, Lien C-Y, Associations of Bcl-2 rs956572 genotype groups in the structural covariance network in early-stage Alzheimer’s disease, Alzheimers. Res. Ther 10 (2018) 17. 10.1186/s13195-018-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].MacHado-Vieira R, Pivovarova NB, Stanika RI, Yuan P, Wang Y, Zhou R, Zarate CA, Drevets WC, Brantner CA, Baum A, Laje G, McMahon FJ, Chen G, Du J, Manji HK, Andrews SB, The Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder, Biol. Psychiatry 69 (2011) 344–352. 10.1016/j.biopsych.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Uemura T, Green M, Corson TW, Perova T, Li PP, Warsh JJ, Bcl-2 SNP rs956572 associates with disrupted intracellular calcium homeostasis in bipolar I disorder, Bipolar Disord. 13 (2011) 41–51. 10.1111/j.1399-5618.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- [214].Berridge MJ, Vitamin D, reactive oxygen species and calcium signalling in ageing and disease, (n.d.). 10.1098/rstb.2015.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Ureshino RP, Bertoncini CR, Fernandes MJS, Abdalla FMF, Porto CS, Hsu Y-T, Lopes GS, Smaili SS, Alterations in calcium signaling and a decrease in Bcl-2 expression: Possible correlation with apoptosis in aged striatum, J. Neurosci. Res 88 (2010) 438–447. 10.1002/jnr.22214. [DOI] [PubMed] [Google Scholar]
- [216].Ghosh D, LeVault KR, Brewer GJ, Dual-energy precursor and nuclear erythroid-related factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species, Neurobiol. Aging 35 (2014) 179–190. 10.1016/j.neurobiolaging.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL, Expression of Nrf2 in neurodegenerative diseases, J. Neuropathol. Exp. Neurol 66 (2007) 75–85. 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E, Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2, J. Neurosci 28 (2008) 3051–3059. 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Karlnoski R, Wilcock D, Dickey C, Ronan V, Gordon MN, Zhang W, Morgan D, Taglialatela G, Up-regulation of Bcl-2 in APP transgenic mice is associated with neuroprotection, Neurobiol. Dis 25 (2007) 179–188. 10.1016/j.nbd.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Asoh S, Mori T, Ohta S, Bcl-x(L) inhibits apoptosis and necrosis produced by Alzheimer’s β-amyloid1–40 peptide in PC12 cells, Neurosci. Lett 272 (1999) 5–8. 10.1016/S0304-3940(99)00525-X. [DOI] [PubMed] [Google Scholar]
- [221].Passer BJ, Pellegrini L, Vito P, Ganjei JK, D’Adamio L, Interaction of Alzheimer’s presenilin-1 and presenilin-2 with Bcl-X(L). A potential role in modulating the threshold of cell death, J. Biol. Chem 274 (1999) 24007–24013. 10.1074/jbc.274.34.24007. [DOI] [PubMed] [Google Scholar]
- [222].Lai J, Hu M, Wang H, Hu M, Long Y, Miao MX, Li JC, Wang XB, Kong LY, Hong H, Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Aβ1–42-induced memory impairment and neuroinflammatory and apoptotic responses in mice, Neuropharmacology. 79 (2014) 707–714. 10.1016/j.neuropharm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- [223].Wang H, Mei ZL, Zhong KL, Hu M, Long Y, Miao MX, Li N, Yan TH, Hong H, Pretreatment with antiasthmatic drug ibudilast ameliorates Aβ1–42-induced memory impairment and neurotoxicity in mice, Pharmacol. Biochem. Behav 124 (2014) 373–379. 10.1016/j.pbb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- [224].Tang SS, Ji MJ, Chen L, Hu M, Long Y, Li YQ, Miao MX, Li JC, Li N, Ji H, Chen XJ, Hong H, Protective effect of pranlukast on Aβ1–42-induced cognitive deficits associated with downregulation of cysteinyl leukotriene receptor 1, Int. J. Neuropsychopharmacol 17 (2014) 581–592. 10.1017/S1461145713001314. [DOI] [PubMed] [Google Scholar]
- [225].Gao HL, Li C, Nabeka H, Shimokawa T, Wang ZY, Cao YM, Matsuda S, An 18-mer Peptide Derived from Prosaposin Ameliorates the Effects of Aβ1–42 Neurotoxicity on Hippocampal Neurogenesis and Memory Deficit in Mice, J. Alzheimer’s Dis 53 (2016) 1173–1192. 10.3233/JAD-160093. [DOI] [PubMed] [Google Scholar]