Abstract
An operationally simple, high yielding three-step cascade process is described for the direct conversion of indole-tethered ynones into functionalized quinolines. A single “multitasking” thiol reagent is used to promote a three-step dearomatizing spirocyclization, nucleophilic substitution, and one-atom ring expansion reaction cascade under remarkably mild conditions. In addition, a novel route to thio-oxindoles is described, which was discovered by serendipity.
Cascade reactions (chemical processes by which two or more consecutive reactions take place in a single pot-process, also known as “tandem” or “domino” reactions) have wide utility in synthetic chemistry.1,2 Incorporating cascade reaction sequences into synthetic routes can significantly improve the speed and ease with which complex target molecules can be prepared and often means that the direct handling of reactive, unstable and/or toxic species can be avoided by forming these intermediates in situ.
This manuscript concerns a three-step cascade reaction sequence, starting from indole-tethered ynones 1 (Scheme 1). In recent years, ynones of this type have emerged as valuable precursors for the preparation of a diverse array of molecular scaffolds.3−6 For example, our groups and others have shown that the activation of the alkyne moiety of 1 promotes efficient dearomatizing spirocyclization7,8 to form medicinally important spirocyclic indolenines 2;9,10 this is most commonly done using π-acidic catalysts (especially Ag(I) species), although Brønsted acids, palladium(II) complexes, and electrophilic halogenation reagents can also be used (1 → 2, Scheme 1a, step 1).3,11,12 Our groups have also shown that dearomatization works well on 2-halogenated indoles (i.e., 1 where X = Cl, Br or I) and that the resulting indoleninyl halide products (i.e., 2 where X = Cl, Br or I) can be transformed further via reaction with nucleophiles, or via Pd-catalyzed cross-coupling, to substitute the halide for various other groups (2 → 3, Scheme 1a, step 2).5 Finally, our groups and others have demonstrated that spirocyclic indolenines of the form 3 will rearrange via a one-atom ring expansion reaction13 to form annulated quinolines, with both acidic and basic reagents able to promote this transformation (3 → 4, Scheme 1a, step 3).6
Scheme 1. Transformations of Indole-Tethered Ynones.
Efficient protocols for each of the individual steps represented in Scheme 1a are therefore established, but three steps are still required to generate functionalized quinolines 4 from ynones 1. Quinolines are found in many marketed drugs, as well as in various other applications.14 On the basis of a growing understanding of each of the three individual processes discussed above,3,5,6 we recognized that certain reagents may be able to promote all three steps and enable the transformation of 1 into 4 via a single-cascade process (Scheme 1b); such a reagent would need to act as an acid to promote step 1, a nucleophile in step 2, and a Brønsted acid to promote step 3. The successful realization of this strategy is reported herein, with thiols emerging as the optimum “multitasking” reagent class capable of promoting the envisaged cascade, under remarkably mild and operationally simple conditions.
We started by exploring the reactivity of model 2-bromo ynone 1aBr with various reagents (NuH) that we thought might have the required acidity and nucleophilicity to promote its conversion into a quinoline of the form 4. Phenol was tested first, and added to a solution of 1aBr in DCE,15 but no reaction was observed after stirring at RT or 60 °C (entries 1 and 2, Table 1). Next, TFA was included as an additive in the reaction, which led to the consumption of the starting material, but the only tractable products observed were oxindole 7a (presumably formed via acid-mediated dearomatizing spirocyclization and hydrolysis of the resulting spirocycle 5a),5 and bromoquinoline 8, which likely formed via a Bronsted acid-mediated rearrangement of 5a (cf. step 3).6b A more acidic NuH reagent, 4-nitrophenol, was tested but no reaction was observed at RT (entry 4), while at 60 °C the same side products 7a and 8 were formed (entry 5). We then decided to move on to species of similarly acidity to phenol, but also more nucleophilic, and pleasingly, thiols16 were found to possess this attractive combination of properties; using n-propanethiol, no conversion was observed at RT (entry 6), but excellent conversion into the desired quinoline 4a was observed upon heating to 60 °C (entry 7). Furthermore, the more acidic thiophenol was able to promote the conversion of 1aBr into quinoline 4b smoothly at RT (entry 8).
Table 1. Initial Optimizationa.
| entry | nucleophile (NuH) | temp | outcomeb |
|---|---|---|---|
| 1 | phenol (Nu = PhO) | RT | no reaction |
| 2 | phenol (Nu = PhO) | 60 °C | no reaction |
| 3 | phenol (Nu = PhO) with 1 equiv of TFA | RT | 7a (62%) 8 (21%) |
| 4 | 4-nitrophenol (Nu = 4-NO2C6H4O) | RT | no reaction |
| 5 | 4-nitrophenol (Nu = 4-NO2C6H4O) | 60 °C | 7a (35%) 8 (45%) |
| 6 | n-propanethiol (Nu = n-PrS) | RT | no reaction |
| 7 | n-propanethiol (Nu = n-PrS) | 60 °C | 4a (95%) |
| 8 | thiophenol (Nu = PhS) | RT | 4b (93%) |
1aBr (1 equiv) and NuH (1.6 equiv) were stirred in DCE (0.1 M, degassed) for 20−24 h at the specified temperature.
Yields are isolated material after column chromatography.
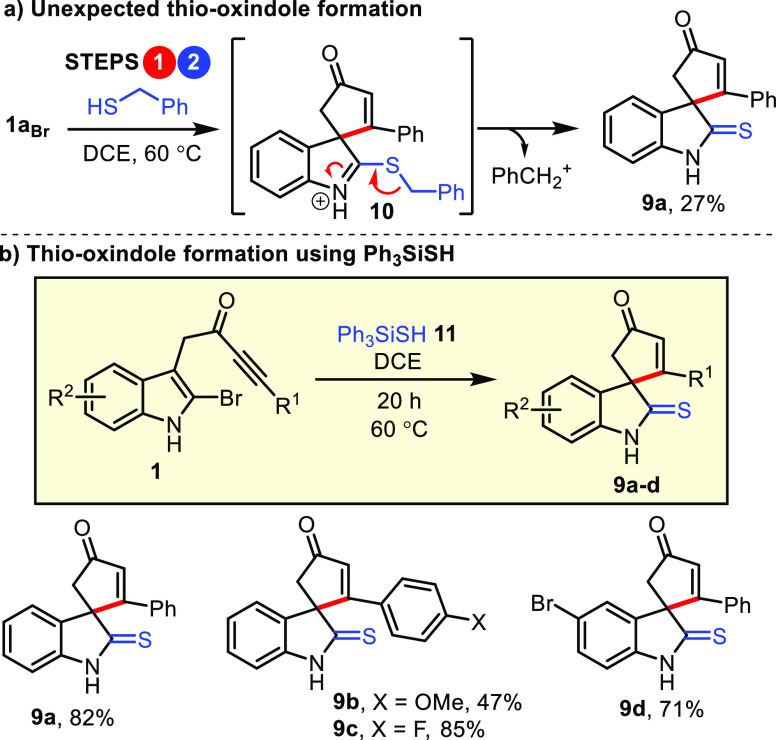
With conditions for the cascade established, attention turned to examining the reaction scope. A range of aromatic thiols were tested (Scheme 2A), and all reacted well with ynone 1aBr; quinolines 4b–k were all prepared in this manner, generally in high yield, under the standard RT conditions using a range of electronically diverse substituted thiophenols. Other aliphatic thiols were also explored, with quinolines 4a and 4l–n prepared, although in this series heating to 60 °C was required. The yield for quinoline 4n was comparatively low (53%), with thio-oxindole 9a also formed in 27% yield; this unexpected side reaction is discussed later in the manuscript (see Scheme 3).17
Scheme 2. Scope of the Three-Step Thiol-Mediated Cascade for the Conversion of Ynones 1 into Quinolines 4.

1 (1 equiv) and RSH (1.6 equiv) were stirred in DCE (0.1 M) for 20 h at RT unless specified.
Reaction performed at 60 °C. HS-Tol = 4-methylbenzenethiol.
Scheme 3. Conversion of Ynones 1 into Thio-Oxindoles 9 via a Desilylative Cascade Process.
1 (1 equiv) and thiol 11 (1.6 equiv) were stirred in DCE (0.1 M) for 20 h at 60 °C.
Next, the 2-halide substituent was varied (Scheme 2B). Thus, 2-chloro (1aCl) and 2-iodo (1aI) analogues of ynone 1aBr were prepared,5 and both reacted smoothly with 4-methylbenzenethiol to form quinoline 4d in high yield, albeit at a higher reaction temperature (60 °C). Finally, we explored variation of the indole-tethered ynone component 1. Four different additional 2-bromo-indole-tethered ynones were successfully tested, with variations to the ynone and the indole motifs explored. For each ynone, a representative aliphatic (n-propanethiol) and aromatic thiol (4-methylbenzenethiol) were tested, with the expected quinoline products 4o–v to be isolated successfully in all cases.18 The only substrate tested that did not deliver the expected quinoline was 4-NMe2-substituted ynone 1fBr; in this case, spirocyclic indoleninyl bromide 5b was isolated in 89% yield.19 Despite not delivering the expected quinoline, the isolation of spirocycle 5b does provide indirect mechanistic evidence for the intermediacy of indoleninyl halides in the reaction cascade (see later for discussion). Finally, by replacing the thiol with benzeneselenol, the analogous selenide product 4bSe was obtained in 62% yield.
The unexpected isolation of thio-oxindole 9a during the synthesis of 4n prompted additional studies, in part to better understand this side reaction, but also to try and harness it productively, as a new way to make thio-oxindoles.20 Our theory for how thio-oxindole 9a formed is summarized in Scheme 3a. The reaction is likely to have started as expected, and thus it proceeded through the normal dearomatizing spirocyclization and nucleophilic substitution steps (i.e., steps 1 and 2). This would generate spirocycle 10, and at this point, it appears that the route diverges, with some of the material going on to form quinoline 4n in the usual way, and the rest undergoing debenzylation, either via an SN1-type pathway as drawn, or the analogous SN2-type cleavage (not shown). To test this idea and improve the yield of thio-oxindole 9a, the reaction was repeated using the silylated thiol Ph3SiSH 11; the idea was that the weak Si–S would cleave more easily than the S–Bn bond in 10, and facilitate thio-oxindole formation via a desilylative mechanism. This idea worked well; the reaction of ynone 1aBr with Ph3SiSH 11 using the standard 60 °C procedure led to the formation of thio-oxindole 9a in 82% isolated yield (Scheme 3b). The same procedure was applied to other 2-halo-indole-tethered ynones, with thio-oxindoles 9b–9d (47–85%) prepared in the same way.
A proposed mechanism for the three-step cascade is outlined in Scheme 4a. The cascade likely initiates with dearomatizing spirocyclization, promoted by the relatively acidic thiol (A → B, step 1, Scheme 4a); protic acids have been shown to promote spirocyclization of related ynones,3b,6b and the isolation of spirocyclic indoleninyl bromide 5b discussed earlier lends further support to this notion. The resulting iminium–thiolate ion pair 2 may then undergo facile nucleophilic substitution to afford substituted spirocycle 12 (step 2).5 The rearrangement of 12 into 17 is then thought to proceed via a previously studied acid-catalyzed one-atom ring-expansion.6c
Scheme 4. Proposed Mechanism and Control Reactions.
Several control experiments were conducted to investigate this mechanism and the ordering of the steps. First, to probe whether the nucleophilic substitution step may proceed before spirocyclization, 2-bromo-indole substrates lacking an ynone substituent (18 and 21) were each reacted under the standard conditions with 4-methylbenzenethiol (Scheme 4b, eq 1). In the case of indole 18, some bromide substitution was indeed observed, with sulfide 19 formed in 31% yield. This confirms that nucleophilic substitution directly on the indole is possible, although the yield was low, and the major product was in fact the reduced product 20. Treating the analogous 3-methylindole 21 in the same way resulted in trace formation of 22 only. In view of these results, and given that no reduction products were observed in any of the synthetic reactions, it seems unlikely that nucleophilic substitution precedes dearomatizing spirocyclization.
We then questioned whether the iminium–thiolate ion pair B might first undergo ring expansion to form a quinoline and that nucleophilic substitution follows this step. To probe this idea, both indoleninyl bromide 5a and 2-bromoquinoline 8 were reacted with 4-methylbenzenethiol under the standard reaction conditions. Interestingly, both reactions afforded the expected quinoline product 4d in high yields (Scheme 4b, eqs 2 and 3), suggesting that the order of steps 2 and 3 could be interchanged.
To investigate this idea further, a discrete sample of the substituted spirocyclic sulfide 6a was reacted with 4-methylbenzenethiol under the standard reaction conditions (eq 4). No conversion into quinoline 4d was observed and only 6a was recovered after stirring for 24 h at both RT and 60 °C. However, the quinoline product 4d could be formed in high yield at RT upon the addition of 1.1 equiv of 48% aq. HBr to spirocyclic sulfide 6a. This result suggests that a strong Brønsted acid is required to promote the ring expansion, and such an acid would only be present in the reaction following the nucleophilic substitution step (which generates HX), thus supporting the originally proposed order of steps. Furthermore, the success of the series of thio-oxindole forming reactions described in Scheme 3 also supports the same pathway, because in these reactions the successful formation of spirocyclic products 9a–d means that nucleophilic substitution must have out-competed ring expansion in these cases.
Considering all these observations, we can be confident that the first step of the cascade is a thiol-promoted dearomatizing spirocyclization (step 1). The next step is most likely to be nucleophilic substitution (step 2) of the resultant iminium–thiolate ion pair, which generates a strong Brønsted acid (HBr) in situ. This acid then promotes a one-atom ring expansion (step 3) to form a stable aromatic quinoline product 4. Some interchange in the ordering of steps 2 and 3 cannot be ruled out once a reasonable concentration of HBr has built up in the reaction, however.
In summary, a three-step cascade process has been developed that allows for the direct conversion of 2-halo-indole-tethered ynones into substituted quinolines. The key to the process is the use of thiols as “multitasking” reagents able to promote dearomatizing spirocyclization and nucleophilic substitution directly, as well promoting a one-atom ring expansion indirectly, via the formation of a strong Brønsted acid (HBr) in situ. The reactions are very simple to perform21 and are typically high yielding, enabling the facile synthesis of a diverse array of functionalized quinolines. They are also easily scalable; for example, quinoline 4d was formed in 97% yield on a 1 mmol scale (see Supporting Information). In addition, a related route to thio-oxindoles was also developed following a serendipitous discovery of an unexpected side reaction.
Acknowledgments
The authors thank the Development and Promotion of Science and Technology Talents Project (DPST), Royal Thai Government (N.I. EP/N035119/1), and the University of York and the Leverhulme Trust (for an Early Career Fellowship, ECF-2019-135, M.J.J.) for financial support. We are also grateful for the provision of an Eleanor Dodson Fellowship (to W.P.U.). We also thank Dr. Victor Chechik (University of York) for helpful discussions concerning the mechanism and Theo Tanner (X-ray crystallography service, University of York) for collecting and processing the X-ray data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c00205.
Experimental procedures, characterization, and copies of 1H and 13C NMR spectra for all compounds (PDF)
Accession Codes
CCDC 2054407 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
The manuscript was written through contributions of all authors
The Leverhulme Trust (ECF-2019-135)
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews of tandem/cascade reactions, see:; a Taylor R. J. K.; Reid M.; Foot J.; Raw S. A. Tandem Oxidation Processes using Manganese Dioxide: Discovery, Applications and Current Studies. Acc. Chem. Res. 2005, 38, 851–869. 10.1021/ar050113t. [DOI] [PubMed] [Google Scholar]; b Nicolaou K. C.; Chen J. S. The art of total synthesis through cascade reactions. Chem. Soc. Rev. 2009, 38, 2993–3009. 10.1039/b903290h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Plesniak M. P.; Huang H.-M.; Procter D. J. Radical cascade reactions triggered by single electron transfer. Nat. Rev. Chem. 2017, 1, 0077. 10.1038/s41570-017-0077. [DOI] [Google Scholar]; d Prabagar B.; Ghosh N.; Sahoo A. K. Cyclization and Cycloisomerization of π-Tethered Ynamides: An Expedient Synthetic Method to Construct Carbo- and Heterocycles. Synlett 2017, 28, 2539–2555. 10.1055/s-0036-1590877. [DOI] [Google Scholar]; e Sperl J. M.; Sieber V. Multienzyme Cascade Reactions–Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. 10.1021/acscatal.7b03440. [DOI] [Google Scholar]; f Huang H.-M.; Garduño-Castro M. H.; Morrill C.; Procter D. J. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 2019, 48, 4626–4638. 10.1039/C8CS00947C. [DOI] [PubMed] [Google Scholar]
- For selected examples from our own groups, see:; a Raw S.; Taylor R. J. K. Cascade Reactions of Substituted 1,2,4-Triazines: Rapid Access to Nitrogen-Containing Polycycles. J. Am. Chem. Soc. 2004, 126, 12260–12261. 10.1021/ja045780g. [DOI] [PubMed] [Google Scholar]; b Edwards M. G.; Kenworthy M.; Kitson R. A. A.; Scott M.; Taylor R. J. K. The Telescoped Intramolecular Michael/Olefination (TIMO) Approach to α-Alkylidene γ-Butyrolactones: Synthesis of (+)-Paeonilactone B. Angew. Chem., Int. Ed. 2008, 47, 1935–1937. 10.1002/anie.200705329. [DOI] [PubMed] [Google Scholar]; c Lawer A.; Rossi-Ashton J. A.; Stephens T. C.; Challis B. J.; Epton R. G.; Lynam J. M.; Unsworth W. P. Internal Nucleophilic Catalyst Mediated Cyclisation/Ring Expansion Cascades for the Synthesis of Medium-Sized Lactones and Lactams. Angew. Chem., Int. Ed. 2019, 58, 13942–13947. 10.1002/anie.201907206. [DOI] [PubMed] [Google Scholar]
- For studies relating to step 1, see:; a Ekebergh A.; Börje A.; Mårtensson J. Total Synthesis of Nostodione A, a Cyanobacterial Metabolite. Org. Lett. 2012, 14, 6274–6277. 10.1021/ol303036j. [DOI] [PubMed] [Google Scholar]; b James M. J.; Cuthbertson J. D.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Silver(I)- or Copper(II)-Mediated Dearomatization of Aromatic Ynones: Direct Access to Spirocyclic Scaffolds. Angew. Chem., Int. Ed. 2015, 54, 7640–7643. 10.1002/anie.201501812. [DOI] [PubMed] [Google Scholar]; c Clarke A. K.; James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Silica-Supported Silver Nitrate as a Highly Active Dearomatizing Spirocyclization Catalyst: Synergistic Alkyne Activation by Silver Nanoparticles and Silica. Angew. Chem., Int. Ed. 2016, 55, 13798–13802. 10.1002/anie.201608263. [DOI] [PubMed] [Google Scholar]; d Liddon J. T. R.; Rossi-Ashton J. A.; Clarke A. K.; Lynam J. M.; Taylor R. J. K.; Unsworth W. P. Divergent reactivity of indole tethered ynones with silver(I) and gold(I) catalysis: a combined synthetic and computational study. Synthesis 2018, 50, 4829–4836. 10.1055/s-0037-1610181. [DOI] [Google Scholar]; e Fedoseev P.; Coppola G.; Ojeda G. M.; Van der Eycken E. V. Synthesis of Spiroindolenines by Intramolecular Ipso-Iodocyclization of Indol Ynones. Chem. Commun. 2018, 54, 3625–3628. 10.1039/C8CC01474D. [DOI] [PubMed] [Google Scholar]; f Han G.; Xue L.; Zhao L.; Zhu T.; Hou J.; Song Y.; Liu Y. Access to CF3-Containing Cyclopentaquinolinone Derivatives from Indolyl-ynones via Silver-Catalyzed One-pot Reaction. Adv. Synth. Catal. 2019, 361, 678–682. 10.1002/adsc.201801482. [DOI] [Google Scholar]; g Rossi-Ashton J. A.; Clarke A. K.; Taylor R. J. K.; Unsworth W. P. Modular Synthesis of Polycyclic Alkaloid Scaffolds via an Enantioselective Dearomative Cascade. Org. Lett. 2020, 22, 1175–1181. 10.1021/acs.orglett.0c00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For radical dearomatizing spirocyclization reactions of indoles of the form 1, see:; a Ho H. E.; Pagano A.; Rossi-Ashton J. A.; Donald J. R.; Epton R. G.; Churchill J. C.; James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Visible-light-induced intramolecular charge transfer in the radical spirocyclisation of indole-tethered ynones. Chem. Sci. 2020, 11, 1353–1360. 10.1039/C9SC05311E. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chengwen Li C.; Xue L.; Zhou J.; Zhao Y.; Han G.; Hou J.; Song Y.; Liu Y. Copper-Catalyzed Trifluoromethylation of Ynones Coupled with Dearomatizing Spirocyclization of Indoles: Access to CF3-Containing Spiro[cyclopentane-1,3′-indole]. Org. Lett. 2020, 22, 3291–3296. 10.1021/acs.orglett.0c01097. [DOI] [PubMed] [Google Scholar]; c Zhou X.-Ji.; Liu H.-Y.; Mo Z.-Y.; Ma X.-L.; Chen Y.-Y.; Tang H.-T.; Pan Y.-M.; Xu Y.-L. Visible-Light-Promoted Selenylative Spirocyclization of Indolyl-ynones toward the Formation of 3-Selenospiroindolenine Anticancer Agents. Chem. - Asian J. 2020, 15, 1536–1539. 10.1002/asia.202000298. [DOI] [PubMed] [Google Scholar]
- For studies relating to step 2, see:Liddon J. T. R.; Clarke A. K.; Taylor R. J. K.; Unsworth W. P. Preparation and Reactions of Indoleninyl Halides: Scaffolds for the Synthesis of Spirocyclic Indole Derivatives. Org. Lett. 2016, 18, 6328–6331. 10.1021/acs.orglett.6b03221. [DOI] [PubMed] [Google Scholar]; and ref (4b).
- For studies relating to step 3, see:; a Liddon J. T. R.; James M. J.; Clarke A. K.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Catalyst-Driven Scaffold Diversity: Selective Synthesis of Spirocycles, Carbazoles and Quinolines from Indolyl Ynones. Chem. - Eur. J. 2016, 22, 8777–8780. 10.1002/chem.201601836. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fedoseev P.; Van der Eycken E. Temperature switchable Brønsted acid-promoted selective syntheses of spiro-indolenines and quinolines. Chem. Commun. 2017, 53, 7732–7735. 10.1039/C7CC02580G. [DOI] [PubMed] [Google Scholar]; c Epton R. G.; Clarke A. K.; Taylor R. J. K.; Unsworth W. P.; Lynam J. M. Synthetic and mechanistic studies into the rearrangement of spirocyclic indolenines into quinolines. Eur. J. Org. Chem. 2019, 2019, 5563–5571. 10.1002/ejoc.201900798. [DOI] [Google Scholar]; and3f,5
- For reviews on dearomatizing spirocyclization reactions, see:; a James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Synthesis of Spirocyclic Indolenines. Chem. - Eur. J. 2016, 22, 2856–2881. 10.1002/chem.201503835. [DOI] [PubMed] [Google Scholar]; b Liang X.-W.; Zheng C.; You S.-L. Dearomatization through Halofunctionalization Reactions. Chem. - Eur. J. 2016, 22, 11918–11933. 10.1002/chem.201600885. [DOI] [PubMed] [Google Scholar]; c Roche S. P.; Youte Tendoung J.-J.; Tréguier B. Advances in Dearomatization Strategies of Indoles. Tetrahedron 2015, 71, 3549–3591. 10.1016/j.tet.2014.06.054. [DOI] [Google Scholar]; d Zhuo C.-X.; Zhang W.; You S.-L. Catalytic Asymmetric Dearomatization Reactions. Angew. Chem., Int. Ed. 2012, 51, 12662–12686. 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]; e Zhuo C.-X.; Zheng C.; You S.-L. Transition-Metal-Catalyzed Asymmetric Allylic Dearomatization Reactions. Acc. Chem. Res. 2014, 47, 2558–2573. 10.1021/ar500167f. [DOI] [PubMed] [Google Scholar]; f Zheng C.; You S.-L. Catalytic Asymmetric Dearomatization by Transition-Metal Catalysis: A Method for Transformations of Aromatic Compounds. Chem. 2016, 1, 830–857. 10.1016/j.chempr.2016.11.005. [DOI] [Google Scholar]; g Zheng C.; You S.-L. Exploring the Chemistry of Spiroindolenines by Mechanistically-Driven Reaction Development: Asymmetric Pictet–Spengler-type Reactions and Beyond. Acc. Chem. Res. 2020, 53, 974–987. 10.1021/acs.accounts.0c00074. [DOI] [PubMed] [Google Scholar]
- For prominent recent examples, see:; a James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Selective synthesis of six products from a single indolyl α-diazocarbonyl precursor. Angew. Chem., Int. Ed. 2016, 55, 9671–9675. 10.1002/anie.201605337. [DOI] [PubMed] [Google Scholar]; b Xia Z.-L.; Zheng C.; Wang S.-G.; You S.-L. Catalytic Asymmetric Dearomatization of Indolyl Dihydropyridines via Enamine Isomerization/Spirocyclization/Transfer Hydrogenation Sequence. Angew. Chem., Int. Ed. 2018, 57, 2653–2656. 10.1002/anie.201712435. [DOI] [PubMed] [Google Scholar]; c Flynn A. R.; McDaniel K. A.; Hughes M. E.; Vogt D. B.; Jui N. T. Hydroarylation of Arenes via Reductive Radical-Polar Crossover. J. Am. Chem. Soc. 2020, 142, 9163. 10.1021/jacs.0c03926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Synthesis of spirocyclic indolenines. Chem. - Eur. J. 2016, 22, 2856–2881. 10.1002/chem.201503835. [DOI] [PubMed] [Google Scholar]
- a Zheng Y.; Tice C. M.; Singh S. B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673. 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]; b Müller G.; Berkenbosch T.; Benningshof J. C. J.; Stumpfe D.; Bajorath J. Charting Biologically Relevant Spirocyclic Compound Space. Chem. - Eur. J. 2017, 23, 703. 10.1002/chem.201604714. [DOI] [PubMed] [Google Scholar]; c Zheng Y.-J.; Tice C. M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discovery 2016, 11, 831. 10.1080/17460441.2016.1195367. [DOI] [PubMed] [Google Scholar]; d Griggs S. D.; Thompson N.; Tape D. T.; Fabre M.; Clarke P. A. A Two-Step Synthesis of 2-Spiropiperidines. Chem. - Eur. J. 2017, 23, 9262. 10.1002/chem.201702467. [DOI] [PubMed] [Google Scholar]
- For related processes involving dearomatizing spirocyclization of ynones tethered to other electron rich aromatics:; a Unsworth W. P.; Cuthbertson J. D.; Taylor R. J. K. Total Synthesis of Spirobacillene A. Org. Lett. 2013, 15, 3306–3309. 10.1021/ol4013958. [DOI] [PubMed] [Google Scholar]; b Clarke A. K.; Liddon J. T. R.; Cuthbertson J. D.; Taylor R. J. K.; Unsworth W. P. Dearomatisation Approaches to Spirocyclic Dienones via the Electrophilic Activation of Alkynes. Org. Biomol. Chem. 2017, 15, 233–245. 10.1039/C6OB02426B. [DOI] [PubMed] [Google Scholar]; c Ho H. E.; James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Ag(I)-catalysed synthesis of azabicyclic alkaloid frameworks from ketimine-tethered ynones: total synthesis of indolizidine 209D. Org. Lett. 2018, 20, 1439–1443. 10.1021/acs.orglett.8b00225. [DOI] [PubMed] [Google Scholar]; d Clarke A. K.; Lynam J. M.; Taylor R. J. K.; Unsworth W. P. ’. Back to front’ indole synthesis using silver(I) catalysis: unexpected C-3 pyrrole activation mode supported by DFT. ACS Catal. 2018, 8, 6844–6850. 10.1021/acscatal.8b00745. [DOI] [Google Scholar]
- For related processes involving metal-mediated transformations of indoles tethered to nonynone alkynes, see:; a James M. J.; Clubley R. E.; Palate K. Y.; Procter T. J.; Wyton A. C.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Silver(I)-Catalyzed Dearomatization of Alkyne-Tethered Indoles: Divergent Synthesis of Spirocyclic Indolenines and Carbazoles. Org. Lett. 2015, 17, 4372–4375. 10.1021/acs.orglett.5b02216. [DOI] [PubMed] [Google Scholar]; b Magné V.; Blanchard F.; Marinetti A.; Voituriez A.; Guinchard X. Synthesis of Spiro[piperidine-3,3′-oxindoles] via Gold(I)-Catalyzed Dearomatization of N-Propargyl- and N-Homoallenyl-2-bromotryptamines. Adv. Synth. Catal. 2016, 358, 3355–3361. 10.1002/adsc.201600398. [DOI] [Google Scholar]; c Magné V.; Marinettim A.; Gandon V.; Voituriez A.; Guinchard X. Synthesis of Spiroindolenines via Regioselective Gold(I)-Catalyzed Cyclizations of N-Propargyl Tryptamines. Adv. Synth. Catal. 2017, 359, 4036–4042. 10.1002/adsc.201700932. [DOI] [Google Scholar]; d He Y.; Li Z.; Robeyns K.; Van Meervelt L.; Van der Eycken E. V. A Gold-Catalyzed Domino Cyclization Enabling Rapid Construction of Diverse Polyheterocyclic Frameworks. Angew. Chem., Int. Ed. 2018, 57, 272–276. 10.1002/anie.201710592. [DOI] [PubMed] [Google Scholar]; e Zhang Z.; Smal V.; Retailleau P.; Voituriez A.; Frison G.; Marinetti A.; Guinchard X. Tethered Counterion-Directed Catalysis: Merging the Chiral Ion-Pairing and Bifunctional Ligand Strategies in Enantioselective Gold(I) Catalysis. J. Am. Chem. Soc. 2020, 142, 3797–3805. 10.1021/jacs.9b11154. [DOI] [PubMed] [Google Scholar]
- a Unsworth W. P.; Donald J. R. Ring expansion reactions in the synthesis of macrocycles and medium sized rings. Chem. - Eur. J. 2017, 23, 8780–8799. 10.1002/chem.201700467. [DOI] [PubMed] [Google Scholar]; b Lawer A.; Rossi-Ashton J. A.; Stephens T. C.; Challis B. J.; Epton R. G.; Lynam J. M.; Unsworth W. P. Internal Nucleophilic Catalyst Mediated Cyclisation/Ring Expansion Cascades for the Synthesis of Medium-Sized Lactones and Lactams. Angew. Chem., Int. Ed. 2019, 58, 13942–13947. 10.1002/anie.201907206. [DOI] [PubMed] [Google Scholar]; c Clarke A. K.; Unsworth W. P. A happy medium: the synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 2020, 11, 2876–2881. 10.1039/D0SC00568A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews and perspective on the applications and uses of functionalized quinolines, see:; a Kumar S.; Bawa S.; Gupta H. Biological Activities of Quinoline Derivatives Mini. Mini-Rev. Med. Chem. 2009, 9, 1648–1654. 10.2174/138955709791012247. [DOI] [PubMed] [Google Scholar]; b Prajapati S. M.; Patel K. D.; Vekariya R. H.; Panchal S. N.; Patel H. D. Recent advances in the synthesis of quinolines: a review. RSC Adv. 2014, 4, 24463–24476. 10.1039/C4RA01814A. [DOI] [Google Scholar]; c Marella A.; Tanwar O. P.; Saha R.; Ali M. R.; Srivastava S.; Akhter M.; Shaquiquzzaman M.; Alam M. M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. 10.1016/j.jsps.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCE (1,2-dichloroethane) was chosen as solvent due to its relatively wide temperature range and efficacy in a recent study involving indole-tethered ynones (see ref (4a)). The cascade reactions also works well when DCM is used in place of DCE (84% isolated yield for the conversion of 1aBr into 4b), but the use of the nonchlorinated solvents THF and acetonitrile for the same reaction was far less effective (no reaction and 20% yield of 6a respectively).
- For an interesting recent study on thiol-mediated cascade reactions of alkyne-based precursors, that operates via a radical mechanism, see:Dutta S.; Mallick R. J.; Prasad R.; Gandon V.; Sahoo A. K. Alkyne Versus Ynamide Reactivity: Regioselective Radical Cyclization of Yne-Ynamides. Angew. Chem., Int. Ed. 2019, 58, 2289–2294. 10.1002/anie.201811947. [DOI] [PubMed] [Google Scholar]
- For examples and perspective on synthetic processes discovered by serendipitous/unforeseen processes, see ref (4a) and; a Zard S. Z. New syntheses of alkynes: a tale of serendipity and design. Chem. Commun. 2002, 1555. 10.1039/b203383f. [DOI] [PubMed] [Google Scholar]; b Grimes R. N. Synthesis and serendipity in boron chemistry: A 50 year perspective. J. Organomet. Chem. 2013, 747, 4. 10.1016/j.jorganchem.2013.04.018. [DOI] [Google Scholar]; c Unsworth W. P.; Taylor R. J. K. Upenamide: trials and tribulations. Org. Biomol. Chem. 2013, 11, 7250–7261. 10.1039/c3ob41519h. [DOI] [PubMed] [Google Scholar]; d Kazim M.; Siegler M. A.; Lectka T. A. Case of Serendipity: Synthesis, Characterization, and Unique Chemistry of a Stable, Ring-Unsubstituted Aliphatic p-Quinone Methide. Org. Lett. 2019, 21, 2326. 10.1021/acs.orglett.9b00615. [DOI] [PubMed] [Google Scholar]; e Strieth-Kalthoff F.; Henkel C.; Teders M.; Kahnt A.; Knolle W.; Gómez-Suárez A.; Dirian K.; Alex W.; Bergander K.; Daniliuc C. G.; Abel B.; Guldi D. M.; Glorius F. Discovery of Unforeseen Energy-Transfer-Based Transformations Using a Combined Screening Approach. Chem. 2019, 5, 2183. 10.1016/j.chempr.2019.06.004. [DOI] [Google Scholar]
- CCDC 2054407 contains the crystallographic data for compound 4v, see www.ccdc.cam.ac.uk/data_request/cif.
- The reason for this difference is not fully clear. Solubility differences and/or changes to the electronic properties of the ynone may both have had an influence, while the relatively basic aniline group may also have altered the pH balance and affected proton transfer in the reaction. Notably, in previous studies we have found that other 4-NMe2-substituted ynones have also reacted differently to other seemingly similar substrates in the series (see ref (6)).
- For background and biological properties of thio-oxindoles and related oxindoles, see:Hurst T. E.; Gorman R. M.; Drouhin P.; Perry A.; Taylor R. J. K. A Direct C–H/Ar–H Coupling Approach to Oxindoles, Thio-oxindoles, 3,4-Dihydro-1H-quinolin-2-ones, and 1,2,3,4-Tetrahydroquinolines. Chem.-Eur. J. 2014, 20, 14603–14703. 10.1002/chem.201403917. [DOI] [PubMed] [Google Scholar]; and reference therein.
- Although degassed solvent was typically used in this study to help ensure consistent results, this level of precaution is generally not needed; for example, the conversion of 1aBr into 4d worked in 98% yield when done without degassing. The insensitivity of the reaction to oxygen also suggests that alternative radical pathways (cf. ref (4a)) are unlikely to operate. In addition, it was found that ynone 1aBr does not react when treated with PhSSPh in place of PhSH under the standard RT conditions, which further reduces the likelihood that the cascade reaction involves thiyl radical intermediates. The analogous reaction with PhSSPh and 1 equiv of HBr led only to the formation of products in which sulfur had not been incorporated: 7a (18%) and 8 (72%).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.