Abstract
Katritzky salts have emerged as effective alkyl radical sources upon metal- or photocatalysis. These are typically prepared from the corresponding triarylpyrylium ions, in turn an important class of photocatalysts for small molecules synthesis and photopolymerization. Here, a flow method for the rapid synthesis of both pyrylium and Katrizky salts in a telescoped fashion is reported. Moreover, several pyrylium salts were tested in the photoinduced RAFT polymerization of vinyl ethers under flow and batch conditions.
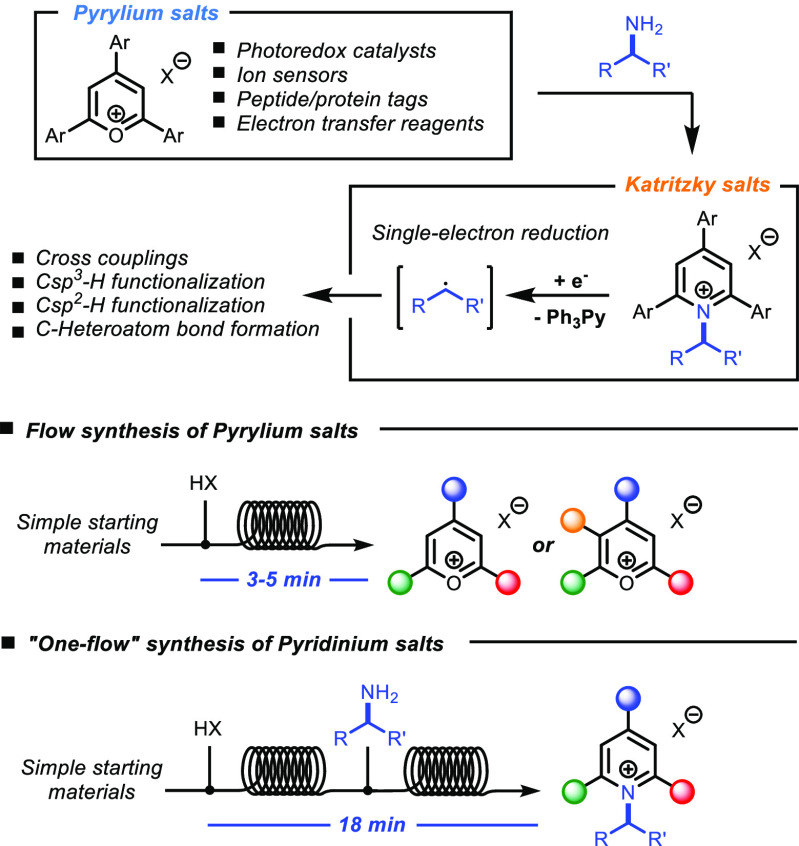
2,4,6-Triarylpyrylium salts have found applications in various fields of chemistry, especially as visible-light photocatalysts for the synthesis of small molecules1 and photoinduced polymerizations.2 In addition to their applications as photocatalysts, pyrylium salts have been used as precursors for a variety of other heterocycles, in particular N-alkylpyridinium salts (Katritzky salts), via reaction with primary amines.3 The pyridinium moiety can act in these compounds as a leaving group in nucleophilic substitution reactions4 or can lead to fragmentation upon single-electron reduction, generating alkyl radicals. Several methods for the single electron reduction of Katritzky salts have been recently investigated. In particular, activation via metal catalysis,5 photoredox catalysis,6 and visible light-activated electron donor–acceptor (EDA) interactions7 have recently found large interest in the chemistry community.
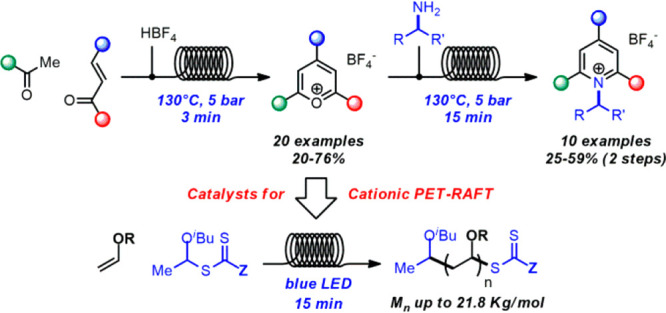
Based on our interest in flow chemistry and photochemical methodologies,8 we developed a flow protocol for the rapid synthesis of pyrylium salts and, in a telescoped fashion, of Katritzky salts, starting from simple starting materials (Scheme 1). As the formation of both pyrylium and Katritzky salts is a thermal reaction, we reasoned that a continuous flow method in superheated conditions would promote much faster reactions than in batch.
Scheme 1. Applications of Pyrylium and Katritzky Salts in Chemistry And Outline of This Work.
Reversible addition–fragmentation chain transfer (RAFT) polymerization has emerged as a powerful method to generate polymeric materials with controlled properties.9 While radical initiation has been traditionally achieved thermally, alternatives have been recently investigated. In particular, the use of photoactivated chain-transfer agents (CTAs) made possible the development of photochemical RAFT methods, which offer milder conditions and higher degrees of control.9b−9d Photocatalytic RAFT polymerizations are often performed in continuous flow conditions, due to the benefits of microflow chemistry in this area.8a−8c,10
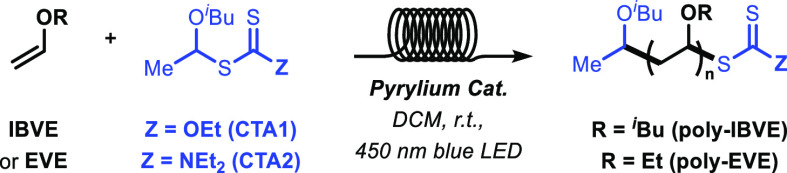
Pyrylium photocatalysts have been recently used in cationic photoinduced electron/energy transfer RAFT processes (PET-RAFT10c,11) for the polymerization of vinyl ethers,2e,2f not always easy to achieve with traditional RAFT.12 Here, we report investigations on the use of pyrylium salts for the photchemical RAFT polymerization of vinyl ethers in flow and comparison with the corresponding batch process. The use of microflow chemistry allowed for much shorter reaction time, higher molecular weight, and lower polydispersity of the polymeric materials.
Pyrylium salts are typically prepared by reaction of an acetophenone and a chalcone derivative in the presence of an acid. This protocol comprises the use of simple starting materials and allows for the synthesis of both symmetrical and unsymmetrical triarylpyryliums, and was therefore chosen for our investigations in flow. As tetrafluoroborate salts are commonly used for both pyryliums and Katritzky salts applications, we focused on these salts.
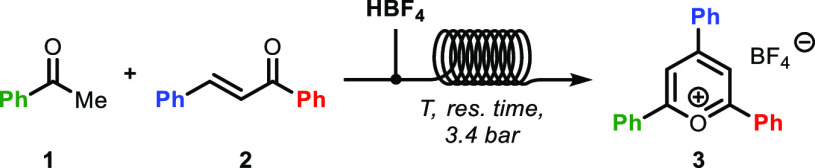
We started our investigation by performing the reaction between chalcone (1), acetophenone (2), and HBF4·Et2O under flow conditions for the synthesis of 2,4,6-triphenylpyrylium tetrafluoroborate (3) in DCE. The reaction was then optimized with respect to temperature and residence time. Reactions were performed above the boiling point of the solvent (82 °C) using a back-pressure regulator set at 3.4 bar at the end of the reactor coil. Screening of temperature showed 110 °C as the optimal temperature for the reaction, giving up to 74% yield over 5 min residence time (entry 3, Table 1). However, reactions conducted at 130 °C over 3 min residence time gave comparable results (entry 9, Table 1).
Table 1. Selected Optimization for the Synthesis of Triphenylpyrylium Tetrafluoroborate in Flowa.
| entry | T (°C) | res time (min) | yield (%) |
|---|---|---|---|
| Temperature Screening | |||
| 1 | 90 | 5 | 57 |
| 2 | 100 | 5 | 65–69b |
| 3 | 110 | 5 | 69–74b |
| 4 | 120 | 5 | 57–67b |
| 5 | 130 | 5 | 63 |
| Residence Time Screening | |||
| 6 | 110 | 2 | 57 |
| 7 | 110 | 3 | 64 |
| 8 | 110 | 7 | 70 |
| 9 | 130 | 3 | 69 |
Conditions: Feed 1: 2.5–5 mmol of acetophenone, 5–10 mmol of chalcone, diluted with DCE to 2–4 mL. Feed 2: 5–10 mmol HBF4·Et2O, diluted with DCE to 2–4 mL. Isolated yields from direct precipitation into Et2O at the outlet of the reactor.
The reported range represent the variation observed within at least two runs. Conditions in entry 3 were run several times, with results always in the reported range.
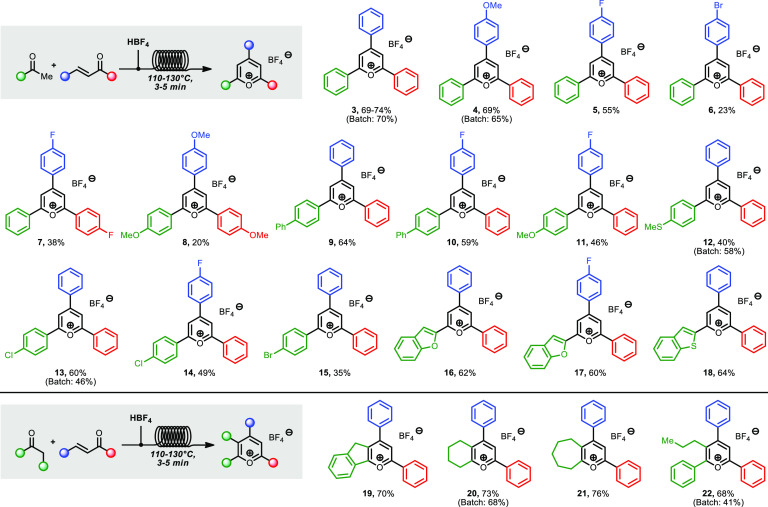
The flow protocol was tested for the synthesis of a variety of triarylpyrylium tetrafluoroborates (Scheme 2). Differently substituted chalcones could be used in the reaction, furnishing halogenated or methoxylated pyryliums 4–8. Different acetophenones, including substituents such as phenyl, methoxy, methylthio, chloro, and bromo, also reacted well, giving products 9–15. Heteroaromatic ketones could also be efficiently employed, and the reaction of chalcones with acetylbenzofuran and acetylbenzothiophene led to pyryliums 16–18. Differently substituted pyrliums, containing alkyl moieties, were then investigated (Scheme 2). Tetrasubstituted and polycyclic pyrylium salts can be prepared by reaction of chalcones with linear or cyclic ketones. For example, indanone, cyclohexa/heptenone, and valerophenone gave pyryliums 19–22 in 68–76% yield.
Scheme 2. Synthesis of Pyrylium Salts.
Conditions: Feed 1: 0.5 g of chalcone (2 equiv), acetophenone (1 equiv), diluted to 2–3 mL with DCE. Feed 2: HBF4·Et2O (2 equiv), diluted to 2–3 mL with DCE; T = 110 or 130 °C, P = 3.4–5.2 bar, residence time = 3–5 min. Products were obtained by direct precipitation into Et2O at the outlet of the reactor, isolated yields after filtration are reported.
The yields obtained for the pyrylium salts 3–22 reflect the electronic properties of the reagents, and analogous trends are observed in the literature for batch reactions.13 Reactions under batch conditions (same scale, higher concentration, 1 h reaction time) for a few of the salts were performed for the sake of comparison and provided similar yields to the flow protocol, further demonstrating the electronic limitations of the synthesis.
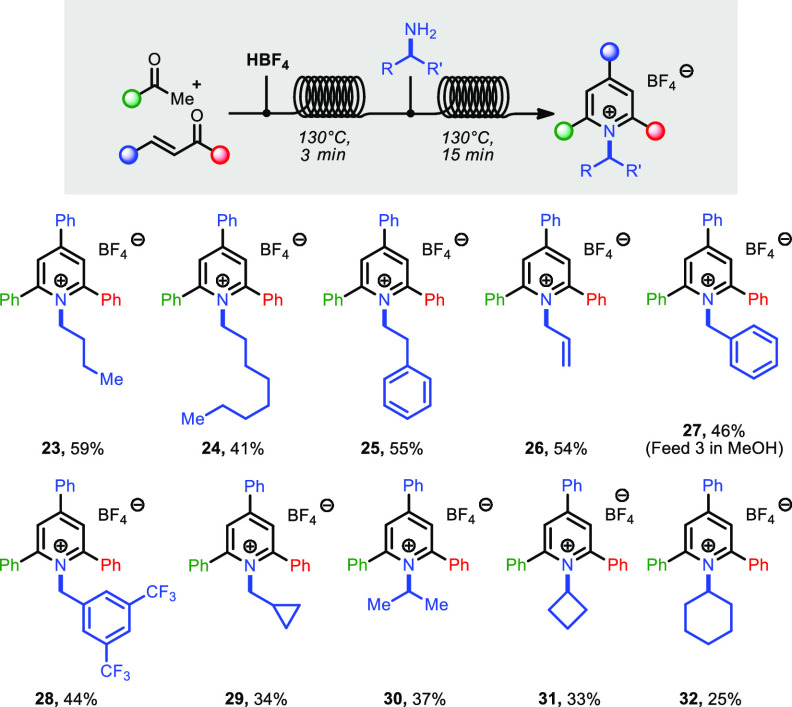
We then set out to investigate the continuous-flow synthesis of N-alkyl triphenylpyridinium compounds (Katritzky salts) in a “one-flow” fashion.14 These compounds are typically prepared in batch by reaction of pyrylium salts with a primary amine in refluxing ethanol for a few hours.5a,5d,7a A one-pot process in batch, starting from the synthesis of triphenylpyrylium 3, followed by addition of an amine solution after 1 h of reflux, resulted in the immediate formation of smoke and deposition of amine salts on the walls of flask and condenser and resulted in a complex mixture of salts, making this process cumbersome and unpractical. A telescoped flow synthesis was therefore envisaged, starting with the initial synthesis of pyrylium, followed by immediate reaction with the desired amine.
The use of DCE for both steps resulted in heavy reactor clogging. Ethanol was therefore selected as the solvent of choice for the second step, as it provides a good medium for the synthesis of pyridinium salts, good miscibility with DCE, and a comparable boiling point.15 The temperature and residence time for the second reactor coil were set respectively at 130 °C and 15 min, making the overall residence time for the two-step process 18 min.
The telescoped protocol was applied to the synthesis of different pyridinium salts (Scheme 3). Pyridinium salts containing linear, primary alkyl chains (23–25) were obtained smoothly in 41–59% yield. Allyl- and benzylamines also reacted with comparable yields (26–28, 44–54%). The reaction with cyclopropylmethyl-, isopropyl-, cyclobutyl-, and cyclohexylamine delivered compounds 29–32, albeit in lower yields (25–37%). As the yield for the triphenylpyrylium precursor 3 (first step) is in average 71%, the second step of the telescoped process results in yields of 58–83% for compounds 23–28. For compounds 29–32, this translates to 35–52% yield. As the reaction of secondary alkyl amines with pyrylium salts (30–32) is known to be much slower than the reaction of primary alkyl amines,15 we suspect individual optimization might be necessary for these compounds, as well as for 29.
Scheme 3. Synthesis of Katritzky Salts.
Conditions: Feed 1: 0.5 g of chalcone (2 equiv), acetophenone (1 equiv), diluted to 2–3 mL with DCE. Feed 2: HBF4·Et2O (2 equiv), diluted to 2–3 mL with DCE. Feed 3: amine (4 equiv), diluted to 2–3 mL with EtOH; T = 130 °C, P = 3.4–5.2 bar, total residence time = 18 min. Products were obtained by direct precipitation into Et2O at the outlet of the reactor (isolated yields after filtration and washing), or purified by chromatography.
Having established the efficiency of the microflow method for the fast preparation of pyrylium salts, we set out to investigate their use in the photopolymerization of vinyl ethers via cationic RAFT.2e,2f We thus selected differently substituted catalysts 3, 4, 6, 8, and 9 to be tested in the polymerization of benchmark monomers isobutyl vinyl ether (IBVE) and ethyl vinyl ether (EVE) under blue light irradiation. Two different CTAs, based on the xanthate and dithiocarbamate moieties, were also compared. Furthermore, a flow and a batch polymerization protocols were compared to evaluate the benefits of microflow in this process.
As shown in Table 2, the flow process resulted in generally higher molecular weight (Mn, in kg/mol) of the polymers than the batch and a slightly lower polydispersity (D). In addition, while full conversion in flow was obtained after a residence time of 15 min, the batch reaction required several hours of irradiation for completion. While the different catalysts tested generally resulted in similar properties of poly-IBVE and poly-EVE, the effect of the CTA is more remarkable. CTA2 resulted, in general, in higher Mn, albeit with slightly higher D values than CTA1, indicating a somewhat lower level of control on the polymerization. The behavior of catalyst 8 in flow is noteworthy. The combination of catalysts 8 and CTA1 resulted in very poor Mn for both polymers, while, on the contrary, its combination with CTA2 resulted in higher Mn than all the other catalysts. This behavior was observed only under flow conditions. As in flow, the photochemical excitation of the catalyst is much enhanced with respect to batch, we suspect the unusual behaviour of catalyst 8 might be related to a faster activation of CTA2 under these conditions. Catalyst 8 appears to have a very rapid response in the presence of both CTA1 and CTA2.16 Despite being a less strong oxidant than, for example, catalysts 3,2f the extinction coefficient of 8 at 450 nm is much higher, resulting in a much faster excitation and more frequent electron transfer. This feature would be enhanced under flow conditions, thus explaining our results. We hypothesize that CTA1 can accommodate a faster electron transfer, generating larger amounts of active CTA species, and the formation of more polymeric chains of lower Mn. CTA2, instead, cannot accommodate the electron transfer as efficiently, and a lower amount of radical initiator is generated, giving less, and longer polymer chains. Further investigation will, however, be needed to confirm this hypothesis.
Table 2. Photoinduced Polymerization of IBVE and EVE under Flow and Batch Conditionsa.
| flow |
batch |
|||||
|---|---|---|---|---|---|---|
| entry | cat. | CTA | Mn | D | Mn | D |
| IBVE (R = iBu) | ||||||
| 1 | 3 | 1 | 14.7 | 1.5 | 9.9 | 1.5 |
| 2 | 3 | 2 | 19.7 | 1.8 | 6.1 | 2.0 |
| 3 | 4 | 1 | 13.4 | 1.3 | 10.4 | 1.3 |
| 4 | 4 | 2 | 19.8 | 1.6 | 14.1 | 1.9 |
| 5 | 6 | 1 | 15.0 | 1.3 | 9.4 | 1.5 |
| 6 | 6 | 2 | 17.6 | 1.7 | 11.7 | 2.3 |
| 7 | 8 | 1 | 5.8 | 1.4 | 9.2 | 1.4 |
| 8 | 8 | 2 | 21.8 | 1.6 | 11.6 | 2.1 |
| 9 | 9 | 1 | 15.8 | 1.3 | 2.9 | 1.4 |
| 10 | 9 | 2 | 18.5 | 1.6 | 12.9 | 1.9 |
| EVE (R = Et) | ||||||
| 11 | 3 | 1 | 12.9 | 1.7 | 6.8 | 1.4 |
| 12 | 3 | 2 | 11.9 | 1.7 | 6.7 | 2.1 |
| 13 | 4 | 1 | 8.7 | 1.3 | 5.8 | 1.6 |
| 14 | 4 | 2 | 9.4 | 1.8 | 6.9 | 2.4 |
| 15 | 6 | 1 | 8.8 | 1.8 | 6.4 | 1.5 |
| 16 | 6 | 2 | 10.8 | 1.9 | 9.2 | 2.2 |
| 17 | 8 | 1 | 2.7 | 1.3 | 7.3 | 1.4 |
| 18 | 8 | 2 | 15.3 | 1.7 | 7.6 | 2.2 |
| 19 | 9 | 1 | 7.9 | 1.4 | 3.2 | 1.3 |
| 20 | 9 | 2 | 13.3 | 1.6 | 7.5 | 2.3 |
Reactions performed at room temperature in DCM, with 24 W 450 nm LED irradiation. The following molecular equivalents were used: 2000:20:1 (monomer/CTA/catalyst). All experiments were run to full conversion (15 min in flow, 8 h in batch), and the properties were obtained from calibration with polystyrene standards. Mn = kg/mol.
The results obtained (in terms of Mn and D values) for the polymerization of vinyl ethers are in line with previous studies on pyrylium catalysts. Polydispersitiy values reported in the literature range between 1.08 and 1.98 for IBVE and 1.08 and 1.89 for EVE, indicating comparable control in our experiments, with higher values observed under flow conditions.2e,2f,17 To our knowledge, no previous photochemical RAFT polymerization of vinyl ethers have been reported under flow conditions. To exclude the possibility of a catalyst-free photoiniferter polymerization, known to occur for example for acrylates and acrylamides,18 control experiments for the polymerization of both monomers were also performed. Under otherwise identical conditions, the absence of catalysts results in essentially no polymerization, with conversions of 4–10% and Mn values about 50 times lower than with the pyrylium catalysts (see ESI).
In conclusion, we reported here the flow synthesis of a range of pyrylium tetrafluoroborate salts and a two-step flow synthesis of their derivatives N-alkylpyridinium salts. The use of flow technology allowed the synthesis of pyrylium salts to be completed in as short as 3 min (1+ hour in batch), while Katritzky salts can be prepared in 18 min (second step alone typically a few hours in batch). Several pyrylium salts were tested in the photochemical RAFT polymerization of vinyl ethers, under batch and flow conditions. The flow protocol resulted in a much shorter reaction time (15 min vs several hours), higher Mn, and lower D of the polymeric materials, compared to the batch procedure. The combination of photoinduced cationic RAFT polymerization and the use of microflow chemistry is in our opinion a very promising method for the fast, mild synthesis of polymeric materials with controlled properties, and although not fully explored at the moment, we believe its study will attract much interest in the near future.
Acknowledgments
C.S. acknowledges the European Union for a Marie Curie European postdoctoral fellowship (FlowAct, Grant No. 794072). M.F. acknowledges the European Union for an Erasmus+ scholarship.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c00178.
Experimental procedures, further optimization, product characterization, UV–vis data, and NMR spectra (PDF)
Author Present Address
∥ T.N.: Flow Chemistry Group, van ’t Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam (UvA), Science Park 904, 1098 XH, Amsterdam, The Netherlands.
The authors declare no competing financial interest.
Supplementary Material
References
- a Alfonzo E.; Alfonso F. S.; Beeler A. B. Redesign of a Pyrylium Photoredox Catalyst and Its Application to the Generation of Carbonyl Ylides. Org. Lett. 2017, 19, 2989. 10.1021/acs.orglett.7b01222. [DOI] [PubMed] [Google Scholar]; b Chandu P.; Ghosh K. G.; Sureshkumar D. Metal-Free Visible-Light-Promoted Trifluoromethylation of Vinylcyclopropanes Using Pyrylium Salt as a Photoredox Catalyst. J. Org. Chem. 2019, 84, 8771. 10.1021/acs.joc.9b01033. [DOI] [PubMed] [Google Scholar]; c Perkowski A. J.; Cruz C. L.; Nicewicz D. A. Ambient-Temperature Newman–Kwart Rearrangement Mediated by Organic Photoredox Catalysis. J. Am. Chem. Soc. 2015, 137, 15684. 10.1021/jacs.5b11800. [DOI] [PubMed] [Google Scholar]; d Perkowski A. J.; You W.; Nicewicz D. A. Visible Light Photoinitiated Metal-Free Living Cationic Polymerization of 4-Methoxystyrene. J. Am. Chem. Soc. 2015, 137, 7580. 10.1021/jacs.5b03733. [DOI] [PubMed] [Google Scholar]; e Miranda M. A.; Garcia H. 2,4,6-Triphenylpyrylium Tetrafluoroborate as an Electron-Transfer Photosensitizer,. Chem. Rev. 1994, 94, 1063. 10.1021/cr00028a009. [DOI] [Google Scholar]; f Reed N. L.; Herman M. I.; Miltchev V. P.; Yoon T. P. Photocatalytic Oxyamination of Alkenes: Copper(II) Salts as Terminal Oxidants in Photoredox Catalysis. Org. Lett. 2018, 20, 7345. 10.1021/acs.orglett.8b03345. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Cruz C. L.; Nicewicz D. A. Mechanistic Investigations into the Cation Radical Newman–Kwart Rearrangement. ACS Catal. 2019, 9, 3926. 10.1021/acscatal.9b00465. [DOI] [Google Scholar]; h Mattay J.; Vondenhof M.; Denig R. Pyrylium Salts as Photosensitizers in Homogeneous and Heterogeneous Electron-Transfer Catalysis. – A Comparison with Cyano Arenes. Chem. Ber. 1989, 122, 951. 10.1002/cber.19891220526. [DOI] [Google Scholar]; i Morse P. D.; Nguyen T. M.; Cruz C. L.; Nicewicz D. A. Enantioselective counter-anions in photoredox catalysis: The asymmetric cation radical Diels-Alder reaction. Tetrahedron 2018, 74, 3266. 10.1016/j.tet.2018.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Goetz A. E.; Boydston A. J. Metal-Free Preparation of Linear and Cross-Linked Polydicyclopentadiene. J. Am. Chem. Soc. 2015, 137, 7572. 10.1021/jacs.5b03665. [DOI] [PubMed] [Google Scholar]; b Ogawa K. A.; Goetz A. E.; Boydston A. J. Metal-Free Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc. 2015, 137, 1400. 10.1021/ja512073m. [DOI] [PubMed] [Google Scholar]; c Pascual L. M. M.; Dunford D. G.; Goetz A. E.; Ogawa K. A.; Boydston A. J. Comparison of Pyrylium and Thiopyrylium Photooxidants in Metal-Free Ring-Opening Metathesis Polymerization. Synlett 2016, 27, 759. 10.1055/s-0035-1561330. [DOI] [Google Scholar]; d Lecompère M.; Allonas X.; Maréchal D.; Criqui A. Mechanistic approach to a photochemical/thermal dual-cure initiating system based on pyrylium salt–hydroperoxide for epoxide cationic polymerization. Polym. Chem. 2017, 8, 388. 10.1039/C6PY01741J. [DOI] [Google Scholar]; e Kottisch V.; Michaudel Q.; Fors B. P. Photocontrolled Interconversion of Cationic and Radical Polymerizations. J. Am. Chem. Soc. 2017, 139, 10665. 10.1021/jacs.7b06661. [DOI] [PubMed] [Google Scholar]; f Michaudel Q.; Chauviré T.; Kottisch V.; Supej M. J.; Stawiasz K. J.; Shen L.; Zipfel W. R.; Abruña H. D.; Freed J. H.; Fors B. P. Mechanistic Insight into the Photocontrolled Cationic Polymerization of Vinyl Ethers. J. Am. Chem. Soc. 2017, 139, 15530. 10.1021/jacs.7b09539. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Krappitz T.; Jovic K.; Feist F.; Frisch H.; Rigoglioso V. P.; Blinco J. P.; Boydston A. J.; Barner-Kowollik C. Hybrid Photo-induced Copolymerization of Ring-Strained and Vinyl Monomers Utilizing Metal-Free Ring-Opening Metathesis Polymerization Conditions. J. Am. Chem. Soc. 2019, 141, 16605. 10.1021/jacs.9b09025. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Kong D.; Moon P. J.; Lundgren R. J. Radical coupling from alkyl amines. Nat. Catal. 2019, 2, 473. 10.1038/s41929-019-0292-9. [DOI] [Google Scholar]; b Pang Y.; Moser D.; Cornella J. Pyrylium Salts: Selective Reagents for the Activation of Primary Amino Groups in Organic Synthesis. Synthesis 2020, 52, 489. 10.1055/s-0039-1690703. [DOI] [Google Scholar]; c Correia J. T. M.; Fernandes V. A.; Matsuo B. T.; Delgado J. A. C.; de Souza W. C.; Paixao M. W. Photoinduced Deaminative Strategies: Katritzky Salts as Alkyl Radical Precursors. Chem. Commun. 2020, 56, 503. 10.1039/C9CC08348K. [DOI] [PubMed] [Google Scholar]
- Unsubstituted pyridinium salts can also be used for this purpose:; a Moser D.; Duan Y.; Wang F.; Ma Y.; O’Neill M. J.; Cornella J. Selective Functionalization of Aminoheterocycles by a Pyrylium Salt. Angew. Chem., Int. Ed. 2018, 57, 11035. 10.1002/anie.201806271. [DOI] [PubMed] [Google Scholar]; b Gómez-Palomino A.; Cornella J. Selective Late-Stage Sulfonyl Chloride Formation from Sulfonamides Enabled by Pyry-BF4. Angew. Chem., Int. Ed. 2019, 58, 18235. 10.1002/anie.201910895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Basch C. H.; Liao J.; Xu J.; Piane J. J.; Watson M. P. Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C–N Bond Activation. J. Am. Chem. Soc. 2017, 139, 5313. 10.1021/jacs.7b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Guan W.; Liao J.; Watson M. P. Vinylation of Benzylic Amines via C–N Bond Functionalization of Benzylic Pyridinium Salts. Synthesis 2018, 50, 3231. 10.1055/s-0037-1610084. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liao J.; Basch C. H.; Hoerrner M. E.; Talley M. R.; Boscoe B. P.; Tucker J. W.; Garnsey M. R.; Watson M. P. Deaminative Reductive Cross-Electrophile Couplings of Alkylpyridinium Salts and Aryl Bromides. Org. Lett. 2019, 21, 2941. 10.1021/acs.orglett.9b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liao J.; Guan W.; Boscoe B. P.; Tucker J. W.; Tomlin J. W.; Garnsey M. R.; Watson M. P. Transforming Benzylic Amines into Diarylmethanes: Cross-Couplings of Benzylic Pyridinium Salts via C–N Bond Activation. Org. Lett. 2018, 20, 3030. 10.1021/acs.orglett.8b01062. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Plunkett S.; Basch C. H.; Santana S. O.; Watson M. P. Harnessing Alkylpyridinium Salts as Electrophiles in Deaminative Alkyl–Alkyl Cross-Couplings. J. Am. Chem. Soc. 2019, 141, 2257. 10.1021/jacs.9b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yi J.; Badir S. O.; Kammer L. M.; Ribagorda M.; Molander G. A. Deaminative Reductive Arylation Enabled by Nickel/Photoredox Dual Catalysis. Org. Lett. 2019, 21, 3346. 10.1021/acs.orglett.9b01097. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Martin-Montero R.; Yatham V. R.; Yin H.; Davies J.; Martin R. Ni-catalyzed Reductive Deaminative Arylation at sp3 Carbon Centers. Org. Lett. 2019, 21, 2947. 10.1021/acs.orglett.9b01016. [DOI] [PubMed] [Google Scholar]; h Yu C.-G.; Matsuo Y. Nickel-Catalyzed Deaminative Acylation of Activated Aliphatic Amines with Aromatic Amides via C–N Bond Activation. Org. Lett. 2020, 22, 950. 10.1021/acs.orglett.9b04497. [DOI] [PubMed] [Google Scholar]
- a Klauck F. J. R.; James M. J.; Glorius F. Deaminative Strategy for the Visible-Light-Mediated Generation of Alkyl Radicals. Angew. Chem., Int. Ed. 2017, 56, 12336. 10.1002/anie.201706896. [DOI] [PubMed] [Google Scholar]; b Klauck F. J. R.; Yoon H.; James M. J.; Lautens M.; Glorius F. Visible-Light-Mediated Deaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal. 2019, 9, 236. 10.1021/acscatal.8b04191. [DOI] [Google Scholar]; c Jiang X.; Zhang M.-M.; Xiong W.; Lu L.-Q.; Xiao W.-J. Deaminative (Carbonylative) Alkyl-Heck-type Reactions Enabled by Photocatalytic C–N Bond Activation. Angew. Chem., Int. Ed. 2019, 58, 2402. 10.1002/anie.201813689. [DOI] [PubMed] [Google Scholar]; d Ociepa M.; Turkowska J.; Gryko D. Redox-Activated Amines in C(sp3)–C(sp) and C(sp3)–C(sp2) Bond Formation Enabled by Metal-Free Photoredox Catalysis. ACS Catal. 2018, 8, 11362. 10.1021/acscatal.8b03437. [DOI] [Google Scholar]; e Zhang M.-M.; Liu F. Visible-light-mediated allylation of alkyl radicals with allylic sulfones via a deaminative strategy. Org. Chem. Front. 2018, 5, 3443. 10.1039/C8QO01046C. [DOI] [Google Scholar]; f Yang Z.-K.; Xu N.-X.; Wang C.; Uchiyama M. Photoinduced C(sp3)–N Bond Cleavage Leading to the Stereoselective Syntheses of Alkenes. Chem. - Eur. J. 2019, 25, 5433. 10.1002/chem.201900886. [DOI] [PubMed] [Google Scholar]; g Zhu Z.-F.; Zhang M.-M.; Liu F. Radical alkylation of isocyanides with amino acid-/peptide-derived Katritzky salts via photoredox catalysis. Org. Biomol. Chem. 2019, 17, 1531. 10.1039/C8OB02786B. [DOI] [PubMed] [Google Scholar]; h Schönbauer D.; Sambiagio C.; Noël T.; Schnürch M. Photocatalytic deaminative benzylation and alkylation of tetrahydroisoquinolines with N-alkylpyridinium salts. Beilstein J. Org. Chem. 2020, 16, 809. 10.3762/bjoc.16.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wu J.; He L.; Noble A.; Aggarwal V. K. Photoinduced Deaminative Borylation of Alkylamines. J. Am. Chem. Soc. 2018, 140, 10700. 10.1021/jacs.8b07103. [DOI] [PubMed] [Google Scholar]; b Wu J.; Grant P. S.; Li X.; Noble A.; Aggarwal V. K. Catalyst-Free Deaminative Functionalizations of Primary Amines by Photoinduced Single-Electron Transfer. Angew. Chem., Int. Ed. 2019, 58, 5697. 10.1002/anie.201814452. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sandfort F.; Strieth-Kalthoff F.; Klauck F. J. R.; James M. J.; Glorius F. Deaminative Borylation of Aliphatic Amines Enabled by Visible Light Excitation of an Electron Donor–Acceptor Complex. Chem. - Eur. J. 2018, 24, 17210. 10.1002/chem.201804246. [DOI] [PubMed] [Google Scholar]; d James M. J.; Strieth-Kalthoff F.; Sandfort F.; Klauck F. J. R.; Wagener F.; Glorius F. Visible-Light-Mediated Charge Transfer Enables C–C Bond Formation with Traceless Acceptor Groups. Chem. - Eur. J. 2019, 25, 8240. 10.1002/chem.201901397. [DOI] [PubMed] [Google Scholar]; e Wang C.; Qi R.; Xue H.; Shen Y.; Chang M.; Chen Y.; Wang R.; Xu Z. Visible-Light-Promoted C(sp3)-H Alkylation by Intermolecular Charge Transfer: Preparation of Unnatural α-Amino Acids and Late-stage Modification of Peptides. Angew. Chem., Int. Ed. 2020, 59, 7461. 10.1002/anie.201914555. [DOI] [PubMed] [Google Scholar]
- a Sambiagio C.; Noël T. Flow Photochemistry: Shine Some Light on Those Tubes!. Trends Chem. 2020, 2, 92. 10.1016/j.trechm.2019.09.003. [DOI] [Google Scholar]; b Cambié D.; Bottecchia C.; Straathof N. J. W.; Hessel V.; Noël T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]; c Su Y.; Straathof N. J. W.; Hessel V.; Noël T. Photochemical Transformations Accelerated in Continuous-Flow Reactors: Basic Concepts and Applications, Chem. Chem. - Eur. J. 2014, 20, 10562. 10.1002/chem.201400283. [DOI] [PubMed] [Google Scholar]; d Govaerts S.; Nyuchev A.; Noel T. Pushing the boundaries of C–H bond functionalization chemistry using flow technology. J. Flow Chem. 2020, 10, 13. 10.1007/s41981-020-00077-7. [DOI] [Google Scholar]
- For reviews, see:; a Moad G.; Rizzardo E.; Thang S. H. RAFT Polymerization and Some of its Applications. Chem. - Asian J. 2013, 8, 1634. 10.1002/asia.201300262. [DOI] [PubMed] [Google Scholar]; b Perrier S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433. 10.1021/acs.macromol.7b00767. [DOI] [Google Scholar]; c Tian X.; Ding J.; Zhang B.; Qiu F.; Zhuang X.; Chen Y. Recent Advances in RAFT Polymerization: Novel Initiation Mechanisms and Optoelectronic Applications. Polymers 2018, 10, 318. 10.3390/polym10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nothling M. D.; Fu Q.; Reyhani A.; Allison-Logan S.; Jung K.; Zhu J.; Kamigaito M.; Boyer C.; Qiao G. G. Progress and Perspectives Beyond Traditional RAFT Polymerization. Adv. Sci. 2020, 7, 2001656. 10.1002/advs.202001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews on photopolymerization in flow, see:; a Junkers T. Precise Macromolecular Engineering via Continuous-Flow Synthesis Techniques. J. Flow Chem. 2017, 7, 106. 10.1556/1846.2017.00030. [DOI] [Google Scholar]; b Junkers T.; Wenn B. Continuous photoflow synthesis of precision polymers. React. Chem. Eng. 2016, 1, 60. 10.1039/C5RE00042D. [DOI] [Google Scholar]; c Zaquen N.; Rubens M.; Corrigan N.; Xu J.; Zetterlund P. B.; Boyer C.; Junkers T. Polymer Synthesis in Continuous Flow Reactors. Prog. Polym. Sci. 2020, 107, 101256. 10.1016/j.progpolymsci.2020.101256. [DOI] [Google Scholar]
- Phommalysack-Lovan J.; Chu Y.; Boyer C.; Xu J. PET-RAFT polymerisation: towards green and precision polymer manufacturing. Chem. Commun. 2018, 54, 6591. 10.1039/C8CC02783H. [DOI] [PubMed] [Google Scholar]
- Sugihara S.; Yoshida A.; Kono T.-a.; Takayama T.; Maeda Y. Controlled Radical Homopolymerization of Representative Cationically Polymerizable Vinyl Ethers. J. Am. Chem. Soc. 2019, 141, 13954. 10.1021/jacs.9b06671. [DOI] [PubMed] [Google Scholar]
- Balaban T. S.; Balaban A. T. In Science of Synthesis - Category 2, Hetarenes and Related Ring Systems; Thomas E. J., Ed.; Georg Thieme Verlag: Stuttgart, 2003; Vol. 14. [Google Scholar]
- Bloemendal V. R. L. J.; Janssen M. A. C. H.; van Hest J. C. M.; Rutjes F. P. J. T. Continuous one-flow multi-step synthesis of active pharmaceutical ingredients. React. Chem. Eng. 2020, 5, 1186. 10.1039/D0RE00087F. [DOI] [Google Scholar]
- Katritzky A. R.; Manzo R. H. Kinetics and mechanism of the reactions of primary amines with pyrylium cations. J. Chem. Soc., Perkin Trans. 2 1981, 2, 571. 10.1039/p29810000571. [DOI] [Google Scholar]
- Upon addition of catalyst 8 to a mixture of monomer and CTA, the mixture immediately increased in temperature, and some solid precipitated. This seems, however, not to influence the polymerization process, and no clogging was observed when performing reactions in flow.
- a Guerre M.; Uchiyama M.; Folgado E.; Semsarilar M.; Améduri B.; Satoh K.; Kamigaito M.; Ladmiral V. Combination of Cationic and Radical RAFT Polymerizations: A Versatile Route to Well-Defined Poly(ethyl vinyl ether)-block-poly(vinylidene fluoride) Block Copolymers. ACS Macro Lett. 2017, 6, 393. 10.1021/acsmacrolett.7b00150. [DOI] [PubMed] [Google Scholar]; b Uchiyama M.; Satoh K.; Kamigaito M. Cationic RAFT Polymerization Using ppm Concentrations of Organic Acid. Angew. Chem., Int. Ed. 2015, 54, 1924. 10.1002/anie.201410858. [DOI] [PubMed] [Google Scholar]
- a Zaquen N.; Yeow J.; Junkers T.; Boyer C.; Zetterlund P. B. Visible Light-Mediated Polymerization-Induced Self-Assembly Using Continuous Flow Reactors. Macromolecules 2018, 51, 5165. 10.1021/acs.macromol.8b00887. [DOI] [Google Scholar]; b McKenzie T. G.; Fu Q.; Wong E. H. H.; Dunstan D. E.; Qiao G. G. Visible Light Mediated Controlled Radical Polymerization in the Absence of Exogenous Radical Sources or Catalysts. Macromolecules 2015, 48, 3864. 10.1021/acs.macromol.5b00965. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.