Abstract
Background: Electrodiagnostic studies (EDX) serve a prominent role in the diagnostic workup of cubital tunnel syndrome (CBTS), but their reported sensitivity varies widely. The goals of our study were to determine the sensitivity of EDX in a cohort of patients who responded well to surgical cubital tunnel release (CBTR), and whether the implementation of the Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) criteria improves the sensitivity. Methods: We identified 118 elbows with clinical CBTS who had preoperative EDX and underwent CBTR. The EDX diagnoses were CBTS, ulnar neuropathy (UN), and normal ulnar nerves. We divided the 118 elbows into those that received above-elbow stimulation (XE group) and those that did not (non-XE group). We calculated the sensitivities for all groups and reinterpreted the results according to the AANEM guidelines. Results: Cubital tunnel release provided significant relief in 93.6% of the elbows. Based on the EDX reports, 11% patients had clear CBTS, 23% had UN, and 66% showed no UN. The sensitivities were 11.7% for CBTS and 34.2% for any UN. In the XE group, the sensitivity of the EDX reports for CBTS and UN climbed to 33.3% and 58.3%, respectively. When we calculated the across-elbow motor nerve conduction velocity, the sensitivity for CBTS and UN was 87.5% and 100%, respectively. The XE and non-XE groups showed no difference except for sex, bilaterality, concomitant carpal tunnel release, and obesity (P < .05). Conclusion: Implementing AANEM guidelines results in significant improvement in correlation of clinical and electrodiagnostic findings of CBTS.
Keywords: cubital tunnel syndrome, electrodiagnostic, nerve conduction study, sensitivity
Introduction
Cubital tunnel syndrome (CBTS) is the second most common focal peripheral neuropathy following carpal tunnel syndrome.1-4 Early symptoms include intermittent paresthesias of the ring and small finger,5,6 frequently at night, that progress into persistent symptoms with development of loss of dexterity, clumsiness, and trouble with fine motor functions.3,7,8
Clinical evaluation of CBTS relies on careful history and physical examination but is frequently tested with electrodiagnostic studies (EDX).9,10 The Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) established specific guidelines for the diagnosis of CBTS in 1996 and reaffirmed them in 2015.11,12 They consider an absolute motor nerve conduction velocity (mNCV) across the elbow below 50 m/s, and a slowing of velocity of greater than 10 m/s between above and below the elbow segments, highly suggestive indicators of CBTS, both evaluated as part of nerve conduction studies. Despite these recommendations, a spectrum of configurations and diagnostic parameters are provided in the literature with variable reported sensitivities.13
Current literature relies on pretest diagnoses of CBTS to determine test sensitivity and sets optimal cut-off based on results of asymptomatic normal populations. The goals of this study were to determine the sensitivity of NCS in the diagnosis of CBTS in a cohort of patients who responded well to surgical cubital tunnel release (CBTR).
Materials and Methods
Institutional review board approval was obtained for this retrospective study. Electronic medical records were queried using Current Procedural Terminology codes for all patients who underwent CBTR between January 1, 2012, and March 9, 2017. Inclusion criteria were a preoperative clinical diagnosis of CBTS and a documented EDX performed preoperatively. Exclusion criteria included revision CBTR, outdated (12 months older) or incomplete EDX, EDX with no available reports, and history of elbow trauma or deformity. Patient demographics including age at the time of surgery, sex, laterality, diabetes mellitus, obesity, and concomitant carpal tunnel release (CTR) were collected.
Patients were diagnosed with CBTS if they described small ± ring finger paresthesias, with or without subjective clumsiness, weakness, and decreased fine motor function and showed one or more positive results on provocative testing such as elbow flexion-compression test and percussion (Tinel) test at the elbow.7,14-17 Cubital tunnel syndrome–specific signs like clawing of the small and ring fingers, Wartenberg or Froment signs, and intrinsic, hypothenar, or first web space atrophy were considered signs of advanced disease.1,5,7,18
All EDXs were performed at a single electrodiagnostic laboratory. The ulnar nerve findings were was diagnosed as one of the following: (1) CBTS; (2) ulnar neuropathy (UN); or (3) normal.
All patients, with the exception of those with signs of advanced disease, had failed a 3-month trial of conservative therapy.19 All patients underwent in-situ release or anterior subcutaneous transposition when the ulnar nerve showed intraoperative anterior instability. Cubital tunnel release was considered successful when patients confirmed improvement in paresthesia, pain, and/or night symptoms in the ulnar nerve distribution.
The sensitivity of EDX reports was calculated based on postoperative outcomes; EDX reports were interpreted based on AANEM criteria. The across-the-elbow mNCV was calculated using the following formula:
The patients were divided into 2 groups based on whether above-elbow stimulation was done (XE) or not (non-XE). The 2 groups were compared regarding age, sex, diabetes, obesity, concomitant CTR, and symptom resolution using χ2 test to determine any significant difference between the 2 groups. Statistical significance was set at P < .05.
Results
We identified a cohort of 98 patients with 118 elbows that underwent CBTR and met the inclusion criteria. The average age was 50 (range, 17-80) years. Men comprised 42.9% of patients, and 20.4% underwent bilateral CBTR (11 men, 9 women). Diabetes mellitus was present in 28.6% of patients, and 60.2% were obese. In-situ releases made up 91.5% of CBTR, and 65.3% had concomitant CTR. Postoperatively, 111 (93.6%) upper extremities showed significant improvement in ulnar nerve symptoms, and their CBTRs were successful.
Based on the EDX reports, 13 elbows (11%) had clear CBTS, 27 (23%) had UN, and 78 (66%) were normal. Twenty-six elbows (22%) received stimulation above the elbow (XE group), whereas the other 72 patients (92 elbows) did not (non-XE group). The reports did not provide justification for the inclusion or exclusion of above-elbow stimulation. None of the reports described the mNCV across the elbow. The cut-off used for normal mNCV was set at 45 m/s.
The sensitivity of EDX reports was 11.7% for the diagnosis of CBTS, which improved to 34.2% where the diagnoses of CBTS and UN were combined.
Twenty-six patients had above-elbow stimulation and were in the XE group; all 26 underwent CBTR. The non-XE group had 72 patients who underwent 92 CBTR. There was no significant difference in age, diabetes, in-situ release, and outcome. The 2 groups differed in sex, bilaterality, concomitant CTR, and obesity using a significance level of P < .05 (Table 1).
Table 1.
Comparison of the XE and Non-XE Groups.
| Group | XE | Non-XE |
|---|---|---|
| Patients | 26 | 72 |
| Bilateral cubital tunnel release | 0 | 20 |
| Average age (range) | 48 (17-68) | 50.8 (26-80) |
| Male | 18 (69.2%) | 24 (33.3%) |
| Diabetes | 7 (22.8%) | 21 (23.7%) |
| Obesity | 12 (55.3%) | 47 (60.2%) |
| In-situ release | 23 (88.5%) | 85 (92.4%) |
| Carpal tunnel release | 11 (42.3%) | 66 (91.7%) |
| Postoperative improvement | 24 (91.7%) | 85 (92.4%) |
Note. XE = across elbow.
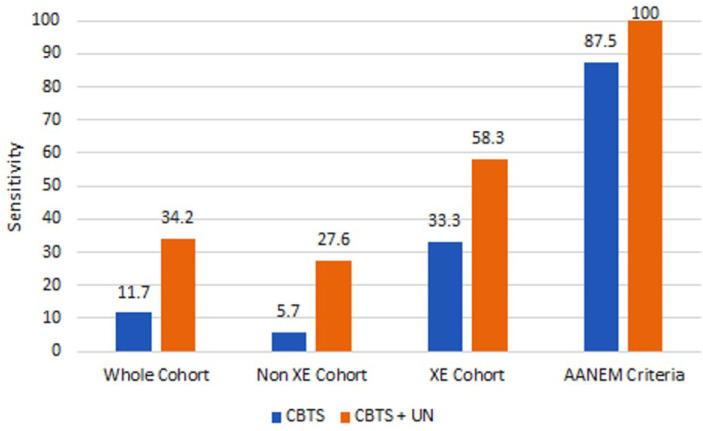
The sensitivity of the EDX reports for the diagnosis of CBTS for the non-XE group was 5.7%, whereas that of the XE group was 33.3%. The sensitivity of our interpretation of the results in the XE group was 87.5%. Figure 1 shows the sensitivities of all groups for CBTS as well as combined CBTS and UN.
Figure 1.
Diagram comparing sensitivities between different groups for CBTS and for combining CBTS and UN. The AANEM columns depict the use of estimated motor nerve conduction velocity and a cut-off of 50 m/s.
Note. CBTS = cubital tunnel syndrome. AANEM = Association of Neuromuscular and Electrodiagnostic Medicine; XE = across elbow; UN = ulnar neuropathy.
Discussion
Electrodiagnostic studies have become a routine component in the workup of hand numbness and tingling. Their results are used to confirm or refute diagnoses, and determine the need, as well as authorization, for further evaluation and treatment. It is common practice to order an EDX before a hand surgery consultation is obtained. Ordering physicians may fail to consider CBTS as a pretest diagnosis or include it on the EDX order which leads to EDX orders with generic, incomplete, or even incorrect clinical pretest diagnoses. With no specific indication of CBTS on the EDX order, EDX technicians may fail to include above-elbow stimulation. Once we realized our low rate of above-elbow stimulation, we investigated and confirmed that our EDX laboratory protocol does not indicate above-elbow stimulation for “hand numbness and tingling” or “carpal tunnel syndrome.” Considering that the vast majority of EDXs are typically ordered prior to surgical or neurological consultations, it became apparent that testing for CBTS was not always implemented when needed. This lack of testing explained our observation that many CBTS patients present with negative EDX reports, which was why we undertook this study. We wanted to examine the utility of obtaining EDX in surgical candidates as we frequently overrule supposedly normal EDXs. Given the greater than 90% rate of clinical improvement after CBTR in our patients, and the unexpectedly low rate of above-elbow stimulation, our suspicion was confirmed which led to a performance improvement initiative at our institution to include XE testing for patients with hand parasthesias, irrespective of the pretest diagnosis.
Only a subset of the literature discusses across-the-elbow testing performed according to AANEM recommended specifications. They report sensitivities in the 60% to 80% range.20-22 Our findings, at 87% sensitivity, are compatible with this literature and confirm that applying the AANEM criteria increases the correlation between the EDX and clinical diagnosis of CBTS.
We are aware of several limitations of our study. They include the retrospective nature of the study and subjective component of CBTS symptoms. Our 2 groups differed in sex, bilaterality, obesity, and concomitant CTR. Of those, concomitant CTR may increase the risk of bias. Patient outcomes following combined carpal tunnel and CBTRs may be difficult to attribute specifically to one release or the other.20,23 Releasing the transverse carpal ligament also decreases pressure on the ulnar nerve in the ulnar tunnel, which effectively provides 2 points of ulnar nerve release. Another limitation is that we were only able to use 2 of several listed AANEM criteria. We elected not to include patients who did not require surgical intervention for several reasons. They are less likely to have had an EDX on presentation and are less likely to follow up after successful treatment. On the contrary, patients who respond to conservative management are more likely to have even less obvious EDX findings, further degrading the test’s sensitivity.
To our knowledge, this study is the only one that compares preoperative EDX diagnoses with postoperative clinical diagnoses. It highlights the importance of above-elbow stimulation testing for CBTS regardless of the pretest diagnoses, and verifies the value of adopting the AANEM guidelines. Based on these findings, we do not routinely repeat EDX to include above-elbow stimulation unless our clinical findings are not convincing. We explain to the patients that our assessment is in conflict with the EDX findings and that the test did not include the suspected segment of the nerve.
Conclusion
Implementing current AANEM standards for CBTS on all patients with reported hand parasthesias that involve the ulnar digits results in significant improvement in correlation of clinical and electrodiagnostic findings of CBTS.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed Consent: Informed consent was not obtained from patients due to the retrospective nature of this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shafic Sraj  https://orcid.org/0000-0003-3873-7369
https://orcid.org/0000-0003-3873-7369
References
- 1. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288. http://www.ncbi.nlm.nih.gov/pubmed/9753755. Accessed October 31, 2018. [DOI] [PubMed] [Google Scholar]
- 2. An TW, Evanoff BA, Boyer MI, et al. The prevalence of cubital tunnel syndrome: a cross-sectional study in a U.S. J Bone Joint Surg Am. 2017;99(5):408-416. doi: 10.2106/JBJS.15.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cutts S. Cubital tunnel syndrome. Postgrad Med J. 2007;83(975):28-31. doi: 10.1136/pgmj.2006.047456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spinner M, Kaplan EB. The relationship of the ulnar nerve to the medial intermuscular septum in the arm and its clinical significance. Hand. 1976;8(3):239-242. http://www.ncbi.nlm.nih.gov/pubmed/976822. Accessed October 31, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Boone S, Gelberman RH, Calfee RP. The management of cubital tunnel syndrome. J Hand Surg Am. 2015;40(9):1897-1904; quiz 1904. doi: 10.1016/j.jhsa.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 6. Root CG, London DA, Schroeder NS, et al. Anatomical relationships and branching patterns of the dorsal cutaneous branch of the ulnar nerve. J Hand Surg Am. 2013;38(6):1131-1136. doi: 10.1016/j.jhsa.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg Am. 2010;35(1):153-163. doi: 10.1016/j.jhsa.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 8. Dy CJ, Mackinnon SE. Ulnar neuropathy: evaluation and management. Curr Rev Musculoskelet Med. 2016;9(2):178-184. doi: 10.1007/s12178-016-9327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. J Hand Surg Am. 2010;35(4):668-677. doi: 10.1016/j.jhsa.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feinberg J. EMG: myths and facts. HSS J. 2006;2(1):19-21. doi: 10.1007/s11420-005-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Practice parameters for electrodiagnostic studies in ulnar neuropathy at the elbow. https://www.aanem.org/getmedia/acf78f99-7f9c-4414-8a4e-afeb96b7103d/UlnarNeur.pdf. Accessed March 20, 2019.
- 12. AAEM Quality Assurance Committee. Literature review of the usefulness of nerve conduction studies and electromyography in the evaluation of patients with ulnar neuropathy at the elbow. https://www.aanem.org/getmedia/acf78f99-7f9c-4414-8a4e-afeb96b7103d/UlnarNeur.pdf. Accessed March 16, 2019. [DOI] [PubMed]
- 13. Landau ME, Campbell WW. Clinical features and electrodiagnosis of ulnar neuropathies. Phys Med Rehabil Clin N Am. 2013;24(1):49-66. doi: 10.1016/j.pmr.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 14. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820. doi: 10.1016/0363-5023(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 15. Rayan GM, Jensen C, Duke J. Elbow flexion test in the normal population. J Hand Surg Am. 1992;17(1):86-89. http://www.ncbi.nlm.nih.gov/pubmed/1538117. Accessed October 31, 2018. [DOI] [PubMed] [Google Scholar]
- 16. Beekman R, Visser LH. Sonography in the diagnosis of carpal tunnel syndrome: a critical review of the literature. Muscle Nerve. 2003;27(1):26-33. doi: 10.1002/mus.10227. [DOI] [PubMed] [Google Scholar]
- 17. Kuschner SH, Ebramzadeh E, Mitchell S. Evaluation of elbow flexion and linel tests for cubital tunnel syndrome in asymptomatic individuals. Orthopedics. 2006;29(4):305-308. http://www.ncbi.nlm.nih.gov/pubmed/16628989. Accessed October 31, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32(6):855-858. doi: 10.1016/j.jhsa.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19. Goldberg BJ, Light TR, Blair SJ. Ulnar neuropathy at the elbow: results of medial epicondylectomy. J Hand Surg Am. 1989;14(2, pt 1):182-188. http://www.ncbi.nlm.nih.gov/pubmed/2703664. Accessed October 31, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Bielawski M, Hallett M. Position of the elbow in determination of abnormal motor conduction of the ulnar nerve across the elbow. Muscle Nerve. 1989;12(10):803-809. doi: 10.1002/mus.880121004. [DOI] [PubMed] [Google Scholar]
- 21. Shakir A, Micklesen PJ, Robinson LR. Which motor nerve conduction study is best in ulnar neuropathy at the elbow. Muscle Nerve. 2004;29(4):585-590. doi: 10.1002/mus.10513. [DOI] [PubMed] [Google Scholar]
- 22. Kothari MJ, Preston DC. Comparison of the flexed and extended elbow positions in localizing ulnar neuropathy at the elbow. Muscle Nerve. 1995;18(3):336-340. doi: 10.1002/mus.880180312. [DOI] [PubMed] [Google Scholar]
- 23. Kincaid JC, Phillips LH, Daube JR. The evaluation of suspected ulnar neuropathy at the elbow. Normal conduction study values. Arch Neurol. 1986;43(1):44-47. http://www.ncbi.nlm.nih.gov/pubmed/3942514. Accessed March 16, 2019. [DOI] [PubMed] [Google Scholar]