Abstract
Background:
Inconsistent associations between long-term exposure to particles with an aerodynamic diameter [fine particulate matter ()] components and mortality have been reported, partly related to challenges in exposure assessment.
Objectives:
We investigated the associations between long-term exposure to elemental components and mortality in a large pooled European cohort; to compare health effects of components estimated with two exposure modeling approaches, namely, supervised linear regression (SLR) and random forest (RF) algorithms.
Methods:
We pooled data from eight European cohorts with 323,782 participants, average age 49 y at baseline (1985–2005). Residential exposure to 2010 annual average concentration of eight components [copper (Cu), iron (Fe), potassium (K), nickel (Ni), sulfur (S), silicon (Si), vanadium (V), and zinc (Zn)] was estimated with Europe-wide SLR and RF models at a scale. We applied Cox proportional hazards models to investigate the associations between components and natural and cause-specific mortality. In addition, two-pollutant analyses were conducted by adjusting each component for mass and nitrogen dioxide () separately.
Results:
We observed 46,640 natural-cause deaths with 6,317,235 person-years and an average follow-up of 19.5 y. All SLR-modeled components were statistically significantly associated with natural-cause mortality in single-pollutant models with hazard ratios (HRs) from 1.05 to 1.27. Similar HRs were observed for RF-modeled Cu, Fe, K, S, V, and Zn with wider confidence intervals (CIs). HRs for SLR-modeled Ni, S, Si, V, and Zn remained above unity and (almost) significant after adjustment for both and . HRs only remained (almost) significant for RF-modeled K and V in two-pollutant models. The HRs for V were 1.03 (95% CI: 1.02, 1.05) and 1.06 (95% CI: 1.02, 1.10) for SLR- and RF-modeled exposures, respectively, per , adjusting for mass. Associations with cause-specific mortality were less consistent in two-pollutant models.
Conclusion:
Long-term exposure to V in was most consistently associated with increased mortality. Associations for the other components were weaker for exposure modeled with RF than SLR in two-pollutant models. https://doi.org/10.1289/EHP8368
Introduction
The Global Burden of Disease (GBD 2015) study estimated that exposure to ambient particles with an aerodynamic diameter [fine particulate matter ()] was the fifth-ranking mortality risk factor, contributing to deaths per year (Cohen et al. 2017). is a mixture of a large number of components related to specific sources. Identifying which components of are main contributors to adverse health effects is important for targeted policy-making. Although some studies have attempted to associate long-term exposure to specific components with mortality risks, the results are inconclusive. The California Teachers Study (Ostro et al. 2015) found an increased risk of ischemic heart disease (IHD) mortality in associations with exposure to nitrate, elemental carbon (EC), copper (Cu), and secondary organics in . The American Cancer Society (ACS) Cancer Prevention Study-II (CPS-II) suggested that long-term exposure from coal combustion and its key emission tracer elements (i.e., selenium and arsenic) were associated with increased IHD mortality risk, whereas exposure to silicon (Si) and potassium (K) was not associated with mortality (Thurston et al. 2013, 2016). In the Medicare population, the excess mortality risk associated with long-term exposure increased with relative concentration of EC, vanadium (V), Cu, calcium, and iron (Fe) and decreased with nitrate, organic carbon, and sulfate (Wang et al. 2017). The large European Study of Cohorts for Air Pollution Effects (ESCAPE) reported a robust relationship between natural-cause mortality and sulfur (S), and some evidence of associations with Fe and Cu in (Beelen et al. 2015). No statistically significant association with components was found for cardiovascular mortality in the ESCAPE study (Wang et al. 2014).
Long-term exposure assessment for particle components is more challenging than for mass because of limited regulatory routine monitoring (with the exception of nitrate, ammonium, and sulfate) and less data on emission rates used as input to dispersion models (Holmes and Morawska 2006). To date, the available epidemiological evidence used different exposure assessment methods, including direct monitoring (Ostro et al. 2010; Thurston et al. 2013, 2016), chemical transport models (CTMs) at a scale (Ostro et al. 2015) and fine spatial scale land-use regression (LUR) models (Beelen et al. 2015; Wang et al. 2014). Different exposure assessment methods may lead to component-specific differences in exposure estimation error, potentially leading to bias (Adams et al. 2015). Studies have suggested that risk estimates of mass differed between exposure assessment methods (Jerrett et al. 2017; McGuinn et al. 2017). Studies comparing exposure assessment methods in their associations with health outcomes mainly focused on the comparison among direct monitoring, satellite products, dispersion/CTMs and LUR models. Recent developments in exposure assessment include combining different methods such as land-use or chemical transport modeling and monitoring data using a variety of approaches including linear regression and machine learning algorithms (Hoek 2017). Comparisons have been made among exposure predictions developed with different algorithms in terms of prediction accuracy (Brokamp et al. 2017; Chen et al. 2019; Kerckhoffs et al. 2019). However, a simulation study suggested that improving the prediction accuracy of exposure models did not always improve the accuracy of health effect estimation, when bias is introduced into health effect estimation by the classical-like measurement error and the impact of Berkson-like measurement error on the health effect estimation error diminishes for large number of subjects (Szpiro et al. 2011). To our knowledge, no studies have compared exposure models developed with different algorithms regarding their relation with health outcomes.
The present study is part of the Effects of Low-level Air Pollution: a Study in Europe (ELAPSE). ELAPSE builds on the elemental composition, mortality and covariate data of ESCAPE (Beelen et al. 2014, 2015; Wang et al. 2014). In ESCAPE, each cohort was analyzed separately, whereas in ELAPSE respective ESCAPE cohorts were pooled to represent a contrast in low-level air pollution exposures. In addition, the follow-up data for mortality were extended from typically up to 2008 in ESCAPE to up to 2011–2017 in ELAPSE, which substantially increased the number of deaths and hence study power. Measurements for black carbon (BC) and elemental composition at individual ESCAPE study areas were pooled to develop Europe-wide exposure models covering combined study areas for application in the ELAPSE (Chen et al. 2020; De Hoogh et al. 2018). The combined ability to do pooled analyses, plus accounting for new insights in the robustness of LUR models related to the number of air pollution monitoring sites (Basagaña et al. 2012; Wang et al. 2012), strengthened the exposure assessment in ELAPSE. The Europe-wide models furthermore allowed better coverage of those ESCAPE cohorts in large study areas of which typically only a fraction was covered by dedicated monitoring campaigns [e.g., only Paris in the national French Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort] (de Hoogh et al. 2013; Tsai et al. 2015). The Europe-wide models for assessing 2010 annual average composition concentrations at a scale were developed using two algorithms—the supervised linear regression (SLR) and the random forest (RF) algorithms, which is a machine learning algorithm (Chen et al. 2020). The RF models outperformed the SLR models at the Europe-wide level by 11–30% across components in hold-out-validation , whereas the two models performed similarly in explaining variability within individual ESCAPE study areas. Despite the similar within-area performance, the exposure predictions at random sites derived from SLR and RF models correlated only moderately at the national level (the average of correlation coefficients at 11 ELAPSE countries range from 0.41 to 0.77 across components). We refer to correlations as low, 0.4–0.7 as moderate, and as high. Although the focus of ELAPSE is on low-level air pollution defined as below the current air quality guidelines and standards, low-level is difficult to define for PM elemental composition because there are currently no guidelines/standards for PM elemental composition.
The first aim of this study was to evaluate whether specific components of were associated with mortality. The second aim was to compare the health effects of components estimated with two different exposure modeling approaches, namely, the SLR and RF algorithms.
Methods
Study Populations
The ELAPSE pooled cohort contains eight cohorts across six European countries able to participate in data pooling, areas with low-level air pollution exposure, and relatively recent recruitment dates (Table 1 and Figure S1). The cohorts are the following: the Cardiovascular Effects of Air Pollution and Noise in Stockholm (CEANS) cohort in Sweden, which was constructed from four subcohorts: the Stockholm Diabetes Prevention Program (SDPP) (Eriksson et al. 2008), the Stockholm Cohort of 60-Year-Olds (SIXTY) (Wändell et al. 2007), the Stockholm Screening Across the Lifespan Twin study (SALT) (Magnusson et al. 2013), and the Swedish National Study on Aging and Care in Kungsholmen (SNACK) (Lagergren et al. 2004); the Diet, Cancer and Health cohort (DCH) (Tjønneland et al. 2007) in Denmark; the Danish Nurse Cohort (DNC) (Hundrup et al. 2012) in Denmark, consisting at baseline of two surveys conducted in 1993 and 1999; the European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL) cohort in the Netherlands, including the Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands (MORGEN) and Prospect (Beulens et al. 2010); the Heinz Nixdorf Recall (HNR) study in Germany (Schmermund et al. 2002); the E3N in France (Clavel-Chapelon and E3N Study Group 2015); the Cooperative Health Research in the Region of Augsburg (KORA) in Germany, consisting at baseline of two cross-sectional population-representative surveys conducted in 1994–1995 (S3) and 1999–2001 (S4); and the Vorarlberg Health Monitoring and Prevention Program (VHM&PP) in Austria (Ulmer et al. 2007). The study areas of most cohorts constituted a large city and its surrounding areas. Some cohorts, such as the French E3N cohort and the Danish DNC cohort, covered large regions of the country. All included cohort studies were approved by the medical ethics committees in their respective countries. Detailed information of each individual cohort is provided in Tables S1–S8. A lot of variable harmonization was done in the ESCAPE collaboration, which formed the basis of the present study (Beelen et al. 2014; Cesaroni et al. 2014; Raaschou-Nielsen et al. 2013; Stafoggia et al. 2014). In ELAPSE, a joint codebook with exact definitions of variables was prepared, starting from the ESCAPE codebook. We asked each cohort to transfer the data to Utrecht University and checked the definition of variables according to the joint codebook. The variables in our confounder models did not require much harmonization. In the E3N cohort, smoking intensity was in classes. We assigned the midpoint as the actual value. Area-level socioeconomic status (SES) was newly collected in ELAPSE. We specified the desired area-level SES variables with respect to spatial scale and variables before pooling the cohort data. The detailed harmonization process for area-level SES variables is described in the Supplemental Material in the section “Area-level socio-economic status (SES) variable harmonization.”
Table 1.
Population characteristics based on the observations included in Model 3.
| Subcohorta | Population size ()b | Persons in main model, Model 3 [ (%)]c | Baseline period | Follow-up | Average years of follow-up | Age at baseline () | Female (%) | Current smokers (%) | Overweight or obese [ (%)] | Married or living with partner (%) | Employed (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled cohort | 381,036 | 323,782 (85.0) | — | — | 19.5 | 66 | 24 | 43 | 72 | 70 | |
| CEANS-SDPP | 7,835 | 7,716 (98.5) | 1992–1998 | 2011 | 15.9 | 61 | 26 | 52 | 84 | 91 | |
| CEANS-SIXTY | 4,180 | 3,965 (94.9) | 1997–1999 | 2014 | 15.5 | 52 | 21 | 64 | 74 | 68 | |
| CEANS-SALT | 6,724 | 6,174 (91.8) | 1998–2003 | 2011 | 10.4 | 55 | 21 | 40 | 68 | 64 | |
| CEANS-SNACK | 3,248 | 2,830 (87.1) | 2001–2004 | 2011 | 7.4 | 62 | 14 | 53 | 46 | 23 | |
| DCH | 56,308 | 52,779 (93.7) | 1993–1997 | 2015 | 18.2 | 53 | 36 | 56 | 71 | 78 | |
| DNC-1993 | 19,664 | 17,017 (86.5) | 1993 | 2013 | 18.7 | 100 | 37 | 28 | 68 | 70 | |
| DNC-1999 | 8,769 | 8,117 (92.6) | 1999 | 2013 | 14.4 | 100 | 29 | 30 | 76 | 95 | |
| EPIC-NL-MORGEN | 20,711 | 18,292 (88.3) | 1993–1997 | 2013 | 16.8 | 55 | 35 | 49 | 65 | 69 | |
| EPIC-NL Prospect | 16,194 | 14,570 (90.0) | 1993–1997 | 2013 | 16.4 | 100 | 23 | 55 | 77 | 51 | |
| HNR | 4,809 | 4,733 (98.4) | 2000–2003 | 2015 | 12.0 | 50 | 24 | 74 | 75 | 40 | |
| E3N | 53,521 | 38,537 (72.0) | 1989–1991 | 2011 | 16.8 | 100 | 13 | 21 | 83 | 68 | |
| KORA-S3 | 4,566 | 2,572 (56.3) | 1994–1995 | 2011 | 15.6 | 51 | 20 | 67 | 80 | 55 | |
| KORA-S4 | 4,257 | 2,281 (53.6) | 1999–2001 | 2014 | 12.9 | 51 | 23 | 69 | 79 | 59 | |
| VHM&PP | 170,250 | 144,199 (84.7) | 1985–2005 | 2014 | 23.1 | 56 | 20 | 42 | 69 | 70 |
Note: —, not applicable; BMI, body mass index; CEANS, Cardiovascular Effects of Air Pollution and Noise in Stockholm; DCH, Diet, Cancer and Health cohort; DNC, Danish Nurse Cohort (1993 and 1999); EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands cohort; E3N, Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale; HNR, Heinz Nixdorf Recall study; KORA, the Cooperative Health Research in the Region of Augsburg [1994–1995 (S3) and 1999–2001 (S4)], MORGEN, Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands; SALT, Stockholm Screening Across the Lifespan Twin study; SD, standard deviation; SDPP, Stockholm Diabetes Prevention Program; SIXTY, Stockholm Cohort of 60-Year-Olds; SNACK, Swedish National Study on Aging and Care in Kungsholmen; VHM&PP, Vorarlberg Health Monitoring and Prevention Program.
The CEANS cohort (including SDPP, SIXTY, SALT, SNACK) is in Sweden; the DCH cohort is in Denmark; the DNC (consisting of two surveys conducted in 1993 and 1999) is in Denmark; the EPIC-NL cohort is in the Netherlands (including MORGEN and Prospect); the HNR study is in Germany; the E3N is in France; the KORA is in Germany (consisting of two surveys S3 and S4); the VHM&PP is in Austria.
Population size is the number of subjects for which information was transferred to Utrecht University for construction of the pooled cohort.
The missing data for individual cohorts are indicated in Table S1–S8.
Air Pollution Exposure Assessment
Eight components were a priori selected in ESCAPE to represent major pollution sources: Cu, Fe, and zinc (Zn) representing non-tailpipe traffic emissions; S representing long-range transport of secondary inorganic aerosols; nickel (Ni) and V representing mixed oil burning/industry; Si representing crustal material; and K representing biomass burning (de Hoogh et al. 2013; Tsai et al. 2015). We assessed exposure to these eight elements in at the participants’ baseline residential addresses using Europe-wide LUR models developed with two algorithms. The models have been described in detail elsewhere (Chen et al. 2020). Briefly, we estimated 2010 annual mean concentrations of elemental composition based on the standardized ESCAPE monitoring data. We offered large-scale satellite-model and CTM estimates of components as predictors to represent background concentrations and land-use, traffic, population, and industrial point source data to model local spatial variability. We applied the SLR (De Hoogh et al. 2018) and the RF algorithms (Chen et al. 2019) to develop models for each component. The models explained a moderate-to-large fraction of the measured concentration variation at the European scale, ranging from 41% to 90% across components. Model performance evaluated by 5-fold hold-out validation reported in Chen et al. (2020) was extracted and is shown in Table S9. The RF models consistently outperformed the SLR models in explaining overall variability, including both between- and within-study area variability. The models explained within-area variability less well, with similar performance for SLR and RF models. The SLR and RF model predictions correlated moderately at the national level (the averaged correlation coefficients at six countries covered by the ELAPSE pooled cohort range from 0.43 to 0.78 across the components).
Exposure to 2010 annual mean concentration of mass and nitrogen dioxide () was assessed by Europe-wide LUR models developed previously (De Hoogh et al. 2018). The models were developed based on the European Environmental Agency AirBase routine monitoring data, with satellite-derived and CTM air pollutants estimates and land-use, traffic, and population data as predictors. The model explained 72% of the measured spatial variation in the annual average concentration across Europe, whereas the model explained 59%.
We applied the exposure models to create grids of the predicted concentrations of the pollutants covering the entire study area and transferred the relevant parts to the participating centers for exposure assignment. Careful procedures were applied to ensure that correct exposure assignment occurred, including clarification of the correct coordinate system. Checking involved exposure assignment to a set of randomly selected coordinates by the participating centers and the coordinating center independently and by comparison of the exposure assignment. After assignment, anonymized data were returned to Utrecht University for checking and pooling.
We selected 2010 as the primary year of exposure modeling because 2009–2010 was the period of ESCAPE monitoring that we used to develop composition models (Chen et al. 2020). For , this was the earliest year of a sufficiently wide coverage of monitoring across Europe (De Hoogh et al. 2018). For consistency, we used the year 2010 for as well. We assumed that the spatial variability of the relevant pollution concentrations remained reasonably stable to the baseline period (1985–2005). We also assumed that, for a mortality outcome, the exposure in the past few years was the most relevant exposure. We considered very high and negative predicted concentrations of elemental composition unrealistic. Truncations were performed to deal with unrealistic predicted concentrations (Chen et al. 2020). We defined a maximum predicted concentration for each component calculated by fitting the SLR model with the maximum predictor values at ESCAPE monitoring sites for positive slopes (or the minimum predictor values for negative slopes). We considered predicted concentrations larger than the maximum modeled values as unrealistic predictions and truncated them to the maximum predicted concentrations. The high unrealistic predictions were mostly related to a close distance to industrial sources. Negative predictions were set to zero. Truncation was performed in the main model population for SLR-modeled exposure: 11.3% for Cu, 0.5% for Fe, 11.6% for Ni, 14.3% for V, and 2.6% for Zn (Table S10). The truncation was mostly performed for predictions below zero and mostly located in the North European cohorts (i.e., CEANS and DNC) and KORA and VHM&PP. The high truncation frequency in some cohorts indicates that these cohorts did not contribute much information to the analyses. Only 2, 24, and 240 observations ( of all observations) were truncated because of high SLR predictions for Cu, Ni, and Zn, respectively. No truncation was needed for RF-modeled exposure because the RF predictions were within the reasonable range, probably due to the flexible nature of the RF algorithm.
Mortality Outcome Definition
Identification of outcomes was based upon linkage to mortality registries. Natural mortality was defined based on the underlying cause of death recorded on death certificates according to the International Classification of Disease, Ninth Revision (ICD 9; WHO 1997) codes 001–779 and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10; WHO 2016) codes A00–R99. We further defined mortality from cardiovascular disease (ICD-9 codes 400–440, ICD-10 codes I10–I70), respiratory disease (ICD-9 codes 460–519, ICD-10 codes J00–J99) and lung cancer (ICD-9 code 162, ICD-10 code C34).
Statistical Analyses
Main analyses.
To estimate HRs and 95% CIs for associations of the component exposure with natural and cause-specific mortality, we applied Cox proportional hazards models following the general ELAPSE analytical framework (Hvidtfeldt et al. 2021b; Liu et al. 2021, 2020; Samoli et al. 2021). We used strata for subcohorts contributing to the pooled cohort to account for differences in baseline hazard between the subcohorts unexplained by the available covariates. We used strata because the assumption of proportional hazards did not hold with respect to subcohort. Strata had a substantially better model performance compared with alternative specifications, such as subcohort indicators. The decision to account for between-cohort heterogeneity using strata implies that we mostly evaluated within-cohort exposure contrasts. Each component was included as a linear function in the Cox models as a reasonable summary of the association, allowing comparison with previous studies. HRs were calculated with a fixed increment for each component following the increments selected in previous publications from ESCAPE and ELAPSE (Beelen et al. 2015; Hvidtfeldt et al. 2021a): Cu, ; Fe, ; K, ; Ni, ; S, ; Si, ; V, ; and Zn, . Censoring occurred at the time of the event of interest, death from other causes, emigration, loss to follow-up for other reasons, or at the end of follow-up, whichever came first. We a priori specified three confounder models with increasing control for individual- and area-level covariates: Model 1 included only age (as the time scale), subcohort (as strata), sex (as strata), and year of enrollment; Model 2 added individual-level covariates, including marital status (married/cohabiting, divorced/separated, single, widowed), smoking status (never, former, current), smoking duration (years of smoking) for current smokers, smoking intensity (cigarettes/day) for current smokers, squared smoking intensity, body mass index (BMI) categories (, 18.5–24.9, 25–29.9, and ), and employment status (employed vs. unemployed); Model 3 further adjusted for neighborhood-level mean income in 2001. We determined the confounder Models 2 and 3 by balancing the need to adjust for a comprehensive set of confounders and the availability in a large number of cohorts. BMI was included as a categorical variable because there is evidence of nonlinear relationships between continuous BMI and mortality (Global BMI Mortality Collaboration et al. 2016). We considered Model 3 as the main model. Participants with missing exposure or incomplete information on Model 3 covariates were excluded from all main analyses to ensure comparability between the model results.
Two-pollutant models were conducted with the main model, Model 3, adjusting each component for mass and separately. We adjusted for mass to investigate whether the association with individual components reflecting specific sources remained after adjustment for generic mass, for which we have strong evidence of associations (Beelen et al. 2014). We adjusted for in an attempt to disentangle the individual component effect from traffic exhaust emission, for which is used as a marker. Adjustment for is especially important when assessing associations with the traffic nonexhaust components Cu, Fe, and Zn. However, two-pollutant models can be difficult to interpret when the two pollutants reflect the same source or are strongly correlated. We did not model combinations of components in two-pollutant models because many were highly correlated (Figure S2) and we preferred to limit the complexity of analyses. The mass and estimates used in the two-pollutant models were developed with the SLR algorithm (De Hoogh et al. 2018). We previously documented that, for mass and separately, SLR and RF models had similar performance, and that SLR- and RF-modeled exposure at external validation sites were highly correlated ( mass: Pearson ; : ) (Chen et al. 2019). Consequently, only the SLR-modeled and exposures were linked to the individual cohorts.
We assessed the shape of the concentration–response functions (CRFs) for components and natural-cause mortality with natural cubic splines with 3 degrees of freedom. The CRFs can be difficult to interpret when there is limited variability in exposure contrasts.
In our interpretations, we attached more importance to two-pollutant models than single-pollutant models, acknowledging the difficulties in interpreting two-pollutant models. Given the similar performance of the SLR and RF model in explaining within-area variation (Table S9), and the fact that our analyses exploited primarily within-cohort exposure contrasts, we interpreted the two exposure methods equally. Therefore, we considered it more convincing when consistent associations between specific components and mortality were observed by applying two different exposure methods.
Sensitivity analyses.
To evaluate the potential bias introduced by excluding participants with missing information on Model 3 covariates, we fitted Model 1 and Model 2 with participants with complete information on Model 1 and Model 2 covariates, respectively. To assess the sensitivity of our findings to using the 2010 exposures, we restricted analyses to follow-up periods starting from 2000, 2005, and 2008, with successively less temporal misalignment of the exposure model at the expense of shorter follow-up and fewer deaths. To address potential residual confounding by SES factors, we further adjusted for individual-level education, occupational status, and additional neighborhood-level SES variables in cohorts that had such information. All sensitivity analyses were performed for natural-cause mortality only.
All analyses were performed in R (version 3.4.0; R Development Core Team), using the packages survival, coxme, Matrix, foreach, glmnet, multcomp, survey, splines, Hmisc, mfp, VIM, ggplot2, frailtySurv, survsim, eha, and stamod. Statistical significance was based on a 95% CI of effect estimate, not including unity.
Results
Characteristics of the Study Population
The total study population in the main model, Model 3 (the most adjusted model), consisted of 323,782 subjects, contributing 6,317,235 person-years at risk. Most of the cohorts started in the mid-1990s with follow-up until 2011–2015. Fifteen percent of the total population was excluded from all main analyses owing to missing exposure (0.5%), individual-level covariates (12.7%), or neighborhood-level mean income (1.8%). The excluded population was slightly younger (baseline age ) than the Model 3 population (baseline age ). The proportion of females in the excluded population (64%) was slightly lower than in the Model 3 population (66%). Table 1 and Tables S1–S8 show the baseline characteristics of the participants in the individual subcohorts. The subcohorts differed in the number of participants, average years of follow-up, mean baseline age, percentage of female participants, lifestyle factors, and neighborhood-level income, supporting the analysis accounting for difference in baseline hazards between subcohorts.
Exposure Distribution and Correlations
For Cu, Fe, K, S, and Zn, concentrations were lower in the North European cohorts (i.e., CEANS, DCH, and DNC; Figure S1) than in the other cohorts (Figure 1 and Table S11). The within-cohort contrast was substantial for Cu, Fe, and Si and limited for K, Ni, S, V, and Zn for both SLR- and RF-modeled exposures. Exposure distributions for the pooled cohort were similar for SLR- and RF-modeled estimates, although for most components the variability was smaller for RF. Our selected fixed increment reflected a larger exposure contrast than the interquartile ranges for most elements. For individual cohorts, large differences between the two algorithms were found, for example, S in the HNR study.
Figure 1.
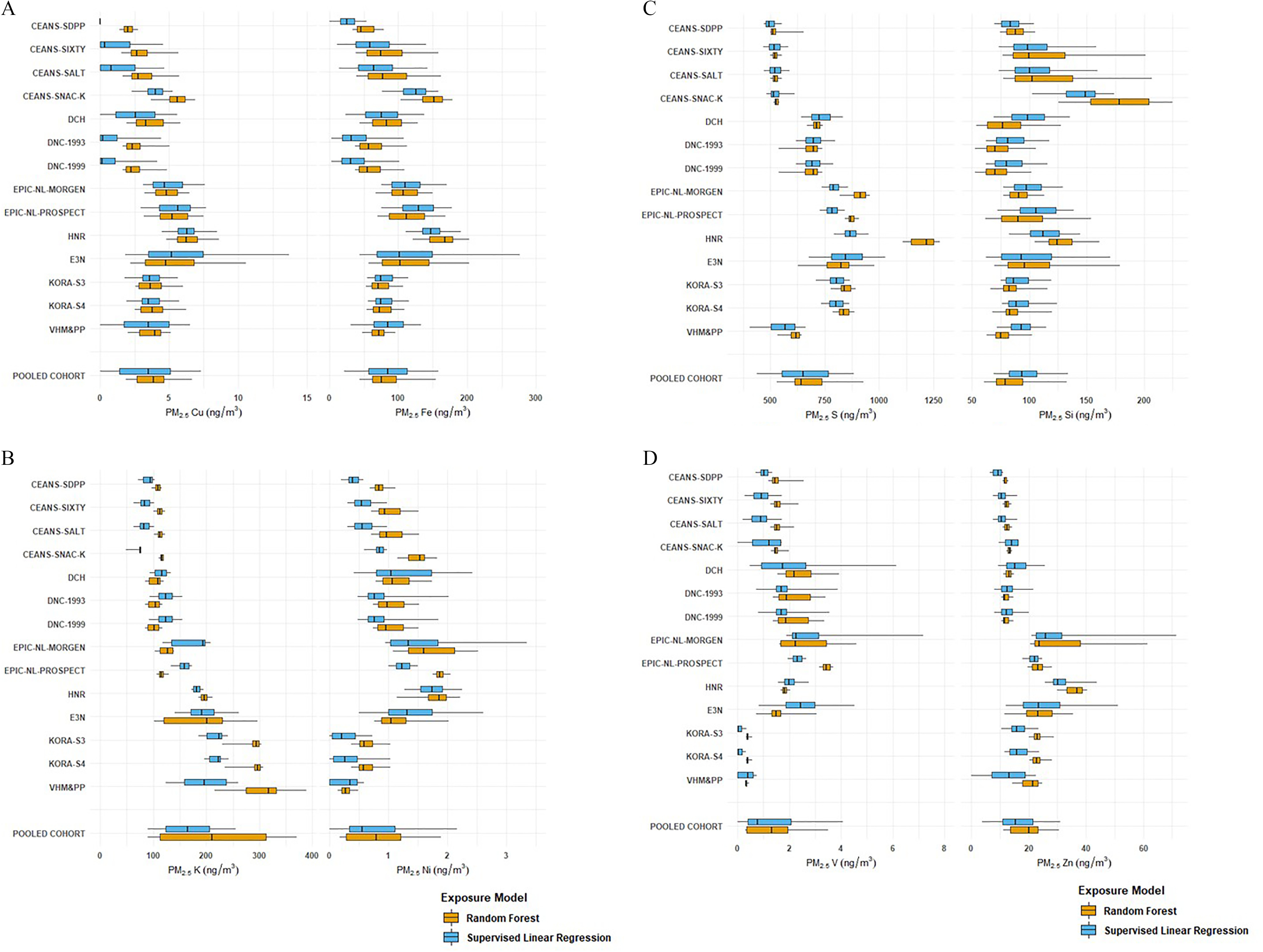
Distribution of component exposure at participant addresses estimated from supervised linear regression and random forest models. (A) copper and iron; (B) potassium and nickel; (C) sulfur and silicon; and (D) vanadium and zinc. The boundary of the box closest to zero indicates P25; the boundary of the box furthest from zero, P75; the bold vertical line inside the box, P50; and the whiskers, P5 and P95. (See Table S11 for exposure distribution of components for the pooled cohort.) Subcohorts are shown from North to South. Note: P, percentile; , fine particulate matter.
Correlations between exposure estimates derived from SLR and RF models were high for Cu and Fe (average within-cohort Spearman for Cu, for Fe) (Table 2). Correlations between SLR- and RF-modeled exposure were moderate for S, Si, and Zn and low for K, Ni, and V, with large variation between cohorts. We focused on within-cohort correlations because the epidemiological analysis exploited mostly within-cohort exposure contrast.
Table 2.
Spearman correlation coefficients between component exposure at participant addresses estimated from supervised linear regression and random forest models ().
| Subcohort | Cu | Fe | K | Ni | S | Si | V | Zn |
|---|---|---|---|---|---|---|---|---|
| Averagea | 0.81 | 0.84 | 0.22 | 0.33 | 0.59 | 0.56 | 0.27 | 0.60 |
| CEANS-SDPP | 0.27 | 0.72 | 0.16 | 0.24 | 0.48 | 0.16 | 0.27 | |
| CEANS-SIXTY | 0.86 | 0.89 | 0.44 | 0.39 | 0.76 | 0.45 | ||
| CEANS-SALT | 0.88 | 0.91 | 0.47 | 0.38 | 0.81 | 0.44 | ||
| CEANS-SNACK | 0.86 | 0.90 | 0.49 | 0.47 | 0.79 | 0.70 | 0.39 | 0.53 |
| DCH | 0.94 | 0.89 | 0.69 | 0.78 | 0.53 | 0.58 | 0.61 | |
| DNC-1993 | 0.80 | 0.79 | 0.31 | 0.45 | 0.72 | 0.43 | 0.35 | 0.63 |
| DNC-1999 | 0.77 | 0.78 | 0.35 | 0.43 | 0.70 | 0.41 | 0.34 | 0.63 |
| EPIC-NL-MORGEN | 0.92 | 0.93 | 0.82 | 0.89 | 0.20 | 0.59 | 0.7 | 0.52 |
| EPIC-NL-Prospect | 0.94 | 0.94 | 0.11 | 0.09 | 0.58 | 0.82 | 0.71 | |
| HNR | 0.81 | 0.70 | 0.53 | 0.56 | 0.72 | 0.53 | 0.79 | |
| E3N | 0.90 | 0.89 | 0.62 | 0.51 | 0.67 | 0.55 | 0.72 | 0.83 |
| KORA-S3 | 0.71 | 0.84 | 0.23 | 0.62 | 0.79 | 0.55 | ||
| KORA-S4 | 0.77 | 0.85 | 0.10 | 0.59 | 0.85 | 0.22 | 0.67 | |
| VHM&PP | 0.88 | 0.74 | 0.89 | 0.79 | 0.22 | 0.74 |
Note: CEANS, Cardiovascular Effects of Air Pollution and Noise in Stockholm; Cu, copper; DCH, Diet, Cancer and Health cohort; DNC, Danish Nurse Cohort (1993 and 1999); EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands cohort; E3N, Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale; Fe, iron; HNR, Heinz Nixdorf Recall study; K, potassium; KORA, the Cooperative Health Research in the Region of Augsburg [1994–1995 (S3) and 1999–2001 (S4)], MORGEN, Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands; Ni, nickel; , fine particulate matter; S, sulfur; SALT, Stockholm Screening Across the Lifespan Twin study; SDPP, Stockholm Diabetes Prevention Program; Si, silicon; SIXTY, Stockholm Cohort of 60-Year-Olds; SNACK, Swedish National Study on Aging and Care in Kungsholmen; V, vanadium; VHM&PP, Vorarlberg Health Monitoring and Prevention Program; Zn, zinc.
Average of cohort-specific correlation coefficients. Cohort-specific correlations are shown because the analyses mostly exploit within-cohort exposure contrasts (i.e., stratified by subcohort identification).
Correlations of composition with mass were mostly low to moderate (average of cohort-specific Spearman ranging from 0.13 to 0.49) for both SLR- and RF-modeled exposures (Table S12). Correlations with were mostly high for Cu and Fe (average of cohort-specific Spearman ) for both methods (Table S13). Correlations with mass and/or differed substantially in magnitude between cohorts, reflecting differences in study area size and the presence of major sources. Average of cohort-specific correlations between Cu and Fe were high, whereas both Cu and Fe were moderately correlated with Zn (Figure S2). Correlation between Ni and V modeled with the same algorithm was moderate, whereas the correlation was low when Ni and V were modeled with different algorithms.
Associations of Composition with Mortality
Natural mortality.
During the follow-up, we observed 46,640 (14.4%) deaths from natural causes. Figure 2 and Table S14 show associations of composition with natural mortality. In the single-pollutant models, all components were significantly associated with natural mortality except for RF-modeled Ni and Si. For Cu, Fe, K, S, V, and Zn, the HR point estimates were similar for SLR- and RF-modeled exposures, with generally wider CIs for RF. For Ni and Si, HRs were above unity for SLR-modeled and essentially unity for RF-modeled exposures.
Figure 2.
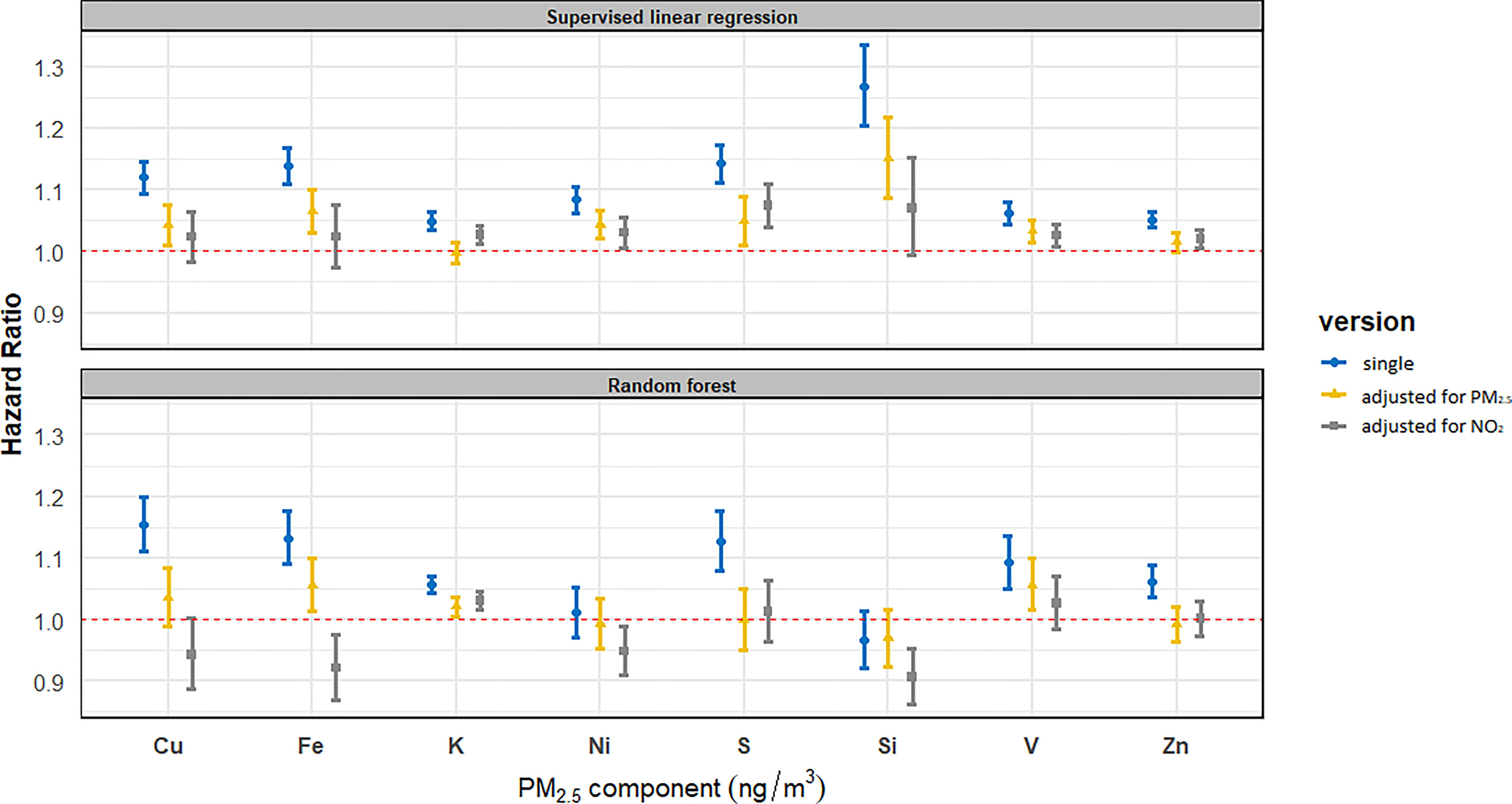
Associations of composition with natural mortality in single-pollutant and two-pollutant models in SLR and RF analyses. Total number of ; person-years at ; number of deaths from natural . HRs (95% CIs) are presented for the following increments: Cu, ; Fe, ; K, ; Ni, ; S, ; Si, ; V, ; Zn, . (See Table S14 for corresponding numeric data.) The main model was adjusted for subcohort identification, age, sex, year of enrollment, smoking (status, duration, intensity, and ), BMI categories, marital status, employment status, and 2001 neighborhood-level mean income. In two-pollutant models, mass and exposures were estimated using SLR only. Note: BMI, body mass index; CI, confidence interval; Cu, copper; Fe, iron; HR, hazard ratio; K, potassium; Ni, nickel; , fine particulate matter; RF, random forest; S, sulfur; Si, silicon; SLR, supervised linear regression; V, vanadium; Zn, zinc.
In two-pollutant models, HRs strongly attenuated for most components, whereas HRs remained stable for mass and (Figure 2 and Table S14). For Cu and Fe, HR point estimates were similar for SLR- and RF-modeled exposures after adjustment for mass, with wider CIs observed for RF-modeled exposures. HRs for Cu and Fe decreased substantially and became mostly nonsignificant after adjustment for , with HRs being above unity for SLR and below unity for RF. HRs for K were attenuated although still significantly above unity for SLR and RF after adjustment for , whereas after adjustment for mass, the HRs reduced to unity for SLR but remained above unity for RF. For Ni, S, Si, and Zn, HRs remained above unity and (almost) significant for SLR in two-pollutant models, whereas HRs reduced to essentially unity for RF. The HRs for V were reduced but remained above unity and (almost) significant in two-pollutant models, with similar estimates observed for SLR- and RF-modeled exposures.
We observed the strongest associations of natural mortality with all components in the minimally adjusted model (Model 1) (Table S15). HRs attenuated substantially after adjusting for individual-level covariates (Model 2), except for K, which remained stable. HRs increased slightly or remained stable after further adjustment for area-level covariates (Model 3). This pattern was observed both for SLR- and RF-modeled exposures. For Cu, Fe, K, S, V, and Zn, the HR point estimates were similar between SLR- and RF-modeled exposures for all three models, with generally wider CIs for RF. For Ni and Si, the effect estimates were larger for SLR- than for RF-modeled exposure in all models.
We generally observed linear or supra-linear concentration–response relationships for SLR-modeled elements and natural mortality (Figure S3). For some RF-modeled elements, there is no strong evidence of linear associations between exposure and mortality, mainly because of the limited variability in exposure concentrations.
Cause-specific mortality.
We observed 15,492 (4.8%) deaths from cardiovascular diseases during the follow-up. HRs were significantly above unity for all components in single-pollutant models except for RF-modeled Ni and Si, for which HRs were (nonsignificantly) below unity (Table S16). The magnitude of HR point estimates was similar to the HRs observed for natural mortality. In two-pollutant models, HRs for most components attenuated substantially, whereas HRs for and remained stable. HRs for and tended to be higher in models with RF-modeled than in SLR-modeled component exposure. With adjustment for , HRs for Cu and Fe remained above unity for SLR but became unity or below unity for RF. HR point estimates for SLR-modeled Ni, S, and Si were above unity in two-pollutant models adjusting for mass or , whereas HRs were unity or below unity for RF. The HRs for V attenuated but remained above unity although nonsignificant after adjustment for mass or , with similar estimates for SLR and RF.
We observed 2,846 (0.9%) deaths from nonmalignant respiratory diseases during the follow-up. HRs above unity were observed for Cu, Fe, Ni, and V, with a similar magnitude for SLR- and RF-modeled exposures in single-pollutant models (Table S17). For S, Si, and Zn, HRs were above unity for SLR-modeled exposures and were higher compared with RF-modeled exposures. HRs were close to unity for RF-modeled Si and Zn. In two-pollutant models, HRs remained stable after adjustment for mass. HRs were nonsignificantly below unity after adjustment for for components modeled with both algorithms except for Ni and V, for which HRs attenuated but were still above unity for both SLR and RF. HRs for were stable in all models. HRs for varied from below to above unity in the different models, most of them were insignificant.
We observed 3,776 (1.2%) deaths from lung cancer during the follow-up. HRs were above unity for all components in single-pollutant models although HRs for RF-modeled exposures were nonsignificant except for K, S, and V (Table S18). In two-pollutant models with adjustment for mass or , HRs stayed stable for SLR-modeled S, whereas HRs reduced substantially although remained nonsignificantly above unity for RF-modeled S. HRs for most other components reduced to unity or below unity and became nonsignificant in two-pollutant models. HRs for mass and remained stable in all models except for reduced HRs for SLR-modeled S.
Sensitivity analyses.
Table S15 shows results derived from Model 1 using Model 1 and Model 3 populations, respectively, and results derived from Model 2 using Model 2 and Model 3 populations, respectively. The effect estimates were almost identical for the same model using a different population, suggesting little selection bias was introduced by excluding subjects with missing covariates. When restricting analyses to participants with follow-up time to after year 2000 (69% of total person-years at risk, 84% of total death), after year 2005 (46% of total person-years at risk, 64% of total death), and after year 2008 (32% of total person-years at risk, 47% of total death), we observed robust associations between composition and natural mortality (Table S19). The effect estimates were not affected by additional adjustment for individual-level education, occupational status (Table S20), and additional neighborhood-level SES variables (Table S21) in cohorts that had such information.
Discussion
We observed an elevated risk of mortality associated with long-term exposure to most elemental components in single-pollutant models. In two-pollutant models with adjustment for mass or , effect estimates were attenuated for almost all component–outcome pairs. Effect estimates for SLR- and RF-modeled exposures agreed well in single-pollutant models, except for Ni and Si, for which effect estimates for RF were lower. Effect estimates for RF-modeled exposures were generally lower than for SLR in two-pollutant models.
Comparison with Previous Studies
Only a limited number of epidemiological studies have assessed associations between mortality and long-term exposure to elemental components. Among the components studied, sulfate has received the most attention. Sulfate is a secondary pollutant produced by atmospheric reactions of sulfur dioxide () emitted by combustion of S-containing liquid and solid fuels. Because sulfate is primarily in the fine particle fraction, sulfate may travel for large distances, resulting in a relatively small within study area variability. Another important source is sea salt sulfate, which is predominately in the coarse fraction but has a small fraction also in that is long-range transported (Belis et al. 2013). The California Teachers Study (Ostro et al. 2010) reported an increased HR of 1.06 (95% CI: 0.97, 1.16) for natural-cause mortality in association with a increase in sulfate concentration, translating into a HR of 1.02 per (the exposure contrast used in our analyses), assuming all S is present as sulfate (sulfate to S ratio of 3). Analyses of ACS CPS-II data suggested that long-term S exposure was associated with all-cause mortality (HR ranged from 1.01 to 1.03 per , depending on the models) (Thurston et al. 2013). In ESCAPE, robust associations of S exposure with natural mortality were found (Beelen et al. 2015). The effect estimate observed in ESCAPE was similar to the estimate in the present study [ (95% CI: 1.06, 1.23) per in ESCAPE; (95% CI: 1.11, 1.17) and (95% CI: 1.08, 1.18) per for SLR- and RF-modeled exposures, respectively, in ELAPSE]. In the present study, we obtained a much narrower CI, probably due to the longer follow-up and the pooling of cohort data. The effect estimate of S in our study was much larger than the estimates from the U.S. cohorts. One major difference is that the U.S. cohorts investigated between-area contrasts only, whereas both ELAPSE and ESCAPE focused on within-area contrasts. Because the transported sulfate has relatively uniform spatial variation at the city scale, the exposure contrast was much smaller in our study than in the U.S. studies, thus a small effect in our study could be inflated when adopting it to the same increment of exposure as in the U.S. studies. Another explanation might be that we measured elemental composition between 2008 and 2011, when emission of had decreased compared with the baseline of all cohorts (EEA 2015). The health effects in our study populations may be partly related to exposure levels and contrasts of 20 y ago (most cohorts have baselines in the 1990s). Therefore, our S-related magnitude of health effect estimates may be overestimated.
In the present study, we also found robust associations between S and lung cancer mortality, which was observed in ACS CPS-II as well (Thurston et al. 2013). The effect estimates for lung cancer mortality were larger than for natural-cause mortality, with wider CIs. In ESCAPE, robust associations were observed for S and lung cancer incidence (Raaschou-Nielsen et al. 2016). We observed elevated associations of S with cardiovascular mortality, which is consistent with previous findings in ESCAPE (Wang et al. 2014; Wolf et al. 2015) and in one of the ELAPSE subcohorts (i.e., the DCH cohort). The latter study reported an elevated risk of cardiovascular mortality associated with long-term exposure to secondary inorganic aerosols (Hvidtfeldt et al. 2019). The Women’s Health Initiative-Observational Study (WHI-OS) found no association of S with cardiovascular deaths [ (95% CI: 0.92, 1.12) per ], but a statistically significant association with cardiovascular events [ (95% CI: 1.05, 1.14) per ] (Vedal et al. 2013). In the California Teachers Study, IHD mortality was associated with long-term exposure to sulfate (Ostro et al. 2010) and high-S content fuel combustion (Ostro et al. 2015).
Both Ni and V are suggested to be tracers of mixed industrial/fuel-oil combustion and derived mainly from shipping emissions in Europe (Viana et al. 2008). Our study found a positive association of natural mortality with long-term exposure to Ni [ (95% CI: 1.06, 1.11) per ] for SLR- and no association for RF-modeled exposures [ (95% CI: 0.97, 1.05) per ]. Our study found positive associations of natural mortality with long-term exposure to V [ (95% CI: 1.04, 1.08) and (95% CI: 1.05, 1.14) per for SLR- and RF-modeled exposures, respectively]. The effect estimates are similar to the estimates in ESCAPE for natural mortality [ (95% CI: 0.97, 1.13) per ; (95% CI: 0.93, 1.23) per ] (Beelen et al. 2015), with much narrower CIs in ELAPSE. In ESCAPE, the accuracy of exposure estimates for Ni and V was limited because of the absence of specific sources of Ni and V in several study areas combined with limited measurement precision, especially in areas with low pollution levels (de Hoogh et al. 2013). The Europe-wide models made use of both within- and between-area measurement contrasts and resulted in models with good performance for Ni and V (Chen et al. 2020). Compared with ESCAPE, the ELAPSE models further added industrial source data as potential predictors, which improved the model performance. The improved exposure assessment may have allowed us to better detect the potential component–mortality associations. Our study also observed consistently positive associations between V and cause-specific mortality. Only a few studies have reported associations of mortality or morbidity with long-term exposure to Ni and V. In ESCAPE, association was found between Ni exposure and lung cancer incidence (Raaschou-Nielsen et al. 2016). In the Medicare population, stronger associations between long-term exposure and mortality were found for with higher V content (Wang et al. 2017). In the ACS CPS-II, associations between IHD mortality and Ni were reported (Thurston et al. 2013). The observed associations of Ni and V with mortality could be due to the components per se or to other components in emissions from oil combustion. Studies have suggested V in can induce oxidative stress, which is considered central to producing many of the negative health effects attributed to PM (Kelly and Fussell 2020; Zhang et al. 2009). However, there is no stronger support from experimental studies for effects of V than for Ni, Fe and Cu, for which oxidative stress is also a major pathway.
In the present study, the effect estimates for the traffic-related components Cu and Fe remained after adjustment for mass but were reduced substantially after adjustment for . The modestly wider CIs for models with mass compared with the single-pollutant models suggest these models provide interpretable results. CIs in two-pollutant models with widened somewhat more, due to the high correlations of Cu and Fe with in our study. Therefore, the substantial attenuation in effect estimates for Cu and Fe should be interpreted with caution because effects of vs. those from Cu or Fe cannot be separated well. The high correlations of Cu and Fe with (average ) in our study are consistent with correlations observed in the measured elemental components () that were used to develop the models (Tsai et al. 2015), suggesting the high correlations were not artificially introduced by the modeling methodology. Previous studies found mixed results regarding associations of mortality with Cu and Fe. Using LUR models developed in ESCAPE, the Rome longitudinal study found associations of mortality with Cu and Fe in as well as tracers of tailpipe emissions (i.e., absorbance) (Badaloni et al. 2017), but in that study (Badaloni et al. 2017), no adjustment for was made. Positive associations were observed in the California Teachers Study between Fe and IHD mortality but not with natural-cause, cardiopulmonary, or pulmonary mortality (Ostro et al. 2010). Although the study by Ostro et al. (2010) did not adjust for or mass, adjustment for organic carbon did substantially reduced HRs. Analyses of ACS CPS-II data showed that traffic-related exposure was less strongly associated with excess mortality compared with coal combustion-related exposure (Thurston et al. 2013). However, the ACS CPS-II study might have underestimated the effects of traffic-related air pollution because it investigated between-city variation, which represents only a small part of the expected overall variation in traffic-related air pollution.
Although Zn was a priori selected in ESCAPE to represent non-tailpipe traffic emissions, our Europe-wide models showed that a large fraction of the variation in the Zn measurements was explained by predictors representing industrial Zn emission (Chen et al. 2020), consistent with Zn being also a tracer for particles from industrial sources. This is consistent with source apportionment analyses in the ACS CPS-II, where Zn was considered as a source identifier for the metals industry (Thurston et al. 2016). The moderate correlations between Zn and (average and 0.54 for SLR- and RF-modeled Zn, respectively), suggest that Zn was not only related to traffic emission. The Rome longitudinal study found positive associations between Zn and mortality from natural causes, cardiovascular diseases, and IHD, using LUR models developed in ESCAPE (Badaloni et al. 2017). The ACS CPS-II also found some evidence of positive associations between Zn and mortality (Thurston et al. 2013). In the California Teachers Study, positive associations between Zn and IHD mortality were reported but not with natural-cause, cardiopulmonary, or pulmonary mortality (Ostro et al. 2010). Our study did not find clear evidence for associations of Zn with natural-cause or cause-specific mortality in two-pollutant models.
K was selected to represent biomass burning emission in ESCAPE (Tsai et al. 2015). Although our new model included a plausible background predictor for biomass combustion (satellite-modeled organic matter), the model may have a limited ability to capture within-area variability of biomass combustion emission because of the lack of reliable fine-scale predictor variables (Chen et al. 2020). Our study found elevated HRs for K exposure associated with mortality from natural-cause, cardiovascular diseases, and lung cancer. HRs decreased after adjustment for mass to close to unity for SLR-based exposure, whereas they remained (significantly) elevated for RF exposures. K was reported to be associated with coronary events in ESCAPE (Wolf et al. 2015). K in ESCAPE was, rather, related to traffic (e.g., from resuspension of road dust) than to biomass burning. The California Teachers Study found positive associations between IHD mortality and K (Ostro et al. 2010), whereas the ACS CPS-II consistently observed null association between K and mortality (Thurston et al. 2013).
Si was selected to represent crustal material, which is abundant in coarse particles (particles with diameters larger than and smaller than, or equal to, ) (). There was little evidence for an association between long-term exposure and mortality (Adar et al. 2014; Hoek et al. 2013). The 2019 Integrated Science Assessment rated the association between exposure and natural-cause mortality as suggestive (U.S. EPA 2019). Our study found positive associations in single- and two-pollutant models for Si based upon SLR models, whereas associations were null or negative for RF models. The ACS CPS-II found that Si was consistently not associated with mortality across all models (Thurston et al. 2013). A negative and marginal association was observed for CVD events with Si in WHI-OS (Vedal et al. 2013). In contrast, analyses in the California Teachers Study showed positive associations of IHD mortality with Si (Ostro et al. 2010).
Effect Estimates Using SLR- and RF-Modeled Exposures
For most components, we observed generally consistent elevated mortality risks for SLR- and RF-modeled exposures in single-pollutant models. However, less consistent associations for exposures by RF than SLR were found in two-pollutant models especially after adjusting for . We do not have a clear explanation for these differences. There is no clear pattern of differences related to the spatial distribution of the components. We found differences both for components with a strong local contribution such as Cu and components with a predominantly large-scale variation such as S. The less consistent association for RF-modeled exposure in two-pollutant models is not due to different correlation of components with mass or , which were similar for SLR- and RF-modeled exposures. The two sets of models had similar performance in explaining within-area variability in internal cross-validations (Chen et al. 2020), which is the exposure contrast primarily exploited in the present analysis. The comparison of performance of the two algorithms is limited because we did not have external validation measurements. We therefore had no prior knowledge of which models had lower biases. We observed that the predicted variability of exposure was less for RF, explaining the wider CIs in the epidemiological analyses using RF-modeled exposures. We note that RF models are more difficult to interpret in terms of how predictor variables act in the models, so a full analysis of the difference of specific predictors in the two algorithms is not possible.
Strengths and Limitations
One important strength is the highly standardized data set used in this study, which was pooled from eight European cohorts with detailed individual- and area-level covariate information, including smoking and BMI, which involved harmonizing variables between cohorts. The pooling of data allowed for more statistical power in our current analyses compared with the previous ESCAPE analyses. Another strength is the improvement in exposure assessment compared with ESCAPE. Analyses in ESCAPE may have had limited ability to detect component-specific mortality associations for Ni and V because of the lack of specific predictors in the exposure models for these components (de Hoogh et al. 2013). The Europe-wide composition models were able to make use of specific predictors representing pollution sources such as industrial sources, which explained a large proportion of the variation in measurements of specific components such as Zn (Chen et al. 2020). The Europe-wide models were developed based on a large number of measurement sites combined from individual ESCAPE study areas. A previous study has suggested that underestimation of the effect estimates was less serious when a large number of measurement sites was used for LUR modeling (Basagaña et al. 2013).
One main limitation of our study is that the exposure models were developed based on measurements made in 2008–2011, whereas most included cohorts started in the mid-1990s. In the present study, we were not able to apply back-extrapolated exposure for components because we had insufficient information on the concentration of components in Europe over time. However, our results were robust when restricting the follow-up period to more recent start dates (years 2000, 2005, and 2008), indicating the impact of temporal misalignment by applying 2010 exposures was limited. Several studies in Europe have reported that the spatial contrast of remained stable for periods up to 10 y (Cesaroni et al. 2012; Eeftens et al. 2011; Gulliver et al. 2013), suggesting that spatial contrast for traffic-related components, such as Cu and Fe, may be stable over time. For Cu and Fe, contrasts may actually be more stable given that non-tailpipe emissions have not been regulated, as opposed to tailpipe emissions. We cannot rule out the possibility that spatial contrast for components from other sources may have been less stable. For example, the magnitude of our S-related health estimates might be overestimated because of decreased emission over the years (EEA 2015), which possibly resulted in a smaller contrast in sulfate exposure. The spatial pattern of major sources has likely not changed in a major way (Belis et al. 2013; Viana et al. 2008). Another limitation of the present study is that we did not consider residential mobility during follow-up. This may have resulted in measurement error, likely nondifferential and resulting in bias toward the null (Armstrong, 1998). The decision to focus on within-cohort exposure contrasts limited our ability to assess associations with components with relatively small within-area exposure contrasts such as S. However, we considered the potential confounding related to unmeasured differences between cohorts more critical. Last, the exposure maps for RF-modeled K, Ni, and V showed strong boundary effects that might affect the exposure estimates for some participants in the E3N cohort (Chen et al. 2020). However, we expected limited impact on the health effect estimation because few people live at the borders and the correlations between SLR- and RF-modeled estimates did not stand out for these three elements, nor the E3N cohort.
Conclusions
Long-term exposures to especially V in was associated with increased mortality risk, with associations observed for both SLR- and RF-modeled exposures. For the other components, associations were generally weaker when exposure was assessed with RF compared with SLR in two-pollutant models. The consistency between SLR and RF could reflect the suitability of the models for estimating components rather than being evidence of a stronger effect on mortality.
Supplementary Material
Acknowledgments
The contributions of the authors were as follows: B.B., G.H., and J.C.: study conceptualization and design; G.H. and B.B.: principal investigators of the ELAPSE project; J.C.: statistical analysis and manuscript writing; G.H. and B.B.: supervision, manuscript review and editing; G.H., B.B., J.C., and MS: ELAPSE project coordination, preparing pooled data for analyses, and providing support with the access to pooled cohort data; S.R., E.S., and K.K.: contribution of statistical analyses strategy and scripts for the statistical analyses; K.d.H., J.C., and G.H.: exposure assessment. All authors contributed to the interpretation of the results. All authors read and revised the manuscript for the important intellectual content and approved the final draft of the manuscript.
We thank M. Tewis for the data management tasks in creating the pooled cohort database.
The research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the U.S. Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of the HEI, or its sponsors, nor do they necessarily reflect the views and policies of the U.S. EPA or motor vehicle and engine manufacturers. The HEI reviewed and approved the study design. HEI was not involved in data collection and analysis, decision to publish, or preparation of the manuscript.
The Swedish Twin Registry is managed by the Karolinska Institutet and receives funding through the Swedish Research Council under grant 2017-00641. The Cooperative Health Research in the Region of Augsburg research platform and the Monitoring Trends and Determinants on Cardiovascular Diseases Augsburg studies were initiated and financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education, Science, Research, and Technology and by the State of Bavaria. Since 2000, the myocardial infarction (MI) data collection has been co-financed by the German Federal Ministry of Health and Social Security to provide population-based MI morbidity data for the official German Health Report (https://www.gbe-bund.de). This work was also supported by a scholarship under the State Scholarship Fund by the China Scholarship Council (File No. 201606010329). None of the abovementioned funding agencies was involved in the study design, data analysis, decision to publish, or preparation of the manuscript.
References
- Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG. 2015. Particulate matter components, sources, and health: systematic approaches to testing effects. J Air Waste Manag Assoc 65(5):544–558, PMID: 25947313, 10.1080/10962247.2014.1001884. [DOI] [PubMed] [Google Scholar]
- Adar SD, Filigrana PA, Clements N, Peel JL. 2014. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr Environ Health Rep 1(3):258–274, PMID: 25152864, 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BG. 1998. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 55(10):651–656, PMID: 9930084, 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaloni C, Cesaroni G, Cerza F, Davoli M, Brunekreef B, Forastiere F, et al. 2017. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ Int 109:146–154, PMID: 28974306, 10.1016/j.envint.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Aguilera I, Rivera M, Agis D, Foraster M, Marrugat J, et al. 2013. Measurement error in epidemiologic studies of air pollution based on land-use regression models. Am J Epidemiol 178(8):1342–1346, PMID: 24105967, 10.1093/aje/kwt127. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Rivera M, Aguilera I, Agis D, Bouso L, Elosua R, et al. 2012. Effect of the number of measurement sites on land use regression models in estimating local air pollution. Atmos Environ 54:634–642, 10.1016/j.atmosenv.2012.01.064. [DOI] [Google Scholar]
- Beelen R, Hoek G, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, et al. 2015. Natural-cause mortality and long-term exposure to particle components: an analysis of 19 European cohorts within the multi-center ESCAPE project. Environ Health Perspect 123(6):525–533, PMID: 25712504, 10.1289/ehp.1408095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. 2014. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383(9919):785–795, PMID: 24332274, 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- Belis CA, Karagulian F, Larsen BR, Hopke PK. 2013. Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmos Environ 69:94–108, 10.1016/j.atmosenv.2012.11.009. [DOI] [Google Scholar]
- Beulens JWJ, Monninkhof EM, Verschuren WMM, van der Schouw YT, Smit J, Ocke MC, et al. 2010. Cohort profile: the EPIC-NL study. Int J Epidemiol 39(5):1170–1178, PMID: 19483199, 10.1093/ije/dyp217. [DOI] [PubMed] [Google Scholar]
- Brokamp C, Jandarov R, Rao MB, LeMasters G, Ryan P. 2017. Exposure assessment models for elemental components of particulate matter in an urban environment: a comparison of regression and random forest approaches. Atmos Environ (1994) 151:1–11, PMID: 28959135, 10.1016/j.atmosenv.2016.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, et al. 2014. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ 348:f7412, PMID: 24452269, 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, et al. 2012. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health 11:48, PMID: 22808928, 10.1186/1476-069X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, de Hoogh K, Gulliver J, Hoffmann B, Hertel O, Ketzel M, et al. 2019. A comparison of linear regression, regularization, and machine learning algorithms to develop Europe-wide spatial models of fine particles and nitrogen dioxide. Environ Int 130:104934, PMID: 31229871, 10.1016/j.envint.2019.104934. [DOI] [PubMed] [Google Scholar]
- Chen J, de Hoogh K, Gulliver J, Hoffmann B, Hertel O, Ketzel M, et al. 2020. Development of Europe-wide models for particle elemental composition using supervised linear regression and random forest. Environ Sci Technol 54(24):15698–15709, PMID: 33237771, 10.1021/acs.est.0c06595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel-Chapelon F, E3N Study Group. 2015. Cohort profile: the French E3N cohort study. Int J Epidemiol 44(3):801–809, PMID: 25212479, 10.1093/ije/dyu184. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389(10082):1907–1918, PMID: 28408086, 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoogh K, Chen J, Gulliver J, Hoffmann B, Hertel O, Ketzel M, et al. 2018. Spatial PM2.5, NO2, O3 and BC models for Western Europe—evaluation of spatiotemporal stability. Environ Int 120:81–92, PMID: 30075373, 10.1016/j.envint.2018.07.036. [DOI] [PubMed] [Google Scholar]
- de Hoogh K, Wang M, Adam M, Badaloni C, Beelen R, Birk M, et al. 2013. Development of land use regression models for particle composition in twenty study areas in Europe. Environ Sci Technol 47(11):5778–5786, PMID: 23651082, 10.1021/es400156t. [DOI] [PubMed] [Google Scholar]
- EEA (European Environment Agency). 2015. Sulphur dioxide (SO2) emissions. https://www.Eea.Europa.Eu/data-and-maps/indicators/eea-32-sulphur-dioxide-so2-emissions-1/assessment-3 [accessed 26 March 2010].
- Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G, et al. 2011. Stability of measured and modelled spatial contrasts in NO2 over time. Occup Environ Med 68(10):765–770, PMID: 21285243, 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG, et al. 2008. Psychological distress and risk of pre‐diabetes and type 2 diabetes in a prospective study of Swedish middle‐aged men and women. Diabet Med 25(7):834–842, PMID: 18513304, 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S, Wormser D, Gao P, Kaptoge S, et al. 2016. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046):776–786, PMID: 27423262, 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver J, de Hoogh K, Hansell A, Vienneau D. 2013. Development and back-extrapolation of NO2 land use regression models for historic exposure assessment in Great Britain. Environ Sci Technol 47(14):7804–7811, PMID: 23763440, 10.1021/es4008849. [DOI] [PubMed] [Google Scholar]
- Hoek G. 2017. Methods for assessing long-term exposures to outdoor air pollutants. Curr Environ Health Rep 4(4):450–462, PMID: 29064065, 10.1007/s40572-017-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. 2013. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health 12(1):43, PMID: 23714370, 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes NS, Morawska L. 2006. A review of dispersion modelling and its application to the dispersion of particles: an overview of different dispersion models available. Atmos Environ 40(30):5902–5928, 10.1016/j.atmosenv.2006.06.003. [DOI] [Google Scholar]
- Hundrup YA, Simonsen MK, Jørgensen T, Obel EB. 2012. Cohort profile: the Danish nurse cohort. Int J Epidemiol 41(5):1241–1247, PMID: 21421694, 10.1093/ije/dyr042. [DOI] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Chen J, Andersen ZJ, Atkinson R, Bauwelinck M, Bellander T, et al. 2021a. Long-term exposure to fine particle elemental components and lung cancer incidence in the ELAPSE pooled cohort. Environ Res 193:110568, PMID: 33278469, 10.1016/j.envres.2020.110568. [DOI] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Severi G, Andersen ZJ, Atkinson R, Bauwelinck M, Bellander T, et al. 2021b. Long-term low-level ambient air pollution exposure and risk of lung cancer—a pooled analysis of 7 European cohorts. Environ Int 146:106249, PMID: 33197787, 10.1016/j.envint.2020.106249. [DOI] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Geels C, Sørensen M, Ketzel M, Khan J, Tjønneland A, et al. 2019. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ Int 133(pt B):105268, PMID: 31675564, 10.1016/j.envint.2019.105268. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Turner MC, Beckerman BS, Pope CA III, van Donkelaar A, Martin RV, et al. 2017. Comparing the health effects of ambient particulate matter estimated using ground-based versus remote sensing exposure estimates. Environ Health Perspect 125(4):552–559, PMID: 27611476, 10.1289/EHP575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ, Fussell JC. 2020. Toxicity of airborne particles—established evidence, knowledge gaps and emerging areas of importance. Philos Trans A Math Phys Eng Sci 378(2183):20190322, PMID: 32981440, 10.1098/rsta.2019.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs J, Hoek G, Portengen L, Brunekreef B, Vermeulen RCH. 2019. Performance of prediction algorithms for modeling outdoor air pollution spatial surfaces. Environ Sci Technol 53(3):1413–1421, PMID: 30609353, 10.1021/acs.est.8b06038. [DOI] [PubMed] [Google Scholar]
- Lagergren M, Fratiglioni L, Hallberg IR, Berglund J, Elmståhl S, Hagberg B, et al. 2004. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 16(2):158–168, PMID: 15195992, 10.1007/BF03324546. [DOI] [PubMed] [Google Scholar]
- Liu S, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, et al. 2020. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J. Preprint posted online 10 December 2020, PMID: 33303534, 10.1183/13993003.030992020. [DOI] [PubMed] [Google Scholar]
- Liu S, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, et al. 2021. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ Int 146:106267, PMID: 33276316, 10.1016/j.envint.2020.106267. [DOI] [PubMed] [Google Scholar]
- Magnusson PKE, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, et al. 2013. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Res Hum Genet 16(1):317–329, PMID: 23137839, 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- McGuinn LA, Ward-Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, et al. 2017. Fine particulate matter and cardiovascular disease: comparison of assessment methods for long-term exposure. Environ Res 159:16–23, PMID: 28763730, 10.1016/j.envres.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, et al. 2015. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study cohort. Environ Health Perspect 123(6):549–556, PMID: 25633926, 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, et al. 2010. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect 118(3):363–369, PMID: 20064787, 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. 2013. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 14(9):813–822, PMID: 23849838, 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B, et al. 2016. Particulate matter air pollution components and risk for lung cancer. Environ Int 87:66–73, PMID: 26641521, 10.1016/j.envint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Samoli E, Rodopoulou S, Hvidtfeldt UA, Wolf K, Stafoggia M, Brunekreef B, et al. 2021. Modeling multi-level survival data in multi-center epidemiological cohort studies: applications from the ELAPSE project. Environ Int 147:106371, PMID: 33422970, 10.1016/j.envint.2020.106371. [DOI] [PubMed] [Google Scholar]
- Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H, et al. 2002. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf Recall Study. Am Heart J 144(2):212–218, PMID: 12177636, 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, et al. 2014. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect 122(9):919–925, PMID: 24835336, 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpiro AA, Paciorek CJ, Sheppard L. 2011. Does more accurate exposure prediction necessarily improve health effect estimates? Epidemiology 22(5):680–685, PMID: 21716114, 10.1097/EDE.0b013e3182254cc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston GD, Burnett RT, Turner MC, Shi Y, Krewski D, Lall R, et al. 2016. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air pollution. Environ Health Perspect 124(6):785–794, PMID: 26629599, 10.1289/ehp.1509777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Ito K, Lall R, Burnett RT, Turner MC, Krewski D, et al. 2013. NPACT Study 4: Mortality and long-term exposure to PM2.5 and its components in the American Cancer Society’s CPS-II Cohort. In: National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components. Lippmann M, Chen LC, Gordon T, Ito K, Thurston, eds. Boston MA: Health Effects Institute, 127–166. [PubMed] [Google Scholar]
- Tjønneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, et al. 2007. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health 35(4):432–441, PMID: 17786808, 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Hoek G, Eeftens M, de Hoogh K, Beelen R, Beregszászi T, et al. 2015. Spatial variation of PM elemental composition between and within 20 European study areas—results of the ESCAPE project. Environ Int 84:181–192, PMID: 26342569, 10.1016/j.envint.2015.04.015. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2019. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, 2019). EPA/600/R-19/188. Washington, DC: U.S. EPA. [PubMed] [Google Scholar]
- Ulmer H, Kelleher CC, Fitz-Simon N, Diem G, Concin H. 2007. Secular trends in cardiovascular risk factors: an age‐period cohort analysis of 6 98 954 health examinations in 1 81 350 Austrian men and women. J Intern Med 261(6):566–576, PMID: 17547712, 10.1111/j.1365-2796.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- Vedal S, Campen MJ, McDonald JD, Kaufman JD, Larson TV, Sampson PD, et al. 2013. National Particle Component Toxicity (NPACT) Initiative Report on Cardiovascular Effects. Research Report No. 178. Boston, MA: Health Effects Institute, 5–8. [PubMed] [Google Scholar]
- Viana M, Kuhlbusch TAJ, Querol X, Alastuey A, Harrison RM, Hopke PK, et al. 2008. Source apportionment of particulate matter in Europe: a review of methods and results. J Aerosol Sci 39(10):827–849, 10.1016/j.jaerosci.2008.05.007. [DOI] [Google Scholar]
- Wändell PE, Wajngot A, de Faire U, Hellénius ML. 2007. Increased prevalence of diabetes among immigrants from non-European countries in 60-year-old men and women in Sweden. Diabetes Metab 33(1):30–36, PMID: 17258927, 10.1016/j.diabet.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang M, Beelen R, Eeftens M, Meliefste K, Hoek G, Brunekreef B, et al. 2012. Systematic evaluation of land use regression models for NO2. Environ Sci Technol 46(8):4481–4489, PMID: 22435498, 10.1021/es204183v. [DOI] [PubMed] [Google Scholar]
- Wang M, Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Hoffmann B, et al. 2014. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects. Environ Int 66:97–106, PMID: 24561271, 10.1016/j.envint.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi L, Lee M, Liu P, Di Q, Zanobetti A, et al. 2017. Long-term exposure to PM2.5 and mortality among older adults in the Southeastern US. Epidemiology 28(2):207–214, PMID: 28005571, 10.1097/EDE.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1997. International Classification of Diseases, Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death. Geneva, Switzerland: WHO. [Google Scholar]
- WHO (World Health Organization). 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 26 March 2021].
- Wolf K, Stafoggia M, Cesaroni G, Andersen ZJ, Beelen R, Galassi C, et al. 2015. Long-term exposure to particulate matter constituents and the incidence of coronary events in 11 European cohorts. Epidemiology 26(4):565–574, PMID: 25978793, 10.1097/EDE.0000000000000300. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chau PYK, Lai HK, Wong CM. 2009. A review of effects of particulate matter-associated nickel and vanadium species on cardiovascular and respiratory systems. Int J Environ Health Res 19(3):175–185, PMID: 20183191, 10.1080/09603120802460392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


