Abstract
The proper storage and release of monoamines contributes to a wide range of neuronal activity. Here, we examine the effects of altered vesicular monoamine transport in the nematode Caenorhabditis elegans. The gene cat-1 is responsible for the encoding of the vesicular monoamine transporter (VMAT) in C. elegans and is analogous to the mammalian vesicular monoamine transporter 2 (VMAT2). Our laboratory has previously shown that reduced VMAT2 activity confers vulnerability on catecholamine neurons in mice. The purpose of this article was to determine whether this function is conserved and to determine the impact of reduced VMAT activity in C. elegans. Here we show that deletion of cat-1/VMAT increases sensitivity to the neurotoxicant 1-methyl-4-phenylpyridinium (MPP+) as measured by enhanced degeneration of dopamine neurons. Reduced cat-1/VMAT also induces changes in dopamine-mediated behaviors. High-resolution mass spectrometry-based metabolomics in the whole organism reveals changes in amino acid metabolism, including tyrosine metabolism in the cat-1/VMAT mutants. Treatment with MPP+ disrupted tryptophan metabolism. Both conditions altered glycerophospholipid metabolism, suggesting a convergent pathway of neuronal dysfunction. Our results demonstrate the evolutionarily conserved nature of monoamine function in C. elegans and further suggest that high-resolution mass spectrometry-based metabolomics can be used in this model to study environmental and genetic contributors to complex human disease.
Keywords: VMAT, vesicle, monoamine, MPP+, neurodegeneration, cat-1, metabolomics, high-resolution mass spectrometry
The synaptic vesicle plays a significant role in the protection of neurons from toxic insult. The vesicular amine transporters (VATs) are members of the toxin extruding antiporter (TEXAN) gene family of proton antiporters, closely related to proteins in prokaryotic organisms that work to exclude antibiotics from the cytoplasm (Parsons, 2000; Schuldiner et al., 1995). In eukaryotes, members of this family reside on the inner vesicular membrane and are instrumental in packaging neurotransmitters into synaptic vesicles, ensuring the transmission of the action potential. Additionally, these transporters have cytoprotective effects, keeping potentially toxic byproducts of neurotransmitters out of the cytoplasm (vesicular monoamine transporter 2, VMAT2) and ensuring the replenishment of amine stores of acetylcholine in the synaptic cleft (vesicular acetylcholine transporter, VAChT). The breakdown in this relationship can have potentially deadly results. The adequate release of acetylcholine into the synapse is dependent on the VAChT. Impairment of acetylcholine release into the synaptic cleft, either through direct inhibition of VAChT (using vesamicol) or indirectly (using botulinum toxin) can lead to respiratory failure and death (Burton et al., 1994; Dressler et al., 2005; Prior et al., 1992). For other members of the TEXAN family, the breakdown of antiporter activity in synaptic vesicles appears to result in direct toxicity to the cell from oxidized amines, like oxidized metabolites of dopamine (Spina and Cohen, 1989).
When its storage is dysregulated, dopamine can accumulate in the cytosol where it is vulnerable to oxidation and enzymatic catabolism. These processes generate reactive metabolites, such as the dopamine quinone, as well as oxidative stress-inducing reactive oxygen species (Stokes et al., 1999) and catechol aldehydes (Goldstein et al., 2013; Vermeer et al., 2012). Through the utility of mouse, in vitro vesicular uptake, and cell culture models, we have established considerable evidence that VMAT2 confers resistance to toxic insult in dopaminergic neurons as a graded response in vesicular function (Caudle et al., 2007; Fumagalli et al., 1999; Lohr et al., 2014, 2015, 2016). We have proposed that increase in VMAT2 leads to a proportional increase in the storage capacity of monoaminergic synaptic vesicles (Lohr et al., 2014). This in turn provides increased protection from both 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and N-methylamphetamine toxicity (Lohr et al., 2014, 2015, 2016). Others have demonstrated similar results including VMAT2-mediated protection against l-DOPA (the precursor to dopamine) and Parkinson’s disease (PD)-associated toxicants (Lawal et al., 2010; Mosharov et al., 2009; Munoz et al., 2012). The hypothesis that increased vesicular sequestration capacity is able to protect against endogenous and exogenous toxicants has been supported in human studies that show a reduction in PD risk associated with gain of function haplotypes in the promoter region of VMAT2 (Brighina et al., 2013; Glatt et al., 2006).
Recent findings highlight VMAT2 as a vulnerable target to environmental toxicants (Caudle, 2015; Caudle et al., 2012). Animal studies suggest that a range of chemical exposures including pesticides, fungicides, polychlorinated biphenyls, polybrominated diphenyl ethers, and perfluorinated compounds reduce the expression and function of VMAT2 and lead to symptoms closely resembling PD (Bradner et al., 2013; Caudle et al., 2005; Choi et al., 2015; Enayah et al., 2018; Inamdar et al., 2013; Miller et al., 1999; Patel et al., 2016; Pham-Lake et al., 2017; Richardson et al., 2006, 2008; Richardson and Miller, 2004; Schuh et al., 2009; Wilson et al., 2014; Xiong et al., 2016). Continued research into the effects of these chemicals on the function of amine transporters should improve our understanding of how they function to disrupt the vesicular integrity of the synaptic vesicle.
Despite the notable benefits of mouse and in vitro cell culture models, changes in behavior and alterations in whole organism metabolic function are difficult and costly to perform in mammalian models. As such, our lab has begun using C. elegans as a model to measure neurotoxic outcomes as mediated by a protein 49% identical to VMAT2, a monoamine transporter encoded by the gene cat-1 (Duerr et al., 1999). In this manuscript, we evaluate the effects of the cat-1 (ok411) mutant allele on cat-1/VMAT expression, dopamine neuron integrity, and monoamine-dependent behaviors. We also determined the effects of reduced cat-1/VMAT on vulnerability to the classical PD-associated toxicant 1-methyl-4-phenylpyridinium (MPP+). Finally, we use high-resolution mass spectrometry-based metabolomics to determine the impact of cat-1/VMAT reduction or MPP+ treatment on metabolism, measured using the whole organism.
MATERIALS AND METHODS
Reagents
Except where noted, all reagents were purchased from Sigma Aldrich (St Louis, Missouri).
Phylogenetic Relationships of Vesicular Amine Transporters
Sequences of VATs (Supplementary Data 1) from various species were subjected to cladistic organization via bioinformatics online tools located at www.phylogeny.fr (Dereeper et al., 2008).
Strains and Culture Conditions
Long established standard methods of culture including the use of normal growth media (NGM) plates, culture temperatures of 20°C, and the OP50 E. coli strain as a food source were followed as described (Brenner, 1974). Deviations in this practice are mentioned in conjunction with the relevant assay. C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). These include the wild-type N2 Bristol strain, dat-1 (ok157) mutant strain, daf-16 (CF1038) mutant strain, cat-2 (CB1112) mutant strain, RB681 [cat-1(ok411)] mutant strain, the fluorescent transgenic strain BZ555 (pDAT::GFP), and a cross between strain RB681 and BZ555 [(pDAT::GFP); cat-1(ok411)] created in our lab.
All nematodes (hereafter referred to as worms) used in experiments were synchronized at the L1 stage using a bleach/sodium hydroxide mixture optimized following standard techniques (Porta-de-la-Riva et al., 2012). Worms reared in liquid culture were maintained on a shaker set to 120 rpm, at 20°C. Liquid cultures were maintained in S-complete media and fed using the UV-sensitive strain NEC937, following UV exposure per N. Stroustrup (CRG, Barcelona, personal communication).
Genotyping
Strains sourced from the CGC and those resulting from an in-house cross were genotyped using standard polymerase chain reaction (PCR) techniques. Primers used to check the cat-1 (ok411) mutant allele are found in the description listing for strain RB681 on the website for the CGC (Caenorhabditis Genetics Center, 2020).
Western Blot
To visualize the cat-1/VMAT protein, C. elegans strains N2, daf-16 (CF1038), cat-1 (ok411), cat-2 (CB1112), and dat-1 (ok157) were cultured on NGM plates. A total of 50 worms of each strain were transferred to a 1.5-ml Eppendorf tube containing 150 µl of M9. After washing 3 times with M9, the worm pellet was suspended in 25 µl of M9, snap frozen in liquid nitrogen, and stored at −80°C until use. Prior to analysis, samples were thawed on ice, after which 25 µl of Laemmli 2× sample buffer and proteinase inhibitor cocktail were added and the sample mixtures were placed in a sonicating water bath at room temperature for 2 min and immediately placed in heat block at 95°C for 5 min. Samples were run on a NuPage 10% bis tris gel (Thermo Fisher, Waltham, Massachusetts) and transferred to a PVDF membrane. Nonspecific antibody binding was blocked with a 7.5% milk in tris-buffered saline plus tween solution for 1.5 h at room temperature. The primary antibody used was a polyclonal rabbit anti cat-1/VMAT serum generated by Dr Richard Nass (IUSM, Indiana) at a dilution of 1:1000. The Secondary antibody used was a goat anti-rabbit HRP-conjugated at a dilution of 1:5000) (Jackson ImmunoResearch, West Grove, Pennsylvania).
Immunohistochemistry
The method was based on a previously published Freeze-Cracking protocol unless otherwise specified (Duerr, 2013). Poly-L-lysine coated slides were prepared to bind worms to slides. Approximately 5000 worms per strain were used to prepare multiple slides. A Fisherbrand Cooling Cartridge (Thermo Fisher; Waltham, Massachusetts) prechilled overnight in −80°C was used to freeze the slides. The slides were lightly fixed using the methanol-acetone method. Nonspecific antibody binding was blocked with a 10% Goat Serum (Thermo Fisher, Waltham, Massachusetts) overnight at 4°C. The primary antibody used was a polyclonal rabbit anti cat-1/VMAT serum generated by Dr Richard Nass (IUSM, Indiana) at a dilution of 1:100. The secondary antibody used was Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Thermo Fisher, Waltham, Massachusetts). VECTASHIELD Antifade mounting medium with DAPI (Vector Laboratories, Burlingame, California) was added prior to sealing the coverslips with nail polish. Images were taken at 40× magnification on a Leica DMi8 (Leica, Wetzlar, Germany) inverted epifluorescent microscope.
Analysis of Monoamine-Derived Behaviors
Reserpine is a pharmacological inhibitor of mammalian catecholamine transporters (Deupree and Weaver, 1984). In vitro studies looking at [3H] dopamine uptake in cell culture have shown reserpine to be a potent inhibitor of C. elegans cat-1/VMAT as well (Duerr et al., 1999). In this paper, reserpine is used to pharmacologically replicate the loss of cat-1/VMAT seen in the cat-1(ok411) mutant strain. To create reserpine assay plates, reserpine was dissolved in 1 M acetic acid to a concentration of 50 mM and diluted 1:80 in M9 for a final concentration of 625 µM. Plates without reserpine were exposed to vehicle only (1 M acetic acid diluted 1:80 in M9).A total of 400 µl was added to the treatment plate and allowed to dry before transferring worms (Duerr et al., 1999). Worms were seeded onto growth plates (6 cm NGM with live OP50) as synchronized L1s and allowed to grow for 48 h. Worms were transferred to reserpine plates and left for 12 h prior to behavioral assays except for egg laying where worms were kept on reserpine plates until the second day of adulthood.
Grazing
Plates for assessing grazing behaviors were created per Duerr et al. (1999). For our assays, worms were rinsed off treatment plates and spotted in 20 µl of M9 with approximately 100 worms per assay. A lint-free tissue was used to wick away the M9 and the worms were recorded using a FLIR chameleon 3 camera from Edmund Optics (Barrington, New Jersey) until either the last worm entered the lawn or a maximum of 30 min. Videos were scored by a researcher blind to treatment conditions, measuring the amount of time it took for each worm to enter the lawn of OP50 (tip of nose to end of tail).
Pharyngeal pumping
Pharyngeal contractions were scored by observation of worms through a standard stereomicroscope, using finger taps on an electronic counting application while a timer was running, a method similar to that previously described (Miller et al., 1996).
Wave initiation rate
The celeST software package was used as described to determine aspects of swim behavior for the N2, cat-1 (ok411) and N2 worms treated with reserpine groups (Restif et al., 2014). Briefly, four worms were placed in 60 μl of M9 on a glass slide. Recordings of swim behavior were made as a series of jpeg images using a chameleon 3 camera (FLIR; Wilsonville, Oregon) for 30 s at a frame rate of 18 f/s. All subsequent analysis was completely automated using the celeST software package.
Egg laying
For the egg laying assay, retention of eggs inside worms was determined by transferring 2 day adult worms, 1 per well, to 96 well plates containing 20% hypochlorite solution. Dissolution of the worm body and release of eggs was monitored by a researcher blinded to experimental condition and eggs were counted manually.
MPP+ Treatment
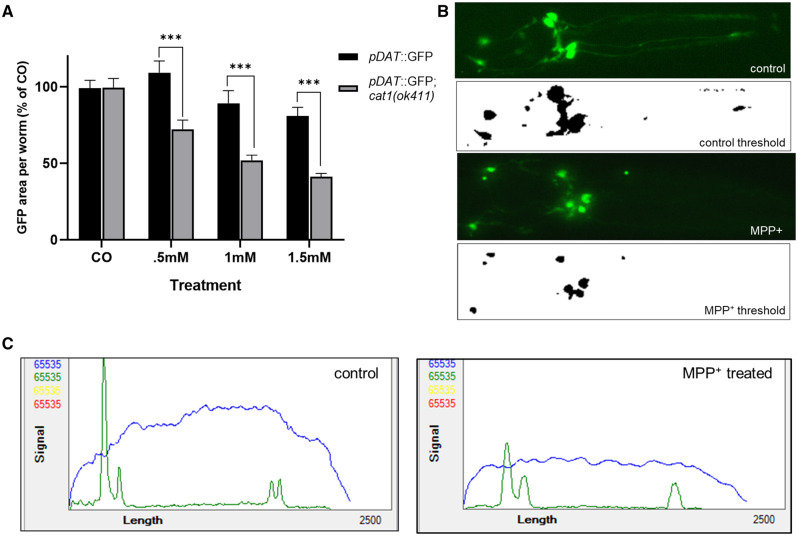
C. elegans strains, pDAT::GFP and pDAT::GFP; cat-1(ok411), were chosen for fluorescent assays due to their intense expression of GFP driven by the dat-1 promoter, highlighting dopamine neurons. Synchronized worms were grown using standard liquid culture (see above) using 125 ml Erlenmeyer flasks until they reached the YA stage of development. At this point, worms were sorted using confirmed gating parameters into 96 well plates using the COPAS FP-250 large particle flow cytometer (Union Biometrica, Massachusetts) to select for fully developed YA worms. Various concentrations of MPP+ dissolved in water were added to the liquid culture (s-complete, UV-treated NEC937, 100 μM Floxuridine), and the worms were left at 20°C on a shaker for an additional 48 h. Worms were then anesthetized in 10 mM sodium azide, mounted on agar (5% in water), and the area surrounding the 6 dopamine neurons in the head were photographed at 20× magnification using a Fisher Scientific EVOS epifluorescence microscope (Waltham, Massachusetts) for later analysis. All photographs were taken using identical objective, light intensity, and exposure settings. We used the Image J software to determine neurodegeneration based on the total area of fluorescence post 48 h of treatment, with the observation that worms treated with MPP+ exhibit overall reductions of dopamine neuron size and branching (Schindelin et al., 2012). First, fluorescent photographs were converted into 8-bit grayscale tiff files. Following conversion, the photographs were batch processed using the Yen-automated thresholding algorithm to isolate areas of GFP fluorescence (Yen et al., 1995) creating a binary image of black objects against a white background (Figure 4B). The total area of these objects, representing areas of GFP fluorescence, was calculated using the particle analysis plugin in Image J. Results were combined over 4 experiments as percent area of untreated worms. Statistical testing using two-way ANOVA followed by Bonferroni’s multiple comparisons tests were designed to compare the extent of degeneration between pDAT::GFP worms and pDAT::GFP worms lacking the cat-1/VMAT protein (pDAT::GFP; cat-1 [ok411]) when treated with equivalent doses of MPP+. A small series of treatments were run through the COPAS biosorter to get representative fluorescent profiles of worms treated with MPP+.
Figure 4.
Dopamine neurons lacking the cat-1/VMAT transporter are more susceptible to MPP+ induced neurodegeneration. A, Image analysis using automated thresholding shows increased sensitivity of the cat-1/VMAT mutant ok411 to MPP+ via reduced fluorescent area of dopamine neurons. B, Representative fluorescent images of an untreated (control) and MPP+ treated worm, followed by their transformation to a binary image using the Yen thresholding method (control threshold and MPP+ threshold) for total area analysis using particle analyzer. C, Representative profile graph using the COPAS large particle flow cytometer, green peaks represent intensity values of dat-1::GFP, blue signal is extinction, representing the body of the worm. GFP fluorescence driven by the dat-1 promoter. Sample size for 2-way ANOVA is n = 587, comprised of 60–100 worms per group over a series of 4 separate experiments *** p <. 0001 using Bonferroni’s multiple comparisons test following a 2-way ANOVA revealing a significant (p < .001) interaction between strain of worm and treatment.
High-Resolution Mass Spectrometry-Based Metabolomics
We applied untargeted high-resolution mass spectrometry (HRMS)-based metabolomics to characterize metabolic effects of the cat-1 mutation and MPP+ exposure in N2 worms. Worms with the cat-1 (ok411) mutation and wild-type N2 worms were grown in liquid culture as described above and 6 replicates per strain were collected in M9 at the L4 stage and snap frozen in liquid nitrogen. Prior to MPP+ exposure, N2 worms were grown in liquid culture and at the L4 stage, 500 worms were sorted into wells of a 24-well plate using the COPAS FP-250. Worms were exposed to 1 mM MPP+ or the control for 4 h, washed and snap frozen. All samples were stored at −80°C until processed. Metabolites were extracted using acetonitrile (in a 2:1 ratio) which was added to samples along with an internal standard (Soltow et al., 2013). Each sample was placed in the bead beater to disrupt the cuticle, and included shaking at 6.5 m/s for 30 s, cooling on ice for 1 min, and placed in the beater for another 30 s, at the same speed (Mor et al., 2020). All processing was performed on ice or in a cold room when necessary. Untargeted high-resolution mass spectrometry analysis was performed using a dual-chromatography and acetonitrile gradient that included HILIC chromatography with positive electrospray ionization (HIILIC-pos) and C18 column with negative electrospray ionization (C18-neg) (Walker et al., 2019). Mass spectral data was generated on a ThermoFischer Q-Exactive HF Orbitrap mass spectrometer operated at 120 000 resolution over mass-to-charge (m/z) scan range 85–1250. Data were extracted using the R packages apLCMS (Yu et al., 2009) and xMSanalyzer (Uppal et al., 2013).
The HILIC positive column measured 21 479 uniquely detected mass spectral features, identified by accurate m/z, retention time and intensity in each sample. To focus on detection of endogenous metabolites and pathways related to neurotransmitter metabolism, data analysis was restricted to the HILIC-pos condition only.
cat-1/VMAT and MPP+ Metabolome-Wide Association Study
We analyzed HRMS results using 3 separate approaches to evaluate metabolic alterations due to the cat-1 mutation and MPP+ exposure: (1) The metabolic effect of the cat-1 mutation was characterized by comparison to wild-type N2 worms grown under identical conditions. (2) Disruption to worm metabolic function following MPP+ exposure for exposed N2 worms and untreated N2 controls and (3) An overlap analysis was completed by identifying features associated with both cat-1 and MPP+ for similarities in disruption of systemic metabolic response due to altered dopaminergic neuronal health. A feature was retained in each of the first 2 analyses if its abundance in at least 1 worm sample was 1.5 times its abundance in the M9 buffer used to wash and collect the worms. Prior to data analysis, missing values for each feature were imputed with half the value of the minimum abundance, quantile normalized, log10 transformed, and auto scaled. Features were analyzed using t tests, partial least squares discriminant analysis (PLS-DA), hierarchical clustering, and pathway analysis using mummichog (Li et al., 2013). All data processing, analysis and visualization was done in R version 3.6.0, using functions: preprocessCore::normalize.quantiles() (Bolstad et al., 2003), (RFmarkerDetector::autoscale() (Palla, 2015), gplots::heatmap2() (Warnes et al., 2009), ggplot2::ggplot() (Wickham, 2016), and mixOmics::plsda() (Le Cao, 2016). Pathway analysis was completed using the Mummichog algorithm (Li et al., 2013) hosted on the MetaboAnalayst (www.metaboanalyst.ca) module “MS peaks to Pathway” (Chong et al., 2019), using the Caenorhabditis elegans metabolic reference map available through KEGG. Using output from the t tests, a nominal p-value cut-off of 0.1 was used to select features for pathway analysis, using a mass tolerance of 5 ppm for cat-1 (ok411) mutant and for wild-type worms treated with MPP+. Features that were different between wild-type and cat-1(ok411) worms at p < .1 (356 features) were compared with features that were different between MPP+ treated and control worms at p < .1 (801 features). To discover overlapping features, we used the getVenn() function in the xMSanalyzer R package with a mass-to-charge tolerance of 5 ppm and retention time tolerance of 5 s. We used the xMSanalyzer::feat.batch.annotation.KEGG() function to determine level 4 annotations (Schymanski rating; Schymanski et al., 2014) for the overlapping features (Uppal et al., 2013).
Statistics
All data excluding metabolomics were analyzed using the Graphpad statistical software package (San Diego, California). Behavioral comparisons in Figure 3 were conducted using 1-way ANOVA followed by Tukey’s multiple comparisons tests in cases where p < .05. Results from MPP+ assays in Figure 4 were conducted using 2-way ANOVA followed by Bonferroni’s multiple comparisons test. All metabolomic analyses were conducted in R (version 4.0.2).
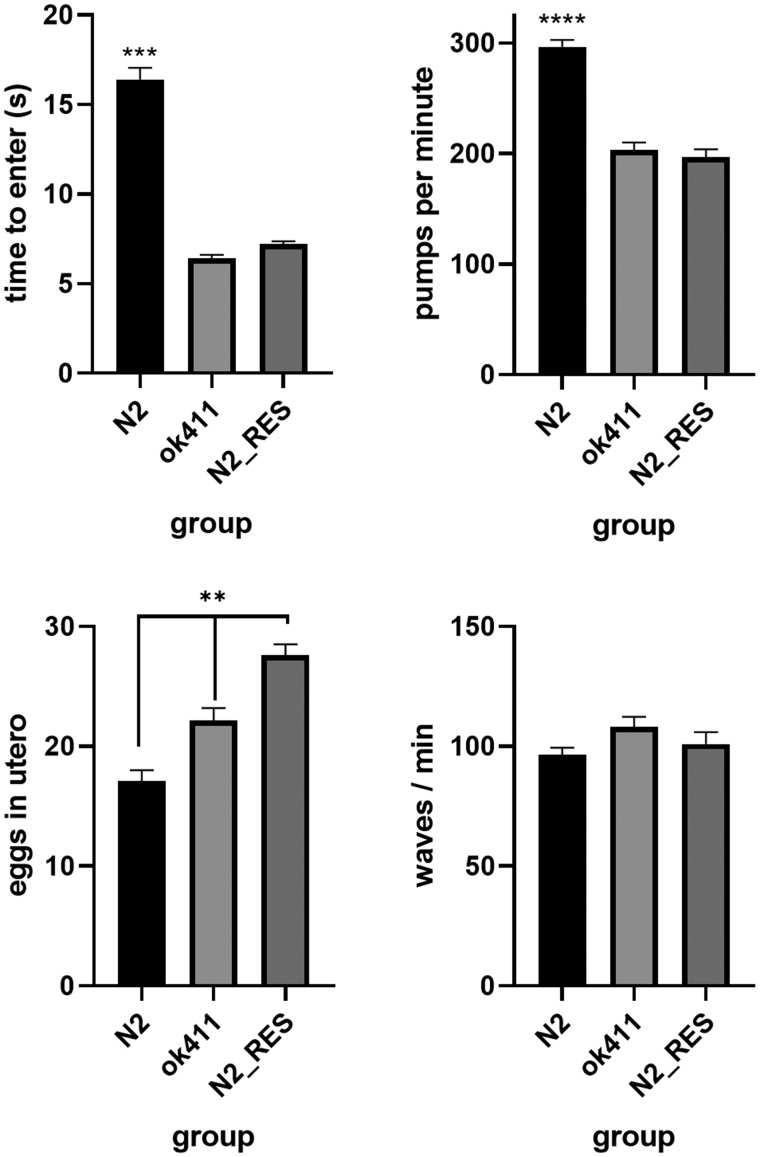
Figure 3.
The loss of cat-1/VMAT protein results in altered monoaminergic behaviors. A, The grazing response is deficient in the cat-1 (ok411) mutant strain and N2 worms treated with reserpine (n = 1289). B, cat-1 (ok411) worms and N2 worms treated with reserpine have impaired pharyngeal pumping compared with N2 (n = 89). C, cat-1 (ok411) and N2 worms treated with reserpine are deficient in egg laying (more eggs in utero) (n = 114). D, There is no difference in wave initiation between N2 and the cat-1 (ok411) mutant (n = 55). N2_res = N2 worms treated with reserpine. All behaviors analyzed using 1-way ANOVA followed by Tukey’s multiple comparisons tests where appropriate. ** p < .01, *** p < .001 vs. all other groups, **** p < .0001 vs. all other groups.
RESULTS
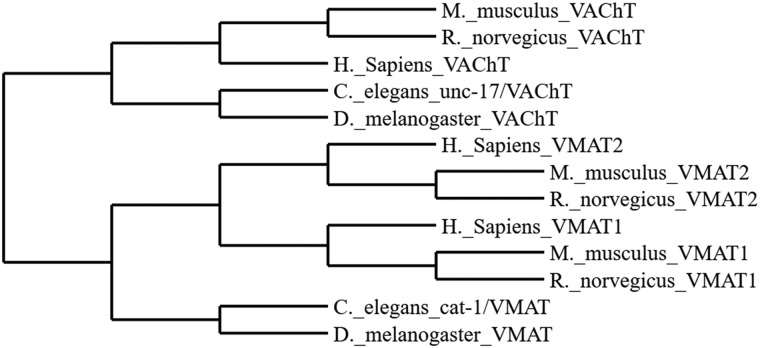
Phylogenetic Relationships of Vesicular Amine Transporters
Cladistic relationships amongst vertebrate and invertebrate species between the VMAT(s) and the VAChT are shown in Figure 1.
Figure 1.
Cladogram representing species specific relationships amongst vesicular amine transporters.
Genotyping and Protein Analysis of cat-1 (ok411)
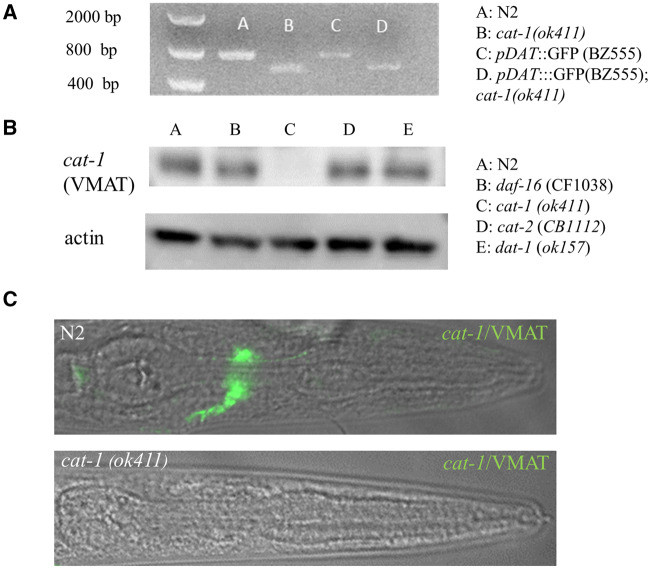
The ok411 mutant allele was produced by the high throughput C. elegans gene knockout consortium (CeDM, 2012). The mutation is a 429 base pair deletion resulting in complete loss of the first coding exon in the cat-1 gene and a loss of the cat-1/VMAT protein by Western blot and immunofluoresence (Figs. 2A–C). A breeding cross of the BZ555 pDAT::GFP with the cat-1(ok411) mutant was successful in producing a transgenic with the mutant cat-1(ok411) allele (Figure 2A). Our efforts at immunostaining support the work of previous labs indicating the expression pattern of cat-1/VMAT in the worm (Duerr et al., 1999; Serrano-Saiz et al., 2017).
Figure 2.
The cat-1 (ok411) strain is deficient in the production of cat-1/VMAT protein. A, Primers specific for cat-1 reveal a 400 bp deletion in the cat-1 (ok411) mutant strain. B, The strain cat-1 (ok411) does not produce cat-1/VMAT protein. C, The cat-1(ok411) strain (B) shows no evidence of cat-1/VMAT immunoreactivity (detail of pharyngeal region).
Monoamine-Derived Behaviors
The cat-1(ok411) mutant strain lacking cat-1/VMAT protein and pharmacological inhibition of cat-1/VMAT in N2 worms treated with reserpine demonstrated defective grazing behavior (Figure 3A) indicative of disruption of dopamine and serotonin signaling. In order to determine if this behavior is due to a general difference in motility, we subjected the cat-1(ok411) strain and the reserpine treated N2 worms to automated swim behavior analysis using the celeST software and found no significant difference between any of the groups using 10 different measures of stroke analysis including a measure (wave initiation rate measured as: waves/minute) analogous to the thrashing assay (Figure 3D). We employed two measures of serotonergic behaviors; egg laying and pharyngeal pumping. Worms with disrupted serotonergic signaling tend to hold their eggs in utero and have a reduced rate of pharyngeal pumping. We found the cat-1(ok411) strain and N2 wild type worms treated with reserpine to be both deficient in egg laying behavior and pharyngeal pumping (Figs. 3B and 3C).
MPP+ Exposure and Neurodegeneration
Worms treated with MPP+ exhibit reductions in total area consistent with the degeneration of dopamine neurons (Figure 4B). Representative fluorescent profiles using the COPAS biosorter support this assertion, MPP+ treated worms had shorter intensity peaks for the green channel (Figure 4C). We found that BZ555 transgenic worms lacking cat-1/VMAT protein are more susceptible to the toxic effects of MPP+ than the BZ555 transgenic worms with cat-1/VMAT protein through analysis with 2-way ANOVA, with a significant interaction between strain and dose (p < .001) (Figure 4A). Reductions in total GFP area occur at lower concentrations of MPP+ in worms with the cat-1(ok411) mutant allele.
Metabolomics
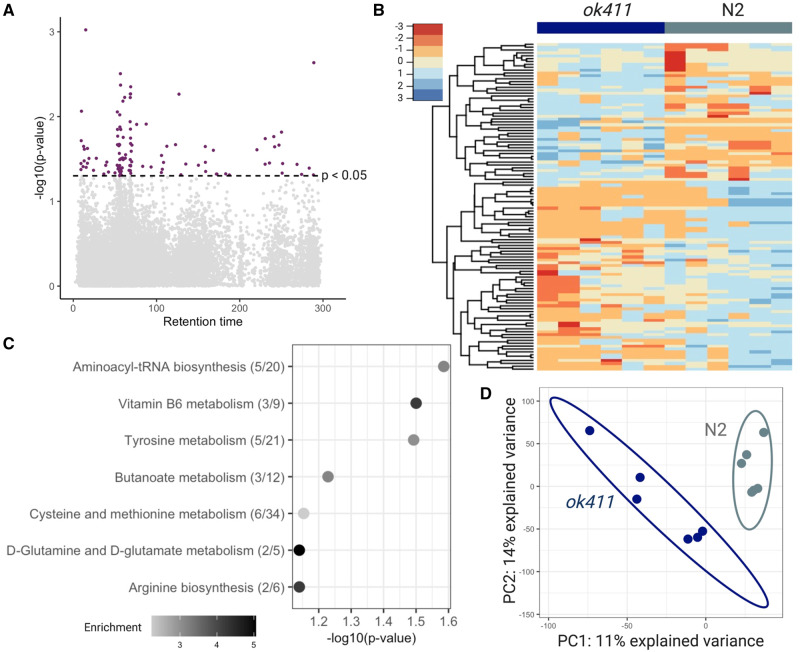
There were 114 features (in red) different between the 2 groups with p < .05 (Figure 5A). There was also clear clustering of the features with p < .05 between the 2 groups (Figure 5B). A metabolome wide association study of phenylalanine metabolism has shown that for initial discovery purposes, raw p combined with metabolic pathway enrichment improves detection of biological effect while reducing identification of false positive biological effects (Go et al., 2015). Thus, for metabolic pathway enrichment, we considered all 356 features with p < 0.1. It revealed several metabolic pathways altered with a fisher exact test p-value < 0.1 in the cat-1(ok411) mutant (Figure 5C), including tyrosine metabolism (Figure 5C). A partial least squares differential analysis showed separation of the wild-type N2 and cat-1(ok411) groups (Figure 5D).
Figure 5.
The cat-1 (ok411) strain shows patterns of altered metabolism. A, Manhattan plot shows features that were different with p < .05 (red) in cat-1 (ok411) mutants compared with wild-type N2 worms. B, Hierarchical clustering of features associated with the cat-1 (ok411) mutant with p < .05. C, Top pathways altered (Fisher’s exact test p-value < 0.1) in cat-1 (ok411) worms, analyzed using the Mummichog software. The overlap size of the pathway is indicated in parentheses (number of significant hits/pathway size). The color of the bubbles represent enrichment, calculated as the quotient of total number of hits in the pathway divided by expected number of hits. D, Partial least squares discriminant analysis (PLS-DA) comparing cat-1 (ok411) mutants to wild-type N2 worms. n = 6 with 500 worms in each sample for both groups.
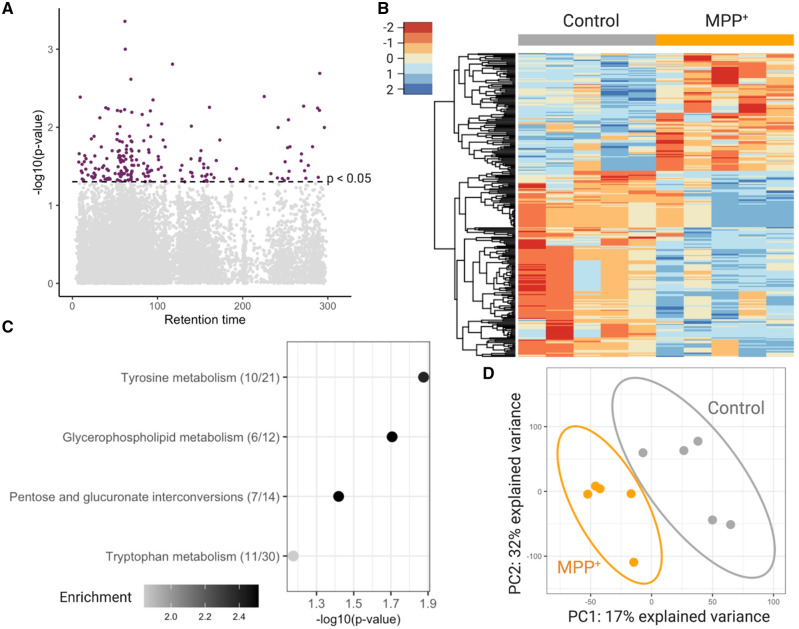
Untargeted metabolomic analysis of worms exposed to MPP+ for 4 h was performed similarly to the cat-1(ok411) mutant. There were 199 features (in red) significantly different between the treated and untreated groups with p < .05 (Figure 6A). These significant features clustered differently in the 2 groups as seen in the heatmap generated using hierarchical clustering analysis (Figure 6B). Pathway analysis using features with p < .1 (801 features) was done using mummichog hosted on MetaboAnalyst. It revealed several pathways of interest (Figure 6C), including the tyrosine and tryptophan metabolism pathway, glycerophospholipid metabolism and the pentose glucuronate interconversions pathways (Figure 6C). A partial PLS-DA was able to differentiate between the treatment groups (Figure 6D). Comparing features with p < .1 in cat-1(ok411) worms and worms exposed to MPP+ revealed 14 features altered in both conditions (Supplementary Figure 1). KEGG annotations were made for 2 of the 14 features (Supplementary Table 1) with level 4 confidence according to Schymanski criteria (Schymanski et al., 2014). See supplemental excel workbook for raw data at https://doi.org/10.5061/dryad.0zpc866x0.
Figure 6.
Worms treated with MPP+ show patterns of altered metabolism. A, Manhattan plot shows features that were different with p < .05 (red) N2 worms treated with MPP+ compared with untreated N2 worms. B, Hierarchical clustering of features associated with treatment with p < .05. C, Top pathways altered (Fisher’s exact test p-value < 0.1) in N2 worms treated with 1 mM MPP+, analyzed using the Mummichog software. The overlap size of the pathway is indicated in parentheses (number of significant hits/pathway size). The color of the bubbles represent enrichment, calculated as the quotient of total number of hits in the pathway divided by expected number of hits. D, Partial least squares discriminant analysis (PLS-DA) comparing N2 worms with N2 worms treated with 1 mM MPP+. n = 5 with 500 worms in each sample for both groups.
DISCUSSION
The importance of VATs in mitigating catecholaminergic toxicity is well established (Guillot and Miller, 2009; Lohr and Miller, 2014; Spina and Cohen, 1989). Multiple in vivo studies have demonstrated the toxic consequences of dopamine including early work that demonstrated toxicity resulting from striatal injections of exogenous dopamine (Hastings et al., 1996). Later studies investigated the consequences of dysregulating endogenous dopamine handling. Overexpression of the plasmalemmal dopamine transporter (DAT) resulted in increased dopamine metabolism and nigrostriatal degeneration thought to result from an accumulation in cytosolic dopamine with insufficient vesicular sequestration capabilities (Masoud et al., 2015). Furthermore, introducing DAT into striatal interneurons that lack the proper machinery to sequester dopamine within vesicles resulted in neurodegeneration, demonstrating the necessity of proper dopaminergic handling for neuronal health (Chen et al., 2008). Mice deficient in VMAT2 display indices of cytosolic dopamine metabolism as well as age-dependent nigrostriatal dopaminergic degeneration (Caudle et al., 2007; Taylor et al., 2009; 2014). This work has been replicated in Drosophila melanogaster deficient in VMAT expression that display fewer dopaminergic neurons (Lawal et al., 2010), and in adult rats that show dopaminergic degeneration resulting from an acquired loss of VMAT2 in adulthood (Bucher et al., 2020). The purpose of this article is to establish the cat-1 knockout specifically and the C. elegans model in general as a viable model for the role of VMAT as a mediator of synaptic function and neuronal vulnerability.
Single worm PCR and the utility of the hermaphroditic reproduction strategy of these worms allows us to readily identify mutants and perform relevant crosses. Simple breeding schemes allow us to create enough reporter strains to examine the effects of vesicular transporter disruption for the entire C. elegans neural network. Western blot and immunofluorescent techniques, though rarely used in C. elegans research, provide a valuable way of confirming the presence or absence of a protein and subsequent distribution in relevant tissues that are consistent with methods used in mammalian models.
Despite their many advantages, studies of C. elegans focused on the cat-1/VMAT protein are limited (Duerr et al., 1999; Young et al., 2018). The importance of cat-1/VMAT in the establishment of food detection, feeding rate, and reproduction have been demonstrated in the past and replicated by the genetic and pharmacological interventions used in this study (Duerr et al., 1999; Young et al., 2018). Previous cell ablation studies demonstrate that the slowing down of a worm when on a food source is dependent on the presence of intact dopamine neuronal architecture (Sawin et al., 2000). It is interesting that the loss of functional cat-1/VMAT replicates this phenotype with the probable effect of system wide catecholaminergic dysfunction (Figure 3A and Duerr et al., 1999; Young et al., 2018). Indeed, one other group has found the addition of either exogenous serotonin or pramipexole can rescue this behavior in cat-1/VMAT mutant strains (Young et al., 2018). Although beyond the scope of this articlee, further exploration of this behavior may reveal synergistic or antagonistic patterns of catecholamine function. Optimal rates of pharyngeal pumping and egg laying require the input of serotonin (Avery and Horvitz, 1990; Trent et al., 1983). The deficits observed in pharyngeal pumping and egg laying rate seen in the cat-1(ok411) mutant and associated pharmacological treatment with the inhibitor reserpine suggest that proper storage and trafficking of endogenous stores of this catecholamine is important for its function.
The synaptic vesicle containing functional VMAT2 provides protection to the cell from both endogenous (oxidized dopamine) and certain exogenous (MPTP) insults (Guillot and Miller, 2009; Spina and Cohen, 1989). The MPTP model of selective toxicity to dopamine neurons is well established and a hallmark of mouse work in the PD field (Meredith and Rademacher, 2011). The C. elegans model has been used many times to demonstrate the selective toxicity of MPP+ (the active metabolite of MPTP) on dopaminergic architecture, however, to our knowledge no laboratory has tested the effects of MPP+ in worms lacking the cat-1/VMAT protein (Braungart et al., 2004; Lu et al., 2010; Pu and Le, 2008; Wang et al., 2007; Yao et al., 2011). These findings suggest what we have previously found in mice, namely that levels of VMAT regulate MPTP vulnerability (Lohr et al., 2016). Given the genetic tractability of C. elegans, the creation of cat-1/VMAT overexpressing worms in future studies is certainly feasible and may facilitate future studies aimed at using enhanced monoamine storage as a therapeutic intervention. It is notable, but not surprising given the evolutionary development of the TEXAN antiporters that the protective effects of monoamine transporters extends across both vertebrate and invertebrate animal models (Figure 1; Schuldiner et al., 1995). This provides further confidence in the utilization of C. elegans as a model for vesicular dysfunction related to monoamine transporter efficacy.
C. elegans is gaining support as a model for toxicity testing, demonstrating conserved LD50 values and similarities in toxic mechanisms with rodent models (Hunt, 2017). Previous research demonstrates a clear connection between a number of environmental pollutants and the expression of VMAT2 in the nigrostriatal system of mouse models. However, mechanistic studies and full-scale screens of Toxcast libraries are lacking due to the absence of an inexpensive, viable model of monoaminergic vesicular dysfunction. The introduction of the COPAS large particle cytometer may help provide the resolution needed to bring high throughput capability to the screening of potential neurotoxic compounds. Indeed, we are currently refining assays in our lab (Figure 4C). The characterization of the C. elegans model of impaired vesicular storage seen in this article is a promising development.
Metabolomics analysis of cat-1(ok411) worms showed altered pathways in tyrosine metabolism, possibly resulting from the vesicular mishandling of dopamine in vesicles lacking cat-1/VMAT. Members of the pathway suggested lower dopamine levels and higher levels of dopamine metabolites (Supplementary Figure 2). Pathway analysis also suggested changes in glutamate metabolism in worms lacking cat-1/VMAT, similar to a previous study that showed altered levels of glutamate detected through NMR metabolomics in the substantia nigra of aged mice expressing low levels of VMAT2 (Salek et al., 2008). MPP+ enters the neuron through the cell membrane via dopamine transporter 1 (DAT1), thus selectively affecting dopaminergic neurons (Gainetdinov et al. 2002). As such, it was telling to see differences in the metabolism of tyrosine—the precursor of dopamine—in wild-type worms treated with MPP+. The finding of differences in tryptophan metabolism is more curious considering that MPP+ has low affinity for the plasma membrane transporter in serotonin neurons. However, cellular crisis in dopaminergic cells could lead to alterations in tryptophan metabolism reflecting the interdependence of both amines. In fact, these changes in tryptophan metabolism might represent a more generalized response to the increased proteotoxicity involved in cellular degeneration (van der Goot et al. 2012). Likewise, changes in the pentose glucuronate interconversion pathway may also be indicative of a mitochondrial crisis (Mullarky and Cantley 2015). Features that are altered in both types of dopaminergic neuronal perturbations could provide information on metabolic pathways that are of interest to neuronal damage and degeneration. Although we were able to discover 14 features overlapping between the two conditions, we did not have sufficient power to determine pathways of interest that were altered between the 2 conditions. Future studies could use spectral fragmentation to identify the chemical structure of these 14 features. Further, chemical entities of metabolic relevance can be studied by using an appropriate mutant worm strain or by employing biochemical assays.
High-resolution mass spectrometry-based metabolomics has been used in several C. elegans studies (Edison et al., 2015; Hastings et al., 2017; Mor, 2020; Witting et al., 2018). The comprehensive molecular understanding of the nematode model makes it amenable to characterizing systemic biochemistry and perturbations to global metabolism as a result of genetic, environmental, and toxicological perturbations. Our initial findings suggest that the use of this technique could add to the systems-level evaluation of toxicity in this model. This information may provide further avenues of exploration when looking at metabolic effects of neurodegeneration. In fact, systems-wide approaches to studying common neurodegenerative diseases like PD are recommended by the variety of circuitry affected (Taylor et al., 2011, 2014, 2009). For example, in the few cases of dopamine-serotonin transport disease, a disease related to a mutant allele in VMAT2 known as P387L, patients exhibit developmental delay, parkinsonism, sleep disturbances, mood disorders and a host of issues related to autonomic dysfunction (Rath et al., 2017). The application of high-resolution mass spectrometry allows us to identify metabolic dysfunction in an untargeted manner. The unbiased nature of this approach has the capability of providing links to associated human diseases previously unrecognized. By replicating the dysfunction of monoamine transport and storage in C. elegans we may yet uncover similar metabolic signatures of relevant disease in humans. Our group has recently reported that the same high resolution mass spectrometry-based metabolomic platform can identify disease-specific alterations in plasma from patients with and without Alzheimer’s disease (Niedzwiecki et al., 2020; Vardarajan et al., 2020) demonstrating the utility of the approach from worms to humans. We expect C. elegans will play an important role in future exposome-level studies by providing a laboratory-based validation of observed metabolomic alterations in human disease (Vermeulen et al., 2020). Overlap in common metabolic signatures could potentially be useful for treatment and early diagnosis in neurodegenerative diseases and a number of addiction and mood disorders.
In summary, our results demonstrate the evolutionary-conserved nature of monoamine function in C. elegans and further suggest that high-resolution mass spectrometry-based metabolomics can be used in this model to study environmental and genetic contributors to complex human disease.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge several members of the C. elegans community for their kind assistance. The Katz lab at Emory University, especially Teresa Lee who got us started in the basics of the C. elegans model system. Nicholas Stroustrup for providing input on assessment of lifespan. Richard Nass for providing several antibodies, including the cat-1/VMAT antibody used in this article. The Edison lab for their hospitality and discussions of worm metabolomics. The Blakely lab for helpful discussions on metabolism in C. elegans. Finally, we would like to thank Shuzhao Li for help with Mummichog pathway analysis, and ViLinh Tran and Michael Orr from the Clinical Biomarkers lab at Emory University for running our metabolomics samples and creating biochemical assays for redox analysis in worms.
FUNDING
National Institute of Environmental Health Sciences (R01ES023839, P30ES019776, P30ES009089, U2CES02656, T32 ES012870, T32 ES007322).
DECLARATION OF CONFLICTING INTERESTS
Dr Miller receives royalties for his book The Exposome.
REFERENCES
- Avery L., Horvitz H. R. (1990). Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 253, 263–270. [DOI] [PubMed] [Google Scholar]
- Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. [DOI] [PubMed] [Google Scholar]
- Bradner J. M., Suragh T. A., Wilson W. W., Lazo C. R., Stout K. A., Kim H. M., Wang M. Z., Walker D. I., Pennell K. D., Richardson J. R., et al. (2013). Exposure to the polybrominated diphenyl ether mixture de-71 damages the nigrostriatal dopamine system: Role of dopamine handling in neurotoxicity. Exp. Neurol. 241, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braungart E., Gerlach M., Riederer P., Baumeister R., Hoener M. C. (2004). Caenorhabditis elegans mpp+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener. Dis. 1, 175–183. [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina L., Riva C., Bertola F., Saracchi E., Fermi S., Goldwurm S., Ferrarese C. (2013). Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson’s disease. Neurobiol. Aging 34, 1712 e1719–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M. L., Barrett C. W., Moon C. J., Mortimer A. D., Burton E. A., Timothy Greenamyre J., Hastings T. G. (2020). Acquired dysregulation of dopamine homeostasis reproduces features of Parkinson’s disease. NPJ Parkinson's Dis. 6, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M. D., Nouri K., Baichoo S., Samuels-Toyloy N., Kazemi H. (1994). Ventilatory output and acetylcholine: Perturbations in release and muscarinic receptor activation. J. Appl. Physiol. 77, 2275–2284. [DOI] [PubMed] [Google Scholar]
- Caenorhabditis Genetics Center (CGC). (2020). University of Minnesota, Minneapolis, MN. https://cgc.umn.edu/strain/RB681. Accessed May 3, 2020.
- Caudle W. M. (2015). Occupational exposures and Parkinsonism. Handb. Clin. Neurol. 131, 225–239. [DOI] [PubMed] [Google Scholar]
- Caudle W. M., Guillot T. S., Lazo C. R., Miller G. W. (2012). Industrial toxicants and Parkinson’s disease. Neurotoxicology 33, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle W. M., Richardson J. R., Wang M., Miller G. W. (2005). Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology 26, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle W. M., Richardson J. R., Wang M. Z., Taylor T. N., Guillot T. S., McCormack A. L., Colebrooke R. E., Di Monte D. A., Emson P. C., Miller G. W. (2007). Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 27, 8138–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Ding Y., Cagniard B., Van Laar A. D., Mortimer A., Chi W., Hastings T. G., Kang U. J., Zhuang X. (2008). Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J. Neurosci. 28, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W. S., Kim H. W., Xia Z. (2015). Jnk inhibition of vmat2 contributes to rotenone-induced oxidative stress and dopamine neuron death. Toxicology 328, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Wishart D. S., Xia J. (2019). Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinformatics 68, e86. [DOI] [PubMed] [Google Scholar]
- CeDM.(2012). Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 2, 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J. F., Guindon S., Lefort V., Lescot M., et al. (2008). Phylogeny.Fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree J. D., Weaver J. A. (1984). Identification and characterization of the catecholamine transporter in bovine chromaffin granules using [3h] reserpine. J. Biol. Chem. 259, 10907–10912. [PubMed] [Google Scholar]
- Dressler D., Saberi F. A., Barbosa E. R. (2005). Botulinum toxin: Mechanisms of action. Arq. Neuropsiquiatr. 63, 180–185. [DOI] [PubMed] [Google Scholar]
- Duerr J. S. (2013). Antibody staining in C. elegans using “freeze-cracking”. J. Vis. Exp. 80, e50664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr J. S., Frisby D. L., Gaskin J., Duke A., Asermely K., Huddleston D., Eiden L. E., Rand J. B. (1999). The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J. Neurosci. 19, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison A. S., Clendinen C. S., Ajredini R., Beecher C., Ponce F. V., Stupp G. S. (2015). Metabolomics and natural-products strategies to study chemical ecology in nematodes. Integr. Comp. Biol. 55, 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayah S. H., Vanle B. C., Fuortes L. J., Doorn J. A., Ludewig G. (2018). Pcb95 and pcb153 change dopamine levels and turn-over in pc12 cells. Toxicology 394, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F., Gainetdinov R. R., Wang Y. M., Valenzano K. J., Miller G. W., Caron M. G. (1999). Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J. Neurosci. 19, 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov R. R., Fumagalli F., Jones S. R., Caron M. G. (2002). Dopamine transporter is required for in vivo MPTP neurotoxicity: Evidence from mice lacking the transporter. J. Neurochem. 69, 1322–1325. [DOI] [PubMed] [Google Scholar]
- Glatt C. E., Wahner A. D., White D. J., Ruiz-Linares A., Ritz B. (2006). Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum. Mol. Genet. 15, 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y. M., Walker D. I., Soltow Q. A., Uppal K., Wachtman L. M., Strobel F. H., Pennell K., Promislow D. E., Jones D. P. (2015). Metabolome-wide association study of phenylalanine in plasma of common marmosets. Amino Acids 47, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. S., Sullivan P., Holmes C., Miller G. W., Alter S., Strong R., Mash D. C., Kopin I. J., Sharabi Y. (2013). Determinants of buildup of the toxic dopamine metabolite dopal in Parkinson’s disease. J. Neurochem. 126, 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot T. S., Miller G. W. (2009). Protective actions of the vesicular monoamine transporter 2 (vmat2) in monoaminergic neurons. Mol. Neurobiol. 39, 149–170. [DOI] [PubMed] [Google Scholar]
- Hastings J., Mains A., Artal-Sanz M., Bergmann S., Braeckman B. P., Bundy J., Cabreiro F., Dobson P., Ebert P., Hattwell J., et al. (2017). Wormjam: A consensus C. elegans metabolic reconstruction and metabolomics community and workshop series. Worm 6, e1373939. [Google Scholar]
- Hastings T. G., Lewis D. A., Zigmond M. J. (1996). Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. USA. 93, 1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P. R. (2017). The C. elegans model in toxicity testing. J. Appl. Toxicol. 37, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar A. A., Hossain M. M., Bernstein A. I., Miller G. W., Richardson J. R., Bennett J. W. (2013). Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proc. Natl. Acad. Sci. USA. 110, 19561–19566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal H. O., Chang H. Y., Terrell A. N., Brooks E. S., Pulido D., Simon A. F., Krantz D. E. (2010). The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 40, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cao K.-A., Rohart F., Gonzalez I., Dejean S.with key contributorsGautier B., Bartolo F. contributions fromMonget P., Coquery J., Yao F.Z., Liquet B. (2016). Mixomics: Omics data integration project. R package version 6.1.1.
- Li S., Park Y., Duraisingham S., Strobel F. H., Khan N., Soltow Q. A., Jones D. P., Pulendran B. (2013). Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 9, e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Bernstein A. I., Stout K. A., Dunn A. R., Lazo C. R., Alter S. P., Wang M., Li Y., Fan X., Hess E. J., et al. (2014). Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc. Natl. Acad. Sci. USA. 111, 9977–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Chen M., Hoffman C. A., McDaniel M. J., Stout K. A., Dunn A. R., Wang M., Bernstein A. I., Miller G. W. (2016). Vesicular monoamine transporter 2 (vmat2) level regulates MPTP vulnerability and clearance of excess dopamine in mouse striatal terminals. Toxicol. Sci. 153, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Miller G. W. (2014). Vmat2 and Parkinson's disease: Harnessing the dopamine vesicle. Expert Rev. Neurother. 14, 1115–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Stout K. A., Dunn A. R., Wang M., Salahpour A., Guillot T. S., Miller G. W. (2015). Increased vesicular monoamine transporter 2 (vmat2; slc18a2) protects against methamphetamine toxicity. ACS Chem. Neurosci. 6, 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. L., Yao X. L., Liu Z., Zhang H., Li W., Li Z., Wang G. L., Pang J., Lin Y., Xu Z., et al. (2010). Protective effects of xyloketal b against MPP+-induced neurotoxicity in Caenorhabditis elegans and pc12 cells. Brain Res. 1332, 110–119. [DOI] [PubMed] [Google Scholar]
- Masoud S. T., Vecchio L. M., Bergeron Y., Hossain M. M., Nguyen L. T., Bermejo M. K., Kile B., Sotnikova T. D., Siesser W. B., Gainetdinov R. R., et al. (2015). Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and L-DOPA reversible motor deficits. Neurobiol. Dis. 74, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith G. E., Rademacher D. J. (2011). MPTP mouse models of Parkinson’s disease: An update. J. Parkinsons Dis. 1, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Kirby M. L., Levey A. I., Bloomquist J. R. (1999). Heptachlor alters expression and function of dopamine transporters. Neurotoxicology 20, 631–637. [PubMed] [Google Scholar]
- Miller K. G., Alfonso A., Nguyen M., Crowell J. A., Johnson C. D., Rand J. B. (1996). A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA. 93, 12593–12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor D. E., Sohrabi S., Kaletsky R., Keyes W., Tartici A., Kalia V., Miller G. W., Murphy C. T. (2020). Metformin rescues Parkinson’s disease phenotypes caused by hyperactive mitochondria. Proc. Natl. Acad. Sci. USA. 117, 26438–26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov E. V., Larsen K. E., Kanter E., Phillips K. A., Wilson K., Schmitz Y., Krantz D. E., Kobayashi K., Edwards R. H., Sulzer D. (2009). Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62, 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarky E., Cantley L. C.. 2015. Diverting glycolysis to combat oxidative stress. In Innovative Medicine: Basic Research and Development (Nakao K., Minato N., Uemoto S., editors), p. 3–23. Tokyo. [PubMed] [Google Scholar]
- Munoz P., Huenchuguala S., Paris I., Segura-Aguilar J. (2012). Dopamine oxidation and autophagy. Parkinsons Dis. 2012, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki M. M., Walker D. I., Howell J. C., Watts K. D., Jones D. P., Miller G. W., Hu W. T. (2020). High-resolution metabolomic profiling of Alzheimer’s disease in plasma. Ann. Clin. Transl. Neurol. 7, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla P. 2015. Information management and multivariate analysis techniques for metabolomics data. Doctoral thesis. University of Cagliari, Cagliari, Sardinia, Italy.
- Parsons S. M. (2000). Transport mechanisms in acetylcholine and monoamine storage. Faseb J. 14, 2423–2434. [DOI] [PubMed] [Google Scholar]
- Patel R., Bradner J. M., Stout K. A., Caudle W. M. (2016). Alteration to dopaminergic synapses following exposure to perfluorooctane sulfonate (PFOS), in vitro and in vivo. Med. Sci. 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Lake C., Aronoff E. B., Camp C. R., Vester A., Peters S. J., Caudle W. M. (2017). Impairment in the mesohippocampal dopamine circuit following exposure to the brominated flame retardant, HBCDD. Environ. Toxicol. Pharmacol. 50, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-de-la-Riva M., Fontrodona L., Villanueva A., Ceron J. (2012). Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 64, e4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior C., Marshall I. G., Parsons S. M. (1992). The pharmacology of vesamicol: An inhibitor of the vesicular acetylcholine transporter. Gen. Pharmacol. 23, 1017–1022. [DOI] [PubMed] [Google Scholar]
- Pu P., Le W. (2008). Dopamine neuron degeneration induced by MPP+ is independent of ced-4 pathway in Caenorhabditis elegans. Cell Res. 18, 978–981. [DOI] [PubMed] [Google Scholar]
- Rath M., Korenke G. C., Najm J., Hoffmann G. F., Hagendorff A., Strom T. M., Felbor U. (2017). Exome sequencing results in identification and treatment of brain dopamine-serotonin vesicular transport disease. J. Neurol. Sci. 379, 296–297. [DOI] [PubMed] [Google Scholar]
- Restif C., Ibanez-Ventoso C., Vora M. M., Guo S., Metaxas D., Driscoll M. (2014). Celest: Computer vision software for quantitative analysis of C. elegans swim behavior reveals novel features of locomotion. PLoS Comput. Biol. 10, e1003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. R., Caudle W. M., Wang M., Dean E. D., Pennell K. D., Miller G. W., Richardson J. R., Caudle W. M., Wang M., Dean E. D., et al. (2006). Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb J. 20, 1695–1697. [DOI] [PubMed] [Google Scholar]
- Richardson J. R., Caudle W. M., Wang M. Z., Dean E. D., Pennell K. D., Miller G. W. (2008). Developmental heptachlor exposure increases susceptibility of dopamine neurons to n-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a gender-specific manner. Neurotoxicology 29, 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. R., Miller G. W. (2004). Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol. Lett. 148, 29–40. [DOI] [PubMed] [Google Scholar]
- Salek R. M., Colebrooke R. E., Macintosh R., Lynch P. J., Sweatman B. C., Emson P. C., Griffin J. L. (2008). A metabolomic study of brain tissues from aged mice with low expression of the vesicular monoamine transporter 2 (vmat2) gene. Neurochem. Res. 33, 292–300. [DOI] [PubMed] [Google Scholar]
- Sawin E. R., Ranganathan R., Horvitz H. R. (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh R. A., Richardson J. R., Gupta R. K., Flaws J. A., Fiskum G. (2009). Effects of the organochlorine pesticide methoxychlor on dopamine metabolites and transporters in the mouse brain. Neurotoxicology 30, 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Shirvan A., Linial M. (1995). Vesicular neurotransmitter transporters: From bacteria to humans. Physiol. Rev. 75, 369–392. [DOI] [PubMed] [Google Scholar]
- Schymanski E. L., Jeon J., Gulde R., Fenner K., Ruff M., Singer H. P., Hollender J. (2014). Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 48, 2097–2098. [DOI] [PubMed] [Google Scholar]
- Serrano-Saiz E., Pereira L., Gendrel M., Aghayeva U., Bhattacharya A., Howell K., Garcia L. R., Hobert O. (2017). A neurotransmitter atlas of the Caenorhabditis elegans male nervous system reveals sexually dimorphic neurotransmitter usage. Genetics 206, 1251–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltow Q. A., Strobel F. H., Mansfield K. G., Wachtman L., Park Y., Jones D. P. (2013). High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics 9, 132– S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M. B., Cohen G. (1989). Dopamine turnover and glutathione oxidation: Implications for Parkinson disease. Proc. Natl. Acad. Sci. USA. 86, 1398–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes A. H., Hastings T. G., Vrana K. E. (1999). Cytotoxic and genotoxic potential of dopamine. J. Neurosci. Res. 55, 659–665. [DOI] [PubMed] [Google Scholar]
- Taylor T. N., Alter S. P., Wang M., Goldstein D. S., Miller G. W. (2014). Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology 76, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T. N., Caudle W. M., Miller G. W. (2011). Vmat2-deficient mice display nigral and extranigral pathology and motor and nonmotor symptoms of Parkinson’s disease. Parkinsons Dis. 2011, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T. N., Caudle W. M., Shepherd K. R., Noorian A., Jackson C. R., Iuvone P. M., Weinshenker D., Greene J. G., Miller G. W. (2009). Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J. Neurosci. 29, 8103–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent C., Tsuing N., Horvitz H. R. (1983). Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104, 619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K., Soltow Q. A., Strobel F. H., Pittard W. S., Gernert K. M., Yu T., Jones D. P. (2013). Xmsanalyzer: Automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot A. T., Zhu W., Vazquez-Manrique R. P., Seinstra R. I., Dettmer K., Michels H., Farina F., Krijnen J., Melki R., Buijsman R. C., et al. (2012). Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc. Natl. Acad. Sci. USA. 109, 14912–14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarajan B., Kalia V., Manly J., Brickman A., Reyes-Dumeyer D., Lantigua R., Ionita-Laza I., Jones D. P., Miller G. W., Mayeux R. (2020). Differences in plasma metabolites related to Alzheimer's disease, APOE epsilon4 status, and ethnicity. Alzheimers Dement. 6, e12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer L. M., Florang V. R., Doorn J. A. (2012). Catechol and aldehyde moieties of 3,4-dihydroxyphenylacetaldehyde contribute to tyrosine hydroxylase inhibition and neurotoxicity. Brain Res. 1474, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R., Schymanski E. L., Barabasi A. L., Miller G. W. (2020). The exposome and health: Where chemistry meets biology. Science 367, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. I., Perry-Walker K., Finnell R. H., Pennell K. D., Tran V., May R. C., McElrath T. F., Meador K. J., Pennell P. B., Jones D. P. (2019). Metabolome-wide association study of anti-epileptic drug treatment during pregnancy. Toxicol. Appl. Pharmacol. 363, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. M., Pu P., Le W. D. (2007). Atp depletion is the major cause of MPP+ induced dopamine neuronal death and worm lethality in alpha-synuclein transgenic C. elegans. Neurosci. Bull. 23, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes G. R., Bolker B., Bonebakker L., Gentleman R., Liaw W. H., Lumley T., Maechler M., Magnusson A., Moeller S., Schwartz M. et al. (2009). gplots: various R programming tools for plotting data, R package version 2.1. Available at: https://CRAN.R-project.org/package=gplots. [Google Scholar]
- Wickham H. 2016. Ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York. [Google Scholar]
- Wilson W. W., Onyenwe W., Bradner J. M., Nennig S. E., Caudle W. M. (2014). Developmental exposure to the organochlorine insecticide endosulfan alters expression of proteins associated with neurotransmission in the frontal cortex. Synapse 68, 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting M., Hastings J., Rodriguez N., Joshi C. J., Hattwell J. P. N., Ebert P. R., van Weeghel M., Gao A. W., Wakelam M. J. O., Houtkooper R. H., et al. (2018). Modeling meets metabolomics—The Wormjam consensus model as basis for metabolic studies in the model organism Caenorhabditis elegans. Front. Mol. Biosci. 5, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Zhang X., Huang J., Chen C., Chen Z., Liu L., Zhang G., Yang J., Zhang Z., Zhang Z., et al. (2016). Fenpropathrin, a widely used pesticide, causes dopaminergic degeneration. Mol. Neurobiol. 53, 995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. L., Wu W. L., Zheng M. Y., Li W., Ye C. H., Lu X. L. (2011). Protective effects of lycium barbarum extract against MPP(+) -induced neurotoxicity in Caenorhabditis elegans and pc12 cells. Zhong Yao Cai 34, 1241–1246. [PubMed] [Google Scholar]
- Yen J. C., Chang F. J., Chang S. (1995). A new criterion for automatic multilevel thresholding. IEEE Trans. Image Process. 4, 370–378. [DOI] [PubMed] [Google Scholar]
- Young A. T., Ly K. N., Wilson C., Lehnert K., Snell R. G., Reid S. J., Jacobsen J. C. (2018). Modelling brain dopamine-serotonin vesicular transport disease in Caenorhabditis elegans. Dis. Model. Mech. 11, dmm035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Park Y., Johnson J. M., Jones D. P. (2009). Aplcms–adaptive processing of high-resolution LC/MS data. Bioinformatics 25, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.