Abstract
The lateral hypothalamic area (LHA) is a heterogeneous brain structure extensively studied for its potent role in regulating energy balance. The anatomical and molecular diversity of the LHA permits the orchestration of responses to energy sensing cues from the brain and periphery. Two of the primary cell populations within the LHA associated with integration of this information are Orexin (ORX) and Melanin Concentrating Hormone (MCH). While both of these non-overlapping populations exhibit orexigenic properties, the activities of these two systems support feeding behavior through contrasting mechanisms. We describe the anatomical and functional properties as well as interaction with other neuropeptides and brain reward and hedonic systems. Specific outputs relating to arousal, food seeking, feeding, and metabolism are coordinated through these mechanisms. We then discuss how both the ORX and MCH systems harmonize in a divergent yet overall cooperative manner to orchestrate feeding behavior through transitions between various appetitive states, and thus offer novel insights into LHA allostatic control of appetite.
1. Introduction
The regulation of energy intake is critical to survival and requires coordination between the peripheral and central nervous system. As such, a complex interplay exists between regulatory mechanisms in order to mediate appropriate feeding-related behaviors. Typically, these regulatory mechanisms influence energy balance by coordinating feeding behaviors depending on perceived needs, which requires transitioning to relevant behavioral states while suppressing the performance of unnecessary inapposite actions. However, these mechanisms are susceptible to dysfunction as homeostatic regulation is vulnerable to biological and environmental factors that influence energy balance. For example, factors arising from an obesogenic environment (e.g., excess consumption of energy-dense hedonic foods) may dampen homeostatic regulation, such that regulatory pathways that normally control energy intake are incapable of defending the body against excess energy storage [1–3]. In the absence of increased expenditure, this leads to weight gain, obesity, and associated comorbidities. In this review, we discuss the role of the lateral hypothalamic area (LHA) as a critical site in the central nervous system (CNS) that integrates both internal homeostatic controls as well as input from environmental and motivational signals to mediate behavioral choices as they relate to the procurement, intake, and metabolism of food.
Early studies on the LHA demonstrated that electrical stimulation of this region profoundly increased food consumption, whereas LHA disruption resulted in dramatic under-consumption of food [4, 5]. Therefore, the LHA was initially characterized as a “feeding hotspot”. However, in addition to influencing energy intake, stimulation to this region also invigorated other motivated behaviors such as drinking, physical activity, and copulation [6, 7]. More recent studies have shown that the LHA expresses a wide variety of molecularly distinct cells that function to coordinate a vast array of motivated behaviors. Within the LHA, Orexin/hypocretin (ORX) and Melanin Concentrating Hormone (MCH) neurons play an important role in driving procurement and ingestion of food: In what follows, we describe the distribution and activity of these populations of LHA neurons and examine how they function to independently and cooperatively mediate the range of behavioral state transitions necessary for energy regulation.
2. Characterization of ORX and MCH neurons in LHA
The lateral hypothalamic area (LHA) is a large diencephalic structure that encompasses close to 50% of the hypothalamus and houses a complex interconnected circuit, in which each cell averages approximately >16,000 synapses [8]. While studies continue to elucidate the molecular diversity of the LHA, within this region, ORX and MCH expressing cells display a similar distribution and project to numerous analogous CNS targets; however, despite this, these two orexigens drive distinct appetitive behaviors.
2.1. Anatomy of LHA
The LHA spans the entire rostral-caudal extent of the hypothalamus—it is an extensive and heterogeneous area, complexly interconnected with mediobasal hypothalamic nuclei including the ventromedial (VMH), ventral premammillary (PMV) and arcuate nucleus (ARC). The LHA emerges anterior to the ventral mesencephalon and is positioned posterior to the preoptic area, though its demarcation is complicated by the lack of a discernable boundary. In the hypothalamus, the LHA borders lateral to the dorsomedial hypothalamus (DMH) and paraventricular nucleus (PVN) and dorsal to the PMV and VMH, and can be divided into rostral (tuberal) and posterior portions [13]. The LHA is ventral to the zona incerta (ZI) and lateral to the mediothalamic tract. The subregion that adjoins the fornix is known as the perifornical hypothalamic area and is located within the ventromedial extent of the LHA. The LHA is ideally located to integrate physiological, gastrointestinal, and sympathetic peripheral signals - such as those relayed from the ARC - with forebrain and mesencephalic motivation and reward signals (for reviews, see [14–16]). Within the LHA, two of the most well characterized neuronal populations are ORX and MCH (Figure 1), which have been shown to influence arousal, energy balance and goal-directed behavior.
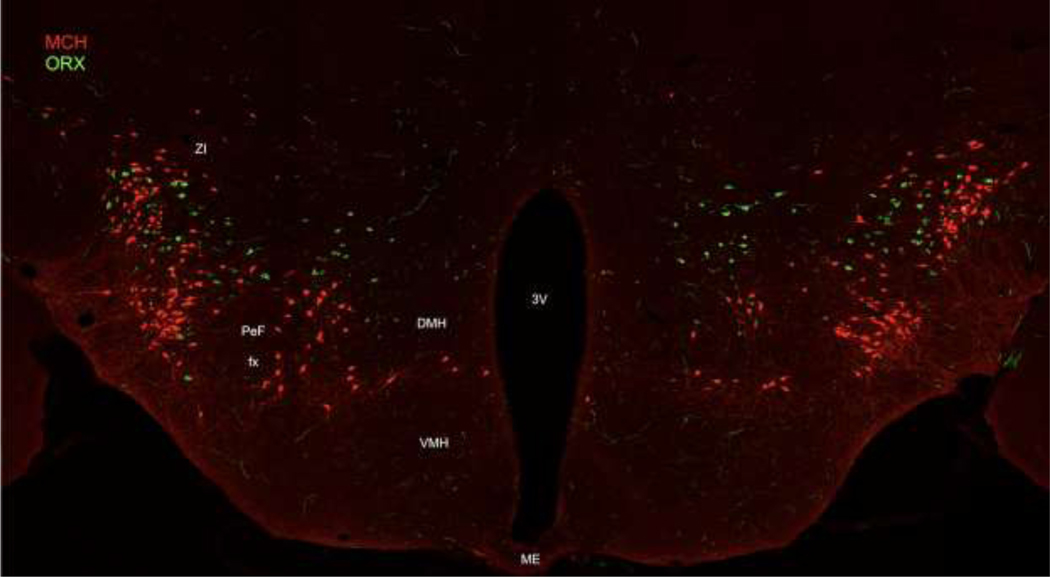
Figure 1: LHA MCH and ORX expressing neurons in the LHA.
Fluorescent labelling of MCH (red) and ORX (green) expressing cells indicates that these neurons form discrete but commingled populations in the LHA. Representative image taken at approximately ~1.7m posterior to bregma from a pMCH-cre mouse crossed with a tdTomato reporter line. Immunohistochemistry was employed to label ORX protein (AlexaFlour 488/ green) and amplify the tdTomato signal (AlexaFluor 568/ red). DMH = dorsomedial hypothalamus; fx = fornix; PeF = perifornical area; VMH = ventromedial hypothalamus; ZI = zona incerta.
2.2. Distribution of ORX and MCH neurons within the LHA
MCH cells were the first population determined to be predominantly located within the ZI and LHA. MCH neurons are widely dispersed in the dorsolateral LHA extending in an anterior to posterior manner where they terminate medial to the subthalamic nucleus. Additionally, a more moderate number of MCH cells are located medial to the internal capsule, whereas a smaller proportion of neurons are located in the perifornical area [18, 24]. ORX expressing cells are located in the DMH and also throughout the LHA, though they exhibit specifically dense concentrations in the perifornical area and the magnocellular nucleus [19, 20]. A small number of cells have been also been revealed in posterior regions of the hypothalamus and subincertal nucleus that demarcates the border between the hypothalamus and thalamus [47]. Thus, both MCH and ORX cells are located in many of the same subdivisions of the LHA, however while both types of cells equivalently populate the LHA [9] and can be intermingled within it, they are non-overlapping (Figure 1).
2.3. Connectivity of LHA ORX and MCH cells
MCH cells receive input from first order feeding neurons originating from the ARC. This includes projections from orexigenic Neuropeptide Y (NPY) as well as Agouti-related peptide (AGRP) neurons. The influence of these orexigenic first order neurons are complex. For example, NPY peptide exerts an inhibitory influence on MCH cells in LHA [21] while administration of MCH peptide increases NPY in hypothalamic section in vitro [22]. These findings suggest that NPY and MCH regulate one another’s activity to coordinate food intake. Unlike NPY, intracerebroventricular (ICV) administration of AGRP peptide enhances LHA MCH mRNA [23]. MCH neurons also receive projections from anorexigenic signals such as α-melanin-stimulating hormone (α-MSH) [17, 19]. Thus, LHA MCH neurons interact with first-order feeding signals in the ARC to coordinate food intake.
MCH has its actions by binding to its receptors, MCH-1R and MCH-2R. Notably however, MCH-2R is either absent or functionally inactive in rodents. MCH-1R is expressed throughout the brain, including cerebral cortex, striatum, hippocampus, amygdala and numerous mesencephalic and rhombencephalic regions [18, 24, 25] (Figure 2). In respect to the regulation of appetite control, MCH-1R expression in the ventral striatal nucleus accumbens shell (ACBs) has been demonstrated to be both necessary and sufficient for food intake [26, 27]. In addition, activation of LHA MCH cells increases dopamine turnover in the ventral striatum, which is thought to encode the nutrient and reward value of sugars [28]. The projection patterns of MCH cells can be distinguished based on co-expression of other molecules, such that the vast majority of efferents to forebrain targets also express both neurokinin 3 and CART [29]. MCH cells also project to other areas important for the regulation of food intake such as PVN [30] and parabrachial nucleus [31]. Furthermore, MCH neurons project to myelencephalic targets that coordinate orofacial movements and mastication, including dorsal motor vagus, facial and hypoglossal nuclei [32]. Additionally, through polysynaptic connections to the hindbrain and spinal cord, MCH neurons also regulate brown adipose tissue (BAT) and influence basal metabolic rate and thermogenesis [33–35]. Conversely, MCH LHA cells display few projections to areas on the CNS that regulate arousal such as the locus coeruleus (LC) and periaqueductal gray (PAG) [36–38].
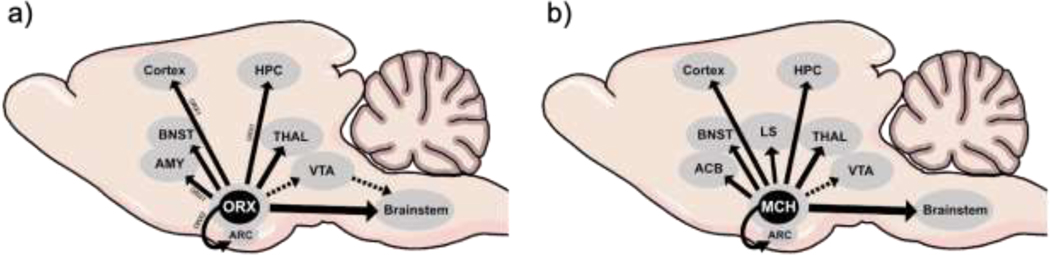
Figure 2: LHA ORX and MCH neuronal projections.
(a) ORX cells project within hypothalamic regions including the arcuate nucleus of the hypothalamus. LHA ORX cells innervate hindbrain regions both through direct connections (solid arrows) to the brainstem as well as indirect ventral tegmental area innervation (dashed arrow). These cells also project to forebrain and limbic regions such as the cortex, amygdala, hippocampus, and thalamus. ORX1 and ORX2 specific projection patterns to specific brain regions are denoted with associated labels. (b) MCH cells project within hypothalamic regions including the arcuate nucleus of the hypothalamus. LHA MCH cells project to the brainstem, cortex, bed nucleus of stria terminalis, lateral septum, nucleus accumbens, and limbic structures such as the thalamus and hippocampus (solid arrows) and debated projection to VTA (dashed arrow). Abbreviations: ACB = nucleus accumbens; AMY= amygdala; ARC = arcuate nucleus of the hypothalamus; BNST = bed nucleus of stria terminalis; HIPP = hippocampus; LS= lateral septum; MCH = melanin concentrating hormone; ORX = orexin; ORX1 = orexin1 receptor; ORX2= orexin 2 receptor; THAL = thalamus VTA = ventral tegmental area.
ORX cells also receive neuronal afferents from the ARC, including from NPY, AGRP and α-MSH cells [17, 19, 39]. In LHA slices NPY also inhibits ORX activity [40], which is unexpected considering both of these neuropeptides evoke feeding. ORX ligands are synthesized into two forms, ORX-A and ORX-B, of which ORX-A shows a tenfold higher affinity for the orexin receptor OxR1, whereas both ligands show similar affinity to the orexin receptor OxR2 [20, 41]. Both OxR1 and OxR2 are expressed in the amygdala, bed nucleus of the stria terminalis (BNST) and ventral tegmental area (VTA). However, only OxR1 is expressed in the medial prefrontal cortex (mPFC) and the hippocampus (HPC), while OxR2 is also expressed in hypothalamic regions such as the ARC and PVN. Orexin neurons drive feeding behavior through their hypothalamic innervations including to the ARC, PVN and DMH [42, 43], as well as the VTA [44] and mPFC [45] to attenuate post-ingestive inhibitory feedback [46] and promote cue-evoked overeating behaviors [1], respectively. Moreover, ORX cells in LHA project to numerous regions that modulate arousal such as the LC, PAG and septal nuclei [47, 48]. Finally, through polysynaptic brainstem projections, LHA ORX cells can regulate BAT [49], and white adipose tissue (WAT), and also influence activity of the gastrointestinal system [50], pancreas and liver [51–53].
To date, it remains to be determined whether subpopulations of LHA MCH and ORX cells preferentially project to specific target regions to influence behavior mediated by the release of their respective peptides [10–12]. We suggest that a combination of influences over their projection targets together with the inherent properties of these LHA neuropeptide expressing cells endows them with the capacity to flexibly mediate a broad appetitive behavioral sequence (Figure 3). That is, we propose ORX plays a critical role in coordinating preparatory responses relevant to acquiring food, whereas MCH orchestrates consummatory behaviors more vital to prolonging food intake and the metabolism of food. This coordinated action is posited to take place via interactions with energetic signals (e.g., glucose), as well as reward and hedonic pathways in the brain (e.g., mesostriatal dopamine).
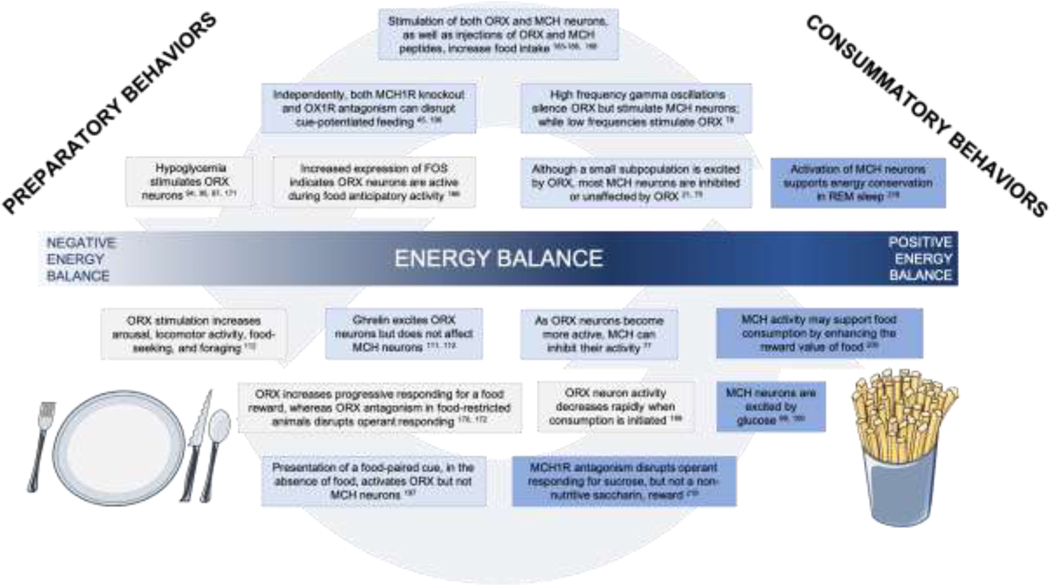
Figure 3. ORX to MCH transitions mediating the appetitive behavioral sequence from food-seeking to consumption.
ICV infusion of MCH [185, 186] increases feeding, as does stimulation of MCH neurons during consumption. Likewise, infusion [183, 184] or chemogenetic stimulation [188] of ORX increases feeding. Knockout of MCH1-R in mice disrupts overeating in cue-potentiated feeding [196] Similarly, antagonism of the OX1R receptor in rats also disrupts cue-potentiated feeding. High frequency gamma oscillations from the lateral septum have the potential to provide input to both ORX and MCH neurons in the LHA; these high frequency oscillations stimulate MCH neurons while silencing ORX neurons [78]. Low blood glucose stimulates orexin neurons [94, 95, 97, 171]. (5) Orexin neurons display increased activation that coincides with food anticipatory activity [166]. MCH neurons are primarily either unaffected or inhibited by ORX [75], although a small (≈ 30%) subpopulation is excited by ORX [21]. MCH neurons are more active during periods of low activity and their activation supports REM sleep [218]. Stimulation of orexin neurons increases overall arousal, as well as feeding-specific behaviors like foraging and food anticipatory activity [112]. Ghrelin, a hunger signal produced in the gut, excites ORX neurons, but appears to have no effect on MCH neurons [111, 112]. MCH neurons can inhibit orexin neurons and appear to do so especially effectively under increased activation of orexin neurons [77]. MCH may support the continued ingestion of food by enhancing its reward value [200]. ICV infusion of ORX-A increased responding for food reward in a progressive ratio task [172], whereas disruption of ORX signaling through ORX antagonism disrupts operant responding under fasted [170] and free-feeding [172]. Orexin neuron activity peaks in anticipation of food consumption but decreases rapidly once consumption is initiated [199]. MCH neurons are excited by glucose [99, 100]. In the absence of food availability, presentation of a food-paired cue results in ORX, but not MCH, neuron excitation [197]. MCH1-R antagonism disrupts operant responding for a sucrose, but not saccharin reward [219]. Fry and dinner plate illustrations were modified from Smart Servier Medical Art on May 20th, 2020, available online at https://smart.servier.com/category/general-items/food/
3. ORX and MCH cells display distinct electrophysiological, molecular and synaptic properties
Although both ORX and MCH exhibit orexigenic properties, in many situations the actions of these two populations of cells are different or even opposing. For instance, MCH cells fire under conditions of paradoxical sleep [54] and drive energy conservation [55–57], whereas ORX neurons have been attributed to invigorating wakefulness [42] and promoting energy expenditure [58]. Differences in the biomolecular properties of these classes of LHA cells may underlie their capacity to appropriately switch between vastly different motivational states.
3.1. Electrophysiological and molecular characteristics of ORX and MCH cells
On binding to OxR1, ORX activates Gs and Gq pathways, activity of which includes triggering intracellular IP3 and DAG cascades, resulting in activation of protein kinase C and Ca2+ signaling and culminating in membrane depolarization [43, 59, 60]. OxR2 signaling is also mediated through Gi/o signaling cascades, which opens K+ channels and inhibits adenylyl cyclase activity to reduces synthesis of cAMP and corresponding cell activity [60–63]. At the same time, both OxR1 and OxR2 are capable of suppressing G-protein regulated inward rectifier channel (GIRK) signaling that typically hyperpolarize and decrease neuronal excitability [64, 65]. Accordingly, the overall result of ORX activity reflects an attenuation of K+ conductance [66–68] and triggering of Na+/Ca2+ exchange currents [43, 59] leading to net excitatory effects of both OxR1 and OxR2 on neuronal activity [60]. MCH binds to MCH-1R activating Gi/o and Gq pathways, triggering a range of downstream effects. This includes enhanced intracellular Ca2+ through IP3 synthesis, reductions in forskolin-mediated elevations in cAMP, and activation of MAPK [25, 69, 70]. Furthermore, in contrast to OxR2, MCH-1R activation has been shown to evoke strong GIRK current [71]. Due in part to this broad competitive intracellular action, cellular effects following MCH-1R activation can be either excitatory (e.g., through the increase in Ca2+), or inhibitory (e.g., by triggering GIRK currents) [37, 69].
Relative to MCH, ORX neurons demonstrate a slightly depolarized resting membrane potential [72]. ORX cells also exhibit spontaneous intrinsic activity by rhythmically generating action potential, whereas MCH containing neurons usually exhibit low activity with few to no spikes at rest [72,73]. Accordingly, MCH neurons are slightly more hyperpolarized than ORX cells recorded under similar physiological conditions and require activation or disinhibition from synapsing cells in order to fire. When stimulated, the vast majority of ORX cells co-release glutamate as well as other regulatory and neurotransmitter signals including Neuronal Pentraxin (NARP) and dynorphin [83]. The co-release of dynorphin in these cells may act in a negative feedback mechanism, dampening the activation of ORX neurons [84]. Conversely, ORX cells can also function in a positive feedback manner, whereby OxR2 activity can itself promote ORX signaling, which may maintain activity of these cells for a prolonged period of time. Transcriptional profiling studies further reveal that almost all ORX containing neurons co-express the protein coding gene implicated in metabolic regulation of nesfastin-1 (NUCB2), the endogenous opioid peptide proenkephalin (PENK) as well as the opioid polypeptide hormone, prodynorphin (PYDN) [85]. Furthermore, ORX neurons express receptors for GABA, glucocorticoids, NPY, melanocortins and leptin [86]. The vast majority of MCH cells also co-express glutamate [87] and vesicular glutamate transporters vGLUT1 and vGLUT2 [86]. A smaller proportion release GABA [88] and contain glutamate decarboxylases, GAD65 and GAD67 [65], the rate limiting enzymes for synthesis of GABA from glutamate. However, MCH neurons do not overlap with vGAT-expressing cells in the LHA, nor do they produce the vesicular transporters vGAT or vMAT [87] that are typically necessary for the synthesis and release of GABA; therefore, the mechanism controlling GABA release via MCH is as yet unknown. MCH neurons also co-release other peptidergic signals including CART, galanin, and nesfatin, and express receptors for GABA glucocorticoids, NPY, melanocortins, and leptin [85,86,90]. In addition to synaptic communication, MCH neurons can influence volume transmission [91], a phenomenon in which peptide release into the extracellular fluid can broadly affect CNS activity via transmission through the ventricles [92].
Interestingly, ORX and MCH containing neurons reciprocally synapse in the LHA and thus can mediate the activity of each other [74]. In slice preparation, application of ORX peptide evokes sustained membrane depolarization and increases spike frequency of MCH cells by elevating glutamate release [21]. However, this activation occurs only in a subpopulation (≈30%) of MCH cells, whereas the remainder are either unaffected or inhibited by ORX peptide administration [75]. The inhibitory influence of ORX over MCH cells reflects increased GABAergic tone, perhaps via local circuit control of GAD65 expressing cells. Additionally, MCH peptide can inhibit ORX neuronal activity, though this occurs only when ORX cells are highly active (i.e., via activity-dependent inhibition) [77]. An additional mechanism in which these signals could be controlled is through network electrical oscillations [76]. At lower oscillation frequencies (<10 Hz), ORX cells show a proclivity for stimulation [78]. Conversely, high frequency gamma oscillations (30–200 Hz) specifically silence ORX cells, while promoting activity in MCH neurons [78]. Thus, reciprocal inhibitory signaling [75, 77] and frequency-dependent modulation [78–80] could enable fast switching between different physiological and behavioral states that are respectively controlled by ORX and MCH cells.
3.2. ORX and MCH responses to energy status
The control of blood glucose is vital for regulating the energy status of an organism and is determined in part by glucose-sensing cells in the hypothalamus [93]. ORX cells are stimulated in response to hypoglycemia, including when evoked by insulin treatment [94–97], and promote glucose utilization under fasted but not ad-libitum testing conditions [98]. Consistent with the reduced activity of the ORX system in response to energy surfeit, approximately 50% of ORX cells are hyperpolarized when glucose levels are high [94, 97, 99]. On the other hand, MCH neurons are excited in response to glucose [99, 100] and are necessary for mediating the metabolic value of sugars [28]. However, this latter finding may reflect the co-release of other neurotransmitter or peptide signals as MCH-1R deletion does not influence glucose-conditioned flavor preferences [223].
In the periphery, the major pre-prandial signal controlling meal initiation is ghrelin, a 28-amino acid peptide that is secreted from gastric mucosa cells in the stomach. Ghrelin works by binding to its receptor, growth hormone secretagogue receptor (GHSR). When binding to GHSR, ghrelin undergoes posttranslational octanoylation via the membrane bound enzyme, ghrelin O-acyltransferase (GOAT) [103, 104]. Two receptor subtypes have been identified – GHS-R type 1a, which is a G-protein coupled receptor, the activation of which leads to phosphorylation and N-linked glycosylation [105, 106]: and GHS-R type 1b, a pharmacologically inert and truncated form of the type 1a receptor [105, 107, 108]. Ghrelin is thought to influence hypothalamic activity either directly via the fenestrated capillaries of the median eminence [109], or indirectly through vagal afferents [110]. Bath application of ghrelin enhances ORX cell firing [97] and influences glucose sensitivity in ORX cells [94], whereas ICV ghrelin infusion leads to an increase in the marker for neuronal activity, FOS (protein product of c-fos gene), in ORX but not MCH cells [111, 112]. Moreover, ghrelin-evoked feeding is dependent upon intact orexin signaling in the LHA [113, 114]. By contrast, ICV ghrelin is still able to evoke food intake in MCH-1R KO mice. Thus, ghrelin-ORX but not MCH interactions appear critical for regulating appetite.
Peripheral satiety signals also differentially influence the ORX and MCH systems. Cholecystokinin (CCK) is secreted in response to nutrients in the intestinal lumen [116, 117] where it delays gastric emptying and inhibits food intake. Vagal afferent neurons are targeted by peripheral signals for the modulatory control of food intake and ORX administration can inhibit the influence of CCK on these neurons [118]. CCK activates ORX cells via the CCK-A receptor [119], whereas it has been shown to directly downregulate MCH-1R on vagal afferent neurons [118] and inhibit MCH release [120–123]. The pancreatic peptide amylin and its receptor salmon calcitonin (sCT) have an inhibitory influence over the LHA; however, while both amylin and sCT decrease mRNA expression of ORX, only sCT reduced expression of MCH mRNA [124]. Finally, the adipokine leptin is synthesized in response to the proportion of body fat and signals energy surfeit. In the LHA, leptin exerts its influence via the receptor, LepRb, which several studies suggest synapses with ORX but not MCH cells [16, 125, 126,127]. Pretreatment with leptin can partially block ORX-A and -B evoked feeding. However, findings are mixed with respect to the mechanism of action of LepRb on ORX cells. That is, while immunoreactivity for LepRb has been noted in ORX cells [128, 129], the antisera used in these studies binds both to the long signaling form of LepRb, as well as its short non-signaling isoform. Furthermore, using genomic approaches that specifically label LepRb-expressing neurons, ORX cells appear not to express LepRb nor are they directly regulated via leptin [127, 130]. By contrast, LepRb is expressed in MCH neurons and may indirectly inhibit MCH activity via disrupting endocannabinoid signaling [86, 131]. Interestingly, the increased adiposity resulting from leptin depletion is rescued in mice deficient in MCH. However, while a drastic reduction in body weight relative to ob/ob mice was revealed, deletion of MCH did not attenuate hyperphagia, but rather reflected a striking increase in energy expenditure.
3.3. ORX and MCH interactions with mesostriatal reward circuitry
Reward circuitry in the brain plays a fundamental role underlying the motivational and hedonic components of food intake and under certain circumstances promoting excess energy intake independent of metabolic need. Dopamine (DA) cells in the VTA are believed to play a particularly important role in modulating reward learning and motivation. In the LHA, ORX cells project to the VTA [47, 132–134] and are known to facilitate DA signaling [135]. Application of ORX-A onto VTA DA cells leads to an increase in NMDAR-mediated synaptic transmission [136] and underlies projection-specific targeting to mesostriatal targets [137]. Moreover, 24 hr fast results in an increase in NMDAR current amplitude in VTA DA cells, which can be blocked by bath application of OxR1 antagonist [95]. Ghrelin can also promote DA signaling in VTA, however these effects are dependent on OxR1 signaling, which suggests a critical role for ORX cells in mediating interactions between ghrelin and DA signaling [114, 138]. When administered into VTA, ORX-A enhances meal size by suppressing post-ingestive negative feedback resulting from palatable food consumption [44]. While OxR1 and OxR2 receptors are expressed in VTA [139–141], the nature of ORX modulation remains debated as the vast majority of LHA ORX fibers project though the VTA with only a small proportion synapsing directly onto cells within this region [142]. This latter finding suggests that modulation via ORX may be volumetric in nature [143, 144]. Interestingly, DA can also directly modulate ORX activity within the LHA in a bidirectional manner, such that low and high concentrations of DA, respectively increase and decrease activity of LHA ORX cells [145]. The ACBs in the ventral striatum is a major target of mesencephalic DA and plays a critical role as an interface between feeding and reward [146]. OxR2 is densely expressed in ACBs [147] and administration of ORX into this region stimulates food intake [148, 149] in part via heightening the hedonic value of food [150].
MCH activity within the VTA is complex and not clearly defined. Earlier studies suggested cell projections from LHA to VTA displayed moderate expression of MCH-1R mRNA and immunoreactive fibers [32, 151]. However, in a transgenic knock-in mouse model, no detectable MCH-1R expression in VTA was noted [152]. Consistent with the latter findings, application of MCH to VTA neurons was shown to have no effect on their activity. With respect to the ACBs, dense LHA MCH innervations have been noted [18] and administration of MCH into this region drives feeding behaviors [26]. Moreover, MCH is required for ventral striatal DA release that typically proceeds sucrose ingestion and reflects the rewarding value of sucrose metabolism [28]. LHA MCH cells that project to ACBs co-express CART [29], which is noteworthy as CART-immunoreactive neurons in ACBs encode drug-associated cues [222]. This may indicate that LHA MCH-CART projections to ACBs encode conditioning and environmental cues associated with reward. MCH-1R is expressed on both D1 and D2 medium spiny neurons (MSNs) where it acts in an inhibitory manner by increasing K+ conductance through Gi/o signaling [27]. Through this mechanism, MCH may inhibit ACBs MSNs to promote ingestive behavior. Moreover, MCH itself can display an antagonistic role on DA signaling in this region, by reducing DARRP-32 phosphorylation [27]. Conversely, it has also been reported that MCH and DA may collectively enhance ACBs firing [220]. While the nature of these discrepant findings requires further characterization, they may reflect different Gq or Gi proteins with which activation of MCH-1R is capable of triggering. Finally, a recent study [222] revealed that selective activation of LHA-MCH neuronal projections to ACBs promoted feeding in male but not female rats. In addition, ovariectomized females treated with vehicle (but not estradiol) displayed increases in food intake upon MCH administration into ACBs. These findings indicate that LHA MCH projections to ACBs may serve as a critical target site for mediating the sex differentiated effects of MCH-dependent feeding behavior [222].
4.0. ORX and MCH modulation of food-seeking and intake
The nuanced properties discussed above position ORX and MCH cells to support an array of orexigenic behaviors necessary for the consumption of food, many of which are functionally distinct. Food-seeking, for example, requires the active procurement of food and expends energy, whereas consumption requires an animal to appropriately terminate food-seeking in order to engage in the act of eating itself. Interactions between ORX and MCH may provide the LHA with the capacity to modulate arousal, food-seeking, and consumption to invigorate energy intake.
4.1. Arousal
Stimulation of the ORX system through either pharmacological or genetic means enhances wakefulness and arousal, whereas it decreases REM sleep [153–155]. Conversely, a loss of ORX neurons greatly augments REM sleep [156] and hypoactivity is noted in response to ORX cell ablation [97]. ORX stimulation also enhances energy expenditure [58] and disruptions in ORX signaling leads to reduced energy expenditure due to decreased locomotor activity [157]. Recently, ORX cell activity has also been shown to predict self-initiated movements [158]. In contrast to ORX, MCH promotes REM sleep [54] and much like MCH gene deletion in ob/ob mice, MCH cell inactivation results in hyperactivity [159, 160] suggesting LHA MCH cells are typically involved in the suppression of locomotion. Moreover, the classical neurotransmitters of the arousal system—noradrenaline and acetylcholine—stimulate ORX but silence MCH activity [161].
4.2. Food anticipation and seeking
In order to survive an organism must successfully forage in their environment, which requires careful selection among a range of complex behaviors and the capacity to anticipate when food will be available. Typically, animals display an increase in locomotor activity in the period preceding predictable food availability [162]. This food-anticipatory activity (FAA) is under the control of a food-entrainable oscillator, a speculated molecular timekeeping mechanism which integrates information of predictable food [163–165]. ORX cells display enhanced FOS activity during FAA [166, 167], whereas neuronal ablation significantly impairs this phenomenon [166]. Although the brain systems underlying FAA remain to be elucidated, it is possible that interactions of ORX neurons with cholinergic and monoaminergic circuitry [168] and LHA ORX projections to the tuberomammillary nucleus [47] underlie ORX modulation of FAA. Conversely, although MCH-1R deletion generally increases locomotor activity during the dark cycle, the MCH system is not required for FAA [169].
In addition to anticipating its availability, animals must also successfully acquire food to assure that their needs for survival are met. In this regard, the effects of ORX can be dependent on the motivational status of the animal—a critical variable when considering whether to engage in food acquisition. Thus, under conditions of food restriction but not free feeding, peripheral treatment with an OxR1 antagonist in rats disrupted operant responding for sucrose under fixed ratio, progressive ratio, as well as cue-induced reinstatement testing conditions [170]. While these results are consistent with electrophysiological findings in which fasting enhances ORX expression [97, 171], food deprivation is not always necessary for ORX modulation of food-seeking. In rats, third ventricle infusion of ORX-A increased progressive ratio responding for sucrose pellets, whereas systemic blockade of OxR1 disrupted performance in this task [172]. The projection targets from that underlie this influence over sucrose responding are unknown, but likely reflect broad interactions of LHA ORX cells with reward circuitry including basal forebrain circuitry and mesencephalic DA [170]. In addition, fourth ventricle infusions of ORX-A also enhanced progressive ratio responding, implicating a role for hindbrain ORX signaling in mediating food-seeking behavior [173]. Given that ORX-1R and immunoreactive ORX-A fibers are noted in the brain stem [47, 174], LHA ORX cell projections to these downstream targets may underlie the influence of ORX on motivated food-seeking [173]. MCH appears to play a less meaningful role in food-seeking as deletion of MCH-1R has no effect on operant responding for sucrose [175, 176]. Similarly, pharmacological blockade of MCH in dorsal hippocampus was found to be without effect on food-seeking in a spatial working memory task [177]. Furthermore, deletion of MCH-1R or systemic treatment with an MCH-1R antagonist does not influence the capacity of a sucrose-paired conditioned stimulus (CS) to augment ongoing operant performance (i.e., Pavlovian-instrumental transfer) [175]. MCH-1R deletion has however been shown to enhance responding under progressive ratio testing conditions [176], suggesting that unlike ORX, the MCH system may generally serve to attenuate this form of incentive-based responding.
Further distinctions between ORX and MCH on their influence over food acquisition are revealed when one considers the nature of the reward being acquired. As mentioned above, ORX effects on sucrose self-administration are mostly observed when animals are tested in a food deprived state [170]. However, when the non-nutritive sweetener saccharin is used as a reinforcer, OxR1 antagonism disrupts operant responding and cue-induced reinstatement regardless of deprivation state [178]. This effect of operant responding for rewards independent of caloric value stands in direct contrast to treatment with the MCH1-R antagonist GW803430, which disrupted sucrose but not saccharin responding. This indicates that the caloric and colligative properties of a food are necessary to any limited role for MCH in acquiring food [179]. This latter finding is in contrast to the effects of targeted deletion of MCH-1R, which leaves intact glucose-conditioned flavor preferences [223]. The basis for this discrepancy might reflect the nature of the disruption in MCH-1R signaling (i.e., congenital ablations, pharmacological blockade), paradigm (i.e., Pavlovian flavor preference, operant responding) or species (mice, rats). Finally, additional differences between ORX and MCH on food seeking have been noted in tests of impulsivity and behavioral inhibition. While systemic antagonism of OxR1 leads to suppressed motivation to engage in a stop-signal reaction time task, it does not influence response inhibition, indicating the ORX system is not important for impulse control [180]. However, LHA MCH containing neurons may mediate response inhibition through descending projections to the ventral hippocampus (vHPC), a region implicated in behavioral impulsivity [181]. Indeed, both pharmacological administration of MCH peptide to the vHPC, as well as selective activation of LHA MCH neuronal projections to this region, increase behavioral impulsivity in a differential reinforcement of low rates of responding task. Interestingly, RNA interference of MCH expression in this pathway also increased behavioral impulsivity, indicating that both increases and decreases in MCH signaling affect impulsivity. Notably, stimulation of MCH signaling did not affect either progressive ratio responding nor interval timing, indicating a selective role for MCH neurons that project to the vHPC in regulating impulsivity [182].
4.3. Consumption of food
Given their labels as orexigens, it is not surprising that ICV administration of either ORX [183, 184] or MCH [185, 186] promotes food intake, whereas systemic pharmacological blockade of their respective receptors leads to intake inhibition [179, 187]. Chemogenetic stimulation of ORX also evokes feeding behavior, and conversely extensive (but not moderate) neuronal ablation attenuates intake of lab chow [188]. Fourth ventricular infusions of ORX-A enhances chow consumption by increasing meal size [189, 190] and this effect is dependent upon intact catecholamine signaling within the hindbrain [189, 191]. In contrast, hindbrain infusions of MCH failed to evoke an orexigenic response for sucrose or saccharin [192] thus, unlike ORX, MCH evoked feeding appears to selectively require forebrain stimulation. Within the LHA, injections of ORX or MCH promotes food intake in a dose-dependent manner [183, 193, 194].
Both ORX and MCH have also been shown to play an important role in learned overeating behaviors, in which a CS can influence eating behavior independent of metabolic need (i.e. cue-potentiated feeding; CPF) [1]. Orexin signaling in the medial prefrontal cortex (mPFC) is implicated in the expression of CPF as systemic blockade of OxR1 prevents both CPF and the expected increase in FOS induction in the mPFC [195]. In addition, targeted mPFC blockade of OxR1 also prevented CPF in sated rats [45]. These findings indicate that projections from LHA ORX cells to mPFC underlie CS evoked feeding responses. Similarly, CPF also appears to require intact MCH-1R signaling [196]. However, when a CS that had been shown to invigorate CPF was presented in the absence of food, it selectively induced FOS activity in LHA ORX but not MCH cells [197].
While the above findings suggest that prolonged activation or long-term disruption of ORX and MCH can broadly influence food intake, when more subtle observations are carried out, the influence of these orexigens on feeding behavior can be readily distinguished from one another. Using in-vivo Ca2+ imaging, Gonzalez et al., recorded dynamic changes in ORX cell activity to reveal a surprising rapid attenuation in cell activity as mice engaged in eating behaviors. This silencing in ORX activity quickly rebound once mice discontinued food consumption [198]. Thus, when dynamic changes in ORX cell activity are tracked, the putative firing rate of these cells becomes weaker as animals engage in food intake. Similar findings were reported using juxtacellular labelling procedures, where ORX cells fired in response to the presentation of an auditory CS associated with subsequent delivery of a sucrose solution. This enhanced firing rate quickly dissipated upon the initiation of licking [199]. By contrast, if acute optogenetic stimulation of LHA MCH cells is timed to coincide with the consumption of food, increased intake is observed [200]. Furthermore, during the consumption of food the activity of MCH neurons communicates nutrient value to provide post-ingestive reward feedback, such that optogenetic stimulation of MCH neurons during consumption of non-caloric sucralose inverts an innate preference for sucrose [28]. This suggests that the pairing of MCH stimulation with sucralose consumption retrieves the positive post-ingestive values associated with nutritive sweeteners such as sucrose. In the same model, stimulation of MCH neurons increases striatal dopamine release in a manner that coincides with the type of post-ingestive reward signaling attributed to dopamine neurons [201]. Interestingly however, optogenetic stimulation of LHA MCH cells does not alone initiate food intake [200], further aligning with the idea that ORX and MCH play distinct roles in the procurement and consumption, respectively, of food.
5.0. Dynamic orchestration of appetitive behaviors via LHA ORX-MCH interactions
We suggest that the ORX system plays an integral role in setting the stage for searching, acquiring, and anticipating the delivery of food. Manipulations of this orexigen robustly influence these preparatory behaviors, which indirectly impact the likelihood that an animal engages in the consumption of food. Alternatively, MCH plays a less prominent role in behaviors that are more distal to food delivery, though it has a direct and far-reaching influence on consummatory behaviors at later stages of the appetitive behavioral sequence (Figure 3).
5.1. From food-seeking to intake: Transitions from LHA ORX to MCH cell activity
Both ORX and MCH neurons in the LHA play a multifaceted role in a range of motivated behaviors including sleep, arousal, maternal caregiving and appetite control [14, 89, 202]. It is unlikely that the same population of cells underlie control of these distinct motivated behaviors. Thus, we suggest that glucose-sensing ORX and MCH cells in particular function to mediate behavioral transitions from seeking to consumption. In this regard, the low glucose levels that accompany hunger could serve to directly stimulate activity in LHA ORX glucose-sensing cells [94–97], which is mediated by activity of the peripheral hunger signal ghrelin [94]. Activity of LHA ORX cells would be expected to facilitate responding to environmental cues predictive of food via projections to mPFC [45]. Moreover, given that ghrelin facilitates phasic firing of DA cells in VTA, which requires OxR1 signaling [114,138], these ghrelin-ORX interactions could enhance DA synthesis and activation of the mesostriatal pathway that is critical for reward-based responding [47, 132–138, 172, 203]. When searching out food it is also advantageous that competing behaviors involved in direct consummatory acts be suppressed. With this in mind, optical activation of LHA ORX cells can inhibit MCH cells via ORX mediated increases in GABAergic signaling [21]. Moreover, ghrelin does not influence MCH [111, 112] and unlike ORX, MCH signaling is not necessary for ghrelin-initiated feeding [115]. Additionally, the DA release resulting directly from ghrelin [204] and/or ORX [47, 132] might also serve to dampen MCH activity [205].
Once food has been acquired, we suggest that a rapid flexible transition from ORX to MCH activity is required, which could be driven by several potential mechanisms (Figure 4). First, MCH can exert an inhibitory influence over ORX in an activity-dependent manner [77]. Thus, when ORX activity is elevated, inhibition via MCH could be critical in allowing for fine-tuning between these two classes of cells. It is also perhaps worthwhile considering that ORX cell activity is greatest immediately prior to contact with food [199]; therefore, MCH would be expected to have its strongest inhibitory influence over ORX at the time when behavioral state transitions (i.e., seeking → feeding) are imminent. Second, descending high frequency gamma oscillations originating in the lateral septum may also have the capacity to flexibly modulate transitions from ORX to MCH activity [78]. Third, it is well known that the endocannabinoid system functions as a pre-synaptic mediator for the release of neuropeptides and neurotransmitters [206]. Activation of the cannabinoid type-1 receptor (CB1) induces a rapid transient though significant suppression of ORX activity. At the same time, CB1-activation also depolarizes MCH cells via presynaptic attenuation of locally synapsing GABAergic neurons [207]. It would be interesting to examine if these are the same population of cells that are proposed to mediate increased ORX-dependent GABAergic drive onto MCH [75]. These effects may underlie the well-documented role of the endocannabinoid system on food intake in general [208], and influence rapid ORX to MCH transitions in particular. Forth, the rising levels of glucose that accompany the metabolism of food serve to inhibit ORX and stimulate MCH cells [99, 100]. Finally, any inhibition to ORX cell activity could disrupt ORX-dependent GABAergic drive onto LHA MCH cells; thus, further disinhibiting them [75]. Collectively, as animals engage in the consumption of food, this ORX to MCH transition in neuronal activity would promote the rewarding post-ingestive [28] and hedonic value of sugars [196, 209] resulting in the maintenance and prolonging of food intake [200].
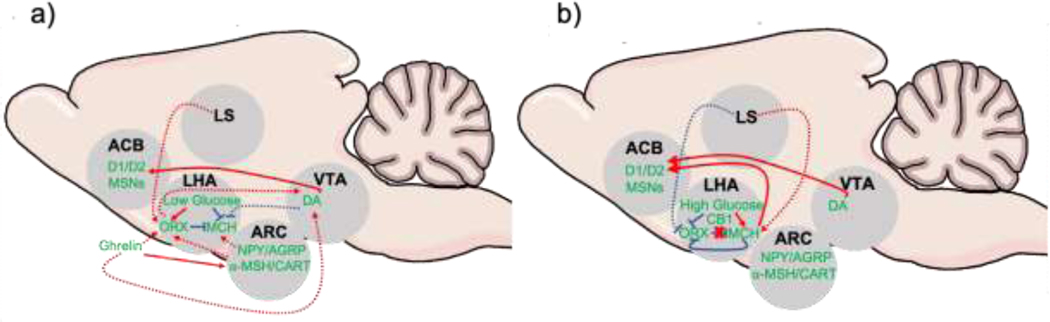
Figure 4. Potential physiological mechanisms underlying ORX to MCH rapid behavioral state transitions for food-seeking and consumption.
(a) Under conditions of hunger, the accompanying low glucose levels stimulates ORX and inhibits MCH. Further, the gastric peptide ghrelin stimulates LHA ORX either indirectly via stimulation of first-order NPY-AGRP neurons in ARC and inhibition of α-MSH and CART signals, or directly via GHSRs in LHA. Increased ghrelin and stimulation of ORX enhances VTA DA, facilitating signaling to ventral striatal ACB MSNs. MCH firing may be inhibited by ORX-dependent increases in GABAergic inhibition and potentially via DA. Finally, lower oscillation frequencies (<10 Hz), ORX cells show a proclivity for stimulation, which could be mediated via LS input. (b) As ORX activity increases, MCH can inhibit LHA ORX in an activity-dependent manner. Increased glucose levels following food consumption further attenuate ORX signaling while heightening activity in LHA MCH cells. The CB1 receptor can also respectively inhibit and excite ORX and MCH cells. This general decrease in ORX activity has the potential to disinhibit MCH cell firing. Activity of MCH leads to increases in reward and hedonic pathways in the brain, such as ACB. Abbreviations: ACB = nucleus accumbens; ARC = arcuate nucleus of the hypothalamus; AGRP = agouti-related peptide; α-MSH = α-melanin-stimulating hormone; CART = cocaine and amphetamine related transcript; CB1 = cannabinoid receptor type 1; DA = dopamine; D1/D2 = dopamine D1-like, D2-like receptors; LS = lateral septum; MCH = melanin concentrating hormone; MSNs = medium spiny neurons; NPY = neuropeptide Y. Solid arrows indicate known excitatory (red) and inhibitory (blue) interactions; dashed red arrows = assumed excitatory (red) and inhibitory (blue) interactions that require further pathway characterization.
5.2. Disruptions to LHA ORX and MCH activity in dietary obesity
Dietary obesity leads to profound changes in physiology with both ORX and MCH displaying many divergent structural and functional alterations. On initial exposure to a high-fat diet, ORX cells displays an increase in spontaneous miniature excitatory post-synaptic current (mEPSC) amplitude immediately upon the animal gaining access to a high-fat diet [210]. This would be expected to augment ORX-dependent appetitive behaviors, including an increase in food anticipatory behaviors [166] and cue-potentiated feeding [45]. Food cues in the environment can promote eating independent of metabolic need, which is thought to contribute to weight gain and obesity [211]. Thus, under this early period of high fat diet exposure, the heightened activity of the ORX system would be expected to enhance vulnerability to food-cues in the environment. Indeed, fMRI studies in humans suggest that enhanced activity of amygdale-hypothalamic CPF circuitry in response to food cue presentation is predictive of susceptibility to weight gain [212]. The increase in ORX cell activity during the initial stages of high fat diet consumption is transient in nature, such that following more prolonged periods of exposure to a high-fat diet ORX cell activity normalizes [210], whereas mRNA levels of prepro-orexin gene and OxR2 decrease [213]. By contrast, limited access to a high-fat diet does not influence MCH cell firing. However, prolonged access leads to an increase in mEPSCs frequency likely driven by an increase in excitatory drive onto MCH cells [210]. In addition, elevated MCH and MCH-1R mRNA in LHA results from prolonged access to a high fat diet [214] consistent with a ramping up of MCH system activity. On the other side of the spectrum, chronic administration of ORX and MCH peptide also differentially impact the onset of dietary obesity. Long-term exposure to ORX has no effect on feeding or body weight [215], whereas chronic administration of MCH enhances food intake and obesity in rodents [185, 216]. Taken together, these results indicate that LHA ORX cells may influence weight gain during the early “dynamic” phase, whereas the increase in excitatory drive and activity of LHA MCH cells would set the conditions for further development and maintenance of weight gain during the “static phase” [5], due to the promotion of energy storage through overconsumption, increased sedentary behaviors and reduced metabolism [159, 160, 216, 217].
6.0. Summary
As we navigate our daily lives, each of us frequently experience some degree of hunger. The severity of this drive is dependent on our ongoing energy needs, with the interoceptive feelings generated differing greatly based on the physiological composition of the individual. Nevertheless, mechanisms underlying the generation of hunger uniformly serve to inform the organism that they should engage in behaviors that drive energy intake. From an evolutionary perspective, mechanisms that maintain homoeostatic balance underwent substantial pressures—after all, if energy needs are not met then the survival of an individual and by consequence the species may be at risk. Accordingly, this suggests conservation across species in the physiological mechanisms underlying body weight control. Interactions between ORX and MCH form a critical physiological allostatic circuit ensuring that these needs for survival can be met. This is achieved in part by flexible and rapid changes in behavioral output that favor relevant and suppress competing appetitive actions.
Highlights.
The lateral hypothalamic area (LHA) plays a critical role in integrating energetic and reward signaling to regulate food intake
Orexin/hypocretin (ORX) and Melanin Concentrating Hormone (MCH) cells are localized in the LHA and play a critical role in appetite regulation
Based on their distribution and functionality, we propose ORX and MCH cells cooperate to respectively mediate flexible transitions from preparatory to consummatory behaviors
A hypothalamic-striatal circuit underlying control of these behavioral state transitions is described
Acknowledgements
This work was in part supported by NIH grant R01 DK111475 to AWJ. We wish to thank the PG&T group for helpful comments on previous versions of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci. 2013;36:101–9. [DOI] [PubMed] [Google Scholar]
- [2].Myers MG, Cowley MA, & Münzberg H Mechanisms of leptin action and leptinresistance. Annu. Rev. Physiol, 2008; 70, 537–556. [DOI] [PubMed] [Google Scholar]
- [3].Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71:521–33. [DOI] [PubMed] [Google Scholar]
- [4].Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–40. [PMC free article] [PubMed] [Google Scholar]
- [5].Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26:541–59. [DOI] [PubMed] [Google Scholar]
- [6].Hess WR, Brugger M. Das subkortikale Zentrum der affektiven Abwehrreaktion (The Subcortical Center of the Affective Defense Reaction). Helvetica Physiologica. 1943;1:33–52. [Google Scholar]
- [7].Hess WR. Das Zwischenhirn. Syndrome, Lokalisationen (The midbrain syndromes, localizations): Basel: Benno Schwabe; 1949. [Google Scholar]
- [8].Palkovits M, Van Cuc H. Quantitative light and electron microscopic studies on the lateral hypothalamus in rat. Cell and synaptic densities. Brain Res Bull. 1980;5:643–7. [DOI] [PubMed] [Google Scholar]
- [9].Brown JA, Bugescu R, Mayer TA, Gata-Garcia A, Kurt G, Woodworth HL, & Leinninger GM. Loss of action via neurotensin-leptin receptor neurons disrupts leptin and ghrelin-mediated control of energy balance. Endocrinology, 2017; 158: 1271–1288.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. [DOI] [PubMed] [Google Scholar]
- [11].Schöne C, Venner A, Knowles D, Karnani MM, Burdakov D. Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol. 2011;589:2767–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183:689–706. [DOI] [PubMed] [Google Scholar]
- [14].Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Petrovich GD. Lateral Hypothalamus as a Motivation-Cognition Interface in the Control of Feeding Behavior. Front Syst Neurosci. 2018;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Broberger C, De Lecea L, Sutcliffe J, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: Relationship to the neuropeptide Y and Agouti gene-related protein systems. Journal of Comparative Neurology. 1998;402:460–74. [PubMed] [Google Scholar]
- [18].Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The Melanin-Concentrating Hormone System of the Rat Brain: An Immuno- And Hybridization Histochemical Characterization. Journal Comp Neuorl. 1992;319:218–45. [DOI] [PubMed] [Google Scholar]
- [19].Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–59. [PubMed] [Google Scholar]
- [20].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli R, Tanaka H, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. [DOI] [PubMed] [Google Scholar]
- [21].van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–52. [DOI] [PubMed] [Google Scholar]
- [22].Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, et al. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144:3943–9. [DOI] [PubMed] [Google Scholar]
- [23].Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T. Differential regulation of melanin-concentrating hormone and orexin genes in the agouti-related protein/melanocortin-4 receptor system. Biochem Biophys Res Commun. 2000;268:88–91. [DOI] [PubMed] [Google Scholar]
- [24].Bittencourt JC. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endocrinol. 2011;172:185–97. [DOI] [PubMed] [Google Scholar]
- [25].Presse F, Conductier G, Rovere C, Nahon JL. The melanin-concentrating hormone receptors: neuronal and non-neuronal functions. Int J Obes Suppl. 2014;4:S31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Georgescu D, Sears R, Hommel J, Barrot M, Bolanos C, Marsh D, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus Accumbens to modulate feeding Behavior and forced-swim performance. Journal of Neuroscience. 2005;25:2933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, et al. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Domingos A, Sordillo A, Dietrich M, Liu Z, Tellez L, Vaynshteyn J, et al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cvetkovic V, Brischoux F, Jacquemard C, Fellmann D, Griffond B, Risold PY. Characterization of subpopulations of neurons producing melanin-concentrating hormone in the rat ventral diencephalon. J Neurochem. 2004;91:911–9. [DOI] [PubMed] [Google Scholar]
- [30].Fekete C, Wittmann G, Liposits Z, Lechan RM. Origin of cocaine- and amphetamine-regulated transcript (CART)-immunoreactive innervation of the hypothalamic paraventricular nucleus. J Comp Neurol. 2004;469:340–50. [DOI] [PubMed] [Google Scholar]
- [31].Touzani K, Tramu G, Nahon JL, Velley L. Hypothalamic melanin-concentrating hormone and alpha-neoendorphin-immunoreactive neurons project to the medial part of the rat parabrachial area. Neuroscience. 1993;53:865–76. [DOI] [PubMed] [Google Scholar]
- [32].Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. [DOI] [PubMed] [Google Scholar]
- [33].Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC. Lateral hypothalamic ‘command neurons’ with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci. 2007;25:2404–12. [DOI] [PubMed] [Google Scholar]
- [34].Bittencourt JC, Elias CF. Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neuronal tracers. Brain Res. 1998;805:1–19. [DOI] [PubMed] [Google Scholar]
- [35].Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, et al. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135:611–25. [DOI] [PubMed] [Google Scholar]
- [36].Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, et al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–5. [DOI] [PubMed] [Google Scholar]
- [37].Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–9. [DOI] [PubMed] [Google Scholar]
- [38].Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–30. [DOI] [PubMed] [Google Scholar]
- [39].Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. [DOI] [PubMed] [Google Scholar]
- [40].Fu L, Acuna-Goycolea C, van den Pol A. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: Tonic depression of the hypothalamic arousal system. Journal of Neuroscience. 2004;24:8741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sakurai T Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–41. [DOI] [PubMed] [Google Scholar]
- [43].Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium--calcium exchanger. J Neurosci. 2003;23:4951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Terrill SJ, Hyde KM, Kay KE, Greene HE, Maske CB, Knierim AE, et al. Ventral tegmental area orexin 1 receptors promote palatable food intake and oppose postingestive negative feedback. Am J Physiol Regul Integr Comp Physiol. 2016;311:R592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cole S, Keefer SE, Anderson LC, Petrovich GD. Medial Prefrontal Cortex Neural Plasticity, Orexin Receptor 1 Signaling, and Connectivity with the Lateral Hypothalamus Are Necessary in Cue-Potentiated Feeding. J Neurosci. 2020;40:1744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Johnson AW. Characterizing ingestive behavior through licking microstructure: Underlying neurobiology and its use in the study of obesity in animal models. International Journal of Developmental Neuroscience, 2018;64, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peyron C, Tighe D, van den Pol A, de Lecea L, Heller H, Sutcliffe J, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;18:9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–59. [PubMed] [Google Scholar]
- [49].Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–26. [DOI] [PubMed] [Google Scholar]
- [50].Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003;549:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stanley S, Pinto S, Segal J, Pérez CA, Viale A, DeFalco J, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci U S A. 2010;107:7024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci. 2012;32:15913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wu X, Gao J, Yan J, Owyang C, Li Y. Hypothalamus-brain stem circuitry responsible for vagal efferent signaling to the pancreas evoked by hypoglycemia in rat. J Neurophysiol. 2004;91:1734–47. [DOI] [PubMed] [Google Scholar]
- [54].Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. Bmc Neuroscience. 2003;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Astrand A, Bohlooly-Y M, Larsdotter S, Mahlapuu M, Andersén H, Tornell J, et al. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol Regul Integr Comp Physiol. 2004;287:R749–58. [DOI] [PubMed] [Google Scholar]
- [56].Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Glick M, Segal-Lieberman G, Cohen R, Kronfeld-Schor N. Chronic MCH infusion causes a decrease in energy expenditure and body temperature, and an increase in serum IGF-1 levels in mice. Endocrine. 2009;36:479–85. [DOI] [PubMed] [Google Scholar]
- [58].Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253:241–5. [DOI] [PubMed] [Google Scholar]
- [59].Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–66. [DOI] [PubMed] [Google Scholar]
- [61].Martin G, Fabre V, Siggins GR, de Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104:111–7. [DOI] [PubMed] [Google Scholar]
- [62].Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–61. [DOI] [PubMed] [Google Scholar]
- [63].Ramanjaneya M, Conner AC, Chen J, Kumar P, Brown JE, Jöhren O, et al. Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J Endocrinol. 2009;202:249–61. [DOI] [PubMed] [Google Scholar]
- [64].Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. [DOI] [PubMed] [Google Scholar]
- [65].Yudin Y, Rohacs T. Inhibitory Gi/O-coupled receptors in somatosensory neurons: potential therapeutic targets for novel analgesics. Mol Pain. 2018;14:1744806918763646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Mühlethaler M, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bayer L, Serafin M, Eggermann E, Saint-Mleux B, Machard D, Jones BE, et al. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J Neurosci. 2004;24:6760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–8. [DOI] [PubMed] [Google Scholar]
- [69].Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26:3122–42. [DOI] [PubMed] [Google Scholar]
- [71].Bächner D, Kreienkamp H, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1). FEBS Lett. 1999;457:522–4. [DOI] [PubMed] [Google Scholar]
- [72].van den Pol A, Patrylo P, Ghosh P, Gao X. Lateral hypothalamus: Early developmental expression and response to hypocretin (orexin). Journal of Comparative Neurology. 2001;433:349–63. [DOI] [PubMed] [Google Scholar]
- [73].Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, et al. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Guan J, Uehara K, Lu S, Wang Q, Funahashi H, Sakurai T, et al. Reciprocal synaptic relationships between orexin- and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. International Journal of Obesity. 2002;26:1523–32. [DOI] [PubMed] [Google Scholar]
- [75].Apergis-Schoute J, Iordanidou P, Faure C, Jego S, Schöne C, Aitta-Aho T, et al. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci. 2015;35:5435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Carus-Cadavieco M, Gorbati M, Ye L, Bender F, van der Veldt S, Kosse C, et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature. 2017;542:232–6. [DOI] [PubMed] [Google Scholar]
- [77].Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kosse C, Burdakov D. Fast and Slow Oscillations Recruit Molecularly-Distinct Subnetworks of Lateral Hypothalamic Neurons. eNeuro. 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. [DOI] [PubMed] [Google Scholar]
- [80].Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci.2012;35:203–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. [DOI] [PubMed] [Google Scholar]
- [83].Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mickelsen LE, Bolisetty M, Chimileski BR, Fujita A, Beltrami EJ, Costanzo JT, ... & Jackson AC (2019). Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nature neuroscience, 22(4), 642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Harthoorn LF, Sañé A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25:1209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Arrigoni E, Chee MJS, Fuller PM. To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology. 2019;154:34–49. [DOI] [PubMed] [Google Scholar]
- [90].Bäckberg M, Ultenius C, Fritschy JM, Meister B. Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16:589–604. [DOI] [PubMed] [Google Scholar]
- [91].Noble EE, Hahn JD, Konanur VR, Hsu TM, Page SJ, Cortella AM, et al. Control of Feeding Behavior by Cerebral Ventricular Volume Transmission of Melanin-Concentrating Hormone. Cell Metab. 2018;28:55–68.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–26. [DOI] [PubMed] [Google Scholar]
- [93].Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda). 2007;22:241–51. [DOI] [PubMed] [Google Scholar]
- [94].Sheng Z, Santiago AM, Thomas MP, Routh VH. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol Cell Neurosci. 2014;62:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Teegala SB, Sheng Z, Dalal MS, Hirschberg PR, Beck KD, Routh VH. Lateral hypothalamic orexin glucose-inhibited neurons may regulate reward-based feeding by modulating glutamate transmission in the ventral tegmental area. Brain Res. 2020;1731:145808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, et al. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50:105–12. [DOI] [PubMed] [Google Scholar]
- [97].Yamanaka A, Beuckmann C, Willie J, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. [DOI] [PubMed] [Google Scholar]
- [98].Tsuneki H, Sugihara Y, Honda R, Wada T, Sasaoka T, Kimura I. Reduction of blood glucose level by orexins in fasting normal and streptozotocin-diabetic mice. Eur J Pharmacol. 2002;448:245–52. [DOI] [PubMed] [Google Scholar]
- [99].Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, et al. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Guyon A, Conductier G, Rovere C, Enfissi A, Nahon JL. Melanin-concentrating hormone producing neurons: Activities and modulations. Peptides. 2009;30:2031–9. [DOI] [PubMed] [Google Scholar]
- [102].Woods SC, Seeley RJ, Porte D, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–83. [DOI] [PubMed] [Google Scholar]
- [103].Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. [DOI] [PubMed] [Google Scholar]
- [105].Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. [DOI] [PubMed] [Google Scholar]
- [106].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. [DOI] [PubMed] [Google Scholar]
- [107].McKee KK, Palyha OC, Feighner SD, Hreniuk DL, Tan CP, Phillips MS, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol. 1997;11:415–23. [DOI] [PubMed] [Google Scholar]
- [108].Smith RG, Feighner S, Prendergast K, Guan X, Howard A. A New Orphan Receptor Involved in Pulsatile Growth Hormone Release. Trends Endocrinol Metab. 1999;10:128–35. [DOI] [PubMed] [Google Scholar]
- [109].Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A. 2013;110:1512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. [DOI] [PubMed] [Google Scholar]
- [111].Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–62. [DOI] [PubMed] [Google Scholar]
- [112].Toshinai K, Date Y, Murakami N, Shimada M, Mondal M, Shimbara T, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–12. [DOI] [PubMed] [Google Scholar]
- [113].Miura T, Maruyama K, Shimakura S, Kaiya H, Uchiyama M, Kangawa K, et al. Regulation of food intake in the goldfish by interaction between ghrelin and orexin. Peptides. 2007;28:1207–13. [DOI] [PubMed] [Google Scholar]
- [114].Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bjursell M, Egecioglu E, Gerdin AK, Svensson L, Oscarsson J, Morgan D, et al. Importance of melanin-concentrating hormone receptor for the acute effects of ghrelin. Biochem Biophys Res Commun. 2005;326:759–65. [DOI] [PubMed] [Google Scholar]
- [116].Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245:323–5. [DOI] [PubMed] [Google Scholar]
- [117].Smith GP, Gibbs J. Are gut peptides a new class of anorectic agents? Am J Clin Nutr. 1992;55:283S–5S. [DOI] [PubMed] [Google Scholar]
- [118].Burdyga G, Lal S, Spiller D, Jiang W, Thompson D, Attwood S, et al. Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology. 2003;124:129–39. [DOI] [PubMed] [Google Scholar]
- [119].Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006;137:1405–15. [DOI] [PubMed] [Google Scholar]
- [122].Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes.2007;14:63–7. [DOI] [PubMed] [Google Scholar]
- [123].Gallmann E, Arsenijevic D, Spengler M, Williams G, Langhans W. Effect of CCK-8 on insulin-induced hyperphagia and hypothalamic orexigenic neuropeptide expression in the rat. Peptides. 2005;26:437–45. [DOI] [PubMed] [Google Scholar]
- [124].Barth SW, Riediger T, Lutz TA, Rechkemmer G. Differential effects of amylin and salmon calcitonin on neuropeptide gene expression in the lateral hypothalamic area and the arcuate nucleus of the rat. Neurosci Lett. 2003;341:131–4. [DOI] [PubMed] [Google Scholar]
- [125].Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–50. [DOI] [PubMed] [Google Scholar]
- [127].Louis G, Leinninger G, Rhodes C, Myers M. Direct Innervation and Modulation of Orexin Neurons by Lateral Hypothalamic LepRb Neurons. Journal of Neuroscience. 2010;30:11278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Håkansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol. 1999;11:653–63. [DOI] [PubMed] [Google Scholar]
- [129].Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19:1072–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Jo Y, Chen Y, Chua S, Talmage D, Role L. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–87. [DOI] [PubMed] [Google Scholar]
- [133].España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl). 2011;214:415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. [DOI] [PubMed] [Google Scholar]
- [137].Baimel C, Lau BK, Qiao M, Borgland SL. Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep. 2017;18:1346–55. [DOI] [PubMed] [Google Scholar]
- [138].Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sakurai T Orexins and orexin receptors: implication in feeding behavior. Regul Pept. 1999;85:25–30. [DOI] [PubMed] [Google Scholar]
- [142].Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–84. [DOI] [PubMed] [Google Scholar]
- [143].Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Tyree SM, de Lecea L. Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front Syst Neurosci. 2017;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Linehan V, Rowe T, Hirasawa M. Dopamine modulates excitatory transmission to orexin neurons in a receptor subtype-specific manner. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2019;316:R68–R75. [DOI] [PubMed] [Google Scholar]
- [146].Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76. [DOI] [PubMed] [Google Scholar]
- [147].Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–5. [DOI] [PubMed] [Google Scholar]
- [148].Mayannavar S, Rashmi KS, Rao YD, Yadav S, Ganaraja B. Effect of Orexin-A infusion in to the Nucleus Accumbens on consummatory behaviour and alcohol preference in male Wistar rats. Indian J Physiol Pharmacol. 2014;58:319–26. [PubMed] [Google Scholar]
- [149].Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl). 2005;182:75–83. [DOI] [PubMed] [Google Scholar]
- [150].Castro DC, Terry RA, Berridge KC. Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose ‘Liking’ and Intake but Scopolamine in Caudal Shell Shifts ‘Liking’ Toward ‘Disgust’ and ‘Fear’. Neuropsychopharmacology. 2016;41:2101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, et al. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–216. [DOI] [PubMed] [Google Scholar]
- [152].Chee M, Pissios P, Maratos-Flier E. Neurochemical characterization of neurons expressing melanin-concentrating hormone receptor 1 in the mouse hypothalamus. Journal of Comparative Neurology. 2013;521:2208–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and −2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6:e20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Willie JT, Takahira H, Shibahara M, Hara J, Nomiyama M, Yanagisawa M, et al. Ectopic overexpression of orexin alters sleep/wakefulness states and muscle tone regulation during REM sleep in mice. J Mol Neurosci. 2011;43:155–61. [DOI] [PubMed] [Google Scholar]
- [156].Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. [DOI] [PubMed] [Google Scholar]
- [157].Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. [DOI] [PubMed] [Google Scholar]
- [158].Karnani MM, Schöne C, Bracey EF, González JA, Viskaitis P, Li HT, et al. Role of spontaneous and sensory orexin network dynamics in rapid locomotion initiation. Prog Neurobiol. 2020;187:101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Shimada M, Tritos N, Lowell B, Flier J, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. [DOI] [PubMed] [Google Scholar]
- [160].Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci. 2013;33:2009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, et al. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–11. [DOI] [PubMed] [Google Scholar]
- [162].Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–95. [DOI] [PubMed] [Google Scholar]
- [163].Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. [DOI] [PubMed] [Google Scholar]
- [165].Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci. 2001;13:1190–6. [DOI] [PubMed] [Google Scholar]
- [166].Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–21. [DOI] [PubMed] [Google Scholar]
- [168].Mieda M, Yanagisawa M. Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr Opin Neurobiol. 2002;12:339–45. [DOI] [PubMed] [Google Scholar]
- [169].Zhou D, Shen Z, Strack AM, Marsh DJ, Shearman LP. Enhanced running wheel activity of both Mch1r- and Pmch-deficient mice. Regul Pept. 2005;124:53–63. [DOI] [PubMed] [Google Scholar]
- [170].Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl). 2013;226:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, et al. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–7. [DOI] [PubMed] [Google Scholar]
- [172].Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. [DOI] [PubMed] [Google Scholar]
- [173].Kay K, Parise EM, Lilly N, Williams DL. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl). 2014;231:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. [DOI] [PubMed] [Google Scholar]
- [175].Sherwood A, Wosiski-Kuhn M, Nguyen T, Holland PC, Lakaye B, Adamantidis A, et al. The role of melanin-concentrating hormone in conditioned reward learning. Eur J Neurosci. 2012;36:3126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Eiler WJ, Chen Y, Slieker LJ, Ardayfio PA, Statnick MA, Witkin JM. Consequences of constitutive deletion of melanin-concentrating hormone-1 receptors for feeding and foraging behaviors of mice. Behav Brain Res. 2017;316:271–8. [DOI] [PubMed] [Google Scholar]
- [177].Sita LV, Diniz GB, Canteras NS, Xavier GF, Bittencourt JC. Effect of intrahippocampala dministration of anti-melanin-concentrating hormone on spatial food-seeking behavior in rats. Peptides. 2016;76:130–8. [DOI] [PubMed] [Google Scholar]
- [178].Cason AM, Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl). 2013;228:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Karlsson C, Zook M, Ciccocioppo R, Gehlert DR, Thorsell A, Heilig M, et al. Melanin-concentrating hormone receptor 1 (MCH1-R) antagonism: reduced appetite for calories and suppression of addictive-like behaviors. Pharmacol Biochem Behav. 2012;102:400–6. [DOI] [PubMed] [Google Scholar]
- [180].Wiskerke J, James MH, Aston-Jones G. The orexin-1 receptor antagonist SB-334867 reduces motivation, but not inhibitory control, in a rat stop signal task. Brain Res. 2020;1731:146222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, et al. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry. 2018;23:1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Noble EE, Wang Z, Liu CM, Davis EA, Suarez AN, Stein LM, et al. Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat Commun. 2019;10:4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–7. [DOI] [PubMed] [Google Scholar]
- [184].Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–12. [DOI] [PubMed] [Google Scholar]
- [185].Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. American Journal of Physiology-Endocrinology and Metabolism. 2003;284:E583-E8. [DOI] [PubMed] [Google Scholar]
- [186].Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–7. [DOI] [PubMed] [Google Scholar]
- [187].Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, et al. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology. 2012;37:1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology. 2014;85:451–60. [DOI] [PubMed] [Google Scholar]
- [189].Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, et al. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology. 2009;150:1202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Parise EM, Lilly N, Kay K, Dossat AM, Seth R, Overton JM, et al. Evidence for the role of hindbrain orexin-1 receptors in the control of meal size. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Li AJ, Wang Q, Davis H, Wang R, Ritter S. Orexin-A enhances feeding in male rats by activating hindbrain catecholamine neurons. Am J Physiol Regul Integr Comp Physiol. 2015;309:R358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Baird JP, Rios C, Loveland JL, Beck J, Tran A, Mahoney CE. Effects of hindbrain melanin-concentrating hormone and neuropeptide Y administration on licking for water, saccharin, and sucrose solutions. Am J Physiol Regul Integr Comp Physiol. 2008;294:R329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. [DOI] [PubMed] [Google Scholar]
- [194].Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–8. [DOI] [PubMed] [Google Scholar]
- [195].Cole S, Mayer HS, Petrovich GD. Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Sci Rep. 2015;5:16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Sherwood A, Holland PC, Adamantidis A, Johnson AW. Deletion of Melanin Concentrating Hormone Receptor-1 disrupts overeating in the presence of food cues. Physiol Behav. 2015;152:402–7. [DOI] [PubMed] [Google Scholar]
- [197].Petrovich GD, Hobin MP, Reppucci CJ. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].González JA, Jensen LT, Iordanidou P, Strom M, Fugger L, Burdakov D. Inhibitory Interplay between Orexin Neurons and Eating. Curr Biol. 2016;26:2486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Hassani OK, Krause MR, Mainville L, Cordova CA, Jones BE. Orexin Neurons Respond Differentially to Auditory Cues Associated with Appetitive versus Aversive Outcomes. J Neurosci. 2016;36:1747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Dilsiz P, Aklan I, Sayar Atasoy N, Yavuz Y, Filiz G, Koksalar F, et al. MCH Neuron Activity Is Sufficient for Reward and Reinforces Feeding. Neuroendocrinology. 2020;110:258–70. [DOI] [PubMed] [Google Scholar]
- [201].de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41. [DOI] [PubMed] [Google Scholar]
- [202].Bonnavion P, Mickelsen L, Fujita A, de Lecea L, Jackson A. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. Journal of Physiology-London. 2016;594:6443–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Abizaid A, Liu Z, Andrews Z, Shanabrough M, Borok E, Elsworth J, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. Journal of Clinical Investigation. 2006;116:3229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Conductier G, Nahon JL, Guyon A. Dopamine depresses melanin concentrating hormone neuronal activity through multiple effects on α2-noradrenergic, D1 and D2-like dopaminergic receptors. Neuroscience. 2011;178:89–100. [DOI] [PubMed] [Google Scholar]
- [206].Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80. [DOI] [PubMed] [Google Scholar]
- [207].Huang H, Acuna-Goycolea C, Li Y, Cheng H, Obrietan K, van den Pol A. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: Implications for cannabinoid actions on food intake and cognitive arousal. Journal of Neuroscience. 2007;27:4870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–9. [DOI] [PubMed] [Google Scholar]
- [209].Lopez CA, Guesdon B, Baraboi ED, Roffarello BM, Hétu M, Richard D. Involvement of the opioid system in the orexigenic and hedonic effects of melanin-concentrating hormone. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1105–11. [DOI] [PubMed] [Google Scholar]
- [210].Linehan V, Fang LZ, Parsons MP, Hirasawa M. High-fat diet induces time-dependent synaptic plasticity of the lateral hypothalamus. Mol Metab. 2020;36:100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2015;58:36–45. [DOI] [PubMed] [Google Scholar]
- [212].Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, et al. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 2015;35:7964–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Yamamoto Y, Ueta Y, Yamashita H, Asayama K, Shirahata A. Expressions of the prepro-orexin and orexin type 2 receptor genes in obese rat. Peptides. 2002;23:1689–96. [DOI] [PubMed] [Google Scholar]
- [214].Elliott JC, Harrold JA, Brodin P, Enquist K, Bäckman A, Byström M, et al. Increases in melanin-concentrating hormone and MCH receptor levels in the hypothalamus of dietary-obese rats. Brain Res Mol Brain Res. 2004;128:150–9. [DOI] [PubMed] [Google Scholar]
- [215].Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–105. [DOI] [PubMed] [Google Scholar]
- [216].Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002;26:1289–95. [DOI] [PubMed] [Google Scholar]
- [217].Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci. 2006;26:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Peyron C, Sapin E, Leger L, Luppi PH, Fort P. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides. 2009;30:2052–9. [DOI] [PubMed] [Google Scholar]
- [219].Karlsson C, Zook M, Ciccocioppo R, Gehlert D, Thorsell A, Heilig M, et al. Melanin-concentrating hormone receptor 1 (MCH1-R) antagonism: Reduced appetite for calories and suppression of addictive-like behaviors. Pharmacology Biochemistry and Behavior. 2012;102:400–6. [DOI] [PubMed] [Google Scholar]
- [220].Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, & Civelli O The melanin-concentrating hormone system modulates cocaine reward. Proceedings of the National Academy of Sciences, 2009; 106(16), 6772–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Mattson BJ, & Morrell JI Preference for cocaine-versus pup-associated cues differentially activates neurons expressing either Fos or cocaine-and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience, 2005; 135(2), 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Terrill SJ, Subramanian K, Lan R, Liu CM, Cortella AM, Noble EE, & Kanoski SE Nucleus accumbens melanin-concentrating hormone signaling promotes feeding in a sex-specific manner. Neuropharmacology, 2020; 108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Sclafani A, Adamantidis A, & Ackroff K MCH receptor deletion does not impair glucose-conditioned flavor preferences in mice. Physiology & behavior, 2020; 163, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]