Abstract
Objectives:
To assess trends, predictors, and perinatal outcomes of ovarian hyperstimulation syndrome (OHSS) associated with in vitro fertilization (IVF) cycles in the United States.
Design:
Retrospective cohort study using National Assisted Reproductive Technology Surveillance System (NASS) data.
Setting:
Not applicable.
Patient(s):
Fresh autologous and embryo-banking cycles performed from 2000 to 2015.
Interventions(s):
None.
Main Outcome Measure(s):
OHSS, first-trimester loss, second-trimester loss, stillbirth, low birth weight, and preterm delivery.
Result(s):
The proportion of IVF cycles complicated by OHSS increased from 10.0 to 14.3 cases per 1,000 from 2000 to 2006, and decreased to 5.3 per 1,000 from 2006 to 2015. The risk of OHSS was highest for cycles with more than 30 oocytes retrieved (adjusted risk ratio [aRR] 3.85). OHSS was associated with a diagnosis of ovulatory disorder (aRR 2.61), tubal factor (aRR 1.14), uterine factor (aRR 1.17) and cycles resulting in pregnancy (aRR 3.12). In singleton pregnancies, OHSS was associated with increased risk of low birth weight (aRR 1.29) and preterm delivery (aRR 1.32). In twin pregnancies, OHSS was associated with an increased risk of second-trimester loss (aRR 1.81), low birth weight (aRR 1.06), and preterm delivery (aRR 1.16).
Conclusion(s):
Modifiable predictive factors for OHSS include number of oocytes retrieved, pregnancy following fresh embryo transfer, and the type of medication used for pituitary suppression during controlled ovarian hyperstimulation. Patients affected by OHSS had a higher risk of preterm delivery and low birth weight. Clinicians should take measures to reduce the risk of OHSS whenever possible.
Keywords: Ovarian hyperstimulation syndrome, OHSS, safety of ART, IVF
Abstract
Objetivo:
investigar las tendencias, predictores y resultados perinatales del síndrome de hiperestimulación ovárica (OHSS) asociado con ciclos de fecundación in vitro (IVF) en los Estados Unidos.
Diseño:
Estudio retrospectivo de cohortes utilizando datos del sistema nacional de vigilancia de técnicas de reproducción asistida (NASS).
Lugar:
No aplica.
Pacientes:
Ciclos en fresco autólogos y de embriones de banco realizados desde 2000 a 2015.
Intervención (es):
Ninguna.
Principales medidas de resultado(s):
OHSS, pérdidas del primer trimestre, pérdidas del segundo trimestre, mortinatos, bajo peso al nacer y parto pretérmino.
Resultados:
La proporción de ciclos de IVF complicados con OHSS incrementó de 10.0 a 14.3 casos por 1000 desde 2000 a 2006, y disminuyó a 5.3 por 1000 desde 2006 a 2015. El riesgo de OHSS fue más alto para ciclos con más de 30 ovocitos recuperados (relación de riesgo ajustado[aRR] 3.85). El OHSS estuvo asociado con un diagnóstico de desorden ovulatorio (aRR 2.61), factor tubárico (aRR 1.14), factor uterino (aRR 1.17), y ciclos que resultaron en gestación (aRR 3.12). En gestaciones únicas, el OHSS estuvo asociado con riesgo incrementado de bajo peso al nacer (aRR 1.29) y parto pretérmino (aRR 1.32). En gemelares, el OHSS estuvo asociado con riesgo incrementado de pérdida en el segundo trimestre (aRR 1.81), bajo peso al nacer (aRR 1.06) y parto pretérmino (aRR 1.16).
Conclusión (es):
Los factores predictivos modificables para OHSS incluyen el número de ovocitos recuperados, la gestación posterior a transferencia de embriones en fresco, y el tipo de medicación empleada para la supresión hipofisiaria durante la hiperestimulación ovárica controlada. Los pacientes afectados de OHSS tuvieron mayor riesgo de parto pretérmino y bajo peso al nacer. Los clínicos deberían tomar medidas para reducir el riesgo de OHSS siempre que sea posible.
Ovarian hyperstimulation syndrome (OHSS) is a potentially life-threatening iatrogenic complication of controlled ovarian hyperstimulation (COH) performed for the treatment of infertility. OHSS is typically classified as mild, moderate, or severe, based on clinical and laboratory findings (1). During the period under study here (2000–2015), moderate OHSS was defined in the National Assisted Reproductive Technology Surveillance System (NASS) as characterized by ‘‘abdominal distention and discomfort as well as nausea, vomiting and/or diarrhea, ovaries enlarged 5–12 cm, and ultrasound evidence of ascites.’’ The definition of severe OHSS included the features of moderate OHSS with ‘‘clinical evidence of ascites and/or hydrothorax, changes in blood volume, increased blood viscosity due to hemoconcentration, coagulation abnormalities, and diminished kidney perfusion and function.’’ These definitions of OHSS generally agree with those put forth in other published studies (2). In patients undergoing in vitro fertilization (IVF), the incidences of moderate and severe OHSS have been estimated to be 3%–6% and 0.1%–2%, respectively (3). In recent years, many strategies have been developed to reduce the incidence of moderate and severe OHSS in patients undergoing IVF (4–7), which may have had an effect on the incidence of OHSS.
Any woman undergoing COH is at risk of developing OHSS, including oocyte donors, but some characteristics may put women at more or less risk. Pretreatment risk factors for OHSS include previous diagnosis of polycystic ovary syndrome (PCOS) (3, 8, 9), a history of OHSS (10), high antimüllerian hormone level (11), young age (8, 12, 13), and low body mass index (BMI) (13). During and after COH, high serum E2 concentrations (14), retrieval of more than 30 oocytes (14), pregnancy following fresh embryo transfer (8), and large number and size of follicles in the ovary (8) are associated with an increased risk of OHSS. The studies in which these risk factors were identified vary substantially in design, sample size, and diagnostic criteria, and many were performed at a time when COH protocols for IVF were substantially different from those used at present. Specific measures aimed at reducing the incidence of OHSS include use of GnRH antagonists in place of GnRH agonists during IVF, use of GnRH agonists for triggering final oocyte maturation, use of embryo banking, and use of cabergoline at the end of COH (4–7).
OHSS occurs only in women who are exposed to LH-receptor stimulation. With rare exceptions, OHSS occurs only after exposure to hCG, which has a significantly longer half-life than LH, and thus causes extensive luteinization in the granulosa cells within the corpus luteum (15). This, in turn, leads to production of vasoactive substances, of which vascular endothelial growth factor (VEGF) is the most important in causing increased vascular permeability and hemoconcentration (16, 17). Interestingly, not all women who respond vigorously to COH develop OHSS. This may be, at least in part, attributable to heterogeneity in the population regarding production of soluble proteins that bind to VEGF and regulate its activity. In a prospective study of women undergoing IVF who were at high risk of developing OHSS, women who developed OHSS produced less soluble VEGF receptor 1 than women who did not develop OHSS (18). In addition to differences in production of soluble regulators of VEGF activity, susceptibility to OHSS may also be mediated by differences in sensitivity to gonadotropins. OHSS has also been reported in the absence of gonadotropins, such as in the case of women with mutations in the FSH receptor that allow hCG to bind avidly to it (17). Thus, although it is possible to identify risk factors for OHSS, it is clear that a woman’s individual risk of OHSS may be determined by factors other than how vigorous her response to COH is.
There is limited and conflicting information regarding pregnancy outcomes in patients who develop OHSS. A single study found that patients who became pregnant and had OHSS experienced higher than expected rates of early pregnancy loss, preterm delivery, low birth weight, pregnancy-induced hypertension, and gestational diabetes mellitus (19). A subsequent study identified a higher risk of preclinical miscarriage (a group that included biochemical pregnancies, anembryonic pregnancies, missed abortions, or spontaneous abortions) in patients with early but not late-onset OHSS (20). A retrospective controlled study of 165 ongoing pregnancies after IVF revealed no difference in the incidence of any adverse obstetric outcome in either the group that experienced OHSS or appropriately matched control subjects (21). A study using data obtained from the Society for Assisted Reproductive Technology database including ~1,500 cycles complicated by OHSS revealed an increased risk of low birth weight (22). More information is therefore needed regarding pregnancy outcomes following IVF cycles complicated by ovarian hyperstimulation.
The present study aimed to quantify national trends in the rate of OHSS and GnRH antagonist cycle use, to evaluate predictive factors for OHSS, and to examine pregnancy outcomes in cycles affected by OHSS with the use of national assisted reproductive technology (ART) surveillance data.
METHODS
Data used in this study were obtained from the NASS, which is maintained by the Centers for Disease Control and Prevention. Reporting to NASS is federally mandated (Fertility Clinic Success Rate and Certification Act of 1992, Public Law No. 102–493, October 24, 1992), and therefore it includes data from an estimated 97% of IVF cycles performed in the United States (23). NASS collects information on patient characteristics, medical and obstetric history, infertility diagnosis, treatment parameters, and outcomes of IVF cycles performed in the United States. Annual data validation, during which data for selected variables are compared with information recorded in medical records, was performed in ~7%–10% of reporting clinics during the period under study (2000–2015). In general, discrepancy rates for 2015 ART cycle data were low (<4%) (24).
Inclusion and exclusion criteria for cycles varied based on the type of analysis being performed. To examine trends in the rate of OHSS, we included all fresh cycles (autologous and donor) and embryo-banking cycles combined that were performed from 2000 to 2015 (n = 1,833,430 cycles). Data regarding trends in GnRH antagonist use were limited to cycles performed from 2004 to 2015 in which embryo banking was not performed, because GnRH antagonist use was not collected before 2004 and is not collected for embryo-banking cycles. We calculated the rates of moderate OHSS, severe OHSS, any OHSS, and GnRH antagonist use per 1,000 IVF cycles. We calculated the percentages of OHSS among fresh autologous and embryo-banking cycles over the entire study period. We used SAS version 9.3 to perform simple linear regression to determine the statistical significance of changes in trends of OHSS diagnosis and GnRH antagonist use over time.
To reflect more recent practice patterns, for all subsequent analyses we included aggregated data from fresh autologous and embryo-banking cycles performed from 2010 to 2015. We compared the distribution of patient and treatment characteristics for cycles complicated by moderate or severe OHSS, complicated by any OHSS, and without OHSS. Among fresh autologous and embryo-banking cycles, we compared the distribution of patient age (years) at oocyte retrieval, race/ethnicity, BMI at time of initiation of IVF cycle, number of previous pregnancies conceived by any means, IVF or otherwise, miscarriages, preterm or full-term births, infertility diagnosis, hospitalization for a complication, and cancellation before retrieval for cycles with and without OHSS. Use of GnRH receptor antagonist or agonist for pituitary suppression was compared for fresh autologous cycles only, because this information is not reported for embryo-banking cycles. Comparisons of number of oocytes retrieved were restricted to fresh autologous and embryo-banking cycles in which oocyte retrieval was performed. The proportion of cycles in which a transfer was attempted was calculated only for fresh autologous cycles with an oocyte retrieval. Among fresh autologous cycles with transfer of at least one embryo, we compared distributions of number of supernumerary embryos cryopreserved, maximum number of fetal hearts before reduction (if any), positive hCG (including intrauterine, biochemical, and ectopic pregnancies), live birth, and plurality for cycles with and without OHSS. Pearson chi-square test was performed to assess homogeneity for distributions of each maternal and treatment characteristic for cycles with any type of OHSS versus those without OHSS.
We used multivariable predicted marginal proportions from logistic regression models to assess predictive factors for OHSS in two sample groups. One group included data from all fresh autologous IVF cycles that resulted in embryo transfer. In this group, multivariable logistic regression was used to calculate adjusted relative risk (aRR) of developing OHSS for the following factors: number of oocytes retrieved, infertility diagnosis, BMI, age, pregnancy following fresh embryo transfer, use of a GnRH antagonist, and history of one or more previous pregnancies conceived by any means, IVF or otherwise. The second group included all noncancelled fresh autologous and embryo-banking cycles. The same variables were included in this second model, with the exception of GnRH antagonist use, pregnancy, and the number of oocytes retrieved per cycle. These variables were excluded because GnRH antagonist use is not reported in embryo-banking cycles, pregnancy is not possible after an embryo-banking cycle, and the number of oocytes retrieved was not reported for 7% of embryo-banking cycles.
To assess pregnancy outcomes associated with OHSS, we restricted the population to fresh autologous IVF cycles resulting in pregnancy and examined outcomes separately for singleton pregnancies and pregnancies with multiple fetuses. The outcomes that we examined were first-trimester pregnancy loss (<14 weeks), second-trimester pregnancy loss (14–20 weeks), stillbirth after 20 weeks of gestation, low birth weight (<2,500 g) for either infant in a multiple pregnancy, and preterm delivery (delivery at <37 completed weeks of gestation) among live births. We calculated the absolute number and percentage of each pregnancy outcome in cycles with OHSS and cycles without OHSS. We used multivariable predicted marginal proportions from logistic regression models to calculate the aRR for each pregnancy outcome in cycles with OHSS compared with cycles without OHSS, adjusting for known confounders. For first- and second-trimester pregnancy losses, we adjusted for maternal age, race/ethnicity, number of previous miscarriages, BMI, and uterine-factor infertility. For low birth weight and preterm delivery, we adjusted for age, race, number of previous preterm deliveries, BMI, and uterine-factor infertility. For stillbirth, we adjusted for maternal age, race, BMI, uterine-factor infertility, and number of previous pregnancies (25–27). We also reported the average birth weight in grams and gestational age in weeks for singleton and multiple gestations in pregnancies in which OHSS occurred.
Approximately 19% of all cycles were missing information regarding BMI, and 25% of cycles did not include data on race/ethnicity. For this reason, we used multiple imputation (using the SUDAAN HOTDECK procedure under the assumption of missing at random) to derive missing BMI and race/ethnicity data to complete all regression analyses. The assumption that BMI data are missing at random is reasonable because, although reporting of BMI and race/ethnicity likely varies from clinic to clinic, there is no reason to suspect that missingness of the data varies systematically within a specific clinic. Use of multiple imputation with the use of the HOTDECK procedure for data contained within the NASS dataset has been reported previously, and studies of other large datasets support the validity of our assumption that data are missing at random (28–30). Five multiply imputed datasets were created to compute within- and between-variations of imputed datasets in parameter estimates, and the SUDAAN RLOGISTIC procedure was applied to fit logistic (28–30) regression models with the use of imputed datasets. Variables used for BMI imputation included state where the cycle was performed, patient race, reason for ART, gestational age, parity, patient age, number of previous preterm births (<37 weeks), number of previous spontaneous abortions (<20 weeks), and total number of embryos transferred.
To further explore whether embryo banking cycles were associated with lower rates of OHSS, we stratified infertility clinics into quartiles based on the percentage of embryo banking cycles that they performed over the study period. We then calculated the rate of OHSS within each quartile and assessed trends in the rate of OHSS by quartile using simple linear regression. SAS version 9.3 and SUDAAN version 11.0 were used for the analyses. SUDAAN was used for ease of analysis of clustered imputed data. Statistical significance was determined using a two-side alpha of 0.05. This study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention (IRB protocol no. 2238).
RESULTS
The rate of any OHSS occurring in women undergoing fresh autologous and embryo-banking cycles increased significantly from 9.96 to 14.34 cases per 1,000 from 2000 to 2006 (P trend < .01) and then decreased significantly to 5.27 cases per 1,000 cycles from 2006 to 2015 (P trend < .0001; Fig. 1). Similar trends were seen in the rates of moderate and severe OHSS. The rate of moderate OHSS increased significantly from 8.10 to 10.51 cases per 1,000 cycles from 2000 to 2006 (P trend < .05) and then decreased significantly to 4.22 cases per 1,000 cycles from 2006 to 2015 (P trend < .001). The rate of severe OHSS increased significantly from 1.87 to 3.83 cases per 1,000 cycles from 2000 to 2006 (P trend < .0001) and then decreased significantly to 1.06 cases per 1,000 cycles from 2006 to 2015 (P trend < .0001). The rate of GnRH antagonist use in IVF cycles increased significantly from ~279 per 1,000 cycles in 2004 to 634 per 1,000 cycles in 2015 (P< .0001; Fig. 1).
FIGURE 1.
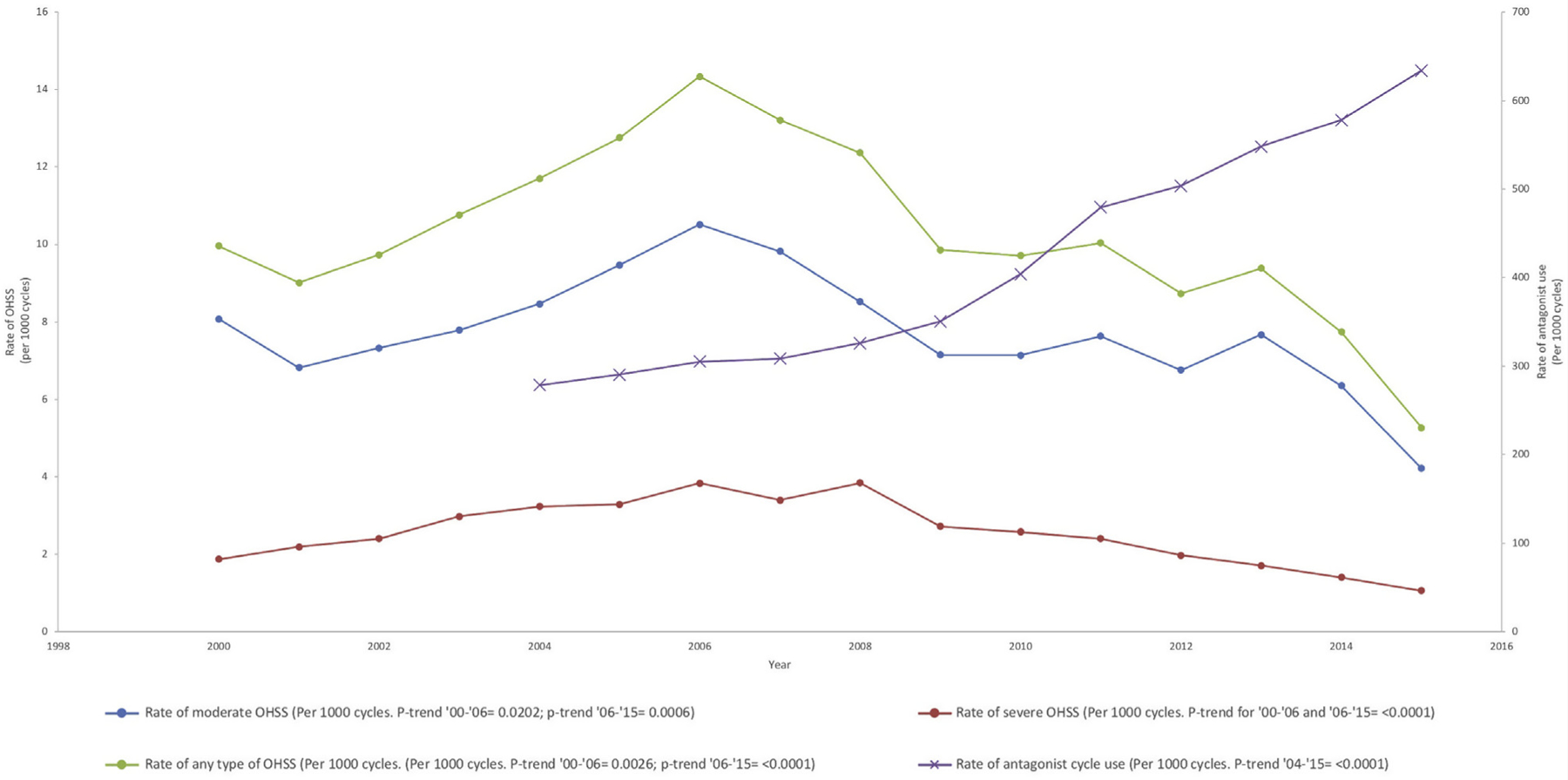
Incidence of moderate and severe OHSS and antagonist cycle use in all fresh cycles (autologous and donor) and embryo-banking cycles from 2000 to 2015. OHSS = ovarian hyperstimulation syndrome.
OHSS was more prevalent, and a higher proportion of IVF cycles that resulted in any type of OHSS occurred among younger women and those who had fewer previous pregnancies, miscarriages, and full-term births than fresh autologous and embryo-banking cycles uncomplicated by OHSS (Table 1; Supplemental Table 1 [available online at www.fertstert.org]). GnRH agonists and GnRH antagonists were used in a greater proportion of IVF cycles that resulted in OHSS than in fresh autologous and embryo-banking cycles that did not result in OHSS. Cycle cancellation occurred in a lower proportion of cycles that resulted in OHSS than in cycles that did not result in OHSS. OHSS was more prevalent in IVF cycles that had a diagnosis of ovulatory disorder, male factor, and diminished ovarian reserve. Twenty or more oocytes were retrieved in a greater proportion of cycles that resulted in OHSS than in cycles that did not result in OHSS. Among cycles in which OHSS occurred, hospitalization was more common, the number of supernumerary embryos that were cryopreserved was higher, pregnancy was more likely to occur, and multiple gestation was more common than in cycles uncomplicated by OHSS (Table 1; Supplemental Table 1).
TABLE 1.
Ovarian hyperstimulation syndrome by maternal characteristics in fresh autologous and embryo banking cycles from 2010 to 2015.
| Cases of any OHSS | Cases of moderate OHSS | Cases of severe OHSS | IVF cycles without OHSS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % | n | % | P value |
| Age at oocyte retrieval,a y | |||||||||
| <30 | 1,680 | 25.83 | 1,334 | 26.20 | 346 | 24.47 | 76,798 | 10.69 | |
| 30–34 | 2,908 | 44.70 | 2,242 | 44.04 | 666 | 47.10 | 202,348 | 28.16 | |
| 35–37 | 1,186 | 18.23 | 929 | 18.25 | 257 | 18.18 | 150,030 | 20.88 | |
| 38–40 | 568 | 8.73 | 459 | 9.02 | 109 | 7.71 | 149,691 | 20.83 | |
| ≥41 | 163 | 2.51 | 127 | 2.49 | 36 | 2.55 | 139,691 | 19.44 | < .0001 |
| Race/ethnicitya | |||||||||
| Non-Hispanic White | 3,404 | 52.33 | 2,657 | 52.19 | 747 | 52.83 | 300,437 | 41.81 | |
| Non-Hispanic Black | 424 | 6.52 | 323 | 6.34 | 101 | 7.14 | 33,008 | 4.59 | |
| Asian/Pacific Islander | 582 | 8.95 | 468 | 9.19 | 114 | 8.06 | 78,531 | 10.93 | |
| Hispanic | 462 | 7.10 | 371 | 7.29 | 91 | 6.44 | 39,848 | 5.55 | |
| Other | 6 | 0.09 | 6 | 0.12 | 0 | 0 | 1,179 | 0.16 | < .0001 |
| Missing | 1,627 | 25.01 | 1,266 | 24.87 | 361 | 25.53 | 265,546 | 36.96 | |
| No. of previous pregnanciesa | |||||||||
| 0 | 3,693 | 56.78 | 2,890 | 56.78 | 803 | 56.79 | 331,320 | 46.28 | |
| 1 | 1,457 | 22.40 | 1,112 | 21.85 | 345 | 24.40 | 179,009 | 25.01 | |
| ≥2 | 1,354 | 20.82 | 1,088 | 21.38 | 266 | 18.81 | 205,553 | 28.71 | < .0001 |
| No. of previous miscarriagesa | |||||||||
| 0 | 5,002 | 76.97 | 3,902 | 76.72 | 1,100 | 77.85 | 498,552 | 69.80 | |
| 1 | 980 | 15.08 | 763 | 15.00 | 217 | 15.36 | 134,083 | 18.77 | |
| ≥2 | 517 | 7.96 | 421 | 8.28 | 96 | 6.79 | 81,583 | 11.42 | < .0001 |
| No. of previous preterm birthsa | |||||||||
| 0 | 6,279 | 96.75 | 4,911 | 96.71 | 1,368 | 96.88 | 690,438 | 96.88 | |
| 1 | 189 | 2.91 | 149 | 2.93 | 40 | 2.83 | 19,737 | 2.77 | |
| ≥2 | 22 | 0.34 | 18 | 0.35 | 4 | 0.28 | 2,509 | 0.35 | .7727 |
| No. of previous full-term birthsa | |||||||||
| 0 | 5,236 | 80.58 | 4,072 | 80.09 | 1,164 | 82.32 | 533,887 | 74.71 | |
| 1 | 948 | 14.59 | 757 | 14.89 | 191 | 13.51 | 133,915 | 18.74 | |
| ≥2 | 314 | 4.83 | 255 | 5.02 | 59 | 4.17 | 46,772 | 6.55 | <.0001 |
| Body mass index,a kg/m2 | |||||||||
| <18.5 | 174 | 2.67 | 138 | 2.71 | 36 | 2.55 | 16,947 | 2.36 | |
| 18.5–24.9 | 3,356 | 51.59 | 2,568 | 50.44 | 788 | 55.73 | 321,666 | 44.77 | |
| 25.0–29.9 | 1,294 | 19.89 | 1,030 | 22.88 | 264 | 18.67 | 136,024 | 18.93 | |
| ≥30.0 | 950 | 14.60 | 766 | 15.05 | 184 | 13.01 | 104,549 | 14.55 | < .0001 |
| Missing | 731 | 11.24 | 589 | 11.57 | 142 | 10.04 | 139,363 | 19.40 | |
| Infertility diagnosisa | |||||||||
| Ovulation disorders | 2,424 | 37.26 | 1,838 | 36.10 | 586 | 41.44 | 89,358 | 12.44 | < .0001 |
| Tubal factor | 940 | 14.45 | 762 | 14.97 | 178 | 12.59 | 95,488 | 13.29 | .006 |
| Male factor | 2,525 | 38.82 | 1,983 | 38.95 | 542 | 38.33 | 193,701 | 33.26 | < .0001 |
| Endometriosis | 606 | 9.32 | 471 | 9.25 | 135 | 9.55 | 63,790 | 8.88 | .2161 |
| Diminished ovarian reserve | 314 | 4.83 | 251 | 4.93 | 63 | 4.46 | 220,975 | 30.75 | < .0001 |
| Uterine factor | 336 | 5.17 | 283 | 5.56 | 53 | 3.75 | 39,140 | 5.45 | .3186 |
| Unexplained | 753 | 11.58 | 572 | 11.24 | 181 | 12.80 | 91,176 | 12.69 | .0072 |
| Complicationsa | |||||||||
| Hospitalization | 894 | 13.74 | 401 | 7.88 | 493 | 34.87 | 346 | 0.05 | < .0001 |
| Cancellation of cycle before retrieval | 146 | 0.22 | 106 | 0.16 | 40 | 0.06 | 6,359 | 0.96 | < .0001 |
| Treatment cycleb | |||||||||
| GnRH-receptor antagonist suppression | 2,607 | 45.02 | 1,999 | 44.76 | 608 | 45.89 | 298,614 | 52.06 | <.0001 |
| GnRH-receptor agonist Suppression | 2,746 | 30.01 | 2,120 | 47.47 | 626 | 47.25 | 172,153 | 47.42 | <.0001 |
| Agonist flare | 262 | 4.52 | 221 | 4.95 | 41 | 3.09 | 70,295 | 12.25 | <.0001 |
| No. of oocytes retrievedc | |||||||||
| 0–10 | 525 | 8.27 | 428 | 8.60 | 97 | 7.07 | 323,542 | 49.78 | |
| 11–15 | 1,059 | 16.67 | 836 | 16.79 | 223 | 16.25 | 149,642 | 23.02 | |
| 16–30 | 3,354 | 52.81 | 2,650 | 53.22 | 704 | 51.31 | 156,740 | 24.12 | |
| >30 | 1,413 | 22.25 | 1,065 | 21.30 | 348 | 25.36 | 20,036 | 3.08 | < .0001 |
| Transfer not attemptedd | |||||||||
| No transfer | 2,916 | 51.61 | 2,366 | 54.20 | 550 | 42.80 | 70,659 | 13.75 | <.0001 |
| No. of supernumerary embryos cryopreservede | |||||||||
| 0 | 690 | 25.28 | 527 | 26.43 | 163 | 22.18 | 254,940 | 57.81 | |
| 1–2 | 532 | 19.49 | 375 | 18.81 | 157 | 21.36 | 80,941 | 18.35 | |
| 3–4 | 521 | 19.09 | 370 | 18.56 | 151 | 20.54 | 51,414 | 11.66 | |
| ≥5 | 986 | 36.13 | 722 | 36.21 | 264 | 35.92 | 53,709 | 12.18 | < .0001 |
| Max. no. of fetal hearts before reductione | |||||||||
| Not pregnant | 551 | 20.86 | 489 | 25.26 | 62 | 8.79 | 244,842 | 57.18 | |
| 1 | 1,149 | 43.51 | 811 | 41.89 | 338 | 47.94 | 128,879 | 30.10 | |
| 2 | 881 | 33.36 | 594 | 30.68 | 287 | 40.71 | 50,651 | 11.83 | |
| ≥3 | 60 | 2.27 | 42 | 2.17 | 18 | 2.55 | 3,840 | 0.90 | < .0001 |
| Live birthe | 1,902 | 69.62 | 1,333 | 66.75 | 569 | 77.41 | 161,621 | 36.47 | < .0001 |
| Pluralitye | |||||||||
| Singleton | 1,160 | 60.99 | 822 | 61.67 | 338 | 59.40 | 118,321 | 73.21 | |
| Twin | 715 | 37.59 | 491 | 36.83 | 224 | 39.37 | 41,604 | 25.74 | |
| Triplet or higher order | 27 | 1.42 | 20 | 1.50 | 7 | 1.23 | 1,696 | 1.05 | < .0001 |
| Positive hCGe,f | 2,306 | 84.53 | 1,617 | 80.97 | 689 | 93.74 | 240,476 | 54.32 | < .0001 |
Note: P value between IVF cycles with any OHSS and those without OHSS. OHSS = ovarian hyperstimulation syndrome.
Among fresh autologous and embryo-banking cycles.
Among fresh autologous cycles only (excluding embryo-banking cycles).
Among fresh autologous and embryo-banking cycles in which an oocyte retrieval was performed.
Among fresh autologous cycles in which a retrieval was performed (excluding embryo-banking cycles).
Among fresh autologous cycles in which a retrieval was performed and at least one embryo was transferred (excluding embryo-banking cycles).
Includes intrauterine, biochemical, ectopic, and heterotopic pregnancy.
Among all fresh autologous and embryo-banking IVF cycles, the risk of OHSS increased with decreasing age (Table 2), with patients under the age of 30 years having the highest risk of OHSS (aRR 6.47, 95% confidence interval [CI] 5.46–7.66) compared with cycles in which the patient was ≥41 years old. Non-Hispanic black (aRR 1.37, 95% CI 1.25–1.49) and Hispanic (aRR 1.11, 95% CI 1.02–1.21) women were at an increased risk of OHSS compared with non-Hispanic white women. There was a significantly lower risk of OHSS in women of Asian/Pacific Islander race (aRR 0.86, 95% CI 0.79–0.92), of other race (aRR 0.49, 95% CI 0.25–0.94), and with diminished ovarian reserve (aRR 0.27, 95% CI 0.24– 0.31). There was also a significantly lower risk of OHSS in women who were overweight (aRR 0.86, 95% CI 0.80–0.92) or obese (aRR 0.70, 95% CI 0.65–0.76) or had previous pregnancy conceived by any means, IVF or otherwise (aRR 0.84, 95% CI 0.80–0.89). The risk of OHSS was also significantly increased in cycles resulting in pregnancy (aRR 3.12, 95% CI 2.81–3.47) compared with cycles that did not result in a pregnancy. There was a significantly increased risk of OHSS in cycles among women with a diagnosis of ovulatory disorder, a diagnostic category which includes PCOS and any other type of ovulatory disorder (aRR 2.61, 95% CI 2.47–2.76), uterine-factor infertility (aRR 1.17, 95% CI 1.05–1.31), or tubal-factor infertility (aRR 1.14, 95% CI 1.07–1.23) compared with cycles among women without these diagnoses, as well as in embryo-banking cycles (aRR 0.66, 95% CI 0.61–0.72) compared with women without these factors (Table 2). Among women undergoing fresh autologous IVF cycles in which an egg retrieval occurred, the aRR of ovarian hyperstimulation increased as the number of oocytes retrieved increased and was highest for cycles in which more than 30 oocytes were retrieved (aRR 3.85, 95% CI 3.36–4.41) compared with cycles in which 11–15 oocytes were retrieved (Table 2). The risk of OHSS was reduced in cycles in which a GnRH antagonist instead of a GnRH agonist was used for pituitary suppression (aRR 0.79, 95% CI 0.73– 0.85).
TABLE 2.
Predictive factors for ovarian hyperstimulation syndrome in fresh autologous and embryo banking cycles from 2010 to 2015.
| Fresh autologous cycles in which embryo transfer occurred | Fresh autologous cycles and embryo-banking cyclesb,c | |||
|---|---|---|---|---|
| Predictive factor | RR (95% CI) | aRRa (95% CI) | RR (95% CI) | aRRa (95% CI) |
| Age, y | ||||
| <30 | 10.17 (7.99–12.95) | 2.27 (1.76–2.92) | 17.58 (14.58–20.24) | 6.47 (5.46–7.66) |
| 30–34 | 7.17 (5.67–9.08) | 2.02 (1.58–2.59) | 11.5 (9.79–13.51) | 4.97 (4.22–5.86) |
| 35–37 | 4.71 (3.68–6.01) | 1.79 (1.39–2.31) | 6.49 (5.49–7.67) | 3.43 (2.89–4.06) |
| 38–40 | 2.67 (2.06–3.46) | 1.49 (1.14–1.94) | 3.17 (2.65–3.78) | 2.12 (1.77–2.54) |
| ≥41 | Reference | Reference | Reference | Reference |
| Race | ||||
| Non-Hispanic White | Reference | Reference | Reference | Reference |
| Non-Hispanic Black | 1.1 (0.96–1.27) | 1.27 (1.10–1.46) | 1.18 (1.08–1.29) | 1.37 (1.25–1.49) |
| Asian/Pacific Islander | 0.88 (0.79–0.98) | 1.01 (0.91–1.14) | 0.72 (0.67–0.77) | 0.86 (0.79–0.92) |
| Hispanic | 1.08 (0.95–1.22) | 1.18 (1.04–1.34) | 1.06 (0.98–1.16) | 1.11 (1.02–1.21) |
| Other | 0.55 (0.21–1.48) | 0.63 (0.24–1.65) | 0.54 (0.28–1.04) | 0.49 (0.25–0.94) |
| Body mass index, kg/m2 | ||||
| <18.5 | 0.94 (0.74–1.20) | 0.95 (0.74–1.21) | 0.99 (0.86–1.15) | 0.99 (0.85–1.15) |
| 18.5–24.9 | Reference | Reference | Reference | Reference |
| 25.0–29.9 | 0.83 (0.76–0.92) | 0.82 (0.75–0.90) | 0.92 (0.86–0.99) | 0.86 (0.80–0.92) |
| ≥30.0 | 0.72 (0.65–0.81) | 0.66 (0.59–0.74) | 0.91 (0.85–0.98) | 0.70 (0.65–0.76) |
| Previous pregnancy | ||||
| Yes | 0.76 (0.70–0.82) | 0.91 (0.84–0.98) | 0.66 (0.63–0.70) | 0.84 (0.80–0.89) |
| No | Reference | Reference | Reference | Reference |
| Current pregnancyb | ||||
| Yes | 4.56 (4.11–5.06) | 3.12 (2.81–3.47) | N/A | N/A |
| No | Reference | Reference | Reference | Reference |
| Ovulatory disorder | ||||
| Yes | 3.18 (2.94–3.44) | 2.01 (1.84–2.19) | 3.96 (3.76–4.16) | 2.61 (2.47–2.76) |
| No | Reference | Reference | Reference | Reference |
| Uterine factor | ||||
| Yes | 1.18 (1.00–1.38) | 1.48 (1.26–1.73) | 0.95 (0.85–1.06) | 1.17 (1.05–1.31) |
| No | Reference | Reference | Reference | Reference |
| Tubal factor | ||||
| Yes | 1.04 (0.94–1.15) | 1.22 (1.10–1.36) | 1.11 (1.04–1.19) | 1.14 (1.07–1.23) |
| No | Reference | Reference | Reference | Reference |
| Endometriosis | ||||
| Yes | 1.14 (1.02–1.29) | 1.21 (1.07–1.36) | 1.08 (0.99–1.17) | 0.94 (0.87–1.03) |
| No | Reference | Reference | Reference | Reference |
| Diminished ovarian reserve | ||||
| Yes | 0.16 (0.13–0.19) | 0.47 (0.39–0.57) | 0.12 (0.11–0.14) | 0.27 (0.24–0.31) |
| No | Reference | Reference | Reference | Reference |
| Unexplained infertility | ||||
| Yes | 0.81 (0.73–0.91) | 0.97 (0.86–1.09) | 0.87 (0.81–0.94) | 0.92 (0.84–1.00) |
| No | Reference | Reference | Reference | Reference |
| No. of oocytes retrievedc | ||||
| 0–10 | 0.25 (0.22–0.29) | 0.37 (0.32–0.42) | N/A | N/A |
| 11–15 | Reference | Reference | N/A | N/A |
| 16–30 | 2.2 (2.01–2.42) | 1.90 (1.73–2.08) | N/A | N/A |
| >30 | 5.15 (4.51–5.87) | 3.85 (3.364.41) | N/A | N/A |
| GnRH antagonist cycle | ||||
| Yes | 0.74 (0.69–0.80) | 0.79 (0.73–0.85) | N/A | N/A |
| No | Reference | Reference | Reference | Reference |
| Embryo-banking cycled | ||||
| Yes | N/A | N/A | 0.45 (0.42–0.49) | 0.66 (0.61–0.72) |
| No | Reference | Reference | Reference | Reference |
Note: aRR = adjusted risk ratio; RR = risk ratio.
Risk ratios were adjusted for age, race, body mass index, previous pregnancy, current pregnancy, diagnosis of ovulatory disorder, uterine factor, tubal factor, endometriosis, diminished ovarian reserve, unexplained infertility, number of oocytes retrieved, GnRH antagonist use for pituitary suppression, and embryo-banking cycle.
Includes embryo-banking cycles, fresh autologous cycles where no transfer was attempted or where no embryos were transferred, and fresh autologous cycles where embryo transfer occurred. Because pregnancy is not possible after an embryo-banking cycle, current pregnancy is not in the model that includes embryo-banking cycles.
Excludes cancelled cycles in which a retrieval was not performed.
Because of missing data, aRR of ovarian hyperstimulation syndrome was not calculated for number of oocytes retrieved or use of a GnRH antagonist in embryo-banking cycles.
In fresh autologous IVF cycles resulting in a singleton pregnancy, OHSS was associated with a significantly higher risk of low birth weight (aRR 1.29, 95% CI 1.10–1.51) and preterm delivery (aRR 1.32, 95% CI 1.15–1.51) compared with cycles without OHSS that resulted in pregnancy (Table 3). The unadjusted mean birth weight for singleton pregnancies resulting from cycles in which OHSS occurred was 3,101 g (95% CI 3,065–3,138 g), and the unadjusted mean gestational age at which delivery occurred was 38.4 weeks (95% CI 38.2–38.6 weeks) of gestation. Among singleton pregnancies in which OHSS did not occur, the unadjusted mean birth weight was 3,230 g (95% CI 3,225–3,237 g) and the unadjusted mean gestational age at which delivery occurred was 38.7 weeks (95% CI 38.7–38.8 weeks). In twin pregnancies, OHSS was associated with a significantly increased risk of preterm delivery (aRR 1.16, 95% CI 1.10– 1.22) and low birth weight (aRR 1.06, 95% CI 1.02–1.12). In all multiple gestations, OHSS was associated with an increased risk of second-trimester loss (aRR 1.81, 95% CI 1.17–2.81). The unadjusted mean gestational age for twin pregnancies resulting from cycles in which OHSS occurred was 34.6 weeks (95% CI 34.3–34.9 weeks) compared with 35.6 weeks (95% CI 35.6–35.6 weeks) for twin pregnancies in which OHSS did not occur. The unadjusted mean birth weight for twin pregnancies resulting from cycles in which OHSS occurred was 2,206 g (95% CI 2,171–2,242 g) compared with 2,352 g (95% CI 2,347–2,356 g) for twin pregnancies resulting from cycles in which OHSS did not occur. All differences in gestational age at delivery and birth weight were tested for statistical significance with the use of the Welch-Sattherwaite t test and were found to be significantly different (P< .0001 in each case). There was no significant difference in the risk of first-trimester loss or stillbirth in IVF cycles affected by OHSS for singleton or multiple gestation.
TABLE 3.
Association between ovarian hyperstimulation syndrome and pregnancy outcomes among fresh autologous cycles, 2010 to 2015.
| Cycles without OHSS | Cycles with OHSS | ||||
|---|---|---|---|---|---|
| Outcome | n (%) | aRR | n (%) | RR | aRR |
| For singleton gestation | |||||
| First-trimester loss (<14 wk)a | 1,4678 (11.50) | Reference | 98 (8.58) | 0.75 (0.62–0.90) | 0.86 (0.71–1.04) |
| Second-trimester loss (14–20 wk)b | 1,362 (1.20) | Reference | 10 (0.96) | 0.80 (0.43–1.48) | 0.87 (0.47–1.61) |
| Stillbirthc | 704 (0.63) | Reference | 7 (0.68) | 1.07 (0.51–2.25) | 1.06 (0.51–2.22) |
| Birth weight <2,500gd | 1,0997 (9.45) | Reference | 139 (12.17) | 1.29 (1.10–1.51) | 1.29 (1.10–1.51) |
| Preterm deliveryd | 1,3916 (11.78) | Reference | 177 (15.30) | 1.3 (1.13–1.49) | 1.32 (1.15–1.51) |
| For multiple gestation | |||||
| First-trimester loss (<14 wk)e | 1,398 (2.59) | Reference | 16 (1.71) | 0.66 (0.41–1.08) | 0.71 (0.43–1.15) |
| Second-trimester loss (14–20 wk)f | 650 (1.23) | Reference | 20 (2.17) | 1.77 (1.14–2.74) | 1.81 (1.17– 2.81) |
| Stillbirthg | 421 (0.81) | Reference | 10 (1.11) | 1.38 (0.74–2.57) | 1.38 (0.74–2.57) |
| Birth weight <2,500gh | 2,8228 (69.33) | Reference | 515 (74.10) | 1.07 (1.02–1.12) | 1.06 (1.02–1.11) |
| Preterm deliveryh | 2,4225 (58.34) | Reference | 483 (67.84) | 1.16 (1.10–1.22) | 1.16 (1.10–1.22) |
Note: aRR = adjusted risk ratio; OHSS = ovarian hyperstimulation syndrome; RR = risk ratio.
Where number of fetal heart beats = 1. Risk ratios adjusted for age, number of prior miscarriages, body mass index, uterine-factor infertility, and race.
Where number of fetal heart beats = 1 and no first-trimester loss. Risk ratios adjusted for age, number of prior miscarriages, body mass index, uterine-factor infertility, and race.
Where number of fetal heart beats = 1 and no first- or second-trimester loss. Risk ratios adjusted for age, body mass index, uterine-factor infertility, primigravid status, and race.
For singleton pregnancies resulting in live birth. Risk ratios adjusted for age, number of previous preterm deliveries, body mass index, uterine-factor infertility, and race.
Where number of fetal heartbeats ≥2. Risk ratios adjusted for age, number of previous miscarriages, body mass index, uterine-factor infertility, and race.
Where number of fetal heartbeats ≥2 and no first-trimester loss. Risk ratios adjusted for age, number of prior miscarriages, body mass index, uterine factor infertility, and race.
Where number of fetal heartbeats ≥2 and no first- or second-trimester loss. Risk ratios adjusted for age, body mass index, uterine-factor infertility, primigravid status, and race.
For twin pregnancies resulting in live birth. Risk ratios adjusted for age, number of previous preterm deliveries, body mass index, uterine-factor infertility, and race.
Stratifying IVF clinics into quartiles by percentage of embryo-banking cycles that they perform revealed a trend of decreasing incidence of severe OHSS as the mean percentage of embryo-banking cycles increased (P=.04; Supplemental Table 2 [available online at www.fertstert.org]). Of note, among clinics in the highest quartile of embryo banking cycles, an average of 37.9% of IVF cycles performed were embryo-banking cycles.
DISCUSSION
The results of this national study indicate that the rate of OHSS increased from 2000 to 2006 and then decreased from 2006 to 2015. The rates of severe OHSS, which is associated with the highest risk of significant morbidity and mortality, were similar at the beginning and the end of the study period. Although the decreasing rate of OHSS from 2006 to 2015 is encouraging, there is considerable room for improvement in preventing OHSS. Determining national trends in the diagnosis of OHSS is a key step in increasing provider awareness of this serious but potentially preventable condition. The NASS dataset provided a rare opportunity to examine pregnancy outcomes associated with OHSS, for which only limited data are available in the published literature.
The cause of the increased rate of OHSS from 2000 to 2006 is unclear; it may reflect changes in medication regimens, changes in patient characteristics, and other factors. The decrease in the rate of OHSS from 2006 to 2015 may reflect the increasing use of GnRH antagonists, which is an effective and well-studied means of preventing OHSS. The use of other measures that reduce the risk of OHSS, many of which were first introduced into practice during the study period, have likely had an impact on the rate of OHSS as well, although it is not possible to study these with the use of the NASS dataset. These measures include use of a GnRH agonist trigger (31), cabergoline administration after COH (5), treatment with metformin before and during COH in patients with PCOS (32, 33), and cryopreserving all embryos to avoid pregnancy immediately after COH (6). These measures have, to varying degrees, been shown to be effective in preventing OHSS but they could not be assessed in the present study because, except for embryo cryopreservation, this information is not collected in the NASS. The rate of moderate OHSS and any type of OHSS declined steadily from 2006 to 2015, although the rates of severe OHSS at the beginning and at the end of the study period were similar. This suggests that although efforts by practitioners of ART to reduce the risk of OHSS during COH have been effective, there is room for improvement in reducing the risk of the form of OHSS that is responsible for the most severe clinical sequelae (33).
We observed a higher rate of OHSS in cycles performed in the United States with the use of the NASS dataset than the rate of OHSS in cycles performed in several European countries according to European IVF Monitoring (EIM) Consortium data. In the most recent report issued by the EIM, the incidence of moderate and severe OHSS in all cycles included in the EIM dataset was 0.3% (34). In 2000, 1.1% of all cycles included in the EIM dataset were affected by OHSS (35). In the most recent data from the International Committee for Monitoring Assisted Reproductive Technology (ICMART), which includes data from 2011, the incidence of OHSS was 0.5% (36). As with NASS data, data regarding OHSS from EIM and ICMART are not validated. Review of validated incidence data for diagnosis of OHSS from the Australian and New Zealand Assisted Reproduction Database revealed that in 2000 2.1% of women were hospitalized for OHSS, and in 2015 0.6% of cycles were affected by OHSS (37). Although NASS, EIM, and ICMART data regarding OHSS are not validated, this would be expected to yield an underestimate of the true incidence of OHSS. Accordingly, the differences in the incidence of OHSS from data obtained from NASS compared with international datasets were somewhat surprising. The differences in reported incidence may be due to several factors, including the subjective nature of diagnosing and reporting cases of OHSS in the United States and, possibly, differences in notification requirements between the United States and Europe. For example, with the introduction of the European Union Tissue and Cells Directive, serious adverse events (SAEs) are reportable to the NOTIFY library, which is a global surveillance database for medical products of human origin. OHSS that requires intensive care is considered a reportable SAE in Europe, whereas no such reporting requirements exist in the United States (38). There may be differences in the practice of ART in the United States and Europe that result in a genuinely reduced incidence of OHSS in the United States. The causes of the observed differences in the reported rates of OHSS between the United States and Europe require further study and reveal a need to validate OHSS data in national ART surveillance systems.
In the present study, the predictive factors for OHSS that are potentially modifiable included retrieval of more than 15 oocytes, pregnancy after fresh embryo transfer, and type of pituitary suppression. Pregnancy after an IVF cycle was associated with an increased risk of OHSS, suggesting that not performing an embryo transfer in patients at high risk of OHSS may reduce the risk of OHSS. These findings are similar to the results of previous studies (3, 8, 9, 14) and are consistent with what is known about the physiologic events that result in OHSS. It is likely the case that as the number of corpus lutea increases, the amount of VEGF that is produced following exposure to hCG increases, therefore increasing the risk of OHSS in women who respond vigorously to COH, such as those who have PCOS.
The finding that retrieval of a large number of oocytes increases the risk of OHSS indicates that careful, limited ovarian hyperstimulation in patients who are likely to be high responders may provide another means of preventing OHSS. There are many factors that may motivate IVF providers to attempt to maximize the oocyte yield of each IVF cycle, including a desire to maintain a high live birth rate per IVF cycle. Recent data have suggested, however, that rather than attempting to maximize oocyte yield, COH with the goal of retrieving 11–15 oocytes per cycle may produce an optimal live birth rate and limit the risk of OHSS. This was demonstrated in at least two large studies showing that the relationship between oocyte yield and live birth rate plateaus in cycles in which more than 15 oocytes were retrieved (39, 40). In our study and in previous studies, the risk of OHSS increases significantly in cycles in which more than 15 oocytes are retrieved (39). Considering the maternal and pregnancy-related risks associated with OHSS, it is important for IVF providers to recognize that limiting COH in high responders may prevent OHSS without sacrificing live birth rate.
We found that use of a GnRH antagonist and embryo banking were associated with a lower risk of OHSS. The efficacy of embryo banking as a means of preventing OHSS has been uncertain (6, 7) owing to conflicting data from previous studies (13, 14). In the present study, cycles in which embryo banking was performed were 35% less likely to result in OHSS than cycles in which embryo banking was not performed. Because some clinics may routinely cryopreserve all embryos without transfer to limit the risk of OHSS, we stratified clinics by the proportion of embryo cryopreservation cycles performed annually. There was a significant trend toward a lower incidence of severe OHSS as the proportion of embryo-banking cycles that a clinic performed increased. Therefore, withholding embryo transfer and cryopreserving all embryos after a cycle of IVF may reduce the risk of developing severe OHSS. Our findings regarding the protective effects of GnRH antagonist use are also consistent with the findings of previous studies (4, 41). However, we were unable to assess whether using a GnRH agonist trigger also reduced the risk for OHSS, which has been shown in other studies (31).
Predictive factors for OHSS that are not readily modifiable included diagnoses of ovulatory disorder/PCOS, uterine factor, tubal factor, endometriosis, and diminished ovarian reserve, age, and BMI. The increased risk of OHSS in women with uterine- or tubal-factor infertility or endometriosis may reflect a higher ovarian reserve in women with these specific diagnoses compared with women without these diagnoses, who may have had diminished ovarian reserve. Whether these diagnoses independently increase the risk of OHSS requires further study. Similar to previous smaller studies (9, 12), we found that the risk of OHSS was increased in younger women and was highest for women under 30 years of age. Low BMI was significantly associated with higher risk of OHSS when embryo-banking cycles were excluded. Over-weight and obese BMI was associated with a decreased risk of OHSS. Given the small variations in relative risk of OHSS, the clinical significance of BMI in determining which patients are at risk of OHSS may be limited.
The decreased risk of OHSS associated with diminished ovarian reserve is consistent with the pathophysiology of OHSS, because the amount of VEGF produced during COH varies with number of follicles and their response to exogenous gonadotropin administration (42). Fewer corpus lutea will likely result in production of less VEGF and a lower risk of developing OHSS.
Although we found that OHSS was associated with an increased risk of preterm delivery and low birth weight, the unadjusted differences in average length of pregnancy and birth weight were relatively small and likely not clinically significant. The one notable exception is that for patients with twins, the average gestational age at delivery was less than 37 weeks for all cycles and, on average, nearly 1 week earlier in cycles with OHSS than in cycles without OHSS. Given that a difference of 1 week may significantly alter morbidity and mortality in babies born before 37 weeks (42–45), the effect of OHSS on preterm delivery and birth weight may be more clinically relevant in twin pregnancies.
There are several limitations inherent in using data from the NASS dataset. Information regarding race/ethnicity and BMI is inconsistently reported, resulting in missing data in a high percentage of cycles. We used multiple imputation to impute race/ethnicity and BMI data for cycles in which it was missing, allowing us to overcome this limitation. We also lacked specific biochemical and hormonal information about each patient, and therefore we were not able to evaluate certain potential risk factors, such as increased ovarian reserve. Similarly, we were not able to evaluate how frequently many measures intended to prevent OHSS were used, including the use of GnRH agonist triggers, cabergoline, or metformin, because data regarding these characteristics and interventions are not included in the NASS dataset. Although this study uses data provided by ART practitioners, it is not possible to determine what criteria were used in establishing a diagnosis of OHSS and to what extent OHSS was reported. Finally, it is possible that residual confounding biased our effect estimates.
In summary, this study demonstrates that OHSS remains a serious complication of ART that affects not only women undergoing ART, but also the outcomes of pregnancies that result from it. Physicians who perform ART have many effective means available to reduce the risk of OHSS in their patients. These include careful controlled ovarian hyperstimulation with a goal of obtaining ~15 mature oocytes in all patients undergoing IVF, use of a GnRH antagonist and, when appropriate, a GnRH agonist trigger in women who are at moderate or high risk of experiencing OHSS, use of metformin and low-dose aspirin in patients who are at high risk of experiencing OHSS, use of cabergoline at the time of trigger, and, when appropriate, embryo cryopreservation and subsequent frozen-thawed embryo transfer in lieu of fresh embryo transfer. Physicians who practice ART should make use of these whenever possible to limit morbidity to patients and the risk of adverse pregnancy outcomes.
Supplementary Material
Acknowledgments
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
D.A.S. has nothing to disclose. A.D.K. has nothing to disclose. Y.Z. has nothing to disclose. J.F.K. has nothing to disclose. S.L.B. has nothing to disclose. D.M.K. has nothing to disclose.
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/65011-28939
REFERENCES
- 1.Golan A, Ron-El R, Herman A, Soffer Y. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv 1989;44:430–40. [DOI] [PubMed] [Google Scholar]
- 2.Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril 1992;58: 249–61. [DOI] [PubMed] [Google Scholar]
- 3.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update 2002; 8:559–77. [DOI] [PubMed] [Google Scholar]
- 4.Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 2016:CD001750. [DOI] [PMC free article] [PubMed]
- 5.Tang H, Hunter T, Hu Y, Zhai SD, Sheng X, Hart RJ. Cabergoline for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev 2012: CD008605. [DOI] [PubMed]
- 6.Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome: efficiency and safety. Hum Reprod 1999;14: 1457–60. [DOI] [PubMed] [Google Scholar]
- 7.d’Angelo A, Amso N. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev 2007:CD002806. [DOI] [PMC free article] [PubMed]
- 8.Enskog A, Henriksson M, Unander M, Nilsson L, Brännström M. Prospective study of the clinical and laboratory parameters of patients in whom ovarian hyperstimulation syndrome developed during controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril 1999;71:808–14. [DOI] [PubMed] [Google Scholar]
- 9.Tummon I, Gavrilova-Jordan L, Allemand MC, Session D. Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review. Acta Obstet Gynecol Scand 2005;84:611–6. [DOI] [PubMed] [Google Scholar]
- 10.Delvigne A, Dubois M, Battheu B, Bassil S, Meuleman C, de Sutter P, et al. The ovarian hyperstimulation syndrome in in-vitro fertilization: a Belgian multicentric study. II. Multiple discriminant analysis for risk prediction. Hum Reprod 1993;8:1361–6. [DOI] [PubMed] [Google Scholar]
- 11.Nakhuda GS, Chu MC, Wang JG, Sauer MV, Lobo RA. Elevated serum müllerian-inhibiting substance may be a marker for ovarian hyperstimulation syndrome in normal women undergoing in vitro fertilization. Fertil Steril 2006;85:1541–3. [DOI] [PubMed] [Google Scholar]
- 12.Delvigne A, Demoulin A, Smitz J, Donnez J, Koninckx P, Dhont M, et al. The ovarian hyperstimulation syndrome in in-vitro fertilization: a Belgian multicentric study. I. Clinical and biological features. Hum Reprod 1993;8: 1353–60. [DOI] [PubMed] [Google Scholar]
- 13.Navot D, Relou A, Birkenfeld A, Rabinowitz R, Brzezinski A, Margalioth EJ. Risk factors and prognostic variables in the ovarian hyperstimulation syndrome. Am J Obstet Gynecol 1988;159:210–5. [DOI] [PubMed] [Google Scholar]
- 14.Asch RH, Li HP, Balmaceda JP, Weckstein LN, Stone SC. Severe ovarian hyperstimulation syndrome in assisted reproductive technology: definition of high risk groups. Hum Reprod 1991;6:1395–9. [DOI] [PubMed] [Google Scholar]
- 15.Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum Reprod Update 2003; 9:275–89. [DOI] [PubMed] [Google Scholar]
- 16.McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV Jr, et al. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet 1994;344:235–6. [DOI] [PubMed] [Google Scholar]
- 17.Soares SR, Gomez R, Simon C, Garcia-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update 2008;14:321–33. [DOI] [PubMed] [Google Scholar]
- 18.Pau E, Alonso-Muriel I, Gómez R, Novella E, Ruiz A, García-Velasco JA, et al. Plasma levels of soluble vascular endothelial growth factor receptor-1 may determine the onset of early and late ovarian hyperstimulation syndrome. Hum Reprod 2006;21:1453–60. [DOI] [PubMed] [Google Scholar]
- 19.Abramov Y, Elchalal U, Schenker JG. Obstetric outcome of in vitro fertilized pregnancies complicated by severe ovarian hyperstimulation syndrome: a multicenter study. Fertil Steril 1998;70:1070–6. [DOI] [PubMed] [Google Scholar]
- 20.Papanikolaou EG, Tournaye H, Verpoest W, Camus M, Vernaeve V, van Steirteghem A, et al. Early and late ovarian hyperstimulation syndrome: early pregnancy outcome and profile. Hum Reprod 2005;20:636–41. [DOI] [PubMed] [Google Scholar]
- 21.Wiser A, Levron J, Kreizer D, Achiron R, Shrim A, Schiff E, et al. Outcome of pregnancies complicated by severe ovarian hyperstimulation syndrome (OHSS): a follow-up beyond the second trimester. Hum Reprod 2005;20: 910–4. [DOI] [PubMed] [Google Scholar]
- 22.Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC 3rd, Stern JE. Factors associated with ovarian hyperstimulation syndrome (OHSS) and its effect on assisted reproductive technology (ART) treatment and outcome. Fertil Steril 2010;94:1399–404. [DOI] [PubMed] [Google Scholar]
- 23.Division of Reproductive Health. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. 2016 assisted reproductive technology fertility clinic success rates report. October 2018. Available at: https://www.cdc.gov/art/reports/2016/fertility-clinic.html.
- 24.Division of Reproductive Health. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. October 2017. 2015 assisted reproductive technology fertility clinic success rates report. Available at: https://www.cdc.gov/art/reports/2015/fertility-clinic.html. Accessed October 3, 2017.
- 25.Agrawal S, Agarwal A, Das V. Impact of grandmultiparity on obstetric outcome in low resource setting. J Obstet Gynaecol Res 2011;37:1015–9. [DOI] [PubMed] [Google Scholar]
- 26.Jacquemyn Y, Senten L, Vellinga S, Vermeulen K, Martens G. Does practice make perfect? An age-matched study on grand multiparity in Flanders, Belgium. J Perinat Med 2006;34:28–31. [DOI] [PubMed] [Google Scholar]
- 27.Roman H, Robillard PY, Verspyck E, Hulsey TC, Marpeau L, Barau G. Obstetric and neonatal outcomes in grand multiparity. Obstet Gynecol 2004;103: 1294–9. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen AB, Mikkelsen EM, Cronin-Fenton D, Kristensen NR, Pham TM, Pedersen L, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol 2017;9:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razzaghi H, Tinker SC, Herring AH, Howards PP, Waller DK, Johnson CY, et al. Impact of missing data for body mass index in an epidemiologic study. Matern Child Health J 2016;20:1497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Crawford S, Boulet SL, Monsour M, Cohen B, McKane P, et al. Using multiple imputation to address the inconsistent distribution of a controlling variable when modeling an infrequent outcome. J Mod Appl Stat Methods 2017;16:744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youssef MA, Abdelmoty HI, Ahmed MA, Elmohamady M. GnRH agonist for final oocyte maturation in GnRH antagonist co-treated IVF/ICSI treatment cycles: systematic review and meta-analysis. J Adv Res 2015;6:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob SL, Brewer C, Tang T, Picton HM, Barth JH, Balen AH. A short course of metformin does not reduce OHSS in a GnRH antagonist cycle for women with PCOS undergoing IVF: a randomised placebo-controlled trial. Hum Reprod 2016;31:2756–64. [DOI] [PubMed] [Google Scholar]
- 33.Tso LO, Costello MF, Albuquerque LE, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2014:CD006105. [DOI] [PMC free article] [PubMed]
- 34.de Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2018;33:1586–601. [DOI] [PubMed] [Google Scholar]
- 35.Nyboe Andersen A, Gianaroli L, Nygren KG. Assisted reproductive technology in Europe, 2000. Results generated from European registers by ESHRE. Hum Reprod 2004;19:490–503. [DOI] [PubMed] [Google Scholar]
- 36.Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, et al. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril 2018;110:1067–80. [DOI] [PubMed] [Google Scholar]
- 37.Chambers GM, Paul RC, Harris K, Fitzgerald O, Boothroyd CV, Rombauts L, et al. Assisted reproductive technology in Australia and New Zealand: cumulative live birth rates as measures of success. Med J Aust 2017;207: 114–8. [DOI] [PubMed] [Google Scholar]
- 38.Kissin DM, Adamson GD, Chambers G, de Geyter C. Assisted reproductive technology surveillance. Cambridge University Press; 2019. [Google Scholar]
- 39.Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril 2014;101:967–73. [DOI] [PubMed] [Google Scholar]
- 40.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 2011;26:1768–74. [DOI] [PubMed] [Google Scholar]
- 41.Toftager M, Bogstad J, Bryndorf T, Lossl K, Roskaer J, Holland T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod 2016; 31:1253–64. [DOI] [PubMed] [Google Scholar]
- 42.Agrawal R, Conway G, Sladkevicius P, Tan SL, Engmann L, Payne N, et al. Serum vascular endothelial growth factor and Doppler blood flow velocities in in vitro fertilization: relevance to ovarian hyperstimulation syndrome and polycystic ovaries. Fertil Steril 1998;70:651–8. [DOI] [PubMed] [Google Scholar]
- 43.Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J. US birth weight/gestational age-specific neonatal mortality: 1995–1997 rates for Whites, Hispanics, and Blacks. Pediatrics 2003;111:e61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouyon J-B, Vintejoux A, Sagot P, Burguet A, Quantin C, Ferdynus C. Neonatal outcome associated with singleton birth at 34–41 weeks of gestation. Int J Epidemiol 2010;39:769–76. [DOI] [PubMed] [Google Scholar]
- 45.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep 2015;64:1–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


