Abstract
Background & Aims:
Ashwagandha (Withania somnifera) is widely used in Indian Ayurvedic medicine. Several dietary supplements containing ashwagandha are marketed in the U.S. and Europe, but only one case of ashwagandha-induced liver injury (DILI) has been published. The aim of this case series was to describe the clinical phenotype of suspected ashwagandha induced liver injury.
Methods:
Five cases of liver injury attributed to ashwagandha-containing supplements were identified; three were collected in Iceland during 2017–2018 and two from the Drug-Induced Liver Injury Network (DILIN) in 2016. Other causes for liver injury were excluded. Causality was assessed using the DILIN structured expert opinion causality approach.
Results:
Among the five patients, three were males; mean age 43 years (range 21–62). All patients developed jaundice and symptoms such as nausea, lethargy, pruritus and abdominal discomfort after a latency of 2–12 weeks. Liver injury was cholestatic or mixed (R ratios 1.4–3.3). Pruritus and hyperbilirubinemia were prolonged (5–20 weeks). No patient developed hepatic failure. Liver tests normalized within 1–5 months in 4 patients. One patient was lost to follow-up. One biopsy was performed, showing acute cholestatic hepatitis. Chemical analysis confirmed ashwagandha in available supplements; no other toxic compounds were identified. No patient was taking potentially hepatotoxic prescription medications, four were consuming additional supplements, in one case rhodiola was a possible causative agent along with ashwagandha.
Conclusions:
These cases illustrate the hepatotoxic potential of ashwagandha. Liver injury is typically cholestatic or mixed with severe jaundice and pruritus, but self-limited with liver tests normalizing in 1–5 months.
Keywords: Liver, Drug-Induced Liver Injury, Dietary Supplements
Lay Summary/Key Points:
Ashwaganda is a plant root which is used in many herbal- and dietary supplements. It seems to be able to cause injury to the liver and the injury has a specific clinical pattern. In this study the characteristics of five such cases are described.
Background & Aims:
Drug-induced injury (DILI) associated with herbal- and dietary supplements (HDS) is an important cause of liver injury and failure.1–4 In a prospective population-based study in Iceland, HDS caused 16% of DILI cases.3
In the Drug-Induced Liver Injury Network (DILIN) prospective study the proportion of DILI cases caused by HDS has been increasing from 7% in 2004–2005 to around 20% in 2010–2014.4 A recent large retrospective study on DILI in Mainland China found Chinese traditional medicine and HDS to be implicated in approximately 27% of cases.5 In other DILI registries or large cohorts, the proportion of DILI cases caused by HDS varies greatly.6–10 Most reported cases of DILI due to HDS are caused by supplements containing multi-ingredient mixtures, thus identifying a single causative agent can be difficult.4 It is reported that half of the U.S. population uses dietary supplements11 and the number of HDS on the market has increased in the last 25 years.2
The root of Withania somnifera, commonly known as ashwagandha, is widely used in Indian Ayurvedic medicine. It’s purported effects are many, including increased strength and vigor, promoting memory and learning abilities as well as decreasing anxiety and counteracting chronic fatigue.12 A population survey showed that Ayurvedic medicine was used by only 0.1% of the adult U.S. population in 2012.13 However, ashwagandha is not limited to Ayurvedic medicine. In fact, many dietary supplements containing ashwagandha are marketed in the U.S.
Only one case of DILI due to ashwagandha has been published. In that report from Japan, a 20 year old man developed cholestatic DILI with jaundice after using ashwagandha in combination with multiple anxiolytic drugs.14
Material and methods:
We present five cases of liver injury in which ashwagandha-containing supplements were implicated. The cases were identified in two different DILI-registries. Three were collected in Iceland during 2017–2018 and two from the DILIN in 2016. The design of the DILIN study has been described in detail in previous publications.15,16
In all cases, a thorough diagnostic workup was performed and other well recognized causes for liver injury were excluded based upon laboratory tests, viral serologies, autoimmune markers and diagnostic imaging. The causality assessment in all cases was based on expert consensus by the DILIN Causality Committee. The strength of the relationship between the implicated agent and the liver injury was scored as definite (≥95% likelihood), very likely (75–94%), probable (50–74%), possible (25–49%) or unlikely (<25%).
The type of liver injury was categorized by R ratios, using initial values of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and their respective upper limits of normal (ULN): R = ALT/ULN ÷ ALP/ULN. R ratio less than 2 was defined as cholestatic, between 2 and 5 as mixed and greater than 5 as hepatocellular.17 R ratio at peak of injury was calculated using ALT and ALP values when bilirubin levels were highest. Time to peak was calculated from onset of lab abnormalities to peak of bilirubin.
Severity was graded with a 4 point severity score as [1] mild, with serum enzyme elevations reaching criteria for DILI but bilirubin concentration <2.5 mg/dL, [2] moderate, with bilirubin ≥2.5 mg/dL or symptomatic hepatitis, [3] severe, with bilirubin concentration ≥2.5 mg/dL and signs of liver failure (INR ≥1.5, ascites and/or encephalopathy) or other organ failure considered to be due to DILI or [4] fatal, with death or liver transplantation due to DILI within 6 months of onset.18 Chemical analysis was performed on products collected from patients using liquid chromatography coupled to quadruple time of flight mass spectrometry (LC-QToF).
Results:
The five cases included three men (ages 21, 24 and 45 years) and two women (ages 60 and 61 years) (Table 1). Viral markers for acute hepatitis A, B, and C as well as cytomegalovirus IgM were negative in all patients. Hepatitis C virus (HCV) RNA PCR was performed in one case and was negative. Hepatitis E virus (HEV) testing was not performed. Antinuclear antibody (ANA) and smooth muscle antibody (SMA) titers were elevated in one patient, ANA 1:40 and SMA 1:40 but the liver tests normalized with no other intervention than stopping the HDS. All patients underwent imaging of the liver and bile ducts, ultrasound in four, computerized tomography (CT) in three and magnetic resonance cholangiopancreatography (MRCP) in two. No patient had evidence of biliary obstruction. The role of ashwagandha in causing liver injury was judged as definite in one case, highly likely in two, probable in one and only possible in one (Table 1). Concise clinical histories and laboratory values of all the cases are available in the supplementary material.
Table 1:
Liver tests and pattern of liver injury. First three cases are from Iceland. ALT: Alanine aminotransferase. Alk P: Alkaline phosphatase. DILIN: Drug-induced liver injury network. F: Female. INR: International normalized ratio. M: Male.
| Case | Product | Age/Sex | Peak ALT (U/L) | Peak AST (U/L) | Peak Alk P (U/L) | Peak Bilirubin (mg/dL) | Peak INR | DILIN Causality |
|---|---|---|---|---|---|---|---|---|
| 1 | “NOW Ashwagandha” | 45/M | 345 | 226 | 279 | 14.4 | 0.9 | Definite |
| 2 | “NOW Ashwagandha” | 24/M | 300 | 177 | 159 | 13.9 | 1.0 | Highly likely |
| 3 | “NOW Ashwagandha” | 62/F | 261 | 226 | 241 | 7.5 | 1.0 | Probable |
| 4 | “Nature’s Way Ashwagandha” | 61/F | 580 | 334 | 187 | 5.9 | 1.0 | Possible |
| 5 | Ashwagandha | 21/M | 317 | 235 | 274 | 8.0 | 1.1 | Highly likely |
Two patients were taking ashwagandha to decrease anxiety, one to counteract stress, one to increase concentration and one for increased energy and vitality. In the three cases from Iceland the patients were taking ashwagandha supplements from the same manufacturer (NOW® Ashwagandha) although one patient was also using another supplement that contained ashwagandha (Infowarslife® Super Male Vitality). In these three cases from Iceland the daily dose varied from 450 to 1350 mg. The implicated product was available for one of the US cases: Nature’s Way®, Standardized Ashwagandha, containing 500 mg of ashwagandha Chemical analysis confirmed the presence of ashwagandha in all supplements, and no other known toxic compounds or trace metals were found. No patient was taking other potentially hepatotoxic prescription medications, although four were consuming one or more other supplements: Super Male Vitality, Spirulina, Chlorella, Rhodiola and Cerenity. In one case rhodiola was implicated as a possible causative agent along with ashwagandha. In other cases, the other HDS products scored as unlikely causes of the hepatic injury.
In all five cases the patients had jaundice as well as other symptoms such as nausea (n=3), abdominal discomfort or pain (n=2), lethargy (n=1) and pruritus (n=2). Latency from the first intake of ashwagandha to the onset of symptoms was 2–12 weeks (table 2).
Table 2.
Summary overview of cases. First three cases are from Iceland. D: Days.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Duration of therapy (d) | 14 | 90 | 14 | 110 | 16 |
| Time to Onset of Symptoms (d) | 7 | 83 | 5 | 116 | 16 |
| Time to Onset of Lab Abnormalities (d) | 14 | 90 | 14 | 116 | 16 |
| Time to Peak of Injury | 36 | 18 | 47 | 3 | 31 |
| Time to Resolution | Within 5 months | Unknown | Within 3 months | Within 2 months | Within 3 months |
| Initial R Ratio | 3.3 (Mixed) | 3.1 (Mixed) | 1.4 (Cholestatic) | 1.6 (Cholestatic) | 2.7 (Mixed) |
| R Ratio at Peak of Injury | 0.3 (Cholestatic) | 1.1 (Cholestatic) | 1.2 (Cholestatic) | 2.0 (Mixed) | 2.0 (Mixed) |
| Symptoms | Jaundice, nausea, pruritus | Jaundice, nausea | Jaundice, nausea, abdominal pain | Jaundice, fatigue, pruritus | Jaundice, abdominal pain |
| Severity | 2: Moderate | 2: Moderate | 2: Moderate | 2: Moderate | 2: Moderate |
| Modal | Bimodal | Bimodal | Bimodal | Unimodal | Bimodal |
Three patients had a mixed pattern of liver injury, two had a cholestatic pattern, R ratios were 1.4–3.3. Hyperbilirubinemia and pruritus were prolonged with elevated levels of bilirubin for 5–20 weeks, the time from onset to peak of liver tests ranging from 9–36 days. One patient was lost to follow up after 32 days. In three patients liver tests had normalized within 1–5 months of stopping ashwagandha. One patient had normal liver tests except for bilirubin of 1.4mg/dL (1.2x ULN) 2.5 months after onset of liver injury, and liver tests had normalized 9 months after onset of injury. Severity was graded as moderate in all cases. One patient was hospitalized, but no patient developed an elevation in INR or other evidence of hepatic failure or impaired liver function. In one case a biopsy was performed 36 days after onset, which showed acute cholestatic hepatitis with mild to moderate inflammation and canalicular cholestasis (figure 1). Rechallenge was not performed in any case.
Figure 1:
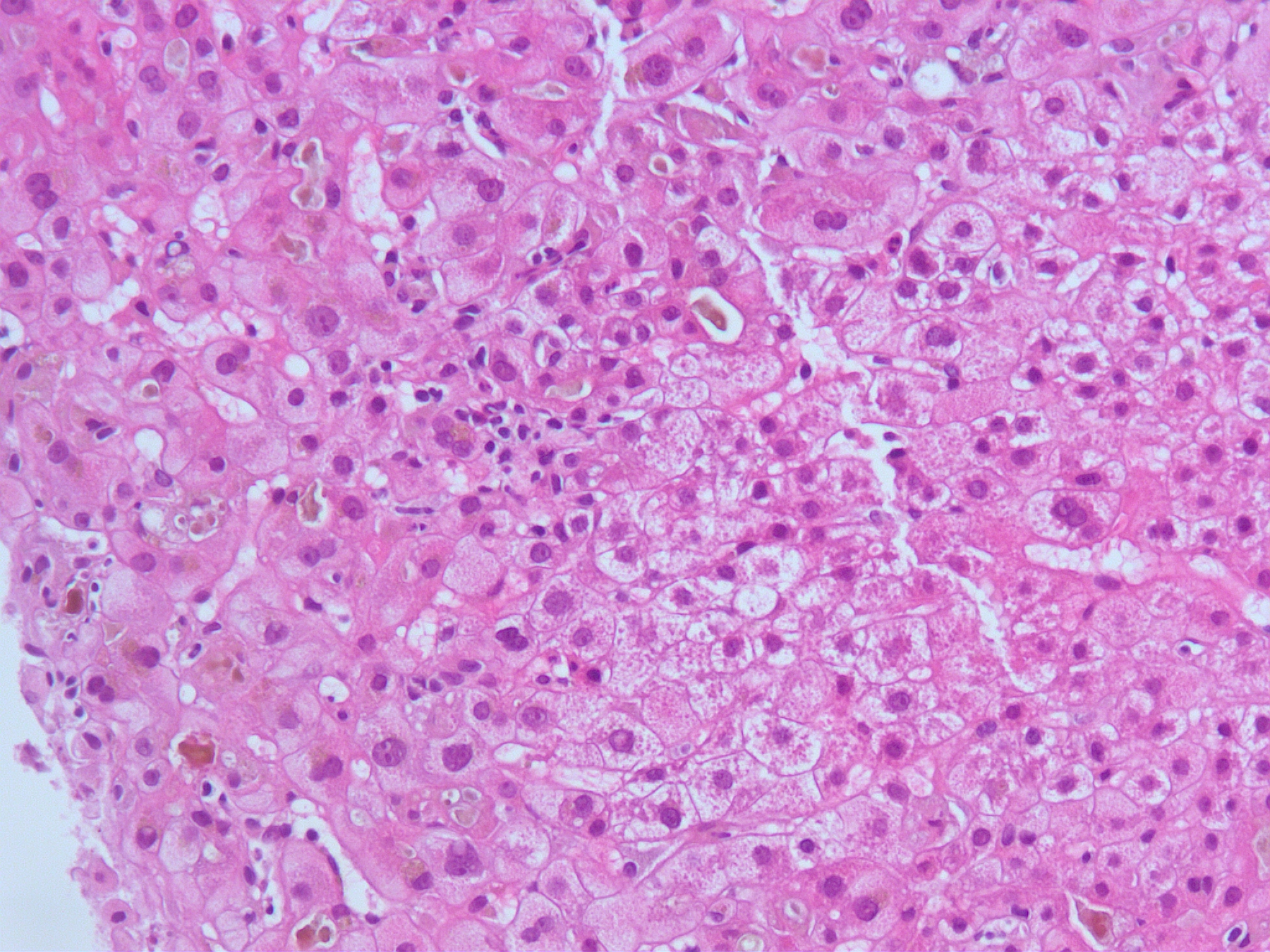
Liver biopsy from case 1 showing liver tissue with mild mononuclear cell inflammatory infiltrate and prominent canalicular cholestasis.
Conclusions:
In the current study, we present five cases of liver injury linked to the herbal- and dietary supplement ashwagandha. The pattern of liver injury was similar in all cases, mixed and cholestatic with notable hyperbilirubinemia. No rechallenge was performed. In this case-series, all patients underwent a thorough and detailed diagnostic work-up, and all the cases were individually confirmed by a committee of experts in the field using the DILIN structured expert opinion causality assessment method.16 A standardized tool such as Roussel Uclaf Causality Assessment Method (RUCAM) was not applied as these measures tend to underestimate the causality of HDS, due to the fact that DILI is usually not listed as an adverse effect. Also RUCAM was designed to assess causality of a single product, whereas herbal products characteristically contain multiple ingredients. One patient was lost to follow up before liver tests had normalized.
Ashwagandha has been reported in the literature as a causative agent in drug-induced liver injury only once before.14 In the case report by Inagaki et. al. the patient developed symptoms after taking ashwagandha with multiple anti-anxiety drugs (alprazolam, lorazepam, escitalopram, quetiapine) for 10 months and increasing the dosage of ashwagandha two- to threefold a month before the onset of symptoms. The dose of ashwagandha was not reported. The liver injury was classified as cholestatic with prominent hyperbilirubinemia, which worsened for some time after intake of ashwagandha was stopped, normalizing in 3.5 months. Other known causes were excluded. The patient was treated with ursodeoxycholic acid and phenobarbital. A biopsy showed canalicular cholestasis without lobular inflammation.14 This is interesting as the histological pattern in the current study also revealed prominent features of cholestasis although with mild to moderate inflammation. Biopsies from more patients would be needed to determine whether this histological appearance is characteristic of ashwagandha-induced liver injury. In all cases, profound hyperbilirubinemia peaked days or weeks after cessation of the offending drug. No patient developed signs or symptoms of liver failure.
In the present study, three cases of liver injury, all due to a single ashwagandha containing product made by the same manufacturer, were identified over a 7-month period in Iceland. This is a relatively high number of cases considering that only 15 cases of DILI due to HDS were identified in a two-year prospective study in Iceland.3
In all cases a thorough diagnostic work-up with imaging, viral- and autoimmune markers was performed to exclude other possible causes of liver injury. All patients had negative anti-HCV antibodies, however HCV RNA PCR was only performed in one case and the diagnostic work-up did not include anti-HEV antibodies or HEV RNA PCR. Although these tests were not performed, clinical suspicion for viral hepatitis was very low. In the cases described in this study, the pattern of liver injury was cholestatic or mixed, with notable elevation in alkaline phosphatase with hyperbilirubinemia that normalized in 1–5 months after discontinuation of ashwagandha. A hepatocellular type of liver injury with prominent elevations in transaminases would be the most likely pattern in liver injury caused by HCV or HEV. Importantly, the prevalence of HEV IgG antibodies has been shown to be very low in Iceland and found almost exclusively in patients who were born in other countries or have lived outside of Iceland for an extended period of time.19
DILI due to HDS is an important cause of liver injury, causing 16% of cases in a prospective study in Iceland3 and around 20% of cases in the U.S.4 In the Acute Liver Failure Study Group database, approximately 16% of DILI cases were due to HDS, with transplantation rates significantly higher (56% vs. 32%) in those with DILI from HDS than from conventional drugs and with lower 21-day transplant-free survival (17% vs. 34%).1 These prevalence and outcome data have led to an increasing appreciation of hepatotoxicity as well as other adverse events due to herbal and dietary supplements. Overall, HDS adverse events are estimated to cause 23,000 emergency department visits annually in the U.S.20
Attribution to specific HDS compounds can be challenging. One of the biggest problems is incorrect labeling of ingredients21, but one of the strengths of our study was the chemical analysis that confirmed the presence of ashwagandha in implicated supplements in four cases. Most cases of DILI due to HDS are caused by supplements containing multi-ingredient mixtures,4 and it can be difficult to prove which one is responsible for the liver injury. Some common ingredients have been identified as hepatotoxic, most notably green tee extract (Camellia sinensis) which was implicated in 25% of HDS hepatotoxicity in the Spanish DILI registry.10 In our cases, the ashwagandha was marketed as the single or predominant ingredient, and chemical analysis did not identify other liver toxic substances. However, clinicians should be aware that ashwagandha is also labeled as a component of multi-ingredient mixtures such as Hydroxycut Platinum®22 and Herbalife Relax Now®23.
In the case report by Inagaki et. al the patient developed DILI after increasing the dosage of ashwagandha two- to threefold. This suggests that liver injury associated with ashwagandha might be dose-related. Idiosyncratic drug-induced liver injury is traditionally not considered to be dose-related, however drugs with a daily dose of ≤ 50mg are less likely to cause liver failure, need for liver transplantation or death.24 The daily dose of ashwagandha ranged from 450 to 1350mg in the present study and no patient had recently increased the dose to our knowledge. However, the dose was recorded according to the label information and no direct dose measurement was performed.
In summary, we present five cases of DILI due to ashwagandha with similar patterns of liver injury and outcome. Clinicians should be aware of ashwagandha’s potential for liver injury and always get a detailed history of HDS use in patients presenting with liver injury.
Supplementary Material
Acknowledgements:
The Authors wish to thank Dr. David Kleiner from the National Institutes of Health for his review of the photomicrograph, figure 1.
Financial support:
The Drug-Induced Liver Injury Network (DILIN) is structured as an U01 cooperative agreement supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) with funds provided by the following grants: U01DK065211 (Indiana University [Purdue]), U01DK065184 (University of Michigan [Ann Arbor]), U01DK065201 (University of North Carolina [Chapel Hill], Asheville, Wake Forest Baptist Medical Center), U01DK083020 (University of Southern California, University of California-Los Angeles [Pfleger Liver Institute]), U01DK083027 (Albert Einstein Medical Center), U01DK100928 (Icahn School of Medicine at Mount Sinai), U01DK065176 (Duke Clinical Research Institute). Additional support was provided by the Intramural Division of the National Cancer Institute (NCI), NIH.
Abbreviations:
- DILI
Drug-induced liver injury
- HDS
Herbal- and dietary supplements
- DILIN
Drug-Induced Liver Injury Network
- HCV
Hepatitis C virus
- HEV
Hepatitis E virus
- ANA
Antinuclear antibody
- SMA
Smooth-muscle antibody
- CT
computerized tomography
- MRCP
magnetic resonance cholangiopancreatography
- RUCAM
Russel Uclaf Causality Assessment Method
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- 1.Hillman L, Gottfried M, Whitsett M, et al. Clinical features and outcomes of complementary and alternative medicine induced acute liver failure and injury. Am J Gastroenterol. 2016;111(7):958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro VJ, Khan I, Bjornsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–1425, 1425.e1411–1413; quiz e1419–1420. [DOI] [PubMed] [Google Scholar]
- 4.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380–1387. [DOI] [PubMed] [Google Scholar]
- 7.Dag MS, Aydinli M, Ozturk ZA, et al. Drug- and herb-induced liver injury: A case series from a single center. Turk J Gastroenterol. 2014;25(1):41–45. [DOI] [PubMed] [Google Scholar]
- 8.Bessone F, Hernandez N, Lucena MI, Andrade RJ. The latin american DILI registry experience: A successful ongoing collaborative strategic initiative. Int J Mol Sci. 2016;17(3):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Yang L, Liao Z, He X, Zhou Y, Guo H. Epidemiology of drug-induced liver injury in China: A systematic analysis of the Chinese literature including 21,789 patients. Eur J Gastroenterol Hepatol. 2013;25(7):825–829. [DOI] [PubMed] [Google Scholar]
- 10.Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, et al. Herbal and dietary supplement-induced liver injuries in the Spanish DILI registry. Clin Gastroenterol Hepatol. 2018;16(9):1495–1502. [DOI] [PubMed] [Google Scholar]
- 11.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J. Steroidal lactones from withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14(7):2373–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8). [Google Scholar]
- 15.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924–1934, 1934 e1921–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: Rationale, design and conduct. Drug Saf. 2009;32(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--i. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323–1330. [DOI] [PubMed] [Google Scholar]
- 18.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–815. [DOI] [PubMed] [Google Scholar]
- 19.Love A, Bjornsdottir TB, Olafsson S, Bjornsson ES. Low prevalence of hepatitis E in Iceland: A seroepidemiological study. Scand J Gastroenterol. 2018;53(3):293–296. [DOI] [PubMed] [Google Scholar]
- 20.Geller AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015;373(16):1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58(9):2682–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hydroxycut. Livertox 2018; https://livertox.nih.gov/Hydroxycut.htm. Accessed September 2, 2019.
- 23.Herbalife. Livertox 2018; https://livertox.nih.gov/Herbalife.htm. Accessed September 2, 2019.
- 24.Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology. 2008;47(6):2003–2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


