Precis:
The MicroShunt was implanted in 23 patients with primary open-angle glaucoma (POAG) in a feasibility study. Reductions in intraocular pressure (IOP) and medications were sustained for up to 5 years with no long-term sight-threatening adverse events (AEs).
Purpose:
The purpose of this study was to assess the long-term effectiveness and safety of the PRESERFLO MicroShunt (8.5 mm long, 70 µm lumen surgical device, formerly known as the InnFocus MicroShunt) in POAG.
Patients and Methods:
In a feasibility study (NCT00772330), patients with POAG inadequately controlled on maximum tolerated therapy with IOP ≥18 to ≤40 mm Hg underwent MicroShunt implantation with adjunctive mitomycin C (0.4 mg/mL), alone or in combination with cataract surgery. Years 1 to 3 findings have previously been reported. Endpoints of this extension study included IOP reduction and success at years 4 and 5 (primary), incidence of AEs, medication use, and reoperations.
Results:
Mean IOP was reduced from 23.8±5.3 mm Hg at baseline to 12.8±5.6 mm Hg (year 4; n=21) and 12.4±6.5 mm Hg (year 5; n=21). Overall success (with/without medication use) was 87.0% (year 4) and 82.6% (year 5). The mean number of medications reduced from 2.4±1.0 at baseline to 0.8±1.3 (year 5). Common (≥5% of patients) AEs included corneal edema (n=4), transient hypotony (n=4), bleb-related complications (n=3), and device touching the iris (n=3). There were 4 reports of serious AEs and 2 reoperations.
Conclusions:
In this extension study, sustained reductions in mean IOP and medications were observed up to 5 years post-MicroShunt implantation. There were no reports of long-term sight-threatening AEs and a low rate of postoperative interventions.
Key Words: primary open-angle glaucoma, surgical implant, intraocular pressure, clinical trial
Glaucoma encompasses a group of diseases that are categorized by retinal ganglion cell death and cupping of the optic nerve head resulting in visual field loss.1,2 The current management of glaucoma focuses on reducing intraocular pressure (IOP) using the fewest possible number of medications.1 Although medical therapy is commonly the first-line treatment for patients with glaucoma,3 medication adherence tends to be suboptimal.4 When medical therapy fails to achieve adequate IOP reduction, laser or incisional surgeries are introduced into the treatment paradigm.1 Trabeculectomy and tube shunt implantation are the most commonly performed incisional glaucoma surgeries5; however, these procedures are often associated with complications and a substantial need for postoperative intervention.5,6 Microinvasive glaucoma surgery (MIGS) has been developed as a less invasive alternative to traditional incisional surgeries7,8; however, more modest IOP reduction results have been seen with the available MIGS devices compared with traditional surgeries.8
There are currently 3 main anatomical categories of MIGS device, including Schlemm’s canal, suprachoroidal, and subconjunctival.8 The PRESERFLO MicroShunt (Santen Inc., Miami, FL; formerly known as the InnFocus MicroShunt) is an example of the latter type of device.9 The MicroShunt is an 8.5 mm long microincisional filtration surgery device with a 350 µm outer diameter and 70 µm lumen (Fig. 1).9 It is composed of poly(styrene—block—isobutylene—block—styrene), or SIBS, which is a highly biocompatible, bioinert material.9 Implantation is carried out via an ab-externo approach, allowing hemostasis control, precise placement, and verification of flow. Aqueous humor flows from the anterior chamber to a posterior bleb formed under the Tenon’s capsule.9,10 At the time of writing, the MicroShunt is an investigational device not yet approved by the Food and Drug Administration.
FIGURE 1.
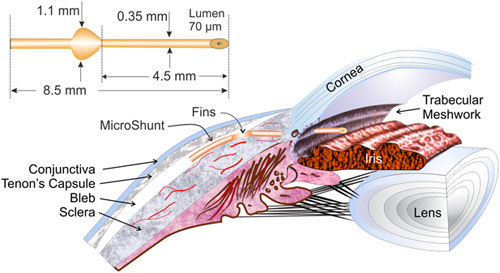
Dimensions of the MicroShunt and placement in the eye. Adapted from Batlle et al.10 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
The MicroShunt was implanted in patients with primary open-angle glaucoma (POAG) using 0.4 mg/mL mitomycin C (MMC) intraoperatively in a feasibility, single-site, nonrandomized study.10,11 Key efficacy and safety outcomes, with a median follow-up period of 3 years, were previously reported.10 At year 3, the qualified success rate (IOP ≤14 mm Hg, IOP reduction ≥20% from baseline, with the use of glaucoma medication) was 95%.10 Few postoperative adverse events (AEs) were reported. The most common AEs reported in ≥5% of patients included the following: device touching the iris [3/23 eyes (13.0%)], transient hypotony [<5 mm Hg; 3/23 (13.0%)], shallow or flat anterior chamber [3/23 (13.0%)], transient choroidal detachment [2/23 (8.7%)], hyphema [2/23 (8.7%)], and exposed Tenon’s capsule [2/23 (8.7%)]. There were no reports of bleb leaks, chronic hypotony, endophthalmitis, or development of peripheral anterior synechiae.10 The goal of this analysis was to assess the long-term efficacy and safety of MicroShunt implantation in patients with POAG; therefore, follow-up 4- and 5-year results are reported herein.
PATIENTS AND METHODS
Study Design
This was a single-center, nonrandomized, single-arm interventional clinical study (ClinicalTrials.gov Identifier: NCT00772330). The study was conducted at Centro Láser, Santo Domingo, Dominican Republic. It was initially a 1-year study, but the protocol was amended to extend the study period to a total of 5 years. The study was conducted in accordance with the Declaration of Helsinki and the requirements for medical device investigations as presented in EN/ISO 14155 (2011), Clinical investigation of medical devices for human subjects—Good clinical practice; Annex X of the European Medical Devices Directive 93/42/EEC, as amended by Directive 2007/47/EEC, MEDDEV 2.7/4, and applicable local regulatory requirements. Institutional review board approval was obtained from CONABIOS, the Dominican Republic National Counsel of Bioethics and Health.
Patients
Eligible patients were 18 to 85 years of age and had POAG inadequately controlled on maximum tolerated medical therapy with medicated IOP of ≥18 and ≤40 mm Hg. All patients provided signed, written informed consent. The full list of exclusion criteria has previously been published10; key exclusion criteria included the following: previous ophthalmic surgery, excluding uncomplicated cataract surgery or corneal refractive surgery.
Treatments and Assessments
The procedure for MicroShunt implantation has been previously described in detail.10 In summary, for patients who required cataract surgery, phacoemulsification was performed before MicroShunt implantation. Following anesthesia, a 6 to 8 mm incision was made to form a fornix-based subconjunctival/Tenon’s flap. The subconjunctival space was treated with topical MMC (0.4 mg/mL), which was applied via 3 MMC-soaked LASIK shields (Network Medical Ltd, UK) for 3 minutes±15 seconds. The subconjunctival space was then rinsed with a balanced salt solution (>20 mL) to flush out any remaining MMC. A location for a scleral pocket was marked 3 mm from the limbus with a 3-mm scleral marker and a marker pen. A triangular pocket was made at the marked point using a 1-mm-wide Mani knife, and a 25 to 27 G needle track was formed from the sclera into the anterior chamber. Forceps were used to insert the proximal tip of the MicroShunt into the anterior chamber. The 1.1-mm wingspan planar fins of the MicroShunt device were wedged into the 1 mm scleral pocket and were positioned so that they were lying flat onto the sclera and not protruding up in a vertical orientation. All implants were placed between the superior and the lateral rectus, that is, in the superotemporal quadrant (Fig. 2). After successful insertion, the distal end of the MicroShunt was observed for droplet formation before it was tucked under the subconjunctival Tenon’s flap. The Tenon’s capsule and conjunctiva were repositioned over the MicroShunt to the limbus and sutured using 10-0 nylon sutures. A check was made for bleb leaks, and a light-pressure patch was used after surgery.
FIGURE 2.
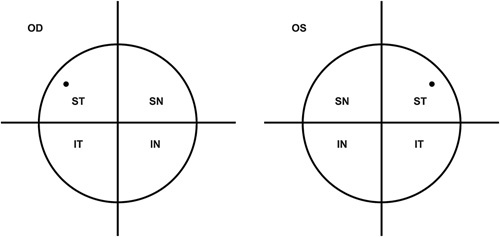
The MicroShunt was placed between the superior and lateral rectus. The dot in the ST quadrant signifies the location of the MicroShunt. IN indicates inferonasal; IT, inferotemporal; OD, oculus dexter; OS, oculus sinister; SN, superonasal; ST, superotemporal.
The primary efficacy endpoint was the reduction in medicated IOP, relative to the preoperative value, which was assessed at each postoperative visit (day 1, day 7, week 3, week 6, month 3, month 6, and at each year following month 12), and the measurement of success, defined as patients who were not pressure or surgical failures. Surgical failures were defined as patients requiring reoperation in the operating room, excluding bleb needling. Pressure failures were defined as patients with IOP outside of the target range (6 to 21 or 6 to 14 mm Hg) or with <20% reduction from baseline on 2 consecutive follow-up visits after 3 months and did not achieve 20% reduction below baseline in the last visit at which the success rate is reported. Success was reported as overall (with or without concomitant use of glaucoma medications), qualified (with glaucoma medications), and complete (without glaucoma medications). IOP was measured using Goldmann applanation tonometry. Visual field assessment was measured preoperatively using the Humphrey Field Analyzer.
The primary safety endpoint was the incidence of all device-related and/or procedure-related AEs during the study. Secondary endpoints included the mean number of glaucoma medications per patient, visual acuity, and the incidence of glaucoma reoperation. Snellen visual acuity was measured by using a standard visual acuity chart; if a patient’s visual acuity was so poor that they could not read the 20/400 line, their ability to count fingers from the examiner’s hand at a 2-foot distance was assessed; if this was unsuccessful, testing for hand motions (stationary, moving back and forth horizontally, and moving up and down vertically) was performed.12 The Snellen visual acuity equivalent score was converted to a logarithm of the minimum angle of resolution (logMAR). Endothelial cell count density was not measured in this study.
Statistical Methods
Statistical analyses were performed with SAS System, Version 9.1 or higher (Buckinghamshire, UK). No formal sample size calculations were conducted; however, 35 patients were to be enrolled to account for a 10% lost to follow-up factor. Quantitative endpoints were reported in terms of mean and SD, whereas qualitative endpoints were reported in terms of number and percentage of each modality. Descriptive summaries were based on observed cases, with the exception of the calculation of success rates and mean IOP. For success and mean IOP, missing data were imputed using the last observed score. Data collected after reoperation (surgical failure) were excluded from the analyses.
RESULTS
Patient Demographics and Baseline Characteristics
The study was initiated on February 1, 2010. Patients were enrolled over a 12-month period, with the first implantation performed in March 2010, and the last year-5 visit was completed in August 2016. A total of 23 patients were enrolled in the study, all of whom had the MicroShunt implanted in only 1 eye. In 9 patients, the MicroShunt was implanted in combination with cataract surgery; the remaining 14 patients received the MicroShunt as a standalone procedure. Of the total 23 patients, follow-up data were available for 16 and 20 patients at years 4 and 5, respectively. Two patients underwent reoperation; therefore, their data are excluded from this analysis. By year 4, in addition to the 2 patients who underwent reoperation, 1 patient was lost to follow up, and 1 patient did not enter the extended study period. Five patients did not attend the year-4 visit (n=14) but returned for the year-5 visit. By year 5, another patient was lost to follow up (n=18) (Fig. 3). All 23 patients are included in the safety analyses. Patient demographics and baseline characteristics are shown in Table 1.
FIGURE 3.

Patient disposition.
TABLE 1.
Demographics and Baseline Characteristics
| Variable | All Patients (N=23) [n (%)] |
|---|---|
| Age (mean±SD) (y) | 59.8±15.3 |
| Male | 15 (65.2) |
| Ethnicity, Hispanic | 23 (100) |
| Lens status | |
| Phakic | 12 (52.2) |
| Combined with cataract | 9 (39.1) |
| Pseudophakic | 2 (8.7) |
| Glaucoma type | |
| Primary open-angle glaucoma | 23 (100) |
| Medicated IOP (mean±SD) (mm Hg) | 23.8±5.3 |
| IOP ≥18 and ≤21 mm Hg | 10 (43.5) |
| IOP >21 mm Hg | 13 (56.5) |
| No. glaucoma medications (mean±SD) | 2.4±1.0 |
| Visual acuity (mean±SD) (logMAR)* | 0.9±1.1 |
| Visual field deviation (mean±SD) (dB)† | −20.1±12.1 |
| >−6 dB (n) | 4 |
| ≤−6 dB and >−12 dB (n) | 3 |
| ≤−12 dB (n) | 13 |
Counting fingers=2; hand motion=3.
Visual field data were missing for 3 patients.
IOP indicates intraocular pressure; logMAR, logarithm of the minimum angle of resolution.
Efficacy
The reduction in mean IOP observed at year 3 was sustained over years 4 and 5 (Fig. 4). Mean IOP±SD was reduced from 23.8±5.3 mm Hg (N=23) to 12.8±5.6 mm Hg at year 4 (n=21) and 12.4±6.5 mm Hg at year 5 (n=21), representing a mean decrease from baseline of 45.7% and 46.7%, respectively.
FIGURE 4.

Mean±SD medicated IOP over 5 years of follow-up. Missing IOP scores were imputed using the last observed IOP score. IOP scores collected after reoperation are excluded. Results at years 1, 2, and 3 have been corrected compared with previously published findings,10 following reanalysis conducted for this manuscript. IOP indicates intraocular pressure.
The mean number of glaucoma medications per patient decreased from 2.4±1.0 (N=23) at baseline to 0.5±1.1 at year 4 (n=14) and 0.8±1.3 at year 5 (n=18), respectively (Table 2). The percentages of medication-free patients at year 4 and year 5 were 78.6% and 61.1%, respectively (Table 2).
TABLE 2.
Glaucoma Medication Use Per Patient Preoperatively and Postoperatively
| No. Patients | No. Glaucoma Medications Per Patient (Mean±SD) | No. Patients Medication Free [n/N (%)] | |
|---|---|---|---|
| Baseline | 23 | 2.4±1.0 | 0/23 (0.0) |
| Year 1 | 23 | 0.3±0.8 | 20/23 (87.0) |
| Year 2* | 22 | 0.3±0.8 | 18/22 (81.8) |
| Year 3* | 21 | 0.6±1.3 | 16/21 (76.2) |
| Year 4 | 14 | 0.5±1.1 | 11/14 (78.6) |
| Year 5 | 18 | 0.8±1.3 | 11/18 (61.1) |
Results at years 2 and 3 have been corrected compared with previously published findings10 following reanalysis conducted for this manuscript.
At years 4 (n=20) and 5 (n=19), >80% of patients (N=23) achieved overall success with an IOP of between ≥6 and <21 mm Hg (Table 3).
TABLE 3.
Success Rates at Years 4 and 5
| Target IOP Zone (mm Hg) | Analysis Visit | Success Rate [n/N (%)] |
|---|---|---|
| Overall success (with or without concomitant use of glaucoma medications) | ||
| ≥6 to <14 | Year 4 | 18/23 (78.3) |
| Year 5 | 19/23 (82.6) | |
| ≥6 to <21 | Year 4 | 20/23 (87.0) |
| Year 5 | 19/23 (82.6) | |
| Qualified success (with glaucoma medications) | ||
| ≥6 to <14 | Year 4 | 2/23 (8.7) |
| Year 5 | 6/23 (26.1) | |
| ≥6 to <21 | Year 4 | 4/23 (17.4) |
| Year 5 | 6/23 (26.1) | |
| Complete success (without glaucoma medications) | ||
| ≥6 to <14 | Year 4 | 16/23 (69.6) |
| Year 5 | 13/23 (56.5) | |
| ≥6 to <21 | Year 4 | 16/23 (69.6) |
| Year 5 | 13/23 (56.5) | |
Missing IOP scores were imputed using the last observed IOP score.
Patients who received any glaucoma reoperation were considered to be surgical failures.
IOP indicates intraocular pressure.
Safety
The most common AEs that occurred up to 5 years are shown in Table 4. Overall, 31 nonserious AEs occurred in 13 patients with a mean resolution time of 29.0±43.9 days.
TABLE 4.
Summary of Procedure-related or Device-related Nonserious AEs on or Before 5 Years of Follow up Reported in ≥5% of the Overall Population
| AE | No. Patients (N=23) [n (%)] | Time to Resolution (Mean±SD) (d) |
|---|---|---|
| Any nonserious AEs | 13 (56.5) | 29.0±43.9 |
| Corneal edema | 4 (17.4) | 11.8±4.4 |
| Transient hypotony* | 4 (17.4) | 8.0±0.0 |
| Bleb-related complications | 3 (13.0) | 47.3±63.1 |
| Device touching the iris | 3 (13.0) | 96.5±115.3 |
| Cornea striae | 2 (8.7) | 15.0±9.9 |
| Flat anterior chamber | 2 (8.7) | 15.0±0.0 |
| Hyphema | 2 (8.7) | 8.5±10.6 |
The summary is based on observed cases. AEs collected after reoperation date were excluded from the summary. AEs related to the procedure and device are presented together to control for double counting.
IOP <6 mm Hg at any time.
AE indicates adverse event; IOP, intraocular pressure.
There were 4 reports of serious procedure-related or device-related AEs in 2 patients, all of which resolved in a mean of 10.5 days and a maximum of 20 days. Serious AEs were posterior capsule opacification (n=2, 8.7%), posterior synechiae (n=1, 4.3%), and pupillary capture (n=1, 4.3%). Reoperation was required for 2 patients (8.7%) because of bleb failures: a second MicroShunt was implanted in one of these patients; the MicroShunt was replaced by a XEN 45 Gel Stent (Allergan, Dublin, Ireland) in the second patient. Needling of the bleb was required in 2 patients (8.7%). There were no reports of device migration or erosion/exposure. In the cohort of patients who were implanted with the MicroShunt in combination with cataract surgery, mean visual acuity scores improved from 1.0±0.9 at baseline (n=9) to 0.2±0.2 at year 4 (n=5) and 0.4±0.7 (n=7). In the MicroShunt alone group, mean visual acuity changed from 0.9±1.3 at baseline (n=13) to 0.6±0.9 at year 4 (n=7) and 0.5±1.1 at year 5 (n=7).
DISCUSSION
The long-term results from this feasibility study conducted in a small patient cohort shows sustained reductions in mean IOP and medications up to 5 years after MicroShunt implantation. At year 5, mean IOP was 12.4±6.5 mm Hg, which represented a 46.7% reduction from baseline, and 61.1% of patients were medication free. A total of 21 nonserious AEs were reported over the first 3 years postsurgery10; an additional 10 nonserious AEs and 4 serious AEs were reported by year 5. In addition, consistent with the previously reported 3-year results,10 there were no reports of chronic hypotony or endophthalmitis; both bleb-related AEs are typically reported following conventional surgeries, such as trabeculectomy.13 These findings provide support for the MicroShunt design (including the suitability of a 70 µm diameter lumen to prevent chronic hypotony and the utility of the planar fin to prevent migration and periannular leakage) and implantation procedure (including the use of a scleral pocket formed with the Mani knife to secure the device and the use of a broad and deep subconjunctival/sub-Tenon’s flap with a high dose of MMC spread throughout).
Moreover, there were no signs of visibly apparent degradation to the MicroShunt after 5 years of placement as confirmed by anterior segment optical coherence tomography. This may be owing to the SIBS material, which was designed specifically for long-term use in the body,9 and has been extensively used as a coating for the drug-eluting coronary stent TAXUS.9,14 Although lack of degradation could not be assessed directly as the MicroShunt devices were still functional for up to 5 years and there was no need to remove the device, observation of the proximal tip through the cornea and lack of inflammation in the distal end, combined with the integrity of the device when needled, indicate a lack of biodegradation in the eye.
Trabeculectomy and tube shunt implantation are the most commonly performed incisional glaucoma surgeries.5 In a large, multicenter, randomized study, which compared the efficacy and safety of trabeculectomy (N=117) with tube shunt implantation (N=125), mean IOP was reduced from 23.9±5.7 mm Hg at baseline to 12.4±4.4 mm Hg at year 1 in the trabeculectomy group and from 23.3±4.9 to 13.8±4.1 mm Hg in the tube shunt group.6 Postoperative interventions and complications were substantial with both techniques6; 41% (n=48) and 29% (n=36) of patients experienced surgical complications following trabeculectomy and tube shunt implantation, respectively.6 In the study reported herein, the need for postoperative management was low for 5 years after MicroShunt implantation, although the sample sizes are not directly comparable.
Other bleb-forming MIGS devices have achieved IOP reductions that are modest in comparison with trabeculectomy and large tube valves; however, these devices exhibited a more favorable safety profile.8 For example, the efficacy and safety of the XEN ab-interno implantation were evaluated in a prospective, nonrandomized, multicenter study (n=64). By the end of the 4-year follow-up period, mean IOP significantly reduced from 22.5±4.2 to 13.4±3.1 mm Hg (P<0.001); similarly the mean number of medications was significantly reduced from 2.4±1.3 at baseline to 1.2±1.3 (P<0.001). The most common reintervention was bleb needling, with 34 eyes (53%) requiring needling during the 4-year period; of these eyes, 13 (72%) required needling before year 1.15 In contrast, in this study, the incidence of needling was low following Microshunt implantation (n=2, 8.7%). Prospective head-to-head studies between conventional surgeries (trabeculectomy) and novel techniques (XEN and the MicroShunt) are yet to be completed; therefore, direct comparisons are currently not possible given that study populations and definitions of success will be different in the existing published trials.
As the study reported here was the first feasibility study of the MicroShunt in patients with POAG, there were several important learnings regarding the surgical technique including (1) the use of a relaxing incision before exposing the sclera and passing scissors beneath the Tenon’s capsule to separate it from the sclera in the formation of a scleral flap; (2) when inserting the needle to create the sclera tunnel, it was important to avoid depression of the surface of the sclera as this creates a U-shape in the needle tract causing the MicroShunt to turn toward the cornea instead of being successfully inserted parallel to the iris; (3) closure of the incision in 2 separate steps by suturing the Tenon’s tissue first before closing the conjunctiva; this is important to ensure that the MicroShunt is placed under the Tenon’s capsule with the distal end not sticking into and being blocked by Tenon’s.
The use of MMC was also believed to be an important element to the success of the surgical procedure. Before the insertion of the MicroShunt, an incision was made, and a dose of 0.4 mg/mL MMC was delivered using the Peng-Khaw technique.16,17 The application and dose of MMC used in this manner ensured that fibrosis did not block the device (as demonstrated by the low reoperation rate in this series of patients) and may have contributed to the long-term efficacy and safety results. There is no clear consensus in the literature on the most efficacious dose of MMC to use during glaucoma surgery,18 and thus the dose and exposure time of MMC during MicroShunt implantation needs to be explored further. Importantly, when MicroShunt implantation was performed in combination with cataract surgery, MMC was applied before phacoemulsification to avoid the antimetabolite entering the anterior chamber during MicroShunt insertion. Bipolar diathermy was also used before the placement of the MMC-soaked sponges to clear the incision site, as human serum factors have been shown to inhibit the antifibrotic activity of MMC on Tenon’s fibroblast cells in vitro.19 The interference of MMC activity by blood factors is supported in the literature as autologous blood injections have been shown to reverse hypotony caused by overfiltration following trabeculectomy with MMC,20 which may in part be explained by the inhibition of MMC.19
There are a few limitations to this study. It was conducted at a single site, with 2 surgeons implanting the MicroShunt, and a small number of patients being enrolled. All patients were diagnosed with POAG; therefore, the results reported here may not be applicable to different types of glaucoma. As this was the first feasibility study, MicroShunt implantation was a new procedure; therefore, the surgical technique was continually being assessed and optimized by the surgeons throughout the study. Finally, the study was not powered or randomized to provide evidence of surgical outcomes compared with other surgical procedures.
In summary, this study, with a long-term follow-up period, demonstrated sustainable and predictable reductions in both mean IOP and number of glaucoma medications up to 5 years post-MicroShunt implantation. In addition, there were no reports of long-term sight-threatening AEs or device degradation. Following the 3-year results of this study, the MicroShunt received CE marking for the treatment of POAG in patients with IOP not controlled on maximum tolerated medical therapy.21 The learnings from this study have contributed to the methodology used in 2 completed European clinical studies (one single-center study22 and one multicenter study23) and 1 European/US multicenter randomized study, with the goal of assessing the efficacy and safety of the MicroShunt compared with trabeculectomy.24
ACKNOWLEDGMENTS
The authors thank Santen for their support in the development of this manuscript, in particular Dr Leonard Pinchuk for his contribution to the development of the PRESERFLO MicroShunt and Zhengyang Shi, Dr Raymund Angeles, and Dr Omar Sadruddin for their support in the development of this manuscript.
Footnotes
The sponsor (InnFocus Inc., Miami, FL, a Santen Pharmaceutical Co. Ltd Company, Osaka, Japan) participated in the design and conduct of the study, data collection, and management. This analysis was also funded by Santen Inc. (Emeryville, CA), who participated in the data analysis, interpretation of the data, preparation, review, and approval of the manuscript. Medical writing support, including preparation of manuscript drafts for critical revision and approval by the authors in accordance with Good Publication Practice (GPP3), was provided by Bethany Broughton, MSc, Helios Medical Communications (Cheshire, UK).
Disclosure: J.F.B. is a consultant for InnFocus Inc. (a Santen Pharmaceutical Co. Ltd company) and received travel grants from Santen. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet. 2017;390:2183–2193. [DOI] [PubMed] [Google Scholar]
- 3.El Hajj Moussa WG, Farhat RG, Nehme JC, et al. Comparison of efficacy and ocular surface disease index score between bimatoprost, latanoprost, travoprost, and tafluprost in glaucoma patients. J Ophthalmol. 2018;2018:1319628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JP, Fong DS, Fang EN, et al. Characterization of glaucoma medication adherence in Kaiser Permanente Southern California. J Glaucoma. 2016;25:22–26. [DOI] [PubMed] [Google Scholar]
- 5.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gedde SJ, Feuer WJ, Shi W, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology. 2018;125:650–663. [DOI] [PubMed] [Google Scholar]
- 7.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saheb H. Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. [DOI] [PubMed] [Google Scholar]
- 9.Pinchuk L, Riss I, Batlle JF, et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J Biomed Mater Res B Appl Biomater. 2017;105:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25:e58–e65. [DOI] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov. NCT00772330. Clinical study of the safety and performance of the Miami InnFocus Drainage Implant to relieve glaucoma symptoms; 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT00772330. Accessed August 6, 2020.
- 12.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30:287–290. [DOI] [PubMed] [Google Scholar]
- 13.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. [DOI] [PubMed] [Google Scholar]
- 15.Lenzhofer M, Kersten-Gomez I, Sheybani A, et al. Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol. 2019;47:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkins MR, Occleston NL, Kotecha A, et al. Sponge delivery variables and tissue levels of 5-fluorouracil. Br J Ophthalmol. 2000;84:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhingra S, Khaw PT. The moorfields safer surgery system. Middle East Afr J Ophthalmol. 2009;16:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Habash A, Aljasim LA, Owaidhah O, et al. A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin Ophthalmol. 2015;9:1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowston JG, Wang XY, Khaw PT, et al. Human serum reduces mitomycin-C cytotoxicity in human Tenon’s fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:946–952. [DOI] [PubMed] [Google Scholar]
- 20.Okada K, Tsukamoto H, Masumoto M, et al. Autologous blood injection for marked overfiltration early after trabeculectomy with mitomycin C. Acta Ophthalmol Scand. 2001;79:305–308. [DOI] [PubMed] [Google Scholar]
- 21.Santen. InnFocus MicroShunt® Glaucoma Drainage System: instructions for use. 2012.
- 22.ClinicalTrials.gov. Safety and performance of Miami InnFocus Drainage Implant (MIDI Arrow) glaucoma drainage implant. NCT01563237; 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT01563237. Accessed August 6, 2020.
- 23.ClinicalTrials.gov. Post market study of the InnFocus MicroShunt. NCT02177123; 2018. Available at: www.clinicaltrials.gov/ct2/show/NCT02177123. Accessed August 6, 2020.
- 24.ClinicalTrials.gov. InnFocus MicroShunt versus trabeculectomy study (IMS). NCT01881425; 2018. Available at: www.clinicaltrials.gov/ct2/show/NCT01881425. Accessed August 6, 2020.


