Abstract
Background
This study evaluated the impact of acute kidney injury (AKI) on posttransplant clinical outcomes for deceased donor (DD) kidney transplantation (KT) using the Kidney Donor Profile Index (KDPI) system.
Methods
Overall, 657 kidney transplant recipients (KTRs) receiving kidneys from 526 DDs from four transplant centers were included. We divided them into the high and low KDPI donor groups by 65%, the KDPI score, and both groups were subdivided into the AKI-DDKT and non-AKI-DDKT subgroups according to AKI in DDs.
Results
There was no significant difference in the incidence of delayed graft function (DGF) between the high and low KDPI-KTR groups; however, the AKI-DDKT subgroup showed significantly higher incidence of DGF than the non-AKI-DDKT subgroup in both groups (p = 0.001, p < 0.001, respectively). The death-censored graft survival rate was significantly lower in the high KDPI-KTR group than in the low KDPI-KTR group (p = 0.005). Only in the high KDPI-KTR group, the death-censored graft survival rate was significantly lower in the KT from DDs with AKI stage 3 than KT from DDs with non-AKI or AKI stage 1 or 2 (p = 0.040). The interaction between AKI stage 3 in DDs and high KDPI on the allograft outcome was significant (p = 0.002).
Conclusion
KTs from DDs with AKI stage 3 showed an adverse impact on the allograft outcome in the high KDPI-KTR group. Therefore, DDs with a high KDPI score should be managed carefully so that severe AKI does not occur prior to KT.
Keywords: Acute kidney injury, Brain death, Graft survival, Kidney transplantation, Mortality
Introduction
Although one of the biggest drawbacks of kidney transplantation (KT) is the problem of organ shortage, the number of discarded kidneys still increases worldwide [1–3]. In 2014, the Kidney Donor Profile Index (KDPI) system, a new allocation system in the United States (US), was introduced, but the number of discarded kidneys in the US is not decreasing, and there is still a disagreement about the utility of this system [1,4–6]. The major factor of this discard is reported as the donor kidney function at the time of KT [7–9]. Recently, the efficacy of conducting procurement biopsy before transplantation has been reported, but it is still not commercialized [10]. Thus, it is important to trust and actively apply the current allocation system [4]. In particular, a kidney injury during donor management affects the condition of the donor kidney, which is mainly affected by the hemodynamic condition [11].
Several researches have recently been published regarding the clinical impact of KT from deceased donors (DDs) with acute kidney injury (AKI) in deceased donor KT (DDKT) [12,13]. AKI is very commonly detected in individuals with brain death state for various causes [14,15]. The shortage of donor kidney has also driven the use of the kidney from DDs with AKI. In addition, some research demonstrated that AKI does not affect the long-term allograft outcome although it results in lower allograft function in the early period after KT [16,17]. Our previous studies also showed that there was no significant difference in the long-term allograft outcome between the AKI-DDKT and non-AKI-DDKT groups or between the expanded criteria donor (ECD)-KT and standard criteria donor-KT groups, but AKI superimposed on ECDs or occurring in elderly DDs has a synergistically adverse impact on the long-term posttransplant allograft outcomes in the corresponding recipients [18]. Finally, the state of donor at the time of KT is important when AKI developed, but this issue is still controversial.
Recently, Koyawala and Parikh [12] have so far addressed this issue and insisted that there was no influence of DD with AKI on the long-term outcome. However, there are various limitations in this study, and it is still difficult to draw a clear conclusion on this issue as the new allocation system, KDPI score, has been published for approximately 4 years. In a previous study, the KDPI score was a useful tool to predict the allograft outcome in DDKT [19], but there was no significant difference in the long-term allograft outcome between the high and low KDPI-kidney transplant recipient (KTR) groups [20]. Based on these studies, we investigated the impact of AKI in DDs with a high or low KDPI score on posttransplant clinical outcomes. The short- and long-term clinical outcomes according to the presence of AKI on DDs in the low and high KDPI-KTR groups were analyzed. In addition, the association between AKI and high KDPI in DDs on posttransplant allograft survival was evaluated.
Methods
Study population
A total of 657 KTRs receiving kidneys from 526 DDs between October 1996 and December 2017 from four transplant centers (Seoul St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, Daejeon St. Mary’s Hospital, and Keimyung University Dongsan Hospital) were included. We divided them into the high and low KDPI donor groups by 65%, which is the median value of the KDPI score, and both groups were subdivided into the AKI-DDKT and non-AKI-DDKT subgroups according to AKI in DDs. AKI in DDs was defined and staged according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria as described in previous reports [21]. The KTRs according to the KDPI score or presence of AKI in DDs are shown in Fig. 1. Among all DDs, there were 257 high KDPI donors (48.9%) and 269 low KDPI donors (51.1%). Among all KTRs, there were 338 high KDPI-KTRs (51.4%) and 319 low KDPI-KTRs (48.6%). In the high KDPI-KTR group, there were 239 cases (36.4%) in the high KDPI-AKI-DDKT subgroup and 99 cases (15.1%) in the high KDPI-non-AKI-DDKT subgroup. In the low KDPI-KTR group, there were 148 cases (22.5%) in the low KDPI-AKI-DDKT subgroup and 171 cases (26.0%) in the low KDPI-non-AKI-DDKT subgroup.
Figure 1. Patient algorithm and distribution in this study.
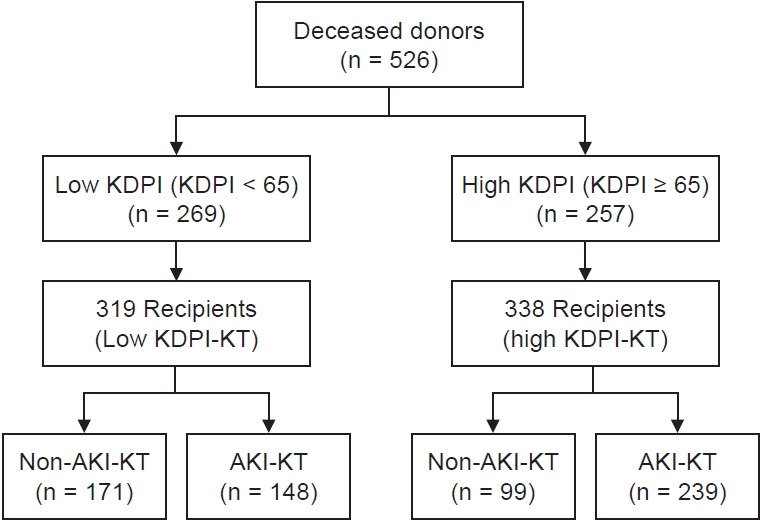
Deceased donors were classified into high KDPI and low KDPI donor groups based on the median value of KDPI of 65%. In addition, KTRs were divided into AKI-KT and non-AKI-KT subgroups.
KDPI, Kidney Donor Profile Index; KTRs, kidney transplant recipients; AKI, acute kidney injury; KT, kidney transplantation.
Clinical and laboratory parameters and clinical outcomes
The medical records of the study population were retrospectively analyzed. We investigated the data of DDs: age, sex, height, weight, body mass index (BMI, kg/m2), ethnicity, history of diabetes mellitus (DM) and hypertension (HTN), causes of brain death, serum creatinine, estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) at baseline and the admission day and prior to KT, hepatitis C virus state, and donation after cardiac death. When serum creatinine was normal at the time of admission, the serum creatinine at admission was defined as baseline creatinine even if there was no previous baseline creatinine, and serum creatinine was measured at least 2 to 3 times until KT. AKI was defined by the KDIGO guideline based on the serum creatinine at the time of admission regardless of the presence or absence of a baseline creatinine. CKD was defined when estimated GFR less than 60 mL/min/1.73 m2 continued for 3 months after measuring baseline creatinine. We used the ECD criteria according to the United Network for Organ Sharing for the definition of marginal donors [22]. We calculated the KDPI score with the terminal serum creatinine level through the KDPI calculator in the website of the Organ Procurement and Transplantation Network [23]. When the KDPI score is calculated, the factors are used as follows; age, height, weight, ethnicity, history of HTN or DM, cause of death, serum creatinine, hepatitis C virus serology, and donation after cardiac death in the DD [6]. The data of KTRs were also investigated; age, sex, height, weight, BMI, ethnicity, dialysis vintage prior to KT, frequency of KT, causes of end-stage renal disease, history of DM and HTN, cold ischemic time, number of human leukocyte antigen (HLA) mismatches, types of immunosuppressive agents for induction and maintenance, and rate of panel-reactive antibodies (PRAs).
Biopsy-proven acute rejection (BPAR) was diagnosed according to the Banff classification [24,25]. Delayed graft function (DGF) was defined as at least one dialysis requirement within the first week after KT [26]. Death-censored allograft survival rate was defined as the proportion considering the return to the dialysis or retransplantation during the study period, except for patient death with a functioning allograft. Patient survival rate was defined as the proportion considering the death from all causes during the study period.
The primary outcome of this study was to investigate the impact of AKI in DDs on the death-censored allograft survival between the high and low KDPI-KTR groups. Therefore, we compared the death-censored allograft survival between the AKI-DDKT and non-AKI-DDKT subgroups in both high and low KDPI-KTR groups and analyzed the interaction between AKI and high KDPI score. The secondary outcomes were to investigate the incidences of DGF and BPAR and changes in allograft function during the first year after KT (1 week, 2 weeks, 1 month, 3 months, 6 months, and 12 months after KT; assessed by eGFR, calculated using the CKD-EPI [27]) between the AKI-DDKT and non-AKI-DDKT subgroups in both high and low KDPI-KTR groups. Patient survival between AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group was compared.
This study was approved by the Institutional Review Boards (IRBs) of Seoul St. Mary’s Hospital (No. XC15RIMI0061K), Uijeongbu St. Mary’s Hospital (No. XC15RIMI0061U), Daejeon St. Mary’s Hospital (No. XC15RIMI0061K), and Dongsan Hospital, Keimyung University School of Medicine (No. 2020-05-047). The requirements for informed consent were waived by the IRBs of the aforementioned four centers because the use of the patient’s data for research was informed to all donors’ families and all recipients prior to KT to protect the personal information. Our study did not contain any distinguishable personal information, and all methods were performed according to the relevant guidelines and regulations.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range) and analyzed using the Student t test or the Mann-Whitney test. Categorical variables are expressed as count and percentage and analyzed using the chi-square test and Fisher exact test. The death-censored graft survival and patient survival rates were analyzed using the Kaplan-Meier curves and log-rank tests. All missing data were excluded. The Cox proportional hazards regression analysis was performed to investigate the relationship of the KDPI score and AKI for the clinical outcomes in DDKT, considering the confounding factors such as recipient age, transplant year (1996–2005 vs. 2006–2010 vs. 2011–2017), transplant center, recipient HTN, and acute rejection. Interaction effects between AKI and high KDPI score were explored by adding interaction terms to the Cox proportional hazards model with backward elimination of variables. In other words, AKI * high KDPI score as an interaction effect was included in the Cox proportional hazards model. The p-values less than 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Comparison of baseline characteristics between the high and low KDPI donors and between the high and low KDPI-KTR groups
The median follow-up duration of the study population was 48.0 months (interquartile range, 22.3–68.0). The mean age of the high KDPI donor group was significantly higher than that of the low KDPI donor group (55.0 ± 8.9 years vs. 35.5 ± 12.2 years, p < 0.001). The proportions of donors with HTN, DM, and death due to cerebrovascular accident (CVA) were significantly higher in the high KDPI donor group than in the low KDPI donor group (37.4% vs. 4.1%, p < 0.001; 17.1% vs. 2.1%, p < 0.001; 76.3% vs. 62.1%, p < 0.001, respectively). The baseline and allocation CKD-EPI eGFRs in DDs were significantly lower in the high KDPI donor group than in the low KDPI donor group (79.7 ± 20.1 mL/min/1.73 m2 vs. 87.8 ± 28.9 mL/min/1.73 m2, p = 0.001; 51.6 ± 29.6 mL/min/1.73 m2 vs. 94.9 ± 44.6 mL/min/1.73 m2, p < 0.001). The proportion of DDs with CKD stage 3 or above at allocation was significantly higher in the high KDPI donor group than in the low KDPI donor group (67.7% vs. 30.9%, p < 0.001). The proportion of AKI was also significantly higher in the high KDPI donor group than in the low KDPI donor group (69.3% vs. 42.8%, p < 0.001). The proportion of ECD donors was significantly higher in the high KDPI donor group than in the low KDPI donor group (57.6% vs. 0.4%, p < 0.001). There were no significant differences in donor sex and BMI between the high and low KDPI donor groups (Table 1).
Table 1.
Comparison of clinical and laboratory parameters between high KDPI donor (or recipient) and low KDPI donor (or recipient)
| Variable | High KDPI | Low KDPI | p-value |
|---|---|---|---|
| Donor | 257 | 269 | |
| Age at KT (yr) | 55.0 ± 8.9 | 35.5 ± 12.3 | <0.001 |
| Sex, male:female | 171:86 | 197:72 | 0.106 |
| BMI (kg/m2) | 23.1 ± 3.2 | 22.6 ± 3.9 | 0.223 |
| HTN | 96 (37.4) | 11 (4.1) | <0.001 |
| DM | 44 (17.1) | 6 (2.2) | <0.001 |
| Cause of donor death, CVA | 196 (76.3) | 167 (62.1) | <0.001 |
| eGFR (mL/min/1.73 m2) | |||
| Baseline | 79.7 ± 20.1 | 87.8 ± 28.9 | 0.001 |
| At allocation | 51.6 ± 29.6 | 94.9 ± 44.6 | <0.001 |
| CKD stage 3 or above stage | 174 (67.7) | 83 (30.9) | <0.001 |
| AKI | 178 (69.3) | 115 (42.8) | <0.001 |
| Stage 1 | 61 (23.7) | 52 (19.3) | |
| Stage 2 | 55 (21.4) | 27 (10.0) | |
| Stage 3 | 62 (24.1) | 36 (13.4) | |
| ECD | 148 (57.6) | 1 (0.4) | <0.001 |
| Recipient | 338 | 319 | |
| Transplant year | 0.004 | ||
| 1996–2005 | 0 (0) | 8 (2.5) | |
| 2006–2010 | 44 (13.0) | 55 (17.2) | |
| 2011–2016 | 294 (87.0) | 256 (80.3) | |
| Age at KT (yr) | 51.3 ± 10.1 | 47.6 ± 9.8 | <0.001 |
| Sex, male:female | 201:137 | 188:131 | 0.937 |
| BMI (kg/m2) | 23.3 ± 3.5 | 23.0 ± 4.1 | 0.297 |
| HTN | 290 (85.8) | 263 (82.4) | 0.242 |
| DM | 82 (24.3) | 55 (17.2) | 0.028 |
| Dialysis duration before KT (yr) | 7.3 ± 11.7 | 8.8 ± 8.6 | 0.289 |
| Previous KT | 25 (7.4) | 45 (14.1) | 0.008 |
| Cause of ESRD | 0.003 | ||
| Glomerulonephritis | 142 (42.0) | 156 (48.9) | |
| DM | 70 (20.7) | 44 (13.8) | |
| HTN | 70 (20.7) | 45 (14.1) | |
| Others | 56 (16.6) | 74 (23.2) | |
| Cold ischemic time (min) | 247.9 ± 118.7 | 254.2 ± 129.8 | 0.531 |
| HLA mismatch number | 3.8 ± 1.5 | 3.5 ± 1.5 | 0.014 |
| Induction | 0.017 | ||
| Basiliximab | 224 (66.3) | 239 (74.9) | |
| ATG | 114 (33.7) | 80 (25.1) | |
| Major immunosuppressant, tacrolimus:cyclosporine | 335 : 3 | 312 : 6 | 0.251 |
| PRA > 50 % | 50 (14.8) | 64 (20.1) | 0.048 |
| Follow-up duration (mo) | 44.1 ± 28.7 | 52.2 ± 39.9 | 0.003 |
Data are expressed as number only, mean ± standard deviation, or number (%).
eGFR is calculated using Chronic Kidney Disease Epidemiology Collaboration.
AKI, acute kidney injury; ATG, antithymocyte globulin; BMI, body mass index; CKD, chronic kidney disease; CVA, cerebrovascular accident; DM, diabetes mellitus; ECD, expanded criteria donor; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HLA, human leukocyte antigen; HTN, hypertension; KDPI, Kidney Donor Profile Index; KT, kidney transplantation; PRA, panel-reactive antibody.
In corresponding recipients, the mean age was also higher in the high KDPI-KTR group than in the low KDPI-KTR group (51.3 ± 10.1 years vs. 47.6 ± 9.8 years, p < 0.001). The proportions of KTRs with DM and use of antithymocyte globulin for induction immunosuppressant were significantly higher in the high KDPI-KTR group than in the low KDPI-KTR group (24.3% vs. 17.2%, p = 0.028; 33.7% vs. 25.1%, p = 0.017), but the proportion of retransplantation was significantly higher in the low KDPI-KTR group than in the high KDPI-KTR group (14.1% vs. 7.4%, p = 0.008). The mean HLA mismatch number was significantly higher in the high KDPI-KTR group than in the low KDPI-KTR group (3.8 ± 1.5 vs. 3.5 ± 1.5, p = 0.014). There was no significant difference in the distribution of recipient sex, dialysis duration before KT, cold ischemic time, and proportion of PRA > 50% between the high and low KDPI-KTR groups (Table 1).
Comparison of baseline characteristics between the high and low KDPI-KTR groups and between the AKI-DDKT and non-AKI-DDKT subgroups
In the high KDPI donor group, the proportions of male sex and cause of donor death by CVA were significantly higher in the AKI donor subgroup compared with those in the non-AKI donor subgroup (71.3% vs. 55.6%, p = 0.016; 78.7% vs. 70.9%, p = 0.024). The proportions of HTN and eGFR at baseline and allocation were significantly lower in the AKI donor subgroup compared with those in the non-AKI donor subgroup (30.9% vs. 51.9%, p = 0.002; 45.4 ± 26.6 vs. 81.0 ± 27.0, p < 0.001; 34.0 ± 21.0 vs. 77.7 ± 24.7, p < 0.001). On the contrary, in the low KDPI donor group, the BMI and proportion of CKD stage 3 or above were significantly higher in the AKI donor subgroup compared with those in the non-AKI donor subgroup (24.1 ± 4.0 vs. 22.2 ± 3.9, p < 0.001; 48.7% vs. 36.4%, p = 0.046), but eGFRs at baseline and allocation were significantly lower in the AKI donor subgroup compared with those in the non-AKI donor subgroup (59.0 ± 36.0 vs. 102.3 ± 34.5, p < 0.001; 50.5 ± 32.0 vs. 106.7 ± 31.4, p < 0.001).
In corresponding recipients, the proportions of antithymocyte globulin were significantly higher in the AKI-DDKT subgroup compared with those in the non-AKI-DDKT subgroup in both high and low KDPI-KTR groups (41.0% vs. 16.2%, p < 0.001; 33.8% vs. 17.5%, p = 0.001, respectively). There was no significant difference in the distribution of recipient age, sex, HTN, DM, dialysis duration before KT, previous KT, cold ischemic time, main immunosuppressant, and proportion of PRA > 50% between the high and low KDPI-KTR groups (Table 2).
Table 2.
Comparison of clinical and laboratory parameters according to AKI in high or low KDPI donor KTR
| Variable | High KDPI-KTR |
Low KDPI-KTR |
||||
|---|---|---|---|---|---|---|
| Non-AKI-DDKT | AKI-DDKT | p-value | Non-AKI-DDKT | AKI-DDKT | p-value | |
| Donor | 79 | 178 | 154 | 115 | ||
| Age at KT (yr) | 56.2 ± 10.0 | 54.5 ± 8.3 | 0.173 | 34.8 ± 13.7 | 36.4 ± 10.7 | 0.261 |
| Sex, male:female | 44:35 | 127:51 | 0.016 | 107:47 | 90:25 | 0.126 |
| BMI (kg/m2) | 23.3 ± 3.3 | 23.1 ± 3.1 | 0.653 | 22.2 ± 3.9 | 24.1 ± 4.0 | <0.001 |
| HTN | 41 (51.9) | 55 (30.9) | 0.002 | 8 (5.2) | 3 (2.6) | 0.362 |
| DM | 14 (17.7) | 30 (16.9) | 0.859 | 4 (2.6) | 2 (1.7) | > 0.999 |
| Cause of donor death, CVA | 56 (70.9) | 140 (78.7) | 0.024 | 92 (59.7) | 75 (65.2) | 0.377 |
| eGFR (mL/min/1.73 m2) | ||||||
| Baseline | 81.0 ± 27.0 | 45.4 ± 26.6 | <0.001 | 102.3 ± 34.5 | 59.0 ± 36.0 | <0.001 |
| At allocation | 77.7 ± 24.7 | 34.0 ± 21.0 | <0.001 | 106.7 ± 31.4 | 50.5 ± 32.0 | <0.001 |
| CKD stage 3 or above stage | 37 (46.8) | 85 (47.8) | 1 | 56 (36.4) | 56 (48.7) | 0.046 |
| ECD | 39 (49.4) | 109 (61.2) | 0.1 | 0 | 1 (0.9) | 0.428 |
| Recipient | 99 | 239 | 171 | 148 | ||
| Transplant year | 0.157 | 0.098 | ||||
| 1996–2005 | 0 (0) | 0 (0) | 7 (4.1) | 1 (0.7) | ||
| 2006–2010 | 17 (17.2) | 27 (11.3) | 34 (19.9) | 21 (14.2) | ||
| 2011–2016 | 82 (82.8) | 212 (88.7) | 130 (76.0) | 126 (85.1) | ||
| Age at KT (yr) | 51.4 ± 10.7 | 51.3 ± 9.9 | 0.878 | 47.0 ± 8.8 | 48.3 ± 10.7 | 0.261 |
| Sex, male:female | 60:39 | 141:98 | 0.809 | 103:68 | 85:63 | 0.649 |
| BMI (kg/m2) | 23.3 ± 3.4 | 23.3 ± 3.5 | 0.981 | 22.7 ± 3.2 | 23.4 ± 4.9 | 0.141 |
| HTN | 84 (84.8) | 206 (86.2) | 0.735 | 141 (82.5) | 122 (82.4) | > 0.999 |
| DM | 18 (18.2) | 64 (26.8) | 0.097 | 29 (17.0) | 26 (17.6) | 0.883 |
| Dialysis duration before KT (yr) | 7.0 ± 4.5 | 8.3 ± 13.6 | 0.895 | 9.0 ± 9.8 | 8.6 ± 7.1 | 0.728 |
| Previous KT | 6 (6.1) | 19 (7.9) | 0.652 | 18 (10.5) | 27 (18.2) | 0.054 |
| Cause of ESRD | 0.290 | 0.022 | ||||
| Glomerulonephritis | 45 (45.5) | 97 (40.6) | 95 (55.6) | 61 (41.2) | ||
| DM | 15 (15.2) | 55 (23.0) | 24 (14.0) | 20 (13.5) | ||
| HTN | 19 (19.2) | 51 (21.3) | 23 (13.5) | 22 (14.9) | ||
| Others | 20 (20.2) | 36 (15.1) | 29 (17.0) | 45 (30.4) | ||
| Cold ischemic time (min) | 250.2 ± 112.6 | 247.0 ± 121.4 | 0.828 | 262.3 ± 124.7 | 245.1 ± 135.2 | 0.257 |
| HLA mismatch number | 3.7 ± 1.6 | 3.8 ± 1.4 | 0.541 | 3.5 ± 1.5 | 3.5 ± 1.4 | 0.634 |
| Induction | <0.001 | 0.001 | ||||
| Basiliximab | 83 (83.8) | 141 (59.0) | 141 (82.5) | 98 (66.2) | ||
| ATG | 16 (16.2) | 98 (41.0) | 30 (17.5) | 50 (33.8) | ||
| Main immunosuppressant, tacrolimus:cyclosporine | 97:2 | 238:1 | 0.206 | 165:6 | 147:1 | 0.222 |
| PRA > 50% | 13 (13.1) | 37 (15.5) | 0.864 | 32 (21.6) | 32 (23.0) | 0.779 |
Data are expressed as number only, mean ± standard deviation, or number (%).
eGFR is calculated using Chronic Kidney Disease Epidemiology Collaboration.
AKI, acute kidney injury; ATG, antithymocyte globulin; BMI, body mass index; CKD, chronic kidney disease; CVA, cerebrovascular accident; DDKT, deceased donor kidney transplantation; DM, diabetes mellitus; ECD, expanded criteria donor; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease, HLA, human leukocyte antigen; HTN, hypertension; KDPI, Kidney Donor Profile Index; KT, kidney transplantation; KTR, kidney transplant recipient; PRA, panel-reactive antibody.
Comparison of the effect of AKI in DDs on the incidences of DGF and BPAR and changes in allograft function between the high and low KDPI-KTR groups
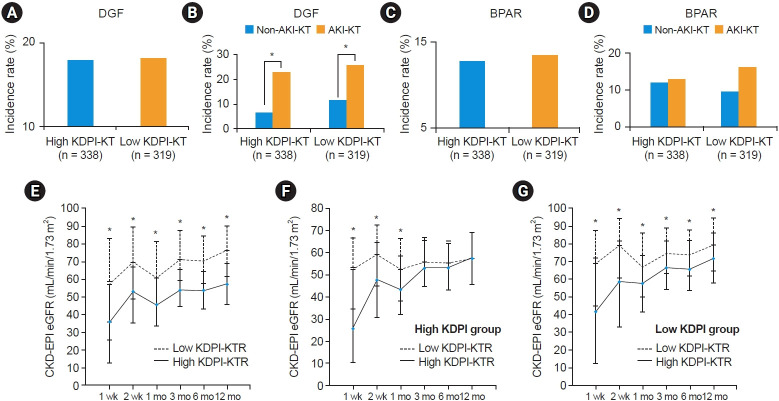
The incidence of DGF was not significantly different between the high and low KDPI-KTR groups (18.0% vs. 18.2%, p > 0.999) (Fig. 2A). In the subgroup analysis, the incidence of DGF was significantly higher in the AKI-DDKT subgroup compared with that in the non-AKI-DDKT subgroup in both high and low KDPI-KTR groups (23.0% vs. 6.1%, p < 0.001; 25.7% vs. 11.7%, p = 0.001) (Fig. 2B).
Figure 2. Comparison of the DGF incidence rates, BPAR incidence rates, and the changes in allograft function after kidney transplant.
(A, B) Comparison of DGF incidence rates (A) between high KDPI-KTR and low KDPI-KTR groups and (B) between AKI-DDKT and non-AKI-DDKT subgroups in the high KDPI-KTR or low KDPI-KTR group. (C, D) Comparison of BPAR incidence rate (C) between high KDPI-KTR and low KDPI-KTR groups and (D) between AKI-DDKT and non-AKI-DDKT subgroups in the high KDPI-KTR or low KDPI-KTR group. (E-G) Comparison of the change in allograft function after kidney transplant (E) between high KDPI-KTR and low KDPI-KTR groups, (F) between AKI-DDKT and non-AKI-DDKT subgroups in the high KDPI-KTR group, and (G) between AKI-DDKT and non-AKI-DDKT subgroups in the low KDPI-KTR group.
AKI, acute kidney injury; BPAR, biopsy-proven acute rejection; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DDKT, deceased donor kidney transplantation; DGF, delayed graft function; KDPI, Kidney Donor Profile Index; KTR, kidney transplant recipient.
*p < 0.001 vs. non-AKI-DDKT.
The incidence of BPAR within the first year after KT did not differ significantly between the high and low KDPI-KTR groups (12.7% vs. 12.5%, p > 0.999) (Fig. 2C). Moreover, there was no significant difference in the incidence of BPAR between the AKI-DDKT and non-AKI-DDKT subgroups in both high and low KDPI-KTR groups (13.0% vs. 12.1%, p > 0.999; 16.2% vs. 9.4%, p = 0.089) (Fig. 2D).
Allograft function for 12 months (1 week, 2 weeks, 1 month, 3 months, 6 months, and 12 months) after KT was significantly lower in the high KDPI-KTR group compared with that in the low KDPI-KTR group (39.4 ± 28.3 vs. 56.2 ± 33.3, p < 0.001; 52.0 ± 24.4 vs. 68.1 ± 29.0, p < 0.001; 48.4 ± 20.8 vs. 63.5 ± 23.6, p < 0.001; 56.0 ± 18.8 vs. 72.5 ± 20.5, p < 0.001; 54.8 ± 18.4 vs. 71.5 ± 20.1, p < 0.001; 57.6 ± 19.7 vs. 75.0 ± 22.5, p < 0.001) (Fig. 2E). In the high KDPI-KTR group, allograft function within 3 months (1 week, 2 weeks, 1 month) after KT was significantly lower in the AKI-DDKT subgroup compared with that in the non-AKI-DDKT subgroup (33.7 ± 27.0 vs. 53.3 ± 26.5, p < 0.001; 48.4 ± 24.7 vs. 60.9 ± 21.5, p < 0.001; 46.1 ± 20.9 vs. 54.2 ± 19.6, p = 0.001), but there was no significant difference between 3 and 12 months (3 months, 6 months, and 12 months) (55.3 ± 18.5 vs. 57.8 ± 19.5, p = 0.273; 54.3 ± 18.0 vs. 56.1 ± 19.4, p = 0.413; 57.6 ± 19.3 vs. 57.4 ± 20.9, p = 0.905) (Fig. 2F). In the low KDPI-KTR group, allograft function for 12 months (1 week, 2 weeks, 1 month, 3 months, 6 months, and 12 months) after KT was significantly lower in the AKI-DDKT subgroup compared with that in the non-AKI-DDKT subgroup (p < 0.001) (45.2 ± 34.6 vs. 65.7 ± 29.1, p < 0.001; 58.5 ± 30.4 vs. 76.4 ± 24.9, p < 0.001; 58.1 ± 23.9 vs. 68.1 ± 22.4, p < 0.001; 68.2 ± 21.6 vs. 76.2 ± 18.7, p < 0.001; 67.5 ± 21.2 vs. 74.8 ± 18.6, p = 0.002; 70.4 ± 23.3 vs. 78.7 ± 21.1, p = 0.001) (Fig. 2G).
Comparison of the impact of AKI in DDs on the death-censored allograft survival between the high and low KDPI-KTR groups
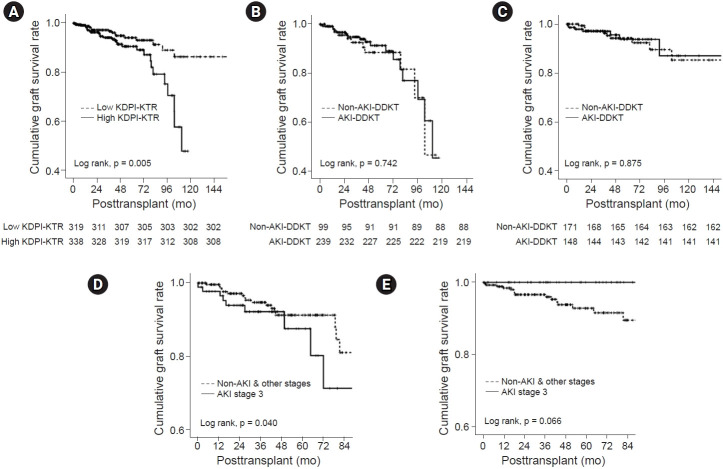
A total of 49 cases (49 of 657, 7.5%) of graft failure developed, including 31 cases (4.7%) in the high KDPI-KTR group (20 and 11 patients in the AKI-DDKT and non-AKI-DDKT subgroups, respectively) and 18 cases (2.7%) in the low KDPI-KTR group (7 and 11 patients in the AKI-DDKT and non-AKI-DDKT subgroups, respectively). There were no significant differences in the distribution of the causes of allograft failure between the AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group (Table 3). The death-censored graft survival rate was significantly lower in the high KDPI-KTR group compared with that in the low KDPI-KTR group (p = 0.005) (Fig. 3A). However, there was no significant difference in the death-censored graft survival rates between the AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group (Fig. 3B, C). In the multivariate analysis, a high KDPI score was an independent risk factor for allograft failure after adjustment for recipient age, transplant year, transplant center, recipient HTN, and acute rejection (hazard ratio [HR], 3.096; 95% confidence interval [CI], 1.642–5.838; p < 0.001), not donor AKI. There was not a significant interaction between AKI in DDs and high KDPI DDs for allograft failure (p for interaction = 0.088). There was no significant difference in the incidence of death-censored graft failure according to the AKI stage in the high and low KDPI-KTR groups (Supplementary Table 1, available online). However, in the high KDPI-KTR group, AKI stage 3 showed the lowest death-censored allograft survival rate in comparison with non-AKI and AKI stages 1 and 2 (P = 0.040) (Fig. 3D), but not in the low KDPI-KTR group (Fig. 3E). In the multivariate analysis, the combination of high KDPI score and AKI stage 3 was an independent risk factor for allograft failure after adjustment for recipient age, transplant year, transplant center, recipient HTN, acute rejection, PRA > 50%, HLA mismatch, and induction immunosuppressant (HR, 2.707; 95% CI, 1.324–5.536; p = 0.006). There was a significant interaction between AKI stage 3 in DDs and high KDPI DDs for allograft failure (p for interaction = 0.002) (Table 4).
Table 3.
Comparison of clinical outcomes according to AKI in high or low KDPI donor KTR
| Variable | High KDPI-KTR |
Low KDPI-KTR |
||||
|---|---|---|---|---|---|---|
| Non-AKI-DDKT | AKI-DDKT | p-value | Non-AKI-DDKT | AKI-DDKT | p-value | |
| Causes of graft failure | 0.170 | 0.952 | ||||
| Acute rejection | 4 (36.4) | 8 (40.0) | 6 (54.5) | 3 (42.9) | ||
| Chronic rejection | 1 (9.1) | 8 (40.0) | 1 (9.1) | 1 (14.3) | ||
| Recurrent glomerulonephritis | 2 (18.2) | 2 (10.0) | 1 (9.1) | 0 (0) | ||
| Ischemia | 0 (0) | 0 (0) | 2 (18.2) | 2 (28.6) | ||
| Infection | 1 (9.1) | 1 (5.0) | 0 | 1 (14.3) | ||
| BK virus-associated nephropathy | 3 (27.3) | 1 (5.0) | 1 (9.1) | 0 (0) | ||
| Causes of death | 0.530 | > 0.999 | ||||
| Cardiovascular disease | 2 (50.0) | 4 (28.6) | 2 (16.7) | 0 (0) | ||
| Cerebrovascular accident | 0 | 1 (7.1) | 0 (0) | 0 (0) | ||
| Infection | 1 (25.0) | 5 (35.7) | 5 (41.7) | 3 (100) | ||
| Malignancy | 0 (0) | 1 (7.1) | 2 (16.7) | 0 (0) | ||
| Gastrointestinal bleeding | 1 (25.0) | 0 | 1 (8.3) | 0 | ||
| Unknown | 0 (0) | 3 (21.4) | 2 (16.7) | 0 | ||
Data are expressed as number (%).
KDPI, Kidney Donor Profile Index; KTR, kidney transplant recipient; AKI, acute kidney injury; DDKT, deceased donor kidney transplantation
Figure 3. Comparison of the death-censored graft survival rates according to the KDPI group and AKI subgroup and the death-censored graft survival rates according to the AKI stage.
(A–C) Comparison of the death-censored graft survival rate (A) between the high KDPI-KTR and low KDPI-KTR groups, (B) between AKI-DDKT and non-AKI-DDKT subgroups in the high KDPI-KTR group, and (C) between AKI-DDKT and non-AKI-DDKT subgroups in the low KDPI-KTR group. (D, E) Comparison of the death-censored graft survival rate according to the AKI stage (D) in the high KDPI-KTR group and (E) in the low KDPI-KTR group.
AKI, acute kidney injury; DDKT, deceased donor kidney transplantation; KDPI, Kidney Donor Profile Index; KTR, kidney transplant recipient.
Table 4.
Odds ratios (OR) for allograft failure on the status of AKI or high KDPI donor in deceased donor
| Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value | p-value for interaction | |
|---|---|---|---|---|---|
| AKI-KT | 0.884 (0.422–1.849) | 0.742 | 1.011 (0.484–2.114) | 0.976 | 0.088b |
| AKI stage 3-KT | 1.349 (0.701–2.596) | 0.370 | 1.357 (0.583–3.155) | 0.479 | 0.002c |
| High KDPI-KT | 2.304 (1.262–4.205) | 0.007 | 3.096 (1.642–5.838) | <0.001 |
AKI, acute kidney injury; CI, confidence interval; KDPI, Kidney Donor Profile Index; KT, kidney transplantation.
Adjusted by recipient age, transplant year, transplant center, recipient hypertension, acute rejection, panel-reactive antibody of >50%, human leukocyte antigen mismatch, and induction immunosuppressant.
An interaction between AKI in deceased donors and high KDPI deceased donors for allograft failure.
An interaction between AKI stage 3 in deceased donors and high KDPI deceased donors for allograft failure.
Comparison of the impact of AKI in DDs on the patient survival between the high and low KDPI-KTR groups
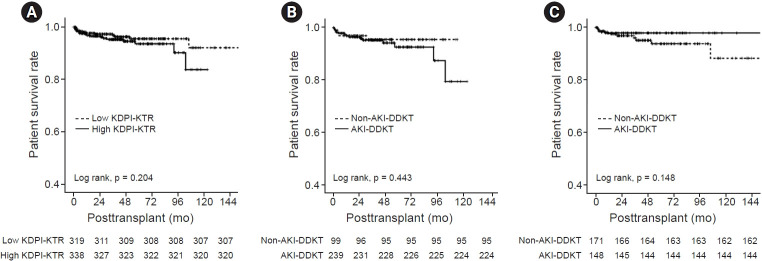
A total of 33 patients (33 of 657, 5.0%) died, 18 cases (2.7%) of whom were in the high KDPI-KTR group (14 and 4 patients in the AKI-DDKT and non-AKI-DDKT subgroups, respectively) and 15 cases (2.3%) of whom were in the low KDPI-KTR group (3 and 12 patients in the AKI-DDKT and non-AKI-DDKT subgroups, respectively). There were no significant differences in the distribution of the cause of patient death between the AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group (Table 3). There was no significant difference in the patient survival rate between the high and low KDPI-KTR groups (Fig. 4A). In the high KDPI-KTR group, there was no significant difference in the patient survival rate between the AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group (Fig. 4B, C).
Figure 4. Comparison of the patient survival rates.
Comparison of (A) between the high KDPI-KTR and low KDPI-KTR groups, (B) between AKI-DDKT and non-AKI-DDKT subgroups in the high KDPI-KTR group, and (C) between AKI-DDKT and non-AKI-DDKT subgroups in the low KDPI-KTR group.
AKI, acute kidney injury; DDKT, deceased donor kidney transplantation; KDPI, Kidney Donor Profile Index; KTR, kidney transplant recipient.
Discussion
For a long time, the ECD criteria have been used to determine to accept or discard DD kidneys. Our previous study reported that the allograft outcome was poor when ECD was accompanied by AKI [18]. Our other study also reported that the elderly donor had a poor allograft outcome when accompanied by AKI [28]. In other words, the poor kidney state of the DDs prior to KT can have a synergistic effect when this situation is accompanied by AKI. Therefore, it is very important to evaluate the kidney state of the DDs prior to KT. We have reported on the usefulness of KDPI for evaluating the kidney state of the DDs prior to KT and predicting allograft outcome [19]. Furthermore, we performed this current study based on the previous one.
First, we compared the clinical characteristics of the high and low KDPI donors. The mean age of donor at KT and proportions of HTN, DM, CVA, AKI, and ECD were significantly higher, and the mean CKD-EPI eGFRs at baseline and allocation were significantly lower in the high KDPI donors than in the low KDPI donors perhaps because the KDPI score contains donor age, creatinine, history of HTN and DM, and cause of death (CVA) [29]. The mean age of recipient at KT was significantly higher in the high KDPI-KTR group than in in the low KDPI-KTR group. Most elderly candidates received KT from marginal donors because of the benefit of old for old KT and the prevention of death prior to KT during the waiting period for DDKT [30–33]. Since the presence of DM or HTN can suggest an underlying chronic tissue injury irrespective of allograft function, such donors could be diagnosed with CKD [34]. In addition, the allograft function at baseline and allocation, as calculated by the CKD-EPI equation, was significantly lower and proportion of donors with CKD stage 3 or above was higher in the high KDPI donor group than in the low KDPI donor group. These findings showed that a significantly higher proportion of DDs with a high KDPI score had underlying CKD compared with DDs with a low KDPI score.
In the short-term clinical outcomes between the high and low KDPI-KTR groups, the occurrence of AKI in high or low KDPI score led to a higher incidence of DGF after KT. These findings suggested that AKI or high KDPI score on DDs was an independent risk factor for DGF, and this result is consistent with previous studies [26,35,36]. Recently, AKI on DDs and the recipients’ factors may have a more significant impact on the development of DGF compared with the baseline chronic damage of the allograft [37]. However, there was no difference in the development of BPAR according to the occurrence of AKI on DDs in the high or low KDPI-KTR groups. This finding suggests that the donor state prior to KT does not significantly affect the immunological response after KT.
A research reported that allograft function in the early period after KT was significantly lower in the KT from marginal DDs [35]. On the contrary, another research reported that the poor kidney state of the DDs prior to KT can cause persistent low graft function after KT [38]. In our previous study, there was no difference in the allograft function among KTs from DDs with a stable kidney state, but KT from DDs with a poor kidney state such as elderly DDs or high KDPI DDs showed persistent low allograft function after KT [19,28]. In our study, the high KDPI-KTR group showed significantly lower allograft function compared with the low KDPI-KTR group. However, the AKI-DDKT subgroup showed lower allograft function within 3 months after KT compared with the non-AKI-DDKT subgroup in the high KDPI-KTR group. On the contrary, the AKI-DDKT subgroup showed lower allograft function for 12 months after KT compared with the non-AKI-DDKT subgroup in the low KDPI-KTR group.
Our main hypothesis is that AKI in DDs has a different impact on the long-term allograft survival in the high and low KDPI-KTR groups. The death-censored graft survival rate was significantly lower in the high KDPI-KTR group than in the low KDPI-KTR group. However, in the subgroup analysis, there was no significant difference in the death-censored graft survival rates between the AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group. Interestingly, in the high KDPI-KTR group, AKI stage 3 was the lowest in the death-censored graft survival rate in comparison with non-AKI and AKI stages 1 and 2 but not in the low KDPI-KTR group. In the multivariate analysis using the Cox proportional hazards regression model, the coexistence of high KDPI and AKI stage 3 in DDs was a significant contributor to allograft failure, and we found a significant interaction between high KDPI and AKI stage 3 in DDs on allograft failure as suggested in Table 4. The aforementioned findings suggest that AKI stage 3 in DDs has a significant impact on the allograft outcomes in the high KDPI-KTR group but not in the low KDPI-KTR group.
In contrast to the death-censored allograft survival rate, the patient survival rate was not significantly different between the high and low KDPI-KTR groups, and the distribution of the cause of death did not depend on the KDPI score. There was also no significant difference in the patient survival rate between the AKI-DDKT and non-AKI-DDKT subgroups in the high or low KDPI-KTR group. It may be because the number of patient death was too small to evaluate the impact of AKI in DDs for the patient survival in the high and low KDPI-KTR groups. Therefore, a large, well-designed prospective study is required to overcome the small sample size.
Both donor and recipient factors are important in the prognosis of DDKT, but donor factors are particularly important for short-term outcomes such as DGF or allograft function at the time of early stage after KT. Therefore, an allocation system for selecting an appropriate donor is currently needed above all. In 2014, the KDPI score was introduced as a new allocation system in the US and it has been studied not only in the US but also in various countries around the world. We demonstrated that the presence of AKI in ECDs significantly impacted the long-term allograft outcomes of KTRs [18]. Furthermore, we also demonstrated that AKI in elderly DDs can significantly affect long-term allograft outcomes of KTRs [28]. In other words, when the underlying kidney status of the donor was bad, the prognosis was poor when AKI occurred. In evaluating these underlying kidney status, KDPI was more effective than the ECD criteria. Comparing ECD criteria with KDPI score, ECD criteria is a binary score that takes into account the factors of four donors, and on the other hand, the KDPI score is a continuous score that considers 10 donor factors. Therefore, the KDPI score can determine the donor status more diversely than the ECD criteria. Finally, among the variables of KDPI score, donor age and kidney function at allocation with chronic change were very important factors according to our studies. Furthermore, AKI in DDs was also an important factor. In addition, we reported that the KDPI score is effective in predicting the long-term prognosis as well as the short-term clinical course. In our previous research, we demonstrated that the KDPI scoring system was useful in predicting allograft outcomes in a Korean DDKT cohort, in particular, KT from DDs with a marginal kidney [19].
Although the KDPI score is helpful to evaluate the effect of the DD factors and predict the prognosis of posttransplant clinical outcomes, there were some limitations in our studies. First, these were retrospective studies, so the KDPI score was calculated retrograde after KT. Therefore, well-designed large-scale prospective study is needed because the KDPI score is a prospective predictor. Second, it is known that the predictive power of the KDPI is only moderate (c-statistic = 0.60). Third, all donor factors associated with graft outcomes are not included with pathologic findings. Fourth, there is a selection bias for the prognosis of KT since clinicians want to selectively perform DDKT with good quality of kidney although they consider the KDPI score.
Our study has some limitations like our previous reports using this cohort. First, because this was a retrospective cohort study, this could have selection bias. However, we analyzed the medical records of four centers considering the characteristics such as multiple transplant centers and transplant year without the loss of KTRs during the study period in the multivariate analysis. Second, because KT from both kidneys in the same transplant center was not performed, the clinical outcome of the contralateral kidney transplanted in the other transplant center could not be known. The tracking system for all transplanted or discarded kidneys is needed to overcome this problem. Lastly, the Korean allocation rule has been applied for the allocation when the brain death donor occurred. Because the KDPI system has not been validated in Korea, it has not been used in the real world. We only used the KDPI score for the research retrospectively. In spite of these limitations, this study is valuable because it is a very useful research as basic data for improving the allocation system, given the reality that the allocation criteria are not clear although DDKT with donor AKI is expanding year by year in Korea.
In conclusion, KTs from DDs with AKI stage 3 showed an adverse impact on the allograft outcome in the high KDPI-KTR group. Therefore, although AKI occurs in DDs with a high KDPI score, it is recommended to perform KT from donor kidney with AKI stages 1 and 2, and it would be better to judge more carefully for donor kidney with AKI stage 3 using additional tools such as procurement biopsy. In addition, donor management should be performed more carefully not to proceed to AKI stage 3 during donor management before allocation.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI20C0317) and also was supported by the First Research Support Project of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT in 2018 (NRF-2017R1C1B5076739).
Authors’ contributions
Conceptualization: All authors
Data curation: WYP, YKC, YSK, KJ, CWY
Funding acquisition: WYP, BHC
Investigation: YKC, YSK, KJ
Writing–original draft: WYP, SH, BHC
Writing–review & editing: WYP, CWY, SH, BHC
All authors read and approved the final manuscript.
Supplementary Materials
References
- 1.Stewart DE, Garcia VC, Rosendale JD, Klassen DK, Carrico BJ. Diagnosing the decades-long rise in the deceased donor kidney discard rate in the United States. Transplantation. 2017;101:575–587. doi: 10.1097/TP.0000000000001539. [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Choi Y, Cho JY, et al. Current status of discarded grafts in Korean organ transplantation. Transplant Proc. 2019;51:1478–1480. doi: 10.1016/j.transproceed.2019.01.119. [DOI] [PubMed] [Google Scholar]
- 3.Jin DC, Yun SR, Lee SW, et al. Current characteristics of dialysis therapy in Korea: 2016 registry data focusing on diabetic patients. Kidney Res Clin Pract. 2018;37:20–29. doi: 10.23876/j.krcp.2018.37.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stallone G, Grandaliano G. To discard or not to discard: transplantation and the art of scoring. Clin Kidney J. 2019;12:564–568. doi: 10.1093/ckj/sfz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelwahab Elhamahmi D, Chaly T, Jr, Wei G, Hall IE. Kidney discard rates in the United States during American transplant congress meetings. Transplant Direct. 2018;5:e412. doi: 10.1097/TXD.0000000000000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae S, Massie AB, Luo X, Anjum S, Desai NM, Segev DL. Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI) Am J Transplant. 2016;16:2202–2207. doi: 10.1111/ajt.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall IE, Schröppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15:1623–1631. doi: 10.1111/ajt.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrero WJ, Naik AS, Friedewald JJ, et al. Predictors of deceased donor kidney discard in the United States. Transplantation. 2017;101:1690–1697. doi: 10.1097/TP.0000000000001238. [DOI] [PubMed] [Google Scholar]
- 9.Mohan S, Chiles MC, Patzer RE, et al. Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int. 2018;94:187–198. doi: 10.1016/j.kint.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentine KL, Naik AS, Schnitzler MA, et al. Variation in use of procurement biopsies and its implications for discard of deceased donor kidneys recovered for transplantation. Am J Transplant. 2019;19:2241–2251. doi: 10.1111/ajt.15325. [DOI] [PubMed] [Google Scholar]
- 11.Kumar L. Brain death and care of the organ donor. J Anaesthesiol Clin Pharmacol. 2016;32:146–152. doi: 10.4103/0970-9185.168266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyawala N, Parikh CR. A review of donor acute kidney injury and posttransplant outcomes. Transplantation. 2020;104:1553–1559. doi: 10.1097/TP.0000000000003144. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Hall IE, Mansour S, Thiessen Philbrook HR, Jia Y, Parikh CR. Association of deceased donor acute kidney injury with recipient graft survival. JAMA Netw Open. 2020;3:e1918634. doi: 10.1001/jamanetworkopen.2019.18634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3:e000933. doi: 10.1161/JAHA.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davenport A. The brain and the kidney: organ cross talk and interactions. Blood Purif. 2008;26:526–536. doi: 10.1159/000167800. [DOI] [PubMed] [Google Scholar]
- 16.Hall IE, Akalin E, Bromberg JS, et al. Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney Int. 2019;95:199–209. doi: 10.1016/j.kint.2018.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon HJ, Bae HJ, Ham YR, et al. Outcomes of end-stage renal disease patients on the waiting list for deceased donor kidney transplantation: a single-center study. Kidney Res Clin Pract. 2019;38:116–123. doi: 10.23876/j.krcp.18.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park WY, Choi MS, Kim YS, et al. Impact of acute kidney injury in expanded criteria deceased donors on post-transplant clinical outcomes: multicenter cohort study. BMC Nephrol. 2019;20:39. doi: 10.1186/s12882-019-1225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Park WY, Kim YS, et al. Clinical significance of the Kidney Donor Profile Index in deceased donors for prediction of post-transplant clinical outcomes: a multicenter cohort study. PLoS One. 2018;13:e0205011. doi: 10.1371/journal.pone.0205011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park WY, Kim JH, Ko EJ, et al. Impact of kidney donor profile index scores on post-transplant clinical outcomes between elderly and young recipients, a multicenter cohort study. Sci Rep. 2020;10:7009. doi: 10.1038/s41598-020-64055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Kim YS, Choi MS, et al. Prediction of clinical outcomes after kidney transplantation from deceased donors with acute kidney injury: a comparison of the KDIGO and AKIN criteria. BMC Nephrol. 2017;18:39. doi: 10.1186/s12882-017-0461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3 Suppl 4:114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 23.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 24.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong HJ. Diagnosis of renal transplant rejection: Banff classification and beyond. Kidney Res Clin Pract. 2020;39:17–31. doi: 10.23876/j.krcp.20.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannon RB. Delayed graft function: the AKI of kidney transplantation. Nephron. 2018;140:94–98. doi: 10.1159/000491558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park WY, Kim JH, Ko EJ, et al. Impact of acute kidney injury in elderly versus young deceased donors on post-transplant outcomes: a multicenter cohort study. Sci Rep. 2020;10:3727. doi: 10.1038/s41598-020-60726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AP, Abramowicz D. Is the Kidney Donor Risk Index a step forward in the assessment of deceased donor kidney quality? Nephrol Dial Transplant. 2015;30:1285–1290. doi: 10.1093/ndt/gfu304. [DOI] [PubMed] [Google Scholar]
- 30.Jay CL, Washburn K, Dean PG, Helmick RA, Pugh JA, Stegall MD. Survival benefit in older patients associated with earlier transplant with high KDPI kidneys. Transplantation. 2017;101:867–872. doi: 10.1097/TP.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez RA, Malek SK, Milford EL, Finlayson SR, Tullius SG. The combined risk of donor quality and recipient age: higher-quality kidneys may not always improve patient and graft survival. Transplantation. 2014;98:1069–1076. doi: 10.1097/TP.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14:2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 33.Foucher Y, Akl A, Rousseau V, et al. An alternative approach to estimate age-related mortality of kidney transplant recipients compared to the general population: results in favor of old-to-old transplantations. Transpl Int. 2014;27:219–225. doi: 10.1111/tri.12241. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee MH, Jeong EG, Chang JY, et al. Clinical outcome of kidney transplantation from deceased donors with acute kidney injury by Acute Kidney Injury Network criteria. J Crit Care. 2014;29:432–437. doi: 10.1016/j.jcrc.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Zens TJ, Danobeitia JS, Leverson G, et al. The impact of kidney donor profile index on delayed graft function and transplant outcomes: a single-center analysis. Clin Transplant. 2018;32:e13190. doi: 10.1111/ctr.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein R, Galante NZ, de Sandes-Freitas TV, de Franco MF, Tedesco-Silva H, Medina-Pestana JO. Transplantation with kidneys retrieved from deceased donors with acute renal failure. Transplantation. 2013;95:611–616. doi: 10.1097/TP.0b013e318279153c. [DOI] [PubMed] [Google Scholar]
- 38.Boffa C, van de Leemkolk F, Curnow E, et al. Transplantation of kidneys from donors with acute kidney injury: friend or foe? Am J Transplant. 2017;17:411–419. doi: 10.1111/ajt.13966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.