Highlights
-
•
BGH is a rare, benign condition to consider in a patient with epigastric pain, dyspepsia, weight loss and upper gastrointestinal bleeding.
-
•
The diagnosis involves multiple modalities including CT, MRI, endoscopic ultrasound and biopsies.
-
•
It is important to determine the correct diagnosis to reduce the risk of overtreating a benign condition.
Keywords: Brunner gland, Hyperplasia, Duodenal neoplasm, Case report
Abstract
Introduction
Brunner’s gland hyperplasia is a rare, benign lesion of the duodenum. The symptomology can range from asymptomatic (as an incidental finding on endoscopy) to gastrointestinal obstruction or haemorrhage.
Case presentation
We report a case of a 60-year-old man presenting with post-prandial vomiting and weight loss. Inpatient evaluation led to the likely diagnosis of a duodenal malignancy for which the patient underwent a laparotomy and proximal duodenectomy.
Clinical discussion
Brunner’s gland hyperplasia is a rare, benign condition that can be overtreated due to the difficulty in obtaining an accurate pre-operative diagnosis. The literature has been reviewed to discuss the approach to diagnosis.
Conclusion
This case highlights the potential for Brunner’s gland hyperplasia mimicking a malignancy.
1. Introduction
Brunner’s glands are exocrine glands that are located in the submucosal layer of the duodenum. Brunner’s gland hyperplasia (BGH) is a rare, benign condition of the duodenum that was first described in 1835. It is also known as Brunner’s gland hamartoma, adenoma or Brunneroma. It has been rarely described in the literature, with less than 200 cases reported [1]. We report an unusual case of a large BGH, presenting to an Australian public hospital with obstructive symptoms, that mimicked duodenal adenocarcinoma requiring a proximal duodenectomy. This case has been reported in line with the SCARE criteria [2].
2. Presentation of case
A 60-year-old male presented to our institution’s emergency department with nausea, post prandial projectile vomiting and a loss of weight of 12 kg in the preceding 3 months. This was associated with intermittent fevers, and nocturnal diaphoresis.
Past medical history included chronic back pain with intermittent prescriptions of Meloxicam and chronic obstructive pulmonary disease. His past surgical history is unremarkable.
On examination, he appeared malnourished. He was haemodynamically stable. His abdomen was mildly distended and a succession splash was present. There was no obvious lymph node enlargement.
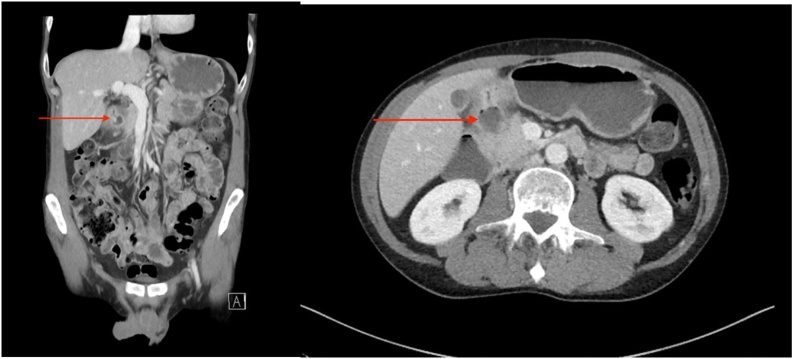
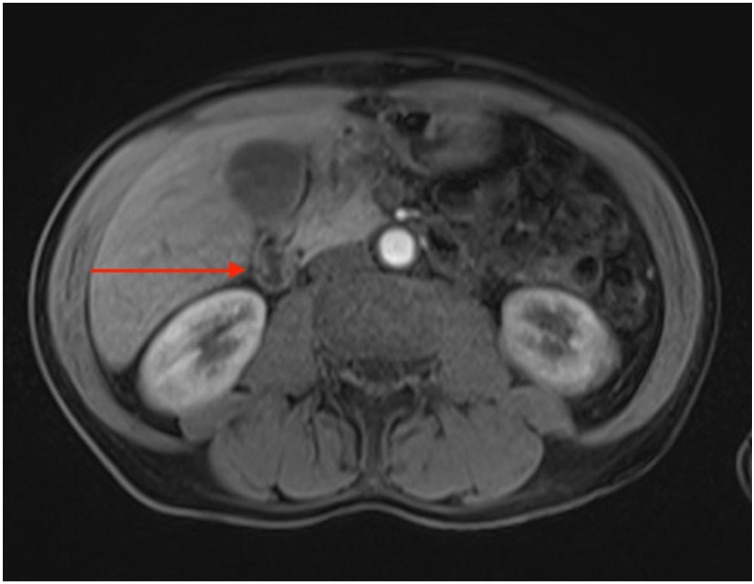
The patient was not anaemic. Tumour markers including carcinoembryonic antigen, cancer antigen 19-9 and alpha-fetoprotein were unremarkable. Liver function tests were unremarkable and there was no obvious biliary obstruction on imaging. A computed tomography (CT) of the abdomen showed a large soft tissue mass at the second part of the duodenum, with loss of fat plane between the duodenum and uncinate process (Fig. 1). Further imaging including magnetic resonance imaging demonstrated mural thickening involving the duodenal bulb, and possibly D2 segment, without any clear focal pancreatic head lesion (Fig. 2).
Fig. 1.
CT demonstrating a mass in the duodenum (red arrow highlighting lesion) a) coronal slice b) axial slice.
Fig. 2.
Axial T2 weighted magnetic resonance image with red arrow highlighting a mass in second part of duodenum.
A gastroscopy showed a large, mucosally denuded regions in the proximal D2, suggestive of an infiltration lesion rather than ulceration. Biopsies were taken and were suggestive, but not diagnostic of a moderately differentiated adenocarcinoma. An endoscopic ultrasound (EUS) was not performed due to deep biopsy and imaging both being indicative of a malignancy.
The patient was managed with nasogastric tube, total parenteral nutrition and anaesthetic optimisation, with a plan for a laparotomy and pancreaticoduodenectomy.
The procedure was performed by a specialist hepatobiliary surgeon. Intra-operatively, findings were of a stricturing area in the first part of the duodenum, approximately 5 cm from the major papilla. A cholecystectomy was performed, and a catheter inserted through the cystic duct to confirm the site of the major papilla. A decision was made to perform a proximal duodenectomy given the location of the lesion, with the rationale that sufficient margins would be obtained with a proximal duodenectomy without the morbidity associated with a pancreaticoduodenectomy. The stomach was divided at the Incisura, and the duodenum was divided at the second part of the duodenum, proximal to the papilla, with a T-Tube inserted into the duodenal stump for decompression. Frozen section was not performed due to the pre-operative biopsies being suggestive of adenocarcinoma. A Billroth II anastomosis was performed with a Braun entero-enterostomy. A feeding jejunostomy was inserted at the time of surgery.
Histopathology showed a prominent and diffuse area of near circumferential Brunner’s gland hyperplasia, expanding the submucosa. The growth pattern of the glands was expansile rather than infiltrative. Associated with this was an area of ulceration approximately 20 × 20 mm, with a focal perforation noted. All associated lymph nodes were benign. No evidence of malignancy was seen. No evidence of Helicobacter infection was noted.
The patient recovered well postoperatively. He was discharged on a puree diet, upgraded as appropriate and on review at six weeks, his jejunostomy and biliary limb t-tube was removed.
3. Discussion
Brunner’s glands are acinotubular exocrine glands that are located in the submucosal layer of the duodenum [3]. The majority of Brunner’s glands are located in the first part of the duodenum, with decreasing prevalence distally through the duodenum. One of their key roles is to secrete mucin, an alkaline fluid which acts to protect the duodenum from the acidic contents entering from the stomach [3].
Brunner’s gland hyperplasia (also referred to as adenoma/hamartoma) is a rare lesion found within the duodenum. A report of over 25,000 upper gastrointestinal endoscopies demonstrated an incidence of duodenal polyps in just 1.5% of cases, and of these, BGH accounted for 6.9% [4]. In a case series by Levine et al., the median age of patients with BGH is 56 years old, with a small female preponderance (4:5 male: female) [5]. This study also reported that BGH most commonly occurs in the first part of the duodenum (70%), with decreasing incidence distally [5]. The mechanism of BGH is not entirely clear. The main hypotheses are that BGH is an adaptive response to increased acidity of the stomach contents, chronic Helicobacter pylori infection or secondary to pancreatic exocrine insufficiency.
Typically, most patients with BGH are asymptomatic. However, patients may present with symptoms including dyspepsia, abdominal pain, obstructive symptoms such as post-prandial vomiting and weight loss, or gastrointestinal bleeding. The diagnosis of BGH is difficult to make, with differential diagnoses including an adenomatous polyp, leiomyoma/leiomyosarcoma, gastrointestinal stromal tumour, or pancreatic tumours [1]. There are cases in the literature reporting BGH simulating a carcinoma of the duodenal-pancreatic region meaning that they can often be overtreated [6,7].
BGH is often an incidental finding on endoscopy. Endoscopic findings are typically mucosal protrusions or polyps. Unfortunately, biopsies of the duodenal mass can be of limited value. Throughout the literature, there are multiple cases of incorrect diagnoses from biopsies – ranging from normal tissue to well differentiated adenocarcinoma [1,[6], [7], [8], [9], [10], [11], [12]]. This is in part due to Brunner’s glands being submucosal, with endoscopic mucosal biopsies often not achieving sufficient depth for diagnosis.
CT with IV contrast imaging of BGH typically demonstrates a duodenal mass that is separate from pancreatic parenchyma, however it is often unable to clearly differentiate between BGH and more concerning malignant lesions [1]. Endoscopic ultrasound (EUS) may be the best imaging modality for the diagnosis of submucosal Brunner’s gland hyperplasia. Matsushita et al. propose that the typical EUS features of BGH are location within the second and third layers of the duodenal wall, an irregular margin, hypoechogenicity, heterogeneous appearance and cystic areas within the lesion [13]. Multiple recent cases demonstrate the role of EUS for BGH, with consistent findings of a hypoechogenic mass in the duodenum [14].
Surgical management of BGH ranges from local endoscopic resection to more extensive surgery. In rare cases patients undergo pancreaticoduodenectomy (Table 1). There is a role for extensive pre-operative work-up, as well as intra-operative assessment to plan for the best management plan for these patients. Thorough examination of the duodenum intra-operatively by the surgeon can reduce the extent of surgery, with cases such as ours initially planned for pancreaticoduodenectomy changed to less extensive surgery based on the intra-operative findings [1,7,15]. Intra-operative frozen section has also been used with varying success to limit the extent of the surgery for a patient with BGH. Reisner and Nava, report an intra-operative frozen section diagnosis of BGH changing management to local tumour resection from the pre-operative plan of pancreaticoduodenectomy [7]. However, Lee et al. were not able to rule out malignancy based on frozen section, so continued with their pre-operative plan for pancreaticoduodenectomy [10].
Table 1.
Clinical features and investigation findings of patients undergoing pancreaticoduodenectomy for Brunner’s gland hyperplasia.
| Age/sex | Symptoms | Pre-op CT scan | EUS findings | Endoscopic findings | Pre-op tissue biopsy | Pre-op diagnosis | |
|---|---|---|---|---|---|---|---|
| Iusco et al. 2005 [6] | 60M | Postprandial epigastric pain | Ectasia of the Wirsung duct, volumetric reduction in the body of the pancreas, thickening of the upper duodenal angle and the second part of the duodenal wall | n/a | Bulky mass which occupied part of the bulb and the second portion of the duodenum | Aspecific phlogosis | Not stated |
| Dhouha et al. 2017 [9] | 72M | Epigastric pain, postprandial vomiting, weight loss | Slightly enhanced circumferential thickening and stenosing mass of the first part of the duodenum, abutting gallbladder and head of pancreas without loss of fat planes | n/a | Obstructive submucosal tumour of the duodenal bulb | Negative for malignancy | Duodenal carcinoma |
| Lee et al. 2008 [10] | 64M | Epigastric pain, dyspepsia, vomiting | Mass in the second portion of duodenum and loss of a fat plane between the mass and pancreas | n/a | Infiltrating type mass on the second portion of the duodenum with luminal narrowing | Moderate and chronically active duodenitis | Duodenal carcinoma with pancreatic invasion |
| Mumtaz et al. 2002 [11] | 50M | Postprandial epigastric pain, vomiting, weight loss | Mass in head of the pancreas extending into the duodenum and causing compression | Mass that seemed to arise from the head of the pancreas without vascular involvement | Mass arising from posterior wall of duodenal bulb that obstructed the lumen | Normal duodenal mucosa | Not stated |
| Sen et al. 2014 [12] | 42M | Epigastric pain, postprandial vomiting | Circumferential thickening of the second part of the duodenum. Abutting head of pancreas with loss of fat planes. | n/a | Nodular stricture at D1/D2 junction | Well differentiated adenocarcinoma | Not stated |
| Rath et al. 2019 [17] | 22M | Epigastric pain, dyspepsia, vomiting | Multiple enhancing polypoidal lesions in duodenum with near complete luminal narrowing along with jejunojejunal intussusception | n/a | Multiple duodenal polyps | Brunner’s gland hyperplasia with foci of high-grade dysplasia | BGH with high-grade dysplasia |
| Bojanapu et al. 2018 [18] | 30M | Vomiting, malaena | Well defined rounded hypodense submucosal lesion in the second part of the duodenum | n/a | Smooth mucosal bulge likely due to submucosal lesion in D2 | Normal duodenal mucosal fragments | Not stated |
| Bojanapu et al. 2018 | 33M | Abdominal pain, vomiting | Poorly circumscribed lesion of head of pancreas, compressing duodenum | n/a | Oedematous folds at D1-D2 junction | Papillary epithelial neoplasm of pancreas | Not stated |
| Hwang et al. 2016 [19] | 44M | Epigastric pain, vomiting | Dilatation of common bile duct and main pancreatic duct due to stenosis at the pancreas head. Mild wall thickening of the proximal duodenum | n/a | Circumferential submucosal oedema. Polypoid mass in the second portion of the duodenum resulting in partial obstruction. | Non-specific moderate duodenitis | Groove pancreatitis vs malignancy |
| Our case | 60M | Postprandial vomiting, weight loss | Large soft tissue mass at the second part of the duodenum, with loss of fat plane between the duodenum and uncinate process | n/a | Large, mucosally denuded regions in the proximal D2 | Suggestive of a moderately differentiated adenocarcinoma | Duodenal carcinoma |
BGH has typically been considered to be a benign condition of the duodenum. Interestingly however, some cases in the literature have demonstrated the malignant potential of Brunner’s gland hyperplasia [16]. This case reports an exceedingly rare entity, and there is debate in the literature whether adenocarcinoma is truly of Brunner’s gland origin or that of surrounding tissue. Further description of the progression from hyperplasia to adenocarcinoma is required.
4. Conclusion
Brunner’s gland hyperplasia is a rare condition that can mimic malignant pathology. As seen in this case, our patient underwent a procedure associated with significant morbidity for a benign condition. It is important to consider Brunner’s gland hyperplasia as a rare pathology that can cause gastrointestinal bleeding or obstructive symptoms. Extensive pre-operative work-up may be required to determine the nature of the pathology in patients with duodenal masses.
Declaration of Competing Interest
The authors report no declarations of interest.
Sources of funding
None.
Ethical approval
Ethical approval was not required for this case as it is an observation report written after the episode of care.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors contribution
Writing, editing and literature research: Jonathan McCafferty and Ashraf Tokhi.
Reviewing and editing: Ashraf Tokhi, Sujith Krishnamoorthy and Girish Pande.
Performing surgery: Girish Pande, Sujith Krishnamoorthy and Ashraf Tokhi.
Registration of research studies
Not Applicable.
Guarantor
Ashraf Tokhi and Jonathan McCafferty.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Peloso A., Vigano J., Vanoli A., Dominioni T., Zonta S., Bugada D., Bianchi C.M., Calabrese F., Benzoni I., Maestri M., Dionigi P., Cobianchi L. Saving from unnecessary pancreaticoduodenectomy. Brunner’s gland hamartoma: case report on a rare duodenal lesion and exhaustive literature review. Ann. Med. Surg. 2017;17:43–49. doi: 10.1016/j.amsu.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 3.2009. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas.http://www.sciencedirect.com/science/book/9781416040590 [Google Scholar]
- 4.Hochter W., Weingart J., Seib H.J., Ottenjann R. Duodenal polyps. Incidence, histologic substrate and significance. Dtsch. Med. Wochenschr. 1984;109:1183–1186. doi: 10.1055/s-2008-1069345. [DOI] [PubMed] [Google Scholar]
- 5.Levine J.A., Burgart L.J., Batts K.P., Wang K.K. Brunner’s gland hamartomas: clinical presentation and pathological features of 27 cases. Am. J. Gastroenterol. 1995;90:290–294. https://www.ncbi.nlm.nih.gov/pubmed/7847303 [PubMed] [Google Scholar]
- 6.Iusco D., Roncoroni L., Violi V., Donadei E., Sarli L. Brunner’s gland hamartoma: “over-treatment” of a voluminous mass simulating a malignancy of the pancreatic-duodenal area. JOP. 2005;6:348–353. https://www.ncbi.nlm.nih.gov/pubmed/16006686 [PubMed] [Google Scholar]
- 7.Reisner R.M., Nava H.R. Large Brunner’s gland hamartoma simulating a pancreatic mass with obstruction and bleeding. Surg. Endosc. 1996;10:341–343. doi: 10.1007/bf00187387. [DOI] [PubMed] [Google Scholar]
- 8.Arena M., Rossi S., Morandi E., Mangiavillano B., Franchi P. Brunner’s glands hyperplasia: diagnosis with EUS-FNA for suspected pancreatic tumor involving the duodenum. JOP. 2012;13:684–686. doi: 10.6092/1590-8577/970. [DOI] [PubMed] [Google Scholar]
- 9.Dhouha B., Ahlem L., Sana B.S., Saadia B., Sabeh M.R. Unexpected cause for duodenal obstruction: Brunner’s gland hyperplasia. Pathologica. 2017;109:414–417. https://www.ncbi.nlm.nih.gov/pubmed/29449737 [PubMed] [Google Scholar]
- 10.Lee W.C., Yang H.W., Lee Y.J., Jung S.H., Choi G.Y., Go H., Kim A., Cha S.W. Brunner’s gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy on pancreatic-duodenal area. J. Korean Med. Sci. 2008;23:540–543. doi: 10.3346/jkms.2008.23.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumtaz R., Shah I.A., Ramirez F.C. Brunner’s gland hamartoma simulating a pancreatic mass with duodenal obstruction. Gastrointest. Endosc. 2002;56:932–934. doi: 10.1067/mge.2002.129591. [DOI] [PubMed] [Google Scholar]
- 12.Sen R., Gupta V., Sharma N., Chawla N., Kumar S., Malik S. Brunner gland hamartoma masquerading as malignancy; a rare case report. Middle East J. Dig. Dis. 2014;6:237–240. https://www.ncbi.nlm.nih.gov/pubmed/25349687 [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita M., Hajiro K., Takakuwa H., Nishio A. Characteristic EUS appearance of Brunner’s gland hamartoma. Gastrointest. Endosc. 1999;49:670–672. doi: 10.1016/s0016-5107(99)70408-6. [DOI] [PubMed] [Google Scholar]
- 14.Bakir M.A., AlYousef M.Y., Alsohaibani F.I., Alsaad K.O. Brunner’s glands hamartoma with pylorus obstruction: a case report and review of literature. J. Surg. Case Rep. 2020;2020:rjaa191. doi: 10.1093/jscr/rjaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung T.T., Ip E.W., Poon R.T., Trendell-Smith N. Brunner’s gland adenoma: unusual cause of duodenal haemorrhage and obstruction. Hong Kong Med. J. 2013;19 doi: 10.12809/hkmj133776. 460.e1-2. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki T., Fujikuni N., Nakadoi K., Nakahara M., Tanabe K., Yonehara S., Noriyuki T. Adenocarcinoma of the duodenum arising from Brunner’s gland resected by partial duodenectomy: a case report. Surg. Case Rep. 2019;5:179. doi: 10.1186/s40792-019-0732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rath M.M., Mohapatra D., Mohanty S.K., Shantisudha S. Brunner’s gland hamartoma with dysplasia, presenting as multiple duodenal polyps: an unexplored entity with literature review. Indian J. Pathol. Microbiol. 2019;62:290–292. doi: 10.4103/IJPM.IJPM_69_18. [DOI] [PubMed] [Google Scholar]
- 18.Bojanapu S., Mangla V., Mehrotra S., Lalwani S., Mehta N., Nundy S. Brunner’s gland hyperplasia: an unusual duodenal submucosal lesion seen in four patients. J. Surg. Case Rep. 2018;2018:rjy305. doi: 10.1093/jscr/rjy305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang I.T., Cho Y.B., Park D.E., Choi K.H., Kim T.H. Large Brunner’s gland hamartoma with annular stricture causing gastric outlet obstruction. Korean J. Intern. Med. 2016;31:392–395. doi: 10.3904/kjim.2014.338. [DOI] [PMC free article] [PubMed] [Google Scholar]