Abstract
Background
The usefulness of bronchodilators in coronavirus diseases 2019 (COVID-19) survivors is still uncertain, especially for patients with a concomitant obstructive lung disease. We aimed at verifying the level of bronchodilator reversibility in COVID-19 patients undergoing multidisciplinary pulmonary rehabilitation after the acute phase.
Methods
We enrolled 105 consecutive patients referring to the Pulmonary Rehabilitation Unit of Istituti Clinici Scientifici Maugeri Spa SB, IRCCS of Telese Terme, Benevento, Italy after being discharged from the COVID-19 acute care ward and after recovering from acute COVID-19 pneumonia. All subjects performed a spirometry before and after inhalation of salbutamol 400 μg to determine the bronchodilation response within 48 h of admission to the unit.
Results
All patients had suffered from a moderate to severe COVID-19, classified 3 or 4 according to the WHO classification, Seventeen patients had concomitant obstructive lung disease (14 suffering from COPD and 3 from asthma). FEV1 after salbutamol improved on average by 41.7 mL in the entire examined sample, by 29.4 mL in subjects without concomitant obstructive lung diseases, by 59.3 mL in COPD patients and by 320.0 mL in asthma patients. Mean FVC after salbutamol improved by 65.7 mL in the entire examined sample, by 52.5 mL in subjects without concomitant obstructive lung diseases, by 120.0 mL in COPD patients, and by 200.0 mL in asthma patients.
Conclusions
This study suggests that a treatment with bronchodilators must always be taken into consideration in post-COVID-19 patients because it can induce a functional improvement that, even if small, can facilitate the breathing of these patients.
Keywords: COVID-19, Bronchodilators, Outcome, Disability, Rehabilitation, Chronic disease
1. Introduction
Currently, there is no data that can help us to clarify the usefulness of bronchodilators in coronavirus diseases 2019 (COVID-19) survivors, especially in patients with a concomitant obstructive lung disease. In particular, the clinical criteria for identifying post-COVID-19 patients who may benefit from bronchodilators are unclear.
Although mentioned in the current treatment recommendations for asthma [1] but not in those for chronic obstructive pulmonary disease (COPD) [2], bronchodilator reversibility testing is commonly used in clinical practice to predict the usefulness of a bronchodilator treatment [3]. However, levels of bronchial reversibility seem to be associated also with functional exercise performance, quality of life, exacerbation frequency, dyspnea, and radiological airway measures and, when focused on forced vital capacity (FVC) instead of forced expiratory volume in 1 s (FEV1), they indicate further clinical and radiological characteristics [4].
The aim of this study was to verify the level of bronchodilator reversibility in post-COVID-19 patients undergoing multidisciplinary pulmonary rehabilitation and to compare the data obtained from patients without a previous pulmonary disease with those from patients who had a history of obstructive lung disease.
2. Materials and methods
This study included consecutive patients admitted to the Pulmonary Rehabilitation Unit of Istituti Clinici Scientifici Maugeri Spa SB, IRCCS of Telese Terme, Benevento, Italy soon after being discharged from the COVID-19 acute care wards of several hospitals in which they were hospitalized for acute pneumonia COVID-19, and always less than 2 months after the onset of COVID-19. All patients had two consecutive negative SARS-CoV-2 swab tests before their admission in our unit. Main demographic and clinical characteristics were collected. We excluded from the subsequent clinical study patients who were unable to perform technically acceptable spirometry upon entering the ward.
At recruitment, which occurred within 48 h of admission to the unit, measurements were made of FEV1 and FVC, and reversibility to salbutamol was assessed irrespective of baseline FEV1 and FVC using an automated equipment (Vyasis FlowScreen II Spirometer, Milan, Italy).
After an initial spirometry in which three FEV1 and FVC measurements were taken, and for analysis the best FEV1 and FVC were chosen regardless of the curve, all subjects inhaled salbutamol 400 μg through a spacer device as recommended by the American Thoracic Society/European Respiratory Sociery Task Force [5] after withdrawing short-acting β2-agonists for ≥6 h, long-acting β2-agonists for 12 h, and long-acting antimuscarinic agents and/or ultra long-acting β2-agonists for 24 h. Subjects then remained seated for 20 min, without smoking or consuming beverages other than water. Then, a repeated spirometry was performed in an identical fashion to determine the bronchodilation response.
The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association. The Institutional Review Board of Istituto Nazionale Tumori, Fondazione Pascale, Naples, Italy approved this study with reference number ICS11/20. All patients provided written informed consent to use their de-identified data for future research.
Statistical analysis was performed with Prism 8 software package (GraphPad Software Inc, USA). Continuous data were expressed as mean and 95% confidence interval (CI) (standard errors have been reported in figures). Analysis of spirometric data for each treatment was performed using the Student's t-test for paired variables. Relationships between continuous variables were examined using simple regressions with Pearson's correlation coefficient (r). All results were expressed as 2-tailed values, and a probability level of P < 0.05 was considered as being of significance for all tests.
3. Results
We recruited 105 patients, 90 men and 15 women. All were Caucasians. Regarding the age groups, 17 were under the age of 50 years, 64 were aged 51–70 years, and 24 were over 70 years. There were 12 current smokers, 44 ex-smokers and 49 never-smokers. The World Health Organization (WHO) cut-off point of body mass index (BMI) for obesity (i.e. 30 kg/m2) [6] indicated that 48 of 105 patients were obese. In particular, 24 had a BMI between 30.00 and 34.99 (obese class I, moderate), 18 a BMI between 35.00 and 39.99 (obese class II, severe), and 6 were suffering from very severe obesity (obese class III, BMI ≥40.00).
All patients had suffered from a moderate to severe COVID-19, classified 3 or 4 by using the WHO classification, which separates patients affected by COVID 19 according to the gravity of their clinical scenario [7]. Seventeen patients had concomitant obstructive lung disease (14 suffering from COPD and 3 from asthma).
The spirometric test showed no formal functional alteration (FEV1 and FVC >80% of the predicted values, and FEV1/VC >70%) in 34 subjects, 6 patients presented an obstructive spirometric pattern (FEV1/VC <70% and FVC >80% of the predicted value), other 6 a restrictive spirometric pattern (FVC <80% predicted value and FEV1 >80% of the predicted value). The remaining patients had a spirometric pattern that was suggestive for a mixed obstructive-restrictive lung syndrome, 53 of them presenting FEV1 and FVC <80% of the predicted values with FEV1/FVC >70%, and 6 FEV1 and FVC <80% of the predicted values with FEV1/FVC <70%.
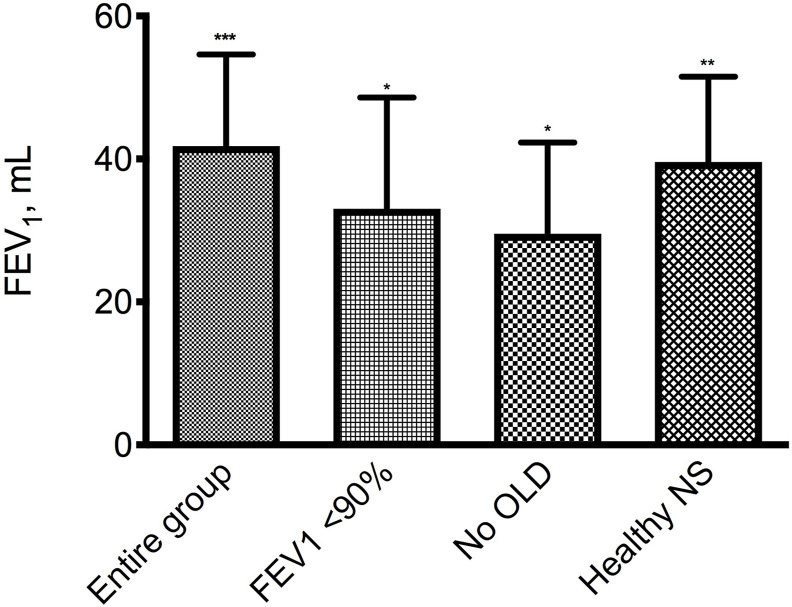
FEV1 after salbutamol improved on average by 41.7 mL (95% CI: 16.3–67.1 mL; P < 0.001) in the entire sample tested (Fig. 1 ). However, given the low probability of a positive response in subjects with normal baseline spirometry [8], we performed a sensitivity analysis excluding 29 patients with pre-bronchodilator FEV1 >90% predicted and the mean improvement in FEV1 was 33.0 mL (95% CI: 19.5–641.0 mL; P < 0.05) (Fig. 1). In subjects without a concurrent obstructive lung disease regardless of whether smokers, former smokers or non-smokers, defined as no OLD patients, FEV1 after salbutamol improved on average by 29.4 mL (95% CI: 4.1–54.8 mL; P < 0.05) (Fig. 1), while in previously healthy asymptomatic non-smoker subjects, defined as healthy NS, the mean increase in FEV1 after salbutamol was 39.4 mL (95% CI: 15.4–63.4 mL; P < 0.01) (Fig. 1).
Fig. 1.
Mean (SE) increase from baseline of FEV1 after salbutamol 400 μg in the entire group (105 subjects), in 76 patients with a basal FEV1 <90% predicted, in 88 subjects without concomitant obstructive lung diseases (No OLD), and in 48 previously healthy asymptomatic non-smokers (Healthy NS). *P < 0.05, **P < 0.01, ***P < 0.001 vs pre salbutamol.
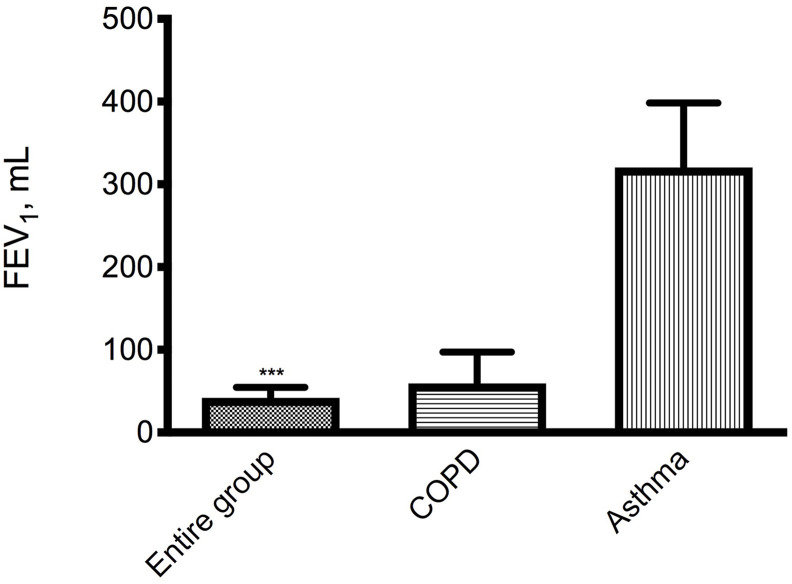
Remaining focused only on patients with a concurrent obstructive lung disease, salbutamol induced average FEV1 improvements of 59.3 mL (95% CI: −22.9−141.4 mL; P = 0.143) in subjects with COPD and 320.0 mL (IC 95%: −16.1−656.1 mL; P = 0.054) in patients with asthma (Fig. 2 ).
Fig. 2.
Mean (SE) increase from baseline of FEV1 after salbutamol 400 μg in the entire group (105 subjects), in 14 patients with preexisting COPD and in 3 asthmatic subjects. ***P < 0.001 vs pre salbutamol.
In those with a nonspecific pulmonary dysfunction pattern (i.e. FEV1 and FVC <80% of the predicted values with FEV1/FVC >70%) [9], the mean FEV1 after salbutamol was 44.1 mL (95% CI: 18.3–70.0 mL; P < 0.01), whereas in those with a prevalent obstructive component (i.e. FEV1 and FVC <80% of the predicted values with FEV1/FVC <70%) it was 201.7 mL (95% CI: 124.4–278.9 mL; P < 0.01).
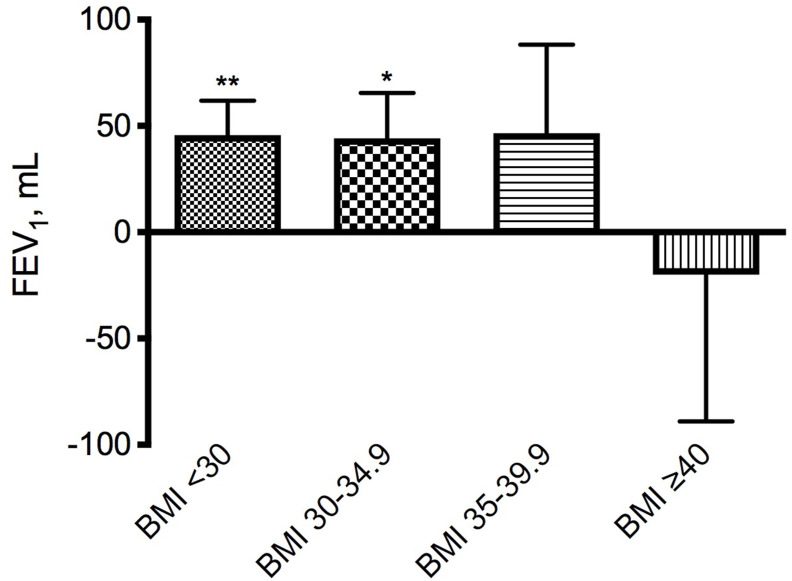
With the only exception of patients with severe obesity (BMI ≥40.00) in whom mean FEV1 after salbutamol decreased by 20.0 mL (95% CI: −197.3−157.3 mL; P = 0.783), mean FEV1 substantially increased in other BMI groups (BMI <30: 45.6 mL, 95% CI: 13.0–78.2 mL, P < 0.01; BMI 30.00–34.99: 44.2 mL, 95% CI: 5.6–88.3 mL, P < 0.05; BMI 35.01–39.99: 46.7 mL 95% CI: −41.0−134.4 mL; P = 0.277) (Fig. 3 ).
Fig. 3.
Mean (SE) increase from baseline of FEV1 after salbutamol 400 μg in the subgroups divided according to the body mass index (BMI) values (BMI <30: 57 subjects; BMI 30.00–34.99: 24 subjects; BMI 35.01–39.99: 18 subjects; BMI ≥40: 6 subjects). *P < 0.05, **P < 0.01 vs pre salbutamol.
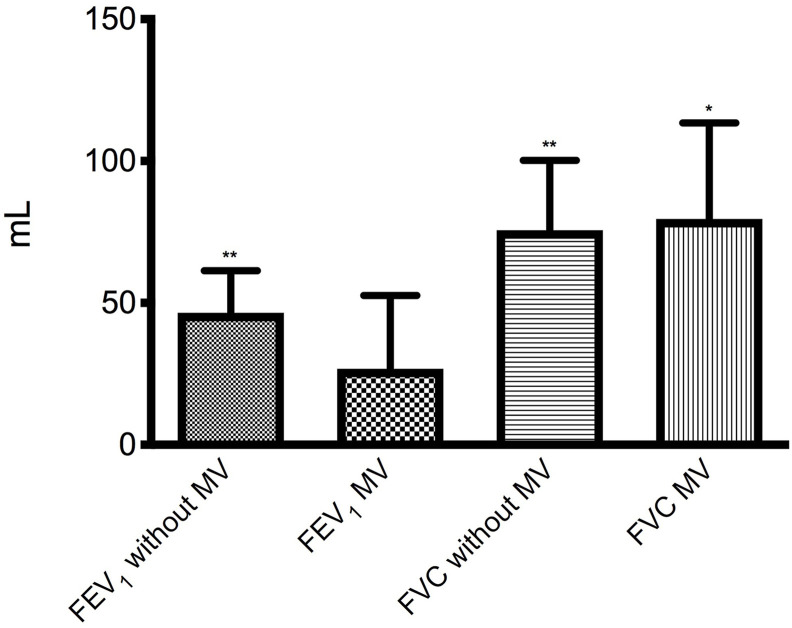
In those who had been mechanically ventilated during hospitalization for COVID pneumonia, the mean increase in FEV1 after salbutamol was 26.4 mL (95% CI: −26.7−79.5 mL; P = 0.315) versus 47.0 mL (95% CI: 17.9–76.2 mL; P < 0.01) in those who had not been mechanically ventilated (Fig. 4 ).
Fig. 4.
Mean (SE) increase from baseline of FEV1 and FVC after salbutamol 400 μg in the group of 80 patients who did not undergo mechanical ventilation (MV) and in that of 25 subjects who had undergone MV. *P < 0.05, **P < 0.01 vs pre salbutamol.
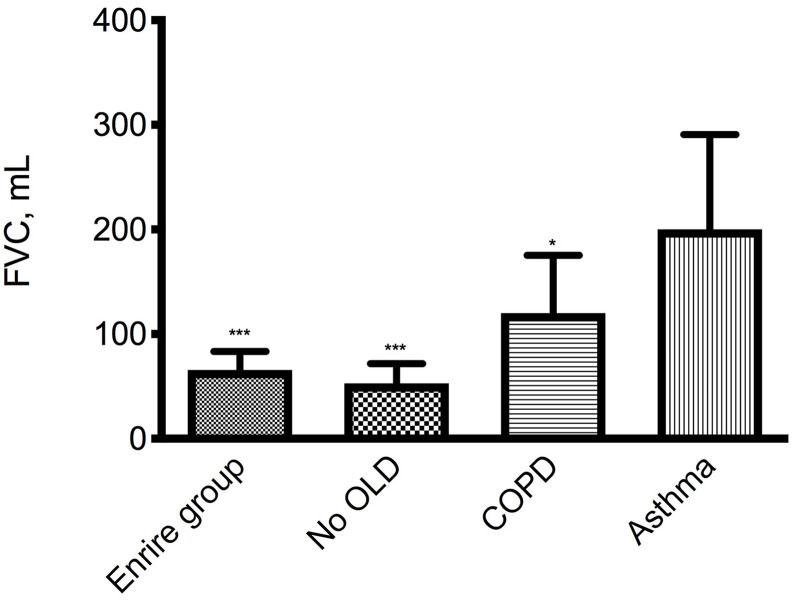
Mean FVC after salbutamol improved by 65.7 mL (95% CI: 30.9–100.6 mL, P < 0.001) in the entire examined sample, by 52.5 mL (95% CI: 15.4–89.6 mL, P < 0.01) in no OLD subjects by 120.0 mL (95% CI: 7.4–239.3 mL, P < 0.05) in COPD patients, and by 200.0 mL (95% CI: −190.4−590.4 mL, P < 0.158) in asthmatic subjects (Fig. 5 ). Mechanical ventilation did not change the magnitude of the increases in FVC (Fig. 4).
Fig. 5.
Mean (SE) increase from baseline of FVC after salbutamol 400 μg in the entire group (105 subjects), in 88 subjects without concomitant obstructive lung diseases (No OLD), in 15 COPD patients and in 3 asthma subjects. *P < 0.05, ***P < 0.001 vs pre salbutamol.
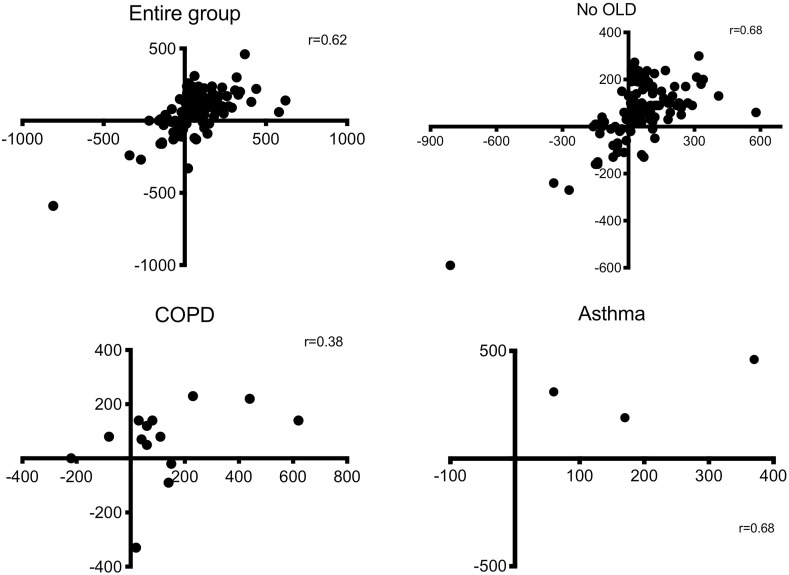
We found a statistically significant positive correlation between the changes of FEV1 and those of FVC (Pearson r = 0.62, P < 0.001), being confirmed also in no OLD subjects (Pearson r = 0.68, P < 0.001) (Fig. 6 ). However, when specifically considering patients with a concomitant obstructive lung disease, no significant correlation between FEV1 and FVC was found both among COPD (Pearson r = 0.38, P = 0.17) and asthma (Pearson r = 0.68, P = 0.52) patients.
Fig. 6.
– Correlation between the changes of FEV1 and those of FVC after salbutamol 400 μg in the entire group, in subjects without concomitant obstructive lung diseases (No OLD), in COPD and in asthma.
4. Discussion
Our data confirm that COVID-19 pneumonia may result in significant alterations in lung function. Although it has been suggested that in post-COVID-19 patients impairment of carbon monoxide diffusion capacity (D LCO) is the most common abnormality of lung function followed by restrictive ventilatory defect, which are both associated with the severity of the disease [10,11], bronchial obstruction can also be present.
It has been shown that, among COVID-19 survivors, 47% had normal findings, 41% presented a restrictive pattern, and 12% an obstructive pattern after 3 months from hospital discharge [12]. Another study revealed that 54% of COVID-19 survivors had abnormal lung function 10 weeks after diagnosis [13]. Restriction was the most prevalent pulmonary function abnormality, with critically ill patients being more prone to this condition and pulmonary function impairment being not related to abnormal imaging results or residual symptoms. A systematic review on five databases aimed at determining the prevalence of restrictive and obstructive patterns in post-COVID-19 patients documented a 15% prevalence of the restrictive pattern and a 7% prevalence of the obstructive pattern, with the diffusion capacity being impaired in close to 40% of patients [14]. In particular, the reduction in D LCO and also dynamic volumes, such as FEV1, seems to be correlated with disease severity and associated with the presence of lung consolidations both during the acute phase and after 6 weeks of follow up [15].
In any case, the reversible restrictive pattern on spirometry can to be a variant of the obstructive lung disease in which early airway closure results in air trapping and low FVC [16]. It has been suggested that the low D LCO characterizing the restrictive pattern is mainly determined by a reduced alveolar volume and not by the residual interstitial lung abnormalities or pulmonary vascular abnormalities caused by COVID-19 [17]. Apparently, in some subjects with airway reversibility, salbutamol tends to significantly affect alveolar volume [18]. However, it also known that β2-agonists can induce an increase in heart rate, and consequently volume per minute, cardiac output and right ventricular ejection also increase, raising D LCO [19]. For this reason we preferred not to measure D LCO before and after the reversibility test although we must admit that, by doing so, we were unable to verify the presence or absence of a correlation between changes in FEV1 induced by salbutamol and values D LCO.
In our study, in which the reversibility tests were conducted less than 2 months after the onset of COVID-19, normal findings were recorded in 32% of the study subjects. Both restrictive and obstructive patterns were found in 6% of the examined subjects, but in the majority of our patients (56%) a mixed obstructive-restrictive lung syndrome was present. The presence of an obstructive component suggested that bronchial reversibility evaluation of post-COVID-19 patients could be clinically important for the treatment planning.
The mean increase in FEV1 after salbutamol in the patient group we studied was 41.8 mL. The increase was lower when we separately considered the previously healthy asymptomatic non-smokers (39.6 mL) and subjects without concomitant obstructive lung diseases (29.6 mL). Contrariwise, in COPD patients, it was larger (59.3 mL). Patients with concomitant asthma showed a good reversibility, although no conclusions can be drawn for them, as in our series only 3 subjects had asthma.
These figures appear to be lower than those reported in large studies conducted in the past, when COVID-19 had not yet appeared, in both the general population and COPD patients. Actually, FEV1 improved on average by 77.2 mL in a general adult urban population and by 62.0 mL in healthy asymptomatic non-smokers [20]. In another study, the mean change in FEV1 after salbutamol was 80 mL in healthy non-smoker controls and 120 mL in COPD patients [21]. A third large worldwide study reported a FEV1 improvement of 72 mL after salbutamol (200 μg) in a healthy general population. The improvement was larger in patients with a concomitant obstructive lung disease without asthma (104 mL) and in patients in which asthma was included (114 mL) [22].
It is likely that the lower values in our series are the expression of persistent lung damage. At present, the long-term pulmonary consequences of COVID-19 remain speculative. We still do not know whether survivors may be truly at risk of developing chronic pulmonary sequelae, although organizing pneumonia and diffuse alveolar damage seem to be, by far, the most common forms of lung injury associated with COVID-19 [23]. It has been reported that breathlessness is an anticipated symptom that can persist long-term after discharge [24] in survivors with COVID-19, and a large proportion of hospitalized patients with COVID-19 have reduction in the functional status 6 months after hospitalization [25].
Looking at our data, it is also worth mentioning that the change in FEV1 induced by salbutamol in subjects who had undergone mechanical ventilation was significantly lower than in those who did not need this procedure, while mechanical ventilation did not change the magnitude of the increases in FVC. It is difficult to determine whether this response was the consequence of COVID-19 severity, thus requiring mechanical ventilation or, instead, mechanical ventilation caused lung damage or, more likely, weakness of the respiratory muscles.
We believe that the positive response we have recorded in patients with previous COPD or asthma or in those where the obstructive component was probably prevalent is very interesting. Although some data suggest a lower than expected prevalence of COVID-19 in patients with asthma and COPD, they are still at greater risk from the long-term consequences of infection [26]. When a patient with asthma or COPD develops COVID-19, the obstructive disease with the possibility of pulmonary emphysema overlaps with pulmonary fibrosis. In such cases, doubts arise about the usefulness of bronchodilators. Regardless of the possible impact of COVID-19, inhaled bronchodilators may be prescribed as for COPD, although the benefit of bronchodilators in combined pulmonary fibrosis and emphysema has not been adequately demonstrated [27]. In any case, several reports suggest that about 50% of patients with combined pulmonary fibrosis and emphysema receive bronchodilators [28,29]. This therapeutic choice is not without foundation if we consider that idiopathic pulmonary fibrosis (IPF) may be comorbid with obstructive lung diseases and furthermore, reversible airflow limitation co-exists in a subgroup of patients with IPF [30].
Our study has however some limitations. First of all, the lack of information on the functional pulmonary status of each patient before the onset of COVID-19, either because the patient had never performed spirometry or because he/she could not provide us with the previous spirometric tests, makes it impossible to establish the real impact of COVID-19 on lung function. The number of patients who were previously suffering from obstructive lung diseases was very small, especially with regard to asthma, and does not allow us to formulate solid conclusions. It could be argued that the number of COPD patients (14 out of 105) in our study resulted in a much higher COPD rate than in the general population [31]. However, the small absolute number led to a large dispersion of the data. Furthermore only one bronchodilator reversibility test was performed in each subject and there is a well-known between-day variability in the classification of the patients as reversible or not [32].
Lung function and bronchial reversibility must be evaluated over time, ideally after 6 months. Since COVID-19 survivors can have an impairment of muscular performance [33], and a decline in muscle strength initiates a chain of events that leads to a reduced pulmonary function [34], it will be important to understand if the multidisciplinary respiratory rehabilitation program will have resulted in an improvement in lung function including bronchial reversibility. Right now, however, this study suggests that a treatment with bronchodilators must always be taken into consideration because it can still induce a functional improvement that, even if small, can facilitate the breathing of post-COVID-19 patients.
CRediT authorship contribution statement
Mauro Maniscalco: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. Pasquale Ambrosino: Data curation, Investigation, Methodology, Writing – review & editing. Salvatore Fuschillo: Investigation. Silvia Stufano: Investigation. Alessandro Sanduzzi: Writing – review & editing. Maria Gabriella Matera: Formal analysis, Validation, Writing – review & editing. Mario Cazzola: Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Global Initiative for Asthma Global strategy for asthma management and prevention. 2020. https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf Update. Available from:
- 2.Global Initiative for Chronic Obstructive Lung Disease Global strategy for prevention, diagnosis and management of COPD. 2021. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.0-16Nov20_WMV.pdf report. Available at:
- 3.Hanania N.A., Celli B.R., Donohue J.F., Martin U.J. Bronchodilator reversibility in COPD. Chest. 2011;140(4):1055–1063. doi: 10.1378/chest.10-2974. [DOI] [PubMed] [Google Scholar]
- 4.Hansen J.E., Dilektasli A.G., Porszasz J., Stringer W.W., Pak Y., Rossiter H.B., Casaburi R. A new bronchodilator response grading strategy identifies distinct patient populations. Ann Am Thorac Soc. 2019;16(12):1504–1517. doi: 10.1513/AnnalsATS.201901-030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrino R., Viegi G., Brusasco V., Crapo R.O., Burgos F., Casaburi R., Coates A., van der Grinten C.P., Gustafsson P., Hankinson J., Jensen R., Johnson D.C., MacIntyre N., McKay R., Miller M.R., Navajas D., Pedersen O.F., Wanger J. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . World Health Organization Geneva; 2000. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity.https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ Available at: [PubMed] [Google Scholar]
- 7.World Health Organization Clinical management of covid-19 - interim guidance. https://www.who.int/publications/i/item/clinical-management-of-covid-19 Available at:
- 8.Hegewald M.J., Townsend R.G., Abbott J.T., Crapo R.O. Bronchodilator response in patients with normal baseline spirometry. Respir. Care. 2012;57(10):1564–1570. doi: 10.4187/respcare.01537. [DOI] [PubMed] [Google Scholar]
- 9.Iyer V.N., Schroeder D.R., Parker K.O., Hyatt R.E., Scanlon P.D. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest. 2011;139(4):878–886. doi: 10.1378/chest.10-0804. [DOI] [PubMed] [Google Scholar]
- 10.Fumagalli A., Misuraca C., Bianchi A., Borsa N., Limonta S., Maggiolini S., Bonardi D.R., Corsonello A., Di Rosa M., Soraci L., Lattanzio F., Colombo D. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection. 2021;49(1):153–157. doi: 10.1007/s15010-020-01474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo X., Jian W., Su Z., Chen M., Peng H., Peng P., Lei C., Chen R., Zhong N., Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latronico N, Peli E, Rodella F, Novelli MP, Rasulo FA, Piva S. Three-month outcome in survivors of COVID-19 associated Acute Respiratory Distress Syndrome. Available at SSRN: 10.2139/ssrn.3749226. [DOI]
- 13.Smet J., Stylemans D., Hanon S., Ilsen B., Verbanck S., Vanderhelst E. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir. Med. 2021;176:106276. doi: 10.1016/j.rmed.2020.106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., Vilaró J. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.013. Nov 2, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santus P., Flor N., Saad M., Pini S., Franceschi E., Airoldi A., Gaboardi P., Ippolito S., Rizzi M., Radovanovic D. Trends over time of lung function and eadiological abnormalities in COVID-19 pneumonia: a prospective, observational, cohort study. J. Clin. Med. 2021;10(5):1021. doi: 10.3390/jcm10051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keddissi J.I., Elya M.K., Farooq S.U., Youness H.A., Jones K.R., Awab A., Kinasewitz G.T. Bronchial responsiveness in patients with restrictive spirometry. BioMed Res. Int. 2013;2013:498205. doi: 10.1155/2013/498205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nusair S. Abnormal carbon monoxide diffusion capacity in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;56(1):2001832. doi: 10.1183/13993003.01832-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Stanton J., Wang L., Beckert L., Frampton C., Burton D., Swanney M.P. Effect of salbutamol on the measurement of single-breath diffusing capacity. Respirology. 2013;18(8):1223–1229. doi: 10.1111/resp.12125. [DOI] [PubMed] [Google Scholar]
- 19.Santus P., Centanni S., Morelli N., Di Marco F., Verga M., Cazzola M. Tiotropium is less likely to induce oxygen desaturation in stable COPD patients compared to long-acting β2-agonists. Respir. Med. 2007;101(8):1798–1803. doi: 10.1016/j.rmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Kainu A., Lindqvist A., Sarna S., Lundbäck B., Sovijärvi A. FEV1 response to bronchodilation in an adult urban population. Chest. 2008;134(2):387–393. doi: 10.1378/chest.07-2207. [DOI] [PubMed] [Google Scholar]
- 21.Albert P., Agusti A., Edwards L., Tal-Singer R., Yates J., Bakke P., Celli B.R., Coxson H.O., Crim C., Lomas D.A., Macnee W., Miller B., Rennard S., Silverman E.K., Vestbo J., Wouters E., Calverley P. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 22.Tan W.C., Vollmer W.M., Lamprecht B., Mannino D.M., Jithoo A., Nizankowska-Mogilnicka E., Mejza F., Gislason T., Burney P.G., Buist A.S., BOLD Collaborative Research Group Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax. 2012;67(8):718–726. doi: 10.1136/thoraxjnl-2011-201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi S., Reddy S., Gholamrezanezhad A. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. J. Thorac. Imag. 2020;35(4):W87–W89. doi: 10.1097/RTI.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 24.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Walshaw C., Kemp S., Corrado J., Singh R., Collins T., O'Connor R.J., Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 25.Taboada M., Cariñena A., Moreno E., Rodríguez N., Domínguez M.J., Casal A., Riveiro V., Diaz-Vieito M., Valdés L., Álvarez J., Seoane-Pillado T. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021;82(4):E31–E33. doi: 10.1016/j.jinf.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes J.P. Considering the long-term respiratory effects of Covid-19. Occup. Med. (Lond.) 2021 Jan 22 doi: 10.1093/occmed/kqaa224. Epub ahead of print. PMID: 33479724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xaubet A., Molina-Molina M., Acosta O., Bollo E., Castillo D., Fernández-Fabrellas E., Rodríguez-Portal J.A., Valenzuela C., Ancochea J. Guidelines for the medical treatment of idiopathic pulmonary fibrosis. Arch. Bronconeumol. 2017;53(5):263–269. doi: 10.1016/j.arbres.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Dong F., Zhang Y., Chi F., Song Q., Zhang L., Wang Y., Che C. Clinical efficacy and safety of ICS/LABA in patients with combined idiopathic pulmonary fibrosis and emphysema. Int. J. Clin. Exp. Med. 2015;8(6):8617–8625. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Zhang C., Dong F., Song Q., Chi F., Liu L., Wang Y., Che C. Combined pulmonary fibrosis and emphysema: a retrospective analysis of clinical characteristics, treatment and prognosis. BMC Pulm. Med. 2016;16(1):137. doi: 10.1186/s12890-016-0300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assayag D., Vittinghoff E., Ryerson C.J., Cocconcelli E., Tonelli R., Hu X., Elicker B.M., Golden J.A., Jones K.D., King T.E., Jr., Koth L.L., Lee J.S., Ley B., Shum A.K., Wolters P.J., Ryu J.H., Collard H.R. The effect of bronchodilators on forced vital capacity measurement in patients with idiopathic pulmonary fibrosis. Respir. Med. 2015;109(8):1058–1062. doi: 10.1016/j.rmed.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cazzola M., Puxeddu E., Bettoncelli G., Novelli L., Segreti A., Cricelli C., Calzetta L. The prevalence of asthma and COPD in Italy: a practice-based study. Respir. Med. 2011;105(3):386–391. doi: 10.1016/j.rmed.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Calverley P.M., Albert P., Walker P.P. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med. 2013;1(7):564–573. doi: 10.1016/S2213-2600(13)70086-9. [DOI] [PubMed] [Google Scholar]
- 33.Zampogna E., Migliori G.B., Centis R., Cherubino F., Facchetti C., Feci D., Palmiotto G., Pignatti P., Saderi L., Sotgiu G., Spanevello A., Zappa M., Visca D. Functional impairment during post-acute COVID-19 phase: preliminary finding in 56 patients. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2020.12.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchman A.S., Boyle P.A., Wilson R.S., Gu L., Bienias J.L., Bennett D.A. Pulmonary function, muscle strength and mortality in old age. Mech. Ageing Dev. 2008;129(11):625–631. doi: 10.1016/j.mad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]