Abstract
A novel series of substituted 4,6-dimethyl-2-oxo-1-(thiazol-2-ylamino)-1,2-dihydropyridine-3-carbonitrile derivatives 6, 9, 13, 15, and 17 was synthesized in a good to excellent yield from the reaction of 1-(3-cyano-4,6-dimethyl-2-oxopyridin-1(2H)-yl)thiourea with 2-oxo-N'-arylpropanehydrazonoyl chloride, chloroacetone, α-bromoketones, ethyl chloroacetate, and 2,3-dichloroquinoxaline, respectively. The potential DNA gyrase inhibitory activity was examined using in silico molecular docking simulation. The novel thiazoles exhibit dock score values between − 6.4 and − 9.2 kcal/mol and they were screened for their antimicrobial activities. Compound 13a shown good antibacterial activities with MIC ranged from 93.7–46.9 μg/mL, in addition, it shown good antifungal activities with MIC ranged from 7.8 and 5.8 μg/mL.
Subject terms: Chemical biology, Drug discovery, Chemistry
Introduction
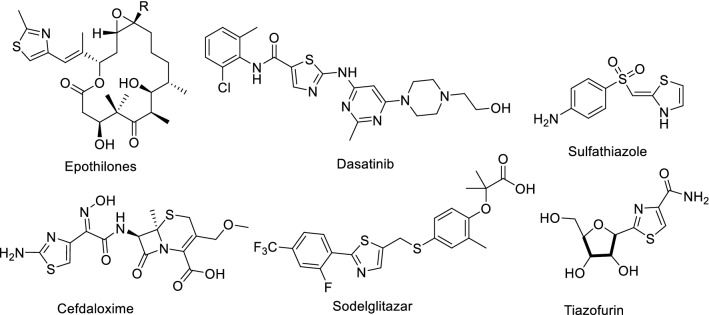
Thiazoles are present in numerous natural products e.g. epithilone, thiostrepton, thiamine pyrophosphate (TPP), carboxylase vitamin B1, and penicillin1. Thiazoles have diverse applications in drug development for treatment allergies2, inflammation3, HIV infections4, hypertension5, bacterial infections6, hypnotics7, schizophrenia8, and pain9, as novel inhibitors of bacterial DNA gyrase B10, and as fibrinogen receptor antagonists with antithrombotic activity11. They exhibited fabulous pharmaceutical activities for instance antifungal12, antimicrobial13–15, anti-inflammatory16,17, analgesic18, and anti-cancer19,20, anticonvulsant activities21. There are several commercial drugs contain thiazole moiety (Fig. 1).
Figure 1.
Commercial drugs contain thiazole moiety.
Pyridines are an important class of heterocyclic compounds because they occur in many natural compounds that have biological activity such as vitamin B3 (niacin) and vitamin B6 (pyridoxin) and natural alkaloids22. Multi substituted pyridines are significant synthons in heterocyclic synthesis23–26. 2-Pyridone derivative appeared as the backbone in over 7,000 drugs27,28 for instance amrinone29 and milrinone30 (Fig. 2) used for treating congestive heart failure (Fig. 2). Compounds containing the pyridine pattern have a wide range of biological profiles including antimicrobial31–36, anti-viral37,38, antioxidant39, antidiabetic40, anticancer41–43, anti-inflammatory agents44,45. For all these benefits related to thiazole and pyridine derivatives and following our work46–50, we report here the synthesis of a new library of thiazole derivatives from 1-(3-cyano-4,6-dimethyl-2-oxopyridin-1(2H)-yl)thiourea 2.
Figure 2.
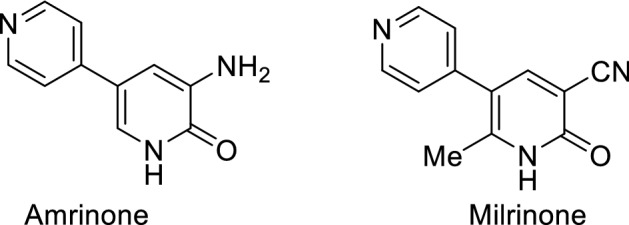
Structure of amrinone and milrinone.
Results and discussion
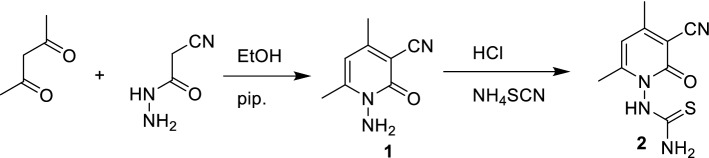
The precursor N-aminopyridone 1 was synthesized from the reaction of acetylacetone with cyanoacetohydrazide in EtOH containing piperidine at reflux temperature36. Solution of 1 in conc. HCl was treated with ammonium isothiocyanate then the mixture was heated at reflux temperature to afford white precipitate in excellent yield and identified as 2-oxopyridinyl thiourea 2 based on elemental analyses and spectral data. IR spectrum of 2 showed absorption bands at 3408, 3261, 3219, 2222, 1662 cm−1 owing to NH, NH2, CN, CO, respectively. 1H NMR spectrum revealed two singlet signals at δ 2.20 and 2.27 ppm owing to 2CH3, one singlet signal at δ 6.32 ppm due to pyridine-H5, two exchangeable signals at δ 7.76 and 10.16 ppm due to NH2 and NH, respectively. Its 13C NMR spectrum displayed the presence 9 carbon peaks. The most important peaks resonate at δ 159.9 (C=O), 185.5 (C=S). Mass spectrum displayed [M+ + 1] ion peak at m/z 223.6 (Scheme 1).
Scheme 1.
Synthesis of 1-(3-cyano-4,6-dimethyl-2-oxopyridin-1(2H)-yl)thiourea 2.
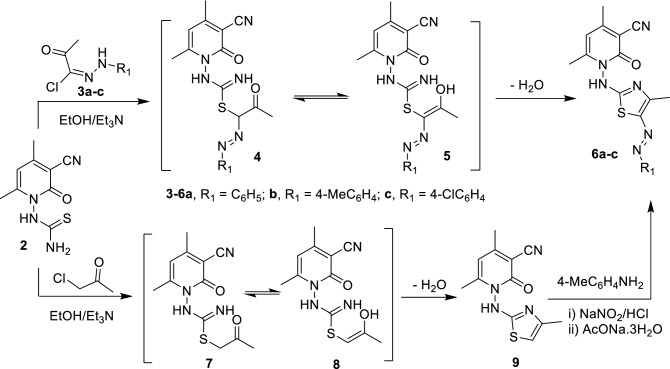
The reactivity of thiourea moiety was tested by the reaction of 2-oxopyridinyl thiourea 2 with different reagents as depicted in Schemes 2, 3, 4. Treatment of compound 2 with hydrazonyl chloride 3a-c in absolute EtOH containing 5 drops of Et3N at reflux temperature to afford the corresponding substituted 1,3-thiazole derivatives 6a-c, in good yields, via nucleophilic substitution followed by cyclization. On the other hand, 4,6-dimethyl-1-((4-methyl-5-(p-tolyldiazenyl)thiazol-2-yl)amino)-2-oxo-1,2-dihydropyridine-3-carbonitrile 6b was prepared by anther rout from the reaction of 2 with chloroacetone to afford 1-(2-thiazolylamino)-2-pyridone 9, in a high yield, followed by diazotization using 4-methylbenzenediazonium chloride (Scheme 2).
Scheme 2.
Synthesis of thiazole derivatives 6 and 9.
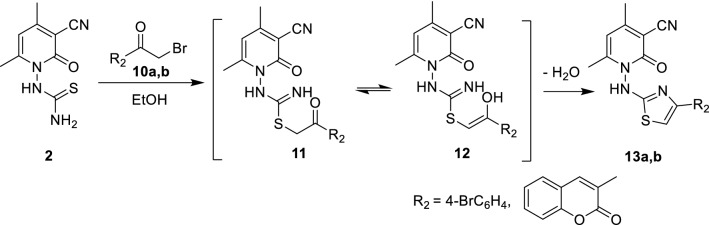
Scheme 3.
Synthesis of thiazole derivatives 13a,b.
Scheme 4.
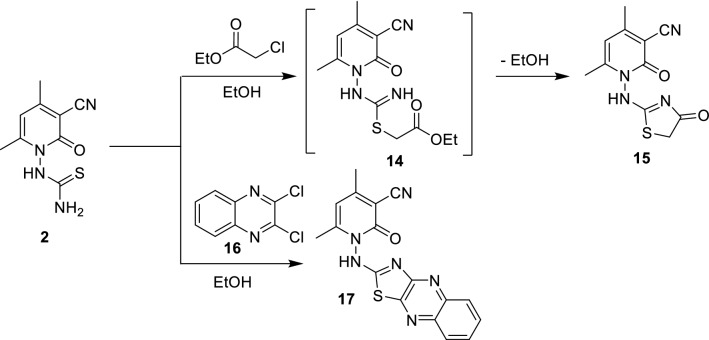
Synthesis of thiazole derivatives 15 and 17.
The structure of the compounds 6a-c and 9 was confirmed. The IR spectrum of compound 6b, as a representative example, exhibited the lack of NH2 and C=S peak at 3261, 3219, and 1269 cm−1. The 1H-NMR spectrum of 6b showed new singlet signals at δ 2.37, 2.49 ppm assigned to two methyl, additionally, two doublet signals at δ 7.20 and 7.55 ppm attributable to 4-methylbenzene. Its 13C-NMR spectrum revealed the lack of C=S signal at 185.5 ppm and appearance 17 carbon signals. Moreover, the mass spectra of 6b revealed [M+-15] ion peak at m/z 383. This clearly indicates the thioamide moiety was involved in cyclization reaction with hydrazonyl chlorides 3a–c to give 1,3-thiazole derivatives 6a-c.
Similarly, treatment compound 2 with an equimolar amount of α-bromoketones, 2-bromo-1-(4-bromophenyl)ethan-1-one 10a and 3-(2-bromoacetyl)-2H-chromen-2-one 10b, in ethanol at reflux temperature afforded 4,6-dimethyl-1-((4-substitutedthiazol-2-yl)amino)-2-oxo-1,2-dihydropyridine-3-carbonitrile 13a,b; respectively (Scheme 3). 1H NMR spectrum of 13a showed singlet signal at δ 7.53 ppm owing to thiazole-H5, in addition, two doublet of doublets signals at δ 7.56 and 7.67 ppm (J = 2 Hz, 9 Hz) due to 4-bromobenzene. Its 13C-NMR spectrum revealed the lack of C=S signal and appearance 15 carbon signals. Its mass spectrum revealed 401 [M+ + 1] (100%).
Next, thiourea derivative 2 was reacted with ethyl chloroacetate and 2,3-dichloroquinoxaline 16 in ethanol at reflux temperature to yield 4,6-dimethyl-2-oxo-1-((4-oxo-4,5-dihydrothiazol-2-yl)amino)-1,2-dihydropyridine-3-carbonitrile 15 and 4,6-dimethyl-2-oxo-1-(thiazolo[4,5-b]quinoxalin-2-ylamino)-1,2-dihydropyridine-3-carbonitrile 17, respectively, in good yields (Scheme 4). The IR spectra of 15 exhibited new strong band corresponding to C=O at 1737 cm−1 and disappearance thioamide moiety. The 1H NMR spectra exhibited new singlet for methylene in thiazole ring at δ 4 ppm. Its 13C NMR spectra showed 11 carbon peaks e.g., CH2 and CO in thiazole ring exhibited at δ 34.4 and 173.7 ppm, respectively. Its mass spectrum displayed [M+] peak at 262 (90%).
Molecular docking studies and antimicrobial activity
The innovative arylthioureas were docked to the active site of DNA gyrase enzyme using Autodock 4. We studied the hypothetical binding approach of 9 derivatives at the clorobiocin binding site via molecular docking. Molecular docking was accomplished for arylthiourea derivatives to comprehend their possible intermolecular interactions with the receptor. Clorobiocin is a based coumarin antibiotics, which prohibits the cell division of bacteria by inhibition of the DNA gyrase enzyme51–54.
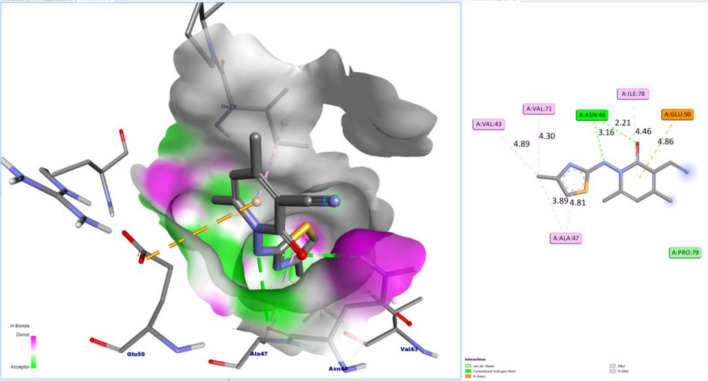
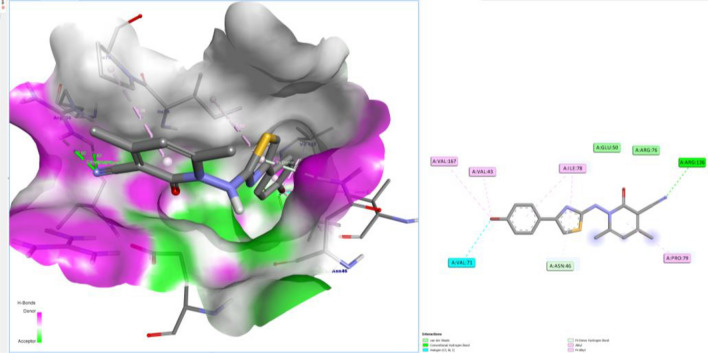
Table 1 summarizes the binding depiction of the arylthioureas with DNA gyrase. The poses obtained from the docking procedure was selected due to their binding energy (~ − 6 – − 9 kcal/mol). Figures 3 and 4 showed 3D schematic interactions of compounds 13a and 9 into the chlorobiocin binding site and showed that the compounds are fit to the binding pocket. These hydrophobic sites and hydrogen bond interactions of the derivatives are conserved in the majority of our compounds (Figs. 3 and 4).
Table 1.
Energy-based interactions and hydrogen bonds of arylthiourea derivatives docked into DNA gyrase.
| No | Estimated free energy of binding (kcal/mol) | Hydrogen bonds (distance) |
|---|---|---|
| 2 | − 6.4 | Arg76 (2.92 A°),Gly77 (2.52 A°), Thr165 (2.39 A°), Asp73 (2.34 A°), Asn46 (2.75 A°) |
| 6a | − 7.7 | Arg76 (2.77 A°), Arg136 (1.95 A°) |
| 6b | − 7.8 | Arg76 (3.02 A°) |
| 6c | − 7.7 | Arg76 (2.93) |
| 9 | − 8.8 | Asn46 (2.21 A°), Asn46 (3.16 A°) |
| 13a | − 9.2 | Arg136 (2.82 A°), Arg136 (2.5 A°) |
| 13b | − 8.5 | Ser121 (2.81 A°), His95 (3.03 A°), Ala96 (2.67 A°), Asn46 (2.14 A°) |
| 15 | − 7.6 | Asn46 (2.6 A°), Asn (2.24 A°) |
| 17 | − 8.3 | Asn46 (2.43 A°) |
Figure 3.
Docked conformation of compound 9 in the binding site of DNA-gyrase. Hydrogen bonds are shown by green dashed line and the other colors represent the hydrophobic interactions.
Figure 4.
Docked conformation of compound 13a in the binding site of DNA-gyrase. Hydrogen bonds are shown by green dashed line and the other colors represent the hydrophobic interactions.
The docking results exhibited that some compounds (9, 13a and 13b) can produce a strong hydrophobic interaction and hydrogen bonds with Arg136 and Asn46 in the binding site. It is exciting that more complex stabilization could result from the hydrogen bonds between these compounds and Arg136 via cyano group in the pyridone ring (Figs. 3 and 4). Although these interactions were also observed for some other derivatives, but we think that the hydrophobic interaction is responsible for the activity variations.
Docked compounds also stabilize the DNA gyrase via hydrophobic interactions with Ala47, Glu50, Val71, Asp73, Arg76, Gly77, Ile78, Pro79, Met91, Val43, Thr165, and Val167. Compounds 9 and 13a were pointedly embedded into the hydrophobic part of the pocket. All compounds showed that the hydrophobic pocket of the inhibitor pocket was occupied by pyridine, phenyl or substituted phenyl.
The docking method approved in this study was validated by redocking of chlorobiocin to the DNA gyrase protein. The residues Asp73, Asn46, and Arg136 are vital in making hydrogen bonds and are very important for the biological activity55 and in our study some compounds also displayed a strong hydrogen bond with Asn46. The highest dock score for our derivatives was − 9.2 and − 8.8 kcal/mol for compounds 13a and 9, respectively. The remainder molecules exhibited a docking scores ranging from − 8.5 to − 6.4 kcal/mol. Thus, the binding model stated here, proposes that arylthiourea derivatives act as DNA gyrase inhibitors and display some key structural points to be used in further optimization.
The biological assay (Tables 2 and 3), some compounds exhibited a strong activity against both the Gram-positive and Gram-negative bacterial. Gained results confirmed that compounds 9 had high activities against E. coli and P. aeruginosa with MIC 93.7 μg/mL. Also, compound 13a showed the superlative activity against E. coli, P. aeruginosa, S. aureus, and B. subtilis with MIC 93.7, 62.5, 46.9, and 62.5 μg/mL, respectively. Also compound 13a has shown the highest activity with MIC 7.8 and 5.8 μg/mL against C. albicans and Aspergillus flavus, respectively.
Table 2.
In vitro antimicrobial activity of the synthesized compounds a,b.
| Diameter of inhibition zone (mm) | ||||||
|---|---|---|---|---|---|---|
| Compounds | E. coli | P. aeruginosa | S. aureus | B. subtilis | C. albicans | A. flavus |
| 1 | NA | NA | NA | NA | 2 ± 0.53 | 4 ± 0.66 |
| 2 | 10 ± 0.92 | 12 ± 1.02 | 16 ± 0.97 | 15 ± 1.36 | 16 ± 1.38 | 19 ± 1.60 |
| 6a | 10 ± 0.87 | 13 ± 1.17 | 17 ± 1.06 | 15 ± 1.41 | 16 ± 1.52 | 21 ± 1.52 |
| 6b | 13 ± 1.13 | 16 ± 1.48 | 20 ± 1.47 | 17 ± 1.61 | 19 ± 1.91 | 22 ± 1.53 |
| 6c | 8 ± 0.79 | 12 ± 1.18 | 14 ± 1.35 | 13 ± 1.42 | 12 ± 1.18 | 16 ± 1.37 |
| 9 | 18 ± 0.94 | 17 ± 0.89 | 21 ± 1.73 | 18 ± 1.74 | 20 ± 1.67 | 23 ± 1.06 |
| 13a | 21 ± 1.36 | 20 ± 1.45 | 22 ± 1.69 | 19 ± 1.68 | 20 ± 0.49 | 24 ± 1.75 |
| 13b | 12 ± 1.24 | 15 ± 1.26 | 19 ± 1.53 | 16 ± 1.48 | 13 ± 1.25 | 17 ± 1.14 |
| 15 | 5 ± 0.53 | 9 ± 0.84 | 7 ± 0.82 | 5 ± 0.74 | 6 ± 0.80 | 7 ± 0.92 |
| 17 | 7 ± 0.86 | 10 ± 0.97 | 13 ± 1.18 | 12 ± 1.19 | 11 ± 1.46 | 15 ± 1.48 |
| Ampicillin | 25 ± 1.48 | 23 ± 1.28 | 24 ± 1.04 | 23 ± 0.93 | – | – |
| Clotrimazole | – | – | – | – | 27 ± 2.37 | 25 ± 1.91 |
a Antimicrobial activity expressed as inhibition diameter zones in millimeters (mm) of synthesized compounds against the pathological strains based on well diffusion assay.
b The experiment was carried out in triplicate and the average zone of inhibition was calculated.
c NA No activity.
Table 3.
Minimum inhibitory concentration (MIC) in (μg/mL) for compounds 2, 6a, 6b, 9, and 13aa.
| Compounds | E. coli | P. aeruginosa | S. aureus | B. subtilis | C. albicans | A. flavus |
|---|---|---|---|---|---|---|
| 2 | 375 ± 3.00 | 250 ± 2.25 | 187.5 ± 8.08 | 250 ± 1.00 | 62.5 ± 0.50 | 31.2 ± 0.62 |
| 6a | 250 ± 3.21 | 187.5 ± 8.23 | 125 ± 0.00 | 187.5 ± 0.50 | 46.9 ± 0.84 | 23.4 ± 0.50 |
| 6b | 187.5 ± 0.50 | 125 ± 1.73 | 125 ± 1.00 | 187.5 ± 0.58 | 31.2 ± 0.62 | 11.7 ± 0.30 |
| 9 | 93.7 ± 0.95 | 93.7 ± 0.95 | 125 ± 0.58 | 187.5 ± 0.50 | 23.4 ± 0.84 | 15.6 ± 0.76 |
| 13a | 93.7 ± 0.95 | 62.5 ± 2.00 | 46.9 ± 0.84 | 62.5 ± 0.50 | 7.8 ± 0.17 | 5.8 ± 0.65 |
| Ampicillin | 125 ± 0.58 | 125 ± 3.51 | 187.5 ± 0.06 | 125 ± 1.73 | – | – |
| Clotrimazole | – | – | – | – | 5.8 ± 0.06 | 3.9 ± 0.06 |
aThe experiment was carried out in triplicate and the average was calculated.
The observed results displayed that compound 13a has better biological results than other arylthioureas. Existence of electron-withdrawing group (bromine) at p-position of the phenyl ring could be accountable for good activities due to its size and inductive effect.
Experiment
General
Melting points were recorded on digital Gallen-Kamp MFB-595 apparatus and are uncorrected. IR spectra were recorded on Schimadzu FTIR 440 spectrometer using KBr pellets. Mass spectra were performed at 70 eV on an MS-50 Kratos (A.E.I.) spectrometer provided with a data system. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker model Ultra Shield NMR spectrometer using CDCl3 or DMSO-d6 with TMS as an internal standard. Chemical shifts are reported as δ ppm units. The monitoring of the progress of reactions and homogeneity of the products was carried out using thin layer chromatography (TLC).
1-(3-Cyano-4,6-dimethyl-2-oxopyridin-1(2H)-yl)thiourea (2). 1-Amino-4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (16.3 g, 0.1 mol) was dissolved in coc. HCl (40 mL) and ammonium isothiocynte was added (7.6 g, 0.1 mol). The mixture was reflux for 1 h. After cooling, the white precipitate was filtered off, washed with ethanol, and dried under reduced pressure. White crystals, yield (95%), mp 249–250° C. IR (KBr) ν (cm−1): 3408 (NH), 3261, 3219 (NH2), 2222 (CN), 1662 (C=O), 1624 (C=C), 1269 (C=S); 1H NMR (500 MHz, CDCl3) δ (ppm): 2.20 (s, 3H, CH3), 2.27 (s, 3H, CH3), 6.32 (s, 1H, pyridine-H5), 7.76 (s, D2O exchangeable, 2H, NH2), 10.16 (s, D2O exchangeable, H, NH); 13C NMR (125 MHz, CDCl3) δC (ppm): 18.6 (CH3), 20.7 (CH3), 101, 108.9, 115.6 (CN), 153.8, 155.5, 159.9 (C=O), 185.5 (C=S); MS m/z (%): 223.6 [M+ + 1] (5%), 204.9 [M+-H2O], 163, 148, 119 (100); Anal. Calcd. for C9H10N4OS (222.27): C, 48.63; H, 4.54; N, 25.21, Found: C, 48.43; H, 4.36; N, 25.03%.
General procedure for synthesis thiazole derivatives 6, 9, 13, 15, and 1756
Equimolar amounts of 2 (1 mmol) and 2-oxo-N-arylpropanehydrazonoyl chloride 3a-c; chloroacetone; α-bromoketones 11a,b; ethyl chloroacetate; and 2,3-dichloroquinoxaline (1 mmol) in absolute ethanol (30 mL) {few drops of triethylamine was added in case of 3a-c and chloroacetone} was heated under reflux for 3–6 h (TLC), then left to cool. The solid was isolated by filtration, washed with ethanol, dried, and recrystallized from (EtOH).
4,6-Dimethyl-1-((4-methyl-5-(phenyldiazenyl)thiazol-2-yl)amino)-2-oxo-1,2-dihydropyridine-3-carbonitrile (6a). Orange crystals, yield (86%), mp 234–235 °C (EtOH); IR (νmax, cm−1): 3219w (NH), 2218 s (CN), 1643 s (C=O), 1578-1485 s (C=C); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.25 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.36 (s, 3H, CH3), 6.33 (s, 1H, pyridine-H5), 7.33 (t, 2H, Ar–H), 7.44 (t, 2H, Ar–H), 7.52 (d, 1H, J = 8.5 Hz, Ar–H), 9.88 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 18.8 (CH3), 20.7 (CH3), 21.5 (CH3), 108, 114, 117, 128.6, 128.8, 129.2, 129.4, 135.7, 138, 147, 154, 160, 167; MS m/z (%): 364 [M+] (3%), 252 (15), 163 (55), 126 (100); Anal. Calcd. for C18H16N6OS (364.43): C, 59.33; H, 4.43; N, 23.06, Found: C, 59.07; H, 4.19; N, 22.89%.
4,6-Dimethyl-1-((4-methyl-5-(p-tolyldiazenyl)thiazol-2-yl)amino)-2-oxo-1,2-dihydropyridine-3-carbonitrile (6b). Orange crystals, yield (85%), mp 245–246 °C (EtOH); IR (νmax, cm−1): 3219w (NH), 2222 s (CN), 1656 s (C=O), 1575–1490 s (C=C); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.26 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.37 (s, 3H, CH3), 2.49 (s, 3H, CH3), 6.31 (s, 1H, pyridine-H5), 7.20 (d, 2H, J = 6.5 Hz, Ar–H), 7.55 (d, 2H, J = 6.5 Hz, Ar–H), 10.08 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 19 (CH3), 20.7 (CH3), 20.8 (CH3), 20.9 (CH3), 100, 108.6, 109, 115, 116, 116.4, 129.4, 135.3, 147, 150.2, 154, 160, 167; MS m/z (%): 363 [M+-15] (4%), 232 (60), 163 (45), 120 (100); Anal. Calcd. for C19H18N6OS (378.45): C, 60.30; H, 4.79; N, 22.21, Found: C, 60.30; H, 4.79; N, 22.21%.
1-((5-((4-Chlorophenyl)diazenyl)-4-methylthiazol-2-yl)amino)-4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (6c). Yellow crystals, yield (85%), mp 238–239 °C (EtOH); IR (νmax, cm−1): 3217w (NH), 2222 s (CN), 1653 s (C=O), 1585–1489 s (C=C); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.24 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.49 (s, 3H, CH3), 6.34 (s, 1H, pyridine-H5), 7.33 (d, 2H, J = 6.5 Hz, Ar–H), 7.44 (d, 2H, J = 6.5 Hz, Ar–H), 11 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 16.83 (CH3), 18.7 (CH3), 20.6 (CH3), 100, 108, 109, 115, 116, 116.4, 129.4, 135.3, 147, 150.2, 154, 160, 167; MS m/z (%): 384 [M+-15] (5%), 252 (15), 163 (55), 126 (100); Anal. Calcd. for C18H15ClN6OS (398.87): C, 54.20; H, 3.79; N, 21.07, Found: C, 53.83; H, 3.61; N, 20.87%.
4,6-Dimethyl-1-((4-methylthiazol-2-yl)amino)-2-oxo-1,2-dihydropyridine-3-carbonitrile (9). White crystals, yield (85%), mp 219–220 °C (EtOH); IR (νmax, cm−1): 3261w (NH), 2216 s (CN), 1654 s (C=O), 1575–1543 s (C=C); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.07 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.49 (s, 3H, CH3), 6.34 (s, 1H, pyridine-H5), 6.42 (s, 1H, thiazole-H5), 10.77 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 16.6 (CH3), 19.4 (CH3), 21.1 (CH3), 100, 101.4, 109, 116, 154.9, 156, 159.4, 181.7, 185.8; MS m/z (%): 260 [M+] (100%), 243 (15), 148 (52); Anal. Calcd. for C12H12N4OS (260.32): C, 55.37; H, 4.65; N, 21.52, Found: C, 55.15; H, 4.32; N, 21.11%.
Method 2
Synthesis of compound 6b from compound 957
To a stirred solution of compound 9 (0.5206 g, 2 mmol) in ethanol (30 mL) sodium acetate trihydrate (0.26 g, 2 mmol) was added. After stirring for 15 min, the mixture was chilled at 0 °C and treated with a cold solution of p-toluidine (0.2 g, 2 mmol) in 6 M hydrochloric acid (1.5 mL) with sodium nitrite solution (0.14 g, 2 mmol) in water (3 mL). The addition of the diazonium salt was stirred for an additional 2 h at 0–5 °C and then left for 8 h in a refrigerator (4 °C). The resulting solid was collected by filtration, washed thoroughly with water and dried. The crude product was crystallized from ethanol.
1-((4-(4-Bromophenyl)thiazol-2-yl)amino)-4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (13a). White crystals, yield (85%), mp 233–235 °C (EtOH); IR (νmax, cm−1): 3261w (NH), 2222 s (CN), 1662 s (C=O), 1593–1537 s (C=C); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.33 (s, 3H, CH3), 2.49 (s, 3H, CH3), 6.47 (s, 1H, pyridine-H5), 7.53 (s, 1H, thiazole-H5), 7.56 (dd, 2H, J = 2 Hz, 9 Hz, Ar–H), 7.67 (dd, 2H, J = 2 Hz, 9 Hz, Ar–H), 10.81 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 19 (CH3), 20.8 (CH3), 100, 106.9, 108.5, 115.6, 120.9, 127.6, 131.6, 133.1, 148.8, 153.8, 158.8, 160, 167.5; MS m/z (%): 401 [M+ + 1] (100%), 256 (70); Anal. Calcd. for C17H13BrN4OS (401.28): C, 50.88; H, 3.27; N, 13.96, Found: C, 50.49; H, 3.11; N, 13.71%.
4,6-Dimethyl-2-oxo-1-((4-(2-oxo-2H-chromen-3-yl)thiazol-2-yl)amino)-1,2-dihydropyridine-3-carbonitrile (13b). White crystals, yield (85%), mp 280–281 °C (EtOH); IR (νmax, cm−1): 3170w (NH), 2223 s (CN), 1739, 1724 (C=O), 1662 s (C=O), 1583–1531 s (C=C); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.41 (s, 3H, CH3), 2.49 (s, 3H, CH3), 6.50 (s, 1H, pyridine-H5), 7.35 (ddd, 1H, J = 1.5 Hz, 8.5 Hz, coumarin-H6), 7.42 (d, 1H, J = 8.5 Hz, coumarin-H8), 7.60 (ddd, 1H, J = 1.5 Hz, 8.5 Hz, coumarin-H7), 7.84 (dd, 1H, J = 1.5 Hz, 9.5 Hz, coumarin-H5), 8.36 (s, 1H, coumarin-H4), 10.85 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 19 (CH3), 20.9 (CH3), 100.2, 108.7, 112.5, 115.4, 115.9, 119, 120.1, 124.7, 129, 131.9, 138.7, 143.5, 152.3, 155, 158.6, 158.8 (CO), 160.2 (CO, lactone), 167.2; MS m/z (%): 390 [M+] (8%), 244 (70), 148 (100), 119 (60); Anal. Calcd. for C20H14N4O3S (390.42): C, 61.53; H, 3.61; N, 14.35, Found: C, 61.28; H, 3.35; N, 14.21%.
4,6-Dimethyl-2-oxo-1-((4-oxo-4,5-dihydrothiazol-2-yl)amino)-1,2-dihydropyridine-3-carbonitrile (15). White crystals, yield (85%), mp 233–235 °C (EtOH); IR (νmax, cm−1): 3250w (NH), 2216 s (CN), 1737s (C=O), 1699 (C=O); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.23 (s, 3H, CH3), 2.33 (s, 3H, CH3), 4 (s, 2H, CH2, thiazole), 6.39 (s, 1H, pyridine-H5), 12.53 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 18.8 (CH3), 20.51 (CH3), 34.4 (CH2), 99.6, 108.1, 115.8, 151, 155.9, 157.5, 171.4 (CO), 173.7 (CO); MS m/z (%): 262 [M+] (90%), 215 (100), 148 (30); Anal. Calcd. for C11H10N4O2S (262.29): C, 50.19; H, 3.53; N, 21.15, Found: C, 50.37; H, 3.84; N, 21.36%.
4,6-Dimethyl-2-oxo-1-(thiazolo[4,5-b]quinoxalin-2-ylamino)-1,2-dihydropyridine-3-carbonitrile (17). Brown powder, yield (85%), mp ˃300 °C (EtOH); IR (νmax, cm−1): 3151w (NH), 2224 s (CN), 1684 (C=O); 1H NMR (500 MHz, CDCl3) δH (ppm): 2.32 (s, 3H, CH3), 2.33 (s, 3H, CH3), 6.34 (s, 1H, pyridine-H5), 7.07–7.10 (m, 2H, Ar–H); 7.89–7.93 (m, 2H, Ar–H); 10.17 (s, D2O exchangeable, 1H, NH); 13C NMR (125 MHz, DMSO) δC (ppm): 18.5 (CH3), 19.2 (CH3), 108.9, 115.1 (CN), 123, 125.6, 128.3, 132, 140, 153.8, 155.5, 158.6, 160, 181.4 (CO); MS m/z (%): 323 [M+-CN] (50%), 322 (100); Anal. Calcd. for C17H12N6OS (348.38): C, 58.61; H, 3.47; N, 24.12, Found: C, 58.23; H, 3.19; N, 23.94%.
Molecular docking studies
The structure of our target enzyme (PDB code 1KZN) was chosen as the protein model for this study58. The heteroatoms were taken away from the protein file and the resulting structure was introduced to AutoDock. The binding image of 9 new arylthioureas with DNA gyrase were assessed in the same way of binding of clorobiocin.
The 3D structures of arylthioureas were optimized using GAMESS (https://www.msg.chem.iastate.edu/gamess). The final forms were calculated with the semi empirical parameterized model number 3 (PM3) method.
Docking was executed by the default parameters of molecular docking AutoDock 4.2 and employed empirical free energy function59. In the docking procedure, compounds were supposed to be flexible and the docking software was allowed to rotate all rotatable bonds of them to obtain the best conformer within the active site of the enzyme. Clorobiocin was redocked to the binding site to evaluate our method.
The grid box was positioned with the coordinates x = 19.172, y = 30.465, z = 34.697 for DNA gyrase (PDB code 1KZN). Grid box sizes were 60 × 60 × 60 with a 0.5 Å grid points space. Grid maps were calculated by Autogrid4. A lamarckian genetic algorithm within the Autodock was used to estimate the diverse ligand conformers. Conformations were clustered by the root mean square deviation tolerance of 2.0 Å and were ranked according to the binding free energy59. Discovery Studio 2020 Visualizer was used to explore the hydrophobic and hydrogen bonding interactions of the compound with DNA gyrase.
Antimicrobial evaluation
The agar well diffusion method is widely used to evaluate the antimicrobial activity of plants or microbial extracts. Similar to the procedure used in disk-diffusion method, the agar plate surface is inoculated by spreading a volume of the microbial inoculum over the entire agar surface then, a hole with a diameter of 6 to 8 mm is punched aseptically with a sterile corkborerora tip A volume (20–100 mL) of the antimicrobial agent or extract solution at desired concentration is introduced into the well and agar plates are then incubated under suitable conditions depending upon the microorganism. The antimicrobial agent diffusion the agar medium and inhibits the growth of the microbial strain60.
Acknowledgements
The authors thank Mr. Ahmed Abbas, researcher at the Drugs Department, Faculty of Pharmacy, Mansoura University, Egypt, for handling the antimicrobial properties.
Author contributions
Two authors participated in the idea of this research. They carried out the synthesis, purification and characterization of all compounds by the different analysis tools. They prepared and wrote the main manuscript text. They read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rizk E. Khidre and Ibrahim Ali M. Radini.
References
- 1.Karam NH, Tomma JH, Al-Dujaili AH. Synthesis and characterization of new derivatives of thiazole with liquid crystalline properties. Chem. Mater. Res. 2013;3(9):162–171. [Google Scholar]
- 2.Haragave KD, Hess FK, Oliver JT. N-(4-Substituted-thiazolyl) oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 1983;26(8):1158–1163. doi: 10.1021/jm00362a014. [DOI] [PubMed] [Google Scholar]
- 3.Sharma RN, Xavier FP, Vasu KK, Chaturvedi SC, Pancholi SS. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: an analogue-based drug design approach. J. Enzyme Inhib. Med. Chem. 2009;24:890–897. doi: 10.1080/14756360802519558. [DOI] [PubMed] [Google Scholar]
- 4.Bell FW, Cantrell AS, Hoegberg M, Jaskunas SR, Johansson NG, Jorden CL, Kinnick MD, Lind P, Morin JM, Jr, Noreen R, Oberg B, Palkowitz JA, Parrish CA, Pranc P, Sahlberg C, Ternansky RJ, Vasileff RT, Vrang L, West SJ, Zhang H, Zhou XX. Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure–activity relationship studies of PETT analogs. J. Med. Chem. 1995;38:4929–4936. doi: 10.1021/jm00025a010. [DOI] [PubMed] [Google Scholar]
- 5.Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG, Jr, Connoly CJC, Doherty AM, Klutchko SR, Sircar I, Steinbaugh BA, Batley BL, Painchaud CA, Rapundalo ST, Michniewiez BM, Olson SCJ. Structure–activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J. Med. Chem. 1992;35:2562–2572. doi: 10.1021/jm00092a006. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji K, Ishikawa H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg. Med. Chem. Lett. 1994;4:1601–1606. doi: 10.1016/S0960-894X(01)80574-6. [DOI] [Google Scholar]
- 7.Ergenc N, Capan G, Gunay NS, Ozkirimli S, Gungor M, Ozbey S, Kendi E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. Pharm. Med. Chem. 1999;332:343–347. doi: 10.1002/(SICI)1521-4184(199910)332:10<343::AID-ARDP343>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Jaen JC, Wise LD, Caprathe BW, Tecle H, Bergmeier S, Humblet CC, Heffner TG, Meltzer LT, Pugsley TA. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: a novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990;33:311–317. doi: 10.1021/jm00163a051. [DOI] [PubMed] [Google Scholar]
- 9.Carter JS, Kramer S, Talley JJ, Penning T, Collins P, Graneto MJ, Seibert K, Koboldt C, Masferrer J, Zweifel B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999;9:1171–1174. doi: 10.1016/S0960-894X(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph J, Theis H, Hanke R, Endermann R, Johannsen L, Geschke F. Seco-cyclothialidines: new concise synthesis, inhibitory activity toward bacterial and human DNA topoisomerases, and antibacterial properties. J. Med. Chem. 2001;44:619–626. doi: 10.1021/jm0010623. [DOI] [PubMed] [Google Scholar]
- 11.Badorc A, Bordes MF, de Cointet P, Savi P, Bernat A, Lale A, Petitou M, Maffrand JP, Herbert JM. New orally active non-peptide fibrinogen receptor (GpIIb-IIIa) antagonists: identification of ethyl 3-[N-[4-[4-[Amino[(ethoxycarbonyl)imino]methyl]phenyl]-1,3-thiazol-2-yl]-N-[1-[(ethoxycarbonyl)methyl]piperid-4-yl]amino]propionate (SR 121787) as a potent and long-acting antithrombotic agent. J. Med. Chem. 1997;40:3393–3401. doi: 10.1021/jm970240y. [DOI] [PubMed] [Google Scholar]
- 12.Lino CI, de Souza IG, Borelli BM, Matos TTS, Teixeira INS, Ramos JP, de SouzaFagundes EM, Fernandes PO, Maltarollo VG, Johann S, et al. Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem. 2018;151:248–260. doi: 10.1016/j.ejmech.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 13.Reddy GM, Garcia JR, Reddy VH, de Andrade AM, Camilo A, Jr, Ribeiro RAP, de Lazaro SR. Synthesis, antimicrobial activity and advances in structure-activity relationships (SARs) of novel tri-substituted thiazole derivatives. Eur. J. Med. Chem. 2016;123:508–513. doi: 10.1016/j.ejmech.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 14.Cushman, M.S., Seleem, M., Mayhoub, A.S. Antimicrobial substituted thiazoles and methods of use. United States Patent No.: US 9, 801, 861 B2, 2017.
- 15.Leoni A, Locatelli A, Morigi R, Rambaldi M. Novel thiazole derivatives: a patent review (2008–2012; Part 1) Expert Opin. Ther. Patents. 2014;24:201–216. doi: 10.1517/13543776.2014.858121. [DOI] [PubMed] [Google Scholar]
- 16.Sinha S, Doble M, Manju SL. Design, synthesis and identification of novel substituted 2-amino thiazole analogues as potential anti-inflammatory agents targeting 5-lipoxygenase. Eur. J. Med. Chem. 2018;158:34–50. doi: 10.1016/j.ejmech.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 17.Kamble RD, Meshram RJ, Hese SV, More RA, Kamble SS, Gacche RN, Dawane BS. Synthesis and in silico investigation of thiazoles bearing pyrazoles derivatives as anti-inflammatory agents. Comput. Biol. Chem. 2016;61:86–96. doi: 10.1016/j.compbiolchem.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Pember SO, Mejia GL, Price TJ, Pasteris RJ. Piperidinyl thiazole isoxazolines: a new series of highly potent, slowly reversible FAAH inhibitors with analgesic properties. Bioorg. Med. Chem. Lett. 2016;26:2965–2973. doi: 10.1016/j.bmcl.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wu C, Zhang Q, Shan Y, Gu W, Wang S. Design, synthesis and biological evaluation of novel β-pinene-based thiazole derivatives as potential anticancer agents via mitochondrial-mediated apoptosis pathway. Bioorg. Chem. 2019;84:468–477. doi: 10.1016/j.bioorg.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Santana TI, Barbosa MO, Gomes PATM, Cruz ACN, Silva TG, LimaLeite AC. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem. 2018;144:874–886. doi: 10.1016/j.ejmech.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Amin KM, Rahman ADE, Al-Eryani YA. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008;16:5377–3588. doi: 10.1016/j.bmc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Paulvannan K, Chen T. solid-phase synthesis of 1,2,3,4-tetrahydro-2-pyridones via aza-annulation of enamines. J. Org. Chem. 2000;65:6160–6166. doi: 10.1021/jo000676c. [DOI] [PubMed] [Google Scholar]
- 23.Elnagdi MH, Ghozlan SA, Abd-Razik FM, Maghraby ASJ. Studies with polyfunctionally substituted heterocycles: synthesis of new thiopyrans, pyridines and pyrans and their fused derivatives with other ring systems. Chem. Res. Synop. 1991;5:116–117. [Google Scholar]
- 24.Attaby FA, Eldin SM, Abd El-Razik M. Reactions with cyanothioacetamide derivatives: synthesis and reactions of some pyridines and thieno[2,3-b]pyridine derivatives. Phosphorus Sulfur Silicon Relat. Elem. 1995;106:21–28. doi: 10.1080/10426509508027885. [DOI] [Google Scholar]
- 25.Krauze A, Verhe R, Duburs G. Concerning reaction of 1,4-dihydropyridine-2(3H)thione with epichlorohydrin. Khim. Geterotsikl. Soedin. 1994;1:139–140. [Google Scholar]
- 26.Jaiprakash NS, Abhay SZ, Firoz AKK, Indrajeet G, Zahid Z. Synthesis and biological activity of substituted-4,5,6,7-tetrahydrothieno pyridines: a review. Mini Rev. Med. Chem. 2014;14:988–1020. doi: 10.2174/1389557514666141106131425. [DOI] [PubMed] [Google Scholar]
- 27.Li AH, Moro S, Forsyth N, Melman N, Ji XD, Jacobsen KAJ. Synthesis, CoMFA analysis, and receptor docking of 3,5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. Med. Chem. 1999;42:706–721. doi: 10.1021/jm980550w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vacher B, Bonnand B, Funes F, Jubault N, Koek W, Assie MB, Cosi C, Kleven M. Novel derivatives of 2-pyridinemethylamine as selective, potent, and orally active agonists at 5-HT1A receptors. J. Med. Chem. 1999;42:1648–1660. doi: 10.1021/jm9806906. [DOI] [PubMed] [Google Scholar]
- 29.Farah AE, Alousi AA. New cardiotonic agents: a search for digitalis substitute. Life Sci. 1978;22:1139–1148. doi: 10.1016/0024-3205(78)90083-8. [DOI] [PubMed] [Google Scholar]
- 30.Alousi AA, Canter JM, Monterano MJ, Fort DJ, Ferrari RAJ. Cardiotonic activity of milrinone, a new and potent cardiac bipyridine, on the normal and failing heart of experimental animals. J. Cardiovasc. Pharmacol. 1983;5:792–803. doi: 10.1097/00005344-198309000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Chavan V, Sonawane S, Shingare M, Karale B. Synthesis, characterization, and biological activities of some 3,5,6-trichloropyridine derivatives. Chem. Heterocycl. Compd. 2006;42:625–630. doi: 10.1007/s10593-006-0137-8. [DOI] [Google Scholar]
- 32.Zav’yalova VK, Zubarev AA, Shestopalov AM. Synthesis and reactions of 3-acetyl-6-methyl-2-(methylthio)pyridine. Russ. Chem. Bull. 2009;58:1939–1944. doi: 10.1007/s11172-009-0265-2. [DOI] [Google Scholar]
- 33.Patel NB, Agravat SN, Shaikh FM. Synthesis and antimicrobial activity of new pyridine derivatives-I. Med. Chem. Res. 2011;20:1033–1041. doi: 10.1007/s00044-010-9440-0. [DOI] [Google Scholar]
- 34.Muthal N, Ahirwar J, Ahriwar D, Masih P, Mahmdapure T, Sivakumar T. Synthesis, antimicrobial and anti-inflammatory activity of some 5-substituted-3-pyridine-1, 2, 4-triazoles. Int. J. Pharm. Tech. Res. 2010;2:2450–2455. [Google Scholar]
- 35.Khidre RE, El-Gogary SR, Mostafa MS. Design, synthesis, and antimicrobial evaluation of some novel pyridine, coumarin, and thiazole derivatives. J. Heterocycl. Chem. 2017;54:2511–2519. doi: 10.1002/jhet.2854. [DOI] [Google Scholar]
- 36.Khidre RE, Radini IAM, Ibrahim DA. Synthesis of a novel heterocyclic scaffold utilizing 2-cyano-N-(3-cyano-4,6-dimethyl-2-oxopyridin-1-yl)acetamide. ARKIVOC. 2016;2016:1–17. doi: 10.3998/ark.5550190.p009.722. [DOI] [Google Scholar]
- 37.El-Hawash SAM, Abdel Wahab AE, El-Demellawy MA. Cyanoacetic acid hydrazones of 3-(and 4-)acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch. Der Pharm. 2006;339:14–23. doi: 10.1002/ardp.200500161. [DOI] [PubMed] [Google Scholar]
- 38.Vrábel M, Hocek M, Havran L, Fojta M, Votruba I, Klepetářová B, Pohl R, Rulíšek L, Zendlová L, Hobza P, Shih IH, Mabery E, Mackman R. Purines bearing phenanthroline or bipyridine ligands and their RuII complexes in position 8 as model compounds for electrochemical DNA labeling-synthesis, crystal structure, electrochemistry, quantum chemical calculations, cytostatic and antiviral activity. Eur. J. Inorg. Chem. 2007;2007:1752–1769. doi: 10.1002/ejic.200700030. [DOI] [Google Scholar]
- 39.Worachartcheewan A, Prachayasittikul S, Pingaew R, et al. Antioxidant, cytotoxicity, and QSAR study of 1-adamantylthio derivatives of 3-picoline and phenyl pyridines. Med. Chem. Res. 2012;21:3514–3522. doi: 10.1007/s00044-011-9903-y. [DOI] [Google Scholar]
- 40.Firke S, Firake B, Chaudhari R, Patil V. Synthetic and pharmacological evaluation of some pyridine containing thiazolidinones. Asian J. Res. Chem. 2009;2:157–161. [Google Scholar]
- 41.Easmon J, Pürstinger G, Thies K-S, Heinisch G, Hofmann J. Synthesis, structure–activity relationships, and antitumor studies of 2-benzoxazolyl hydrazones derived from alpha-(N)-acyl heteroaromatics. J. Med. Chem. 2006;49:6343–6350. doi: 10.1021/jm060232u. [DOI] [PubMed] [Google Scholar]
- 42.Kovala-Demertzi D, Alexandratos A, Papageorgiou A, Yadav PN, Dalezis P, Demertzis MA. Synthesis, characterization, crystal structures, in vitro and in vivo antitumor activity of palladium(II) and zinc(II) complexes with 2-formyl and 2-acetyl pyridine N(4)-1-(2-pyridyl)-piperazinyl thiosemicarbazone. Polyhedron. 2008;27:2731–2738. doi: 10.1016/j.poly.2008.04.009. [DOI] [Google Scholar]
- 43.Illán-Cabeza NA, Jiménez-Pulido SB, Martínez-Martos JM, Ramírez-Expósito MJ, Moreno-Carretero MN. New 2,6-bis-[uracil-imino] ethylpyridine complexes containing the CdN6 core: synthesis, crystal structures, luminescent properties and antiproliferative activity against C6 glioma cells. J. Inorg. Biochem. 2009;103:1176–1184. doi: 10.1016/j.jinorgbio.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Márquez-Flores YK, Campos-Aldrete ME, Salgado-Zamora H, et al. Acute and chronic anti-inflammatory evaluation of imidazo[1,2-a]pyridine carboxylic acid derivatives and docking analysis. Med. Chem. Res. 2012;21:3491–3498. doi: 10.1007/s00044-011-9870-3. [DOI] [Google Scholar]
- 45.Sondhi SM, Dinodia M, Kumar A. Synthesis, anti-inflammatory and analgesic activity evaluation of some amidine and hydrazone derivatives. Bioorg. Med. Chem. 2006;14:4657–4663. doi: 10.1016/j.bmc.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Khidre RE, Ameen TA, Salem MAI. Tetrazoloquinolines: synthesis, reactions, and applications. Curr. Org. Chem. 2020;24:439–464. doi: 10.2174/1385272824666200217095341. [DOI] [Google Scholar]
- 47.Mohamed HA, Khidre RE, Kariuki BM, El-Hiti GA. Synthesis of novel heterocycles using 1,2,3-triazole-4-carbohydrazides as precursors. J. Heterocycl. Chem. 2020;57:1055–1062. doi: 10.1002/jhet.3840. [DOI] [Google Scholar]
- 48.Khidre RE, Mohamed HA, Kariuki BM, El-Hiti GA. Facile, mild and efficient synthesis of azines using phosphonic dihydrazide. Phosphorus Sulfur Silicon Relat. Elem. 2020;195:29–36. doi: 10.1080/10426507.2019.1633531. [DOI] [Google Scholar]
- 49.Elgogary SR, Khidre RE, El-Telbani EM. Regioselective synthesis and evaluation of novel sulfonamide1,2,3-triazole derivatives as antitumor agents. J. Iran. Chem. Soc. 2020;17:765–776. doi: 10.1007/s13738-019-01796-y. [DOI] [Google Scholar]
- 50.Khidre RE, Radini IAM. Synthesis and antimicrobial activity of novel heterocycles utilizing 3-(1,4-dioxo-3,4-dihydrophthalazin-2(1H)-yl)-3-oxopropanenitrile as precursors. J. Heterocycl. Chem. 2019;56:850–858. doi: 10.1002/jhet.3463. [DOI] [Google Scholar]
- 51.Thomsen R, Christensen MH. MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 52.Nanda AK, Ganguli S, Chakraborty R. Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones: synthesis and preliminary QSAR studies. Molecules. 2007;12:2413–2426. doi: 10.3390/12102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bansal S, Kumar S, Aggarwal V, Joseph A. Design, synthesis, docking study & antibacterial evaluation of 1, 3-diarylpyrazolyl substituted indolin-2-ones. Indo Glob. J. Pharm. Sci. 2014;4:1–7. [Google Scholar]
- 54.Jayashree BS, Thomas S, Nayak Y. Design and synthesis of 2-quinolones as antioxidants and antimicrobials: a rational approach. Med. Chem. Res. 2010;19:193–209. doi: 10.1007/s00044-009-9184-x. [DOI] [Google Scholar]
- 55.Rahimi H, Najafi A, Eslami H, Negahdari B, Moghaddam MM. Identification of novel bacterial DNA gyrase inhibitors: an in-silico study. Res. Pharm. Sci. 2016;11:250–258. [PMC free article] [PubMed] [Google Scholar]
- 56.Radini IAM, Khidre RE, El-Telbani EM. Synthesis and antimicrobial evaluation of new pyrazoline and pyrazolinyl thiazole derivatives bearing tetrazolo[1,5-a]quinoline moiety. Lett. Drug Design Discov. 2016;13:921–931. doi: 10.2174/1570180813666160712234454. [DOI] [Google Scholar]
- 57.Shawali AS, Elsheikh S, Párkányi C. Cyclization of thiohydrazonate esters and azo-hydrazone tautomerism of 2-arylhydrazono-3-oxo-1,4-benzothiazines. J. Heterocycl. Chem. 2003;40:207–212. doi: 10.1002/jhet.5570400202. [DOI] [Google Scholar]
- 58.Lafitte D, Lamour V, Tsvetkov PO, Makarov AA, Klich M, Deprez P, et al. DNA gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5'-methyl group of the noviose. Biochemistry. 2002;41:7217–7223. doi: 10.1021/bi0159837. [DOI] [PubMed] [Google Scholar]
- 59.Mansourian M, Fassihi A, Saghaie L, Madadkar-Sobhani A, Mahnam K, Abbasi M. QSAR and docking analysis of A2B adenosine receptor antagonists based on non-xanthine scaffold. Med. Chem. Res. 2015;24:394–407. doi: 10.1007/s00044-014-1133-7. [DOI] [Google Scholar]
- 60.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]