Abstract
N6-methyladenosine (m6A), the most abundant internal modification in eukaryotic mRNA, is installed by a multi-component writer complex; however, the exact roles of each component remain poorly understood. Here we show that a potential E3 ubiquitin ligase Hakai colocalizes and interacts with other m6A writer components, and Hakai mutants exhibit typical m6A pathway defects in Drosophila, such as lowered m6A levels in mRNA, aberrant Sxl alternative splicing, wing and behavior defects. Hakai, Vir, Fl(2)d and Flacc form a stable complex, and disruption of either Hakai, Vir or Fl(2)d led to the degradation of the other three components. Furthermore, MeRIP-seq indicates that the effective m6A modification is mostly distributed in 5’ UTRs in Drosophila, in contrast to the mammalian system. Interestingly, we demonstrate that m6A modification is deposited onto the Sxl mRNA in a sex-specific fashion, which depends on the m6A writer. Together, our work not only advances the understanding of mechanism and regulation of the m6A writer complex, but also provides insights into how Sxl cooperate with the m6A pathway to control its own splicing.
Subject terms: Development, Epigenetics, RNA splicing, RNA modification
Drosophila m6A writer complex regulates alternative splicing of the Sex-lethal gene. Here the authors show that a potential E3 ligase Hakai interacts with the fly m6A writer complex and that m6A level is reduced in Hakai mutant flies.
Introduction
There are a variety of chemical modifications on biological macromolecules, such as proteins, nucleic acids, and glycolipids. Like DNA methylation and histone modification, RNA modification represents an extra layer of epigenetic regulatory mechanism1,2. More than 150 chemical modifications in RNA have been discovered, and their biological functions are only starting to be revealed3. Chemical modifications of RNA exist in all organisms and for all forms of RNA, including tRNA, rRNA, mRNA, and long noncoding RNA. Common RNA modifications include N6-methyladenosine (m6A), N6,2’-O-dimethyladenosine (m6Am), N1-methyladenosine (m1A), 5-methylcytidine (m5C), N4-acetylcytidine (ac4C), 7-methylguanosine (m7G), and pseudouridine (Ψ), etc4,5. Among them, m6A is the most abundant internal modification of mRNA in eukaryotes. Although m6A in mRNA was found more than 40 years ago6,7, it was only recently that the field has made extensive progress owing to technological and experimental breakthroughs. By combining m6A-specific antibody and high-throughput sequencing, MeRIP-Seq or m6A-Seq allows the m6A mapping at the whole transcriptome level, thereby providing the possibility to correlate RNA modifications with their biological functions8,9. These and subsequent studies revealed that m6A sites contain a consensus motif RRACH (R = G/A; H = U/A/C), and m6A peaks are enriched in the 3′ untranslated region (UTR) and near the stop codon in yeast and mammals8–10. In Arabidopsis, m6A is enriched not only in 3′UTRs and near the stop codon but also in 5′UTRs and around the start codon11. In mammalian cells, m6A also accumulates in the 5′UTR region in response to stress conditions such as heat shock12,13. The distribution of m6A is important since it implies the mechanism by which m6A modification regulates its mRNA.
Another major breakthrough is the gradual elucidation of the m6A modification pathway by biochemical and genetic studies. The m6A is deposited by a multicomponent methyltransferase complex (“writers”)14–16, mainly recognized by YTH domain-containing “readers”17, and can be removed by FTO and ALKBH5 “erasers”18,19, although FTO was also indicated as an m6Am demethylase20. The key catalytic component of the m6A writer complex, Mettl3, was purified and cloned in 1990s21,22. Since then, studies from yeast, Arabidopsis, Drosophila, and mammalian cells have identified several core components of the writer complex23,24, including Mettl1425,26, WTAP (Fl(2)d)27–29, VIRMA (Virilizer)30,31, RBM15/15B (Spenito)32,33, ZC3H13 (Flacc or Xio)34–36, and Hakai37. Interestingly, Fl(2)d38, Virilizer (Vir)39, Spenito (Nito)40, and Xio36 were first identified from Drosophila sex determination screens and later realized as part of the writer complex. They regulate Drosophila sex determination by controlling the alternative splicing of the master regulatory gene Sex-lethal (Sxl)41–46. Recently, Mettl3, Mettl14, as well as the reader Ythdc1, were also shown to be involved in this process33,47,48. However, the detailed mechanism of how the m6A modification cooperates with Sxl protein to modulate its own splicing is still unclear. Thus, Drosophila can serve as a unique system to screen components in the m6A pathway and pinpoints a critical role for m6A in regulating splicing. Other than Sxl splicing, Drosophila m6A genes are highly expressed in the nervous system and exhibit similar wing and behavior defects when mutated33,36,47,48. Mutants of several fly m6A factors are viable and thus provide an ideal model to study other processes, such as metabolism and immunity, in the future.
Hakai, also known as CBLL1, was found as an interacting protein with several m6A writer components in proteomic studies23,31,49. It encodes a RING finger-type E3 ubiquitin ligase and was originally identified as an E-cadherin-binding protein in human cell lines50. It was proposed that Hakai ubiquitinates E-cadherin at the plasma membrane and induces its endocytosis, thus playing a negative role post-translationally. Due to the key role of E-cadherin in tumor metastasis, especially epithelial–mesenchymal transition, Hakai has been extensively studied mainly using cell culture and overexpression system51, but a previous study using the Drosophila model did not observe an increase of E-cadherin level in Hakai mutants52. In Arabidopsis, Hakai mutants show partially reduced m6A levels and the mutant phenotypes are weaker than other writer components37. Importantly, the in vivo role of Hakai as a core m6A writer component has not been studied in any animal species. Here, we analyzed the role of Hakai in the Drosophila m6A modification pathway. Our results demonstrated that Hakai is a bona fide member of the m6A writer complex, with its mutants showing reduced global m6A levels, typical m6A mutant phenotypes, and commonly-regulated gene sets. We also obtained a high-quality fly m6A methylome using stringent MeRIP-seq, discovered a female-specific m6A methylation pattern for Sxl mRNA, characterized the role of Hakai in the m6A writer complex, and finally revisited the function of Hakai in E-cadherin regulation.
Results
Hakai interacts and colocalizes with known m6A writer complex subunits
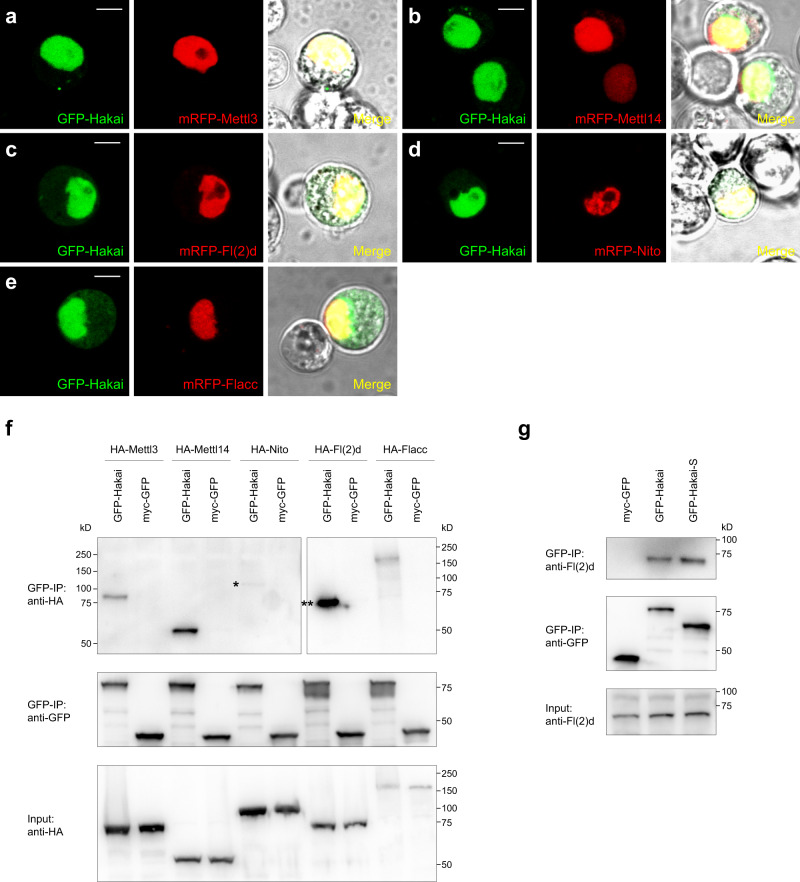
Since Hakai was found as an interacting protein with other m6A writer components in the mammalian proteomic study23, we searched the large-scale Drosophila Protein interaction Map (DPiM) database53. Hakai as bait can pull down Fl(2)d, Vir, and Nito in affinity purification and mass spectrometry experiments; on the other hand, Flacc as a bait can pull down Hakai (Supplementary Fig. 1b). Similarly, our own previous mass-spec study using Fl(2)d or Nito as bait can reciprocally pull down Hakai (Supplementary Fig. 1a)36. To confirm these interactions, we performed both co-localization and co-immunoprecipitation (Co-IP) assays. GFP-Hakai localized to nucleus in live S2 cells and co-localized well with mRFP-Mettl3, mRFP-Mettl14, mRFP-Fl(2)d, mRFP-Nito, and mRFP-Flacc (Fig. 1a–e). Next, we transfected GFP-Hakai and different HA-tagged constructs in S2 cells, and used myc-GFP as a control. In the Co-IP experiments, GFP-Hakai, but not myc-GFP, was able to pull down HA-Mettl3, HA-Mettl14, HA-Nito, HA-Fl(2)d, and HA-Flacc (Fig. 1f). Interestingly, the pulldown between Hakai and Fl(2)d was particularly strong compared to other factors, suggesting that Hakai may directly interact with Fl(2)d, while the interaction between Hakai and Nito was the weakest (Fig. 1f). We then further examined the ability of GFP-Hakai to pull down endogenous Fl(2)d proteins using the available monoclonal antibody42. Hakai transcript is alternatively spliced, producing long and short protein isoforms (Fig. 2a), both of which contain a C3HC4 RING finger domain and a C2H2 zinc-finger domain (Supplementary Fig. 2a). We also included the short isoform in our assay and found that both GFP-Hakai (long isoform) and GFP-Hakai-S (short isoform) can robustly pull down Fl(2)d to a similar extent (Fig. 1g). Together, these data suggest that Hakai is a conserved core component of the m6A writer complex and its N-terminal domains are important for its interaction.
Fig. 1. Hakai colocalizes and interacts with other m6A writer components.
a–e GFP-Hakai and mRFP-Mettl3, mRFP-Mettl14, mRFP-Fl(2)d, mRFP-Nito, or mRFP-Flacc were co-transfected into S2 cells and their subcellular localization examined in live conditions. All the proteins were predominantly nuclear and GFP-Hakai showed strong co-localization with other factors. Scale bars: 5 μm. f GFP-Hakai or myc-GFP and HA-Mettl3, HA-Mettl14, HA-Nito, HA-Fl(2)d, HA-Flacc were co-transfected into S2 cells. Cell lysates were immunoprecipitated using GFP nanobody and analyzed by western blot. myc-GFP was used as a control. GFP-Hakai can pull down HA-Mettl3, HA-Mettl14, HA-Nito, HA-Fl(2)d, and HA-Flacc. Note that much more HA-Fl(2)d was co-IPed than other factors (double asterisk), while the interaction with Nito was the weakest (asterisk). g myc-GFP, GFP-Hakai, or GFP-Hakai-S (short isoform) were transfected into S2 cells. Cell lysates were immunoprecipitated using GFP nanobody and analyzed by western blot. GFP-Hakai and GFP-Hakai-S can pull down endogenous Fl(2)d protein to a similar level. Source data are provided as a Source Data file. The experiments in a–g were repeated at least twice independently with similar results.
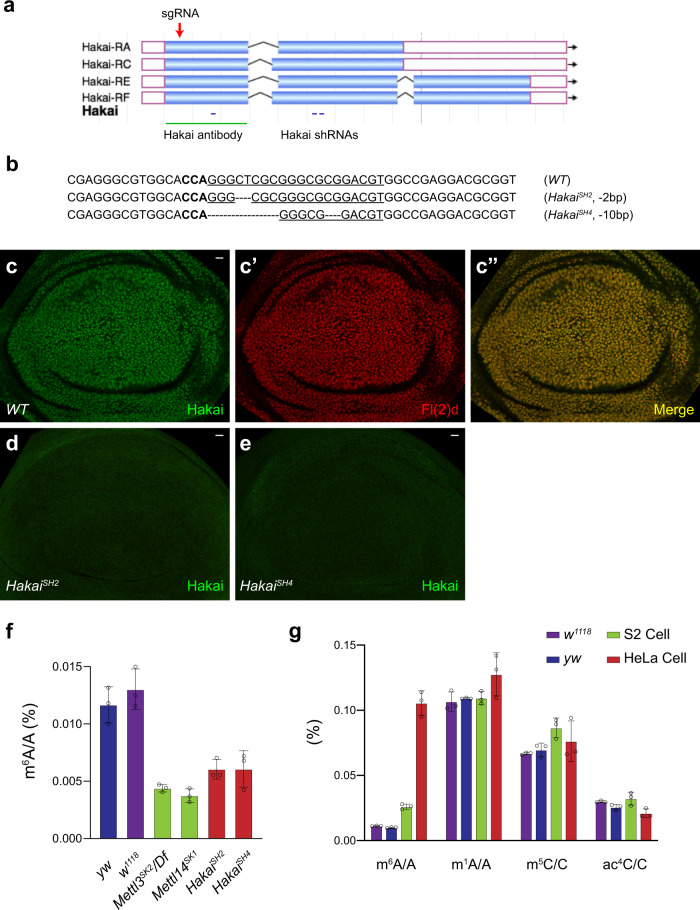
Fig. 2. Hakai is required to maintain proper levels of m6A methylation.
a Flybase JBrowse view of the Hakai gene locus. Hakai has four transcripts due to alternative splicing that generates two long and two short protein isoforms. The positions of three independent shRNAs, Hakai sgRNA, and the protein region used to generate a Hakai antibody are indicated. b Sequencing results showing frameshift indels in HakaiSH2 and HakaiSH4 flies generated by CRISPR/Cas9-mediated mutagenesis. The targeted genomic DNA sequence is underlined and the NGG PAM sequence is in bold type. c–c” Hakai and Fl(2)d antibody staining in WT wing discs showing a high degree of co-localization. Hakai antibody staining was strongly reduced in HakaiSH2 (d) or HakaiSH4 (e) homozygous mutant wing discs. The experiments in c–e were repeated at least twice independently with similar results, and each time around 30 wing discs for any genotype were examined. Scale bars: 10 μm. f Quantifications of m6A relative to A in mRNA extracted from male adult flies by LC-MS. Compared to yw and w1118 controls, m6A levels dropped to about 30% in Mettl3 or Mettl14 mutants and to <50% in HakaiSH2 or HakaiSH4 mutants. g Quantifications of m6A/A, m1A/A, m5C/C, and ac4C/C levels in mRNA extracted from yw and w1118 male flies, Drosophila S2 cells, and human HeLa cells. Note the substantial difference between fly and human m6A levels. f, g Data are presented as mean ± SD from three biological replicates. Source data are provided as a Source Data file.
Hakai is required to maintain proper levels of m6A methylation
Hakai transcript shows a similar expression pattern to those of other m6A writers and readers36, with high expression in the CNS, ovary, fat body and imaginal discs (Supplementary Fig. 2b; modENCODE developmental and tissue expression database54). During development, its expression is high in early embryos, decreases during larval stages, and rises again at pupal stages (Supplementary Fig. 2b), which coincides with the reported m6A levels33. To monitor endogenous protein expression, we raised an antibody against Hakai (Fig. 2a) and found that it is a ubiquitously-expressed nuclear protein that strongly colocalizes with Fl(2)d (Fig. 2c–c”). To further investigate Hakai function, we generated a sgRNA and constructed or obtained three nonoverlapping shRNA lines (Fig. 2a)55,56. By crossing the U6:3-Hakai sgRNA with nanos-Cas957, we generated a series of Hakai mutants with various small deletions and/or insertions. We chose two alleles, HakaiSH2 and HakaiSH4, for further analysis, since they represent different early frameshift mutations that are expected to disrupt translation (Fig. 2b). Indeed, only the background level of Hakai antibody staining remained in Hakai homozygous mutant wing discs compared to wild-type (Fig. 2d, e, compared to c), suggesting these are null or strong loss-of-function alleles. Hakai homozygous mutants are semi-lethal and only produce viable adult flies in noncrowded conditions, which have delayed developmental time, smaller size, and reduced lifespan.
We then measured N6-methyladenosine levels in Hakai mutant adults by quantitative liquid chromatography–mass spectrometry (LC-MS) and used yw and w1118 as wild-type controls. We used an external calibration curve prepared with A and m6A standards to determine the absolute quantities of each ribonucleoside (Supplementary Fig. 3). After two rounds of polyA selection, m6A levels dropped to around 30% in Mettl3 or Mettl14 mutant flies, while m6A levels reduced more than half in HakaiSH2 or HakaiSH4 mutants (Fig. 2f). These results clearly indicate a critical role of Hakai in m6A methylation. During our analysis, we found that the m6A levels we measured in Drosophila (0.01–0.02% of adenosine after two rounds of polyA purification) were one magnitude lower than those in mammals (0.1–0.4% of adenosine)1. This result is consistent with a previous study showing that m6A accounts for 0.04% of adenosine after one round of polyA selection in yw flies47. Thus, we further measured the m6A level, as well as the level of several other RNA modifications such as m1A, m5C, and ac4C, in w1118, yw flies, S2 cells, and human HeLa cells. To our surprise, m6A level was five to ten times higher in human cells than those in Drosophila, while m1A, m5C, and ac4C levels were comparable (Fig. 2g). These results imply that the function and mechanism of the m6A pathway may be quite different between human and fly.
Sxl alternative splicing and adult fly behavior were defective in Hakai mutant
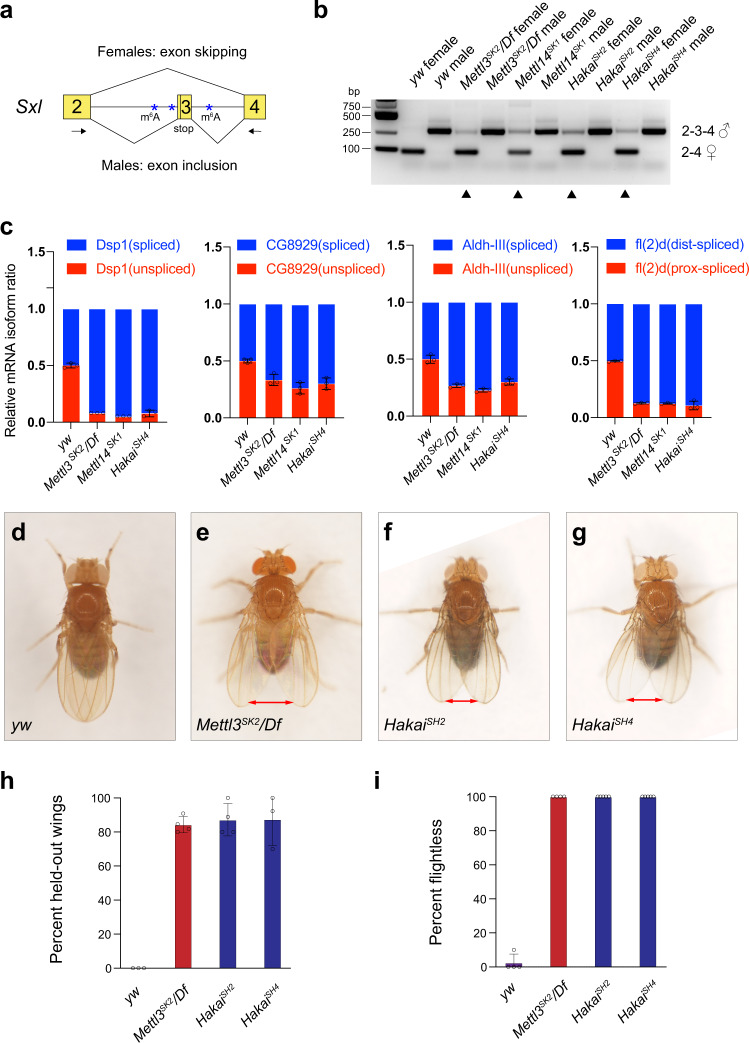
In Drosophila, Sxl pre-mRNA is the best-characterized example of m6A-modified transcripts. Sxl transcripts are alternatively spliced. While the male form includes exon3 that contains a stop codon and leads to early termination of Sxl protein translation, the female form skips exon3 and thus produces a functional Sxl protein (Fig. 3a)58. Previously multiple m6A sites have been mapped in introns on both sides of exon3 and these modifications were proposed to facilitate the alternative splicing of Sxl in female flies47,48. Indeed, the switch from female form to male form of Sxl splicing occurs in all m6A mutants, including Mettl3, Mettl14, Ythdc1, Fl(2)d, Vir, Nito, Flacc, thus representing a gold standard to validate components in this pathway33,34,36,38,41,43,47,48. To monitor Sxl splicing pattern, we used a pair of primers flanking exon3 that detects the small female and large male spliced Sxl products in RT-PCR (Fig. 3a, b, lanes 1, 2)59. As positive controls, Sxl splicing was partially shifted from the female form to the male form in Mettl3 or Mettl14 mutant females (Fig. 3b, lanes 3–6, arrowheads). In HakaiSH2 or HakaiSH4 female flies, a large band corresponding to the male-specific spliced form was clearly detected (Fig. 3b, last 4 lanes, arrowheads), similar to Mettl3 or Mettl14 mutants.
Fig. 3. Hakai regulates Sxl alternative splicing and adult fly behavior.
a Diagram showing the alternative splicing event that produces male- or female-specific Sxl transcripts. The arrows indicate primers used for RT-PCR. b Sxl splicing was analyzed by RT-PCR using RNAs extracted from adult flies of indicated genotypes. Note the appearance of male-specific bands in Mettl3SK2/Df, Mettl14SK1, HakaiSH2, HakaiSH4 females (arrowheads). Male-specific bands: 2–3–4. Female-specific bands: 2–4. c Relative isoform quantification for Dsp1, CG8929, Aldh-III and fl(2)d by qPCR in control or m6A mutant flies. Hakai is involved in m6A-dependent splicing events. Data are presented as mean ± SD from three biological replicates. d yw flies have their wings properly folded. (e) Mettl3SK2/Df, (f) HakaiSH2, (g) HakaiSH4 flies cannot fold their wings and exhibit a held-out wing phenotype (marked by the double arrows). The frequency of flies showing held-out wings were quantified in (h). i Flies of the indicated genotypes were tested for their flight abilities, and the number of flightless flies was quantified. All flies used from (c) to (i) were males. h–i Data are presented as mean ± SD from three to five biologically independent groups (h, n = 3, 4, 4, 3; i, n = 4, 4, 5, 5). Source data are provided as a Source Data file.
Disruption of several m6A components Fl(2)d, Vir, Nito or Flacc leads to not only aberrant Sxl splicing but also strongly reduced Sxl protein levels, thus generating a striking female-to-male transformation phenotype in adult flies34,36,38,41,43,60. Mutation of other factors Mettl3, Mettl14, or Ythdc1 alone does not affect Sxl protein levels and does not exhibit the transformation phenotype33,47,48. We found that Sxl protein level was not reduced in HakaiSH2 or HakaiSH4 female discs (Supplementary Fig. 4a–d). Furthermore, we expressed three Hakai RNAi using dome-Gal4 and did not observe any transformation phenotype in females, as evidenced before36,41.
Other than Sxl, it was reported that splicing of several other genes, including Dsp1, CG8929, Aldh-III, and fl(2)d, depends on the m6A pathway33,34. We then analyzed the splicing isoforms for these transcripts by RT-qPCR. In HakaiSH4 mutants, the splicing patterns for all four genes were affected similarly to those in Mettl3 or Mettl14 mutants (Fig. 3c, see Supplementary Fig. 9f–i for positions of primers used). It is worth noting that in all four cases, the spliced isoforms were increased while the unspliced forms were reduced in m6A pathway mutants.
Besides sex determination, m6A writer and reader mutants show characteristic adult defects. The most prominent ones are the held-out wings and flightless phenotypes in Mettl3, Mettl14, Ythdc1, or Flacc mutants36,47,48, likely due to loss of m6A functions in the nervous system33. Wild-type flies normally keep their wings in a folded position (Fig. 3d, h), however, the majority of Mettl3 mutant flies cannot fold their wings correctly and exhibit held-out wings (Fig. 3e, h), and 100% Mettl3 mutant flies cannot fly (Fig. 3i). Interestingly, HakaiSH2 and HakaiSH4 mutants phenocopy Mettl3 adult defects in terms of the strong held-out wing (Fig. 3f–h) and 100% flightless phenotypes (Fig. 3i). Together, these data suggest that Hakai plays an important role in m6A-modification pathway in vivo.
Hakai, Vir, Fl(2)d, and Flacc form a stable complex
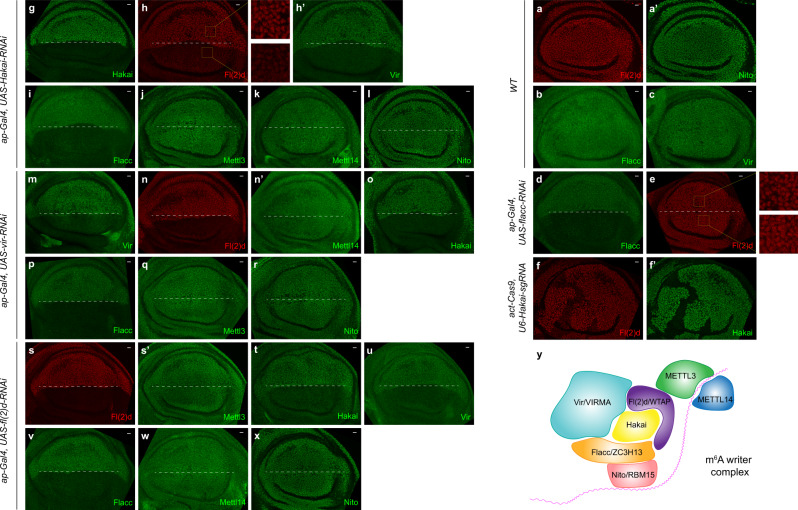
We aim to investigate further the mechanisms of Hakai in m6A methylation. Since Hakai is a potential E3 ubiquitin ligase, we examined the protein distribution of other m6A writer factors in the absence of Hakai. For this purpose, we generated antibodies against Mettl3, Mettl14, and Vir, constituting a full toolkit for all seven Drosophila m6A writers. In wild-type wing discs, all seven m6A writer components appeared as ubiquitous nuclear proteins that colocalizes extensively with each other (Figs. 4a–c, 2c–c” and Supplementary Fig. 5a, c). Expression of Hakai RNAi in the dorsal half of the wing disc using ap-Gal4 led to no effect on Mettl3, Mettl14, and Nito protein levels (Fig. 4j–l), but the strong reduction of Fl(2)d, Vir, and Flacc levels (Fig. 4h–i). In addition, we crossed actin-Cas9 with U6-Hakai-sgRNA flies to generate random Hakai loss-of-function clones. These clones were marked by the loss of Hakai staining, and the Fl(2)d level was reduced in these clones as well (Fig. 4f–f’). Since the roles of several other m6A writer components are not fully understood, we extended our immunostaining assays to those genes. Interestingly, knocking down vir by RNAi resulted in no effect on Mettl3, Mettl14, and Nito protein levels (Fig. 4q, n’, r), but strong reduction of Fl(2)d, Hakai, and Flacc levels (Fig. 4n, o, p). Similarly, depletion of fl(2)d by RNAi did not change the protein levels of Mettl3, Mettl14, and Nito (Fig. 4s’, w, x), but strongly reduced Hakai, Vir, and Flacc levels (Fig. 4t–v). Together, these results suggest that Fl(2)d, Vir, Hakai, and Flacc form a stable complex, and disruption of either Fl(2)d, Vir, or Hakai leads to degradation of the whole complex, while Mettl3, Mettl14, and Nito are not part of this complex. In cell culture, ZC3H13 plays a role in anchoring the writer complex in the nucleus35. Consistent with this, we observed more diffusive and less nuclear staining of Fl(2)d upon flacc RNAi knockdown in the wing discs (Fig. 4d, e, compare insets in 4e with 4h). Based on our data, we proposed a working model for the m6A writer complex (Fig. 4y, see “Discussion”).
Fig. 4. Hakai, Vir, Fl(2)d, and Flacc form a stable complex.
In wild-type wing discs, Fl(2)d (a), Nito (a’), Flacc (b), and Vir (c) show ubiquitous nuclear staining patterns. g–l Expressing Hakai RNAi in the dorsal half of the disc (below the dashed line) using ap-Gal4 resulted in a strong reduction of Fl(2)d (h, squared areas are magnified on the right), Vir (h’), and Flacc (i) levels, but not Mettl3 (j), Mettl14 (k), and Nito staining (l). f–f’ Fl(2)d staining is similarly reduced in Hakai mutant clones generated by actin-Cas9/U6-Hakai-sgRNA (f), which are marked by the loss of Hakai staining (f’). m–r Expressing vir RNAi in the dorsal half of the disc (below the dashed line) using ap-Gal4 resulted in a strong reduction of Fl(2)d (n), Hakai (o), and Flacc (p) levels, but not Mettl3 (q), Mettl14 (n’), and Nito staining (r). s–x Expressing fl(2)d RNAi in the dorsal half of the disc (below the dashed line) using ap-Gal4 resulted in a strong reduction of Hakai (t), Vir (u), and Flacc (v) levels, but not Mettl3 (s’), Mettl14 (w), and Nito staining (x). d, e Expressing Flacc RNAi in the dorsal half of the disc (below the dashed line) using ap-Gal4 led to more diffusive and less nuclear staining of Fl(2)d (e, squared areas are magnified on the right). The experiments in a–x were repeated at least twice independently with similar results, and each time around 30 wing discs for any genotype were examined. Scale bars: 10 μm. y A working model of the m6A writer complex comprised of seven core components.
Hakai does not mediate E-cadherin levels in wing discs
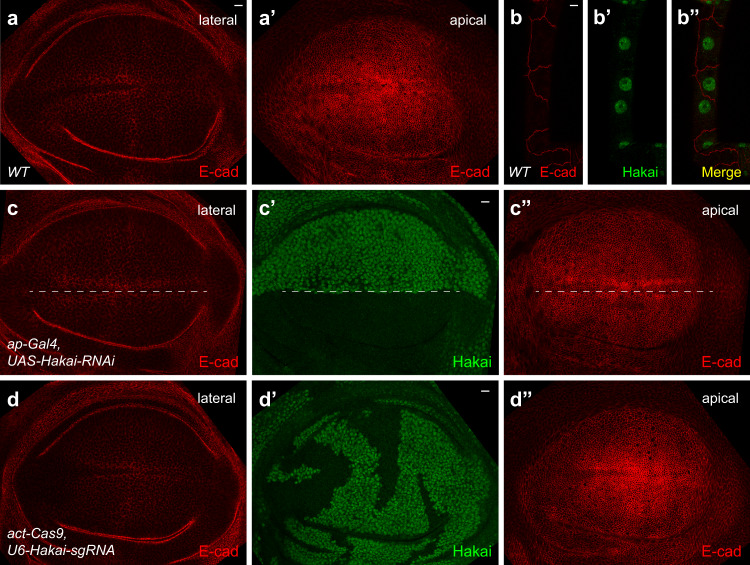
Hakai was first demonstrated as an E-cadherin interaction protein and its role in E-cadherin endocytosis and down-regulation was extensively studied in cell cultures50,51. However, a previous study in Drosophila failed to observe a major role for Hakai in E-cadherin regulation52. Thus, we addressed this question using our genetic toolset. In wild-type wing-disc epithelia, E-cadherin showed a membrane distribution and accumulates in the adherens junction (Fig. 5a, a’). First, we used ap-Gal4 to drive the expression of Hakai RNAi in the dorsal half of the wing disc. Although Hakai level was effectively knocked down (Fig. 5c’), E-cadherin level did not change in either the apical or the lateral section (Fig. 5c, c”). Second, in Hakai mutant clones generated by crossing actin-Cas9 with U6-Hakai-sgRNA, E-cadherin level was not affected either (Fig. 5d–d”). Finally, we examined the localization of E-cadherin and Hakai in detail using large tracheal cells. As shown in Fig. 5b–b”, most E-cadherin staining was on the cell membrane, while most Hakai staining was in the nucleus, and we did not observe co-localization between these two proteins. In conclusion, Hakai is not important for E-cadherin levels in Drosophila, and its major function likely happens in the nucleus.
Fig. 5. Hakai does not mediate E-cadherin levels in wing discs.
a–a’ Lateral (a) and apical (a’) section of E-cadherin staining in wild-type wing-disc epithelia. b–b” E-cadherin is mainly localized in the cell membrane (b), while Hakai is mostly in the nucleus (b’) in the large tracheal cells. c–c” Expression of Hakai RNAi in the dorsal half of the disc (below the dashed line) using ap-Gal4 led to a strong reduction of Hakai (c’), but E-cadherin staining was not affected either laterally (c) or apically (c”). d–d” Lateral (d) and apical (d”) E-cadherin distribution is not changed in Hakai mutant clones marked by the absence of Hakai staining (d’), when crossing actin-Cas9 with U6-Hakai-sgRNA. The experiments in a-d” were repeated at least twice independently with similar results, and each time around 30 wing discs for any genotype were examined. Scale bars: 10 μm.
The m6A modifications are deposited onto the Sxl mRNA in a sex-specific fashion
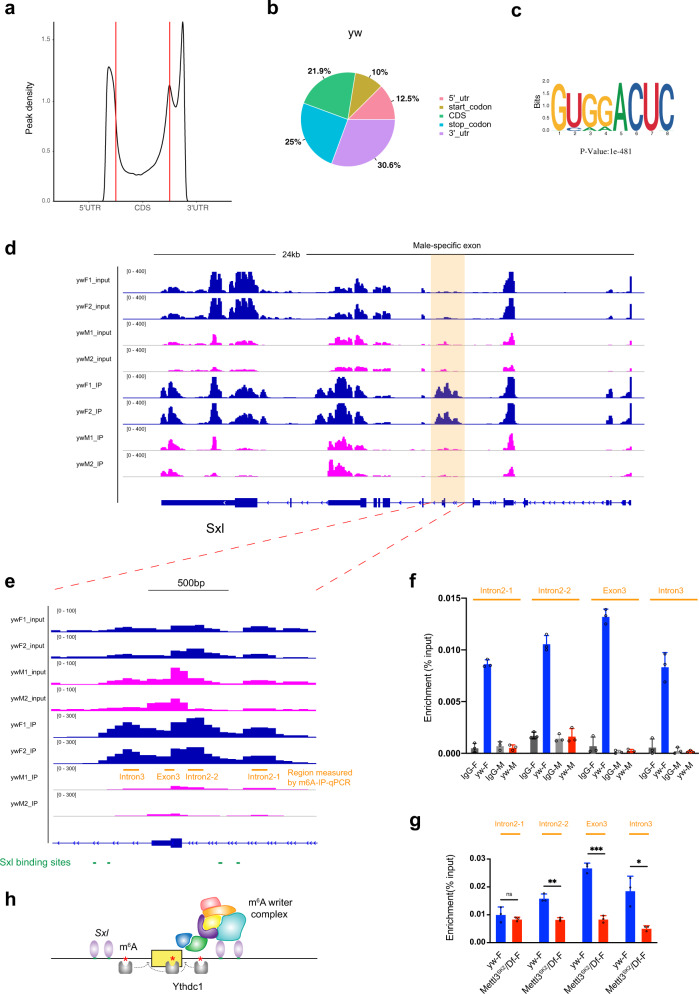
Previously, only two studies were reported to map global m6A methylation pattern in Drosophila, one being MeRIP-seq in S2R + cells and the other being miCLIP in embryos33,47. To obtain a high-stringent m6A methylome in adult flies, we performed methylated RNA immunoprecipitation sequencing (MeRIP-seq) in yw male and female flies. We included two replicates for each MeRIP-seq and the mapped reads were enriched around TSS and TES in IP samples compared to inputs (Supplementary Fig. 6). A peak detection algorithm was used to identify m6A peaks (P < 0.05, Supplementary Data 1), which were enriched in the 3′UTR and close to the stop codon, and to a lesser extend enriched in the 5′UTR and around the start codon (Fig. 6a, b). De novo motif analysis using HOMER identified the consensus sequence RRACH (Fig. 6c), consistent with those in the mammalian system8,9.
Fig. 6. Female-specific deposition of m6A on the Sxl mRNA.
a MeRIP-seq shows that the normalized density of m6A peaks across 5′UTR, CDS, and 3′UTR of mRNA in yw male adult flies. b Pie charts depicting m6A peak distribution in different transcript segments in yw flies. c Sequence motif identified from m6A peaks in yw flies by HOMER program. d Integrative Genomics Viewer (IGV) tracks displaying MeRIP-seq (lower panels, IP) and RNA-seq (upper panels, input) reads along Sxl locus in yw male (ywM) and female flies (ywF). Two replicates are shown. The region around male-specific exon3 is shaded and enlarged in (e) highlighting 3–4 m6A peaks on and around exon3 only in female flies. Adjacent Sxl-binding sites are also shown. f m6A-IP-qPCR showing enrichments over Sxl mRNA in yw male or female flies IPed with m6A or IgG antibody. Regions measured are indicated in (e). g m6A-IP-qPCR showing enrichments over Sxl mRNA in yw or Mettl3SK2/Df female flies IPed with m6A antibody. Data are presented as mean ± SD from three biological replicates. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant, two-sided unpaired t test. In g, P = 0.3679, 0.0012, 0.0001, 0.0114. Source data are provided as a Source Data file. h A working model on how the m6A modifications cooperate with Sxl to regulate its mRNA splicing.
We then zoomed in on the Sxl locus, especially around the male-specific exon3, for potential m6A sites (Fig. 6d). Although the mRNAs used for MeRIP-seq were through polyA selection, we were able to detect 3–4 m6A peaks in and around exon3 (Fig. 6e). Strikingly, these m6A peaks only exist in female flies, but not males (Fig. 6e, compare ywF_IP with ywM_IP). We next validated the MeRIP-seq results with independent m6A-immunoprecipitation (IP)-qPCR. Four regions on Sxl mRNA, intron2–1, intron2–2, exon3, and intron3, were measured by qRT-PCR (Fig. 6e), and substantial enrichment was observed only in female mRNA IPed with m6A-specific antibody, but not in female mRNA IPed with control IgG, nor in male mRNA IPed with either m6A or IgG antibody (Fig. 6f). These results demonstrated that the m6A modifications are deposited in a sex-specific manner, which has not been shown in Drosophila or any other species before. We further investigated whether m6A modifications around Sxl exon3 are dependent on the m6A writer. In Mettl3 mutant females, the enrichments on intron2-2, exon3, and intron3, but not intron2-1, were significantly reduced, probably implying the importance of the former three m6A sites (Fig. 6g).
How can these m6A modifications be installed only in females? In fact, Sxl protein itself is the master sex determination factor that is only expressed in females. Sxl binds to polyU sites located in Sxl intron2 and intron361,62 and interestingly our mapped m6A peaks were close to those Sxl-binding sites (Fig. 6e). In addition, it was known that Sxl physically interacts with four m6A writer components, Fl(2)d, Vir, Nito, and Flacc36,41,42,45,46. Therefore, based on our data, we developed a model to explain how the m6A modifications cooperate with Sxl protein to regulate its mRNA splicing (Fig. 6h). Sxl in females recruits the m6A writer complex that in turn methylates m6A sites located in exon3 and nearby introns. Since these sites are quite close to exon/intron junction regions, m6A readers may bind to these sites and interfere with the splicing machinery, forcing the exon3 to be skipped in females (Fig. 6h).
Effective m6A modification occurs in 5′UTR and around start codon in Drosophila
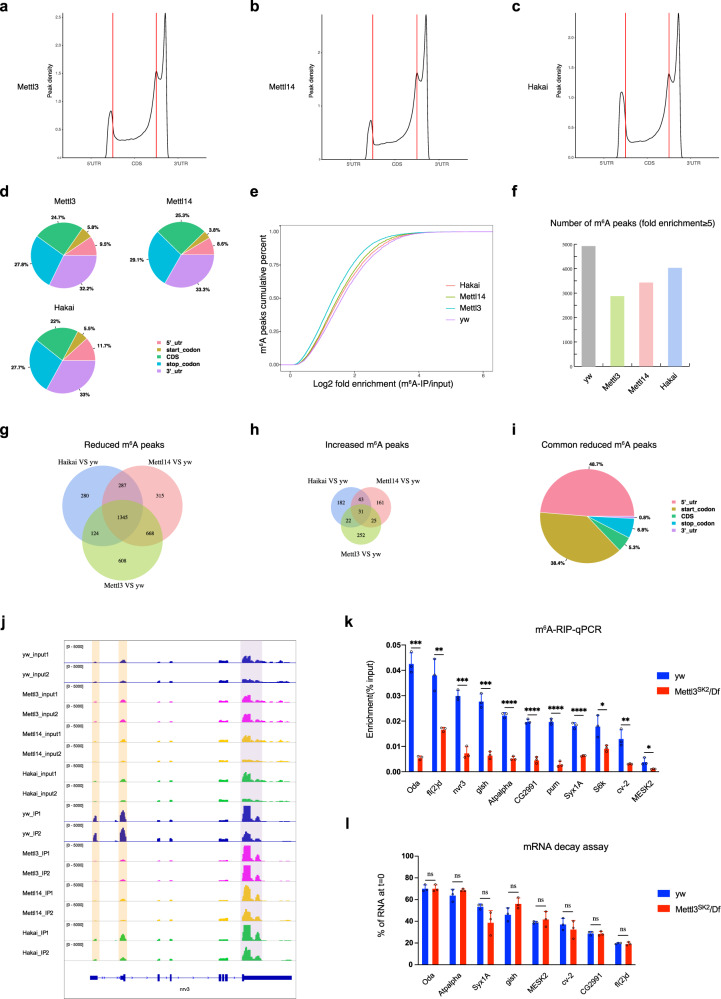
Since previous m6A-Seq data in Drosophila were generated only in wild-type cells or tissues33,47, we extended our MeRIP-seq to Mettl3, Mettl14, and Hakai mutants in order to find out the effective m6A modifications that depend on the m6A writer (Supplementary Fig. 6 and Supplementary Data 1). We used previously reported alleles Mettl3SK2 and Mettl14SK1 for our experiments47, since they are strong or null alleles as evidenced by the complete loss of Mettl3 and Mettl14 antibody staining in these mutant discs, respectively (Supplementary Fig. 5). Peak density plots indicated that m6A peaks were primarily enriched in the 3′UTR and near the stop codon, and enrichment in the 5′UTR and around the start codon seems to be reduced in these mutants compared to yw (Fig. 7a–d, compare to 6a). The cumulative distribution plot demonstrated that there is less fold enrichment (m6A-IP/input) in m6A mutants than yw, implying less methylation levels (Fig. 7e). Indeed, when we filtered m6A peaks with higher stringency (fold enrichment ≥5), the number of m6A peaks dropped >40% in Mettl3 mutant compared with yw (Fig. 7f).
Fig. 7. Effective m6A modification in Drosophila is distributed in 5′UTRs.
a–c MeRIP-seq shows the normalized density of m6A peaks across 5′UTR, CDS, and 3′UTR of mRNA in Mettl3, Mettl14, and Hakai mutant male adult flies. d Pie charts depicting m6A peak distribution in different transcript segments in Mettl3, Mettl14, and Hakai flies. e Cumulative percent distribution of log2 fold enrichment (m6A-IP/input) in yw, Mettl3, Mettl14, and Hakai flies. f When filtered with fold enrichment ≥5, the number of m6A peaks dropped in Mettl3, Mettl14, or Hakai mutant compared with yw. g Venn diagram showing the overlap of reduced m6A peaks (P < 0.05 and fold change ≤0.5) between Mettl3, Mettl14, and Hakai flies versus yw control. h Venn diagram showing the overlap of increased m6A peaks (P < 0.05 and fold change ≥2) between Mettl3, Mettl14, and Hakai flies versus yw control. i Pie chart depicting the distribution of 1345 common reduced m6A peaks in different transcript segments. j IGV tracks displaying MeRIP-seq (lower panels, IP) and RNA-seq (upper panels, input) reads along nrv3 mRNA in yw, Mettl3, Mettl14, and Hakai male flies. Note the reduced peaks in the 5′UTR (shaded in yellow) and peaks in the 3′UTR (shaded in purple) are not changed. k m6A-IP-qPCR validation for selected mRNAs in yw or Mettl3SK2/Df male flies. l The m6A-containing mRNAs were measured by qPCR in yw or Mettl3SK2/Df imaginal discs, at time = 0 or after four hours actinomycin D treatment to block transcription. k, l Data are presented as mean ± SD from three biological replicates. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant, two-sided unpaired t test. In k, P = 0.0001, 0.0043, 0.0003, 0.0003, <0.0001, <0.0001, <0.0001, <0.0001, =0.0273, 0.0073, 0.0276 for Oda, fl(2)d, nvr3, gish, Atpalpha, CG2991, pum, Syx1A, S6k, cv-2, MESK2. In l, P > 0.9999, =0.2282, 0.0832, 0.0939, 0.5032, 0.455, 0.8416, 0.7415 for Oda, Atpalpha, Syx1A, gish, MESK2, cv-2, CG2991, fl(2)d. Source data are provided as a Source Data file.
More importantly, we focused on those peaks changed upon m6A writer depletion. Interestingly, many more m6A peaks were reduced (Mettl3, 2745; Mettl14, 2615; Hakai, 2036; P < 0.05 and fold change ≤0.5) than increased (Mettl3, 330; Mettl14, 260; Hakai, 278, P < 0.05 and fold change ≥2) in these mutants, validating their role as m6A writers (Fig. 7g, h and Supplementary Data 2). Only in reduced m6A peaks, a significant overlap between the three mutants was found (1345 common peaks), suggesting that Hakai plays a similar role to Mettl3 and Mettl14 in m6A methylation. The common reduced peaks in three writer mutants likely represent high-confidence m6A modification sites in Drosophila. We focused on common reduced m6A peaks for further analysis. Surprisingly, around 90% of these peaks were located in the 5′UTR and close to the start codon, and only a small portion of them occurred in the 3′UTR (Fig. 7i). A few examples of loss of m6A peaks in the three mutants were shown in Fig. 7j and Supplementary Fig. 7, which almost always happened in the 5′UTR, while m6A peaks in the 3′UTR were generally not changed. We then used m6A-IP-qPCR to validate the reduction of m6A peaks in the 5′UTR region for 11 genes and found a significant reduction of m6A signal in Mettl3 mutant versus yw (Fig. 7k). These results indicate that although most m6A sites map to 3′UTRs in Drosophila, the peaks responding to the loss of m6A writers, and thus the effective ones, are distributed in 5′UTRs and this is different from the mammalian system. The majority of the peaks in 3′UTRs may be mediated by another methyltransferase or come from a non-specific background.
Next, we analyzed gene expression regulated by m6A writer components by RNA-seq (Supplementary Data 3). In Mettl3, Mettl14, and Hakai mutant adult flies, 987, 954, and 886 genes were differentially expressed (P < 0.05 and fold change ≥2 or ≤0.5), respectively (Supplementary Fig. 8a and Supplementary Data 4). These genes substantially overlapped with each other (Supplementary Fig. 8b) and common differentially expressed genes generally changed in the same pattern in the three mutants (Supplementary Fig. 8c), arguing that they act in the same pathway. Interestingly, differentially expressed genes were strongly enriched for immune response genes in the GO analysis and for metabolic pathways in the KEGG analysis (Supplementary Fig. 8d, e). By exhibiting MeRIP-seq together with RNA-seq data, we observed the reduction of m6A peaks in Mettl3, Mettl14, or Hakai mutant, and no obvious trend for change of mRNA expression correlated with differential m6A peaks (Supplementary Fig. 8f–h and Supplementary Data 5). We further divided the genes into m6A targets or nontargets, and the cumulative plot shows that there was no obvious difference between m6A targets and nontargets in Hakai or Mettl3 mutant flies (Supplementary Fig. 8i, j), while there was a slight positive effect of m6A on mRNA levels in Mettl14 mutant (Supplementary Fig. 8k), consistent with a previous report33. Moreover, we performed RNA decay assay for validated m6A-containing transcripts. Four hours after transcription inhibition by actinomycin D, we did not observe the significant difference in mRNA levels for these genes between Mettl3 and yw imaginal discs (Fig. 7l). These findings are consistent with the notion that effective m6A modifications are located in 5′UTRs in Drosophila, and thus do not mediate mRNA degradation as in the mammalian system.
Finally, we performed a similar analysis for splicing changes. In Mettl3, Mettl14, and Hakai mutant flies, 445, 364, and 340 genes were differentially spliced (FDR < 0.05, IncLevelDifference ≥0.2 or ≤ −0.2), respectively, and they overlapped substantially with each other (Supplementary Fig. 9a, c and Supplementary Data 6–8). The differential alternative spliced events occurred in all splicing categories (Supplementary Fig. 9b) and were functionally enriched for mRNA splicing and signaling transduction, etc (Supplementary Fig. 9d, e). We then examined the alternatively spliced transcripts of Dsp1, CG8929, Aldh-III, and fl(2)d, which were regulated by the m6A pathway (Fig. 3c). In all four cases, we found heavily methylated peaks surrounding the splicing junctions that depended on Mettl3, suggesting that these modifications may regulate splicing directly (Supplementary Fig. 9f–i). Interestingly, it seemed that the role of m6A modification is to repress splicing in these events. Even in the case of Sxl, the m6A modification plays a role to inhibit splicing as well, whether this is a general mechanism needs to be determined in the future.
Discussion
m6A modification has been known for more than 40 years6 but has recently gained great attention due to the emergence of technologies to map m6A methylome8,9,63, as well as the identification of the writers, readers, and erasers in this pathway4,14–16. Since the initial purification of the key methyltransferase Mettl321, other components of the writer complex were gradually identified through biochemical experiments and genetic screens. We now know that m6A writer complex is comprised of multiple components including Mettl3, Mettl14, WTAP, VIRMA, RBM15/15B, ZC3H13. Hakai was first indicated as a WTAP interaction protein23 and was shown later to be required for full m6A methylation in Arabidopsis37; however, its role in the m6A pathway in animals has not been studied. Here, we show that Hakai interacts with other m6A writer subunits, and Hakai mutants exhibit characteristic m6A pathway phenotypes, such as lowered m6A levels in mRNA, aberrant alternative splicing of Sxl and other genes, held-out wings, and flightless flies, as well as reduced m6A peaks shared with Mettl3 and Mettl14 mutants in MeRIP-seq. Altogether, these data unambiguously argue that Hakai is the seventh, and likely last core component of the conserved m6A writer complex.
Each component in the m6A writer complex plays a role in mRNA methylation but their exact roles are not well understood64. Our systematic analysis of several m6A writer subunits has provided insights into the mechanism of this important complex. We found that Fl(2)d, Vir, Hakai, and Flacc form a stable complex, and knocking down either of Fl(2)d, Vir, or Hakai led to the degradation of the other three components. Mettl3, Mettl14, and Nito were not affected by the disruption of Fl(2)d, Vir or Hakai, suggesting that they have separate functions. Knocking down Flacc resulted in less nuclear staining of Fl(2)d, consistent with a role in nuclear localization of the writer complex35. Based on these results, we proposed a model for the m6A methyltransferase complex (Fig. 4y). Mettl3 and Mettl14 form a stable heterodimer to catalyze the addition of the methyl group to mRNA. Nito/RBM15 contains three RRM domains and binds to positions adjacent to m6A sites, thus may provide target specificity for the m6A writer complex32. Fl(2)–Vir–Hakai–Flacc form a platform to connect different components and may integrate environmental and cellular signals to regulate m6A methylation.
Hakai is a potential E3 ubiquitin ligase with an intact C3HC4 RING domain and a C2H2 domain. Its absence led to the degradation, rather than the accumulation of other m6A writer subunits, indicating that it may not act as an E3 ubiquitin ligase in this complex. Hakai was initially identified as an E-cadherin binding protein to downgrade its levels50, and the role of Hakai in cell proliferation and tumor progression was extensively studied in cell culture51. However, our in vivo analysis using various genetic tools did not find a role of Hakai in E-cadherin regulation. In addition, Hakai appeared as a ubiquitous nuclear protein showing little co-localization with E-cadherin in the membrane. Consistently, Hakai was shown to interact with PTB-associated splicing factor (PSF), a nuclear protein, and to affect its RNA-binding ability65. Thus, the role of Hakai in E-cadherin regulation needs to be further investigated using the knockout mouse model and whether Hakai has other substrates for its E3 ligase activity also needs to be determined.
Recent emerging studies suggest that m6A is involved in numerous developmental processes and human diseases16,66, mainly by regulating mRNA stability, translation, or splicing. Pioneer work from three labs has established the framework for the m6A pathway in Drosophila33,47,48. However, only published Drosophila m6A methylome was performed in S2R + cells or embryos33,47 and was not done against writer mutants. Other than Sxl, few m6A target loci have been firmly mapped. By performing MeRIP-seq in wild-type adult flies as well as Mettl3, Mettl14, and Hakai mutants, we demonstrated that although most m6A peaks are distributed in 3′UTRs, the functional peaks responding to the loss of m6A writers are mainly located in 5′UTRs. This finding indicates a major difference between Drosophila and mammalian m6A methylome, which mainly occurs in 3′UTRs, and is in agreement with a recently published manuscript using miCLIP67. Interestingly, our LC-MS data show that the overall level of m6A modification in Drosophila only accounted for 10–20% of that in mammalian cells. Mettl3 or Mettl14 mutants are embryonic lethal in mice while they develop into adults in flies. It is possible that the m6A pathway acquires additional functions during evolution.
m6A modification in 3′UTRs usually causes mRNA instability and m6A in 5′UTRs is linked to translation enhancement1,4. In agreement with the view that functional m6A peaks are located in 5′UTRs in Drosophila, we did not observe an increase in mRNA half-life of m6A targets in Mettl3 mutants compared to wild-type. These results imply that the major role of m6A modification in Drosophila is not on mRNA degradation, but possibly on translation upregulation, which can be tested by combining ribosome profiling68 and functional analysis of a single transcript in the future. Our data by combining MeRIP-seq and splicing analysis shed light on how the m6A modification contributes to splicing regulation. In all five cases, we analyzed, four (Dsp1, CG8929, fl(2)d, Aldh-III) in 5′UTRs and one (Sxl) in exon/intron, reduction of m6A modification was correlated with enhanced splicing, arguing that the normal role of these modifications might be to repress splicing events nearby.
Last but probably the most interesting finding from our work is to demonstrate the female-specific m6A modification around Sxl exon3. Sxl is a textbook paradigm to study alternative splicing and has been intensively investigated for more than thirty years69. Sxl protein binds to its own mRNA to control the alternative splicing, but its binding sites are located >200 nucleotides downstream or upstream of the male exon, meaning other regulators should be involved. Recently, the m6A modification pathway was shown to modulate Sxl alternative splicing, but the detailed mechanism has not been resolved33,47,48. Our MeRIP-seq data revealed that several m6A peaks were deposited only in females on and around Sxl exon3, and they were in the vicinity of Sxl-binding sites (Fig. 6e). We further validated this finding by independent m6A-IP-qPCR and showed that these modifications were reduced in Mettl3 mutant females. This unexpected finding suggests a model that one main function of Sxl may be to recruit the m6A writer complex that methylates nearby m6A sites. The m6A reader Ythdc1 in turn binds to these sites and might interact with the splicing machinery to repress splicing (Fig. 6h). Future experiments, such as interactions between Sxl and Mettl3/Mettl14, interactions between Ythdc1 and general splicing factors, mapping of the exact m6A methylation site in Sxl at the single nucleotide level, comparison of transcriptome-wide binding sites of Sxl with m6A modification sites, will be required to firmly prove our model.
Methods
Fly strains and genetics
Flies were grown on standard cornmeal food and experiments were performed at 25 °C. The following stocks were used: w1118 (used as wild-type, WT), yw, Mettl3SK2, Mettl14SK1 47, Df(3R)Exel6197 (Mettl3 deficiency, Bloomington 7676), ap-Gal4, Hakai shRNA (VDRC330548), vir shRNA (HMC03908, Bloomington 55694), fl(2)d shRNA (HMC03833, Bloomington 55674), flacc shRNA (VDRC 35212GD), actin-Cas9 (Bloomington 54590)55, nanos-Cas9 (Bloomington 78782)57. To generate U6-Hakai-sgRNA, target sequence “ACGTCCGCGCCCGCGAGCCC” was picked by DRSC Find CRISPRs57 and cloned into pCFD3 vector55 (Addgene 49410). The construct was inserted at attP40 site by standard PhiC31-mediated transformation to make transgenic flies in UniHuaii. U6-Hakai-sgRNA was crossed with nanos-Cas9 flies to generate a series of indel mutations, and HakaiSH2 and HakaiSH4 were chosen for further analysis. Two additional Hakai shRNAs were made based on the pNP vector by Tsinghua Fly Center56. TH14422.S with target sequence “GAGCTCGACAAGGACGGCGAA” was inserted at attP2 site and TH14423.S with target sequence “CGGCCGCATGATACCCTGCAA” was inserted at attP40 site. The three Hakai shRNAs show similar knockdown efficiency as examined by Hakai antibody staining. VDRC330548 was used in Figs. 4g, j, k, 5c–c”, TH14423.S was used in Fig. 4h–h’, 4i, and TH14422.S was used in Fig. 4l. To test adult flight ability, cohorts of ten male flies were tapped down into a Petri dish, and the number of flies that flew away within 2 min was recorded.
Immunostaining
Wing discs from 3rd instar larvae were dissected in PBS and fixed in 4% formaldehyde (Sigma) in PBST (PBS + 0.1% Triton X-100) for 15 min. After blocking in 1% normal donkey serum (Jackson Immuno) in PBST for 1 h, the samples were incubated with the primary antibody in the same solution at 4 °C overnight. After three washes in PBST, samples were incubated with the secondary antibody for 2 h at room temperature, washed in PBST three times, and subsequently mounted in Antifade Mounting Medium (Beyotime). All images were taken on a Zeiss LSM 880 microscope.
The following antibodies were used: mouse anti-Fl(2)d (1:10) (9G2, DSHB), rat anti-Ecad (1:5) (DCAD2, DSHB), mouse anti-Sxl (1:10) (M18, DSHB), rabbit anti-Flacc (1:200)36, rabbit anti-Hakai (1:200), rabbit anti-Vir (1:200), rabbit anti-Mettl3 (1:200), rabbit anti-Mettl14 (1:200); Alexa 488-conjugated anti-mouse and anti-rabbit IgG (1:1000) (Thermo Fisher, A21202, A21206), Cy3-conjugated anti-mouse, and anti-rabbit IgG (1:400) (Jackson Immuno, 715-165-150, 711-165-152). Hakai antibody was generated in rabbits against a recombinant protein containing amino acids 1–120. Vir antibody was generated in rabbits against a recombinant protein containing amino acids 750–1005. Mettl3 antibody was generated in rabbits against a recombinant protein containing amino acids 110–300. Mettl14 antibody was generated in rabbits against a recombinant protein containing amino acids 1–205. All four antibodies were generated and affinity-purified at ABclonal.
Molecular cloning and co-immunoprecipitation
To generate the GFP-, mRFP-, or HA-tagged plasmids, full-length cDNAs for Hakai (the long and the short isoform amplified from a cDNA library), Flacc (GH14795), Nito (GH11110), Fl(2)d (LD21616), METTL3 (AT20169), and METTL14 (LD06016) were cloned into the pENTR vector (Invitrogen), and transferred into the Drosophila Gateway vector pAGW, pARW, and pAHW. GFP was cloned into pAWM as a control.
Drosophila S2 cells were maintained in Schneider’s medium (Gibco) supplemented with 10% FBS (Gibco) at 25 °C. In all, 4 μg of total DNA was transfected into S2 cells in a 100-mm dish with Effectene (QIAGEN). After 48 h, cells were lysed in IP lysis buffer (Beyotime, 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 1× protease inhibitor) on ice for 30 min, and cleared at 20,000×g for 10 min at 4 °C. Supernatants were incubated with anti-GFP nanobody agarose beads (Allele Biotechnology) for 2 h at 4 °C. The beads were washed three to four times with 1 ml lysis buffer and resuspended in 2× SDS sample buffer. Eluted proteins were detected by western blotting using rabbit anti-GFP (1:1000, A6455, Molecular Probes), rat anti-HA (1:1000, 3F10, Roche) or mouse anti-Fl(2)d (1:100, 9G2, DSHB) primary antibodies, and HRP-conjugated secondary antibodies (1:3000, Santa Cruz Biotech). Uncropped western blots can be found in the Source Data file. For co-localization, S2 cells were grown on Lab-Tek chamber slides and imaged in live conditions 2 days after transfection. HeLa cells were cultured at 37 °C with 5% CO2 in Dulbecco’s modified eagle’s medium (TransGen) supplemented with 10% FBS (Gibco).
Analyzing m6A levels by LC-MS
Total RNAs were extracted from adult flies or cultured cells using TRIzol (Invitrogen), and then subjected to two rounds of poly(A) selection using the GenElute mRNA Miniprep kit (Sigma). Before LC-MS analysis, all RNA samples were hydrolyzed enzymatically to ribonucleosides. Briefly, 600 ng RNA of each sample was digested by nuclease P1 (Sigma) and snake venom phosphodiesterase (Sigma) at 37 °C for 2 h, and followed by digestion with fast alkaline phosphatase (Thermo Fisher) for another 1 h. Then, the digested samples were used for the following LC-MS analysis.
Quantitative LC-MS analyses of m6A and adenosine were achieved using a Waters UPLC coupled to Thermo Q Exactive mass spectrometer in positive ion mode using dynamic multiple reaction monitoring. The ribonucleosides in the hydrolyzed RNA samples were resolved on an Acquity UPLC HSS T3 column (1.8 µm particle size, 100 Å pore size, 2.1 × 100 mm, 30 °C) at 300 µl min−1 using a solvent system of 0.1% formic acid in H2O (A) and acetonitrile (B). The elution profile was 2% B for 2 min, 2–11% B over 4 min, then to 11–80% B over 4 min, followed by a column washing at 80% B and column equilibration. The quantification of a ribonucleoside can be achieved using m/z of the parent ribonucleoside ion and m/z of the deglycosylated ion product. Nucleosides were quantified based on the transition of the parent ribonucleoside to the deglycosylated base ion: m/z 268.1–136.1 for A, m/z 282.1–150.1 for m6A, m/z 282.1–150.1 for m1A, m/z 244.1–112 for C, m/z 258.1–126 for m5C, and m/z 286.1–154 for ac4C. Absolute quantities of each ribonucleoside were determined using an external calibration curve prepared with A (Sigma), m6A (Selleck), m1A (J&K), C (Sigma), m5C (Sigma), and ac4C (J&K) standards.
MeRIP-seq
MeRIP-seq was performed following previous protocols70,71. The total RNA was isolated from yw (male and female), Mettl3SK2/Df (male), Mettl14SK1 (male), and HakaiSH4 (male) adult fly about 1–2 days old using TRIzol (Invitrogen). The RNA amount of each sample was quantified using NanoDrop ND-1000 and the RNA integrity was assessed by Bioanalyzer 2100 (Agilent) with RIN number >7.0, and confirmed by electrophoresis with denaturing agarose gel. Poly(A) RNA was purified from 50 μg of the total RNA using Dynabeads Oligo (dT)25 (Thermo Fisher) by two rounds of purification and then was fragmented into small pieces using Magnesium RNA Fragmentation Module (NEB) under 86 °C for 7 min. The cleaved RNA fragments were incubated for 2 h at 4 °C with Dynabeads (Dynabeads Antibody Coupling Kit, Thermo Fisher) coupled with m6A-specific antibody (202003, Synaptic Systems) in IP buffer (50 mM Tris-HCl, 750 mM NaCl, and 0.5% Igepal CA-630). Then the IP RNA fragments and untreated input control fragments are converted to the final cDNA library in accordance with a strand-specific library preparation by the dUTP method. The average insert size for the final cDNA library was 200 ± 50 bp. At last, we performed the 2 × 150 bp paired-end sequencing (PE150) on an Illumina Novaseq 6000 (LC-Bio Technology) following the vendor’s recommended protocol.
Bioinformatic analysis
fastp (v0.19.4) software was used to remove the reads that contained adaptor contamination, low-quality bases, and undetermined bases with default parameters. Then sequence quality of IP and Input samples were also verified using fastp. We used HISAT2 (v2.0.4)72 to map reads to the genome of Drosophila_melanogaster (Version: v96) with default parameters. Mapped reads of IP and input libraries were provided for R package exomePeak (v1.9.1)73, which identifies m6A peaks with bed or bigwig format that can be adapted for visualization on the IGV software. HOMER (v4.1)74 was used for de novo and known motif finding followed by localization of the motif with respect to peak summit. Called peaks were annotated by the intersection with gene architecture using R package ChIPseeker (v1.18.0)75. A common peak was picked if the peaks from different groups overlap in the genome >50% of the smallest of the peaks. StringTie (v1.3.4)76 was used to perform expression levels for all mRNAs from input libraries by calculating FPKM. The differentially expressed mRNAs were selected with log2 (fold change) ≥1 or log2 (fold change) ≤−1 and P value <0.05 by R package edgeR (v4.1)77. rMATS (v4.0.1) was used for differential splicing analysis with a filter for FDR < 0.05 and IncLevelDifference ≥0.2 or ≤ −0.278.
RT-PCR
Total RNAs were extracted using TRIzol (Invitrogen) and cDNAs were generated from 0.5 μg of RNA using Hifair II 1st Strand cDNA Synthesis Kit with gDNA digester plus (Yeason). 2xHieff PCR Master Mix (Yeasen) was used for regular PCR, and Hieff qPCR SYBR Green Master Mix (Yeasen) was used for qPCR. All primers used are listed in Supplementary Data 9. Sxl primers used in Fig. 3b are described in ref. 59 and Dsp1, CG8929, Aldh-III and fl(2)d primers used in Fig. 3c are described in refs. 33,34. Statistical analysis was performed using GraphPad Prism (v9.0.0).
m6A-IP-qPCR
m6A-IP-qPCR was performed following a protocol with minor modifications70. Total RNAs were extracted using TRIzol (Invitrogen) and purified through GenElute mRNA Miniprep kit (Sigma). In total, 5 µg of purified mRNAs were fragmented into ~200 300 nt fragments by incubation in RNA fragmentation reagent (Thermo Fisher) at 94 °C for 30 s and then stopped with stop solution. Ten percent of the fragmented mRNAs were kept as input. The remaining mRNAs were incubated with 6.25 µg m6A-specific antibody (202003, Synaptic Systems) or rabbit IgG (Beyotime) in IP buffer (50 mM Tris-HCl (pH 7.4), 750 mM NaCl, 0.5% Igepal CA-630, 0.4 U/µl RNasin (Promega)) for 2 h at 4 °C. Then the mixture was incubated with 25 µl Dynabeads Protein A (Thermo Fisher) for another 2 h at 4 °C. After extensive washing, the bound mRNAs were eluted with 6.7 mM N6-methyladenosine (Sigma) in IP buffer and then recovered with ethanol precipitation. The immunoprecipitated mRNAs and input mRNAs were processed as in RT-PCR.
mRNA decay assay
To assay for mRNA stability, yw or Mettl3 mutant L3 larval imaginal discs were dissected and treated with 20 µg/ml Actinomycin D (Sigma) in Schneider’s medium (Gibco) for 4 h at 25 °C. Total RNA was extracted at T = 0 h and T = 4 h and processed as in RT-PCR.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of additional supplementary files
Acknowledgements
We thank Eric Lai, Matthias Soller, Bloomington Drosophila Stock Center, TsingHua Fly Center, and Vienna Drosophila Resource Center for fly stocks; Developmental Studies Hybridoma Bank for antibodies; Xiaoyan Xu, Wenli Hu, and Shuining Yin from the mass-spec and imaging core facility of Institute of Plant Physiology and Ecology for technical support; Siyuan Liang for help in bioinformatic analysis; Ning Zheng for critical reading of the paper; the referees for suggestions regarding methylation patterns over the Sxl gene. This work is supported by the National Key R&D Program of China (2018YFA0800100) and National Natural Science Foundation of China (91857114, 31970786, 31771586) to D.Y.
Source data
Author contributions
Y.W. and D.Y. designed the research; Y.W., L.Z., H.R., L.M., J.G., D.M., L.L., and D.Y. performed the experimental studies; Y.W., Z.L., and D.Y. carried out bioinformatic analysis; Y.W. and D.Y. analyzed the data; and D.Y. wrote the paper.
Data availability
High-throughput m6A-seq and RNA-Seq data are deposited into the Gene Expression Omnibus with accession number GSE155662. The data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks James Erickson and other, anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yanhua Wang, Lifeng Zhang, Hang Ren.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22424-5.
References
- 1.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert WV, Bell TA, Schaening C. Messenger RNA modifications: form, distribution, and function. Science. 2016;352:1408–1412. doi: 10.1126/science.aad8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boccaletto P, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 5.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry R, Kelley DE. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. doi: 10.1016/0092-8674(74)90153-6. [DOI] [Google Scholar]
- 8.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz S, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo GZ, et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 2014;5:5630. doi: 10.1038/ncomms6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, et al. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer KD, et al. 5’ UTR m(6)A promotes Cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer KD, Jaffrey SR. Rethinking m(6)A readers, writers, and erasers. Annu. Rev. Cell Dev. Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patil DP, Pickering BF, Jaffrey SR. Reading m(6)A in the transcriptome: m(6)A-binding proteins. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauer J, et al. Reversible methylation of m(6)Am in the 5’ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J. Biol. Chem. 1994;269:17697–17704. doi: 10.1016/S0021-9258(17)32497-3. [DOI] [PubMed] [Google Scholar]
- 22.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 23.Horiuchi K, et al. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 2013;288:33292–33302. doi: 10.1074/jbc.M113.500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hongay CF, Orr-Weaver TL. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Natl Acad. Sci. USA. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong S, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ping XL, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732. doi: 10.1371/journal.pgen.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz S, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue Y, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil DP, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lence T, et al. m(6)A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 34.Knuckles P, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen J, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol. Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Tang HW, Li J, Perrimon N, Yan D. Xio is a component of the Drosophila sex determination pathway and RNA N(6)-methyladenosine methyltransferase complex. Proc. Natl Acad. Sci. USA. 2018;115:3674–3679. doi: 10.1073/pnas.1720945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruzicka K, et al. Identification of factors required for m(6) A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. N. Phytol. 2017;215:157–172. doi: 10.1111/nph.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granadino B, Campuzano S, Sanchez L. The Drosophila melanogaster fl(2)d gene is needed for the female-specific splicing of Sex-lethal RNA. EMBO J. 1990;9:2597–2602. doi: 10.1002/j.1460-2075.1990.tb07441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilfiker A, Nothiger R. The temperature-sensitive mutation vir (ts)(virilizer) identifies a new gene involved in sex determination of Drosophila. Roux Arch. Dev. Biol. 1991;200:240–248. doi: 10.1007/BF00241293. [DOI] [PubMed] [Google Scholar]
- 40.Yan D, et al. A regulatory network of Drosophila germline stem cell self-renewal. Dev. Cell. 2014;28:459–473. doi: 10.1016/j.devcel.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan D, Perrimon N. spenito is required for sex determination in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2015;112:11606–11611. doi: 10.1073/pnas.1515891112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penn JK, et al. Functioning of the Drosophila Wilms’-tumor-1-associated protein homolog, Fl(2)d, in Sex-lethal-dependent alternative splicing. Genetics. 2008;178:737–748. doi: 10.1534/genetics.107.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilfiker A, Amrein H, Dubendorfer A, Schneiter R, Nothiger R. The gene virilizer is required for female-specific splicing controlled by Sxl, the master gene for sexual development in Drosophila. Development. 1995;121:4017–4026. doi: 10.1242/dev.121.12.4017. [DOI] [PubMed] [Google Scholar]
- 44.Penalva LO, et al. The Drosophila fl(2)d gene, required for female-specific splicing of Sxl and tra pre-mRNAs, encodes a novel nuclear protein with a HQ-rich domain. Genetics. 2000;155:129–139. doi: 10.1093/genetics/155.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niessen M, Schneiter R, Nothiger R. Molecular identification of virilizer, a gene required for the expression of the sex-determining gene Sex-lethal in Drosophila melanogaster. Genetics. 2001;157:679–688. doi: 10.1093/genetics/157.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega A, et al. Biochemical function of female-lethal (2)D/Wilms’ tumor suppressor-1-associated proteins in alternative pre-mRNA splicing. J. Biol. Chem. 2003;278:3040–3047. doi: 10.1074/jbc.M210737200. [DOI] [PubMed] [Google Scholar]
- 47.Kan L, et al. The m(6)A pathway facilitates sex determination in Drosophila. Nat. Commun. 2017;8:15737. doi: 10.1038/ncomms15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haussmann IU, et al. m(6)A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 49.Wan C, et al. Panorama of ancient metazoan macromolecular complexes. Nature. 2015;525:339–344. doi: 10.1038/nature14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujita Y, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 51.Aparicio LA, Valladares M, Blanco M, Alonso G, Figueroa A. Biological influence of Hakai in cancer: a 10-year review. Cancer Metastasis Rev. 2012;31:375–386. doi: 10.1007/s10555-012-9348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaido M, Wada H, Shindo M, Hayashi S. Essential requirement for RING finger E3 ubiquitin ligase Hakai in early embryonic development of Drosophila. Genes Cells. 2009;14:1067–1077. doi: 10.1111/j.1365-2443.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 53.Guruharsha KG, et al. A protein complex network of Drosophila melanogaster. Cell. 2011;147:690–703. doi: 10.1016/j.cell.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graveley BR, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl Acad. Sci. USA. 2014;111:E2967–E2976. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao HH, et al. An efficient and multiple target transgenic RNAi technique with low toxicity in Drosophila. Nat. Commun. 2018;9:4160. doi: 10.1038/s41467-018-06537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren X, et al. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc. Natl Acad. Sci. USA. 2013;110:19012–19017. doi: 10.1073/pnas.1318481110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell LR, Horabin JI, Schedl P, Cline TW. Positive autoregulation of Sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991;65:229–239. doi: 10.1016/0092-8674(91)90157-T. [DOI] [PubMed] [Google Scholar]
- 59.Johnson ML, Nagengast AA, Salz HK. PPS, a large multidomain protein, functions with Sex-lethal to regulate alternative splicing in Drosophila. PLoS Genet. 2010;6:e1000872. doi: 10.1371/journal.pgen.1000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.SchuLtt C, Hilfiker A, Nothiger R. virilizer regulates Sex-lethal in the germline of Drosophila melanogaster. Development. 1998;125:1501–1507. doi: 10.1242/dev.125.8.1501. [DOI] [PubMed] [Google Scholar]
- 61.Samuels ME, et al. RNA binding by Sxl proteins in vitro and in vivo. Mol. Cell Biol. 1994;14:4975–4990. doi: 10.1128/MCB.14.7.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakamoto H, Inoue K, Higuchi I, Ono Y, Shimura Y. Control of Drosophila Sex-lethal pre-mRNA splicing by its own female-specific product. Nucleic Acids Res. 1992;20:5533–5540. doi: 10.1093/nar/20.21.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linder B, et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcias Morales D, Reyes JL. A birds’-eye view of the activity and specificity of the mRNA m(6) A methyltransferase complex. Wiley Interdiscip. Rev. RNA. 2021;12:e1618. doi: 10.1002/wrna.1618. [DOI] [PubMed] [Google Scholar]
- 65.Figueroa A, et al. Novel roles of Hakai in cell proliferation and oncogenesis. Mol. Biol. Cell. 2009;20:3533–3542. doi: 10.1091/mbc.e08-08-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu PJ, Shi H, He C. Epitranscriptomic influences on development and disease. Genome Biol. 2017;18:197. doi: 10.1186/s13059-017-1336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kan, L. et al. A neural m6A/Ythdf pathway is required for learning and memory in Drosophila. Nat. Commun.12, 1458 (2021). [DOI] [PMC free article] [PubMed]
- 68.Zhang H, et al. Genome-wide maps of ribosomal occupancy provide insights into adaptive evolution and regulatory roles of uORFs during Drosophila development. PLoS Biol. 2018;16:e2003903. doi: 10.1371/journal.pbio.2003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salz HK, Erickson JW. Sex determination in Drosophila: the view from the top. Fly. 2010;4:60–70. doi: 10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G. Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013;8:176–189. doi: 10.1038/nprot.2012.148. [DOI] [PubMed] [Google Scholar]
- 71.Molinie B, Giallourakis CC. Genome-wide location analyses of N6-methyladenosine modifications (m(6)A-Seq) Methods Mol. Biol. 2017;1562:45–53. doi: 10.1007/978-1-4939-6807-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng J, et al. A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods. 2014;69:274–281. doi: 10.1016/j.ymeth.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- 76.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen S, et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl Acad. Sci. USA. 2014;111:E5593–E5601. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of additional supplementary files
Data Availability Statement
High-throughput m6A-seq and RNA-Seq data are deposited into the Gene Expression Omnibus with accession number GSE155662. The data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.