Summary
Androgens have a robust effect on skeletal muscles to increase muscle mass and strength. The molecular mechanism of androgen/androgen receptor (AR) action on muscle strength is still not well known, especially for the regulation of sarcomeric genes. In this study, we generated androgen-induced hypertrophic model mice, myofiber-specific androgen receptor knockout (cARKO) mice supplemented with dihydrotestosterone (DHT). DHT treatment increased grip strength in control mice but not in cARKO mice. Transcriptome analysis by RNA-seq, using skeletal muscles obtained from control and cARKO mice treated with or without DHT, identified a fast-type muscle-specific novel splicing variant of Myosin light-chain kinase 4 (Mylk4) as a target of AR in skeletal muscles. Mylk4 knockout mice exhibited decreased maximum isometric torque of plantar flexion and passive stiffness of myofibers due to reduced phosphorylation of Myomesin 1 protein. This study suggests that androgen-induced skeletal muscle strength is mediated with Mylk4 and Myomesin 1 axis.
Subject areas: Animal Physiology, Molecular Physiology, Molecular Biology, Endocrinology
Graphical abstract
Highlights
-
•
DHT increases muscle strength through myofiber AR
-
•
Myofiber AR increases a fast-type muscle-specific novel splicing variant of Mylk4
-
•
MYLK4 regulates muscle strength and muscle stiffness
-
•
MYLK4 induces phosphorylation of MYOM1
Animal Physiology; Molecular Physiology; Molecular Biology; Endocrinology
Introduction
Androgens are steroid hormones, which are ligands of the androgen receptor (AR) (Chang et al., 2013). Testosterone is produced mainly in the testis and has weak ligand activity against AR. 5α-reductase converts testosterone to 5α-dihydrotestosterone (5α-DHT), which is a more potent metabolite to AR compared with testosterone (Burd et al., 2006). While DHT is more potent, it is synthesized almost exclusively in the skin, liver, and gonads such that the major circulating androgen is testosterone. Androgen binding to AR leads to nuclear translocation of AR, and a ligand-bound AR protein forms a complex with transcriptional coregulators to regulate target gene transcription. AR is expressed in various tissues to achieve specific physiological functions. One target of androgens is skeletal muscle, and supraphysiological doses of androgens increase muscle mass and strength (Basaria et al., 2010; Bhasin et al., 2001).
Skeletal muscle contraction is achieved by concerted action of sarcomeric proteins. During a resting state, a troponin complex, consisting of troponin T, troponin C (TNNC), and troponin I, inhibits myosin heavy chain (MyHC) ATPase activity. Motoneuron firing stimulates intramyofibrillar Ca2+ release from sarcoplasmic reticulum, and Ca2+ binds to TNNC, which induces conformational change and enables MyHC ATPase to act as a motor protein (Schiaffino and Reggiani, 2011). Four isoforms of MyHC proteins (MyHCI, MyHCIIA, MyHCIIX, and MyHCIIB) are present in the adult mouse limb. These MyHC proteins potentiate different ATPase activity, which results in different muscle contraction force. MyHC proteins are bound by two essential myosin light chains (MyLCs), essential MyLC and regulatory MyLC, which play a modulatory role in maximum shortening velocity. Cytosolic Ca2+ also forms a complex with calmodulin, and a Ca2+∕calmodulin complex further binds to myosin light-chain kinase 2 (MLCK2), which phosphorylates regulatory MyLC to increase frequency-dependent potentiation of skeletal muscle contraction (Stull et al., 2011; Zhi et al., 2005). Thus, skeletal muscle strength is regulated by the amounts or functional status of sarcomeric proteins.
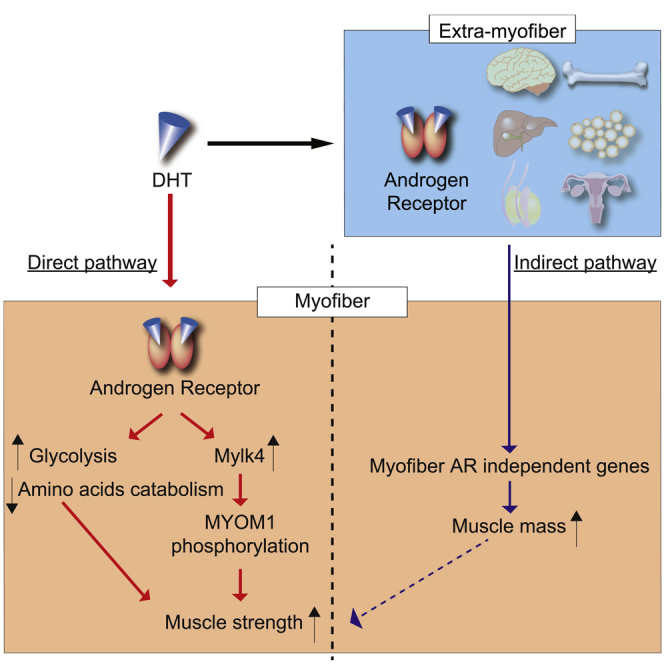
Dozens of studies about androgen action on skeletal muscles have been reported (De Gendt and Verhoeven, 2012). Global AR-deficient male mice exhibited low muscle mass and low muscle contraction force in hindlimb muscles, which indicated that AR is essential for muscle mass and strength in male mice (MacLean et al., 2008; Ophoff et al., 2009). Several lines of skeletal muscle-specific AR knockout (ARKO) mice have been generated by crossing three lines of AR floxed mice (Artm2Ska, Artm1Verh, or Artm1Jdz) and transgenic mice expressing Cre recombinase under the control of the human skeletal actin promoter (HSA-Cre, myocytes), muscle creatine kinase (MCK-Cre, myocytes), or Myod promoter (Myod-iCre, myogenic progenitor cells) (De Gendt and Verhoeven, 2012). Almost all the skeletal muscle-specific ARKO mice showed reduced LA (levator ani) muscle mass and muscle grip strength or tibialis anterior (TA) muscle contraction force but did not show reduction of limb muscle mass (Chambon et al., 2010; Dubois et al., 2014; Ferry et al., 2014; Fraysse et al., 2014; Ophoff et al., 2009; Rana et al., 2016). These results of various muscle-specific ARKO mice showed that although LA muscle mass and limb muscle strength is regulated by myofiber AR, most limb muscle mass (gastrocnemius, TA, extensor digitorum longus, soleus muscle) is not regulated by myofiber AR. Some studies reported that myofiber AR could have a role in mitochondrial maintenance, but a molecular mechanism of AR action on muscle strength in limb muscles still remains elusive, especially for the regulation of sarcomeric genes.
In this paper, we established muscle-specific AR knockout mice and a fast-type muscle hypertrophic model using DHT pellet implantation. RNA sequencing provided a key to understand the molecular mechanism of AR function in fast-type skeletal muscle. We propose a new model of androgen/AR actions on fast-type skeletal muscle strength.
Results
AR deficiency in myofiber impairs muscle strength
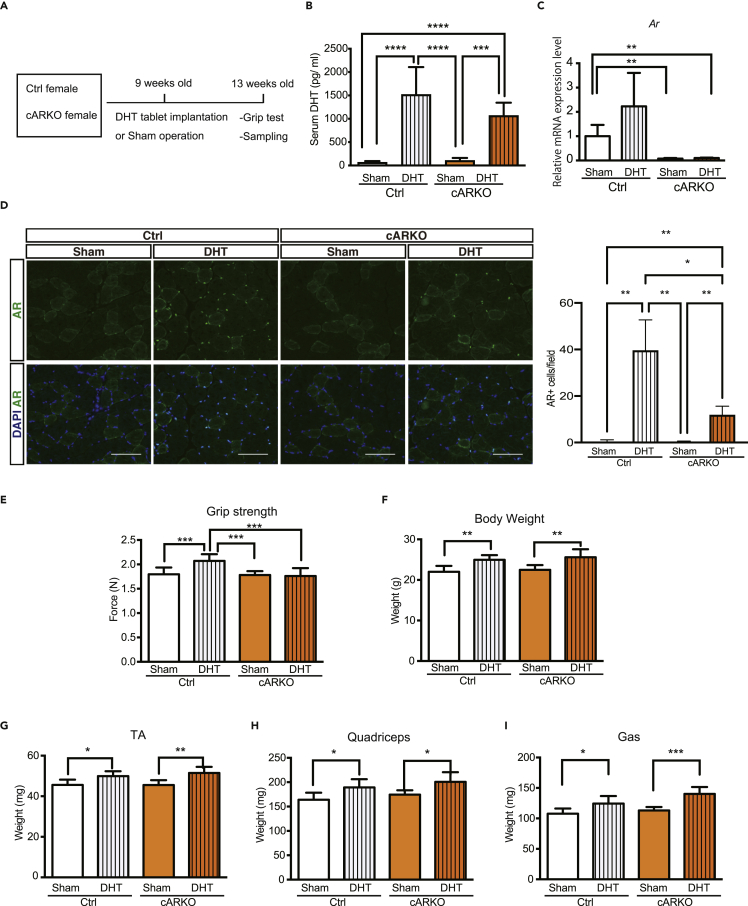
To characterize the role of AR in adult skeletal muscles, we generated myofiber-specific AR knockout (cARKO) mice and implanted a biodegradable pellet containing 10 mg DHT in 9-week-old female mice to avoid the effects of endogenous androgens. One month after implantation, grip strength and muscle weight were measured (Figure 1A). Serum DHT levels were significantly increased by 20 fold in mice supplemented with DHT (Figure 1B). Deletion of AR mRNA was confirmed by quantitaive reverse transcription PCR (RT-qPCR) (Figure 1C). Nuclear AR signals were observed in TA muscles of Ctrl mice supplemented with DHT, which is strongly decreased in TA muscles of cARKO mice supplemented with DHT (Figure 1D). Consistent with previous reports (Chambon et al., 2010; Ferry et al., 2014; Fraysse et al., 2014), Ctrl mice increased grip strength by implantation of a DHT pellet, but grip strength in cARKO mice did not increase (Figure 1E). Body weight and skeletal muscle weight were increased by DHT treatment at a similar level even in cARKO mice (Figures 1F and 1G). These results are consistent with previous reports (Chambon et al., 2010; Ferry et al., 2014; Fraysse et al., 2014) and suggested that grip strength is regulated by myofiber AR and DHT, and muscle mass is regulated by DHT but not through myofiber AR.
Figure 1.
cARKO female mice showed impaired muscle strength induced by DHT treatment
(A) Experimental scheme. Nine-week-old female control and cARKO mice were implanted with a pellet containing 10 mg DHT. Grip strength and tissue weight were measured at 13 weeks old.
(B) Serum DHT levels were determined in 13-week-old female Control_Sham (n = 8), Control_DHT (n = 7), cARKO_Sham (n = 5), and cARKO_DHT (n = 6) mice.
(C) Ar mRNA expression levels in gastrocnemius muscles of 13-week-old female Control_Sham (n = 4), Control_DHT (n = 3), cARKO_Sham (n = 4), and cARKO_DHT (n = 5) mice.
(D) Immunostaining of AR in TA muscles. Scale bars: 100 μm.
(E) Grip strength were measured in 13-week-old female Control_Sham (n = 9), Control_DHT (n = 7), cARKO_Sham (n = 9), and cARKO_DHT (n = 7) mice.
(F–I) body weight (F), TA muscle weight (G), quadriceps muscle weight (H), and gastrocnemius muscle weight (I) were measured in 13-week-old female Control_Sham (n = 8), Control_DHT (n = 7), cARKO_Sham (n = 5), and cARKO_DHT (n = 6) mice. Data are represented as mean ± standard deviation. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. One-way analysis of variance followed by Student Newman-Keuls tests.
See also Figure S1.
To clarify the responsible cell type for androgen-dependent muscle mass regulation, we generated Pax7CreERT2+; ARflox mice (satARKO) and implanted a pellet containing 10 mg DHT in 9-week-old female mice, as in cARKO mice. TA, quadriceps, and gastrocnemius muscle mass increased in satARKO mice (Figure S1). This result indicated that AR in satellite cells is not important for the regulation of muscle mass under the conditions of the experiment.
RNA-seq analysis revealed direct targets of AR in skeletal muscle
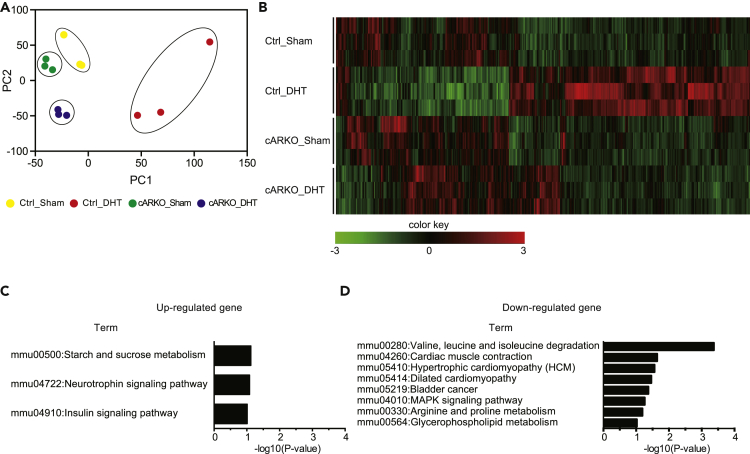
To clarify the mechanism of DHT- and AR-dependent enhancement of muscle strength, AR target genes in skeletal muscles were analyzed by RNA-seq in the gastrocnemius muscles of Ctrl and cARKO mice implanted with a DHT pellet (Figure 2A). As a result, 529 genes were identified as differentially expressed genes (DEGs) with statistical significance (q < 0.05) and were classified by their expression patterns into two major groups: myofiber AR- and DHT-dependent genes and myofiber AR-independent DHT-dependent genes (Figure 2B). Considering the phenotypes of cARKO mice, myofiber AR- and DHT-dependent genes regulate muscle strength and myofiber AR-independent DHT-dependent genes regulate muscle mass. To analyze the direct effects of AR in a skeletal muscle, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed with AR- and DHT-dependent upregulated or downregulated genes (Figures 2C and 2D). Sucrose metabolism was identified as an upregulated pathway, and amino acid catabolism and cardiac muscle contraction were identified as downregulated pathways. These results suggested that glucose/amino acid metabolism and/or muscle sarcomeric gene regulation is a key factor for muscle strength.
Figure 2.
RNA-seq analysis revealed AR- and DHT-dependent transcription
(A and B) (A) Principal components analysis with RNA-seq analysis of gastrocnemius muscles of 13-week-old female Control_Sham, Control_DHT, cARKO_Sham, and cARKO_DHT mice (n = 3, each group) (B) Hierarchical clustering of 529 DEGs in RNA-seq analysis of gastrocnemius muscles of 13-week-old female Control_Sham, Control_DHT, cARKO_Sham, and cARKO_DHT mice (n = 3, each group).
(C and D) KEGG pathway analysis for AR- and DHT-dependent upregulated genes (C) and downregulated genes (D).
See also Figures S2 and S3.
To understand the metabolic change, we visualized DEGs as a metabolic map (Figure S2A). This map clearly indicates that AR increased the gene expression for glycolysis through Slc2a3, Hk2, and Agl and for amino acid import through Slc38a2, Sla38a4, and Slc15a5, but the genes for branched chain amino acid degradation (Bckdha, Dbt, and Ivd) were decreased. Notably, polyamine synthesis-related genes (Odc1, Amd1, Mtr, and Smox) were highly upregulated by AR, which is consistent with previous reports (Lee and MacLean, 2011), and this was confirmed by RT-qPCR (Figure S2B). To validate gene expression in wild-type male and female mice, the expression levels of Odc1, Amd1, and Smox were higher in fast-twitch skeletal muscles of male mice compared with those of female mice, although there was no difference in slow-twitch skeletal muscles (Figure S2C). These results suggested that metabolic changes associated with AR prevent amino acid catabolism and provide amino acids for protein production.
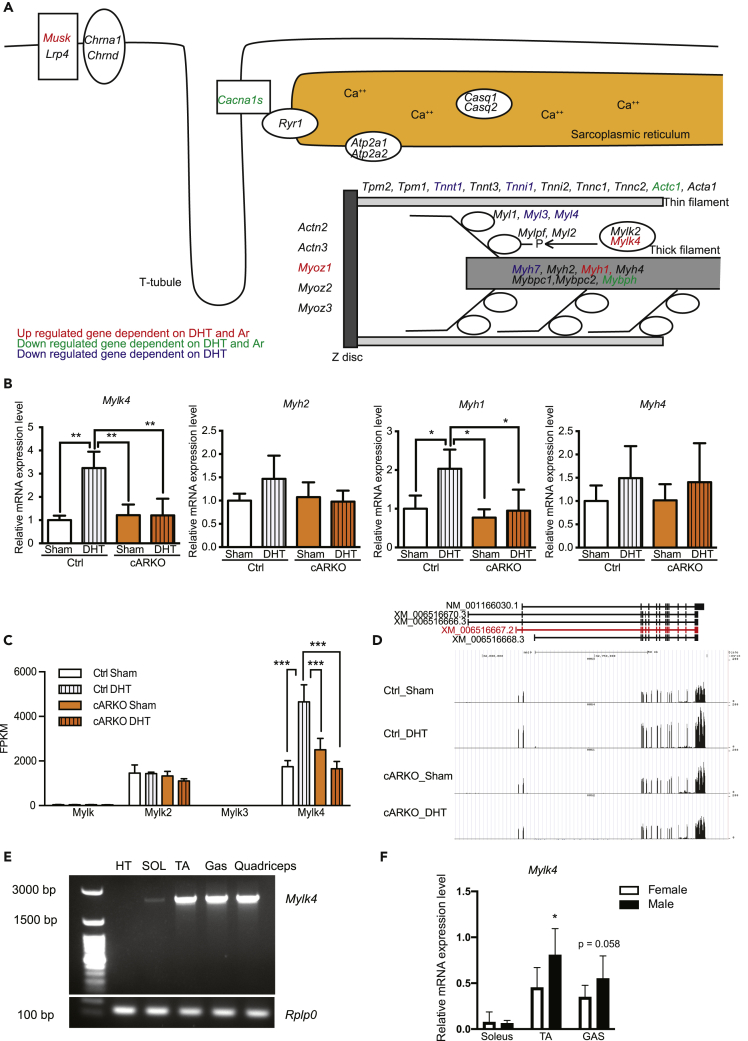
Mylk4 was induced by muscle AR
Androgen-induced metabolic changes especially for polyamine synthesis in muscle have been already reported (Lee and MacLean, 2011). In addition to the metabolic pathways, pathways related with sarcomeres were also identified from KEGG pathway analysis. Muscle sarcomere structure is critical to achieve muscle contraction, so the DEGs related to muscle sarcomere structure were visualized in a sarcomere model (Figure 3A). Mylk4, Myh1, Musk, and Myoz1 were upregulated, and Actc1, Mybph, Cacna1s were downregulated by DHT and AR. Given that the muscle strength in control mice treated with DHT was increased, we focused on the upregulated genes. Among these genes, the upregulation of Mylk4 and Myh1 was confirmed by RT-qPCR in an AR- and DHT-dependent manner (Figure 3B). Among them, we hypothesized that Mylk4 is a possible candidate responsible for increased muscle strength because Mylk2 is known as a positive regulator of muscle strength (Stull et al., 2011; Zhi et al., 2005). There are four Mylk genes in the mouse genome (Mylk1-4), and the expression levels of Mylk genes were shown from RNA-seq analysis (Figure 3C). Both Mylk2 and Mylk4 were expressed in skeletal muscles at similar fragments per kilobase of exon per million mapped reads values, but only Mylk4 was regulated by AR and DHT. Several splicing variants of Mylk4 have been found, and a cardiac splicing variant of Mylk4 has been reported (Chang et al., 2016). To identify splicing variants expressed in skeletal muscles, the Mylk4 sequence read by RNA-seq was visualized by a genome browser and matched to the Mylk4 splicing variant (XM_006516667.2, skmMylk4), which is different from the cardiac splicing variant of Mylk4 (Figure 3D). Recently, the existence of the same Mylk4 splicing variant (XM_006516667.2) also was reported by an RNA-seq analysis of various skeletal muscles (Terry et al., 2018). The expression of the Mylk4 splicing variant (XM_006516667.2) was confirmed by real-time PCR with specific primers for the Mylk4 splicing variant (XM_006516667.2) (Figure 3E). We also examined Mylk4 expression in male and female tissues (Figure 3F). Mylk4 is mainly expressed in fast-twitch skeletal muscles (TA, Gas, and quadriceps muscles) and not in hearts or slow-twitch muscles (soleus muscles), and the expression levels of Mylk4 in male mice are higher than those in female mice. In addition, AR-binding capacity to the promotor region of the Mylk4 gene was confirmed by registered genome-wide chromatin immunoprecipitaton sequencing data against AR in mouse prostates (Figure S4), suggesting that Mylk4, especially the skeletal muscle splicing variant, could be a direct target of myofiber AR in skeletal muscles.
Figure 3.
AR induced skeletal muscle-specific splicing variant of Mylk4 gene expression
(A) A muscle sarcomere model showing AR- and DHT-dependent upregulated or downregulated genes.
(B) mRNA expression levels of Mylk4, Myh2, Myh1, and Myh4 in gastrocnemius muscles of 13-week-old female Control_Sham (n = 4), Control_DHT (n = 3), cARKO_Sham (n = 4), and cARKO_DHT (n = 5) mice. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
(C) FPKM (fragments per kilobase of exon per million reads mapped) values of Mylk genes.
(D) Schematic image of Mylk4 gene locus.
(E) Real-time-PCR analysis of skeletal muscle-specific splicing variants of Mylk4.
(F) Mylk4 gene expression was determined in soleus muscles, TA muscles, and gastrocnemius muscles of male or female mice by RT-qPCR (n = 8, each tissue). Data are represented as mean ± standard deviation. ∗P < 0.05. One-way analysis of variance followed by Student Newman-Keuls tests.
See also Figures S4 and S5.
Decreased isometric torque and impaired passive stiffness in Mylk4 knockout mice
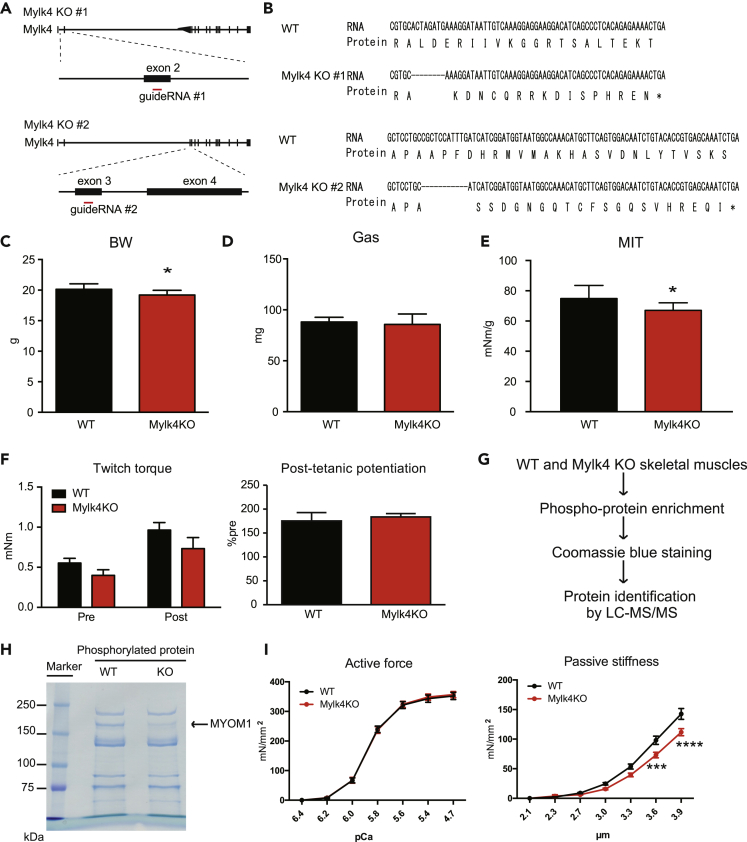
To clarify the Mylk4 role in muscle strength, we generated Mylk4 knockout (KO) mice by a CRISPR/CAS9 system. The guide RNAs were designed at exon 2 and exon 3 of the Mylk4 gene (Figure 4A), and deletion of the Mylk4 genome DNA sequence was confirmed by sequencing (Figure 4B). Mylk4 KO mice were viable and born at the expected Mendelian ratio. The body weight of Mylk4 KO mice was slightly decreased (Figure 4C), while muscle weight of the gastrocnemius muscle was not different (Figure 4D). However, maximum isometric torque normalized by the whole weight of the PF muscles of Mylk4 KO mice was significantly decreased compared with wild-type littermate control mice (Figure 4E). These results supported the hypothesis that Mylk4 contributes to AR-induced muscle strength increase.
Figure 4.
Mylk4 KO mice showed decreased isometric torque and passive stiffness mediated by phosphorylation of MYOM1
(A) Strategy for generation of Mylk4 KO mice #1 and #2 by the Cas9 system.
(B) Sequence of mRNA and protein of Mylk4 KO mice #1 and #2.
(C–E) Body weight (C), gastrocnemius muscle (Gas) weight (D), and maximum isometric torque (MIT) of in situ plantar flexion (E) were measured in 10-week-old female WT (n = 10) and Mylk4 KO mice (n = 8). ∗P < 0.05.
(F) Post-tetanic potentiation of in situ plantar flexion was measured in 10-week-old female WT (n = 5) and Mylk4 KO mice (n = 3).
(G) Proteomic strategy for substrate identification.
(H) Phosphorylated proteins were enriched from WT and Mylk4 KO mice and analyzed by SDS-PAGE.
(I) Active force and passive stiffness were measured with chemically skinned fibers from tibialis anterior muscles of 10-week-old female WT (active force, n = 28; passive force, n = 23) and Mylk4 KO mice (active force, n = 20; passive force, n = 24). ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. Two-tailed, unpaired Student's t tests. Data are represented as mean ± standard deviation.
See also Figures S6–S8.
As Mylk2 is known to regulate post-tetanic potentiation (Stull et al., 2011; Zhi et al., 2005), we first hypothesized that Mylk4 KO mice showed reduced muscle strength through post-tetanic potentiation-dependent manner. However, to our surprise, post-tetanic potentiation was not changed in Mylk4 KO mice (Figure 4F). Also, the amino acid sequence of Mylk4 is largely distinguished from Mylk2 except for the kinase domain (Figure S5). These results suggested that Mylk4 has a distinct role compared with Mylk2. Next, we focused on substrate preference of a skeletal muscle splicing variant of Mylk4. A cardiac splicing variant of Mylk4 has been reported to have kinase activity against a regulatory MyLC (Chang et al., 2016). Meanwhile, the N terminal amino acid sequence is completely different between a cardiac splicing variant and a skeletal muscle splicing variant of Mylk4 (Figure S5), which might affect substrate preference of a skeletal muscle splicing variant of Mylk4. To identify substrates of Mylk4 in skeletal muscles, we performed phospho-proteomics analysis with TA muscles from Mylk4 KO mice (Figure 4G). Phosphorylated proteins were enriched from the lysates of the TA muscles and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie brilliant blue (CBB) staining, and we found that the signal of one band at around 200 kDa was apparently decreased in Mylk4 KO mice (Figure 4H). Mass spectrometry analysis identified the band as MYOM1 (Myomesin 1) protein, which is a structural protein of the sarcomeric M band (Obermann et al., 1997). MYOM1 protein level in skeletal muscle was comparable between wild type and Mylk4 KO mice (Figure S6), suggesting that Mylk4 affects phosphorylation but not protein level of MYOM1. To confirm this result, we introduced the in vivo siRNA against Mylk4 into TA muscles (Figure S7A). The efficiency of Mylk4 knockdown was confirmed by RT-qPCR (Figure S7B). Phosphorylated proteins were enriched from the lysates of the TA muscles and SDS-PAGE followed by CBB staining showed the same as for Mylk4 KO mice (Figure S7C). As a result, a decreased protein level of phosphorylated MYOM1 protein also was confirmed by in vivo siRNA for Mylk4. Next, to examine the stiffness of sarcomeric structure because MYOM1 is a component of the sarcomeric M band, we performed skinned fiber analysis with TA muscles from Mylk4 KO mice and WT littermates. Active force was not changed but passive stiffness was significantly decreased in Mylk4 KO mice (Figure 4I), suggesting deteriorating function of MYOM1. These results indicate that the skeletal muscle splicing variant of Mylk4 has a distinct physiological role from both Mylk2 and the cardiac splicing variant of Mylk4 and contributes to androgen/AR-induced skeletal muscle strength (Figure S8).
Discussion
In this study, we analyzed androgen function of skeletal muscles mediated by AR. We showed that androgens have two different modes of action on skeletal muscles, a direct pathway and an indirect pathway. DHT treatment increased grip strength in Ctrl mice, whereas DHT treatment failed to increase grip strength in the cARKO mice (Figure 1). Therefore, muscle strength is regulated by AR in skeletal muscles, referred as a direct pathway. In the direct pathway, androgens activate AR in the myofiber to regulate muscle strength. DHT treatment increased skeletal muscle mass both in Ctrl mice and in cARKO mice (Figure 1). Therefore, muscle mass is not regulated by AR in skeletal muscles, referred as an indirect pathway. In the indirect pathway, androgens activate AR in extra-myofiber cells or tissues to regulate muscle mass. RNA-seq analysis revealed direct and indirect target genes of AR, among which we found Mylk4 as a responsible gene for the regulation of muscle strength. Deletion of Mylk4 resulted in impaired muscle strength, and phospho-proteomics analysis identified MYOM1 as a substrate of MYLK4. Altogether, our analysis indicated the molecular mechanism of AR-dependent muscle strength through Mylk4 and MYOM1.
Muscle strength and stiffness
Consistent with previous reports, we also found that androgens directly regulate muscle strength. AR increased the skeletal muscle splicing variant of Mylk4 gene expression, and Mylk4 KO mice showed decreased isometric torque. AR-induced muscle strength is, at least in part, achieved by Mylk4. In addition, Mylk4 KO mice had decreased passive stiffness of skeletal muscles. Men's skeletal muscle stiffness is known to be higher than that of women's (Wu et al., 2016). The higher stiffness in men might be due to AR-induced transcriptional regulation of Mylk4 and phosphorylation of MYOM1 by Mylk4. MYOM1 is known as an important molecule to be a structural linker between the thick filaments and serves as the major cross-linking protein of the M-line and plays a role in organization of sarcomere proteins (Lange et al., 2005; Sweeney and Hammers, 2018). In addition, in vitro biochemical analyses revealed that the interaction between myomesin and titin is regulated by the phosphorylation of myomesin (Obermann et al., 1997). Also, MYOM1 is reported as one of the responsible genes of myotonic dystrophy (Koebis et al., 2011). Thus, MYOM1 and its phosphorylation would have a significant role in regulation of skeletal muscle strength. Still, the physiological significance of phosphorylation of MYOM1 and the relationship between muscle strength and muscle stiffness is not clearly understood, and future study of these topics would be valuable. We would like to note that Mylk4 is also expressed at low level in the central nervous system, lung, mammary gland, and thymus (Yue et al., 2014); therefore, MYLK4 could phosphorylate an unknown protein in the central nervous system and affect skeletal muscle through a motoneuron or an endocrine system. In addition, we used female mice which have the estrous cycle, so estrogen, progesterone, follicle-stimulating hormone, and luteinizing hormone could also affect the expression of Mylk4.
Androgen-induced metabolic change
RNA-seq analysis revealed that muscle AR increased gene expression of glycolysis and decreased gene expression of amino acid catabolism (Figures 2 and S2). This metabolic shift preserves amino acids for protein synthesis. In addition to glycolysis, genes for polyamine metabolism (Odc1, Amd1, Smox) also were induced by myofiber AR in this report, and these genes are well-known targets of AR (Lee and MacLean, 2011). Polyamines decrease with aging, and supplementation with spermidine promoted longevity mediated by induction of autophagy in yeast (Eisenberg et al., 2009). Myopathy in Col6a1 null mice was ameliorated by spermidine treatment (Chrisam et al., 2015). Spermidine supplementation coupled with exercise ameliorated skeletal muscle atrophy induced by intraperitoneal injection of D-galactose (Fan et al., 2017). These reports suggested that AR-induced polyamine synthesis could increase autophagy flux in skeletal muscles to maintain muscle quality, which might affect consequent muscle strength.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a), a key regulator of mitochondrial biogenesis, is induced by synthetic androgen in prostate cancer cells (Tennakoon et al., 2014). However, our RNA-seq analysis did not detected upregulation of Ppargc1a in skeletal muscles by DHT treatment. AR would activate its target depending on the context, such as availability of protein complex or epigenetic modification.
The expression of Mylk4, Odc1, Amd1, and Smox in soleus muscles, slow-type muscles, was not significantly different between male and female (Figures 3F and S2C). These results suggested that androgen action in slow-type muscles is different from that of fast-type muscles. Further investigation is required to understand androgen action in slow-type muscles.
Indirect pathways
Previous studies focusing on AR and skeletal muscles were mainly conducted on male extragenital muscles such as LA and the bulbocavernosus muscles, which are well known as androgen-sensitive muscles. However, in this study, limb muscle mass was increased by DHT treatment even in cARKO mice (Figure 1). This suggested that increased limb skeletal muscle mass by androgen/AR would be regulated through endocrinological secretion or neuronal pathway by extra-muscle organs expressing Ar. Recently, neuron-specific Ar knockout mice were reported to have decreased hindlimb muscle mass but muscle strength was not affected (Davey et al., 2017). When testosterone acted on neurons in the dorsal striatum, locomotor activity was increased via dopaminergic pathways (Jardi et al., 2018). These reports indicated muscle mass regulation by a neural circuit. As an effector of muscle mass in skeletal muscles, Igf1 mRNA expression was decreased in gastrocnemius muscles of neuron-specific AR knockout mice (Davey et al., 2017). In our RNA-seq analysis, Igf1 was not included in DEGs, but RT-qPCR analysis showed that Igf1 mRNA was slightly increased by DHT treatment even in cARKO mice (Figure S3). In a C2C12 cell line, testosterone supplementation directly increased Igf1 mRNA expression (Sculthorpe et al., 2012). So, the regulation of Igf1 mRNA expression would also be context dependent.
Our RNA-seq analysis revealed that expression levels of 65 genes were increased by DHT treatment even in cARKO mice, which were categorized as DHT-dependent and myofiber AR-independent genes. Among 65 genes, Ace, which encodes an angiotensin-1 converting enzyme, showed a clear expression pattern for muscle AR independence and DHT dependence. A human ACE gene is known to have a polymorphism characterized by the presence (ACE-I allele) or absence (ACE-D allele) of a silencer sequence in intron 16 of the ACE gene. These polymorphisms of ACE allele are correlated with exercise endurance performance (Montgomery et al., 1998; Myerson et al., 1999), suggesting that ACE-angiotensin signaling could regulate skeletal muscle mass. On the other hand, hyperactivity of classical renin-angiotensin system in skeletal muscles is also known to be associated with insulin resistance, atrophy, and fibrosis (Cabello-Verrugio et al., 2015). The physiological role of increased Ace expression is still controversial.
In conclusion, this study explored, at least in part, the molecular function underlying the effects of androgen/AR on skeletal muscle, especially for its direct effects (Figure S8). We identified a splicing variant of Mylk4 as a target of AR in skeletal muscle and showed that Mylk4 regulates muscle strength through phosphorylation of MYOM1. Our results will have a significant impact on the understanding muscle strength and contribute to develop a medicine to treat sarcopenia in future. Further studies are needed to fully understand the entire mechanisms of androgen-induced augmentation of skeletal muscle mass and strength.
Limitation of the study
The limitation of our experiment is the small sample size for the quantitative PCR experiment, which might prevent the discovery of AR target genes.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, M.D., Ph.D. Yuuki Imai, y-imai@m.ehime-u.ac.jp.
Material availability
This study did not generate nor use any new or unique reagents.
Data and code availability
RNA sequencing data have been deposited in the Gene Expression Omnibus as accession no. GSE152756.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank Dr. Naohito Tokunaga, the staff of the Division of Analytical Bio-Medicine and the Division of Laboratory Animal Research, the Advanced Research Support Center (ADRES), Ehime University. Ms. Aya Tamai and Ms. Sayoko Nakanishi and the members of the Division of Integrative Pathophysiology, Proteo-Science Center (PROS), Ehime University, for their technical assistance and helpful support. We also thank Prof. Ken-Ichiro Morohashi, Prof. Hirotoshi Tanaka, Prof. Yasuyuki Ohkawa, and Dr. Takashi Baba for their helpful discussion. Our study was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grants (JP15K16517, JP18K11069, JP17H06427, JP15H04961, JP19H03786), Ehime University PROS, the Osaka Medical Research Foundation for Intractable Diseases, The Nakatomi Foundation (2016ws42), and Takeda Science Foundation.
Author contributions
I.S. and Y.I. conceived and managed the project. Y.Y., K.H., T.Y., and Y.S. generated Mylk4 KO mice and analyzed the muscle function of Mylk4 mice. H.S. performed immunostaining of AR. I.S., H.T., A.Y., and T.S. performed proteomics analysis. I.S. and N.S. performed qPCR experiments. H.H. helped the RNA-seq analysis. S.F. advised all aspect of the study. I.S., T.Y., S.F., and Y.I. wrote the manuscript. All the authors discussed and edited the manuscript.
Declaration of interests
All authors declare no competing interests.
Inclusion and diversity
We worked to ensure diversity in experimental samples through the selection of the cell lines.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102303.
Supplemental information
References
- Basaria S., Coviello A.D., Travison T.G., Storer T.W., Farwell W.R., Jette A.M., Eder R., Tennstedt S., Ulloor J., Zhang A. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S., Woodhouse L., Storer T.W. Proof of the effect of testosterone on skeletal muscle. J. Endocrinol. 2001;170:27–38. doi: 10.1677/joe.0.1700027. [DOI] [PubMed] [Google Scholar]
- Burd C.J., Morey L.M., Knudsen K.E. Androgen receptor corepressors and prostate cancer. Endocr. Relat. Cancer. 2006;13:979–994. doi: 10.1677/erc.1.01115. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C., Morales M.G., Rivera J.C., Cabrera D., Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med. Res. Rev. 2015;35:437–463. doi: 10.1002/med.21343. [DOI] [PubMed] [Google Scholar]
- Chambon C., Duteil D., Vignaud A., Ferry A., Messaddeq N., Malivindi R., Kato S., Chambon P., Metzger D. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc. Natl. Acad. Sci. U S A. 2010;107:14327–14332. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A.N., Mahajan P., Knapp S., Barton H., Sweeney H.L., Kamm K.E., Stull J.T. Cardiac myosin light chain is phosphorylated by Ca2+/calmodulin-dependent and -independent kinase activities. Proc. Natl. Acad. Sci. U S A. 2016;113:E3824–E3833. doi: 10.1073/pnas.1600633113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Yeh S., Lee S.O., Chang T.M. Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: lessons learned from mice lacking AR in specific cells. Nucl. Recept. Signal. 2013;11:e001. doi: 10.1621/nrs.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisam M., Pirozzi M., Castagnaro S., Blaauw B., Polishchuck R., Cecconi F., Grumati P., Bonaldo P. Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice. Autophagy. 2015;11:2142–2152. doi: 10.1080/15548627.2015.1108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R.A., Clarke M.V., Russell P.K., Rana K., Seto J., Roeszler K.N., How J.M.Y., Chia L.Y., North K., Zajac J.D. Androgen action via the androgen receptor in neurons within the brain positively regulates muscle mass in male mice. Endocrinology. 2017;158:3684–3695. doi: 10.1210/en.2017-00470. [DOI] [PubMed] [Google Scholar]
- De Gendt K., Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol. Cell. Endocrinol. 2012;352:13–25. doi: 10.1016/j.mce.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Dubois V., Laurent M.R., Sinnesael M., Cielen N., Helsen C., Clinckemalie L., Spans L., Gayan-Ramirez G., Deldicque L., Hespel P. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 2014;28:2979–2994. doi: 10.1096/fj.14-249748. [DOI] [PubMed] [Google Scholar]
- Eisenberg T., Knauer H., Schauer A., Buttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Fan J., Yang X., Li J., Shu Z., Dai J., Liu X., Li B., Jia S., Kou X., Yang Y. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget. 2017;8:17475–17490. doi: 10.18632/oncotarget.15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry A., Schuh M., Parlakian A., Mgrditchian T., Valnaud N., Joanne P., Butler-Browne G., Agbulut O., Metzger D. Myofiber androgen receptor promotes maximal mechanical overload-induced muscle hypertrophy and fiber type transition in male mice. Endocrinology. 2014;155:4739–4748. doi: 10.1210/en.2014-1195. [DOI] [PubMed] [Google Scholar]
- Fraysse B., Vignaud A., Fane B., Schuh M., Butler-Browne G., Metzger D., Ferry A. Acute effect of androgens on maximal force-generating capacity and electrically evoked calcium transient in mouse skeletal muscles. Steroids. 2014;87:6–11. doi: 10.1016/j.steroids.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Jardi F., Laurent M.R., Kim N., Khalil R., De Bundel D., Van Eeckhaut A., Van Helleputte L., Deboel L., Dubois V., Schollaert D. Testosterone boosts physical activity in male mice via dopaminergic pathways. Sci. Rep. 2018;8:957. doi: 10.1038/s41598-017-19104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebis M., Ohsawa N., Kino Y., Sasagawa N., Nishino I., Ishiura S. Alternative splicing of myomesin 1 gene is aberrantly regulated in myotonic dystrophy type 1. Genes Cells. 2011;16:961–972. doi: 10.1111/j.1365-2443.2011.01542.x. [DOI] [PubMed] [Google Scholar]
- Lange S., Himmel M., Auerbach D., Agarkova I., Hayess K., Furst D.O., Perriard J.C., Ehler E. Dimerisation of myomesin: implications for the structure of the sarcomeric M-band. J. Mol. Biol. 2005;345:289–298. doi: 10.1016/j.jmb.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Lee N.K., MacLean H.E. Polyamines, androgens, and skeletal muscle hypertrophy. J. Cell. Physiol. 2011;226:1453–1460. doi: 10.1002/jcp.22569. [DOI] [PubMed] [Google Scholar]
- MacLean H.E., Chiu W.S., Notini A.J., Axell A.M., Davey R.A., McManus J.F., Ma C., Plant D.R., Lynch G.S., Zajac J.D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- Montgomery H.E., Marshall R., Hemingway H., Myerson S., Clarkson P., Dollery C., Hayward M., Holliman D.E., Jubb M., World M. Human gene for physical performance. Nature. 1998;393:221–222. doi: 10.1038/30374. [DOI] [PubMed] [Google Scholar]
- Myerson S., Hemingway H., Budget R., Martin J., Humphries S., Montgomery H. Human angiotensin I-converting enzyme gene and endurance performance. J. Appl. Physiol. 1999;87:1313–1316. doi: 10.1152/jappl.1999.87.4.1313. [DOI] [PubMed] [Google Scholar]
- Obermann W.M., Gautel M., Weber K., Furst D.O. Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997;16:211–220. doi: 10.1093/emboj/16.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff J., Callewaert F., Venken K., De Gendt K., Ohlsson C., Gayan-Ramirez G., Decramer M., Boonen S., Bouillon R., Verhoeven G. Physical activity in the androgen receptor knockout mouse: evidence for reversal of androgen deficiency on cancellous bone. Biochem. Biophys. Res. Commun. 2009;378:139–144. doi: 10.1016/j.bbrc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Rana K., Chiu M.W., Russell P.K., Skinner J.P., Lee N.K., Fam B.C., Zajac J.D., MacLean H.E. Muscle-specific androgen receptor deletion shows limited actions in myoblasts but not in myofibers in different muscles in vivo. J. Mol. Endocrinol. 2016;57:125–138. doi: 10.1530/JME-15-0320. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Reggiani C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Sculthorpe N., Solomon A.M., Sinanan A.C., Bouloux P.M., Grace F., Lewis M.P. Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med. Sci. Sports Exerc. 2012;44:610–615. doi: 10.1249/MSS.0b013e318237c5c0. [DOI] [PubMed] [Google Scholar]
- Stull J.T., Kamm K.E., Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch. Biochem. Biophys. 2011;510:120–128. doi: 10.1016/j.abb.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H.L., Hammers D.W. Muscle contraction. Cold Spring Harb. Perspect. Biol. 2018;10:a023200. doi: 10.1101/cshperspect.a023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennakoon J.B., Shi Y., Han J.J., Tsouko E., White M.A., Burns A.R., Zhang A., Xia X., Ilkayeva O.R., Xin L. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene. 2014;33:5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry E.E., Zhang X., Hoffmann C., Hughes L.D., Lewis S.A., Li J., Wallace M.J., Riley L.A., Douglas C.M., Gutierrez-Monreal M.A. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife. 2018;7:e34613. doi: 10.7554/eLife.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Delahunt E., Ditroilo M., Lowery M., De Vito G. Effects of age and sex on neuromuscular-mechanical determinants of muscle strength. Age (Dordr) 2016;38:57. doi: 10.1007/s11357-016-9921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi G., Ryder J.W., Huang J., Ding P., Chen Y., Zhao Y., Kamm K.E., Stull J.T. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc. Natl. Acad. Sci. U S A. 2005;102:17519–17524. doi: 10.1073/pnas.0506846102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data have been deposited in the Gene Expression Omnibus as accession no. GSE152756.