Abstract
Codeine stimulates skin mast cells and is therefore used in skin tests and as an inducer of experimental itch. MRGPRX2 responds to various drugs, including opioids, to elicit pseudoallergic reactions, but whether it represents the main opiate receptor of skin mast cells remains unknown. By combining a number of approaches, including the silencing of MRGPRX2, we now report that MRGPRX2 is indeed the dominant codeine receptor of dermal mast cells. Activation by codeine displayed profound subject variability and correlated with secretion elicited by compound 48/80 or substance P but not by FcεRI aggregation. Degranulation by codeine was attenuated by stem cell factor, whereas the opposite was found for FcεRI. Compound 48/80 or codeine alone was able to achieve maximum MRGPRX2 activation. MRGPRX2 was rapidly internalized on codeine binding in a β-arrestin-1‒dependent manner. Codeine-triggered β-arrestin activation was also established by the Tango assay. Prestimulation with MRGPRX2 agonists (but not C3a or FcεRI aggregation) resulted in refractoriness to further stimulation by the same or another MRGPRX2 ligand (cross desensitization). This was duplicated in a cell line (RBL-MRGPRX2). Collectively, codeine degranulates skin mast cells through MRGPRX2, at which it acts as a balanced ligand. It has yet to be determined whether codeine-induced refractoriness could be exploited to desensitize MRGPRX2 to prevent severe pseudoallergic reactions.
INTRODUCTION
Opiates, such as codeine and morphine, and their synthetic counterparts (opioids) can produce severe hypersensitivity reactions in susceptible individuals, which depend on degranulating mast cells (MCs) (Barke and Hough, 1993; Golembiewski, 2002; Swerlick et al., 1989).
Accordingly, opiates have long been known to trigger the activation of MCs in vitro, not only in mixed cell cultures but also when purified to (near) homogeneity, demonstrating that MCs are directly targeted by this group of analgesics (Benyon et al., 1989; Grosman, 1981; Hermens et al., 1985; Tharp et al., 1987).
Only certain MCs, especially those in the skin, are responsive to opioids and release preformed mediators, whereas those in the gut, lung, adenoids, tonsils, and heart are refractory to opiate stimulation (Lawrence et al., 1987; Lowman et al., 1988; Rees et al., 1988; Tharp et al., 1987). This differential responsiveness is reflected by the prevalence of cutaneous symptoms over those in other organs (Prieto-Lastra et al., 2006; Scherer et al., 2007).
Although morphine has been commonly employed in vitro, codeine is the most frequently used opiate in skin prick and intradermal tests (Gollhausen et al., 1985; Humphreys and Hunter, 1991; Jutel et al., 1995; Krause et al., 2013; Kupczyk et al., 2007; Nancey et al., 2004; Nielsen et al., 2001; Scherer et al., 2007; Theunis et al., 2008; Varney et al., 2003; Zweiman et al., 1997).
Both opiates are equipotent (Casale et al., 1984; Grosman, 1981), and equipotency is also found between opiate- and FcεRI-induced degranulation in vivo (Casale et al., 1984; McBride et al., 1989; Petersen et al., 1996).
Moreover, codeine is associated with the sensation of itch (over pain), a quality exploited in pruritus research (Blunk et al., 2004; Charney et al., 2008; Herde et al., 2007; Kupczyk et al., 2007; Namer et al., 2008; Steinhoff et al., 2003; Theunis et al., 2008; von Muhlendahl et al., 1976; Weidner et al., 2000).
The mechanism by which opiates elicit degranulation specifically in the skin MCs but not in other MCs has remained a mystery. Several scenarios have been proposed, including an IgE-dependent mechanism (Dybendal et al., 2003), IgE-independent but receptor-dependent activation (e.g., through canonical opioid receptors) (Casale et al., 1984; Grosman, 1981; Tharp et al., 1987; Yamasaki et al., 1982), or a direct activation of G-proteins (Barke and Hough, 1993; Blunk et al., 2004; Shanahan et al., 1984). Yet, experimental evidence to support any of the above has remained poor. Especially, the involvement of classical opioid receptors has been much debated (Benyon et al., 1987; Blunk et al., 2004; Hermens et al., 1985; Schmidt-Rondon et al., 2018; Shanahan et al., 1984; Sheen et al., 2007). In addition, because opiates degranulate the skin MCs in nearly every individual (Casale et al., 1984; Lin et al., 1990; Nasser and Ewan, 2001), a universal mechanism appeared to be the likely explanation.
MRGPRX2 was recently uncovered as the principal receptor of IgE-independent MC activation (McNeil et al., 2015), and its significance in clinically relevant drug hypersensitivity is being gradually recognized (Babina, 2020). MRGPRX2 can operate as an unusual opioid receptor in cell lines ectopically or natively expressing MRGPRX2 (Lansu et al., 2017), which made us hypothesize that codeine mainly acts through MRGPRX2 in the skin MCs, as also supported by the exclusive expression of MRGPRX2 and lack of canonical opioid receptors in these cells (Fujisawa et al., 2014; Motakis et al., 2014).
Using a variety of approaches, we now report that MRGPRX2 indeed constitutes the dominant (or only) opiate receptor of dermal MCs. Responsiveness can vary substantially across individuals in a way unrelated to FcεRI stimulability. Besides activation, codeine elicits internalization and desensitization of its receptor in a β-arrestin-1‒dependent manner. Although the stimuli activating alternative receptors (FcεRI and C3aR) fail to desensitize MRRGPRX2, MRRGPRX2 ligands are able to cross desensitize their receptor, signifying clinical potential.
RESULTS
Codeine degranulates human skin MCs by activating MRGPRX2
We first confirmed that codeine triggers histamine release in human skin MCs in a dose-dependent manner (Figure 1a). This was accompanied by the rapid and transient translocation of the degranulation marker CD107a to the cell surface (Guhl et al., 2005) in ≈50% of the cells (all-or-nothing response [Joulia et al., 2017]) (Figure 1b). Finally, β-hexosaminidase release could further ascertain codeine-triggered degranulation and served to calculate the half maximal effective concentration as 24.0 µg/ml (corresponding to 29.5 µM) (Figure 1c).
Figure 1. MRGPRX2 is the codeine receptor on human skin MCs.
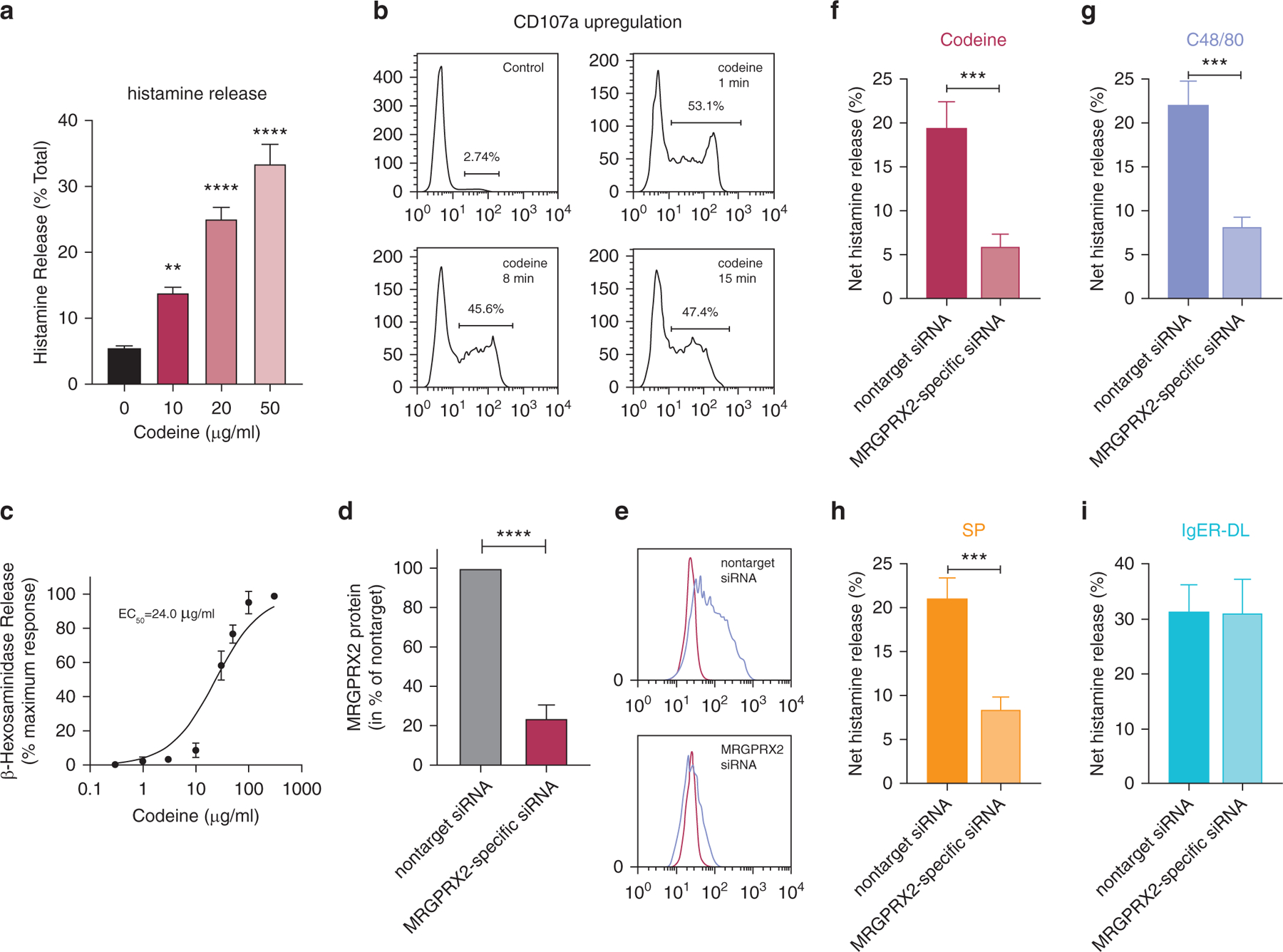
(a–c) Skin MCs ex vivo were stimulated with codeine, and (a) net histamine release (stimulated release–spontaneous release; in percentage of total histamine) was assessed after 30 minutes; mean ± SEM and n = 8. (b) CD107a externalization was determined by flow cytometry after the indicated times, representative of two independent experiments. (c) β-Hexosaminidase release served to calculate EC50. The net release for each concentration was normalized to the maximum; mean ± SD, n = 6. (d–i) Cells were subjected to MRGPRX2-specific siRNA or nontarget siRNA for 2 days. (d) MRGPRX2 surface expression; mean ± SEM of net MFI (MFI MRGPRX2–MFI isotype) for MRGPRX2 siRNA normalized to nontarget siRNA; n = 10. (e) Representative histograms, blue: MRGPRX2, red: isotype. (f–i) Net histamine release triggered by C48/80 (10 µg/ml), SP (30 µM), codeine (50 µg/ml), and IgER-CL (29C6, 0.5 µg/ml); mean ± SEM and n = 7–8. MRGPRX2-specific siRNA reduced degranulation to 29.8 ± 12.8% (codeine), 36.2 ± 12.0% (C48/80), 40.0 ± 11.9% (SP). **P < 0.01, ***P < 0.001, ****P < 0.0001. C48/80, compound 48/80; CL, crosslinking; EC50, half maximal effective concentration; IgER, IgE receptor; MC, mast cell; MFI, mean fluorescence intensity; min, minute; siRNA, small interfering RNA; SP, substance P.
To explore whether MRGPRX2 constitutes the relevant receptor in skin MCs, we utilized RNA interference (Hazzan et al., 2017a), by which MRGPRX2 expression was suppressed down to 24.0 ± 6.6% (mean ± SEM) of the nontarget control at protein level (Figure 1d and e) and similarly at transcript level (not depicted). The reduction of MRGPRX2 effectively attenuated histamine release triggered by codeine (Figure 1f). Degranulation by compound 48/80 (C48/80) and substance P (SP) was likewise curtailed (Figure 1g and h), but responsiveness to codeine was reduced to the same (or slightly greater) extent. Conversely, responses to FcεRI aggregation remained unperturbed (Figure 1i). Even though skin MCs expressed none of the classical opioid receptors (Motakis et al., 2014), we employed naloxone as a pan-opioid receptor antagonist to confirm that opioid receptors are not involved in the response of skin MCs to codeine (Supplementary Figure S1).
In a complementary strategy, the MC line (RBL-2H3) was employed, which only becomes responsive to MRGPRX2 agonists on transfection of the human gene (RBL-MRGPRX2) (Roy et al., 2019a). In accordance, RBL-MRGPRX2 were responsive and released histamine similarly to the skin MCs, whereby codeine mimicked C48/80 and SP (Supplementary Figure S2a). RBL-MRGPRX2 also released β-hexosaminidase with a half maximal effective concentration of 6.3 µM (Supplementary Figure S2c and e). Codeine triggered calcium ion influx in RBL-MRGPRX2 cells but not in RBL-2H3 cells (Supplementary Figure S2f), duplicating the response pattern to C48/80 and SP (Alkanfari et al., 2018; Roy et al., 2019a).
Thus, codeine generally targets MRGPRX2 to trigger MC degranulation. Importantly, MRGPRX2 represents the codeine receptor in skin MCs, the clinically relevant responder cells.
Responsiveness to codeine varies interindividually in correlation with C48/80 and SP but not with FcεRI-triggered degranulation
Using MCs from a substantial number of individuals, we found that responsiveness to codeine varied in the studied population between 6.3% and 63.4% (net histamine release, Figure 2a, x-axis). Donor variability and maximum secretion were similar for the three ligands codeine, C48/80, and SP (Figure 2a, y-axis) (see reference Babina et al. [2018a] for C48/80 vs. SP).
Figure 2. Responses to codeine by skin MCs are highly divergent across individuals with no correlation with allergic degranulation and inhibition by SCF.

(a) HR was stimulated by C48/80 (10 µg/ml), SP (30 µM), codeine (50 µg/ml), and IgER CL (29C6 at 0.5 µg/ml) on skin MCs ex vivo; each dot corresponds to a single donor. The net release was pairwise plotted. R denotes the Spearman correlation coefficient. (b) Ex vivo skin MCs were pretreated with SCF for 15 minutes and then subjected to the different stimuli for 30 minutes. Note the opposite mode of regulation caused by MRGPRX2 ligands (codeine, C48/80, and SP) compared with IgER-triggered degranulation. ***P < 0.001, ****P < 0.0001. C48/80, compound 48/80; CL, crosslinking; HR, histamine release; IgER, IgE receptor; MC, mast cell; SCF, stem cell factor; SP, substance P.
Pairwise comparisons between C48/80 or SP and codeine revealed nearly perfect correlations, substantiating that the three ligands signal through MRGPRX2 in the skin MCs (Figure 2a). In contrast, there was no correlation with the degranulation triggered by the allergic route, corroborating the independence of the two secretory networks (Babina et al., 2018a; Gaudenzio et al., 2016).
Furthermore, codeine-triggered histamine secretion was attenuated by stem cell factor, replicating findings for SP and C48/80, whereas FcεRI-mediated degranulation was enhanced (Figure 2b). Collectively, codeine mimics the behavior of C48/80 and SP in the skin MCs, supporting action at the same receptor.
Codeine stimulation of MRGPRX2 triggers the downregulation of the receptor
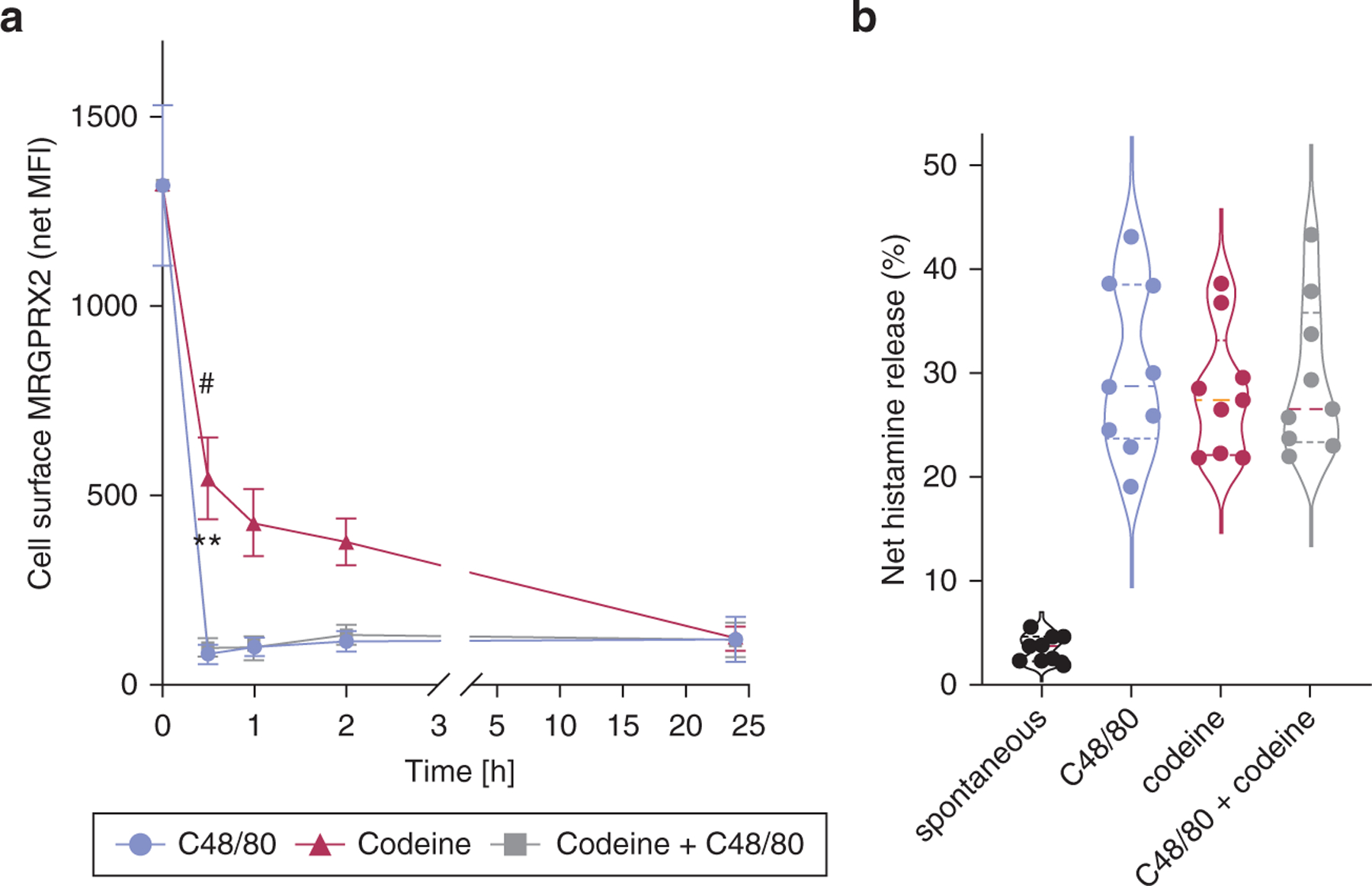
Many G-protein‒coupled receptors, including MRGPRX2, incur β-arrestin‒initiated internalization on ligand binding (Roy et al., 2019a). MRGPRX2 was rapidly diminished in response to C48/80 on the skin MCs (Figure 3a, red curve), and less pronounced downregulation was also found for SP (Figure 3a, green line). The curve obtained for codeine stimulation (Figure 3a, magenta) had a shape between SP and C48/80, yet resemblance was stronger with C48/80 because despite slower initiation, the downregulation reached a similar minimum after 24 hours (Figure 3a and b).
Figure 3. C48/80, codeine, and SP trigger rapid downregulation of MRGPRX2 cell surface expression in skin-derived MCs intracellular accumulation of MRGPRX2.

(a, b) Cultured skin-derived MCs were stimulated for the times indicated (10 µg/ml of C48/80, 30 µM of SP, 50 µg/ml of codeine). (a) MRGPRX2 surface expression (net MFI, Figure 1) plotted as a function of time; mean ± SEM and n = 9. *P < 0.05; **P < 0.01 versus C48/80; #P < 0.05 versus codeine. (b) Representative histograms of a; red: isotype, blue: MRGPRX2. (c) Intracellular MRGPRX2 expression by flow cytometry and fluorescence microscopy (on cell permeabilization, left panel) in comparison with MRGPRX2 at the cell surface (intact cells, right panel) at 1 hour after codeine or PBS treatment (control); one representative of n = 3. Bar = 25 µm. C48/80, compound 48/80; h, hour; MC, mast cell; MFI, mean fluorescence intensity; SP, substance P.
Using flow cytometry on permeabilized versus intact cells, we found that codeine only reduced MRGPRX2 at the cell surface, whereas total expression (visible on permeabilization) remained unchanged over the observation period, indicating internalization (Figure 3c). The same result was found by immunofluorescence, whereby bright surface staining was only found on nonstimulated MCs; conversely, naive and codeine-stimulated MCs displayed intracellular MRGPRX2 staining, typically in clusters, which was more pronounced in the stimulated cells (Figure 3c).
Downregulation of MRGPRX2 was also observed in skin MCs shortly after isolation (ex vivo setting), the latter expressing higher levels of MRGPRX2 (Babina et al., 2018b) (Supplementary Figure S3a and b). Although less potently and rapidly, codeine likewise reduced MRGPRX2 in RBL-MRGPRX2 cells (Supplementary Figure S3d and e), suggesting universality across MC types.
Codeine causes the activation of β-arrestin internalization of MRGPRX2, which is β-arrestin-1 dependent
The above data insinuated that codeine, similar to C48/80, activates both the G-protein and the β-arrestin pathway. To further prove this, we made use of an assay dubbed Tango, which involves transcriptional activation on ligand binding (Lansu et al., 2017). To assess β-arrestin‒mediated gene expression (indicative of β-arrestin activation), HTLA cells stably expressing FLAG-tagged MRGPRX2-Tango were incubated with graded doses of codeine and C48/80 overnight. Besides C48/80, codeine induced robust gene expression at 10 µg/ml, which was further increased at 100 µg/ml (Figure 4a and b).
Figure 4. Codeine and C48/80 cause the activation of β-arrestin MRGPRX2 internalization, which depends on β-arrestin-1 in skin MCs.
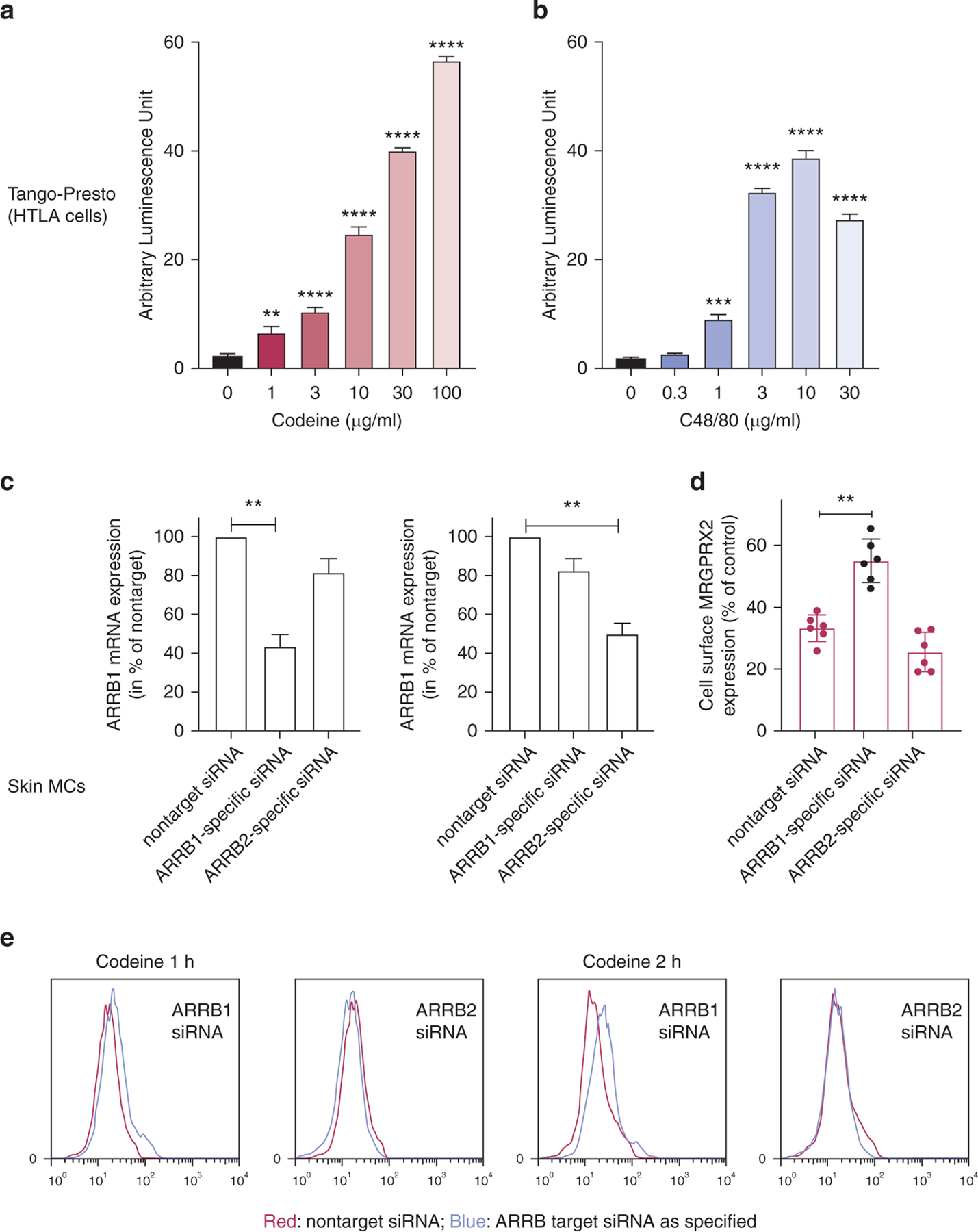
(a, b) HTLA cells were stimulated overnight with the indicated stimuli, and luminescence units were measured on a luminometer after substrate addition; mean ± SEM and n = 3. (c–e) Skin MCs were treated for 48 hours with ARRB1 selective, ARRB2 selective, or nontarget siRNA. (c) ARRB1 and ARRB2 mRNA expression. (d) MRGPRX2 cell surface expression after codeine (100 µg/ml), normalized to the matching unstimulated control (by net MFI, see Figure 1). Mean ± SEM and n = 6 for c and d. (e) Corresponding representative histograms showing MRGPRX2 expression after codeine triggering in ARRΒ-silenced versus control siRNA-treated cells. Color code is as explained in the figure. **P < 0.01, ***P < 0.001, ****P < 0.0001. C48/80, compound 48/80; h, hour; MC, mast cell; MFI, mean fluorescence intensity; siRNA, small interfering RNA.
To identify the relevance of the β-arrestin pathway in skin MCs, we utilized ARRB1- and ARRB2-selective small interfering RNAs (siRNAs), resulting in an efficient reduction of their respective targets (Figure 4c). Silencing of β-arrestin-1 attenuated MRGPRX2 internalization, whereas interference with β-arrestin-2 had no effect (Figure 4d and e). We conclude that the internalization of MRGPRX2 in skin MCs chiefly depends on β-arrestin-1 and that codeine (such as C48/80) constitutes a balanced ligand that elicits G-protein‒dependent degranulation and β-arrestin‒mediated receptor internalization.
Codeine alone can cause complete MRGPRX2 activation
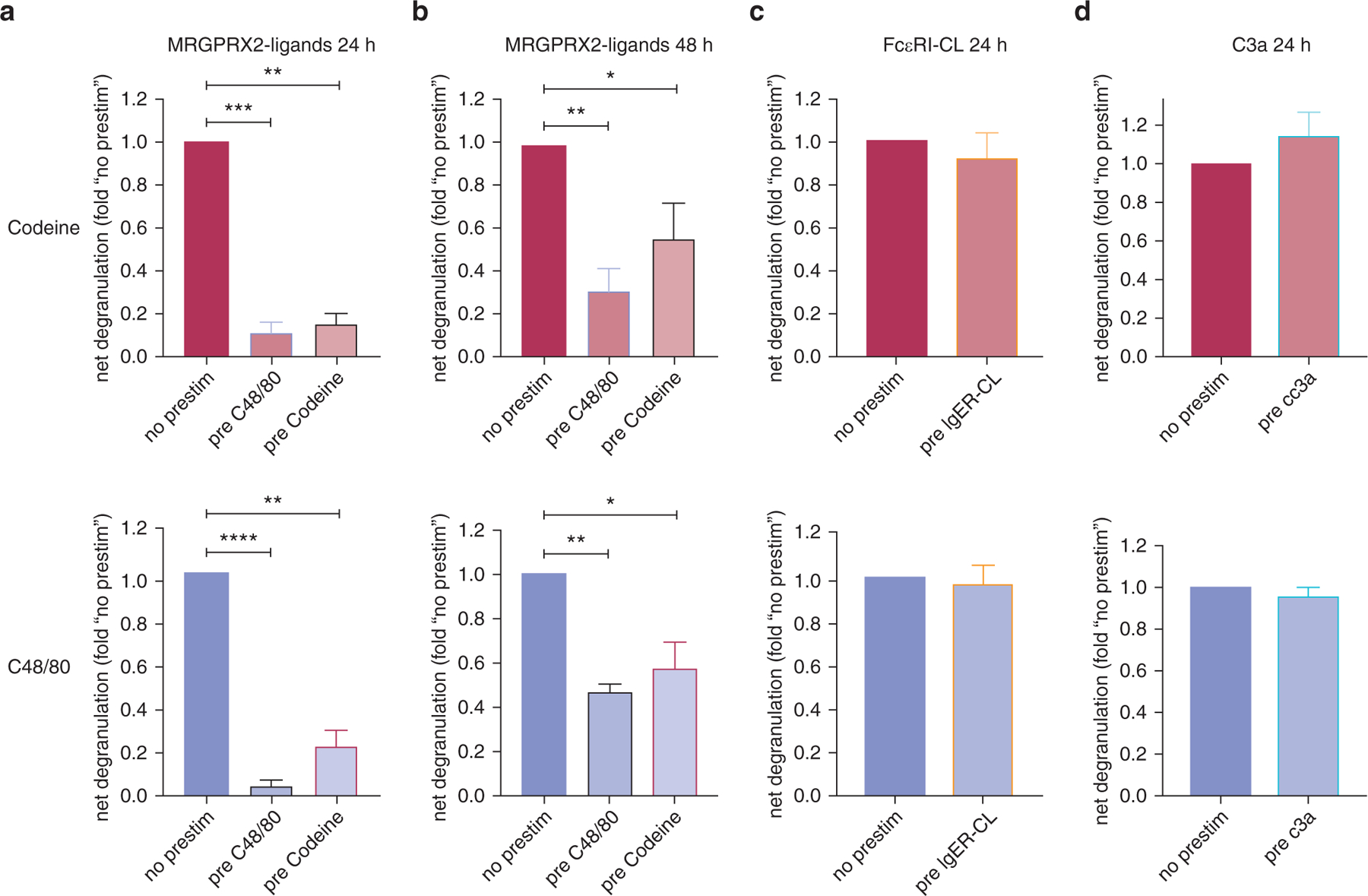
To further ascertain that MRGPRX2 is the codeine receptor on skin MCs, we reasoned that a single ligand would be sufficient to fully activate MRGPRX2. In fact, C48/80 plus codeine did not further promote internalization because the curve depicted in Figure 5a was virtually identical to the one obtained for C48/80 alone. Similarly, the combination of C48/80 with codeine did not increase histamine liberation compared with the individual ligands (Figure 5b). Together, the pseudoallergic route can be saturated by a single ligand, corroborating that codeine and C48/80 target the same receptor, that is, MRGPRX2.
Figure 5. A combination of C48/80 with codeine does not increase MRGPRX2 internalization or degranulation over each stimulus alone.
(a) Cultured skin-derived MCs were triggered by C48/80 (10 µg/ml), codeine (50 µg/ml), or C48/80 + codeine for different times. Cell surface MRGPRX2 expression was determined as in Figure 3. The data are presented as mean ± SEM of n = 8 individual experiments. **P < 0.01 for codeine versus c48/80, #P < 0.05 for codeine versus codeine + c48/80. (b) Ex vivo skin MCs were stimulated, and HR was measured as in Figure 1. C48/ 80, compound 48/80; h, hour; HR, histamine release; MC, mast cell; MFI, mean fluorescence intensity.
Induction of refractoriness: cross desensitization of MRGPRX2 by codeine or C48/80
β-arrestin‒mediated G-protein‒coupled receptor internalization contributes to receptor desensitization commonly observed in G-protein‒coupled receptor signaling. Having found that C48/80 and codeine both lead to receptor internalization (Figure 3) and β-arrestin activation (Figure 4), we finally asked whether the reduction of MRGPRX2 at the cell surface attenuates its function and whether the two ligands are interchangeable.
Prestimulation of skin MCs with C48/80 for 24 hours indeed resulted in nearly complete inhibition of degranulation on second stimulation by the same ligand (Figure 6a). The same result was obtained when codeine was used for the first and second stimulation. Interestingly, prestimulation with codeine heterologously inhibited C48/80-triggered degranulation and vice versa; preincubation with C48/80 inhibited subsequent codeine-induced secretion (Figure 6a).
Figure 6. Codeine and c48/80 cross desensitize MRGPRX2, but no desensitization on prestim through unrelated receptors.
Cultured skin-derived MCs were subjected to different pretreatments at time point zero: C48/80 (10 µg/ml), codeine (50 µg/ml), IgER CL (AER-37, 0.1 µg/ml), or C3a (10 nM) versus no stimulus (no prestim). All pretreatments induced degranulation: AER-37, most strongly (30–50% net release); C3a, least strongly (5–10%), as determined separately. Cells were washed after 1 hour and cultured in regular medium. After (a, c, d) 24 or (b) 48 hours, cells were subjected to a second stimulation with codeine (top panel) or C48/80 (bottom). Net β-hexosaminidase release was assessed and normalized to the no prestim group; mean ± SEM and n = 5–8 different cultures. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. C48/80, compound 48/80; CL, crosslinking; h, hour; IgER, IgE receptor; MC, mast cell; prestim, prestimulation.
Desensitization and cross desensitization were still fairly pronounced after 48 hours, albeit stimulability was partially recovered vis-à-vis 24 hours (Figure 6b).
We next assessed whether the observed effects were selectively induced by MRGPRX2 agonists. In fact, neither FcεRI aggregation nor C3a (signaling through an unrelated G-protein‒coupled receptor, C3aR) had an effect on subsequent MRGPRX2-triggered degranulation (Figure 6c and d), suggesting that desensitization is only achieved by MRGPRX2 activation.
It was not only cultured skin-derived MCs that showed the behavior of heterologous desensitization (Figure 6) but also skin MCs ex vivo (Supplementary Figure S3c) and RBL-MRGPRX2 cells (Supplementary Figure S3f), substantiating a shared concept across MC types. Thus, previous stimulation by a balanced ligand will attenuate subsequent responses to other MRGPRX2 agonists, an aspect of clinical significance if transferable to the situation in vivo.
DISCUSSION
After its discovery as a major receptor system of the MCTC-type MC, MRGPRX2 has rapidly evolved into a premier focus in MC research (Ali, 2017; Babina, 2020; Kim et al., 2020; Olivera et al., 2018). This study demonstrates that MRGPRX2 likely serves as the sole opiate receptor on skin MCs. The conclusion is based on the following observations. First (and most direct), the silencing of MRGPRX2 in skin MCs leads to a strong reduction in codeine-triggered degranulation. Second, responses to codeine show perfect correlations with those triggered by other MRGPRX2 agonists. Third, the combination of codeine with C48/80 does not increase degranulation over each stimulus alone, as also reported for SP + C48/80 vis-à-vis the individual agonists (Babina et al., 2018a). Fourth, codeine leads to rapid internalization of its receptor similarly to C48/80, and the combination of codeine and C48/80 does not accelerate downregulation. Fifth, responsiveness to codeine is lost in cells that have been desensitized not only with codeine but also with C48/80 (yet not with FcεRI crosslinking or C3a), corroborating that both molecules target the same receptor. Sixth, skin MCs do not express any of the classical opioid receptors (OPRD1, OPRK1, OPRM1) (Motakis et al., 2014), nor does the pan-opioid antagonist naloxone interfere with codeine-triggered degranulation (Supplementary Figure S1). In summary, if there is an MRGPRX2-independent component, it may be assumed as small and insignificant under most circumstances and in the majority of individuals.
Our findings are in accordance with reports identifying MRGPRX2 as an atypical opioid-like receptor in cell lines ectopically or naturally expressing human MRGPRX2 (Lansu et al., 2017; Navinés-Ferrer et al., 2018). The ability of MRGPRX2 to act as a codeine receptor is also demonstrated in RBL-MRGPRX2 cells in this study (Supplementary Figure S2).
Using MCs of multidonor origin, we also demonstrate that MRGPRX2 and FcεRI are, on average, equipotent at eliciting degranulation, even though responsiveness at the individual level depends on donor specificities. Comparable potency of the two routes is in accordance with previous literature obtained long before the discovery of MRGPRX2 (Petersen et al., 1996). Moreover, interindividual variability has been described in skin tests, for example, in titrated skin prick tests (Nasser and Ewan, 2001) or a microdialysis study showing that codeine responsiveness could differ by a factor of ≈ 10 across subjects (Krause et al., 2013). Because the factor is replicated in this study for skin MCs (Figure 2a), the variance in intact skin may result, to an essential part, from the altered proneness of an individual’s MCs to become activated by codeine. Differential MRGPRX2 activity may stem from different variants in the MRGPRX2 gene (Alkanfari et al., 2018; Chompunud Na Ayudhya et al., 2019; Yang et al., 2005).
As mentioned, codeine is a common nonimmunologic MC secretagogue in clinical practice, and its use in skin tests has been encouraged to rate MC reactivity in addition to tissue reactivity, with the latter being judged from the histamine control, which bypasses MC activation. Codeine has also been associated with pruritus and is used experimentally to provoke itch (see Introduction section). The sensation of pure itch over pain is a hallmark of opiates, and although pain is effectively suppressed by the potent analgesic effects of opiates, m-opioid antagonists can conversely suppress pruritus (Akiyama and Carstens, 2013), highlighting the dichotomy between itch and pain, in which both the µ-opioid receptor (on neurons) and MRGPRX2 (on MCs) seem to be involved. It is notable that other members of the MRGPR family, expressed by sensory neurons, also play crucial roles in the generation of itch (Akiyama and Carstens, 2013; Reddy et al., 2015; Sharif et al., 2020; Xing et al., 2020; Yosipovitch et al., 2019).
Our findings have relevance to the use of opiates in skin tests, including (re)interpretation of findings for opiates and allergens utilized side by side. The fact that skin responses to codeine will not make predictions on the patient’s response to FcεRI aggregation and vice versa perfectly matches the two well-distinctive modes of granule fusion and discharge (Gaudenzio et al., 2016). It remains to be seen whether opiates can capture an individual’s propensity to react to MRGPRX2 ligands in general and therefore become suitable to predict the development of adverse reactions not only to opioids themselves but also to other MRGPRX2 ligands. This is important in view of the sheer number of agents targeting MRGPRX2, which could be in the hundreds (Grimes et al., 2019; Hou et al., 2019; Lansu et al., 2017; McNeil et al., 2015; Navinés-Ferrer et al., 2018; Tatemoto et al., 2006) with their potential of adverse reactions. Considering the association between nonallergic MC activation and conditions such as sudden infant death (Gold et al., 2000) and fatality in heroin addicts (Edston and van Hage-Hamsten, 1997), this connection, if proven, may also help identify individuals at risk for these conditions. The nearly perfect correlations between codeine, C48/80, and SP (this study and Babina et al. [2018a]) may imply so, but skin prick or intradermal tests with multiple identified MRGPRX2 ligands will be required to prove or disprove this possibility. This is also important in view of the growing list of chronic diseases, in which MRGPRX2 seems to be involved beyond mere hypersensitivity reactions, especially urticaria, atopic dermatitis, and rosacea (Babina, 2020; Muto et al., 2014).
We also found that the best-established MC-supportive factor stem cell factor (Olivera et al., 2018) dampens responsiveness to codeine, replicating its effect on C48/80- and SP-triggered exocytosis (Figure 2b and Babina et al., 2018a). An opposite influence on allergic versus pseudoallergic stimulation was likewise found for IL-4 (Babina et al., 2018b), another supportive factor of skin MCs (Babina et al., 2016), and for retinoic acid (Babina et al., 2017). In contrast, IL-33 seems to affect both routes in a consistent fashion, that is, downregulation on chronic treatment but intensification upon acute priming (Babina et al., 2019; Wang et al., 2019), Combined however, the data emphasize the uncoupled nature of MRGPRX2- and FcεRI-triggered activation of skin MCs.
MRGPRX2 can incur β-arrestin‒initiated internalization on ligand binding, but internalization does not apply to all ligands alike (Roy et al., 2019a). In this study, we first show that in cutaneous MCs, C48/80 potently elicits MRGPRX2 internalization, whereas SP-triggered internalization is less rapid and complete, indicating less profound β-arrestin coupling. Codeine likewise prompted internalization, and the pattern more strongly resembled C48/80 than SP. That the receptor was truly internalized and not degraded or shed was confirmed by equal levels in nonstimulated and codeine-stimulated cells after permeabilization.
The combination of codeine with C48/80 did not increase either internalization or degranulation versus each ligand alone (Figure 5), demonstrating the saturable nature of MRGPRX2 by a single ligand. Downregulation in skin MCs was more complete and rapid than in RBL-MRGPRX2. We assume that the overall coupling to β-arrestin may be less efficient in the human and/or rat hybrid. Collectively, in cutaneous MCs, the physiological producers of MRGPRX2, receptor sequestration occurs rapidly on ligand binding and depends on β-arrestin-1 but not on β-arrestin-2. Conversely, β-arrestin-2 seems to be the more dominant entity in other types of MCs and in the mouse (Roy et al., 2019b).
In summary, our study demonstrates that MRGPRX2 serves as the codeine receptor on skin MCs. Codeine-MRGPRX2 versus allergen-FcεRI constitute two well-separated systems capable of eliciting comparable skin MC degranulation at the population level. Codeine acts as a balanced ligand at MRGPRX2, initializing degranulation by G-protein activation but also internalization through the β-arrestin route, making the receptor refractory to second stimulation. The phenomenon of cross desensitization across ligands may lay the basis for a clinical instrument to relieve receptor activity through controlled activation and/or desensitization not only in flareups of MRGPRX2-associated skin diseases but also as a proactive measure in circumstances under which misplaced MRGPRX2 activation needs to be strictly avoided such as in surgery.
MATERIALS AND METHODS
Skin samples
Donor foreskins, which otherwise would be disposed of, were obtained from circumcisions and provided to the study authors in an anonymous way, as described (Babina et al., 2018a, 2004). Written informed consent of the patients or their legal guardians was obtained. The study was approved by the Ethics Committee of the Charité–Universitätsmedizin Berlin (Berlin, Germany), and experiments were conducted according to the Declaration of Helsinki Principles.
MC purification and culture
MCs were purified from skin samples according to a routine method employed in our laboratory (Babina et al., 2019; Hazzan et al., 2019). Skin MCs were either used ex vivo or on culture as described (Babina et al., 2018b, 2016; Guhl et al., 2011). Further details are specified in the online Supplementary Materials.
RNA interference
RNA interference in MCs was performed according to a recently established protocol (Hazzan et al., 2017b) using the Accell siRNA technology (Dharmacon, Lafayette, CO). Briefly, MCs were washed with Accell siRNA medium (supplemented with nonessential amino acids and L-glutamine), plated at 1 × 106/ml, and treated with 1 µM MRGPRX2-targeting siRNA (E-005666–00–0050) or -nontargeting siRNA (D-001910–10–50) for 48 hours. For internalization experiments, ARRB1-targeting (E-011971–00–0050) and ARRB2-targeting (E-007292–00–0050) siRNAs were utilized. After incubation, cells were harvested for RT-qPCR, flow cytometry, histamine release, or MRGPRX2 internalization.
Histamine release assay
Histamine release assays were performed according to a method routinely employed in our laboratory (Babina et al., 2018a; Guhl et al., 2014). Further specifications can be found in the online Supplementary Materials.
β-Hexosaminidase release assay
β-Hexosaminidase assays were performed as described (Roy et al., 2019a; Wang et al., 2019). Further specifications are given in the online Supplementary Materials.
Calcium ion mobilization
Calcium ion mobilization in RBL-2H3 and RBL-2H3 cells stably expressing MRGPRX2 was performed as described (Chompunud Na Ayudhya et al., 2019). The method is specified in the online Supplementary Materials.
Tango assay
β-Arrestin activation assay was performed similarly as stated earlier (Roy et al., 2019a). The method is detailed in the online Supplementary Materials.
RT-qPCR
RT-qPCR for MRGPRX2 was performed exactly as described (Wang et al., 2019). Further details are provided in the online Supplementary Materials.
Flow cytometry
Flow cytometry on intact and permeabilized MCs was performed as described (Guhl et al., 2010; Wang et al., 2019). The online Supplementary Materials gives further details.
Immunofluorescence
MCs were fixed and stained as detailed in the online Supplementary Materials.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 8 (San Diego, CA). P < 0.05 was considered statistically significant. Details on the different statistical tests are specified in the online Supplementary Materials.
Data availability statement
No datasets were generated or analyzed during this study.
Supplementary Material
ACKNOWLEDGMENTS
We thank von Grüner and Heßler for excellent technical assistance. This work was funded by the Deutsche Forschungsgemeinschaft (BA-3769/4-1) to MB. ZW was funded by a scholarship from the Chinese Government Scholarship. This work was also supported by the National Institutes of Health grant R01 AI124182 to HA. The study also received funding from the European Center for Allergy Research Foundation to TZ.
Abbreviations:
- C48/80
compound 48/80
- MC
mast cell
- siRNA
small interfering RNA
- SP
substance P
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2020.09.017.
REFERENCES
- Akiyama T, Carstens E. Neural processing of itch. Neuroscience 2013;250: 697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H Emerging roles for MAS-related G protein-coupled receptor-X2 in host defense peptide, opioid, and neuropeptide-mediated inflammatory reactions. Adv Immunol 2017;136:123–62. [DOI] [PubMed] [Google Scholar]
- Alkanfari I, Gupta K, Jahan T, Ali H. Naturally occurring missense MRGPRX2 variants display loss of function phenotype for mast cell degranulation in response to substance P, hemokinin-1, human beta-defensin-3, and icatibant. J Immunol 2018;201:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babina M The pseudo-allergic/neurogenic route of mast cell activation via MRGPRX2: discovery, functional programs, regulation, relevance to disease, and relation with allergic stimulation. Itch 2020;5:e32. [Google Scholar]
- Babina M, Artuc M, Guhl S, Zuberbier T. Retinoic acid negatively impacts proliferation and MCTC specific attributes of human skin derived mast cells, but reinforces allergic stimulability. Int J Mol Sci 2017;18:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babina M, Guhl S, Artuc M, Zuberbier T. IL-4 and human skin mast cells revisited: reinforcement of a pro-allergic phenotype upon prolonged exposure. Arch Dermatol Res 2016;308:665–70. [DOI] [PubMed] [Google Scholar]
- Babina M, Guhl S, Artuc M, Zuberbier T. Allergic FcεRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018a;73:256–60. [DOI] [PubMed] [Google Scholar]
- Babina M, Guhl S, Stärke A, Kirchhof L, Zuberbier T, Henz BM. Comparative cytokine profile of human skin mast cells from two compartments–strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J Leukoc Biol 2004;75:244–52. [DOI] [PubMed] [Google Scholar]
- Babina M, Wang Z, Artuc M, Guhl S, Zuberbier T. MRGPRX2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture. Exp Dermatol 2018b;27:1298–303. [DOI] [PubMed] [Google Scholar]
- Babina M, Wang Z, Franke K, Guhl S, Artuc M, Zuberbier T. Yin-Yang of IL-33 in human skin mast cells: reduced degranulation, but augmented histamine synthesis through p38 activation. J Invest Dermatol 2019;139:1516–25.e3. [DOI] [PubMed] [Google Scholar]
- Barke KE, Hough LB. Opiates, mast cells and histamine release. Life Sci 1993;53:1391–9. [DOI] [PubMed] [Google Scholar]
- Benyon RC, Lowman MA, Church MK. Human skin mast cells: their dispersion, purification, and secretory characterization. J Immunol 1987;138: 861–7. [PubMed] [Google Scholar]
- Benyon RC, Robinson C, Church MK. Differential release of histamine and eicosanoids from human skin mast cells activated by IgE-dependent and non-immunological stimuli. Br J Pharmacol 1989;97:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg 2004;98:364–70. [DOI] [PubMed] [Google Scholar]
- Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol 1984;73:775–81. [DOI] [PubMed] [Google Scholar]
- Charney RL, Yan Y, Schootman M, Kennedy RM, Luhmann JD. Oxycodone versus codeine for triage pain in children with suspected forearm fracture: a randomized controlled trial. Pediatr Emerg Care 2008;24:595–600. [DOI] [PubMed] [Google Scholar]
- Chompunud Na Ayudhya C, Roy S, Alkanfari I, Ganguly A, Ali H. Identification of gain and loss of function missense variants in MRGPRX2’s transmembrane and intracellular domains for mast cell activation by substance P. Int J Mol Sci 2019;20:5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybendal T, Guttormsen AB, Elsayed S, Askeland B, Harboe T, Florvaag E. Screening for mast cell tryptase and serum IgE antibodies in 18 patients with anaphylactic shock during general anaesthesia. Acta Anaesthesiol Scand 2003;47:1211–8. [DOI] [PubMed] [Google Scholar]
- Edston E, van Hage-Hamsten M. Anaphylactoid shock–a common cause of death in heroin addicts? Allergy 1997;52:950–4. [DOI] [PubMed] [Google Scholar]
- Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol 2014;134:622–33.e9. [DOI] [PubMed] [Google Scholar]
- Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest 2016;126:3981–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold Y, Goldberg A, Sivan Y. Hyper-releasability of mast cells in family members of infants with sudden infant death syndrome and apparent life-threatening events. J Pediatr 2000;136:460–5. [DOI] [PubMed] [Google Scholar]
- Golembiewski JA. Allergic reactions to drugs: implications for perioperative care. J Perianesth Nurs 2002;17:393–8. [DOI] [PubMed] [Google Scholar]
- Gollhausen R, Kaidbey K, Schechter N. UV suppression of mast cell-mediated wealing in human skin. Photodermatol 1985;2:58–67. [PubMed] [Google Scholar]
- Grimes J, Desai S, Charter NW, Lodge J, Moita Santos R, Isidro-Llobet A, et al. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol Res Perspect 2019;7:e00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman N Histamine release from isolated rat mast cells: effect of morphine and related drugs and their interaction with compound 48/80. Agents Actions 1981;11:196–203. [DOI] [PubMed] [Google Scholar]
- Guhl S, Artuc M, Neou A, Babina M, Zuberbier T. Long-term cultured human skin mast cells are suitable for pharmacological studies of anti-allergic drugs due to high responsiveness to FcεRI cross-linking. Biosci Biotechnol Biochem 2011;75:382–4. [DOI] [PubMed] [Google Scholar]
- Guhl S, Babina M, Neou A, Zuberbier T, Artuc M. Mast cell lines HMC-1 and LAD2 in comparison with mature human skin mast cells–drastically reduced levels of tryptase and chymase in mast cell lines. Exp Dermatol 2010;19:845–7. [DOI] [PubMed] [Google Scholar]
- Guhl S, Neou A, Artuc M, Zuberbier T, Babina M. Skin mast cells develop non-synchronized changes in typical lineage characteristics upon culture. Exp Dermatol 2014;23:933–5. [DOI] [PubMed] [Google Scholar]
- Guhl S, Stefaniak R, Strathmann M, Babina M, Piazena H, Henz BM, et al. Bivalent effect of UV light on human skin mast cells-low-level mediator release at baseline but potent suppression upon mast cell triggering. J Invest Dermatol 2005;124:453–6. [DOI] [PubMed] [Google Scholar]
- Hazzan T, Eberle J, Worm M, Babina M. Apoptotic resistance of human skin mast cells is mediated by Mcl-1. Cell Death Discov 2017a;3:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzan T, Eberle J, Worm M, Babina M. Thymic stromal lymphopoietin interferes with the apoptosis of human skin mast cells by a dual strategy involving STAT5/Mcl-1 and JNK/Bcl-xL. Cells 2019;8:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzan T, Guhl S, Artuc M, Franke K, Worm M, Zuberbier T, et al. An efficient method for gene knock-down by RNA interference in human skin mast cells. Exp Dermatol 2017b;26:1136–9. [DOI] [PubMed] [Google Scholar]
- Herde L, Forster C, Strupf M, Handwerker HO. Itch induced by a novel method leads to limbic deactivations a functional MRI study. J Neurophysiol 2007;98:2347–56. [DOI] [PubMed] [Google Scholar]
- Hermens JM, Ebertz JM, Hanifin JM, Hirshman CA. Comparison of histamine release in human skin mast cells induced by morphine, fentanyl, and oxymorphone. Anesthesiology 1985;62:124–9. [DOI] [PubMed] [Google Scholar]
- Hou Y, Che D, Wei D, Wang C, Xie Y, Zhang K, et al. Phenothiazine anti-psychotics exhibit dual properties in pseudo-allergic reactions: activating MRGPRX2 and inhibiting the H1 receptor. Mol Immunol 2019;111: 118–27. [DOI] [PubMed] [Google Scholar]
- Humphreys F, Hunter JA. The effects of astemizole, cetirizine and loratadine on the time course of weal and flare reactions to histamine, codeine and antigen. Br J Dermatol 1991;125:364–7. [DOI] [PubMed] [Google Scholar]
- Joulia R, L’Faqihi FE, Valitutti S, Espinosa E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J Allergy Clin Immunol 2017;140:497–509.e10. [DOI] [PubMed] [Google Scholar]
- Jutel M, Skrbic D, Pichler WJ, Müller UR. Ultra rush bee venom immunotherapy does not reduce cutaneous weal responses to bee venom and codeine phosphate. Clin Exp Allergy 1995;25:1205–10. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Res 2020;9. F1000 Faculty Rev-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K, Giménez-Arnau A, Martinez-Escala E, Farré-Albadalejo M, Abajian M, Church MK, et al. Platelet-activating factor (PAF) induces wheal and flare skin reactions independent of mast cell degranulation. Allergy 2013;68:256–8. [DOI] [PubMed] [Google Scholar]
- Kupczyk M, Kupryś I, Górski P, Kuna P. The effect of montelukast (10mg daily) and loratadine (10mg daily) on wheal, flare and itching reactions in skin prick tests. Pulm Pharmacol Ther 2007;20:85–9. [DOI] [PubMed] [Google Scholar]
- Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 2017;13:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence ID, Warner JA, Cohan VL, Hubbard WC, Kagey-Sobotka A, Lichtenstein LM. Purification and characterization of human skin mast cells. Evidence for human mast cell heterogeneity. J Immunol 1987;139: 3062–9. [PubMed] [Google Scholar]
- Lin RY, Erlich ER, Don PC. Skin prick test responses to codeine, histamine, and ragweed utilizing the multitest device. Ann Allergy 1990;65: 222–6. [PubMed] [Google Scholar]
- Lowman MA, Rees PH, Benyon RC, Church MK. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung, adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol 1988;81:590–7. [PubMed] [Google Scholar]
- McBride P, Jacobs R, Bradley D, Kaliner M. Use of plasma histamine levels to monitor cutaneous mast cell degranulation. J Allergy Clin Immunol 1989;83:374–80. [DOI] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015;519:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014;123:e58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol 2014;134:2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namer B, Hilliges M, Orstavik K, Schmidt R, Weidner C, Torebjörk E, et al. Endothelin 1 activates and sensitizes human C-nociceptors. Pain 2008;137: 41–9. [DOI] [PubMed] [Google Scholar]
- Nancey S, Freymond N, Catelain A, Cousin F, Rozieres A, Nicolas JF. Effects of local corticosteroids on acute experimental urticaria. Eur J Dermatol 2004;14:323–6. [PubMed] [Google Scholar]
- Nasser SM, Ewan PW. Opiate-sensitivity: clinical characteristics and the role of skin prick testing. Clin Exp Allergy 2001;31:1014–20. [DOI] [PubMed] [Google Scholar]
- Navinés-Ferrer A, Serrano-Candelas E, Lafuente A, Muñoz-Cano R, Martín M, Gastaminza G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Rep 2018;8:11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PN, Skov PS, Poulsen LK, Schmelz M, Petersen LJ. Cetirizine inhibits skin reactions but not mediator release in immediate and developing late-phase allergic cutaneous reactions. a double-blind, placebo-controlled study. Clin Exp Allergy 2001;31:1378–84. [DOI] [PubMed] [Google Scholar]
- Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J Allergy Clin Immunol 2018;142:381–93. [DOI] [PubMed] [Google Scholar]
- Petersen LJ, Brasso K, Pryds M, Skov PS. Histamine release in intact human skin by monocyte chemoattractant factor-1, RANTES, macrophage inflammatory protein-1 alpha, stem cell factor, anti-IgE, and codeine as determined by an ex vivo skin microdialysis technique. J Allergy Clin Immunol 1996;98:790–6. [DOI] [PubMed] [Google Scholar]
- Prieto-Lastra L, Iglesias-Cadarso A, Reaño-Martos MM, Pérez-Pimiento A, Rodríguez-Cabreros MI, García-Cubero A. Pharmacological stimuli in asthma/urticaria. Allergol Immunopathol (Madr) 2006;34:224–7. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA. Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nat Commun 2015;6:7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees PH, Hillier K, Church MK. The secretory characteristics of mast cells isolated from the human large intestinal mucosa and muscle. Immunology 1988;65:437–42. [PMC free article] [PubMed] [Google Scholar]
- Roy S, Ganguly A, Haque M, Ali H. Angiogenic host defense peptide AG-30/5C and bradykinin B2 receptor antagonist icatibant are G protein biased agonists for MRGPRX2 in mast cells. J Immunol 2019a;202:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Gupta K, Ganguly A, Ali H. β-Arrestin2 expressed in mast cells regulates ciprofloxacin-induced pseudoallergy and IgE-mediated anaphylaxis. J Allergy Clin Immunol 2019b;144:603–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer K, Grize L, Schindler C, Surber C, Bircher AJ. Reaction pattern to histamine and codeine in a human intradermal skin test model. Clin Exp Allergy 2007;37:39–46. [DOI] [PubMed] [Google Scholar]
- Schmidt-Rondon E, Wang Z, Malkmus SA, Di Nardo A, Hildebrand K, Page L, et al. Effects of opioid and nonopioid analgesics on canine wheal formation and cultured human mast cell degranulation. Toxicol Appl Pharmacol 2018;338:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F, Lee TD, Bienenstock J, Befus AD. The influence of endorphins on peritoneal and mucosal mast cell secretion. J Allergy Clin Immunol 1984;74:499–504. [DOI] [PubMed] [Google Scholar]
- Sharif B, Ase AR, Ribeiro-da-Silva A, Séguéla P. Differential coding of itch and pain by a subpopulation of primary afferent neurons. Neuron 2020;106: 940–51.e4. [DOI] [PubMed] [Google Scholar]
- Sheen CH, Schleimer RP, Kulka M. Codeine induces human mast cell chemokine and cytokine production: involvement of G-protein activation. Allergy 2007;62:532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003;23:6176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick RA, Yancey KB, Lawley TJ. Inflammatory properties of human C5a and C5a des Arg/ in mast cell-depleted human skin. J Invest Dermatol 1989;93:417–22. [PubMed] [Google Scholar]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 2006;349:1322–8. [DOI] [PubMed] [Google Scholar]
- Tharp MD, Kagey-Sobotka A, Fox CC, Marone G, Lichtenstein LM, Sullivan TJ. Functional heterogeneity of human mast cells from different anatomic sites: in vitro responses to morphine sulfate. J Allergy Clin Immunol 1987;79:646–53. [DOI] [PubMed] [Google Scholar]
- Theunis J, Black D, Degouy A, Schmitt AM, Misery L. Comparison of perceived itch induced by skin prick-tests with histamine and codeine. Acta Derm Venereol 2008;88:455–7. [DOI] [PubMed] [Google Scholar]
- Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy 2003;33:1076–82. [DOI] [PubMed] [Google Scholar]
- von Muhlendahl KE, Scherf-Rahne B, Krienke EG, Baukloh G. Codeine intoxication in childhood. Lancet 1976;2:303–5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Guhl S, Franke K, Artuc M, Zuberbier T, Babina M. IL-33 and MRGPRX2-triggered activation of human skin mast cells-elimination of receptor expression on chronic exposure, but reinforced degranulation on acute priming. Cells 2019;8:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, et al. Acute effects of substance P and calcitonin gene-related peptide in human skin–a microdialysis study. J Invest Dermatol 2000;115:1015–20. [DOI] [PubMed] [Google Scholar]
- Xing Y, Chen J, Hilley H, Steele H, Yang J, Han L. Molecular signature of pruriceptive MrgprA3+ neurons. J Invest Dermatol 2020;140: 2041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y, Shimamura O, Kizu A, Nakagawa M, Ijichi H. IgE-mediated 14C-serotonin release from rat mast cells modulated by morphine and endorphins. Life Sci 1982;31:471–8. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu Y, Lin AA, Cavalli-Sforza LL, Zhao Z, Su B. Adaptive evolution of MRGX2, a human sensory neuron specific gene involved in nociception. Gene 2005;352:30–5. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Misery L, Proksch E, Metz M, Ständer S, Schmelz M. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol 2019;99:1201–9. [DOI] [PubMed] [Google Scholar]
- Zweiman B, Atkins PC, Moskovitz A, von Allmen C, Ciliberti M, Grossman S. Cellular inflammatory responses during immediate, developing, and established late-phase allergic cutaneous reactions: effects of cetirizine. J Allergy Clin Immunol 1997;100:341–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during this study.


