Abstract
Background:
Previous studies in the field of organ transplantation have shown a possible association between nighttime surgery and adverse outcomes. We aim to determine the impact of nighttime lung transplantation on postoperative outcomes, long-term survival, and overall cost.
Methods:
We performed a single center retrospective cohort analysis of adult lung transplant recipients who underwent transplantation between January 2006 and December 2017. Data were extracted from our institutional Lung Transplant Registry and Mid-America Transplant services database. Patients were classified into two strata (daytime 5AM to 6PM; nighttime 6PM to 5AM) based upon time of incision. Major postoperative adverse events, 5-year overall survival and 5-year BOS-free survival were examined after propensity score matching. Additionally, we compared overall cost of transplantation between nighttime and daytime groups.
Results:
Of the 740 patients included in this study, 549 (74.2%) patients underwent daytime transplantation (DT) and 191 (25.8%) patients underwent nighttime transplantation (NT). Propensity score matching yielded 187 matched pairs. NT was associated with a higher risk of having any major postoperative adverse event (adjusted OR=1.731, 95% CI 1.093-2.741, P=0.019), decreased 5-year overall survival (adjusted HR=1.798, 95% CI 1.079–2.995, P=0.024), as well as decreased 5-year BOS-free survival (adjusted HR=1.556, 95% CI 1.098-2.205, P=0.013) in doubly robust multivariable analyses following propensity score matching. Overall cost for NT and DT were similar.
Conclusions:
NT was associated with higher risk of major postoperative adverse events, decreased 5-year overall survival and decreased 5-year BOS-free survival. Our findings suggest potential benefits of delaying NT to daytime.
In the field of transplantation, nighttime surgery poses a unique challenge. Unlike most other emergent operations where the decision to operate expeditiously is dictated by the patient’s medical condition, the timing of organ transplant operations is predicated almost entirely upon availability of organ donors. In the complex environment of multiorgan donation that benefits several recipients, donor optimization, organ function preservation, the coordination of multiple procuring teams as well as the availability of operating rooms at the donor hospital outweighs any specific requests to avoid nighttime surgery. In an effort to maximize recipient benefit and minimize ischemic times, transplanting teams often perform long, technically challenging operations in very sick organ recipients either partially or entirely at nighttime.
Nighttime surgery can be complicated by several factors, including but not limited to surgeon fatigue, fewer hospital staff, reduced attending physician coverage in the ICU, and patient circadian rhythm1-5. Studies have shown that fatigue and sleep deprivation in residents and surgeons were associated with higher rate of perceived medical errors and decreased cognitive and psychomotor skills1,2. Increased mortality rate was also found to be associated with nighttime ICU admission even after adjusting for patient acuity3. Additionally, timing of operation was shown to be significantly associated with the extent of myocardial injury after cardiac surgery due to the difference in expression of circadian rhythm genes4. A similar study in lung transplantation (LT) reported that the highest incidence of primary graft dysfunction was observed when reperfusion took place between 4AM and 8AM5. Because reperfusion is a late event in lung transplantation, operations with reperfusion happening between 4AM to 8AM likely started at late night. With those potential drawbacks of nighttime surgery, providers are often faced with the question “Can the operation be safely delayed until the next morning?”
For transplantation, previous studies have shown a possible association between nighttime surgery and adverse postoperative outcomes6-8. Fechner et al. demonstrated a higher likelihood of operative complications and graft failure in kidney transplants performed at nighttime6. Similarly, in a study examining nighttime liver transplants, Lonze et al. reported a twofold greater risk of early death in comparison to daytime operations7. For LT specifically, a United Network for Organ Sharing database analysis found an association between 90-day mortality and NT while other outcomes were not significantly correlated with operation timing8. However, this study was limited as the authors used the time of organ reperfusion to categorize the time of transplant. Organ reperfusion is a late event in the operation and the majority of the operation including recipient lung dissection, explantation, and donor lung implantation has already occurred before organ reperfusion. Additionally, the impact of NT on long-term survival as well as resource utilization remains largely unknown. Therefore, we performed a single center retrospective cohort analysis to examine the clinical and financial implications of NT. Our primary goals were to determine whether NT was associated with an increased risk of postoperative adverse outcomes and decreased long-term survival. Additionally, we examined the cost of NT compared with DT.
Patients and Methods
Study Design
We performed a single center retrospective cohort analysis of adult LT recipients who underwent surgery between January 21, 2006 and December 31, 2017. Data were extracted from our institutional Lung Transplant Registry and Mid-America Transplant database. Re-transplants and heart-lung transplants were excluded. Patients were stratified into two subgroups based upon time of surgical incision. Daytime was defined as 5AM to 6PM; nighttime was defined as 6PM to 5AM. This study was approved by the Institutional Review Board.
We examined the relationship between NT and the overall incidence of having any major postoperative adverse event, including delayed chest closure, unexpected return to operating room, pneumonia, airway complications, bronchopleural fistula, pulmonary embolus, tracheostomy, reintubation, and grade 3 primary graft dysfunction (PGD) within 72 hours postoperatively. Airway complication was defined as the need for any intervention for bronchial anastomosis, including debridement, balloon dilation, stent or surgery. Adverse events evaluated were limited to those occurring within 30 days of transplantation or during the entire hospitalization if the patient was not discharged within 30 days after LT. In addition, we analyzed the association between nighttime operation and 5-year overall survival, 5-year bronchiolitis obliterans syndrome-free (BOS-free) survival, as well as the overall cost of hospitalization for transplantation. BOS-free survival is a composite outcome of death or BOS, whichever occurred first.
Statistical Analysis
Baseline characteristics were compared using Chi-squared test or Fisher exact test for categorical variables and Student’s t-test or Wilcoxon rank-sum test for continuous variables. Categorical variables are reported using count (%); continuous variables are reported using mean (± standard deviation) for those normally distributed and median (quartile 1 – quartile 3) for those highly skewed. P value < 0.05 was considered statistically significant in all analyses.
Propensity score matching using nearest neighbor algorithm without replacement and with a caliper width of 0.25 was performed to produce 1:1 matched pairs. Variables used for matching were selected based on both the imbalance between daytime and nighttime group as well as the potential relationship with posttransplant outcomes. Recipient age, lung allocation score (LAS), diagnosis grouping, body mass index (BMI), preoperative extracorporeal membrane oxygenation (ECMO)/mechanical ventilation (MV), organ ischemic time, singe lung transplant, prior cardiothoracic (CT) surgery, hypertension, DCD (donor after circulatory death), and operation after 2009 were used for propensity matching. Standardized mean differences (SMD) were used to examine post-match balance for all baseline characteristics. A multivariable logistic regression model with generalized estimating equation (GEE) accounting for the pairwise nature of matched pairs was used to examine the composite of major postoperative adverse events in the matched cohort9. Covariables for multivariable analysis were determined based on clinical experience and literature review and included nighttime operation, recipient age, gender, LAS, hypertension, restrictive lung disease, ischemic time, preoperative ECMO/MV, single lung transplant, donor age, and operation after 2009. Operation after 2009 was incorporated as a variable of interest because an innovative lung protective management plan that optimizes lung function in potential brain-dead donors was initiated at our specialized donor care facility in 200810. January 1, 2009 was chosen as the cutoff for this binary variable to allow for the time to full adoption of this new strategy. The definitions of all baseline characteristics were included in Supplemental Table 1. Kaplan-Meier curves and stratified multivariable Cox proportional hazards models were used to analyze the association between nighttime operation and 5-year overall and BOS-free survival in the matched cohort. Stratified log-rank test was used to examine the significance of survival difference in Kaplan-Meier curves. Covariables for constructing the Cox models were the same as those used in the logistic regression model.
Cost of hospitalization for transplantation was calculated as the sum of fixed cost and variable cost over the entire hospitalization starting from the transplantation event and was adjusted to 2019 US dollars. Patients who underwent LT before December 2009 were excluded from the cost analysis because cost data was not collected. Patients with missing cost data were also excluded. Wilcoxon rank-sum test was used to compare the median cost between two subgroups as the cost is right skewed. A generalized linear regression model with log-link function was constructed to analyze the association between NT and overall cost. Variables in the model were determined a priori based on clinical experience and literature review and included nighttime operation, recipient age, gender, LAS, diagnosis grouping, hypertension, CAD, preoperative ECMO/MV, prior CT surgery, single lung transplant, and distant donor. Distant donor was defined as donor from non-local organ procurement organizations (OPOs). Percent change of cost from the reference was estimated by the exponentiated coefficient and was shown with 95% CI.
Statistical analyses were performed using R version 3.5.3 (R Core Team, Vienna, Austria) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 740 patients included in this study, 549 (74.2%) underwent DT and 191 (25.8%) underwent NT. The overall median follow-up was 43 months (Q1-Q3, 21-80 months; nighttime 52 months [Q1-Q3, 19-90] vs. daytime 43 months [Q1-Q3, 23-76]). Median follow-up was 104 months (Q1-Q3, 22-134 months) for patients transplanted before 2009 and 40 months (Q1-Q3, 21-72 months) for patients transplanted after 2009. Compared with DT, NT patients were more likely to be on preoperative ECMO/MV (P=0.017), had longer organ ischemic time (P<0.001), and more often received distant donor organs (P<0.001; Table 1). Duration of operation was slightly longer in NT (nighttime 324 minutes [Q1-Q3, 284-389] vs. daytime 314 minutes [Q1-Q3, 273-366], P=0.033).
Table 1.
Baseline characteristics before and after propensity score matching.
| Pre-Match | Day (N=549) | Night (N=191) | SMD | P value |
|---|---|---|---|---|
| Recipient Factors | ||||
| Age (years) | 53.0 (±14.3) | 53.2 (±14.0) | 0.017 | 0.839 |
| Male | 322 (58.7%) | 105 (55.0%) | 0.074 | 0.375 |
| White | 503 (91.6%) | 175 (91.6%) | 0.000 | 0.999 |
| LAS | 39.1 (34.4 – 49.0) | 42.1 (34.6 – 63.3) | 0.224 | 0.105 |
| BMI | 23.8 (20.1 – 27.3) | 23.7 (20.4 – 27.6) | 0.034 | 0.904 |
| O2 supplement at rest (L/min) | 3 (2 - 6) | 4 (2 - 6) | 0.066 | 0.236 |
| Diabetes | 123 (22.4%) | 50 (26.2%) | 0.088 | 0.289 |
| Hypertension | 141 (25.7%) | 56 (29.3%) | 0.082 | 0.327 |
| Preoperative ECMO/MV | 34 (6.2%) | 22 (11.5%) | 0.188 | 0.017 |
| CAD | 79 (14.4%) | 29 (15.2%) | 0.022 | 0.789 |
| CVD | 5 (0.9%) | 2 (1.0%) | 0.014 | 0.867 |
| Diagnosis | 0.126 | 0.545 | ||
| Obstructive lung disease | 160 (29.1%) | 63 (33.0%) | ||
| Pulmonary vascular disease | 14 (2.6%) | 3 (1.6%) | ||
| Cystic fibrosis | 98 (17.9%) | 28 (14.7%) | ||
| Restrictive lung disease | 277 (50.5%) | 97 (50.8%) | ||
| Prior CT surgery | 155 (28.2%) | 42 (22.0%) | 0.144 | 0.093 |
| Donor Factors | ||||
| Age (years) | 35.3 (±14.7) | 35.7 (±14.8) | 0.031 | 0.709 |
| Male | 335 (61.0%) | 118 (61.8%) | 0.016 | 0.853 |
| Extended-criteria donor | 77 (14.0%) | 23 (12.0%) | 0.059 | 0.490 |
| DCD | 9 (1.6%) | 2 (1.0%) | 0.051 | 0.560 |
| Chest trauma | 53 (9.7%) | 15 (7.9%) | 0.064 | 0.477 |
| X-ray abnormalities | 304 (55.4%) | 107 (56.0%) | 0.013 | 0.877 |
| Surgical Factors | ||||
| Single lung transplant | 18 (3.3%) | 9 (4.7%) | 0.073 | 0.363 |
| Ischemic time (minutes) | 257 (±66.3) | 301 (±64.8) | 0.660 | <0.001 |
| Distant donor | 207 (37.7%) | 132 (69.1%) | 0.663 | <0.001 |
| Operation after 2009 | 458 (83.4%) | 149 (78.0%) | 0.138 | 0.093 |
| Post-Match | Day (N=187) | Night (N=187) | SMD | P value |
| Recipient Factors | ||||
| Age (years) | 51.9 (±13.9) | 53.0 (±14.1) | 0.081 | 0.434 |
| Male | 110 (58.8%) | 104 (55.6%) | 0.065 | 0.531 |
| White | 168 (89.8%) | 171 (91.4%) | 0.055 | 0.594 |
| LAS | 41.9 (34.2 – 60.5) | 42.1 (34.6 – 55.6) | 0.040 | 0.651 |
| BMI | 23.5 (19.7 – 27.1) | 23.6 (20.3 – 27.6) | 0.015 | 0.616 |
| O2 supplement rest (L/min) | 4 (2 - 6) | 4 (2 - 6) | 0.133 | 0.574 |
| Diabetes | 43 (23.0%) | 49 (26.2%) | 0.075 | 0.471 |
| Hypertension | 56 (29.9%) | 54 (28.9%) | 0.023 | 0.820 |
| Preoperative ECMO/MV | 16 (8.6%) | 19 (10.2%) | 0.055 | 0.594 |
| CAD | 28 (15.0%) | 28 (15.0%) | 0.000 | 1.000 |
| CVD | 2 (1.1%) | 1 (0.5%) | 0.060 | 1.000 |
| Diagnosis | 0.093 | 0.805 | ||
| Obstructive lung disease | 67 (35.8%) | 61 (32.6%) | ||
| Pulmonary vascular disease | 2 (1.1%) | 3 (1.6%) | ||
| Cystic fibrosis | 31 (16.6%) | 28 (15.0%) | ||
| Restrictive lung disease | 87 (46.5%) | 95 (50.8%) | ||
| Prior CT surgery | 38 (20.3%) | 41 (21.9%) | 0.039 | 0.704 |
| Donor Factors | ||||
| Age (years) | 35.4 (±15.7) | 35.9 (±14.8) | 0.034 | 0.745 |
| Male | 116 (62.0%) | 114 (61.0%) | 0.022 | 0.832 |
| Extended-criteria donor | 25 (13.4%) | 21 (11.2%) | 0.065 | 0.529 |
| DCD | 3 (1.6%) | 2 (1.1%) | 0.047 | 1.000 |
| Chest trauma | 16 (8.6%) | 15 (8.0%) | 0.019 | 0.851 |
| X-ray abnormalities | 96 (51.3%) | 106 (56.7%) | 0.107 | 0.299 |
| Surgical Factors | ||||
| Single lung transplant | 11 (5.9%) | 9 (4.8%) | 0.048 | 0.646 |
| Ischemic time (minutes) | 294 (±66.3) | 299 (±64.1) | 0.073 | 0.478 |
| Distant donor | 115 (61.5%) | 129 (69.0%) | 0.158 | 0.128 |
| Operation after 2009 | 142 (75.9%) | 146 (78.1%) | 0.051 | 0.623 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CT, cardiothoracic; CVD, cerebrovascular disease; DCD, donor after circulatory death; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score; MV, mechanical ventilation; SMD, standardized mean difference.
SMD > 0.2 was considered significant imbalance.
Transplant cost
A total of 546 patients were included in the cost analysis (daytime 412 [75.5%] vs. nighttime 134 [24.5%]). In unadjusted analysis, the median cost for NT was higher than that of DT (nighttime $211,493 [Q1-Q3, $177,809-$294,416] vs. daytime $197,543 [Q1-Q3, $166,986-$270,815]; P=0.031). However, multivariable analysis revealed an insignificant difference between the two subgroups (nighttime −4.5%; 95% CI −12.7%-4.7%, P=0.324; Table 2). Each unit of LAS multiplicatively increased overall cost by 0.8% (95% CI 0.5%-1.1%, P<0.001); preoperative ECMO/MV increased overall cost by 19.9% (95% CI 4.0%-38.9%, P=0.013); diagnosis of pulmonary vascular disease increased overall cost by 49.5% (95% CI 13.0%-102.1%, P=0.007).
Table 2.
Generalized linear regression model for overall cost of hospitalization.
| Percent change (95% CI) | P value | |
|---|---|---|
| Nighttime | −4.5% (−12.7%–4.7%) | 0.324 |
| Recipient age (per year) | −0.2% (−0.6%–0.2%) | 0.246 |
| Male recipient | −6.9% (−14.0%-0.7%) | 0.076 |
| LAS (per unit) | 0.8% (0.5%–1.1%) | <0.001 |
| Diagnosis | ||
| Obstructive lung disease | Reference | |
| Pulmonary vascular disease | 49.5% (13.0%–102.1%) | 0.007 |
| Cystic fibrosis | −3.8% (−18.0%–12.9%) | 0.633 |
| Restrictive lung disease | 3.0% (−6.8%–13.8%) | 0.566 |
| Hypertension | 8.3% (−0.5%–18.1%) | 0.068 |
| Preoperative ECMO/MV | 19.9% (4.0%–38.9%) | 0.013 |
| Prior CT surgery | 5.7% (−2.9%–15.1%) | 0.201 |
| CAD | 9.3% (−2.0%–22.2%) | 0.113 |
| Single lung transplant | 6.0% (−14.8%-34.0%) | 0.614 |
| Distant donor | 8.6% (0.0%–17.9%) | 0.051 |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; CT, cardiothoracic surgery; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score; MV, mechanical ventilation.
Univariable analysis in the overall cohort
In the overall cohort, incidence of having any major postoperative adverse event was higher in NT (nighttime 70.2% vs. daytime 61.2%, P=0.027; Supplemental Table 2). More specifically, delayed chest closure (nighttime 35.6% vs. daytime 25.0%, P=0.005) and pneumonia (nighttime 22.5% vs. daytime 14.6%, P=0.011) occurred more frequently in the NT group. However, NT had a lower incidence of airway complications (nighttime 5.2% vs. daytime 10.7%, P=0.024). Kaplan-Meier analysis revealed both decreased 5-year overall survival (Log-rank P=0.027; Supplemental Figure 1) and decreased 5-year BOS-free survival (Log-rank P=0.030; Supplemental Figure 2) in NT.
Propensity score matched analysis
A total of 187 matched pairs were obtained using 1:1 propensity score matching, with all baseline characteristics well balanced (Table 1, Figure 1). Multivariable analysis showed a significant association between NT and risk of having any major postoperative adverse event (adjusted OR=1.731, 95% CI 1.093-2.741, P=0.019; Table 3). Longer ischemic time (adjusted OR=1.006, 95% CI 1.002-1.010, P=0.004) and preoperative ECMO/MV (adjusted OR=6.214, 95% CI 1.928-20.029, P=0.002) were also associated with higher risk of having any postoperative adverse event.
Figure 1.
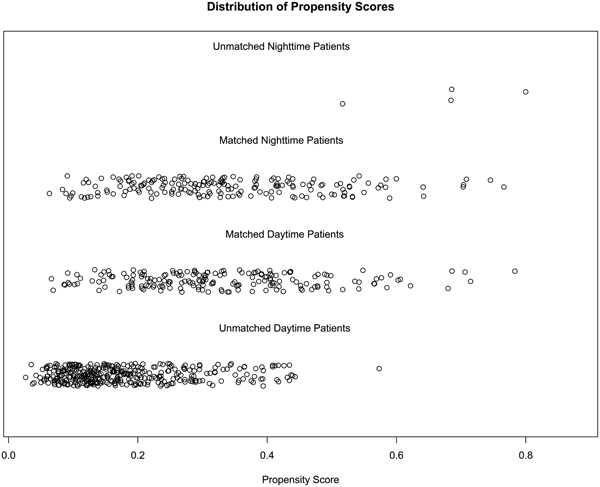
Propensity score distribution before and after match.
Table 3.
Multivariable logistic regression with GEE for having any major postoperative adverse event.
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Nighttime | 1.731 (1.093–2.741) | 0.019 |
| Recipient age (per year) | 1.002 (0.983-1.021) | 0.853 |
| Male recipient | 0.546 (0.342-0.870) | 0.011 |
| LAS (per unit) | 1.006 (0.992–1.020) | 0.424 |
| Restrictive lung disease | 1.679 (0.967-2.916) | 0.066 |
| Ischemic time (per minute) | 1.006 (1.002–1.010) | 0.004 |
| Hypertension | 1.444 (0.836–2.492) | 0.187 |
| Single lung transplant | 1.338 (0.444-4.034) | 0.605 |
| Preoperative ECMO/MV | 6.214 (1.928–20.029) | 0.002 |
| Donor age | 1.002 (0.983-1.021) | 0.853 |
| Operation after 2009 | 1.689 (0.979-2.879) | 0.060 |
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score; MV, mechanical ventilation.
Kaplan-Meier analysis using matched pairs showed decreased 5-year overall survival in NT (stratified log-rank P=0.033; Figure 2). Furthermore, this association remained significant in a stratified multivariable Cox model (adjusted HR=1.798, 95% CI 1.079–2.995, P=0.024; Table 4). Operation after 2009 was associated with better 5-year survival (adjusted HR=0.378, 95% CI 0.159–0.897, P=0.027). 5-year BOS-free survival was significantly lower in NT in Kaplan-Meier analysis (stratified log-rank P=0.027; Figure 3). Stratified multivariable Cox modeling showed similar negative effects of NT on 5-year BOS-free survival (adjusted HR=1.556, 95% CI 1.098-2.205, P=0.013; Table 5).
Figure 2.
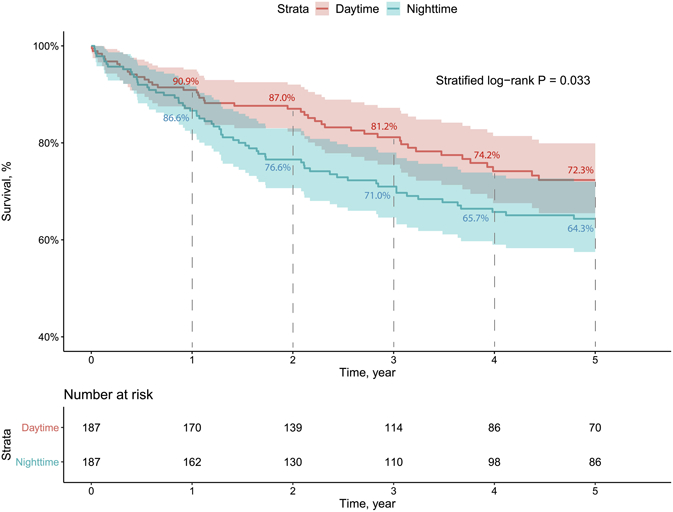
Kaplan-Meier curve for 5-year overall survival using matched pairs.
Table 4.
Stratified Cox proportional hazards model for 5-year overall survival.
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Nighttime | 1.798 (1.079-2.995) | 0.024 |
| Recipient age (per year) | 1.020 (0.986-1.055) | 0.247 |
| Male recipient | 0.846 (0.374-1.911) | 0.688 |
| LAS (per unit) | 1.002 (0.979–1.026) | 0.884 |
| Restrictive lung disease | 0.571 (0.242-1.349) | 0.201 |
| Ischemic time (per minute) | 1.000 (0.986–1.014) | 0.964 |
| Hypertension | 0.840 (0.341-2.070) | 0.705 |
| Single lung transplant | 3.319 (0.460-23.965) | 0.234 |
| Preoperative ECMO/MV | 1.384 (0.284-6.733) | 0.687 |
| Donor age | 1.020 (0.986-1.055) | 0.247 |
| Operation after 2009 | 0.378 (0.159-0.897) | 0.027 |
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score; MV, mechanical ventilation.
Figure 3.
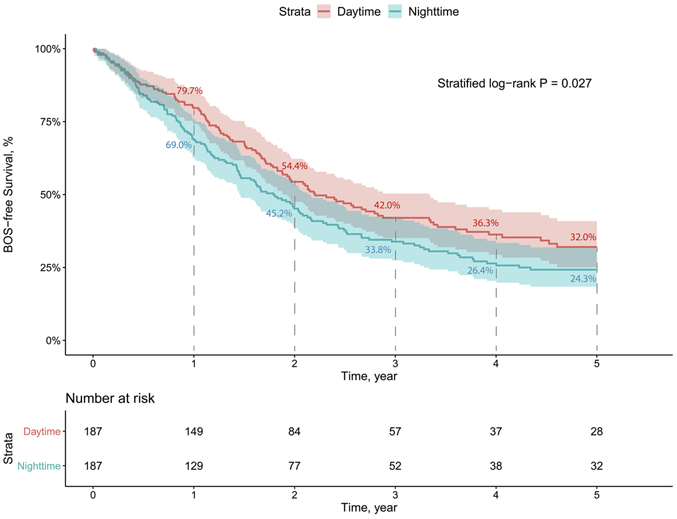
Kaplan-Meier curve for 5-year BOS-free survival using matched pairs.
Table 5.
Stratified Cox proportional hazards model for 5-year BOS-free survival.
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Nighttime | 1.556 (1.098-2.205) | 0.013 |
| Recipient age (per year) | 1.001 (0.980-1.023) | 0.900 |
| Male recipient | 0.950 (0.534-1.689) | 0.861 |
| LAS (per unit) | 1.002 (0.987–1.017) | 0.812 |
| Restrictive lung disease | 0.677 (0.381-1.205) | 0.185 |
| Ischemic time (per minute) | 0.998 (0.990–1.007) | 0.690 |
| Hypertension | 1.014 (0.554-1.858) | 0.964 |
| Single lung transplant | 1.271 (0.300-5.381) | 0.745 |
| Preoperative ECMO/MV | 0.459 (0.151-1.391) | 0.169 |
| Donor age | 1.001 (0.980-1.023) | 0.900 |
| Operation after 2009 | 0.645 (0.368-1.131) | 0.126 |
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score; MV, mechanical ventilation.
Comment
Our study demonstrated an association between NT and higher risk of major postoperative adverse events, decreased 5-year overall and BOS-free survival. On the other hand, consistent with prior observations by George et al., short-term mortality was similar between nighttime and daytime subgroups (Figure 2, Supplemental Figure 1)8.
In the overall cohort of this study, the incidence of major postoperative adverse events was approximately 10% higher after NT. More specifically, risk of delayed chest closure and pneumonia was significantly higher in NT in univariable comparison. Delayed chest closure in LT has been associated with surgical site infection, greater need for postoperative transfusion, worse pulmonary function, and decreased long-term survival11-13. Additionally, multiple studies have shown that postoperative complications after major surgery are associated with decreased long-term survival even when early postoperative deaths are excluded14,15. We also noted decreased 5-year BOS-free survival after NT. The higher risk of BOS in NT potentially explains the lower 5-year survival rate in NT; however, with our current knowledge it is unclear why NT would predispose to BOS. It is possible that the disruption of recipients’ circadian rhythm played a role in the immunologic response after transplantation 5, or that perioperative adverse events including pneumonia triggered the immune cascade. Further investigation is warranted to understand the pathophysiology behind this observation.
Cost of hospitalization for transplantation was not significantly different between the two subgroups after recipient and donor covariables were adjusted, though the unadjusted analysis revealed a higher median cost for NT. The unadjusted cost difference was likely due to the higher prevalence of preoperative ECMO/MV in NT, and more distant donors being utilized for NT. However, the cost we evaluated did not include a detailed breakdown of personnel-related cost, which is an important component when assessing resource utilization. NT likely incurs additional personnel-related cost as nighttime providers are often paid at a higher rate. Furthermore, there are unmeasured personal and social costs to medical providers who work overnight. From the perspective of physician wellness, nighttime surgery has been associated with burnout and decreased career satisfaction16. Reducing nighttime surgery will undoubtedly improve transplant surgeons’ quality of life.
Organ transplantation is entirely dependent upon the availability of organ donors and the coordination of timing of the donor procurement operation. Our findings indicate improved patient outcomes with DT and prior studies also indicate a better quality of care in procurement operations during daytime17. Any changes to the status quo will require balancing optimal resource utilization and the coordination of a complex event involving multiple teams. Our transplant center has had the privilege of collaborating with Mid-America Transplant, our local OPO, to address this particular aspect of donor and recipient care. Our collective team has actively pursued policies to schedule donor procurements early in the morning and thus allow recipient operations to be conducted in daytime hours. With this effort, we have noted that over 30% of transplants were performed at nighttime at the beginning of the analyzed cohort, while this proportion has gradually decreased to approximately 15% in our most recent experience, including a time period beyond the conclusion of this study. While we understand that such joint efforts between transplant centers and OPOs pose practical challenges, our experience shows that these hurdles can be overcome and may improve patient outcomes as well as provider experience.
It is important to note some limitations in this study. First, stratifying patients into daytime group and nighttime group based upon skin incision time inevitably forced some borderline cases into one of the two categories. In this study, we aimed to define nighttime as the period that encompassed not only the start of operation, but also the majority of operation. Because factors such as patient circadian rhythm may play a role in transplant outcome, using 5AM as the cutoff would better confine the duration of operation to nighttime based on literature in the field5. Second, the nature of question we posed only allows for a retrospective observational study. Finally, as a single center study, our results might not be representative across the nation. In our analysis, we attempted to adjust for the confounding incurred by the gradual trend towards greater proportion of daytime transplantation, which coincided with the establishment of new donor care protocols at our local OPO10, by dichotomizing the cohort into pre and post 2009 eras. It is possible that our single center cohort may be unable to adjust for all confounding factors contributing towards outcomes after a complex care pathway. On the other hand, our study also has important strengths. We used a combined longitudinal database that has been rigorously maintained by our institution and our local OPO. The database includes a comprehensive record of postoperative outcomes, which allowed us to analyze the association between nighttime operation and specific postoperative adverse events.
In conclusion, our findings suggest potential benefits of delaying NT to the following morning and underscore the need of further evaluation to find institution-specific practices that maximize patient safety.
Supplementary Material
Abbreviations.
- BMI
Body mass index
- BOS
Bronchiolitis obliterans syndrome
- CAD
Coronary artery disease
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- CT
Cardiothoracic
- CVD
Cerebrovascular disease
- DCD
Donor after circulatory death
- DT
Daytime transplantation
- ECMO
Extracorporeal membrane oxygenation
- GEE
Generalized estimating equation
- HR
Hazard ratio
- LAS
Lung allocation score
- LT
Lung transplantation
- MV
Mechanical ventilation
- NT
Nighttime transplantation
- OR
Odds ratio
- PGD
Primary graft dysfunction
- SMD
Standardized mean difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Southern Thoracic Surgical Association (STSA) 66th Annual Meeting oral presentation, Marco Island, FL, November 6-9, 2019.
References
- 1.West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of Resident Fatigue and Distress with Perceived Medical Errors. JAMA. 2009;302(12): 1294–1300. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes J, Kahol K, Smith M, Leyba MJ, Ferrara JJ. Jack Barney Award: the Effect of Fatigue on Cognitive and Psychomotor Skills of Trauma Residents and Attending Surgeons. Am J Surg. 2008;196(6):813–9. [DOI] [PubMed] [Google Scholar]
- 3.Laupland KB, Shahpori R, Kirkpatrick AW, Stelfox HT. Hospital Mortality Among Adults Admitted to and Discharged from Intensive Care on Weekends and Evenings. J Crit Care. 2008;23(3):317–24. [DOI] [PubMed] [Google Scholar]
- 4.Montaigne D, Marechal X, Modine T, et al. Daytime Variation of Perioperative Myocardial Injury in Cardiac Surgery and Its Prevention by Rev-Erbα Antagonism: A Single-Centre Propensity-Matched Cohort Study and a Randomized Study. Lancet. 2018;391(10115):59–69. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham PS, Maidstone R, Durrington HJ, et al. Incidence of Primary Graft Dysfunction after Lung Transplantation is Altered by Timing of Allograft Implantation. Thorax. 2019;74(4):413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fechner G, Pezold C, Hauser S, et al. Kidney’s Nightshift, Kidney’s Nightmare? Comparison of Daylight and Nighttime Kidney Transplantation: Impact on Complication and Graft Survival. Transplantation Proceedings. 2008;40(5): 1341–1344. [DOI] [PubMed] [Google Scholar]
- 7.Lonze BE, Parsikia A, Feyssa EL, et al. Operative Start Time and Complications After Liver Transplant. American Journal of Transplant. 2010; 10(8): 1842–1849. [DOI] [PubMed] [Google Scholar]
- 8.George TJ, Arnaoutakis GJ, Merlo CA, et al. Association of Operative Time of Day with Outcomes After Thoracic Organ Transplant. JAMA. 2011;305(21):2193–2199. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thoracic Cardiovasc Surg. 2007;134(5): 1128–35. [DOI] [PubMed] [Google Scholar]
- 10.Chang SH, Kreisel D, Marklin GF, et al. Lung Focused Resuscitation at a Specialized Donor Care Facility Improves Lung Procurement Rates. Ann Thorac Surg. 2018; 105(5): 1531–1536. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar PR, Bemiss BC, Witt C, et al. Impact of Delayed Chest Closure on Surgical Site Infection After Lung Transplantation. Ann Thorac Surg. 2017;104(4):1208–1214. [DOI] [PubMed] [Google Scholar]
- 12.Rafiroiu S, Hassouna H, Ahmad U, et al. Consequences of Delayed Chest Closure During Lung Transplantation. Ann Thorac Surg. 2019; [DOI] [PubMed] [Google Scholar]
- 13.Shigemura N, Orhan Y, Bhama JK, et al. Delayed Chest Closure After Lung Transplantation: Techniques, Outcomes, and Strategies. J Heart Lung Transplant. 2014;33(7):741–8. [DOI] [PubMed] [Google Scholar]
- 14.Nathan H, Yin H, Wong SL. Postoperative Complications and Long-Term Survival After Complex Cancer Resection. Ann Surg Oncol. 2017;24(3):638–644. [DOI] [PubMed] [Google Scholar]
- 15.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of Long-Term Survival After Major Surgery and the Adverse Effect of Postoperative Complications. Ann Surg. 2005;242(3):326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon Distress as Calibrated by Hours Worked and Nights on Call. J Am Coll Surg. 2010;211(5):609–19. [DOI] [PubMed] [Google Scholar]
- 17.de Boer JD, Van der Bogt KEA, Putter H, et al. Surgical Quality in Organ Procurement During Day and Night: an Analysis of Quality Forms. BMJ Open. 2018;8(11):e022182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


