Abstract
Elevated plasma triglyceride levels are associated with metabolic disease. Angiopoietin-like protein 4 (ANGPTL4) regulates plasma triglyceride levels by inhibiting lipoprotein lipase (LPL). Our aim was to investigate the role of adipocyte-specific deficiency of ANGPTL4 in mice during high fat diet feeding. Adipocyte-specific ANGPTL4 deficient mice were fed a high fat diet (60% kCal from fat) for either 12 weeks or 6 months. We performed plasma metabolic measurements, triglyceride clearance and uptake assays, LPL activity assays, and assessed glucose homeostasis. Mice lacking adipocyte ANGPTL4 recapitulated the triglyceride phenotypes of whole-body ANGPTL4 deficiency, including increased adipose LPL activity, lower plasma triglyceride levels, and increased uptake of triglycerides into adipose tissue. When fed a high fat diet (HFD), these mice continued to display enhanced adipose LPL activity and initially had improved glucose and insulin sensitivity. However, after 6 months on HFD, the improvements in glucose homeostasis were largely lost. Moreover, despite higher adipose LPL activity levels, mice lacking adipocyte ANGPTL4 no longer had increased triglyceride uptake into adipose compared to littermate controls after chronic high-fat feeding. These observations suggest that after chronic high-fat feeding LPL is no longer rate-limiting for triglyceride delivery to adipocytes. We conclude that while adipocyte-derived ANGPTL4 is an important regulator of plasma triglyceride levels and triglyceride partitioning under normal diet conditions, its role is diminished after chronic high-fat feeding.
Subject terms: Fat metabolism, Metabolic diseases
Introduction
Elevated plasma triglyceride levels have been implicated in the pathology of a variety of cardiovascular and metabolic diseases. Circulating triglycerides are hydrolyzed by lipoprotein lipase (LPL), releasing fatty acids that are taken up and utilized by tissues. Angiopoietin-like protein 4 (ANGPTL4), a secreted factor induced by fasting and expressed in several metabolically active tissues, inhibits LPL activity and thereby regulates plasma triglyceride levels1–5. Genetic loss of ANGPTL4 leads to decreased fasting plasma triglyceride levels in both humans and mice, and humans deficient in ANGPTL4 appear to be protected from cardiovascular disease6–8. Moreover, a recent study found that human carriers of the common ANGPTL4 inactivating mutant E40K were protected against obesity-associated dyslipidemia9. However, whether these protections are conferred by loss of ANGPTL4 in a specific tissue or require systemic loss of ANGPTL4 remains unclear.
ANGPTL4 is expressed highly in adipose depots3. In the last twenty years the work of many labs has led to development of a model where, during fasting, adipose-derived ANGPTL4 is rapidly induced and acts locally to inhibit adipose LPL activity and triglyceride uptake, diverting triglycerides to other tissues3,5,10–12. Recent studies using Angptl4–/– or adipose-specific Angptl4 knockout mice strongly support this model by showing that fasted mice lacking ANGPTL4 have increased uptake of triglycerides specifically in adipose tissue13,14. The role of ANGPTL4 in mediating or alleviating metabolic disturbances induced by high-fat feeding has not been fully studied. Such studies have been hindered by the fact that Angptl4–/– mice fed a high fat diet (HFD) develop a lethal intestinal injury and lymphatic nodule inflammation15.
In this study, we sought to define the adipose-specific actions of ANGPTL4 more clearly. To this end, we generated adipocyte-specific Angptl4 knockout mice and studied their triglyceride and metabolic phenotypes when fed normal chow and high-fat diets. The effects of adipocyte-specific ANGPTL4-deficiency on triglyceride and glucose homeostasis were determined after 12 weeks of high-fat feeding, as well as after 6 months of high-fat feeding to model a chronic state of obesity.
Results
Effects of adipocyte-specific ANGPTL4 deficiency on plasma TG levels and TG uptake into tissues
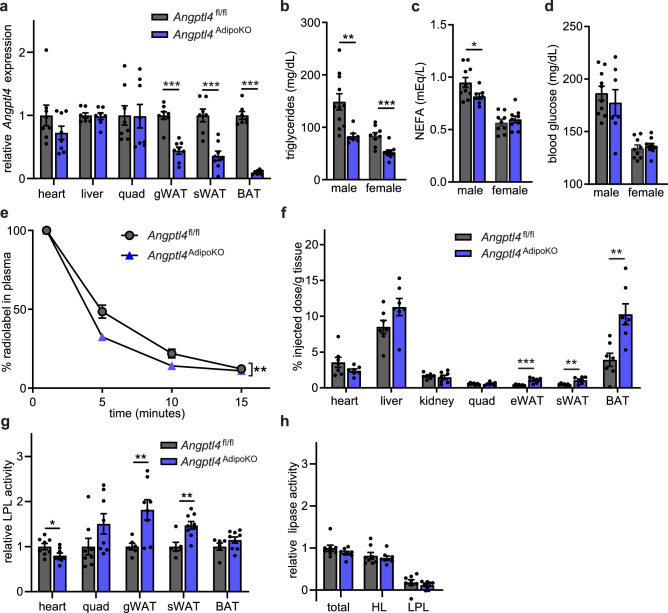
To examine the adipose-specific role of ANGPTL4, we generated adipocyte-specific Angptl4 knockout mice. To do so, we first generated Angptl4-floxed mice (Angptl4fl/fl) by utilizing CRISPR/Cas9 to insert LoxP sites in the introns between exons 1 and 2 and between exons 3 and 4 (Supplemental Fig. 1a). Recombination between LoxP sites generates a frame-shift resulting in a truncated (145 aa) protein in which the final 35 amino acids do not match the native sequence. Adipocyte-specific (Angptl4AdipoKO) mice were generated by crossing Angptl4fl/fl mice with mice expressing the adiponectin-Cre. To assess the impact of Cre-mediated recombination on Angptl4 expression, we performed qPCR with 7 different primer sets on brown adipose tissue (BAT) from Angptl4AdipoKO mice (Supplemental Fig. 1b). Compared to Cre negative controls, significantly reduced expression was observed with all 7 primer sets (Supplemental Fig. 1c). qPCR signal was further reduced when using primer sets targeting exons 2 and 3, the exons removed by recombination (Supplemental Fig. 1c). Together these data implied that Cre-mediated recombination was successful and that the recombined gene resulted in greatly reduced RNA expression of Angptl4.
Ideally, levels of ANGPTL4 protein would be assessed to ensure knockout of ANGPTL4, however no currently available antibodies are specific for mouse ANGPTL4. To assess if our recombined allele would still make protein, we generated a plasmid construct mimicking the predicted protein product of our Angptl4 flox allele after Cre-mediated recombination. We also generated a plasmid construct mimicking the post-recombination protein product of the Angptl4 flox allele generated by the KOMP mouse repository and used in other studies of the tissue-specific actions of ANGPTL413,16,17. 293 T cells were transfected with these constructs or a construct expressing full-length wildtype mouse ANGPTL4 and both cell lysate and the media were subjected to Western blot analysis. As expected, in cells transfected with the full-length construct, we observed ANGPTL4 protein in both the cell lysate and secreted in the media (Supplemental Fig. 1d). We observed no protein expression in either the lysate or the media from the construct representing our flox allele, suggesting that after Cre recombination our flox allele does not produce protein. Interestingly, we did observe substantial protein expression in the lysate from the construct representing the KOMP allele, though very little of this protein appeared to be secreted (Supplemental Fig. 1d). When the conditioned media from these constructs were tested for their ability to inhibit LPL, neither the media from our allele, nor the media containing the small amount of secreted protein from the KOMP flox allele were able to inhibit LPL activity (Supplemental Fig. 1e).
We examined Angptl4 expression in metabolically active tissues in our adipocyte-specific Angptl4–deficient mice. As expected, expression of Angptl4 in gonadal, subcutaneous, and brown adipose tissues (gWAT, sWAT, BAT) was significantly reduced in Angptl4AdipoKO mice, while being preserved in other tissues (Fig. 1a). Fasting plasma triglyceride levels were significantly lower in 8-week–old male and female Angptl4AdipoKO mice compared to Angptl4fl/fl mice (Fig. 1b). These results suggest that the lower plasma TG levels observed in Angptl4 whole-body knockout mice2,14,18 is due to the loss of adipocyte-derived ANGPTL4. We observed a decrease in fasting plasma free fatty acid levels in male, but not female Angptl4AdipoKO mice compared to Angptl4fl/fl mice (Fig. 1c). There were no genotype-specific differences in fasting blood glucose levels between groups (Fig. 1d).
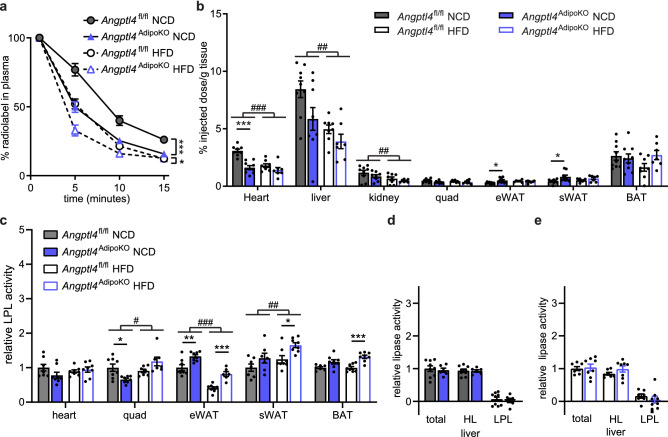
Figure 1.
Characterization of mice with adipocyte-specific deletion of Angptl4. (a) mRNA expression of Angptl4 in liver, heart, quadriceps muscle (quad), gonadal white adipose tissue (gWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissues (BAT) from 8–12 week old female Angptl4fl/fl (n = 8) and Angptl4AdipoKO (n = 8) mice following a 6 h fast (mean ± SEM). (b–d) Fasting plasma triglyceride (b), non-esterified fatty acid (c), and blood glucose (d) levels in 8–12 week male and female Angptl4fl/fl and Angptl4AdipoKO mice following a 6 h fast (mean ± SEM; n = 7–11). (e, f) Fasted (6 h) female Angptl4fl/fl (n = 8) and Angptl4AdipoKO (n = 7) mice were injected intravenously with 3H-triglyceride–containing chylomicrons. (e) Clearance of radiolabel from the plasma 1, 5, 10, and 15 min after injection. Points represent percentage of radiolabel remaining in the plasma at the indicated time points compared to the 1 min time point (mean ± SEM). (f) Uptake of radiolabel after 15 min (% injected dose/g tissue) into the indicated tissues (mean ± SEM). (g) Heart, quadricep muscle (quad), gonadal white adipose tissue (gWAT), subcutaneous adipose tissue (sWAT), and brown adipose (BAT) tissue from fasted (6 h) female Angptl4fl/fl and Angptl4AdipoKO mice were harvested and lipase activity was measured (n = 6–9/group). (h) Liver was harvested from fasted (6 h) female Angptl4fl/fl and Angptl4AdipoKO mice (n = 8/group). Lipase activity was measured in the presence or absence of 1 M-NaCl to distinguish between hepatic and lipoprotein lipase. Bars show relative lipase activity in each tissue normalized to Angptl4fl/fl (mean ± SEM). *p < 0.05, **p < 0.01, ***p < 0.001 by t-test analysis (panels a–c, f, g) or repeated measures ANOVA (panel e).
We previously observed increased plasma triglyceride clearance, increased adipose uptake of triglycerides, and increased adipose LPL activity in whole-body Angptl4–/– mice14. In that study we inferred that the increased clearance was due to ANGPTL4 deficiency specifically in the adipose tissue14. To determine if this was indeed the case, we performed triglyceride clearance assays on female Angptl4AdipoKO mice using radiolabeled chylomicrons. We also assessed uptake of radiolabeled chylomicrons into specific tissues. After an intravenous injection of radiolabeled chylomicrons, Angptl4AdipoKO mice cleared the radiolabeled TGs from the plasma faster than Angptl4fl/fl mice (Fig. 1e). Similar to what we had observed with whole-body Angptl4–/– mice14, Angptl4AdipoKO female mice had increased radiolabel uptake into adipose depots compared to Angptl4fl/fl mice (Fig. 1f). As with whole-body Angptl4–/– mice, LPL activity was increased in white adipose tissue of female Angptl4AdipoKO mice compared to Angptl4fl/fl mice (Fig. 1g,h). Additionally, there was a decrease in LPL activity in heart tissue of female Angptl4AdipoKO mice compared to Angptl4fl/fl mice. These data support our previous supposition that deficiency in adipose ANGPTL4 is responsible for the increased triglyceride uptake into adipose tissue.
Effects of high-fat feeding on body phenotypes and plasma parameters in adipocyte-specific ANGPTL4–deficient mice
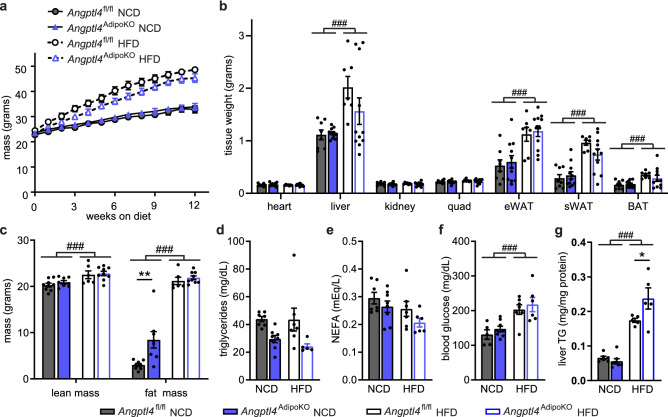
Given the importance of ANGPTL4 in directing triglycerides away from adipose tissue14, we sought to determine how high-fat feeding would alter metabolic phenotypes in Angptl4AdipoKO and Angptl4fl/fl mice. We randomly assigned Angptl4AdipoKO and Angptl4fl/fl mice at 8 weeks of age to a normal chow (NCD) or high fat diet (HFD; 60% kCal/fat) and fed them the respective diet for 12 weeks. On HFD, body weight gain was similar between all groups (Fig. 2a). No genotype-specific differences in tissue weights were observed (Fig. 2b). Adiposity, as determined by NMR, was greater in all mice fed a HFD compared to mice fed a NCD (Fig. 2c). Despite no differences in body weight, some Angptl4AdipoKO mice on NCD had significantly more total fat mass compared to Angptl4fl/fl mice (Fig. 2c). This genotype-specific difference in fat mass was not seen in HFD-fed Angptl4AdipoKO mice (Fig. 2c).
Figure 2.
Body weights, fat mass, and metabolic phenotypes of Angptl4fl/fl and Angptl4AdipoKO mice. (a) Weekly body weights of male Angptl4fl/fl and Angptl4AdipoKO mice fed either a normal chow diet (NCD) or a high fat diet (HFD; 60% by kCal) for 12 weeks starting at 8 weeks of age (mean ± SEM; n = 12–19/group). (b) Tissues weights of heart, liver, kidney, quadriceps muscle (quad), epididymal white adipose (eWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissue (BAT) in male Angptl4fl/fl and Angptl4AdipoKO mice after 12 weeks on diet (mean ± SEM; n = 7–11group). (c) Lean muscle and fat mass of male Angptl4fl/fl and Angptl4AdipoKO mice as measured by NMR after 12 weeks on diet (mean ± SEM; n = 6–9/group). Plasma triglycerides (d), plasma non-esterified fatty acids (e), blood glucose (f), and liver triglycerides (g) of fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice at the end of 12 weeks on diet (mean ± SEM; n = 5–8/group). ###p < 0.001 for dietary differences by two-way ANOVA. *p < 0.05, **p < 0.01 for individual genotype-specific differences by multiple comparison after two-way ANOVA (Tukey correction).
As before, Angptl4AdipoKO mice had lower fasting plasma TG levels compared to Angptl4fl/fl mice (Fig. 2d). Neither HFD nor adipocyte ANGPTL4 deficiency altered plasma non-esterified fatty acid levels (Fig. 2e). HFD feeding for 12 weeks increased plasma glucose and liver triglyceride levels in all groups, and the increase in liver triglycerides was higher in Angptl4AdipoKO mice than that in Angptl4fl/fl mice. (Fig. 2f,g).
Tissue expression of Angptl4 was influenced both by genotype and by diet. As expected, expression of Angptl4 in gonadal, subcutaneous, and brown adipose tissues (eWAT, sWAT, BAT) was much lower in Angptl4AdipoKO mice compared to Angptl4fl/fl mice (Supplemental Fig. 2a). Surprisingly, Angptl4AdipoKO mice on NCD had greatly reduced Angptl4 expression in the heart compared to Angptl4fl/fl mice (Supplemental Fig. 2a), suggesting that loss of ANGPTL4 in the adipose tissue had a systemic effect leading to downregulation of ANGPTL4 in other tissues. Interestingly, HFD alone significantly decreased Angptl4 expression in several tissues, including the heart, liver, and white adipose tissue (Supplemental Fig. 2a). These data indicate that high fat feeding can regulate expression of Angptl4. We also measured tissue gene expression of Lpl and found no genotype-specific differences (Supplemental Fig. 2b).
As changes in energy expenditure play a role in the pathogenesis of obesity, we asked if adipocyte-specific loss of ANGPTL4 would lead to changes in energy expenditure, oxygen consumption (VO2), carbon dioxide production (VCO2), or respiratory quotient (RQ; VCO2/VO2). Using the Promethion metabolic caging system, we observed no differences in energy expenditure, VO2, VCO2, or RQ in Angptl4AdipoKO mice on either a NCD or HFD compared to Angptl4fl/fl mice (Supplemental Fig. 3).
Effects of high-fat feeding on TG uptake and LPL activity in adipocyte-specific ANGPTL4 deficient mice
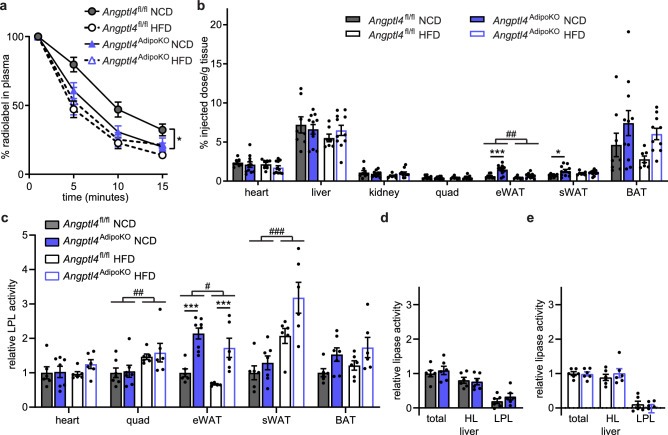
To assess if high-fat diet feeding altered triglyceride partitioning in an ANGPTL4-dependent manner, we performed plasma triglyceride clearance and tissue uptake assays on male Angptl4AdipoKO mice at the end of 12 weeks of either NCD or HFD feeding. On a NCD, Angptl4AdipoKO mice cleared radiolabeled TGs from the plasma faster than Angptl4fl/fl mice (Fig. 3a). Mice fed a HFD had faster clearance of plasma triglycerides than those fed a NCD, but on HFD there were no genotype-specific differences in clearance rates (Fig. 3a). Consistent with our observations in female mice, on NCD Angptl4AdipoKO male mice had increased radiolabel uptake into epididymal and subcutaneous adipose depots compared to floxed controls (Fig. 3b). Interestingly, on HFD, the differences in radiolabel uptake into adipose tissues between Angptl4AdipoKO mice and Angptl4fl/fl littermates were reduced (Fig. 3b). These data again support the idea that on a NCD, deficiency in adipose ANGPTL4 results in increased triglyceride uptake into adipose tissue, but also suggest that this difference decreases in the face of high-fat feeding.
Figure 3.
Chylomicron clearance and uptake and lipase activity in Angptl4AdipoKO mice. (a, b) At the conclusion of 12 weeks of either normal chow diet (NCD) or high fat diet (HFD) Angptl4fl/fl and Angptl4AdipoKO male mice (n = 7–10/group) were fasted (6 h) and injected intravenously with 3H-triglyceride–containing chylomicrons. (a) Clearance of radiolabel from the plasma 1, 5, 10, and 15 min after injection. Points represent percentage of radiolabel remaining in the plasma at the indicated time points compared to the 1-min time point (mean ± SEM). *p < 0.05 by repeated measures ANOVA. (b) Uptake of radiolabel (% injected dose/g tissue) into the indicated tissues after 15 min (mean ± SEM). ##p < 0.01 for dietary differences by two-way ANOVA. *p < 0.05, ***p < 0.001 for individual genotype-specific differences by multiple comparison after two-way ANOVA (Tukey correction). (c) Heart, quadricep muscle (quad), epididymal adipose tissue (eWAT), subcutaneous adipose tissue (sWAT), and brown adipose (BAT) tissue from fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice were harvested and lipase activity was measured (n = 6–8/group). (d, e) Liver was harvested from fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice (d, NCD groups and e, HFD groups) (n = 6/group). Lipase activity was measured in the presence or absence of 1 M NaCl to distinguish between hepatic and lipoprotein lipase. Bars show relative lipase activity in each tissue normalized to Angptl4fl/fl (mean ± SEM). #p < 0.05, ##p < 0.01, ###p < 0.001 for dietary differences by two-way ANOVA. ***p < 0.001 for individual genotype-specific differences by multiple comparison after two-way ANOVA (Tukey correction).
To elucidate if the increased uptake of triglyceride-derived fatty acids into adipose tissue in Angptl4AdipoKO mice was the result of increased LPL activity we measured tissue lipase activity. LPL activity was greater in the gonadal adipose tissue of Angptl4AdipoKO mice on both NCD or HFD compared to Angptl4fl/fl mice (Fig. 3c). HFD itself altered LPL activity in some tissues, increasing activity in quadricep muscle and slightly decreasing activity in epididymal white adipose tissue (Fig. 3c). We also assessed if loss of ANGPTL4 resulted in changes in liver lipase activity. As expected, LPL activity in the liver was low compared to total lipase activity. We found no genotype-specific differences in total lipase, hepatic lipase, or LPL activity on either diet in the Angptl4AdipoKO mice compared to Angptl4fl/fl mice (Fig. 3d,e). Together, TG clearance and LPL activity in Angptl4AdipoKO mice closely resemble those we have previously reported in whole-body ANGPTL4 knockout mice14, supporting the idea that the lack of adipose-derived ANGPTL4 is the primary driver of the increased adipose LPL activity and TG clearance in those mice.
Effects of high-fat feeding on glucose tolerance and insulin sensitivity in adipocyte-specific ANGPTL4–deficient mice
A previous report found that an independent strain of adipocyte-specific ANGPTL4 knockout mice had improved glucose tolerance compared to wild-type mice after being fed a HFD for 4 weeks13. To assess if the loss of adipocyte-derived ANGPTL4 altered glucose metabolism in our mice, we performed glucose (GTT) and insulin tolerance (ITT) tests. On a normal chow diet, no differences were observed in glucose tolerance or insulin sensitivity in Angptl4AdipoKO mice compared to floxed controls (Fig. 4). However, glucose tolerance and insulin sensitivity were markedly improved in HFD-fed Angptl4AdipoKO mice compared to HFD-fed floxed controls (Fig. 4a,b). These data support a protective role for loss of adipose-derived ANGPTL4 on systemic glucose homeostasis. Chronic inflammation has been linked with insulin resistance during obesity and type 2 diabetes19. To test whether the changes we observed in glucose tolerance and insulin sensitivity were due to changes in tissue inflammation we measured tissue expression (liver, eWAT, sWAT, and BAT) of inflammatory markers C–C Motif Chemokine Ligand 2 (Ccl2), Cd68, and tumor necrosis factor α (Tnfα). As expected, there was an increase in inflammation in all tested tissues of HFD-fed mice. However, no major genotype-specific differences were observed (Supplemental Fig. 4).
Figure 4.
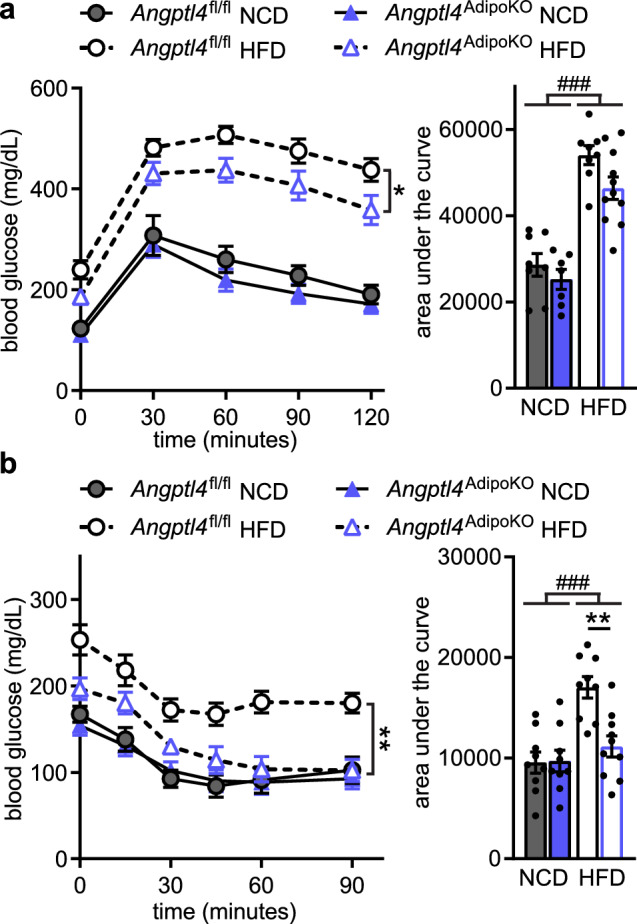
Glucose tolerance and insulin sensitivity of Angptl4AdipoKO mice. (a) Glucose tolerance tests were performed on fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice after 11 weeks of either a normal chow diet (NCD) or high fat diet (HFD). Blood glucose concentrations were measured over 2 h after injection with glucose. Points represent glucose levels (mean ± SEM; n = 8–11) at each respective time point. *p < 0.05 by repeated measures ANOVA. Bar graphs represent area under the curve (mean ± SEM) for all time points. ###p < 0.001 for dietary differences by two-way ANOVA. (b) Insulin tolerance tests were performed on fasted (4 h) male Angptl4fl/fl and Angptl4AdipoKO mice after 12 weeks on diet. Blood glucose concentrations were measured over 90 min after injection with insulin. Points represent glucose levels (mean ± SEM; n = 9–10) at each respective time point. **p < 0.01 for individual genotype-specific differences by repeated measures ANOVA. Bar graphs represent area under the curve (mean ± SEM) for all time points. ###p < 0.001 for dietary differences by two-way ANOVA. **p < 0.01 for individual genotype-specific differences by multiple comparison after two-way ANOVA (Tukey correction).
Effects of adipocyte-specific ANGPTL4 deficiency in mice after a chronic high-fat diet
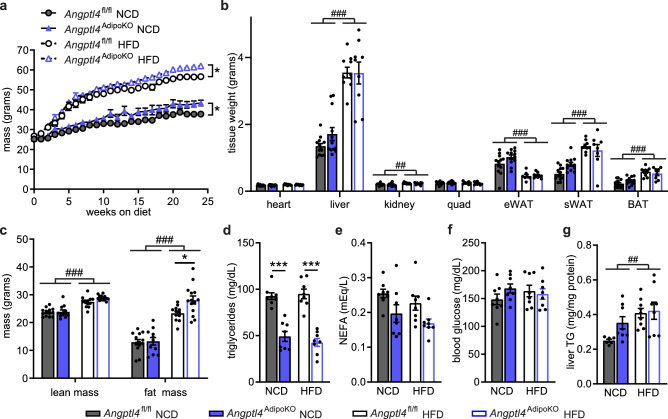
To better characterize the role of ANGPTL4 in the setting of chronic obesity, we fed Angptl4AdipoKO, and Angptl4fl/fl mice a NCD or HFD (60% kCal/fat) for 6 months. Initially, there were no genotype-specific differences in weight gain between any of the diet groups (Fig. 5a). However, after 18 weeks on diet, the body weight curves began to diverge, with Angptl4AdipoKO mice gaining modestly more weight than littermate Angptl4fl/fl mice on both a NCD or and a HFD (Fig. 5a). There were no genotype-specific differences in tissue weights (Fig. 5b). Adiposity, as determined by NMR, was greater in all mice fed a HFD compared to mice fed a NCD, and there was a significant increase in total fat mass in the high fat fed Angptl4AdipoKO mice compared to high fat fed Angptl4fl/fl controls (Fig. 5c). After 6 months on diet, Angptl4AdipoKO mice continued to have significantly lower fasting plasma triglyceride levels compared to Angptl4fl/fl mice (Fig. 5d). At this age, mice with ANGPTL4-deficiency in adipocytes tended to have lower plasma non-esterified fatty acids, but there were no genotype-specific differences in blood glucose or liver triglycerides levels (Fig. 5e–g). Interestingly, at this time point, the diet induced differences in blood glucose had disappeared and the diet-induced differences in liver TG had decreased (Fig. 5f,g).
Figure 5.
Body weights, fat mass, and metabolic phenotypes of Angptl4fl/fl and Angptl4AdipoKO mice after chronic high-fat feeding. (a) Weekly body weights of male Angptl4fl/fl and Angptl4AdipoKO mice fed either a normal chow diet (NCD) or high fat diet (HFD) for 6 months starting at 8 weeks of age (mean ± SEM; n = 10–17/group). *p < 0.05 by repeated measures ANOVA. (b) Tissues weights of heart, liver, kidney, quadriceps muscle (quad), epididymal white adipose (eWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissue (BAT) in male Angptl4fl/fl and Angptl4AdipoKO mice after 6 months on diet (mean ± SEM; n = 8–13/group). (c) Lean muscle mass and fat mass of male Angptl4fl/fl and Angptl4AdipoKO mice as measured by NMR after 24 weeks on diet. (mean ± SEM; n = 11–14/group). Plasma triglycerides (d), plasma non-esterified fatty acids (e), blood glucose (f), and liver triglycerides (g) of fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice after 6 months on diet (mean ± SEM; n = 8–9/group). ##p < 0.01, ###p < 0.001 for dietary differences by two-way ANOVA. *p < 0.05,***p < 0.001 for individual genotype-specific differences by multiple comparison after two-way ANOVA (Tukey correction).
As expected, expression of Angptl4 in the gonadal, subcutaneous and brown adipose tissues (eWAT, sWAT, BAT) of Angptl4AdipoKO mice was much lower than in Angptl4fl/fl littermates (Supplemental Fig. 5a). Similar to what we observed after 12 weeks on diet, expression of Angptl4 was reduced in Angptl4AdipoKO mice in the heart and quadricep muscle compared to Angptl4fl/fl. Also similar to what we observed after 12 weeks of high-fat feeding, Angptl4 expression was reduced in heart, liver, and white adipose tissue of Angptl4fl/fl mice fed a HFD for 6 months, again supporting the idea that Angptl4 expression in these tissues is regulated by diet (Supplemental Fig. 5a). We also measured tissue gene expression of Lpl and found no genotype-specific differences (Supplemental Fig. 5b). As we consistently observed lower Angptl4 expression in the hearts of NCD-fed Angptl4AdipoKO mice, we asked if this reduction was due to off-target cre recombination in the heart. To test this idea, we assessed recombination of the Angptl4 flox allele by performing PCR on genomic DNA isolated from the heart, eWAT, sWAT and BAT of Angptl4AdipoKOand Angptl4fl/fl mice fed a NCD for 6 months (Supplemental Fig. 5c). Angptl4 genomic recombination was seen in the fat pads of the Angptl4AdipoKO mice but not Angptl4fl/fl mice. Furthermore, no recombination was observed in the hearts of either genotype (Supplemental Fig. 5c), supporting the idea that the reduced expression of Angptl4 in heart was the result of some kind of feedback regulation.
To assess liver health in our mice we measured plasma activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). All mice fed a HFD for 6 months had elevated AST and ALT activity compared to NCD fed mice, but no genotype-specific differences were observed (Supplemental Fig. 6a,b). We also measured inflammatory protein amyloid A in the plasma of our mice. Although HFD-fed mice had an increase in plasma amyloid A there were no genotype-specific differences (Supplemental Fig. 6c). Livers of mice fed a HFD were larger than those from mice fed NCD, but again no genotype-specific differences were observed (Supplemental Fig. 6d).
After 6 months on diet, Angptl4AdipoKO mice continued to clear radiolabeled triglycerides from the plasma faster than littermate Angptl4fl/fl mice on NCD (Fig. 6a). Angptl4AdipoKO mice also cleared triglycerides faster when fed a HFD, though the difference was less pronounced (Fig. 6a). Again, Angptl4AdipoKO male mice fed a NCD had increased uptake of radiolabeled fat into white adipose depots compared to floxed controls, but on HFD those differences largely disappeared (Fig. 6b). This was true even when we corrected for the somewhat faster plasma TG clearance in Angptl4AdipoKO mice (Supplemental Fig. 7). To understand why adipose tissue ANGPTL4 deficiency no longer resulted in improved adipose clearance of triglycerides after a 6-month high-fat diet, we next measured tissue-specific LPL activity. After 6 months of high-fat feeding, LPL activity was still greater in the adipose depots of Angptl4AdipoKO mice compared to those of Angptl4fl/fl mice (Fig. 6c). These data suggest that during the setting of chronic high-fat feeding, increased LPL activity in the adipose tissue of Angptl4AdipoKO mice no longer leads to increased adipose triglyceride uptake. We hypothesized that this could be that after chronic high feeding, LPL activity is no longer rate-limiting for triglyceride uptake. To assess if a chronic high-fat diet reduces expression of proteins involved in fatty acid uptake we measured the gene expression of Cd36, fatty acid transporters Fatp1 and Fapt4, and Acyl-CoA Synthetase Long Chain family members Acsl1 and Acsl4 in adipose tissues (Supplemental Fig. 8). Although we did observe a small decrease in Cd36 and Fatp1 expression in eWAT and decrease in Fatp1 expression in BAT following chronic high-fat feeding, we also observed increases in Cd36, Fatp4, and Acsl4 in sWAT, and Cd36 and Acsl1 in BAT (Supplemental Fig. 8). Thus, there was no clear indication that fatty acid uptake was downregulated at the gene expression level. Similar to what was observed after 12 weeks on HFD, there were no genotype-specific differences in total lipase, hepatic lipase, or LPL activity in the livers of Angptl4AdipoKO mice after chronic high-fat diet feeding (Fig. 6d,e).
Figure 6.
Chylomicron clearance and uptake and lipase activity in Angptl4AdipoKO mice after chronic high-fat feeding. At the conclusion of 6 months of either normal chow diet (NCD) or high fat diet (HFD) Angptl4fl/fl and Angptl4AdipoKO male mice (n = 7–9/group) were fasted (6 h) and injected intravenously with 3H-triglyceride containing chylomicrons. (a) Clearance of radiolabel from the plasma 1, 5, 10, and 15 min after injection. Points represent percentage of radiolabel remaining in the plasma at the indicated time points compared to the 1-min time point (mean ± SEM). ***p < 0.001 by repeated measures ANOVA. (b) Uptake of radiolabel (% injected dose/g tissue) into the indicated tissues after 15 min (mean ± SEM). #p < 0.05, ##p < 0.01, ###p < 0.001 for dietary differences by two-way ANOVA. *p < 0.05, ***p < 0.001 for individual genotype-specific differences by multiple comparison after two-way ANOVA. (c) Heart, quadricep muscle (quad), epididymal adipose tissue (eWAT), subcutaneous adipose tissue (sWAT), and brown adipose (BAT) tissue from fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice were harvested and lipase activity was measured (n = 6–8/group). (d, e) Liver was harvested from fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice (d, NCD groups and e, HFD groups)(n = 6–9/group). Lipase activity was measured in the presence or absence of 1 M NaCl to distinguish between hepatic and lipoprotein lipase. Bars show relative lipase activity in each tissue normalized to Angptl4fl/fl (mean ± SEM). #p < 0.05, ##p < 0.01, ###p < 0.001 for dietary differences by two-way ANOVA. *p < 0.05, **p < 0.01 ***p < 0.001 for individual genotype-specific differences by multiple comparison after two-way ANOVA.
Finally, we asked if the disappearance of increased adipose triglyceride clearance after 6 months on HFD affected the improved glucose tolerance we had observed in Angptl4AdipoKO mice after 12 weeks on HFD (see Fig. 6). Indeed, we found that Angptl4AdipoKO mice no longer had improved glucose tolerance after 6 months on HFD (Fig. 7a). And while Angptl4AdipoKO mice remained more insulin sensitive than Angptl4fl/fl mice after 6 months on HFD, the improvement was greatly reduced compared to that observed after 12 weeks of HFD (Fig. 7b). As before, we measured tissue expression (liver, eWAT, sWAT, and BAT) of inflammatory markers Ccl2, Cd68 and Tnfα. Once again, there was an increase in inflammation in HFD-fed mice in tested tissues, but no major genotype-specific differences were observed (Supplemental Fig. 9).
Figure 7.
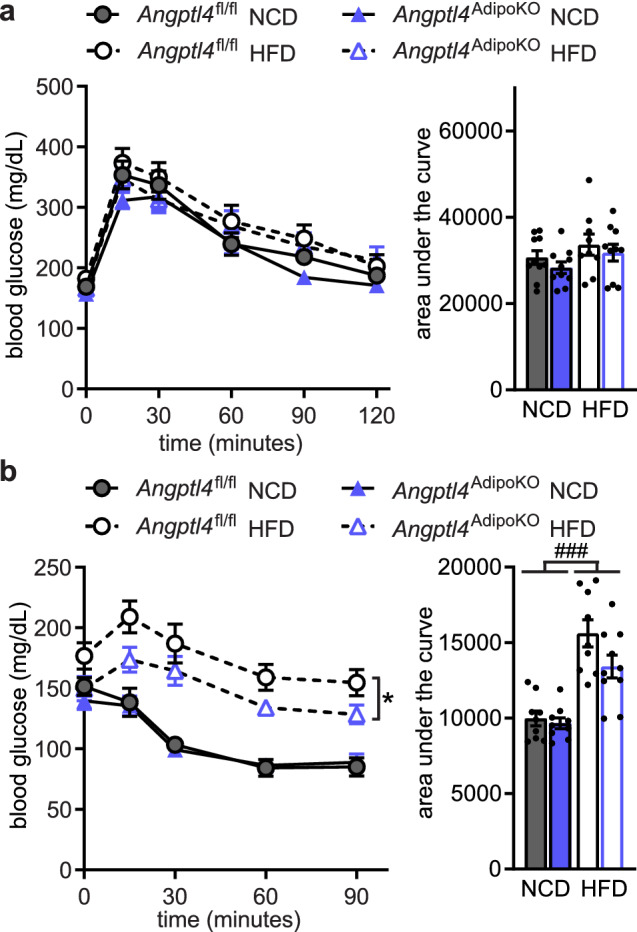
Glucose tolerance and insulin sensitivity of Angptl4AdipoKO mice after chronic high fat feeding. (a) Glucose tolerance tests were performed on fasted (6 h) male Angptl4fl/fl and Angptl4AdipoKO mice after 24 weeks of either a normal chow diet (NCD) or high fat diet (HFD). Blood glucose concentrations were measured over 2 h after injection with glucose. Points represent glucose levels (mean ± SEM; n = 9–10) at each respective time point. Bar graphs represent area under the curve (mean ± SEM) for all time points. (b) Insulin tolerance tests were performed on fasted (4 h) male Angptl4fl/fl and Angptl4AdipoKO mice after 25 weeks of either a normal chow diet (NCD) or high fat diet (HFD). Blood glucose concentrations were measured over 90 min after injection with insulin. Points represent glucose levels (mean ± SEM; n = 9–10) at each respective time point. *p < 0.05 by repeated measures ANOVA. Bar graphs represent area under the curve (mean ± SEM) for all time points. ###p < 0.001 for dietary differences by two-way ANOVA.
Discussion
Proteins made and secreted by the adipose tissues play a vital role in lipid and glucose homeostasis during obesity. ANGPTL4 is highly expressed by adipose tissues in mice and humans3,20–22. In this study we investigated the triglyceride phenotypes of adipocyte-specific ANGPTL4 knockout mice. We also investigated how adipocyte-specific loss of ANGPTL4 affected body weight, glucose tolerance, and insulin resistance in the setting of either 3 or 6 months of high-fat diet feeding and obesity. We found that adipocyte-specific loss of ANGPTL4 resulted in lower plasma triglycerides and increased uptake of triglyceride-fatty acids into adipose tissue when mice were fed a normal chow diet. When mice were fed a high-fat diet, we found that adipocyte-specific ANGPTL4 deletion initially resulted in improved glucose tolerance and insulin sensitivity, but over time this improvement disappeared, as did the increased adipose triglyceride uptake observed in normal chow fed mice.
The role of ANGPTL4 in regulating triglyceride metabolism through LPL inhibition has been well established. Human carriers of the E40K mutation in ANGPTL4 have lower triglycerides and a lower risk of coronary artery disease6 and dyslipidemia during obesity9. In mice, genetic loss of ANGPTL4 leads to decreased fasting plasma TG levels and increased uptake of triglycerides into adipose tissue3,13,14,18. The specific increase in adipose tissue LPL activity and TG uptake in whole body ANGPTL4 knockout mice strongly supported the idea that adipose derived ANGPTL4 was responsible for the triglyceride phenotypes in whole-body ANGPTL4 knockout mice. Here, adipocyte-specific loss of ANGPTL4 in mice fed a chow diet phenocopies the triglyceride phenotypes observed in whole-body Angptl4–/– mice. Angptl4AdipoKO mice had lower plasma triglyceride levels, increased uptake of triglycerides into adipose tissue, and increased adipose tissue LPL activity. This was true in both male and female mice. The triglyceride phenotypes observed in Angptl4AdipoKO mice were consistent with those recently reported by Aryal et al. using an independently derived adipocyte-specific ANGPTL4 knockout mouse13. Both studies support the hypothesis that it is primarily loss of adipose-derived ANGPTL4 that is responsible for the alterations in plasma triglyceride levels and triglyceride partitioning observed in Angptl4–/– mice.
Angptl4–/– mice fed a high-fat diet with high levels of saturated fat develop a lethal intestinal injury and lymphatic nodule inflammation15. In these mice serum amyloid A levels are extremely elevated after 8 weeks on diet (compared to wild-type mice) and Angptl4–/– mice die after 15 to 25 weeks on diet15. Lethality on a high-fat diet was also observed in mice given an anti-ANGPTL4 antibody16. This phenotype appears to be mediated by the lack of macrophage ANGPTL4, allowing excessive lipid uptake into lymphatic macrophages15. In our study, we used adipose-specific ANGPTL4 conditional knockout mice in hopes of circumventing the high-fat feeding intestinal pathophysiology observed in whole-body knockout mice. As we did not observe increased serum amyloid A levels, increased hepatic inflammation, or increased mortality in HFD-fed Angptl4AdipoKO mice compared to Angptl4fl/fl mice, we conclude that adipose ANGPTL4 is not the primary protector against the lethal phenotype observed in HFD-fed Angptl4–/– mice.
Loss of adipocyte ANGPTL4 in the context of HFD had an interesting effect on LPL activity and adipose triglyceride uptake. On a chow diet, the increase in adipose LPL activity in Angptl4AdipoKO mice led to a corresponding increase in adipose tissue uptake of triglycerides. This connection between increased LPL activity and increased triglyceride uptake in adipose tissue was lost in mice fed a HFD. Adipose tissue LPL activity remained higher in Angptl4AdipoKO mice fed a HFD compared to floxed control mice, but the enhanced adipose tissue uptake observed on chow diet was largely lost. These data suggest that on a chronic high-fat diet, LPL activity is no longer rate-limiting for the entry of triglyceride-derived fatty acids into adipose tissues. Why this would be the case is not clear. One possibility is that LPL-mediated lipolysis continues to occur, but uptake of these fatty acids into tissues is now limiting. The mechanisms by which fatty acids released by vascular lipolysis traverse capillary endothelial cells and are then taken up by adipocytes are not well understood. We measured gene expression of several genes associated with fatty acid uptake, but did not observe changes in expression that would clearly indicate a reduction in fatty acid uptake. However, our analysis did not encompass all genes associated with uptake, nor does gene expression necessarily corelate with functionality. Moreover, in our analysis we did not differentially analyze cell types within adipose tissues. A dramatic change in fatty acid uptake genes in endothelial cells might be masked by the lack of change or even increase in these same genes in adipocytes. Further, more targeted research will be needed to understand how fatty acid uptake is altered by long term high fat feeding and whether this may contribute to adipose dysfunction. Alternatively, it could be that changes in fatty acid uptake pathways are not responsible for the similar uptake in Angptl4fl/fl and Angptl4AdipoKO mice. It is possible that in Angptl4AdipoKO mice other tissues uptake sufficient TGs to reduce the supply available to white adipose tissue. Although we did not see evidence of this in our uptake assays, it is important to note that we did not measure uptake in all tissues. The fact that Angptl4AdipoKO mice fed a chronic HFD cleared plasma TGs somewhat faster and had lower plasma TG levels than Angptl4fl/fl mice on the same diet suggest that there may indeed be tissues in Angptl4AdipoKO mice that take up more TGs.
High-fat feeding experiments performed by Janssen et al.23 suggested that ANGPTL4 deficiency not only impacts triglyceride metabolism, but also glucose metabolism. Their work demonstrated that Angptl4–/– mice fed a diet rich in unsaturated fatty acids, gained more weight than wildtype mice, but had lower blood glucose levels and improved glucose tolerance. Indeed, human studies found that carriers of the inactivating E40K mutation in Angptl4 had lower fasting glucose levels than non-diabetic participants and had a lower odds ratio (0.89) for developing type 2 diabetes24. One hypothesis is that improved glucose homeostasis is due to relief of LPL inhibition and improved triglyceride clearance to adipose tissues23. Indeed, Aryal et al. found that after 4 weeks on HFD, adipose-specific Angptl4 knockout mice had improved glucose tolerance and insulin sensitivity13. We likewise found that Angptl4AdipoKO mice had increased clearance of triglycerides to adipose tissues, and that after 12 weeks on HFD these mice have improved glucose tolerance and are protected from insulin resistance compared to control mice. However, in both our study and the Aryal study, the improved glucose tolerance and protection from insulin resistance were mostly lost after long-term HFD feeding. As discussed above, we found that chronically HFD fed Angptl4AdipoKO mice did not have increased uptake of triglycerides into adipose tissue. We suspect that increasing TG partitioning to adipose tissues improves glucose homeostasis, but that when the increased TG uptake to adipose tissues is lost, improved glucose tolerance is also lost. Therapeutically, these findings imply that targeting ANGPTL4 to improve glucose tolerance may not be effective over the long-term, at least not in the situation of continued overconsumption of calories.
The current study is not without limitations. We chose a 60% (kCal) high fat diet, a diet that is widely used experimentally to induce an obese and insulin resistant phenotype. Other diets may more closely reflect the typical American diet, such as the “Western diet” comprised of 42% (kCal) high fat, high sucrose and high cholesterol. Alternative diets that induce different pathological states might illuminate tissue-specific roles for ANGPTL4 that were missed in our study. Another limitation to our study is that we studied adipocyte-specific Angptl4 knockout, but Angptl4 is expressed in other metabolically active tissues such as the liver. Therefore, we could have missed any synergistic effects of ANGPTL4 derived from adipose tissues with ANGPTL4 derived from other tissues. ANGPTL4 is expressed in liver1,3,25, where its function is unknown. It is also expressed, although at lower levels, in skeletal muscle and heart26 and could have important actions during exercise27 or situations of lipotoxicity28. ANGPTL4 expressed in the intestine has also been implicated in the effects of the intestinal microbiota on metabolism23. Generating additional tissue-specific Angptl4 knockout mice could identify the roles of ANGPTL4 in these and other tissues.
Our studies highlight the importance of studying age and diet duration going forward. HFD studies in mice are generally performed in mice that are less than 1 year old and who have been fed a high-fat diet for 4 to 16 weeks. However, both Aryal et al.13 and our current study clearly showed that improved glucose tolerance in adipocyte-specific ANGPTL4 knockout mice disappeared with chronic high-fat feeding. Given that metabolic disease is most prevalent in older and obese populations, these observations highlight the need and importance of performing long-term feeding studies in aged mice if we are to fully understand the roles for different proteins in dyslipidemia and metabolic disease.
Methods
Mice
Mice with floxed alleles of the Angptl4 gene were generated by the University of Iowa genome editing facility using CRISPR/Cas9. B6SJLF1/J mice were purchased from Jackson Labs (100012; Bar Harbor, ME). Male mice older than 8 weeks were bred with 3 to 5–week–old super-ovulated females to produce zygotes for microinjection. Female ICR (Envigo; Hsc:ICR(CD-1)) mice were used as recipients for embryo transfer. Chemically modified CRISPR-Cas9 crRNAs and tracrRNAs were purchased from IDT (Alt-R CRISPR-Cas9 crRNA; Alt-R CRISPR-Cas9 tracrRNA (Cat# 1072532)). crRNA sequences were CCCTTTCACAGTCTGCTCTG and TGTGTCTAGTCTAGGAGCCG. The crRNAs and tracrRNA were suspended in T10E0.1 buffer (10 mM Tris pH 8, 0.1 mM EDTA) and combined to 1 μg/μl (~ 29.5 μM) final concentration in a 1:2 (μg:μg) ratio. The RNAs were heated at 98 °C for 2 min and allowed to cool slowly to 20 °C in a thermal cycler. The annealed cr:tracrRNAs were aliquoted to single-use tubes and stored at − 80 °C. Cas9 nuclease was also purchased from IDT (Alt-R S.p. HiFi Cas9 Nuclease). Individual cr:tracr:Cas9 ribonucleoprotein complexes were made by combining Cas9 protein and cr:tracrRNA in T10E0.1 (final concentrations: 100 ng/ul (~ 0.6 μM) Cas9 protein and 100 ng/μl (~ 2.9 μM) cr:tracrRNA). The Cas9 protein and annealed RNAs were incubated at 37 °C for 10 min. The two RNP complexes were mixed resulting in a final concentration of 100 ng/μl (~ 0.6 μM) Cas9 protein and 50 ng/μl (~ 1.5 μM) each cr:tracrRNA. Long single stranded DNA was prepared by digesting the Angptl4 target vector (524 bp 5′ arm of homology; 911 bp floxed region; 636 bp 3′ arm of homology) with the nicking enzyme Nb.Bsm1 (New England Biolabs) and purification from an alkaline-agarose gel. The final concentrations in the micro-injection mix were 20 ng/μl each cr:tracr RNA (~ 0.6 μM), 40 ng/μl Cas9 protein (~ 0.3 μM), and 10 ng/μl long ssDNA. Pronuclear-stage embryos were collected using methods described in29. Embryos were collected in KSOM media (Millipore; MR101D) and washed 3 times to remove cumulous cells. Microinjection into the male pronucleus was performed and embryos incubated overnight. Two cell zygotes were implanted into pseudo-pregnant ICR females.
All pups were tested for the appropriate insertion of 5′ and 3′ LoxP sites by PCR amplification of the genomic region and subsequent sequencing. The above procedures resulted in the successful generation of a single male mouse with both 5′ and 3′ LoxP sites. This mouse was bred to a C57Bl/6 female mouse and the heterozygous progeny were interbred to generate homozygous floxed mice. During breeding, the presence of the LoxP Sites was confirmed by genotyping PCR using oligonucleotides TAGGCGCATCTACTAGGACTC (forward) and AGATATGCAAGGCTAGTGAAGAC (reverse) to detect the 5′ LoxP site and oligonucleotides CCTCCAACATCTCTTGATGTAAC (forward) and ATATGTGTATGTGACTGGATGG (reverse) to detect the 3′ LoxP site. Adipocyte-specific knockout mice (Angptl4AdipoKO) were generated by breeding Angptl4fl/fl mice with C57/BL6 transgenic mice containing the adiponectin promoter–driven Cre recombinase (kindly provided by Dr. Matthew Potthoff, Jackson stock 01080330). The presence of adiponectin-Cre was verified by genotyping PCR using GGATGTGCCATGTGAGTCTG (forward) and ACGGACAGAAGCATTTTCCA (reverse) as primers. In all studies Cre-negative Angptl4fl/fl littermates were used as controls.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa and were carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals. The experiments complied with the ARRIVE guidelines31. All animals were maintained in a climate-controlled environment at 25 °C with a 12:12 h light–dark cycle. Water was provided ad libitum. During non-fasting conditions mice were given standard mouse chow (NIH 7951) until 8 weeks of age when high-fat diet studies began. At 8 weeks of age mice were randomly assigned to either remain on standard mouse chow (NIH 7951) or given high-fat diet (60% by kCal; Research Diets, 12592). Mice were fed their respective diets for either 12 weeks or 6 months. Experiments were unblinded.
Production of Angptl4 and LPL conditioned media
A construct expressing full-length mouse ANGPTL4 (pXC4) was generated from full-length mouse ANGPTL4 cDNA (MMM1013-202763591). A V5 tag was appended to the C terminus of the open reading frame using Phusion site-directed mutagenesis (New England Biolabs) to generate a V5 tagged version of mouse ANGPTL4 (pHS5). To generate a mouse ANGPTL4 construct mimicking the predicted protein product of our Angptl4 flox allele after Cre-mediated recombination (pKMS22) we deleted residues 111–187 (Exons 2 and 3) from full-length mouse ANGPTL4 by site-directed mutagenesis. A V5 tag was appended to the new C terminus of the altered open reading frame using site directed mutagenesis (pKMS25). We also generated a mouse ANGPTL4 construct to mimic the predicted protein product of a flox allele generated by the EUCOMM/KOMP consortium and used by several previous studies13,16,17. This flox Angptl4 allele uses a knock-out first strategy and has LoxP sites inserted into the Angptl4 gene flanking Exons 4,5, and 6. To generate a construct mimicking the predicted protein after Cre-mediated recombination (pXC32) we deleted the residues coded by exons 4 through 6 from full-length mouse ANGPTL4 by site-directed mutagenesis. A V5 tag was appended to the C terminus also using site directed mutagenesis (pXC34). To produce conditioned media containing either V5-tagged mANGPTL4, V5-tagged Flox mANGPTL4 or V5-tagged KOMP allele mANGPTL4, 293 T cells were transiently transfected with the respective constructs using the transfection agent PEI. 24 h post-transfection the media was switched to serum-free DMEM containing 1 × protease inhibitors and grown for 48 h. Mock transfected 293 T cells were used as a control. The media was then collected for use in Western blot analysis and LPL activity assays. FLAG-tagged human LPL was concentrated from the medium of a Chinese hamster ovary cell line (CHO-K1) stably expressing FLAG-tagged human LPL as previously described32,33. The presence of LPL in the conditioned media was confirmed through Western blotting using a mouse antibody against the FLAG tag (1:5000; Sigma). LPL activity was assessed through an LPL activity assay (see below).
Western blot
Lysate and media samples collected from 293 T cells transfected with V5-tagged mANGPTL4, V5-tagged Flox mANGPTL4, or V5-tagged KOMP allele mANGPTL4 constructs were size fractionated on 12% SDS–polyacrylamide gels and then transferred to nitrocellulose membranes. Membranes were blocked with casein before primary antibodies were added (1:3000 dilution of mouse monoclonal antibody against the V5 tag (ThermoFisher MA5-15253)) in a casein buffer containing Tween. Membranes were rocked overnight at 4 °C. Following incubation, the primary antibody was removed and membranes washed 3 times with PBS containing 0.1% Tween. Membranes were then incubated with anti-mouse Dylight800-labeled secondary antibody (Invitrogen) diluted 1:5000 in casein buffer. After washing with PBS containing Tween, antibody binding was detected using an Odyssey Infrared Scanner (LI-COR).
Analysis of fasting plasma parameters
Fasting plasma parameters were measured in fasted (6 h) Angptl4fl/fl and Angptl4AdipoKO mice. Blood was collected into EDTA-coated capillary tubes following a tail-nick and centrifuged to collect plasma (1500 × g, 15 min, 4 °C). Plasma TG, NEFA, and glucose measurements were taken at 8 weeks of age before beginning diet and 12 weeks or 6 months following the start of diet studies. For plasma TG analysis, plasma (2 μl, technical duplicates using the same aliquot of plasma per individual mouse were used) was combined with Infinity Triglyceride Reagent (200 μl, Thermo Scientific TR22421) in a 96 well plate. Samples were incubated for 10 min at 37 °C, gently tapped to ensure proper mixing of reagent with sample, and absorbance was read at 500 nm and 660 nm. Triglyceride concentrations were determined using a standard curve prepared from Triolein standards (Nu-Chek Prep, Lot T-235-N13-Y). For plasma NEFA analysis, plasma (2 μl, technical duplicates) was combined with Color Reagent A (112.5 μl, Wako HR Series NEFA-HR 999–34691/995–34791) and incubated for 10 min at 37 °C. Following incubation, Color Reagent B was added to samples (37.5 μl, Wako HR Series NEFA-HR 991–34891/993–35191) and incubated for 5 min at 37 °C. Absorbance was read at 560 nm and 670 nm. Non-esterified fatty acid concentrations were determined using a standard curve prepared from Oleic Acid standard (TCI, O0180, Lot Z8RVM). Fasting plasma glucose concentration was measured once per mouse immediately following a tail nick using a glucometer (One Touch Ultra). Plasma amyloid A was measured using an ELISA kit (Crystal Chem #80659) following manufacturer’s protocol. Plasma activity of aspartate aminotransferase (AST, Sigma-Aldrich, MAK055) and alanine aminotransferase (ALT, Sigma-Aldrich, MAK052) were measured according to manufacturer’s protocols.
RNA extraction and qPCR analysis
Mouse tissues (heart, liver, gonadal and epididymal white adipose tissue, quadriceps muscle, subcutaneous white adipose tissue and brown adipose tissue) were harvested following a 6 h fast. Tissue was snap frozen in liquid nitrogen and stored at − 80 °C until processed. Tissues were pulverized before homogenization. Total RNA was extracted using Trizol (Invitrogen) according to manufacturer’s instructions. 2 μg of RNA was used to prepare cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, 4368813) according to manufacturer’s instructions. Prepared cDNA was used for qPCR analysis using primers across different exons of Angptl4 (Supplemental Table 1), or against Angptl4, Lpl, Ccl2, Cd68, Tnfa, Cd36, Fatp1, Fatp4, Acsl1, Acsl4, U36b4, or CycloA (Supplemental Table 2). Prepared diluted cDNA, primers, and SYBR Green ER qPCR Supermix reagent (Invitrogen, 11762100) were combined, and PCR was performed on the QuantStudio 6 Flex system (3 technical replicates per mouse, Applied Biosystems, Iowa Institute of Human Genetics). Gene expression was calculated using the ΔΔct method34 using either CycloA or U36B4 as the reference gene.
DNA extraction and PCR analysis
Heart, epididymal white adipose tissue, subcutaneous white adipose tissue and brown adipose tissue were harvested from male Angptl4fl/fl and Angptl4AdipoKO mice and placed in ice cold PBS. DNA was extracted from approximately 25 g of tissue using a DNeasy Blood and Tissue Kit (Qiagen, 69504) according to manufacturer’s instructions. Extracted genomic DNA was amplified by PCR using primers approximately 500 basepairs upstream and downstream of the LoxP sites in the Angptl4 locus (Supplemental Table 3). Following PCR, amplified DNA was subjected to gel electrophoresis on a 2% agarose gel containing EtBr and imaged.
Preparation of 3H-labeled chylomicrons
3H-labeled chylomicrons were prepared as previously described14. Briefly, glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1) knock out mice were orally gavaged with 100 μCi of [9,10-3H(N)]-Triolein (Perkin Elmer, NET431001MC) suspended in olive oil. After 4 h, blood was collected via cardiac puncture and placed into a tube containing 0.5 M EDTA and placed on ice. The blood was then centrifuged at 1,500 × g for 15 min and plasma was collected. The plasma was then centrifuged at 424,000 × g twice for 2 h at 10 °C. The chylomicrons were collected from the upper layer of the resulting supernatant, resuspended in sterile saline and stored at 4 °C. Radioactivity was measured using a Beckman-Coulter Liquid Scintillation Counter.
Triglyceride uptake assay
TG clearance and uptake assays were performed in mice as previously described14. Briefly, mice were fasted for 6 h and then injected retro-orbitally with 100 μl of 3H-labeled chylomicron suspension. Blood samples were taken 1, 5, 10, and 15 min after injection. For each time point, 10 μl of blood was assayed for radioactivity in BioSafe II scintillation fluid using a Beckman-Coulter Scintillation Counter. Following the final timepoint mice were anesthetized with isoflurane and perfused with 20 mL of cold 0.5% Tyloxapol in PBS solution. Tissues (heart, liver, kidney, gonadal or epidydimal white adipose tissue, quadriceps, subcutaneous white adipose tissue, and brown adipose tissue) were harvested and weighed. 40–90 mg of each tissue was placed into a glass vial containing a 2:1 chloroform:methanol solution and stored overnight at 4 °C. 1 mL of 2 M CaCl2 was added to each vial to separate the organic and aqueous phases. Samples were centrifuged for 10 min at 1,000 rpm and the upper aqueous layer was assayed for radioactivity in BioSafe II scintillation fluid using a Beckman-Coulter Scintillation Counter. The organic layer was allowed to evaporate overnight in an empty scintillation vial, then resuspended in scintillation fluid and counted. The counts per million (CPM) from the aqueous and organic fractions were combined to obtain the total uptake CPMs. To normalize radiolabel across mice, CPM values were normalized to the CPMs of the injected dose (measured by assaying 10% of the chylomicron suspension injected into the mouse).
Liver triglyceride measurements
Liver triglyceride measurement was performed using a modified Folch extraction method35. Liver tissues (approximately 100 mg) were homogenized in 500 µl of ice-cold PBS. From this homogenate 50 µl was removed and analyzed for protein content. 1.5 ml of a 2:1 (chloroform:methanol) mixture was added to each sample. Samples were vortexed briefly and incubated for 10–15 min. Samples were then centrifuged for 10 min at 2,050 × g at 4 °C. The lower organic phase was separated with a glass Pasteur pipette and placed into a 7 mL plastic vial (RPI 125509) and allowed to dry. The dried lipid was then dissolved with 500 µl of 2% Triton X-100 in chloroform and allowed to dry. The dried lipid was resuspended in 100 µl of molecular grade water. The sample was diluted 1 to 20 for triglyceride assay analysis. Analysis was performed using 2 μl of diluted lipid (technical duplicates) which was combined with Infinity Triglyceride Reagent (200 μl, Thermo Scientific TR22421) in a 96 well plate. The plate was incubated for 10 min at 37 °C, gently tapped to ensure proper mixing of reagent with sample, and absorbance was read at 500 nm and 660 nm. Triglyceride concentrations were determined using a standard curve prepared from Triolein standard (Nu-Chek Prep, Lot T-235-N13-Y). Triglyceride levels were normalized to milligram of protein as measured in the original homogenate. Protein content was assessed by performing a DC Protein Assay (Bio-Rad) according to manufacturer’s instructions.
Lipoprotein lipase activity assays
LPL activity in tissues was measured as previously described14. Briefly, frozen tissue samples were crushed using a metal tissue pulverizer. The tissue was resuspended in LPL assay buffer (25 mM NH4Cl, 5 mM EDTA, 0.01% SDS, 45 U/mL heparin, 0.05% 3-(N,N-Dimethylmyristylammonio) propanesulfonate zwittergent detergent (Acros Organics, 427740050)) containing protease inhibitor (Mammalian ProteaseArrest-APExBIO K1008). The tissue was then vortexed and incubated on ice for 30 min with occasional further disruption with surgical scissors. The lysate was then clarified by centrifugation at 15,000 × g for 15 min (4 °C). Protein concentrations were equalized prior to assaying activity. Supernatants were combined with a buffer comprised of 0.6 M NaCl, 80 mM Tris–HCl pH 8, 6% fatty-acid free BSA, and 1% of the EnzChek lipase fluorescent substrate (Molecular Probes, E33955). Fluorescence was measured from technical duplicates of each lysate (30 min, 37 °C) on a SpectraMax i3 plate reader (Molecular Devices). Relative lipase activity was determined following calculation of the linear slope of the curve and subtraction of background (assay buffer) slope readings. For liver lipase activity assays each lysate was treated with either vehicle (PBS) or NaCl (1 M final concentration) to differentiate the roles of hepatic lipase (NaCl insensitive) from LPL (NaCl sensitive) before the addition of the assay buffer.
The LPL activity of LPL conditioned media treated with either V5-tagged mANGPTL4, V5-tagged Flox mANGPTL4, or V5-tagged KOMP allele mANGPTL4 constructs was measured as previously described36. Briefly, ANGPTL4 containing conditioned media was combined with LPL conditioned media and samples were incubated at 37 °C for 30 min. After incubation, 50 µl of each assay samples was combined with 25 µl of assay buffer (0.6 M NaCl, 80 mM Tris–HCl pH 8, 6% fatty-acid free BSA), following which 25 µl of substrate solution (1% of the EnzChek lipase fluorescent substrate (Molecular Probes, E33955) 0.05% 3-(N,N-dimethylmyristylammonio) propanesulfaonate Zwittergent detergent (Acros Organics) in 1% methanol) was then added to each sample. Fluorescence was measured from technical duplicates of each lysate (30 min, 37 °C) on a SpectraMax i3 plate reader (Molecular Devices). Lipase activity was determined as stated above.
Glucose and insulin tolerance tests
Glucose tolerance tests (GTT) were performed after fasting mice for 6 h on week 10 on diet in the 12-week diet cohort or week 24 on diet in the 6-month diet cohort. Blood samples were collected before and 30, 60, 90, and 120 min following an intraperitoneal injection with glucose (12-week HFD study: 2 g/kg for mice fed NCD, 1.3 g/kg for mice fed HFD; 6-month HFD study: 1 g/kg for all mice). For mice fed a HFD for 12 weeks, blood was collected into an EDTA-coated capillary tube and stored on ice. Plasma was collected following centrifugation of sample tubes at 1,500 × g for 20 min. Plasma glucose concentration was assessed using the Autokit Glucose kit (Wako-997–03001). 2.5 μl of plasma was combined with 200 μl of the Autokit glucose buffer solution and incubated at 37 °C for 5 min. Absorbance was read at 505 nM and 600 nM. The 600 nM reading was subtracted from the 505 nM read. Glucose concentrations were determined using a standard curve prepared from glucose standards provided by the kit. For mice fed a HFD for 6 months, blood glucose readings were taken following a tail nick, before injection and 30, 60, 90, and 120 min following glucose injection using a glucometer (OneTouch Ultra).
Insulin tolerance tests (ITT) were performed following a 4 h fast in mice following 11 weeks on diet in the 12-week diet cohort and 25 weeks on diet in the 6-month diet cohort. Glucose readings were taken following a tail-nick bleed before and 15, 30, 60, and 90 min after an intraperitoneal injection with insulin (0.75U/kg, Humalin-R 100) using a glucometer (OneTouch Ultra).
Metabolic cage studies
Mice were individually housed in Promethion cage systems from Sable Systems International at the University of Iowa Metabolic Phenotyping Core. Mice were allowed to acclimate to their housing for 48 h. Metabolic measurements were then taken for 48 h. Measurements were taken during the duration of two light cycles (6 a.m.–6 p.m.) and two dark cycles (6 p.m.–6 a.m.). Measurements were recorded as energy expenditure (kcal/hr), oxygen consumption (VO2 (ml/min)), carbon dioxide production (VCO2 (ml/min)) and respiratory quotient (RQ, VCO2/VO2).
Body composition
Body composition (fat and lean mass) was determined using nuclear magnetic resonance (NMR) in mice following 11 weeks on diet in the 12-week diet cohort and 25 weeks on diet in the 6-month diet cohort. Mice were weighed before being placed in a restraint tube without anesthesia and placed into either a Bruker LF50 (for mice under 50 g) or a Bruker LF90 (for mice over 50 g). Following NMR scanning mice were immediately returned to their cages. Body composition measurements were performed in the Fraternal Order of the Eagles Diabetes Research Center Metabolic Phenotyping Core.
Statistics and outlier identification
Results are expressed as means ± SEM. Bar graphs also show individual values. Outlier identification was performed on all mouse datasets using ROUT analysis in GraphPad Prism. An unpaired Student’s t-test with Welch’s correction was used to determine statistical significance of samples with two groups (Figs. 1 and 2). For groups of three or more statistical significance was determined by 2-way ANOVA followed by multiple comparison with Tukey correction. Repeated measured ANOVA was utilized for body weights, chylomicron clearance, GTT, and ITT assays. Statistical analysis was performed in GraphPad Prism.
Supplementary Information
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01HL130146 [BSJD]). We thank the University of Iowa Genome Editing Facility for assistance in generating the ANGPTL4 floxed allele and the Fraternal Order of Eagles Diabetes Research Center Metabolic Phenotyping Core Laboratory for assistance is collecting metabolic caging data.
Author contributions
B.S.J.D. and K.M.S. conceived the study and designed the experiments. K.M.S. conducted most of the experiments. S.K.S., E.M.C. and K.L.D.S. conducted some experiments, acquired data and gave helpful suggestions. K.M.S. and B.S.J.D. performed data analysis. K.M.S. and B.S.J.D. wrote and edited the manuscript. All authors interpreted data, participated in manuscript review, and approved the final manuscript.
Data availability
The data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87020-5.
References
- 1.Ge H, Cha J-Y, Gopal H, Harp C, Yu X, Repa JJ, Li C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J. Lipid Res. 2005;46:1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 3.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 2002;43:1770–1772. doi: 10.1194/jlr.C200010-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Dijk W, Beigneux AP, Larsson M, Bensadoun A, Young SG, Kersten S. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. J. Lipid Res. 2016;57:1670–1683. doi: 10.1194/jlr.M067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai K-MV, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 2016;374:1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genetics, M. I. et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N. Engl. J. Med.374, 1134–1144 (2016). [DOI] [PMC free article] [PubMed]
- 8.Lotta LA, Stewart ID, Sharp SJ, Day FR, Burgess S, Luan J, Bowker N, Cai L, Li C, Wittemans LBL, Kerrison ND, Khaw K-T, McCarthy MI, O’Rahilly S, Scott RA, Savage DB, Perry JRB, Langenberg C, Wareham NJ. Association of genetically enhanced lipoprotein lipase-mediated lipolysis and low-density lipoprotein cholesterol-lowering alleles with risk of coronary disease and type 2 diabetes. JAMA Cardiol. 2018;3:957–966. doi: 10.1001/jamacardio.2018.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailetti D, Bertoccini L, Mancina RM, Barchetta I, Capoccia D, Cossu E, Pujia A, Lenzi A, Leonetti F, Cavallo MG, Romeo S, Baroni MG. ANGPTL4 gene E40K variation protects against obesity-associated dyslipidemia in participants with obesity. Obes. Sci. Pract. 2019;5:83–90. doi: 10.1002/osp4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergö M, Wu G, Ruge T, Olivecrona T. Down-regulation of adipose tissue lipoprotein lipase during fasting requires that a gene, separate from the lipase gene, is switched on. J. Biol. Chem. 2002;277:11927–11932. doi: 10.1074/jbc.M200325200. [DOI] [PubMed] [Google Scholar]
- 11.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroupa O, Vorrsjö E, Stienstra R, Mattijssen F, Nilsson SK, Sukonina V, Kersten S, Olivecrona G, Olivecrona T. Linking nutritional regulation of Angptl4, Gpihbp1, and Lmf1 to lipoprotein lipase activity in rodent adipose tissue. BMC Physiol. 2012;12:13. doi: 10.1186/1472-6793-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aryal B, Singh AK, Zhang X, Varela L, Rotllan N, Goedeke L, Chaube B, Camporez J-P, Vatner DF, Horvath TL, Shulman GI, Suárez Y, Fernández-Hernando C. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight. 2018;3:e97918. doi: 10.1172/jci.insight.97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushing EM, Chi X, Sylvers KL, Shetty SK, Potthoff MJ, Davies BSJ. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 2017;6:809–818. doi: 10.1016/j.molmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Köster A, Tamsma JT, Tan NS, Müller M, Kersten S. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12:580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oteng A-B, Ruppert PMM, Boutens L, Dijk W, van Dierendonck XAMH, Olivecrona G, Stienstra R, Kersten S. Characterization of ANGPTL4 function in macrophages and adipocytes using Angptl4-knockout and Angptl4-hypomorphic mice. J. Lipid Res. 2019;60:1741–1754. doi: 10.1194/jlr.M094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AK, Aryal B, Chaube B, Rotllan N, Varela L, Horvath TL, Suárez Y, Fernández-Hernando C. Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis. Mol. Metab. 2018;11:59–69. doi: 10.1016/j.molmet.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E-C, Desai U, Gololobov G, Hong S, Feng X, Yu X-C, Gay J, Wilganowski N, Gao C, Du L-L, Chen J, Hu Y, Zhao S, Kirkpatrick L, Schneider M, Zambrowicz BP, Landes G, Powell DR, Sonnenburg WK. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL) J. Biol. Chem. 2009;284:13735–13745. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA-K, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 2000;20:5343–5349. doi: 10.1128/MCB.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai U, Lee E-C, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc. Natl. Acad. Sci. USA. 2007;104:11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen AWF, Katiraei S, Bartosinska B, Eberhard D, Willems van Dijk K, Kersten S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia. 2018;61:1447–1458. doi: 10.1007/s00125-018-4583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gusarova V, O’Dushlaine C, Teslovich TM, Benotti PN, Mirshahi T, Gottesman O, Van Hout CV, Murray MF, Mahajan A, Nielsen JB, Fritsche L, Wulff AB, Gudbjartsson DF, Sjögren M, Emdin CA, Scott RA, Lee W-J, Small A, Kwee LC, Dwivedi OP, Prasad RB, Bruse S, Lopez AE, Penn J, Marcketta A, Leader JB, Still CD, Kirchner HL, Mirshahi UL, Wardeh AH, Hartle CM, Habegger L, Fetterolf SN, Tusie-Luna T, Morris AP, Holm H, Steinthorsdottir V, Sulem P, Thorsteinsdottir U, Rotter JI, Chuang L-M, Damrauer S, Birtwell D, Brummett CM, Khera AV, Natarajan P, Orho-Melander M, Flannick J, Lotta LA, Willer CJ, et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat. Commun. 2018;9:2252. doi: 10.1038/s41467-018-04611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, Cohen JC. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See L-H, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu Y-C, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang H, Kwon O, Shin M-S, Kang GM, Leem YH, Lee CH, Kim SJ, Roh E, Kim H-K, Youn B-S, Kim M-S. Role of Angptl4/Fiaf in exercise-induced skeletal muscle AMPK activation. J. Appl. Physiol. 2018;125:715–722. doi: 10.1152/japplphysiol.00984.2016. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. PNAS. 2005;102:1767–1772. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinkert CA. Transgenic Animal Technology: A Laboratory Handbook. 2. Academic Press; 2002. [Google Scholar]
- 30.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percie du Sert, N., V. Hurst, A. Ahluwalia, S. Alam, M. T. Avey, M. Baker, W. J. Browne, A. Clark, I. C. Cuthill, U. Dirnagl, M. Emerson, P. Garner, S. T. Holgate, D. W. Howells, N. A. Karp, S. E. Lazic, K. Lidster, C. J. MacCallum, M. Macleod, E. J. Pearl, O. H. Petersen, F. Rawle, P. Reynolds, K. Rooney, E. S. Sena, S. D. Silberberg, T. Steckler, and H. Würbel. 2020. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18: e3000410. [DOI] [PMC free article] [PubMed]
- 32.Beigneux AP, Gin P, Davies BSJ, Weinstein MM, Bensadoun A, Fong LG, Young SG. Highly conserved cysteines within the Ly6 domain of GPIHBP1 are crucial for the binding of lipoprotein lipase. J. Biol. Chem. 2009;284:30240–30247. doi: 10.1074/jbc.M109.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi X, Shetty SK, Shows HW, Hjelmaas AJ, Malcolm EK, Davies BSJ. Angiopoietin-like 4 modifies the interactions between lipoprotein lipase and its endothelial cell transporter GPIHBP1. J. Biol. Chem. 2015;290:11865–11877. doi: 10.1074/jbc.M114.623769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 36.Basu D, Manjur J, Jin W. Determination of lipoprotein lipase activity using a novel fluorescent lipase assay. J. Lipid Res. 2011;52:826–832. doi: 10.1194/jlr.D010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analyzed during this study are available from the corresponding author upon reasonable request.