Abstract
Purpose
The purpose of this study was to compare the efficacy of intra-articular injection of platelet-rich plasma after arthrocentesis versus arthrocentesis alone as a treatment modality in patients with internal derangement of temporomandibular joint.
Methods
Twenty-four patients suffering from internal derangement of temporomandibular joint were included in the study. The patients were randomly divided into two groups as follows—twelve patients underwent arthrocentesis followed by intra-articular injection of platelet-rich plasma (study group) and the other twelve were treated by arthrocentesis alone (control group). Pain intensity was recorded on visual analogue scale (VAS); maximum mouth opening and joint sound were measured before and after intervention. The patients were clinically evaluated at the intervals of 1 month, 3 and 6 months subsequently.
Results
There was no statistically significant difference in all the parameters between the groups. Intra-group analysis showed statistically significant improvement in all the parameters.
Conclusion
In both groups, improvement of pain, maximum mouth opening and TMJ sound were observed at all intervals, but there was no statistically significant improvement in arthrocentesis with PRP group when compared with arthrocentesis alone.
Keywords: Internal derangement, Temporomandibular joint disorders, Platelet-rich plasma, Arthrocentesis
Introduction
Temporomandibular joint disorders (TMDs) are chronic musculoskeletal debilitating conditions due to the involvement of temporomandibular joint (TMJ), masticatory muscles and associated structures [1]. These disorders affect the quality of life of the patient with prevalence of 10–70% in the population [2]. Internal derangement is among the most common TMDs. It can be defined as an abnormal functional relationship of the disc with mandibular condyle, glenoid fossa and articular eminence. It ranges from joint inflammation (synovitis), adhesions, disc displacements and often disc perforations [3, 4]. Most of the time the etiopathogenesis is not specific. Common aetiologies proposed so far are joint overloading, micro- or macro-trauma to the joint with stretching or tearing of the disc, joint hyperlaxity and some joint infections [5]. Most of the patients with internal derangement improve clinically with conservative methods like analgesics, muscle relaxants, occlusal splints and physiotherapy [6]. Patients refractory to the conservative methods require minimally invasive techniques like arthrocentesis, intra-articular steroids and arthroscopy with lysis and lavage. These methods have shown good clinical results in relieving pain and improving functional outcomes [6–10].
Platelet-rich plasma (PRP) consists of various growth factors that have strong regenerative capacity, anti-inflammatory and analgesic action leading to improvement in functional outcome [1, 11]. Cerza et al. have reported intra-articular PRP resulted in rapid reduction of pain and recovery of functional capability in knee joints [12]. Various clinical trials in regenerative medicine have reported anabolic effects on chondrocytes, proliferation of mesenchymal stem cell and synoviocytes, cartilaginous extracellular matrix accumulation and increase in hyaluronic acid secretion in joints [13–15]. So the present study was undertaken to evaluate the synergistic effect of platelet-rich plasma when used along with arthrocentesis as a treatment modality in internal derangement of temporomandibular joints.
Materials and Methods
The study population comprised of twenty-four patients with internal derangement of temporomandibular joint (Wilkes Stage II and III) who were refractory to the conservative methods (analgesics, splints and physiotherapy) for minimum of 6 months. The diagnosis of internal derangement was based on clinical and radiological findings as suggested by Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) [16]. The approval of institutional ethical committee was taken, and all patients provided written informed consent before entering into the study.
Inclusion criteria was pain located in the involved TMJ, restricted mouth opening (< 35 mm), pain on wide oral opening, protrusive and lateral excursion movements, joint sound (clicking or popping) and refractory to the conservative methods for at least 6 months. Exclusion criteria included patients with immunocompromised status, pregnancy, haematological disorders, previous TMJ surgery, trauma, refusal for written consent and follow-up.
The patients who fulfilled the inclusion criteria were randomly divided into two groups, group I—arthrocentesis followed by intra-articular injection of PRP—and group II—arthrocentesis alone.
PRP Preparation
Platelet-rich plasma was prepared from 6 ml autologous blood sample for single joint. The blood was drawn from the antecubital vein and placed in the test tubes containing 3.2% sodium citrate. Isolation of PRP from blood was done by centrifugation for 8 min at 460 g (approximately 2000 rpm). There were three blood fractions: the upper layer, consisting of platelet poor plasma (PPP), the middle layer consisting of large and dense liquid phase platelet-rich plasma (PRP) and the lower layer, consisting of red blood cells (RBCs). It was aspirated carefully into a separate syringe and then injected into the upper joint space [17].
Operative Technique
All operative procedures were performed by the same surgeon. The preauricular region was prepared with the antiseptic solution. The canthotragal line (CTL) was marked. The first point was marked 10 mm anterior to the tragus and 2 mm below the CTL; the second point was marked 20 mm anterior and 10 mm below the CTL. After auriculotemporal nerve block, two 18 gauge syringes were inserted through the marked points and 100 ml Ringer’s lactate solution was injected for joint lavage. After arthrocentesis, 1 ml of PRP was injected into the joint space (group I). The control group was treated with the conventional arthrocentesis from the same markings as mentioned above (group II). Soft diet was advised for 1 week, and analgesics (aceclofenac 100 mg with acetaminophen 500 mg) were given twice daily on first day followed by as per requirement.
The parameters used in the study were pain which was measured using ten point visual analogue scale (VAS); maximum oral opening was recorded by measuring the inter-incisal distance with digital calipers, and the presence or absence of TMJ sound was recorded. These parameters were measured at baseline, 1-month, 3-month and 6-month interval. So, all the patients were followed up for 6 months.
The data were entered in Microsoft excel, and all statistical analyses were performed using IBM SPSS statistics for windows (version 17.0). The intra-group comparison of parametric data (pain and maximum mouth opening) was analysed using repeated measures ANOVA test followed by post hoc analysis. Cochran’s test was used for intra-group analysis of nonparametric data (TMJ sound). Intergroup analysis for pain and oral opening was analysed using unpaired student’s t test, and for TMJ sound Mann–Whitney test was used. p value less than 0.05 was considered statistically significant.
Results
The sample comprised of 24 subjects with internal derangement; the study group contained 12 subjects with a mean age of 34.75 ± 10.83 years; the control group had 12 subjects with a mean age of 36.41 ± 11.15. Group I consisted of 4 male patients and 8 female patients, whereas group II had 2 male patients and 10 female patients.
Pain
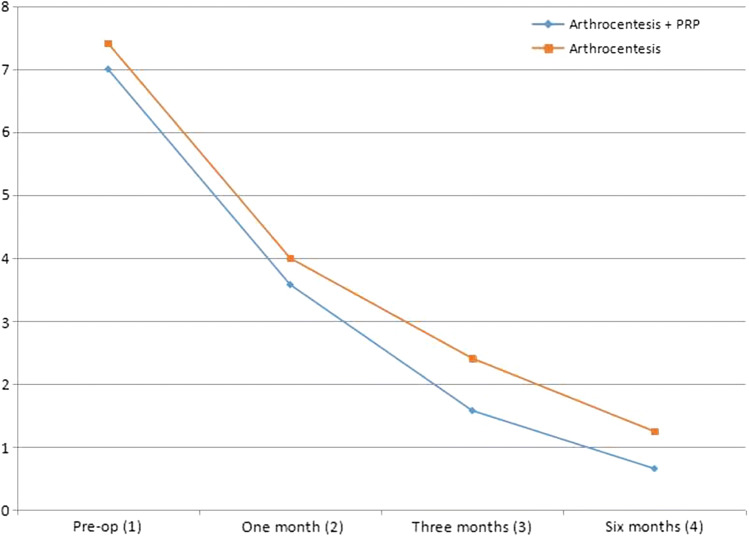
At baseline, there was no significant difference (p < 0.501) between the groups; this made the comparison possible. At 1-month, 3-months and 6-months follow-up, the p value was 0.483, 0.164 and 0.193, respectively, which indicates there was no significant difference in pain score between the groups at various time intervals. However, when intra-group analysis was conducted, there was gradual and significant improvement in pain along with time in both groups (p < 0.000). Duncan’s post hoc analysis showed least pain was recorded after 6 months, and it was statistically less than 1-month and 3-months follow-up (Table 1, Fig. 1).
Table 1.
Inter- and intra-group analyses of pain score
| Interval | Arthrocentesis + PRP (group I) | Arthrocentesis (group II) | Unpaired t test (t value) | p value |
|---|---|---|---|---|
| Pre-op (1) | 7 ± 1.65 | 7.41 ± 1.31 | 0.684 | 0.501 (NS) |
| 1 month (2) | 3.58 ± 1.37 | 4 ± 1.47 | 0.714 | 0.483 (NS) |
| 3 months (3) | 1.58 ± 1.16 | 2.41 ± 1.62 | 1.44 | 0.164 (NS) |
| 6 months (4) | 0.66 ± 0.88 | 1.25 ± 1.21 | 1.34 | 0.193 (NS) |
| Repeated measures ANOVA |
F = 55.76 p = 0.000 (HS) |
F = 43.02 p = 0.000 (HS) |
||
| Duncan’s post hoc analysis | 4 < 3, 4,3 < 2,1, 2 ≤ 1 | 4 < 3, 4,3 < 2,1, 2≤1 |
Fig. 1.
Graphical analysis of pain score
Maximum Mouth Opening
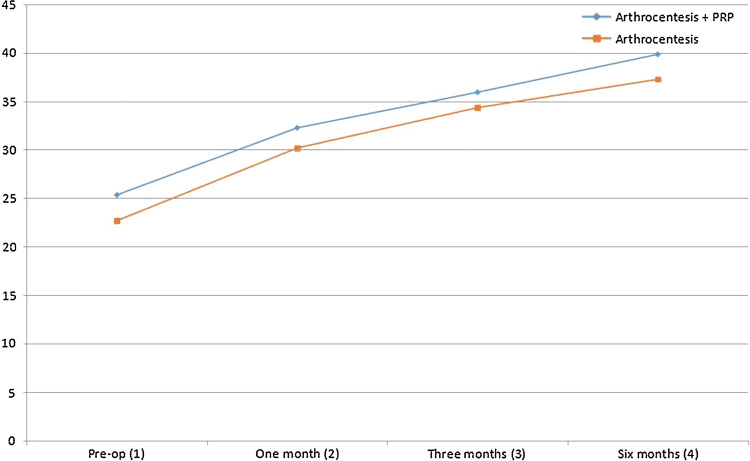
At baseline, there was no significant difference (p < 0.098) between the groups, and this made the comparison possible. At 1-month, 3-months and 6-months follow-up, the p value was 0.34, 0.496 and 0.28, respectively, which indicates there was no significant difference in mouth opening between the groups at various time intervals. However, when intra-group analysis was conducted, there was gradual and significant improvement along with time in both groups (p < 0.000). Tukey’s post hoc analysis showed maximum mouth opening was observed after 6 months, and it was statistically more than 1-month and 3-months follow-up (Table 2, Fig. 2).
Table 2.
Inter- and intra-group analyses of maximum mouth opening
| Interval | Arthrocentesis + PRP (group I) | Arthrocentesis (group II) | Unpaired t test (t value) | p value |
|---|---|---|---|---|
| Pre-op (1) | 25.33 ± 3.44 | 22.67 ± 4.09 | 1.725 | 0.098 (NS) |
| 1 month (2) | 32.25 ± 5.13 | 30.17 ± 5.32 | 0.976 | 0.34 (NS) |
| 3 months (3) | 35.92 ± 6.14 | 34.33 ± 5.01 | 0.692 | 0.496 (NS) |
| 6 months (4) | 39.83 ± 5.89 | 37.25 ± 5.52 | 1.108 | 0.28 (NS) |
| Repeated measures ANOVA |
F = 16.494 p = 0.000 (HS) |
F = 19.083 p = 0.000 (HS) |
||
| Tukey’s post hoc analysis | 4 ≥ 3, 3 ≥ 2, 4 > 2,1, 3 > 1 | 4 ≥ 3, 3 ≥ 2, 4 > 2,1, 3 > 1 |
Fig. 2.
Graphical analysis of maximum mouth opening
TMJ Sound
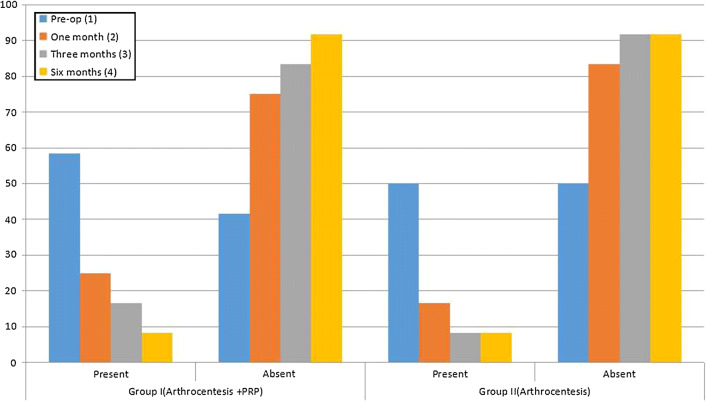
This parameter was recorded as present or absent. At baseline, 1-month, 3-months and 6-months follow-up, there was no significant difference between the groups (p = 0.688, 0.628, 0.688, 1, respectively). Cochran’s intra-group analysis showed significant reduction along with the follow-ups in both the groups (p = 0.004 and p = 0.005 for group I and group II, respectively) (Table 3, Fig. 3).
Table 3.
Inter- and intra-group analyses of TMJ sound
| Arthrocentesis + PRP (Group I) N (%) | Arthrocentesis (Group II) N (%) | Mann–Whitney test | |||
|---|---|---|---|---|---|
| Present | Absent | Present | Absent | ||
| Pre-op (1) | 7 (58.4) | 5 (41.6) | 6 (50) | 6 (50) | 66.00, p = 0.688 (NS) |
| 1 month (2) | 3 (25) | 9 (75) | 2 (16.67) | 10 (83.33) | 66.00, p = 0.628 (NS |
| 3 months (3) | 2 (16.67) | 10 (83.3) | 1 (8.33) | 11 (91.67) | 66.00, p = 0.688 (NS) |
| 6 months (4) | 1 (8.33) | 11 (91.67) | 1 (8.33) | 11 (91.67) | p = 1 (NS) |
| Cochran test |
Cochran’s Q = 13.105 p = 0.004 (S) |
Cochran’s Q = 12.75 p = 0.005 (S) |
|||
Fig. 3.
Graphical analysis of TMJ sound
No loco-regional complications were observed during the treatment and follow-up periods.
Discussion
Temporomandibular joint disorders are one of the most common diseases leading to chronic orofacial pain. They are progressive in nature if left untreated. In early stages like disc displacement and adhesions, arthrocentesis has been the time-trusted procedure. It removes the catabolites and inflammatory cells and thus helps in improvement of oral opening, reduces pain and other symptoms [18].
Recent literature suggests that various therapeutic agents like sodium hyaluronate, steroids and platelet-rich plasma, etc., provide superior results if combined with arthrocentesis. So in the present study, we evaluated the synergistic effect of platelet-rich plasma with arthrocentesis in comparison with arthrocentesis alone in the management of internal derangement of TMJ.
Platelet-rich plasma (PRP) is a concentrate of platelet-derived growth factors which modifies the natural healing pathway in many ways. The activated platelets release a group of biologically active proteins that bind to the transmembrane receptors of their target cells, thus leading to the expression of gene sequences that promote cellular recruitment, growth and morphogenesis [19]. Growth factors and cytokines promote neovascularization, fibroblast proliferation and reduces inflammation [20].
In the present study, improvement in pain score was observed in both the groups, and maximum pain reduction was recorded at the end of 6 months. There was no significant difference in between the groups. Pihut et al. reported that application of the intra-articular injections of PRP had a positive impact on the reduction of the intensity of pain experienced by patients treated for TMJ dysfunction [21]. Lee et al. explained the analgesic effect of PRP. The study described that PRP has augmentation effect on the cannabinoid receptors CB1 and CB2 leading to improvement in the joint pain [22]. Kon et al. reported improvement in knee function and reduction in pain, thereby improving quality of life in patients of osteoarthritic knee [23].
Hossameldin et al. studied the efficacy of PRP versus hyaluronic acid in the management of Wilkes V temporomandibular joint patients. There was no statistical significant difference between the two groups regarding the success rates (65.6% in HA group and 69.6% in the PRP group) [24]. Lin et al. compared the efficacies of treatment using arthrocentesis plus PRP (A + PRP) and PRP alone for TMJ osteoarthritis (TMJ-OA). They concluded that both the approaches effectively treated TMJ-OA and did not show statistically significant differences in improving the joint crepitus sounds, reparative remodelling and TMJ arthralgia, but A + PRP treatment was superior to PRP alone in terms of the performance in improving symptoms such as TMD-associated headache, jaw range of motion <6 mm, myofascial pain with referral, and pain when chewing most foods [25]. Our study also showed that although PRP is more effective in reducing pain and symptoms, the results are not statistically significant in comparison with arthrocentesis alone.
Our study showed increase in mouth opening and reduction in joint sounds in both the groups and highest was observed after 6 months. This shows that minimum 6-month follow-up is required to observe definitive results. Few authors have reported that the effects of PRP are temporary and the improvement may diminish during the long-term follow-up [23]. So further studies with long-term follow-up are required to evaluate prolonged effect of PRP in temporomandibular disorders.
Conclusion
Various in vivo and in vitro clinical trials in past two decades have shown drastic improvement in TMJ functions with the use of PRP. But in our study, there was no statistically significant difference among these two groups. Thus, we can infer that although PRP is an abundant reservoir of growth factors, arthrocentesis alone is capable of treating TMJ disorders with similar outcome. However, further studies with larger sample size are required.
Funding
Nil.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cooper BC, Kleinberg I. Establishment of a temporomandibular physiological state with neuromuscular orthosis treatment affects reduction of TMD symptoms in 313 patients. Cranio. 2008;26:104–117. doi: 10.1179/crn.2008.015. [DOI] [PubMed] [Google Scholar]
- 2.Barros-Vde M, Seraidarian PI, Cortes MI, de Paula LV. The impact of orofacial pain on the quality of life of patients with temporomandibular disorder. J Orofac Pain. 2009;23(1):28–37. [PubMed] [Google Scholar]
- 3.Wilkes CH. Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg. 1989;115:469–477. doi: 10.1001/archotol.1989.01860280067019. [DOI] [PubMed] [Google Scholar]
- 4.Kirk WS., Jr Morphological differences between superior and inferior disc surfaces in chronic internal derangement of the temporomandibular joint. J Oral Maxillofac Surg. 1990;48:455–460. doi: 10.1016/0278-2391(90)90230-Y. [DOI] [PubMed] [Google Scholar]
- 5.Ogren M, Faltmars C, Lund B, Holmlund A. Hypermobility and trauma as etiologic factors in patients with disc derangements of the temporomandibular joint. Int J Oral Maxillofac Surg. 2012;41:1046–1050. doi: 10.1016/j.ijom.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Reston JT, Turkelson CM. Meta analysis of surgical treatments for temporomandibular articular disorders. J Oral Maxillofac Surg. 2003;61:737–738. doi: 10.1053/joms.2003.50169. [DOI] [PubMed] [Google Scholar]
- 7.Monje-Gil F, Nitzan D, Gonzalez-Garcia R. Temporomandibular joint arthrocentesis. Review of the literature. Med Oral Patol Oral Cir Bucal. 2012;17:575–581. doi: 10.4317/medoral.17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long X, Chen G, Cheng AH, Cheng Y, Deng M, Cai H, et al. A randomized controlled trial of superior and inferior temporomandibular joint space injection with hyaluronic acid in treatment of anterior disc displacement without reduction. J Oral Maxillofac Surg. 2009;67:357–361. doi: 10.1016/j.joms.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Guarda-Nardini L, Olivio M, Ferronato G, Salmaso L, Bonnini S, Manfredini D. Treatment effectiveness of arthrocentesis plus hyaluronic acid injections in different age groups of patients with temporomandibular joint osteoarthritis. J Oral Maxillofac Surg. 2012;70:2048–2056. doi: 10.1016/j.joms.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Sanroman J. closed lock (MRI fixed disc): a comparison of arthrocentesis and arthroscopy. Int J Oral Maxillofac Surg. 2004;33:344–348. doi: 10.1016/j.ijom.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Lana J, Weglein A, Vicente E, Perez A, Rodrigues A, Luzo Â, et al. Platelet-rich plasma: regenerative medicine: sports medicine, orthopedic, and recovery of musculoskeletal injuries. New York: Springer; 2014. [Google Scholar]
- 12.Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 13.Akeda K, An H, Okuma M, Attawia M, Miyamoto K, Thonar E, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation. Osteoarthr Cartil J. 2006;14:1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair and matrix biosynthesis. Arthritis Res Ther. 2014;16:20419. doi: 10.1186/ar4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Osch GJ, Bernsen MR, van Buul GM, Koevoet WL, Kops N, Bos PK, Verhaar JA, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–2370. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. International RDC/TMD Consortium network, international association for dental research; Orofacial pain special interest group, international association for the study of pain. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network and orofacial pain special interest group. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415–421. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- 18.Nitzan DW, Dolwick MF, Martinez GA. Temporomandibular joint arthrocentesis: a simplified treatment for severe, limited mouth opening. J Oral Maxillofac Surg. 1991;49:1163–1167. doi: 10.1016/0278-2391(91)90409-F. [DOI] [PubMed] [Google Scholar]
- 19.Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev. 2010;62(7–8):741–752. doi: 10.1016/j.addr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 21.Pihut M, Szuta M, Ferendiuk E, Zeńczak-Więckiewicz D. Evaluation of pain regression in patients with temporomandibular dysfunction treated by intra-articular platelet-rich plasma injections: a preliminary report. Biomed Res Int. 2014;2014:132369. doi: 10.1155/2014/132369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release. 2012;159(3):332–337. doi: 10.1016/j.jconrel.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, Cenacchi A, Fornasari PM, Giannini S. 472 Marcacci M: Platelet-rich plasma: intra-articular knee injections produced favorable results on 473 degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18:472. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 24.Hossameldin RH, McCain JP. Efficacy of platelet-rich plasma versus hyaluronic acid intraarticular injection in arthroscopic management of Wilkes V temporomandibular joint patients. Int J Oral Maxillofac Surg. 2017;46(1):229–230. doi: 10.1016/j.ijom.2017.02.776. [DOI] [Google Scholar]
- 25.Lin SL, Tsai CC, Wu SL, Ko SY, Chiang WF, Yang JW. Effect of arthrocentesis plus platelet-rich plasma and platelet-rich plasma alone in the treatment of temporomandibular joint osteoarthritis: a retrospective matched cohort study. Medicine. 2018;97(16):e477–e484. doi: 10.1097/MD.0000000000010477. [DOI] [PMC free article] [PubMed] [Google Scholar]