Abstract
Background
Medication management requires complex cognitive functioning, and therefore, difficulty taking medications might be an early sign of cognitive impairment and could be a risk factor for Alzheimer’s disease and related dementias (ADRD). Accordingly, people with difficulty taking medications may benefit from more detailed cognitive screening, potentially aiding in the diagnosis of ADRD, which is underdiagnosed. We are unaware of evidence on medication management difficulties that precede a real-world ADRD diagnosis in the USA.
Objective
Examine the association between difficulty taking medications and subsequent real-world ADRD diagnoses.
Design
Case-control study, using Health and Retirement Study (HRS) survey data linked to Medicare claims.
Participants
A total of 1461 HRS respondents with an ADRD diagnosis observed from 1993 to 2012 (cases), matched by year of birth, wave of HRS entry, and sex to 3771 controls with no ADRD diagnosis.
Main Measures
We examined the association between diagnosis of ADRD and self-reported difficulty taking medications in the preceding years (1–2 and 3–4 years prior to case definition). Control individuals were assigned the index date from their matched case. Conditional logistic regressions adjusted for age, sex, race, education, and comorbidities.
Key Results
Compared with matched controls, cases had higher prevalence of difficulty taking medications 1–2 years prior to diagnosis (11.0% versus 2.3%), and 3–4 years prior to diagnosis (5.8% versus 2.3%). Adjusted analyses showed that compared with individuals without ADRD, those with an ADRD diagnosis had more than four times higher odds of difficulty taking medications 1–2 years prior (OR = 4.56 (CI 3.30–6.31)), and more than two times higher odds of difficulty taking medications 3–4 years prior (OR = 2.41 (CI 1.61–3.59)).
Conclusions
Odds of medication difficulty 1–2 years prior were more than four times greater for individuals with ADRD diagnoses compared with those without ADRD. Medication management difficulties may prompt further cognitive screening, potentially aiding in earlier recognition of ADRD.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-06279-y) contains supplementary material, which is available to authorized users.
KEY WORDS: Alzheimer’s disease and related dementias, medication management, cognitive screening
INTRODUCTION
Alzheimer’s disease and related dementias (ADRD) are underdiagnosed,1, 2 which is concerning because of the missed opportunities to inform care and facilitate planning by patients and their families. Accordingly, prediction models have been developed with the aim of improving detection, including those that assess everyday memory deficits, executive function, and financial capacity.3–6 Unfortunately, many of these models are complex, onerous, require data that makes them difficult or impossible for primary care providers to use, and no single model is universally applicable.3 Simply observable risk factors that can be easily and quickly discerned as part of routine primary care might serve as an initial tool for primary care providers, which could suggest that detailed and validated cognitive screening might be beneficial.
Instrumental activities of daily living (IADL) are a measure of functional status among older adults, some of which may be easily discernible for providers in their routine care.7 Many IADLs require complex cognitive functioning, and a small body of research has shown that functional status declines for individuals in the years prior to a formal ADRD diagnosis.8, 9 In a notable prospective cohort study in France, Peres et al. found that future cases of dementia had greater restriction in four IADLs: telephone use, transportation, medication management, and managing finances. Restrictions in finances were the first to manifest in future ADRD cases, occurring 10 years prior to diagnosis.10 Another study showed the utility of IADLs in screening tools, by combining money and medication management with demographic and comorbidity data in order to identify subgroups to target for cognitive screening.11 These results suggest that IADL restrictions could aid in ADRD risk prediction, but the suggested measures remain relatively complex and burdensome on a primary care provider.
In this study, we focused on one IADL, difficulty taking medications, as an early hint of cognitive impairment, because of the high prevalence of use of prescription drugs among older adults in the USA, and the simplicity of discerning these difficulties. Prescription drugs are used by 91% of adults over the age of 65 in the USA,12 and problems with medication management and adherence are common, especially for those with cognitive impairment.13 Additional research has found decision-making regarding medication management to be impaired for people with mild cognitive impairment, which is defined as cognitive problems that are greater than normal age-related changes, but not as serious as dementia.14 Medication is considered one of the four core concepts of geriatric care in an “age-friendly health system,” and routinely inquiring about medication use is important for ensuring safe and effective medication use.15 The widespread and frequent use of prescription drugs, coupled with the ease of inquiry for primary care physicians and pharmacists as a part of routine care, suggest that difficulty taking medications could be an especially important risk factor for ADRD. These signals could improve risk prediction, and potentially be used as a simple and inexpensive way to target additional cognitive screening, and therefore facilitate earlier diagnosis of ADRD. To our knowledge, existing work has not studied the relationship between medication difficulties and real-world diagnoses of ADRD. To this end, we examined the association between difficulty taking medications and subsequent ADRD diagnoses among Medicare enrollees in the USA.
METHODS
Data and Sample
We conducted a nested case-control study in Health and Retirement Survey (HRS) respondents with linked Medicare claims data in the years 1993–2012.16 The HRS is conducted by the University of Michigan (grant number NIA U01AG009740), with survey waves every 2 years.16 After in-person baseline interviews, most follow-up interviews are conducted via telephone. Among HRS respondents, 80% consent to have their data linked with Medicare claims, which allows observation of all diagnoses and health care utilization.
Selection of Cases and Controls
We compared difficulty taking medications for cases with an ADRD diagnosis (1997–2012) to matched controls without diagnosed ADRD. Cases were selected by the incidence of ADRD as defined in real-world claims data, according to the Chronic Conditions Warehouse (CCW) ICD-9 codes of ADRD (Supplementary Text S1). Index date for cases was the date of incident ADRD (from CCW), and control individuals were assigned an index date of their matched case. Control individuals were required to have three consecutive years of fee-for-service enrollment at their index date, to ensure that all recent diagnoses were observable. We excluded individuals living in nursing homes, and those with missing medication difficulty information. A total of 1461 eligible case individuals were randomly assigned at most four control individuals (N = 3771) with same year of birth, sex, and wave of HRS entry, to create an analytic sample of 5232 individuals, all of whom were using medications. We matched on these three variables in order to select appropriate comparators for our cases, while still maximizing sample size. The process of selection for the sample is in Supplementary Text S1.
Measures
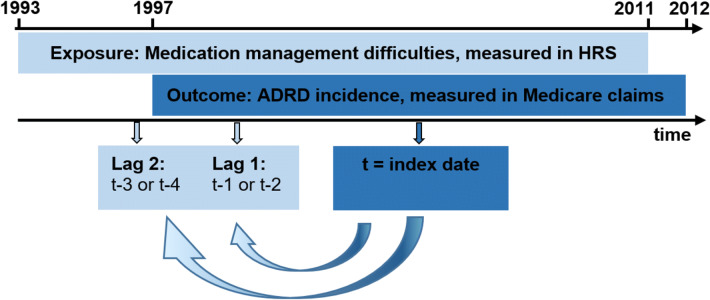
The primary exposure was an indicator for if the individual self-reported difficulty taking medications, as measured in the HRS Instrumental Activities of Daily Living (IADL) assessment (HRS question reported in Supplementary Text S1), and compiled by RAND for the RAND HRS.17 The question asked if, “(Because of a health or memory problem do you have) any difficulty taking medications?” Proxy responses were not used. For an individual with index date t, we examined difficulty taking medications at two points in time, looking back from 90 days prior to date t: 1 or 2 years prior, and 3 or 4 years prior (Fig. 1). The 90-day washout was imposed in order to exclude medication difficulty measures that were concurrent with a diagnosis. The time intervals of measurement correspond to waves of HRS data collection, which occurs every 2 years. Covariates were measured in the HRS (year of birth, sex, wave of HRS entry, race/ethnicity, and years of education), except for comorbidities, which were measured in the Medicare claims (hypertension, stroke, acute myocardial infarction (AMI), diabetes, atrial fibrillation, and hyperlipidemia).
Fig. 1.
Study design and data sources. Notes: Index date is incident ADRD for cases, and for controls, it is the diagnosis date of their matched case. Year t-1 ends 90 days before index date; lag 1 is captured 1 or 2 years before that, and lag 2 is captured 3 or 4 years before. The timing of the lags corresponds to the waves of HRS (Health and Retirement Study) data collection, which occur every 2 years. ADRD, Alzheimer’s disease and related dementia diagnoses
Statistical Analysis
We described cases and controls according to difficulty taking medications, sociodemographic characteristics, and the presence of comorbidities. Conditional logistic regression models were used to examine if difficulty taking medications in the years t-4 to t was a significant predictor of an ADRD diagnosis in year t (t = 1997–2012). Relative to their index date, t, we ran two separate analyses for medication difficulties in the years t-1 or t-2 (lag 1) and t-3 or t-4 (lag 2). The dependent variable in both analyses was a binary indicator for if the individual was diagnosed with ADRD in year t.
We used two models to control for different sets of covariates. Model 1 captured the association of medication difficulties and ADRD with only parsimonious adjustment (race/ethnicity indicators and quartiles of education years). Model 2 included additional covariates for six comorbidities frequently associated with ADRD (hypertension, stroke, AMI, diabetes, atrial fibrillation, and hyperlipidemia); these comorbidities are also a proxy for overall medication burden. In a robustness check, to control for the possibility that some individuals with cognitive impairment may not accurately report their difficulties taking medication, we analyzed a subsample restricted to individuals whose cognitive screening (measured in HRS as the sum of Total Word Recall and Mental Status scores18, 19) did not suggest ADRD prior to index date (as far back as their first wave in HRS).
RESULTS
The sample for individuals included in analyses of medication difficulties 1–2 years prior to ADRD diagnosis is described in Table 1 (N = 5232). The analytic sample for analyses of medication difficulties 3–4 years prior to diagnosis is partially overlapping, corresponding to the set of individuals with the medication management difficulty question observed 3–4 years prior to time t (N = 4595; this sample is described in Supplementary Table S1, and does not vary meaningfully from Table 1).
Table 1.
Sample Characteristics of Cases and Controls
| Total | ADRD | No ADRD | |
|---|---|---|---|
| N | 5232 | 1461 | 3771 |
| Age (mean, SD) | 78.1 (7.3) | 79.2 (7.5) | 77.7 (7.1) |
| Female (%) | 59.4 | 60.4 | 59.0 |
| Race/ethnicity (%) | |||
| White | 81.7 | 75.8 | 84.0 |
| Black | 14.0 | 18.6 | 12.2 |
| Hispanic | 2.8 | 3.8 | 2.3 |
| Other | 1.1 | 1.2 | 1.1 |
| Asian | 0.4 | 0.5 | 0.3 |
| Education years (mean, SD) | 11.2 (3.2) | 10.6 (3.6) | 11.4 (3.0) |
| Medication difficulties (%) | |||
| Index date (t) | 8.4 | 21.5 | 3.8 |
| 1 or 2 years prior to index date | 4.7 | 11.0 | 2.3 |
| 3 or 4 years prior to index date | 3.3 | 5.8 | 2.3 |
| Diagnoses (%) | |||
| Hypertension | 74.7 | 83.9 | 71.1 |
| Stroke | 17.0 | 29.2 | 12.3 |
| AMI | 6.2 | 7.5 | 5.6 |
| Diabetes | 32.0 | 39.3 | 29.2 |
| Atrial fibrillation | 14.0 | 19.1 | 12.0 |
| Hyperlipidemia | 61.0 | 66.0 | 59.1 |
Characteristics of HRS respondents with linked Medicare claims (1993–2012). Cases had a claims-based diagnosis of ADRD in year t, and were matched to 1–4 controls who had the same sex, age, and wave of entry. Sample restricted to community-dwelling individuals with non-missing medication difficulty 1 or 2 years prior index date. Diagnoses are assessed prior to index date. ADRD Alzheimer’s disease and related dementias, HRS Health and Retirement Study, AMI acute myocardial infarction
In the sample for analyses of medication difficulties 1–2 years prior to ADRD diagnosis (Table 1), 1461 cases were diagnosed with ADRD, 5.8% of whom had difficulty managing their medications 3–4 years prior to diagnosis, as opposed to 2.3% of their matched controls. When examining 1–2 years prior to diagnosis, 11.0% of cases had difficulties, compared with 2.3% of controls. The cases were slightly older than controls (mean, 79.2 vs 77.7 years), more likely to be Black (18.6% vs 12.2%) or Hispanic (3.8% vs 2.3%), had fewer years of education (10.6 vs 11.4), and had higher rates of hypertension (83.9% vs 71.1%), stroke (29.2% vs 12.3%), acute myocardial infarction (AMI) (7.5% vs 5.6%), diabetes (39.3% vs 29.2%), atrial fibrillation (19.1% vs 12.0%), and hyperlipidemia (66.0% vs 59.1%).
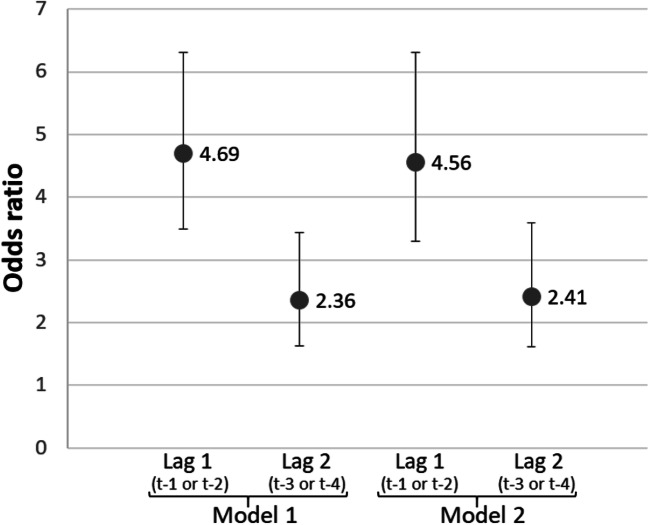
The adjusted odds ratios (OR) for the associations between difficulty taking medications and subsequent ADRD are reported in Fig. 2. Model 1, which controlled for race/ethnicity and education, had very similar results to model 2, with additional adjustment for comorbidities. Model 2 showed that cases with ADRD had 456% higher odds of difficulty taking medications 1–2 years prior (OR = 4.56, CI 3.30–6.31, p < 0.001, and 241% higher odds of difficulty taking medications 3–4 years prior (OR = 2.41, CI 1.61–3.59, p < 0.001). The ORs for specific comorbidities are featured in Supplementary Table S2, along with results from robustness checks.
Fig. 2.
Association of ADRD incidence (year t) and medication management difficulties 1–2 years prior (lag 1) and 3–4 years prior (lag 2). Notes: Odds ratios from conditional logistic regressions of ADRD incidence (year t) as related to medication management difficulties in previous years (lag 1 is difficulties measured 1 or 2 years before index date, and lag 2 is difficulties measured 3 or 4 years before index date). Model 1 adjusts for race/ethnicity and education; model 2 adjusts for race/ethnicity, education, and indicators for if the person was diagnosed with hypertension, stroke, acute myocardial infarction, diabetes, atrial fibrillation, and hyperlipidemia. Sample is HRS respondents with linked Medicare claims (1993–2012). Cases had a claims-based diagnosis of ADRD in year t, and were matched to 1–4 controls who had the same sex, age, and wave of entry. Sample restricted to community-dwelling individuals with non-missing medication difficulty 1 or 2 years prior to year t (N = 5232) and 3 or 4 years prior to year t (N = 4595). ADRD, Alzheimer’s disease and related dementias; HRS, Health and Retirement Study
DISCUSSION
We used HRS survey data and linked Medicare claims to examine difficulty taking medications for individuals in the years preceding an ADRD diagnosis, comparing them to matched controls who did not receive a diagnosis. ADRD diagnosis was significantly associated with past difficulty taking medications, with more than four times the odds of difficulty 1–2 years prior to diagnosis, and more than twice the odds 3–4 years prior. These findings suggest that medication management difficulties are a risk factor for ADRD. The presence of difficulties may help identify older adults with early signs of ADRD, potentially prompting the subsequent use of validated cognitive screening tests.
Difficulty taking medications is associated with cognitive impairment,20, 21 and it is plausible that the medication difficulties measured among those who subsequently developed ADRD in our study represent an unnoticed worsening of cognitive status. Importantly, self-reported medication difficulties are easily discernible by primary care physicians and pharmacists. Other IADL measures associated with future ADRD incidence, like financial capacity or credit score,5, 6 are not easily apparent to healthcare providers. Additional measures used in ADRD prediction models, such as comorbidities and genetics, are more burdensome on the provider to ascertain, and are therefore harder to include in routine care. Medication difficulties, on the other hand, are simple and fast to discern, and therefore could be easily used to improve risk prediction, and possibly also identify individuals for validated cognitive screening tests. Furthermore, better identification of medication difficulties could have additional benefits related to non-adherence and polypharmacy.
Our results build on earlier evidence that found declines in functional status in the years prior to ADRD diagnosis,8, 9 including the work on IADLs by Peres et al.10 This French study with regularly scheduled neurologist follow-up visits found that in addition to restrictions related to telephone use, transportation, and management of finances, restrictions in medication responsibilities were significantly associated with dementia diagnosis 5 years later (OR = 3.49, CI 1.63–7.48, p = 0.001), and 2 years later (OR = 3.13, 1.70–5.75, p = 0.002).10 Our findings in the American context are similar, except we found that the association increased with proximity to diagnosis date. A difference in our study is that our index date referred to a real-world clinical diagnosis, compared with a research-based diagnosis (part of regularly scheduled neurologist follow-ups) in the French study.
Our study has limitations, including residual confounding. Self-reported measures may not accurately reflect medication management difficulties, especially for individuals experiencing cognitive impairment. We therefore conducted a sensitivity analysis in a subsample where we removed anyone whose cognitive screening in HRS showed signs of ADRD prior to index date, and these results confirmed the main results. It is also important to acknowledge that HRS responses about difficulty taking medications that were provided via telephone may differ from responses given in-person to a clinician, which may reduce the generalizability of our findings. Another limitation is that we did not know the cause of difficulty taking medications. While it seems probable that the difficulties were related to cognition, evidence has shown difficulty taking medications is associated with worse health status,22 and lower medication refill persistence for individuals taking a greater number of medications.23 Indeed, Table 1 shows that diagnostic risk factors for ADRD are more prevalent in cases, as would be expected. However, model 2 adjusts for these factors (e.g., comorbidities), which also proxy for medication burden; this model gives us confidence that our results are unlikely to be driven by underlying comorbidities or medication burden. We also note that severity of illness could be a confounder, which is only partially addressed by including comorbidity indicators that may be correlated with severity of illness. A final limitation is that since our lagged analyses used slightly different samples (according to non-missing medication difficulty information at the time of the lag), the ORs for the different lags are not directly comparable.
In conclusion, this study makes an important contribution to understanding how information that can be easily and quickly collected as part of routine clinical care is related to future diagnosis of ADRD. Given that formal diagnosis of ADRD is often delayed beyond its actual onset, leading to underdiagnosis of ADRD, it is important to identify signs of early cognitive impairment. Such hints could improve risk prediction, and serve as one of many aids to primary care providers to prompt the subsequent use of validated cognitive screening, which could ultimately improve diagnostic timing and accuracy. This is especially important for racial/ethnic minorities with elevated risk of underdiagnosis.24 Benefits of improved diagnostic timing could include benefits from pharmaceuticals and other therapies, including those yet to be developed, which can provide modest delays in the progression of ADRD for some patients.25 Better informed patients and caregivers can plan more adequately for the cognitive decline that will accompany progression, optimize care for comorbidities, and possibly experience reduced stress and anxiety. Future work should continue to examine how to improve early recognition of cognitive decline, without overburdening providers and caregivers.
Electronic Supplementary Material
(DOCX 32 kb)
Acknowledgments
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. Everyone who significantly contributed to the work is listed as an author. Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number K76AG059929 (Marcum). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Funding
• Douglas Barthold is supported by NHLBI R01HL126804, Kaiser/NIH R01HL130462, NIH R01MH121424-01A1, NHLBI-1OT3HL152448-01
• Zachary Marcum is supported by the National Institute on Aging of the National Institutes of Health under Award Number K76AG059929.
• Shuxian Chen is supported by the Comparative Health Outcome, Policy, and Economics (CHOICE) Institute in the Department of Pharmacy at the University of Washington.
• Lindsay White is supported by the National Institute on Aging 1R01AG049815.
• Nagham Ailabouni is supported by the Plein Center for Geriatric Pharmacy Fellowship.
• Anirban Basu has no funding support to report.
• Norma Coe is supported by the National Institute on Aging 1R01AG049815.
• Shelly Gray is supported by Centers for Disease Control and Prevention U01CE002967 and NIA U01AG006781.
Compliance with Ethical Standards
Conflict of Interest
All authors have no conflict of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 2.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Disease and Associated Disorders. 2009;23(4):306. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang EY, Harrison SL, Errington L, et al. Current developments in dementia risk prediction modelling: an updated systematic review. PLOS ONE. 2015;10(9). [DOI] [PMC free article] [PubMed]
- 4.Farias ST, Lau K, Harvey D, Denny KG, Barba C, Mefford AN. Early functional limitations in cognitively normal older adults predict diagnostic conversion to mild cognitive impairment. Journal of the American Geriatrics Society. 2017;65(6):1152–1158. doi: 10.1111/jgs.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spreng RN, Karlawish J, Marson DC. Cognitive, social, and neural determinants of diminished decision-making and financial exploitation risk in aging and dementia: A review and new model. Journal of Elder Abuse & Neglect. 2016;28(4-5):320–344. doi: 10.1080/08946566.2016.1237918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean LT, Nicholas LH. Using Credit Scores to Understand Predictors and Consequences of Disease. American Jounal of Public Health. 2018;108(11):1503–1505. doi: 10.2105/AJPH.2018.304705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millán-Calenti JC, Tubío J, Pita-Fernández S, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Archives of Gerontology and Geriatrics. 2010;50(3):306–310. doi: 10.1016/j.archger.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Gaugler JE, Hovater M, Roth DL, Johnston JA, Kane RL, Sarsour K. Depressive, functional status, and neuropsychiatric symptom trajectories before an Alzheimer’s disease diagnosis. Aging & Mental Health. 2014;18(1):110–116. doi: 10.1080/13607863.2013.814100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farias ST, Chou E, Harvey DJ, et al. Longitudinal trajectories of everyday function by diagnostic status. Psychology and Aging. 2013;28(4):1070. doi: 10.1037/a0034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérès K, Helmer C, Amieva H, et al. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. Journal of the American Geriatrics Society. 2008;56(1):37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 11.Barnes DE, Beiser AS, Lee A, et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimer’s & Dementia. 2014;10(6):656-665. e651. doi: 10.1016/j.jalz.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health, United States, 2016: With Chartbook on Long-term Trends in Health. In. Hyatsville, MD: National Center for Health Statistics; 2017. [PubMed]
- 13.Smith D, Lovell J, Weller C, et al. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLOS ONE. 2017;12(2):e0170651. doi: 10.1371/journal.pone.0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lui VW-C, Lam LC-W, Chau RC-M, et al. Capacity to make decisions on medication management in Chinese older persons with mild cognitive impairment and mild Alzheimer’s disease. International Psychogeriatrics. 2012;24(7):1103–1111. doi: 10.1017/S1041610212000129. [DOI] [PubMed] [Google Scholar]
- 15.Fulmer T, Mate KS, Berman A. The age-friendly health system imperative. Journal of the American Geriatrics Society. 2018;66(1):22–24. doi: 10.1111/jgs.15076. [DOI] [PubMed] [Google Scholar]
- 16.Health and Retirement Study, RAND HRS Longitudinal File 2014 (V2) public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Ann Arbor, MI, 2014.
- 17.RAND HRS Longitudinal File 2014 (V2). Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration. Santa Monica, CA (February 2018).
- 18.Fisher GG, Hassan H, Faul JD, Rodgers WL, Weir DR. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992 – 2014 (Final Release Version) Data Description. Survey Research Center, University of Michigan, 2017.
- 19.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(suppl_1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sino CG, Sietzema M, Egberts T, Schuurmans M. Medication management capacity in relation to cognition and self-management skills in older people on polypharmacy. The Journal of Nutrition, Health & Aging. 2014;18(1):44–49. doi: 10.1007/s12603-013-0359-2. [DOI] [PubMed] [Google Scholar]
- 21.Millán-Calenti JC, Tubío J, Pita-Fernández S, Rochette S, Lorenzo T, Maseda A. Cognitive impairment as predictor of functional dependence in an elderly sample. Archives of Gerontology and Geriatrics. 2012;54(1):197–201. doi: 10.1016/j.archger.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Morgan AL, Masoudi FA, Havranek EP, et al. Difficulty taking medications, depression, and health status in heart failure patients. Journal of Cardiac Failure. 2006;12(1):54–60. doi: 10.1016/j.cardfail.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Grant RW, O’Leary KM, Weilburg JB, Singer DE, Meigs JB. Impact of concurrent medication use on statin adherence and refill persistence. Archives of Internal Medicine. 2004;164(21):2343–2348. doi: 10.1001/archinte.164.21.2343. [DOI] [PubMed] [Google Scholar]
- 24.Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2019;5:891–898. doi: 10.1016/j.trci.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 32 kb)