Abstract
Background
Melanotic neuroectodermal tumour of infancy (MNTI) is a rare benign neoplasm. MNTI appears most often during the first year of life, arises predominantly in the maxilla and tends to recur. We discuss possible therapeutic options given in the literature and within our experience in three cases.
Patients
In our recent case, we used an intraoral approach to perform resection of the right-sided maxilla. Despite tumour-positive margins, there was no recurrence over the course of one year. In a previous case of MNTI, two recurrences occurred and 6 months after last resection patient received a rib graft for maxillary reconstruction. However, at the age of 7 years, the infant displayed severe maxillary hypoplasia. In a third case of MNTI, the patient was followed up after initial therapy for two decades and underwent multiple reconstruction procedures to achieve successful rehabilitation.
Conclusion
Surgical treatment of MNTI should respect vital anatomic structures to avoid gross mutilation. The need for extended and repetitive tumour resection in early childhood can lead to growth disturbances and to further multiple reconstruction procedures in adulthood. Because of the rarity of MNTI, an international database is warranted to evaluate therapies and clinical courses over decades.
Keywords: Melanotic neuroectodermal tumour of infancy; Recurrence, growth disturbance; Maxillary reconstruction; Free fibula flap
Background
Melanotic neuroectodermal tumour of infancy (MNTI) is well described by anecdotes, small surveys, and the literature reviews, even though it is a rare tumour. However, factors that could have an impact on its clinical course, prognosis, and outcome are still disputable. Since it was first described by Krompecher in 1918, there have been reports of a total of more than 470 cases [1, 2]. In general, the tumour is categorized as a melanotic neuroectodermal neoplasm since staining against melanoma marker antibody and neuron-specific enolase are positive, and histology reveals a typical biphasic cell pattern with neuroblast-like cells and epithelioid cells containing melanin. Furthermore, high levels of urinary vanillylmandelic acid (VMA) can be measured in most cases [3, 4]. MNTI appears most frequently during the first year of life at the maxillary site (60%). Other locations are the skull base (18%), the mandible (10%), and in the remaining 12% the peripheral bones and even the testis and epididymis [2]. The tumour is of firm consistency and has an almost reddish to bluish-glowing colour. Similar presentations may be seen with diverse odontogenic and gingival tumours, which are the most important differential diagnoses [5].
Generally, MNTI has to be treated surgically, with treatments comprising a spectrum from curettage to segmental resection, thus causing wide-ranging defects. However, MNTI has a high rate of recurrence and in some rare cases; primary malignancy or metastases of MNTI primarily classified as benign have been observed with even fatal courses [6]. Hence, radical surgical treatments and adjuvant therapy including chemotherapy are still worth discussing.
Few reports describing the further clinical courses over several years after therapy for MNTI are available [7, 8]. Case reports covering the period of time from first diagnosis to the end of reconstruction procedures upon the end of adolescence simply do not exist in the literature. In the years 1992 and 1998, we treated two infants with MNTI. Against the background of a recent case, we reviewed the further clinical courses of all our cases and present, to the best of our knowledge, the first report of successful reconstruction of one case followed up for 20 years after surgical therapy for MNTI.
Patients
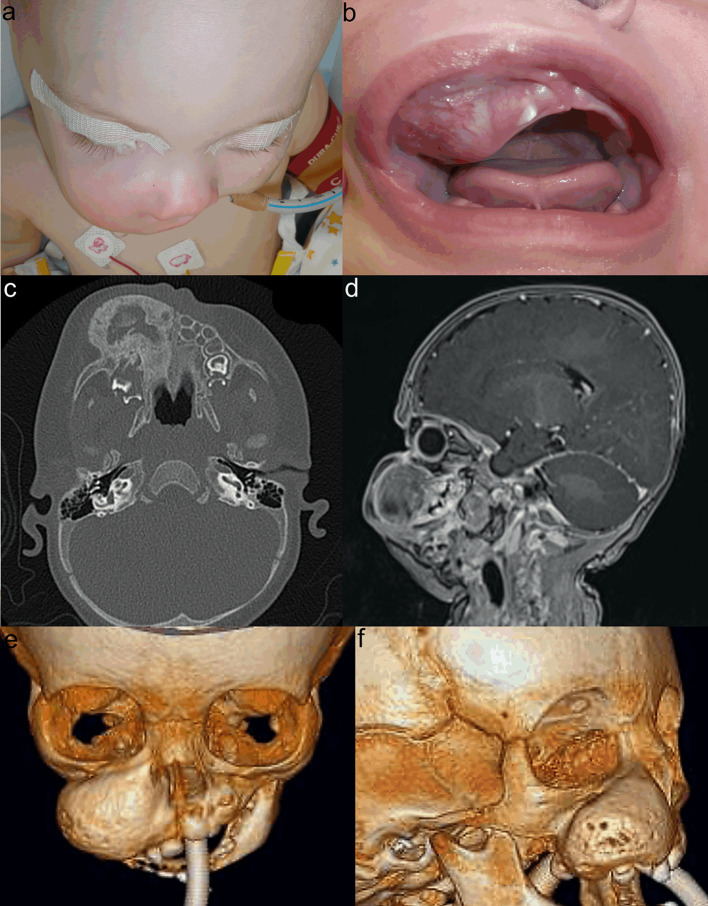
Our recent case of MNTI was identified in the right maxilla of a five-month-old male infant (case 1). The tumour mass involved almost all teeth of the right upper jaw, infiltrated the apertura pirifomis and was close to the orbital rim and floor but presented no clinical or radiological signs of invasion of these structures (Fig. 1). Biopsies revealed the pathohistological diagnosis of MNTI (Fig. 2). The level of VAS was high at 4.6 mg/L. Surgery was performed using an intraoral approach to avoid extraoral scarring and lip splitting. The infraorbital nerve, nasolacrimal duct, and hard palate were preserved, but the infiltrated alveolar ridge containing tooth germs had to be removed. The surgical approach was then closed primarily with a mucosal flap. The infant stayed for overnight intubation and spent the following 5 days at the intensive care unit. VAS levels decreased beyond the lower detection limit of 0.2 mg/L even though definite histology revealed positive tumour margins at the orbital floor and maxillary tuber. Magnetic resonance imaging (MRI) and computed tomography (CT) scans were first performed 6 weeks after surgery. Neither clinical nor MRI or CT findings showed signs of residual tumour masses or recurrences. The face did not appear grossly asymmetric at this time. Further MRI and CT scans after 6 months also revealed no evidence of recurrence. The infant grew and thrived and is re-admitted for close follow-ups at 5 to 6 months intervals.
Fig. 1.
Initial findings of MNTI in case 1. Swelling of the right midface caused gross asymmetry due to the bulging tumour (a, b). CT scan revealed expanded tumour mass with ossified borders (c). MRI showed close relationship between tumour and orbital floor (d). 3D CT scan revealed total tumour volume (e, f)
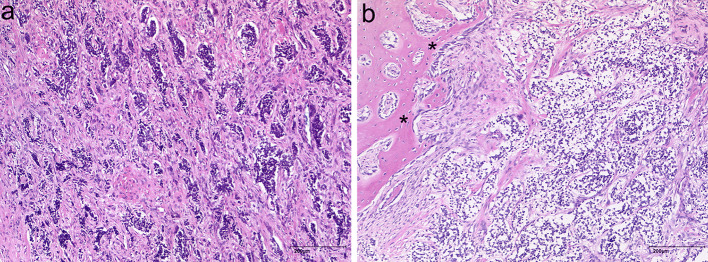
Fig. 2.
Pathohistological findings in MNTI. A biphasic cell pattern consisting of nests of dark-staining neuroblast-like round cells within a stroma containing larger epithelioid cells with melanin pigmentation (a). Tumour branches infiltrate osseous structures (asterisks, b) (H&E)
Our earliest documented case of MNTI was seen in 1992. It involved the right maxilla in a two-month-old male infant (case 2). Tumour resection was performed via an intra- and extraoral approach with lip splitting. Marginal resection included parts of the right-sided alveolus and parts of the apertura pirifomis at the level of the alveolus. After 2 weeks and again after 3 months, recurrences of MNTI appeared and were resected. Six months after the last surgery, the infant underwent osseous reconstruction using a free transplant from the sixth rib. Upon the further course, the residual defect became relatively small comprising a wedge-shaped defect over a distance of two centimetres in the canine area. However, by the age of 7 years the patient had developed severe maxillary hypoplasia with crossbite even on the unaffected side (Fig. 3). Shortly thereafter, the patient and his family moved to another town and were lost to follow-up.
Fig. 3.
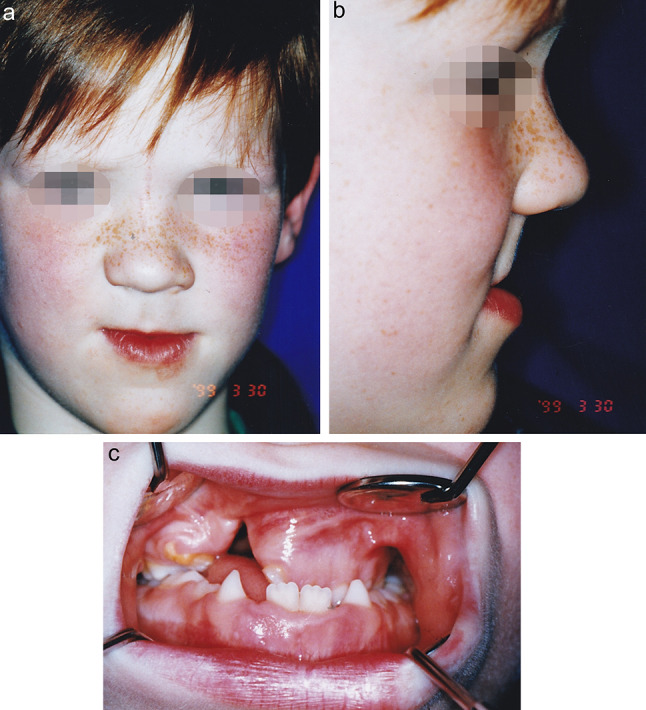
Infant of case 2 at the age of 7 years. Midface hypoplasia (a, b) and crossbite even on the unaffected side were present with a persistent wedge-shaped defect at the right alveolus (c)
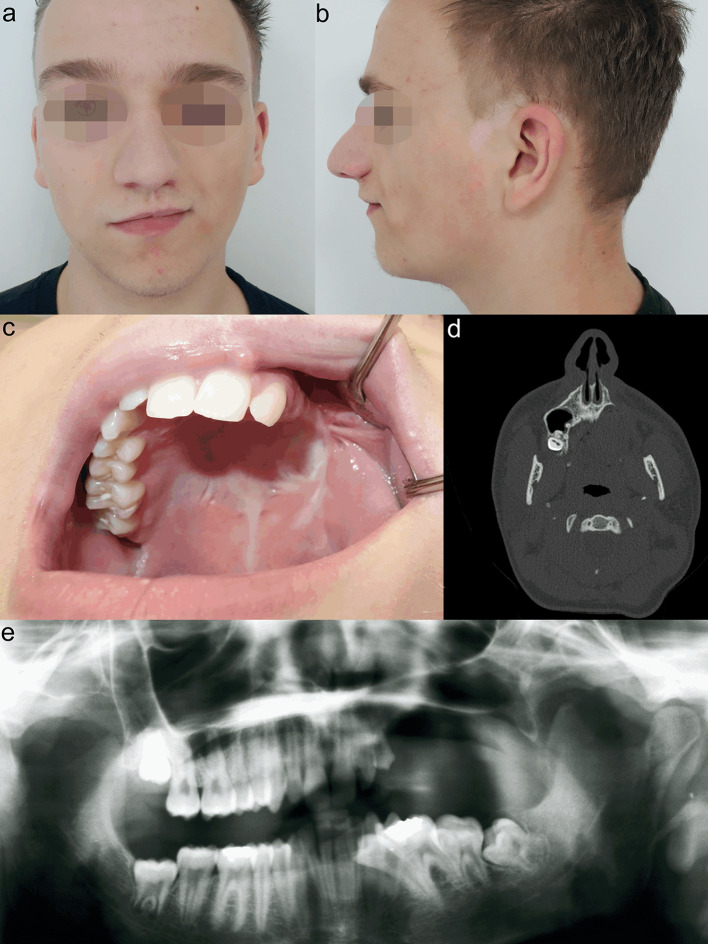
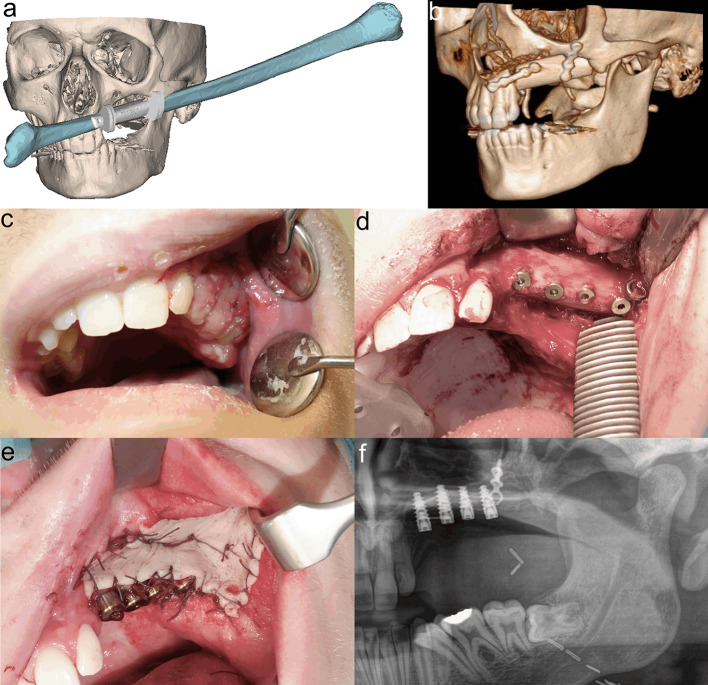
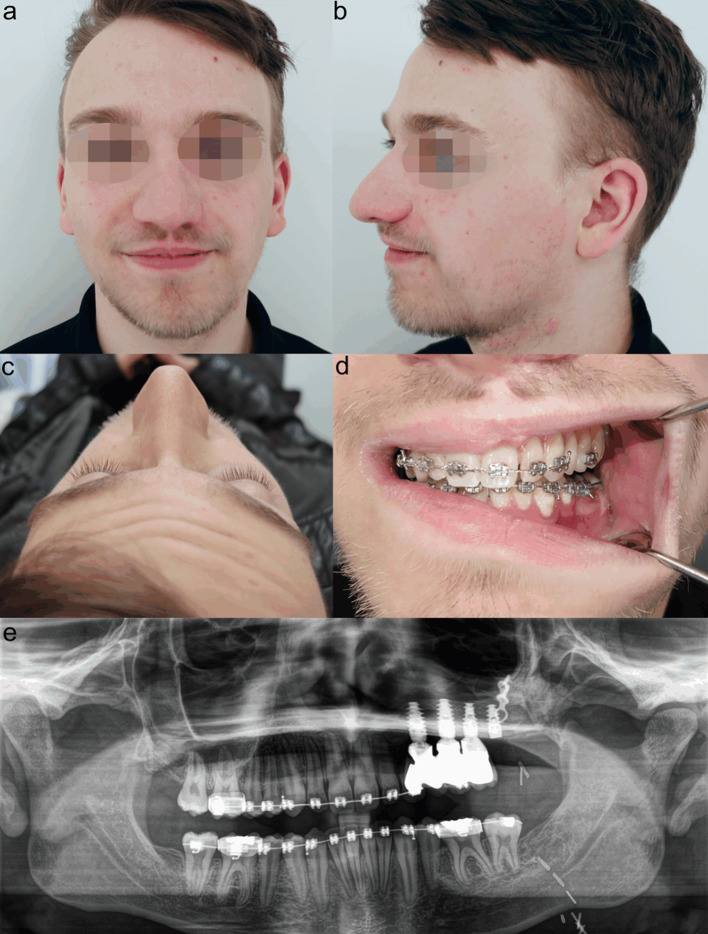
In 1998, we treated MNTI in a six-week-old male infant (case 3). A left-sided maxillectomy was performed through an intra- and extraoral approach including lip splitting. Segmental resection comprised an area beginning one centimetre lateral to the midline and ending distally beyond the maxillary tuber. The orbital rim and floor were preserved. The defect was packed with gauze and healed secondarily after definite removal of the gauze package. However, after 3 months, clinical and MRI findings confirmed a four by three centimetre recurrence. After resection again, no more recurrence was apparent upon the further course. During the next months, the intraoral defect closed spontaneously with development of a wide and rigid scar. In the yearly follow-up sessions, increasingly asymmetric growth of the midface with hypoplasia of the left midface could be observed but did not affect the patient`s well-being. At the age of 12 years, provisional dentures were manufactured. However, the patient complained about the comfort of the dentures and refused to wear them anymore. At the age of 17 years, when no further growth was expected, the patient agreed to our suggestion of osseous reconstruction as a requirement for further prosthodontic dental rehabilitation (Fig. 4). For maxillary reconstruction of the Okay Class II defect [9], we used a free osseous fibula flap with a surrounding flexor halluces muscle cuff. Pre-surgical virtual planning and computer-aided design (CAD)/computer-aided manufacturing (CAM) technique were performed including maxillary and fibula cutting guides for exact harvesting and positioning of the fibula flap. Six months after surgery, four implants were loaded into the healthy fibula flap. After another period of six months, three implants were exposed and received healing abutments. We decided not to use the fourth, most distal, implant, because of rigid scarring even after the set in of a split-skin graft for vestibuloplasty (Fig. 5). Shortly before completing orthodontic treatment, the definitive prosthodontic dental restoration was inserted and the residual minimal lateral open bite was closed. At the end of treatment, the patient had no further complaints and was very satisfied with the functional outcome and facial appearance (Fig. 6).
Fig. 4.
Patient of case 3 before undergoing reconstructive procedures at the age of 17 years. Hypoplasia of the left midface (a, b). Okay Class II defect (c). CT scan showing maxillary bony loss (d). Orthopantomogram illustrating the elongated and tilted teeth of the third quadrant (e)
Fig. 5.
Reconstruction procedures in patient of case 3. Virtual planning of fibula flap (a). 3D-CT scan post-surgery (b). Healing of fibula flap with flexor halluces muscle cuff becoming a neo-mucosa after 4 weeks (c). Implant loading after 6 months (d). Vestibuloplasty with split-skin graft (e). Orthopantomogram showing osseointegrated implants (f)
Fig. 6.
Final outcome in patient of case 3 at the age of 20 years. Good projection of left-sided maxilla without gross asymmetry (a–c). Dental occlusion and prosthodontic dental rehabilitation before removal of orthodontic appliances (d). Final orthopantomogram. Posterior implant was not involved in prosthetic restoration (e)
Discussion
The previous literature reviews report high recurrence rates dependent, among others, upon the radicalness of the primary tumour excision. An overview of results of recent review studies is given in Table 1. Curettage alone seems to be inappropriate, with recurrence rates reaching up to 60%. Regarding resection, on the one hand, marginal resection and segmental resection offer the lowest rates of recurrence at about 10% [10] and this fact contributed the most to our decision-making. On the other hand, histological clear margins or safety margins of five millimetres or more seem not to be appropriate in every case since too much soft and hard tissue would be sacrificed, possibly causing extensive facial deformity and mutilation [2, 8]. To minimize both effects, we aimed to resect gross tumour masses while also achieving macroscopic clear margins, but in addition, in first-line treatment, aimed to save eminently important anatomical structures such as the orbital cavity and its bony boundaries. In turn, however, the intention to minimize mutilation has its restriction when surgical overview is limited and decision-making for or against further resection is not possible without extension of the approach. In our early cases in 1992 (case 2) and 1998 (case 3), we had to choose an extraoral approach and lip splitting for exactly this reason. In the recent case (case 1), we were able to avoid an extraoral approach without any restrictions of overview and safety, essentially due to the fact that although presenting comparable tumour masses, the infant in case 1 was twice the age of the infants in the previous cases and consequently the surgical field was wider.
Table 1.
Overview of results of recent review studies
| Authors | Cases (n) | Age at diagnosis (months) | Site (%) | Treatment (%) | Recurrence (%) |
|---|---|---|---|---|---|
| Chrcanovic and Gomez [10] | 429 | Male 5.9 ± 13.3 | Maxilla 89.9 | Enucleation 67.2 | 22.4 |
| Female 5.1 ± 7.5 | Mandible 9.4 | Resection 24.5 | 10.7 | ||
| Others 0.7 | Curettage 3.5 | 61.5 | |||
| Chemoth. 2.5 | 30 | ||||
| Radioth. 1.5 | 33.3 | ||||
| Rachidi et al. [2] | 427 | 10.4 ± 23.1 | Maxilla 60.3 | Resection 64.6 | In total: Female 25, Male 22 |
| Mandible 10.3 | Curettage 24.7 | ||||
| Others 29.3 | Chemoth. 1.8 | ||||
| Rickart et al. [8] | 11 | 5 (median age) | Maxilla 72.7 | Resection 90.9 | In total: 27 |
| Mandible 9.1 | Resection and Chemoth 9.1 | ||||
| Others 18.2 |
For recurrences of MNTI, not only previous surgery but also the age at first manifestation plays an important role. The earlier MNTI is diagnosed, the higher the chance of recurrence. When diagnosis occurs at 6 months of age, the risk of recurrence is minimal [2, 11]. However, some authors recommend that patients should be followed up at frequent intervals just as if they had a malignant tumour and suggest monthly controls for the first post-operative year [12]. We are in agreement with this suggestion, independent of age at first diagnosis, and based on our own experience, we additionally recommend MRI and CT scans after 5 to 6 weeks and at 6 months post-surgery.
At first sight, chemotherapy might be an alternative to surgery and might avoid mutilation. Two regimes are recommended for MNTI therapy. The first includes cyclophosphamide and vincristine for benign but recurrent tumours. The second regime of OPEC/OJEC (vincristine (O), cisplatin (P), etoposide (E), cyclophosphamide (C), and carboplatin (J)) should be reserved for persistently progressing tumours [8, 13]. In the literature, chemotherapy is used if malignancy is diagnosed, surgical margins are not clear or uncertain, functional or vital anatomical structures are invaded such as the nasal cavity, the orbit, and the skull base, and in cases of multiple recurrences [6, 12]. Chrcanovic and Gomez found recurrences in three cases (30%) in their systematic review of ten patients who underwent chemotherapy [10]. However, the rate was higher than that in surgical cases of marginal resection, and furthermore, it remains unclear if, in the seven cases without recurrence, this was due to the chemotherapy itself or to the typical characteristic of the MNTI not to develop recurrence after a mean of one year. Hence, the effect of chemotherapy is at least disputable not only for this reason, but also because patients are at significant risk of long-term complications including subsequent malignancies and cardiovascular diseases [14, 15].
As an undesired effect of more or less radical ablative surgery including the maxillary bone, primary and permanent teeth, infants, and adolescents suffer from growth disturbances with asymmetric facial appearance causing aesthetic as well as functional deficits affecting chewing, swallowing, and speaking to varying degrees. Hence, management of facial growth is needed to minimize above mentioned effects and should be adapted to the management of cleft lip and palate patients. Early functional orthodontic treatment is one of the most decisive procedures to develop dental arches and to counteract growth disturbances following scaring after surgery [16].
However, in our experience, moderate aesthetic and functional deficits did not cause a great burden of disease during adolescence. This observation led us to the decision to perform definitive reconstructive procedures in our third case as late as at the end of adolescence when no further growth was expected and patient compliance was much higher than before. This latter factor is decisive since multiple procedures including free osseous flap transplantation, loading of dental implants, orthodontic treatment, prosthodontic dental restoration, and coordination of all features were necessary to achieve full rehabilitation 20 years after initial diagnosis and therapy of MNTI.
Up to the time-point when reconstruction starts, patience and perseverance are required from patients and medical staff to achieve a successful outcome. In particular, follow-up visits at regular intervals over a period of 18–20 years are necessary to support patient compliance and retain their confidence. However, nowadays it is difficult to retain patients at one clinical centre over the long-term since it could be expected that family and occupation-related reasons lead to a loss to follow-up, as we experienced in our earliest case. Hence, close cooperation between the involved clinical centres is desirable.
Conclusion
Surgical treatment of MNTI should comprise marginal resection but should also respect vital anatomical structures to avoid gross mutilation. Dependent on the extension of resection of maxillary MNTI and of its frequent recurrences in early childhood, growth disturbances during adolescence and the need for defect reconstruction could lead to further multiple procedures as late as adulthood. It is therefore particularly important to carry out regular follow-up examinations so as not to miss the most appropriate moment for surgical or non-surgical interventions. Even 100 years after its first description and due to its rarity, by far not all of aspects of MNTI are elucidated yet. Hence, an international database is warranted to evaluate further therapeutic options and clinical courses over decades.
Funding
No funding received.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krompecher E. Zur Histogenese und Morphologie der Adamantinome und sonstiger Kiefergeschwülste. Beitr Path Anat. 1918;64:165. [Google Scholar]
- 2.Rachidi S, Sood AJ, Patel KG, Nguyen SA, Hamilton H, Neville BW, Day TA. Melanotic neuroectodermal tumor of infancy: a systematic review. J Oral Maxillofac Surg. 2015;73:1946–1956. doi: 10.1016/j.joms.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Borello ED, Gorlin RJ. Melanotic neuroectodermal tumor of infancy: a neoplasma of neural crest origin. Cancer. 1966;19:196–206. doi: 10.1002/1097-0142(196602)19:2<196::AID-CNCR2820190210>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Howell RE, Cohen MM. Pathological case of the mouth. Arch Pediatr Adolesc Med. 1996;150:1103–1104. doi: 10.1001/archpedi.1996.02170350105021. [DOI] [PubMed] [Google Scholar]
- 5.Puchalski R, Shah UK, Carpentieri D, McLaughlin R, Handler SD. Melanotic neuroectodermal tumor of infancy (MNTI) of the hard palate: presentation and management. Int J Pediatr Otorhinolaryngol. 2000;53:163–168. doi: 10.1016/S0165-5876(00)00315-3. [DOI] [PubMed] [Google Scholar]
- 6.Nicosia G, Spennato P, Aliberti F, Cascone D, Quaglietta L, Errico ME, Muto M, Ionna F, Cinalli G. Giant melanotic neuroectodermal tumor of infancy (melanotic progonoma) of the head and neck: report of a malignant case. J Neurosurg Pediatr. 2017;19:538–545. doi: 10.3171/2016.11.PEDS16509. [DOI] [PubMed] [Google Scholar]
- 7.Eckardt A, Swennen G, Teltzrow T. Melanotic neuroectodermal tumor of infancy involving the mandible: 7-year follow-up after hemimandibulectomy and costochondral graft reconstruction. J Craniofac Surg. 2001;12:349–354. doi: 10.1097/00001665-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Rickart AJ, Drummond-Hay V, Suchak A, Sadiq Z, Sebire NJ, Slater O, Mills C. Melanotic neuroectodermal tumour of infancy: refining the surgical approach. Int J Oral Maxillofac Surg. 2019;48:1307–1312. doi: 10.1016/j.ijom.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Okay DJ, Genden E, Buchbinder D, Urken M. Prosthodontic guidelines for surgical reconstruction of the maxilla: a classification system of defects. J Prosthet Dent. 2001;86:352–363. doi: 10.1067/mpr.2001.119524. [DOI] [PubMed] [Google Scholar]
- 10.Chrcanovic BR, Gomez RS. Melanotic neuroectodermal tumour of infancy of the jaws: an analysis of diagnostic features and treatment. Int J Oral Maxillofac Surg. 2019;48:1–8. doi: 10.1016/j.ijom.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Gupta R, Gupta R, Kumar S, Saxena S. Melanotic neuroectodermal tumor of infancy: review of literature, report of a case and follow up at 7 years. J Plast Reconstr Aesthet Surg. 2015;68:e53–e54. doi: 10.1016/j.bjps.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Kruse-Lösler B, Gaertner C, Bürger H, Seper L, Joos U, Kleinheinz J. Melanotic neuroectodermal tumor of infancy: systematic review of the literature and presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:204–216. doi: 10.1016/j.tripleo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Béogo R, Nikiéma Z, Traoré SS, Bouletreau P. Maxillary melanotic neuroectodermal tumor of infancy management: is conservative surgery the best approach? J Craniofac Surg. 2013;24:e338–e340. doi: 10.1097/SCS.0b013e31828a7c4c. [DOI] [PubMed] [Google Scholar]
- 14.Woessmann W, Neugebauer M, Gossen R, Blütters-Sawatzki R, Reiter A. Successful chemotherapy for melanotic neuroectodermal tumor of infancy in a baby. Med Pediatr Oncol. 2003;40:198–199. doi: 10.1002/mpo.10135. [DOI] [PubMed] [Google Scholar]
- 15.Baker KS, Syrjala KL. Long-term complications in adolescent and young adult leukemia survivors. Hematology Am Soc Hematol Educ Program. 2018;30:146–153. doi: 10.1182/asheducation-2018.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farronato G, Kairyte L, Giannini L, Galbiati G, Maspero C. How various surgical protocols of the unilateral cleft lip and palate influence the facial growth and possible orthodontic problems? Which is the best timing of lip, palate and alveolus repair? Literature review. Stomatologija. 2014;16:53–60. [PubMed] [Google Scholar]