Summary
Mucosal-associated invariant T (MAIT) cells recognize microbial riboflavin metabolites presented by MR1 and play role in immune responses to microbial infections and tumors. We report here that absence of the transcription factor (TF) Bcl11b in mice alters predominantly MAIT17 cells in the thymus and further in the lung, both at steady state and following Salmonella infection. Transcriptomics and ChIP-seq analyses show direct control of TCR signaling program and position BCL11B upstream of essential TFs of MAIT17 program, including RORγt, ZBTB16 (PLZF), and MAF. BCL11B binding at key MAIT17 and at TCR signaling program genes in human MAIT cells occurred mostly in regions enriched for H3K27Ac. Unexpectedly, in human MAIT cells, BCL11B also bound at MAIT1 program genes, at putative active enhancers, although this program was not affected in mouse MAIT cells in the absence of Bcl11b. These studies endorse BCL11B as an essential TF for MAIT cells both in mice and humans.
Subject areas: Immunology, Systems Biology, Transcriptomics
Graphical abstract
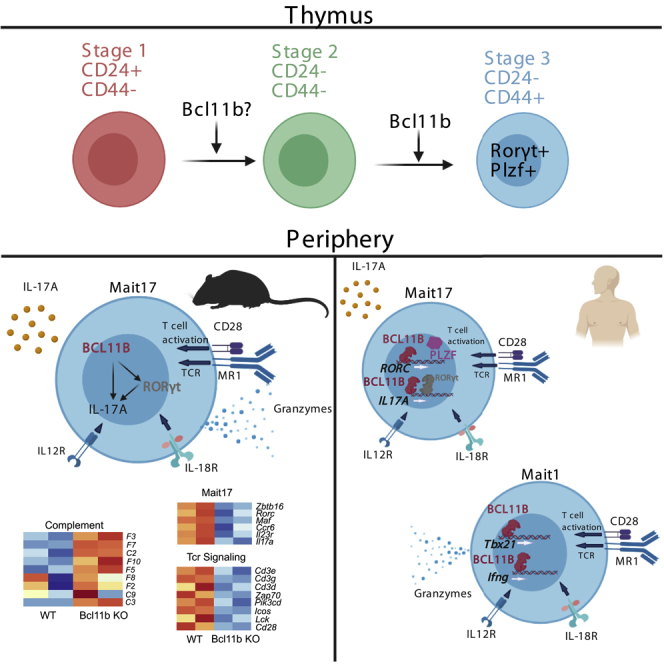
Highlights
-
•
BCL11B controls MAIT cell development in mice, predominantly MAIT17 lineage
-
•
BCL11B sustains MAIT17 and TCR signaling programs at steady state and in infection
-
•
BCL11B binds at MAIT17 and TCR program genes in human MAIT cells
-
•
Many BCL11B binding sites at MAIT17 and TCR genes are at putative active enhancers
Immunology; Systems Biology; Transcriptomics
Introduction
Mucosal-associated invariant T (MAIT) cells reside at mucosal sites and respond to bacterial infections. They have an invariant TCRα chain paired with a TCRβ chain of limited diversity that recognizes riboflavin metabolites produced by bacteria and yeast and presented in the context of the evolutionary conserved MHC class I-like molecule MR1 (Tilloy et al., 1999; Treiner et al., 2003; Gold et al., 2010; Le Bourhis et al., 2010; Kjer-Nielsen et al., 2012; Corbett et al., 2014). MAIT cells are abundant in humans, including in peripheral blood, and are known to produce IL17 and IFNγ (Dusseaux et al., 2011; Le Bourhis et al., 2010; Gherardin et al., 2018). In common laboratory mice, MAIT cells are in reduced numbers and are predominantly Rorγt+ IL17-producing (MAIT17), while Tbet + MAIT1 cells are barely detectable (Koay et al. 2016, 2019; Godfrey et al., 2019; Rahimpour et al., 2015). MAIT cell precursors are selected by thymocytes which present riboflavin precursors in the context of MR1. Commensal bacteria producing riboflavin metabolites are necessary for their development, and numbers of MAIT cells are much lower in germ free mice (Koay et al., 2016; Martin et al., 2009; Constantinides et al., 2019; Legoux et al., 2019a). MAIT cell development in the thymus consists of three sequential stages: stage 1 (CD24 + CD44-), stage 2 (CD24-CD44-), and stage 3 (CD24-CD44+) (Koay et al. 2016, 2019). Several transcription factors (TFs) have been found essential for MAIT cell development and function. Absence of the TF Tcf7, expressed early in thymic MAIT cells and downregulated at stage 3 (Koay et al., 2019), causes a major deficiency in MAIT cells (Mielke et al., 2019), and so does Bcl6 (Gioulbasani et al., 2020). The TF Zbtb16 (PLZF) reaches the highest levels at stage 3, remaining elevated mostly in MAIT17 cells in common laboratory C57B6/L mice (Koay et al., 2019), different from iNKT cells where PLZF is expressed very early and then downregulated in mature iNKT cells, with the exception of iNKT2 cells (Kovalovsky et al., 2008; Savage et al., 2008; Lee et al., 2013). A recent study using Cast/C57BL/6 mice, which have increased iVα19 -Jα33 rearrangements and thus elevated numbers of MAIT cells (Cui et al., 2015), proposed that SAP signaling initiated by selection mediated by DP thymocytes promotes expression of PLZF, which remains expressed in both MAIT1 and MAIT17 cells, albeit at higher levels in MAIT17 (Legoux et al., 2019b). The same study showed that Cast/C57BL/6 mice have, in addition to the CD44+ PLZF+ MR1t-5-OP-RU+ MAIT cells, a population of MR1t-5-OP-RU+ cells selected on thymic epithelial cells, which do not express PLZF and have a naive phenotype (Legoux et al., 2019b), similar to the transgenic mice with TRAV1 TRAJ33 (reviewed in (Godfrey et al., 2019)). It is unclear if such MR1t-5-OP-RU+ cells selected on thymic epithelial cells exist in NON-Cast/C57BL/6 mice.
In common laboratory C57BL/6 mice, stage 3 MAIT cells comprise mostly RORγt+ MAIT17 cells (Rahimpour et al., 2015; Koay et al. 2016, 2019). The essential role of RORγt in MAIT cells is supported by the fact that biallelic RORC functional loss in humans results in absence of MAIT cells (Okada et al., 2015). RORγt is essential for MAIT17 program, including in controlling expression of IL17A at steady state and in infection (Dusseaux et al., 2011; Koay et al. 2016, 2019; Chen et al., 2017). Another TF, c-Maf, also upregulated at stage 3, is part of the MAIT17 signature (Koay et al., 2019). Although MAIT1 population is scarce in mice at steady state, following bacterial infection, when MAIT cells expand, Tbet is upregulated and a considerable number of MAIT cells become Rorγt+ Tbet+ (Wang et al., 2019; Chen et al., 2017). In humans, MAIT cells respond to viral infection by producing IFNγ and granzyme B (Gzmb) to lyse infected cells (Le Bourhis et al., 2013; van Wilgenburg et al. 2016, 2018; Kurioka et al., 2015; Sattler et al., 2015; Slichter et al., 2016; Ussher et al., 2014).
MAIT cells have been also suggested to play a role in tumor immunity, responding by cytotoxic activity to myeloma cells loaded with 5-OP-RU (Gherardin et al., 2018). However, a reduction of these cells was observed in peripheral blood of patients with mucosal tumors and an accumulation in tumors, and not always with a favorable outcome (reviewed in (Godfrey et al., 2019; Vacchini et al., 2020)).
Bcl11b is a TF expressed in all T cells and innate lymphoid cells. It plays a fundamental role in T cell lineage commitment in mice and humans (Li, Leid and Rothenberg 2010a; Li et al., 2010b; Garcia-Perez et al., 2020; Roels et al., 2020; Dolens et al., 2020), and in beta and positive selection and survival of thymocytes (Wakabayashi et al., 2003; Albu et al., 2007; Avram and Califano 2014). Bcl11b is essential for development of CD4+ and CD8+ T cells (Albu et al., 2007; Kastner et al., 2010; Kojo et al., 2017), as well as of Treg cells (Vanvalkenburgh et al., 2011; Drashansky et al., 2019; Hasan et al., 2019) and iNKT cells (Albu et al., 2011; Kastner et al., 2010; Uddin et al., 2016). Absence of Bcl11b in DP thymocytes results in lack of iNKT cells due to Bcl11b's role in the control of glycolipid processing and presentation by thymocytes, as well as intrinsic alterations in iNKT cell precursors (Albu et al., 2011). Specific ablation of Bcl11b in iNKT cells results in reduction of iNKT1 and iNKT2 subsets and increased expression of iNKT17 program genes (Uddin et al., 2016). Bcl11b also controls the function of mature effector CD4+ T cells and their identity (Fang et al., 2018; Califano et al., 2014; Lorentsen et al., 2018), as well as Ag-specific expansion of CD8+ T cells (Zhang et al., 2010; Abboud et al., 2016). Thus, in addition to a common essential function of Bcl11b in maintaining T cell identity, Bcl11b plays specific and complex roles in each of the T cell lineages and subsets. In addition to T cells, Bcl11b is essential for ILC2 development and identity maintenance and function (Califano et al., 2015; Walker et al., 2015; Yu et al., 2015) and NK cell immunity (Holmes et al., 2021). In this study, we show an essential intrinsic role of Bcl11b in MAIT cell development and function. Transcriptomics analysis revealed major alterations in the MAIT17 program, CD3 and TCR signaling and cytotoxic granule exocytosis (CGE) programs, but not in MAIT1 program in murine Bcl11b KO MAIT cells. BCL11B and H3K27Ac ChIP-seq analyses in human MAIT cells revealed binding of BCL11B at key genes of the MAIT17 program, many of them associated with putative active enhancers. BCL11B also bound at CD3 complex and at essential genes of TCR signaling program, and surprisingly at MAIT1 program genes in regions with enriched H3K27Ac. Many of the human genes where BCL11B bound are the homologs of the mouse genes regulated by Bcl11b, with the exception of MAIT1 genes, which were not found dysregulated in mouse MAIT cells by absence of Bcl11b. This study thus demonstrates an essential role of Bcl11b in the development of MAIT cells and in the control of MAIT17 cell program at steady state and in infection and positions Bcl11b in the context of critical TFs that regulate these cells. The role of Bcl11b in positively supporting MAIT17 program is in contrast to its role in iNKT cells, where Bcl11b was required to sustain iNKT1 and iNKT2 programs, but restrained the iNKT17 program (Uddin et al., 2016), further supporting the concept that Bcl11b, in addition to regulating common programs in T cells, exerts also complex lineage and subset specific roles.
Results
Bcl11b is essential for MAIT cell development and MAIT17 subset
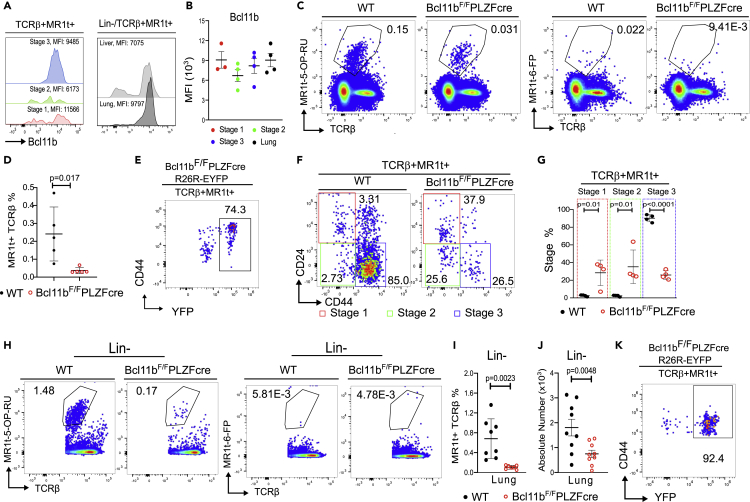
Bcl11b is expressed in all αβ T cell populations that derive at the DP stage of T cell development (Albu et al. 2007, 2011; Zhang et al., 2010; Lorentsen et al., 2018; Drashansky et al., 2019; Fang et al., 2018; Hasan et al., 2019; Vanvalkenburgh et al., 2011). Considering that MAIT cells are also generated at this stage, we evaluated Bcl11b in thymic and peripheral MAIT cells and found it expressed at an equivalent level throughout all MAIT cell developmental stages, as well as in the lung MAIT cells (Figures 1A and 1B). The TF PLZF is known to be expressed in MAIT cells and iNKT cells, but not in DP thymocytes or other αβ T cell populations (Kovalovsky et al., 2008; Savage et al., 2008). We thus made use of Bcl11bF/F/PLZFcre mice (Zhang et al., 2015) to study the role of Bcl11b in MAIT cells. Results show that Bcl11bF/F/PLZFcre mice (non-Cast/C57BL/6) had a marked reduction in the thymic TCRβ+MR1t-5-OP-RU+ MAIT cells compared to WT (Figures 1C and 1D). The small number of TCRβ+MR1t-5-OP-RU+ cells in the thymus of Bcl11bF/F/PLZFcre/R26R-EYFP mice were largely YFP+ (Figure 1E), demonstrating that they are dependent on PLZF expression, and thus not mainstream T cells.
Figure 1.
MAIT cells in the thymus and lung in the absence of Bcl11b at steady state
(A) Representative histograms of Bcl11b in TCRβ+MR1t-5-OP-RU+ MAIT cells. Thymic developmental stages (left), liver and lung (right).
(B) Bcl11b MFI in MAIT cells in thymic developmental stages and lung (n = 4).
(C) Representative dot plots of MR1t-5-OP-RU and control MR1t-6-FP staining in thymi of Bcl11bF/F/PLZFcre and Bcl11bF/F (WT) mice.
(D) Frequencies of TCRβ+MR1t-5-OP-RU+ MAIT cells from thymi of Bcl11bF/F/PLZFcre and Bcl11bF/F (WT) mice (n = 5). The data from thymus are derived from 10 pairs of mice in which two FACS files from the same type of mice (KO or WT) were concatenated.
(E) Representative dot plot of YFP and CD44 in TCRβ+MR1t-5-OP-RU+ MAIT cells isolated from thymi of Bcl11bF/F/PLZFcre/R26R-EYFP mice.
(F and G) Representative dot plots (F) and frequencies (G) of Bcl11b KO and WT TCRβ+MR1t-5-OP-RU+ MAIT cell developmental stages in thymi of Bcl11bF/F/PLZFcre and WT mice (n = 4). The data are derived from 8 pairs of mice in which two FACS files from the same type of mice (KO or WT) were concatenated.
(H) Representative dot plots of MR1t-5-OP-RU and control MR1t-6-FP staining in lung of BCL11BF/F/PLZFcre and BCL11BF/F (WT) mice.
(I and J) Frequencies (I) and absolute numbers (J) of TCRβ+MR1t-5-OP-RU+ MAIT cells in lung of Bcl11bF/F/PLZFcre and WT mice. Each dot represents the data from one mouse.
(K) Representative dot plot of YFP and CD44 in TCRβ+MR1t-5-OP-RU+ MAIT cells isolated from the lung of Bcl11bF/F/PLZFcre/R26R-EYFP mice at steady state.
Data are represented as mean ± SEM. Student's t-test was used for statistical analysis. p < 0.05 was considered significant.
Analysis of thymic developmental stages revealed a severe decrease of MAIT cell stage 3 frequency in Bcl11bF/F/PLZFcre thymi compared to WT (Figures 1F and 1G). Further evaluation of TCRβ+MR1t-5-OP-RU+ MAIT cells in the lung, where they are known to localize (Rahimpour et al., 2015; Salou et al., 2019), showed that their frequency and absolute numbers were further reduced in the absence of Bcl11b (Figures 1H–1J). The small number of TCRβ+MR1t-5-OP-RU+ CD44+ MAIT cells in the lung of Bcl11bF/F/PLZFcre/R26R-EYFP mice were over 90% YFP+ (Figure 1K), demonstrating again that they are dependent on PLZF expression. Importantly, TCRβ+ MR1t-5-OP-RU+ PLZF-Cre-YFP+ were all CD44 positive at steady state (Figure 1K), and thus not main-stream T cells, which are known to be CD44 negative.
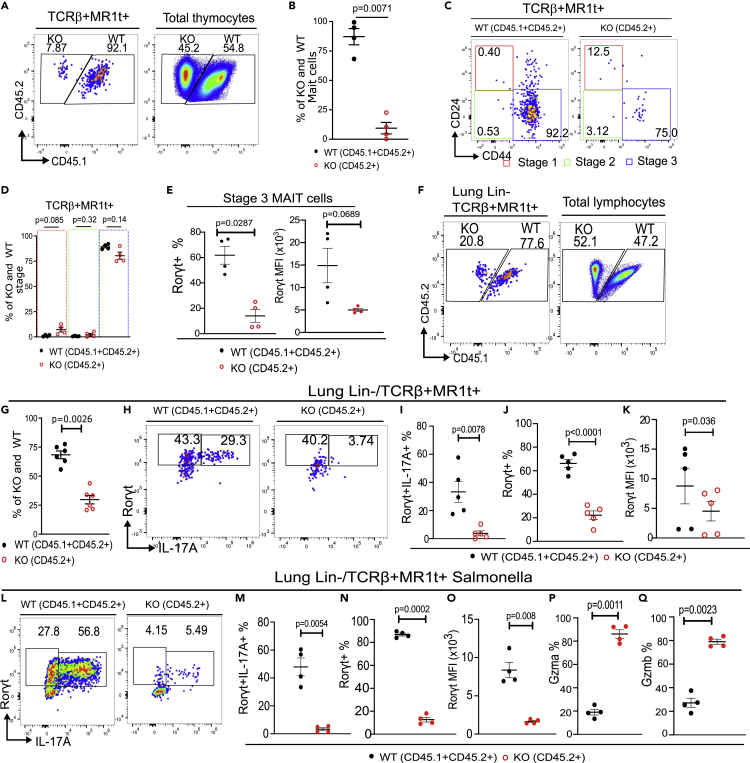
Mixed bone marrow chimeras (1:1, Bcl11bF/F/PLZFcre:WT) further showed reduction of the Bcl11b KO TCRβ+MR1t-5-OP-RU+ MAIT cell frequency both in the thymus and in the lung (Figures 2A, 2B, 2F, and G), however the frequency of thymic developmental stage 3 was in large restored (Figures 2C and 2D). These results demonstrate that the numerical reduction is cell intrinsic, however cell extrinsic factors may contribute to the developmental defects. We previously showed that Bcl11bF/F/PLZFcre mice had also reduced numbers of iNKT cells, with a major reduction in iNKT1 and iNKT2 subsets and reduced production of IL4 and IFNγ, including in the thymus (Uddin et al., 2016). Importantly, IL4Ra is expressed on thymic MAIT cells, being downregulated from early developmental stages to mature thymic MAIT cells (Koay et al., 2019). Thus, the reduction in IL4 in the thymus of Bcl11bF/F/PLZFcre mice may negatively impact the transition of Bcl11b KO MAIT cells to mature stage 3, while in chimeric mice, when IL4 was provided by wild type iNKT2 cells, the transition of Bcl11b KO MAIT cells to stage 3 was largely restored, however not the reduced frequency of Bcl11b KO MAIT cells. Of note, IL4Ra was normally expressed by Bcl11b KO MAIT cells (Figure S1).
Figure 2.
Defects in MAIT cells in the absence of Bcl11b are cell intrinsic
Mixed bone marrow (BM) chimeras were generated by 1:1 ratio of BM cells from Bcl11bF/F/PLZFcre (KO) (CD45.2+) mice and Bcl11bF/F (WT) (CD45.1+ CD45.2+) mice, transplanted into lethally irradiated CD45.1 mice.
(A and B) Representative dot plot (A) and frequencies (B) of TCRβ+MR1t-5-OP-RU+ MAIT cells in the thymus, 8 weeks post transfer versus total transplanted thymocytes.
(C and D) Representative dot plot (C) and frequencies (D) of Bcl11b KO (CD45.2) and WT (CD45.1+ CD45.2+) TCRβ+MR1t-5-OP-RU+ MAIT cells in the three developmental stages in thymi of mixed BM chimeras.
(E) Frequencies and MFI of RORγt in developmental stage 3.
(F and G) Representative dot plots (F) and frequencies (G) of transplanted Bcl11b KO (CD45.2) and WT (CD45.1+ CD45.2+) TCRβ+MR1t-5-OP-RU+ MAIT cells versus TCRβ+MR1t-5-OP-RU- cells in the lung of mixed BM chimeric mice.
(H–K) Representative contour plots (H) and frequencies (I) of RORγt and IL17A, frequencies (J) and MFI (K) of RORγt in the lung Bcl11b KO (CD45.2) and WT (CD45.1+ CD45.2+) TCRβ+MR1t-5-OP-RU+ MAIT cells in transplanted BM chimeric mice at steady state.
(L–Q) Representative contour plots (L) and frequencies (M) of RORγt and IL17A, frequencies (N) and MFI (O) of RORγt, and Gzma (P) and Gzmb (Q) frequencies in the lung Bcl11b KO (CD45.2) and WT (CD45.1+ CD45.2+) TCRβ+MR1t-5-OP-RU+ MAIT cells in transplanted BM chimeric mice infected with 106 cfu of Salmonella enterica ser. Typhimurium BRD509 (Salmonella BRD509) intranasally. Two mouse files were concatenated using FlowJo for each point on the statistical graphs.
Data are represented as mean ± SEM. All statistics were conducted as paired t test. p < 0.05 was considered significant.
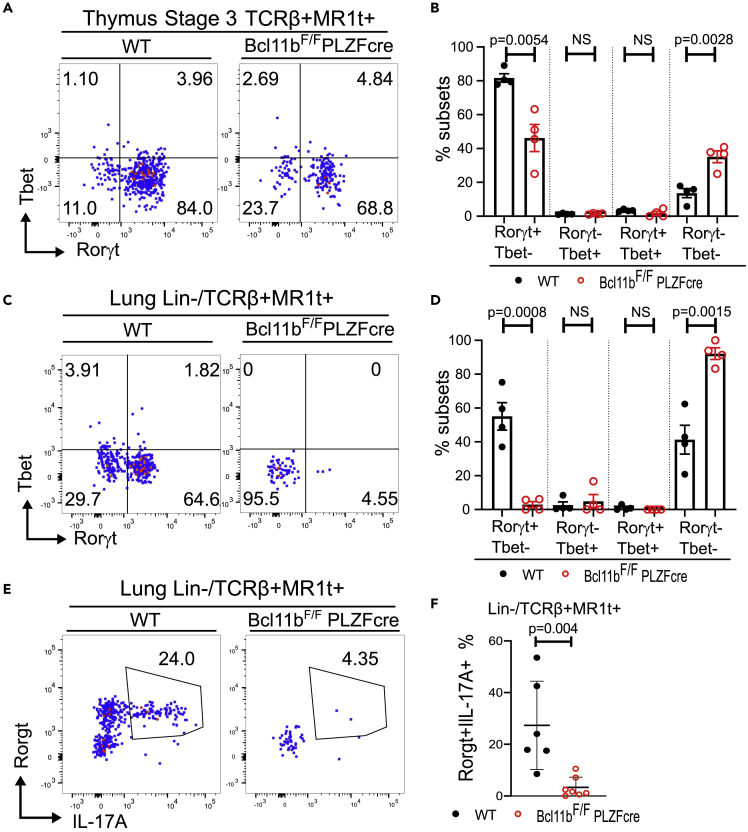
At thymic stage 3, two functional MAIT cell subsets have been identified, namely MAIT17, known to express Rorγt and numerically predominant in common lab C57BL/6 mice, and MAIT1, expressing Tbet and much lower numerically (Koay et al. 2016, 2019) (and Figures 3A and 3B). The Rorγt+ population was significantly decreased in stage 3 Bcl11b KO MAIT cells, however without resulting in an increase in the Tbet+ population, but rather in a population negative for both TFs (Figures 3A and 3B).
Figure 3.
MAIT17 cells are reduced in the absence of Bcl11b in the thymus and periphery and have reduced IL17A production at steady state
(A) Representative dot plots of RORγt and Tbet within stage 3 thymic TCRβ+MR1t-5-OP-RU + cells of Bcl11bF/F/PLZFcre and WT mice.
(B) Frequencies of RORγt + Tbet-, RORγt-Tbet+, RORγt + Tbet+, and RORγt-Tbet-within developmental stage 3 TCRβ+MR1t-5-OP-RU + MAIT cells of Bcl11bF/FPLZFcre and WT mice. The data are derived from 8 pairs of mice in which two FACS files from the same type of mice (Bcl11bF/F/PLZFcre or WT mice) were concatenated.
(C) Representative dot plots of RORγt and Tbet within lin-TCRβ+MR1t-5-OP-RU+ in the lung of Bcl11bF/F/PLZFcre and WT mice.
(D) Frequencies of Bcl11b KO and WT RORγt+Tbet-, RORγt-Tbet+, RORγt+Tbet+ and RORγt-Tbet-lin-TCRβ+MR1t-5-OP-RU + MAIT cell subsets in the lung.
(E) Representative dot plots of Bcl11b KO and WT RORγt+ IL17A+ lin-TCRβ+MR1t-5-OP-RU+ MAIT cells in lung.
(F) Frequencies of Bcl11b KO and WT RORγt+ IL17A+ TCRβ+MR1t-5-OP-RU+ MAIT cells in the lung. Each point represents a concatenated file of 2 lung samples run independently.
Data are represented as mean ± SEM. All statistics were conducted as paired t test. p < 0.05 was considered significant.
In the lung at steady state, as expected the Rorγt+ MAIT17 population was predominant, with barely detectable Tbet+ MAIT1 cells, and around 30% of MAIT cells negative for both Rorγt and Tbet (Figures 3C and 3D). Bcl11b KO Rorγt+ MAIT17 cell frequency was even more markedly diminished in the lung, representing only approximately 10% of that of WT. There was no increase in the Bcl11b KO Tbet+ MAIT1 population, but rather in the Rorγt-Tbet- MAIT cells, which represented over 90% (Figures 3C and 3D). Of note, Bcl11b levels were slightly but significantly higher in MAIT17 cells in the thymus and lung of WT mice compared to both MAIT1 and Rorγt-Tbet-MAIT cells (Figures S2A and S2B). Further investigation of Rorγt downstream target IL17A, part of the MAIT17 cell signature and known to be produced by MAIT cells even at steady state (Rahimpour et al., 2015), showed that Bcl11b KO TCRβ+MR1t-5-OP-RU+ cells failed to produce IL17A at steady state (Figures 3E and 3F). However, the frequency of IFNγ-producing TCRβ+MR1t-5-OP-RU+ MAIT cells was the same in Bcl11b KO versus WT MAIT cells, in both very low (Figure S3). In mixed BM chimeric mice, although frequency of stage 3 MAIT cells was in large recovered in the absence of Bcl11b (Figures 2C and 2D), frequency of Rorγt+ population and Rorγt MFI remained reduced (Figure 2E). Similarly, the frequency of Rorγt+ cells and Rorγt+ MFI was reduced in the Bcl11b KO TCRβ+MR1t-5-OP-RU+ MAIT cells in the lung of the chimeric mice, as was the frequency of Rorγt+ IL17A+ population (Figures 2H–2K).
These results taken together demonstrate that absence of Bcl11b causes a severe reduction in MAIT cell frequencies and numbers in the thymus and in the lung, with alteration in MAIT17 subset and inability to produce IL17 at steady state, caused by MAIT cell intrinsic defects.
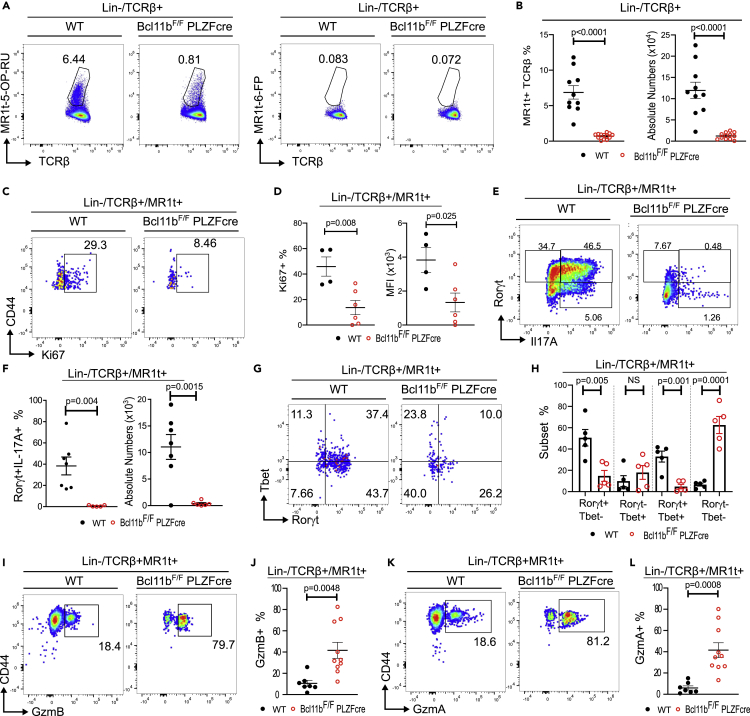
Bcl11b-deficient MAIT cells expand poorly and fail to upregulate IL17A in response to Salmonella BRD509 infection, but have elevated granzyme levels
MAIT cells respond to riboflavin metabolites produced by infectious bacteria. We used an established murine infection model with Salmonella enterica ser. Typhimurium BRD509 (Salmonella BRD509), known to induce a MAIT cell response (Chen et al., 2017). While WT MAIT cells expanded vigorously following infection, Bcl11b-deficient TCRβ+MR1t-5-OP-RU+ MAIT cells remained at much lower frequency and absolute numbers (Figures 4A and 4B). Along this line, frequency of Ki67+ TCRβ+MR1t-5-OP-RU+ MAIT cells was significantly lower in the absence of Bcl11b (Figures 4C and 4D), demonstrating that these cells expand poorly following bacterial infection. However, Bcl11b KO TCRβ+MR1t-5-OP-RU+ MAIT cells did not die more compared to WT following infection (Figure S4A).
Figure 4.
Bcl11b KO MAIT cells fail to expand and produce IL17A during Salmonella infection but have elevated granzyme levels
In all experiments Bcl11bF/F/PLZFcre and control mice were infected with 106 cfu of Salmonella enterica ser. Typhimurium BRD509 (Salmonella BRD509) intranasally. Mice were euthanized on day 7 post infection.
(A) Representative dot plots of lin- TCRβ+ MR1t-5-OP-RU and control MR1t-6-FP staining in lung of infected Bcl11bF/F/PLZFcre and WT mice.
(B) Frequencies and absolute numbers of lin- TCRβ+ MR1t-5-OP-RU+ MAIT cells in lung of infected Bcl11bF/F/PLZFcre and WT mice.
(C and D) Representative dot plots (C), frequencies and MFI (D) of Ki67+ CD44+ lung Bcl11b KO and WT lin-TCRβ+MR1t-5-OP-RU+ MAIT cells from infected mice.
(E and F) Representative dot plots (E), frequencies and absolute numbers (F) of Bcl11b KO and WT RORγt+ IL17A+ lin-TCRβ+MR1t-5-OP-RU+ MAIT cells from lung of infected mice.
(G) Representative dot plots of RORγt and Tbet within lin-TCRβ+MR1t-5-OP-RU+ in the lung of Bcl11bF/F/PLZFcre and WT infected mice.
(H) Frequencies of Bcl11b KO and WT RORγt+ Tbet-, RORγt-Tbet+, RORγt+Tbet+ and RORγt-Tbet-lin-TCRβ+MR1t-5-OP-RU+ MAIT cell subsets in the lung of Bcl11bF/F/PLZFcre and WT infected mice.
(I–L) Representative dot plots (I, K) and frequencies (J, L) of GzmB+ CD44+ (I, J) and GzmA+ CD44+ (K, L) lin-TCRβ+MR1t-5-OP-RU+ MAIT cells in the lung of infected Bcl11bF/F/PLZFcre and WT mice.
Data are represented as mean ± SEM. Statistics were conducted as paired t test. p < 0.05 was considered significant.
It has been established that in response to Salmonella BRD509 infection, MAIT cells increase production of IL17A (Chen et al., 2017). While a large proportion of WT TCRβ+MR1t-5-OP-RU+ MAIT cells produced IL17A and upregulated Rorγt following Salmonella infection, Bcl11b KO MAIT cells only modestly upregulated RORγt and failed to produce IL17A (Figures 4E and 4F). Similarly, in mixed BM chimeric mice, Bcl11b-deficient TCRβ+MR1t-5-OP-RU+ MAIT cells failed to upregulate Rorγt and produce IL17A following infection (Figures 2L–2O), demonstrating that the defect is intrinsic.
At steady state in the lung, Tbet+ MAIT cells are in very low number. However following bacterial infection, Tbet is known to be upregulated (Wang et al., 2019). Upregulation of Tbet occurred both in WT and Bcl11b KO TCRβ+MR1t-5-OP-RU+ MAIT cells after Salmonella infection (Figures 4G and 4H versus Figures 3C and 3D). However while in WT TCRβ+MR1t-5-OP-RU+ MAIT cells, a large proportion of Tbet+ cells also expressed RORγt, the Tbet+ Bcl11b KO MAIT remained largely negative for Rorγt (Figures 4G and 4H), suggesting that Bcl11b KO MAIT cells upregulate Tbet, but fail to express Rorγt. In fact, the largest proportion of Bcl11b KO MAIT cells was negative for both TFs (Figures 4G and 4H). Bcl11b KO and WT Tbet+ TCRβ+MR1t-5-OP-RU+ MAIT cells produced on average similar levels of IFNγ post infection (Figure S4B).
Granzymes, including GzmA and GzmB, are known to be produced by MAIT cells to kill sensitized targets (Kurioka et al., 2015; Le Bourhis et al., 2010). Both in WT and Bcl11b KO TCRβ+MR1t-5-OP-RU+ MAIT cells, it was the Tbet+ and RORγt-Tbet- MAIT cell subsets which produced granzymes, and not the RORγt + population (Figure S4C). A significantly higher frequency of Bcl11b KO MAIT cells produced GzmA and GzmB compared to WT (Figures 4I–4L), including in mixed chimeras (Figures 2P and 2Q), suggesting an intrinsic defect.
These results taken together demonstrate that Bcl11b is required for MAIT cell expansion during Salmonella infection and for supporting MAIT17 cell functional response; however, its absence results in increase in granzyme levels, but not in Tbet or IFNγ.
Bcl11b regulates expression of MAIT17 cell program, CD3 complex and TCR signaling genes at steady state and following Salmonella infection
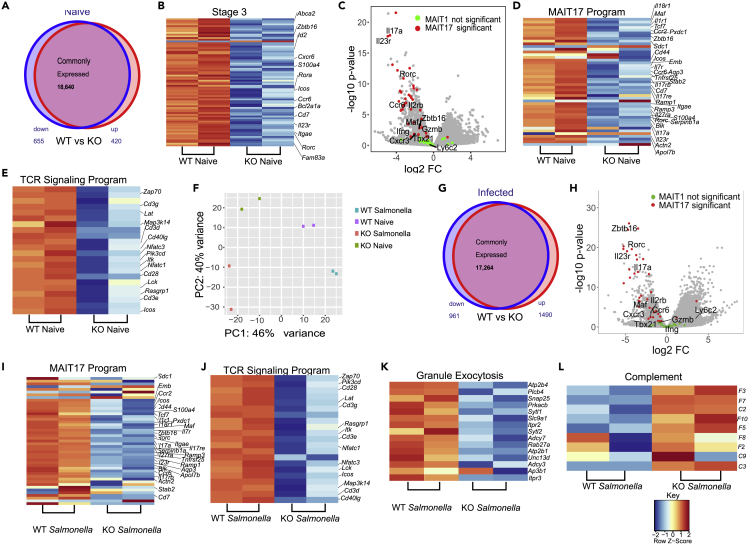
Given the scarce numbers of Bcl11b KO TCRβ+MR1t-5-OP-RU+ MAIT cells in the thymus and the fact that MAIT cells in the periphery resemble thymic stage 3, which are predominantly MAIT17 in NON-Cast C57BL/6 mice (Koay et al. 2016, 2019), we performed RNA-seq on lung Bcl11b KO TCRβ+MR1t-5-OP-RU+ YFP+ (from Bcl11bF/F/PLZFcre/R26R-EYFP mice) and WT TCRβ+MR1t-5-OP-RU+ MAIT cells at steady state and following Salmonella infection. In our analysis we used as reference for MAIT subsets and developmental stages the results from (Koay et al., 2019), given that this study used MAIT cells from NON-cast C57BL/6 mice, same as our study. As expected, lung WT MAIT cells expressed stage 3 program genes, with a predominant MAIT17 profile (Figures 5A–5D) (Koay et al. 2016, 2019). Lung Bcl11b KO MAIT cells have reduced expression of some of these genes, including Zbtb16, Rorc and other genes mostly of the MAIT17 program (Figures 5A–5D, Tables S1 and S2).
Figure 5.
Bcl11b sustains MAIT17, TCR signaling and cytotoxic granule exocytosis programs but represses complement genes in MAIT cells
All RNA-seq experiments were performed on sorted lin-TCRβ+MR1t-5-OP-RU+ MAIT cells from lung of Bcl11bF/F/PLZFcre/R26R-EYFP mice and WT control mice at steady state (A-F) or infected with Salmonella Typhimurium BRD509 (Salmonella) intranasally, day 7 post-infection (F-L). For Bcl11bF/F/PLZFcre/R26R-EYFP mice, in addition to lin-TCRβ+ MR1t-5-OP-RU+, sorting was conducted on YFP+ cells.
(A) Venn diagram depicting differentially expressed genes (significantly downregulated in blue and significantly upregulated in red) in Bcl11b KO and WT MAIT cells from uninfected mice.
(B) Heatmaps of differentially expressed stage 3 genes.
(C) Volcano plot of MAIT1 and MAIT17 genes in Bcl11b KO versus WT MAIT cells from mice at steady state.
(D) Heatmap of differentially expressed MAIT17 program.
(E) Heatmap of differentially expressed TCR signaling program genes in Bcl11b KO and WT MAIT cells from lung of uninfected mice. (n = 2).
(F) Principal component analysis (PCA) of MAIT cell RNA-seq from lung of Bcl11bF/F/PLZFcre/R26R-EYFP and WT mice at steady state or infected with Salmonella. (n = 2).
(G) Venn diagram depicting differentially expressed genes (significantly downregulated in blue and significantly upregulated in red) from Bcl11b KO and WT MAIT cells from Salmonella-infected mice.
(H) Volcano plot of MAIT17 and MAIT1 cells in Bcl11b KO versus WT MAIT cells from lung of mice infected with Salmonella.
(I–L) Heatmaps of MAIT17 program (I), TCR signaling (J), cytotoxic granule exocytosis (K) and complement (L) genes differentially expressed in Bcl11b KO and WT MAIT cells from Salmonella-infected mice. (n = 2). False discovery rate (FDR)–adjusted p values were calculated using the Benjamini-Hochberg method, with a significance cutoff of adjusted p < 0.05.
Some genes belonging to early thymic stages of MAIT cell development, including Bcl6 were elevated, however others, including Satb1 and Tox (also part of stage 1), were reduced (Figure S5) (Table S5), suggesting a complex role of Bcl11b in regulation of early stage program in mature MAIT cells.
As mentioned above, it was predominantly the MAIT17 cell program genes which were reduced in the absence of Bcl11b while MAIT1 program genes were mostly similarly expressed in Bcl11b KO and WT MAIT cells, and in both at very low levels (Figures 5C and 5D, Tables S3 and S4), supporting the observation that Bcl11b mainly promotes the MAIT17 program at steady state. The differentially expressed genes of MAIT17 cell program included genes for essential TFs (Rorc, c-Maf and Zbtb16). Of note, Zbtb16 (PLZF) is categorized as belonging to MAIT17 program, given that it is predominantly expressed in in these cells in common mouse laboratory strains (Koay et al., 2019). Essential MAIT17 genes, including those for Il17a, Il23r, CCR6, cytokines and their receptors (Il7ra, Il18r1, Il1r1, Il17rb, Il17re, Il27ra), chemokine receptors (Ccr2), specific markers, receptors and signaling molecules (Cd44, Icos, Itgae, Sdc1 (CD138), Blk, Pxdc1, Emb, Tnfrsf25, Aqp3, Stab2, Cd7, Ramp1, Ramp3, S100a4, Actn2 and Apol7b) (Koay et al., 2019) have reduced expression in lung Bcl11b KO MAIT cells at steady state (Figures 5C and 5D, and Table S3). From the few genes classified as belonging to the MAIT1 program downregulated in Bcl11b KO MAIT cells were Klrk1, Fgl2, Il2rb and Cd28 (Table S4), however mRNA levels for Tbx21, Ifnγ and Tnf remained similar between Bcl11b KO and WT MAIT cells at steady state (Figure S6).
In addition, RNA-seq analysis showed that Bcl11b KO MAIT cells have reduced mRNA levels encoding numerous genes belonging to TCR signaling, including for: CD3 complex, Cd28, Icos, Pdcd1, Lck, Itk, Zap70, Rasgrp1, Grap2, Card11, Lat, Map3k14, Nfatc1, Nfatc3, Nfkbie, Pik3cd, Pik3cg, Pdcd1, Ptprc, and Cd40l (Figures 5E and Table S6), demonstrating a critical role for Bcl11b in sustaining TCR signaling program.
We further conducted RNA-seq analysis of Bcl11b KO TCRβ+MR1t-5-OP-RU+ YFP+ (from Bcl11bF/F/PLZFcre/R26R-EYFP mice) and WT TCRβ+MR1t-5-OP-RU+ MAIT cells following infection with Salmonella BRD509. Principal component analysis (PCA) clearly separated WT MAIT cells from infected and uninfected mice, Bcl11b KO MAIT cells of uninfected mice and infected mice, and additionally the Bcl11b KO versus WT MAIT cells from both uninfected and infected mice (Figure 5F). Analysis of differentially expressed genes between Bcl11b KO and WT TCRβ+MR1t-5-OP-RU+ MAIT cells from Salmonella-infected mice shows that MAIT17 cell program genes further remained reduced in the Bcl11b KO MAIT cells compared to WT MAIT cells from infected mice, while MAIT1 genes were similarly expressed for the most, with a trend in Tbx21 downregulation (Figures 5G–5I and S6, Tables S3 and S6). Reduced MAIT17 mRNAs included those for the TF Rorc, Zbtb16, c-MAF, essential MAIT17 cytokines and receptors Il17a, IL17f, Il23r, CCR6, cytokines and receptors (Il7ra, Il18r1, Il1r1, Il17rb, Il17re, Il27ra), chemokine receptors (Ccr2), specific markers, other receptors and signaling molecules (Cd44, Icos, Blk, Itgae, Pxdc1, Emb, Tnfrsf25, Ramp1, Ramp3, S100a4, Serpinb1a, Actn2, and Apol7b) (Figures 5H and 5I, Table S3). Expression of TCR signaling genes also remained significantly diminished in Bcl11b KO MAIT cells following Salmonella infection, including mRNAs for CD3 complex, Cd28, Icos, Pdcd1, Lck, Zap70, Lat, Itk, Rasgrp1, Grap2, Card11, CD40l, Map3k14, Nfatc1, Nfatc2 and Nfatc3 (Figures 5J and Table S6).
Given the increased levels of GzmA and GzmB in Bcl11b KO cells MAIT cells from infected mice, we further evaluated granzyme mRNAs and found their levels not differentially expressed between Bcl11b KO and WT MAIT cells (Figure S7). These results suggest that the accumulation of GzmA and GzmB in the KO MAIT cells may occur through a distinct mechanism than regulation of their expression. In line with this, multiple genes belonging to cytotoxic CGE pathway, including Atp2b4, Plcb4, Snap25, Prkacb, Sytl1, Slc9a1, Itpr2, Sytl2, Adcy7, Rab27a, Atp2b1, Unc13d, Adcy3, and Itpr3 (Haddad et al., 2001; Kurioka et al., 2015; Kwong et al., 2000; Ménasché et al., 2008; Stinchcombe et al., 2001; Holt et al., 2008), were downregulated in the absence of Bcl11b (Figure 5K), suggesting that accumulation of granzymes in the absence of Bcl11b in TCRβ+MR1t-5-OP-RU+ MAIT cells may be caused by this reduction.
Among genes upregulated in the absence of Bcl11b were those belonging to the complement pathway, but not to NK or myeloid genes (Figure 5L and data not shown), known to be derepressed in other mature T cells in the absence of Bcl11b (Drashansky et al., 2019). Complement genes are derepressed in the absence of Bcl11b in other T cells as well (Helm and Avram, unpublished).
These results demonstrate that Bcl11b sustains MAIT17 and TCR signaling programs at steady state and following Salmonella infection. Additionally, Bcl11b is required to support expression of CGE program genes with role in granzyme release, and restrains complement gene expression in lung MAIT cells following infection.
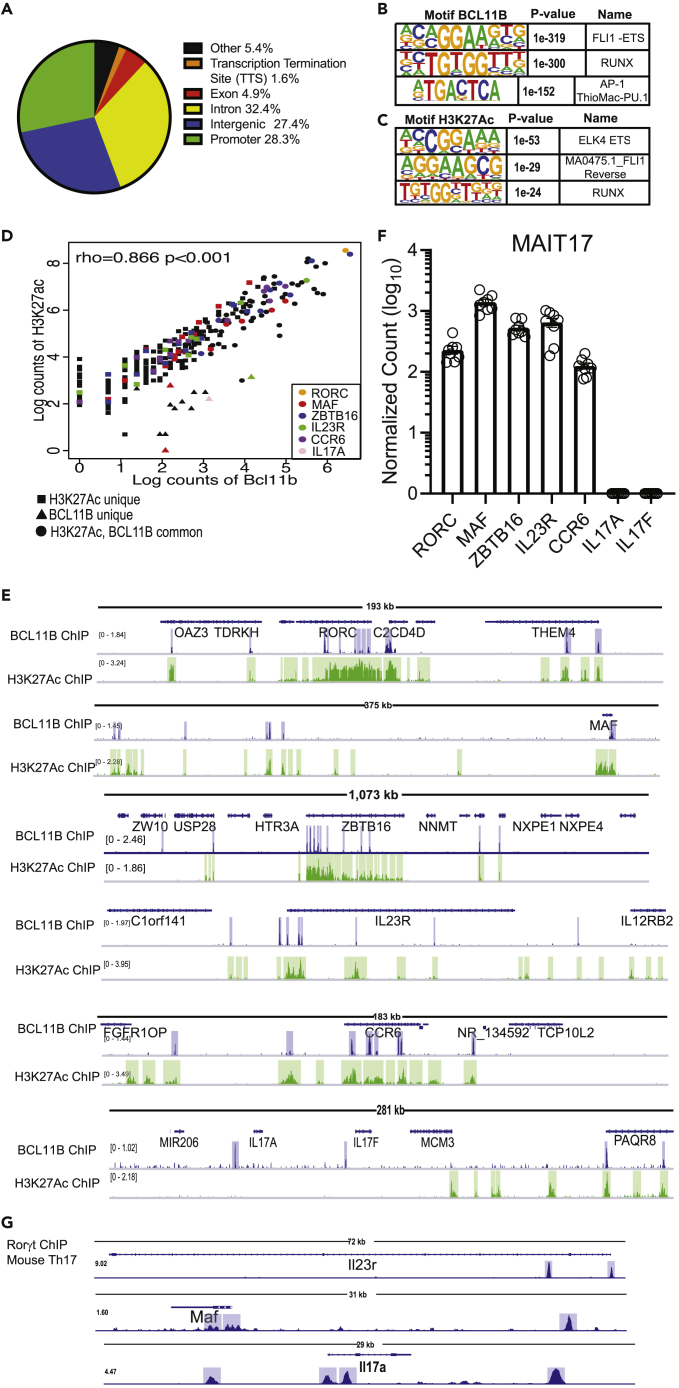
BCL11B binds at genomic regions with enriched H3K27ac of the MAIT17 and MAIT1 programs and at core TCR signaling genes in human MAIT cells
BCL11B is highly conserved between humans and mice (Avram et al. 2000, 2002; Avram and Califano 2014). Our previous results showed that BCL11B bound at many common genes in human and mouse Treg cells, and moreover the binding regions were situated at similar locations within the homologous gene (Drashansky et al., 2019). Given these similarities and the fact that MAIT cells are in reduced numbers in mice, we performed BCL11B ChIP-seq and H3K27Ac ChIP-seq on human peripheral blood CD3+CD161+TCRVα7.2+ MAIT cells, to identify the genes bound by BCL11B and the associated regulomes. Peak annotation showed BCL11B binding at intergenic regions, introns and promoters in human MAIT cells (Figure 6A). Just as we previously reported in CD4+ T cells and Treg cells (Lorentsen et al., 2018; Drashansky et al., 2019), the top binding motif for BCL11B was ETS, with a preference for Fli1, the second was RUNX, while AP-1 was the third (Figure 6B). Analysis of BCL11B-binding motifs correlated with H3K27Ac peaks revealed two ETS motifs as top (Elk4, the first, and Fli1 reverse, the second), while RUNX was the third (Figure 6C), suggesting that BCL11B uses ETS and RUNX motifs to control putative enhancer activity in MAIT cells.
Figure 6.
BCL11B binds at H3K27Ac-rich regions of key genes of MAIT17 program in human MAIT cells from healthy donors
(A) Peak distribution of BCL11B binding sites genome-wide in human MAIT cells sorted as CD3+CD161+TCRVα7.2+ from PBMCs of healthy donors. (B) Top 3 BCL11B binding motifs in human MAIT cells.
(C) Top 3 BCL11B motifs overlapping with H3K27Ac peaks. CD3+CD161+TCRVα7.2+ were sorted from PBMCs derived from nine donors (BCL11B ChIP-seq) and two donors (H3K27Ac ChIP-seq).
(D) Spearman's Correlation plot of BCL11B binding intensity with H3K27Ac peaks at MAIT17 signature genes. Several genes of the MAIT17 program are listed.
(E) Integrative Genomics Viewer (IGV) visualization at RORC, MAF, ZBTB16, IL23R, CCR6 and IL17A and IL17F loci of BCL11B ChIP-seq (track 1) and H3K27Ac ChIP-seq (track 2) in human MAIT cells sorted from PBMCs of healthy donors.
(F) Normalized counts in log10 format for RORC, MAF, ZBTB16, IL17A, IL17F, IL23R, CCR6 in blood MAIT cells of healthy donors. Data analyzed from (Dias et al., 2018). n = 8. Data are represented as mean ± SEM.
(G) IGV visualizations of Rorγt ChIP-seq in murine Th17 cells at IL23r, Maf, and IL17a loci. Data analyzed from (Ciofani et al., 2012). Blue and green highlights indicate significant ChIP-seq peaks. ChIP-seq scale bar normalized to sequences per million reads.
We focused on genomic regions associated with essential programs of MAIT cells. Analysis using Spearman's Correlation Algorithm showed high correlation of BCL11B binding and H3K27Ac at human MAIT17 signature genes (Figure 6D). RORC and ZBTB16 showed the highest correlation, followed by CCR6, IL23R, and c-MAF (Figure 6D). These MAIT17 program genes had decreased expression in mouse Bcl11b-deficient MAIT cells both at steady state and in infection (Figures 5C, 5D, 5H, and 5I). RORC, ZBTB16, c-MAF, IL23R, and CCR6 were all expressed in MAIT cells from healthy donors (Figure 6F, compiled from (Dias et al., 2018)). While BCL11B bound upstream of IL17A and IL17F genes, H3K27ac was very low at these loci, including in regions where BCL11B bound (Figure 6E), and neither IL17A or IL17F genes were expressed in the blood MAIT cells of healthy donors (Figure 6F, compiled from (Dias et al., 2018)). Thus, IL17 genes are not expressed in human MAIT cells from healthy volunteers, and though BCL11B binds at these genes, additional factors are needed for expression of these genes in human MAIT cells.
While no data is available on Rorγt ChIP-seq in MAIT cells, the analysis of Rorγt ChIP-seq in murine Th17 cells (Ciofani et al., 2012) showed that Rorγt bound at homologs genes to those bound by BCL11B in human MAIT17 cells, including at c-MAF, IL23R and IL17A (Figure 6G), suggesting that BCL11B and Rorγt may cooperate in regulating gene expression of key type 3 (17) program genes.
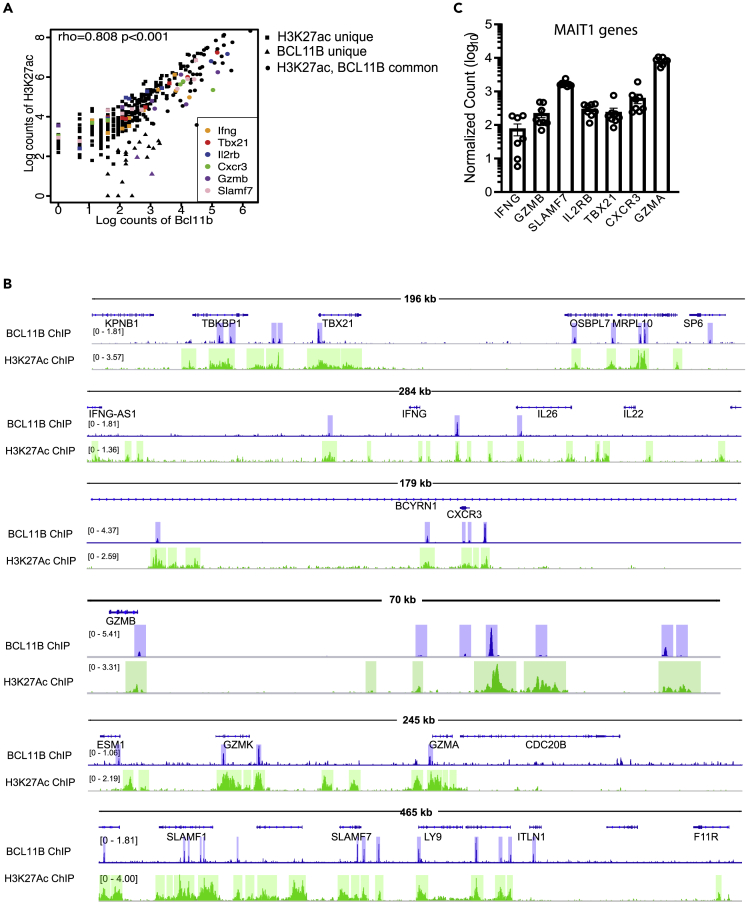
Surprisingly, Spearman's Correlation analysis showed significant correlation of BCL11B binding and H3K27Ac at human MAIT1 signature genes (Figure 7A), despite that in the absence of BCL11B, these genes were not significantly changed in expression in murine MAIT cells (Figures 5C and 5H). Binding was observed at TBX21, CXCR3, GZMA, GZMB, GZMK, SLAMF7 and IFNγ, correlated with enriched H3K27Ac (Figure 7B). All MAIT1 program genes are expressed in human MAIT cells from healthy donors (Figure 7C) (compiled from (Dias et al., 2018)). These results thus suggest that in human MAIT cells, in addition to control MAIT17 program, BCL11B may be implicated in the control of essential genes of the MAIT1 program, dissimilar to mouse.
Figure 7.
BCL11B binds at H3K27Ac-rich regions of key genes of MAIT1 program in human MAIT cells from healthy donors
(A) Spearman's Correlation plot of BCL11B binding intensity with H3K27Ac peaks at MAIT1 signature genes. Several genes of the MAIT1 program are listed.
(B) Integrative Genomics Viewer (IGV) visualization at TBX21, IFNγ, CXCR3, SLAMF7, GZMB, GZMA, and GZMK loci of BCL11B ChIP-seq (track 1) and H3K27Ac ChIP-seq (track 2) in human MAIT cells sorted from PBMCs of healthy donors. CD3+CD161+TCRVα7.2+ were sorted from PBMCs derived from nine donors (BCL11B ChIP-seq) and two donors (H3K27Ac ChIP-seq).
(C) Normalized counts in log10 format for TBX21, IFNγ, CXCR3, GZMB, GZMA, and SLMF7 in blood MAIT cells of healthy donors. Data analyzed from (Dias et al., 2018). n = 8. Data are represented as mean ± SEM. Blue and green highlights indicate significant ChIP-seq peaks. ChIP-seq scale bar normalized to sequences per million reads.
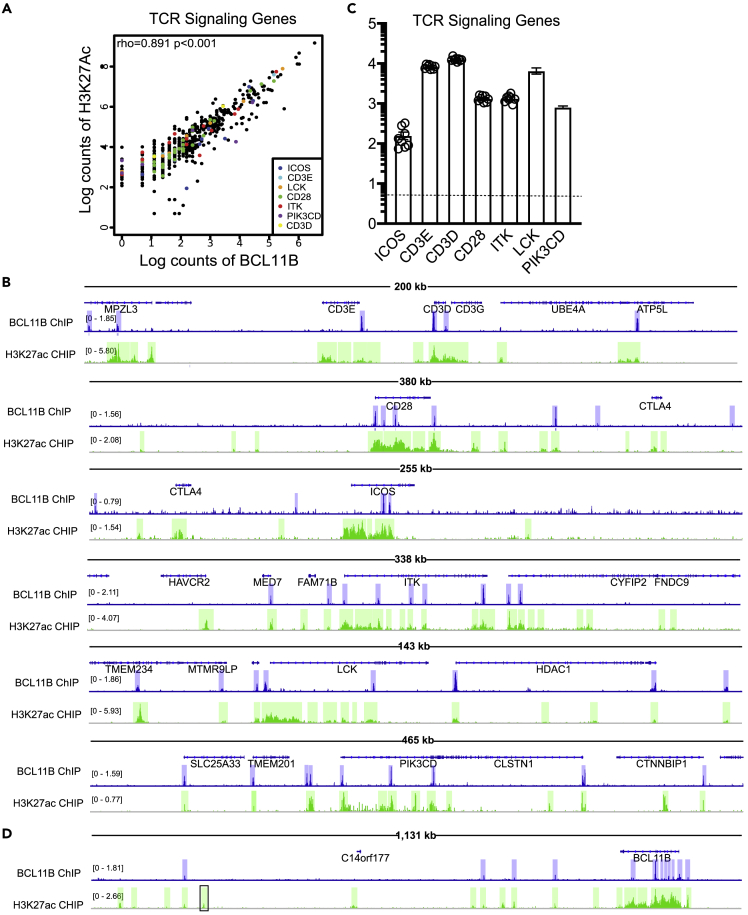
Genes encoding the CD3 complex and TCR signaling were downregulated in the absence of BCL11B in murine MAIT cells at steady state and in infection (Figures 5E and 5J). In human MAIT cells we found a strong correlation between BCL11B binding and H3K27Ac peaks at CD3 complex and TCR signaling program genes (Figure 8A). Among the bound genes which showed strong correlations, were CD3 complex genes, CD28, ICOS, ITK, LCK, and PIK3CD (Figure 8B). These genes are all expressed in MAIT cells isolated from peripheral blood of healthy donors (Figure 8C) (compiled from (Dias et al., 2018)).
Figure 8.
BCL11B binds at essential genes of TCR signaling program and at BCL11B locus in human MAIT cells from healthy donors at regions with high H3K27Ac
(A) Spearman's Correlation plot of BCL11B binding intensity with H3K27Ac peaks at TCR Signaling genes. Several genes of the TCR program are listed.
(B) Integrative Genomics Viewer (IGV) visualization at TCR signaling loci: CD3 complex, CD28, ICOS, ITK, LCK, and PIK3CD of BCL11B ChIP-seq (track 1) and H3K27Ac ChIP-seq (track 2) in human MAIT cells sorted from PBMCs of healthy donors.
(C) Normalized counts in log10 format for TCR signaling mRNAs from blood MAIT cells of healthy donors. Data analyzed from (Dias et al., 2018). n = 8.
(D) IGV at BCL11B locus of BCL11B ChIP-seq (track 1) and H3K27Ac ChIP-seq (track 2) in human MAIT cells. In B and D, blue and green highlights indicate significant ChIP-seq peaks. In D, the position of +845kb enhancer is indicated in black rectangular. ChIP-seq scale bar normalized to sequences per million reads.
In addition to genes implicated in MAIT17, MAIT1 and TCR signaling programs, BCL11B showed significant binding at intron 3 of its own locus, as well as at a region located approximately 200kb from its 3′ end in a gene empty island, as well as at a location in close vicinity of the +845kb enhancer, also conserved in Treg cells (data not shown). While binding at the 3′ downstream locations showed minimal H3K27Ac, binding at the intron overlapped with high H3K27Ac (Figure 8D), suggesting that BCL11B may play a role in sustaining its own expression in these cells.
In addition, BCL11B bound at flanking regions of C2 and C3 complement genes, depressed in the absence of BCL11B in murine MAIT cells, in areas with H3K27Ac peaks, despite that overall H3K27Ac was almost absent at C2 and C3 complement genes (Figure S8), suggesting a putative role of BCL11B in repressing these innate genes in MAIT cells.
These results taken together show that BCL11B binds in human MAIT cells at genes homologous to the mouse genes that have the expression dysregulated in its absence, including at essential MAIT17, CD3 complex and TCR signaling programs, as well as at some complement genes. Many of the bound regions are associated with increased H3K27Ac, suggesting a critical role of BCL11B in the control of regulomes with roles in these programs. Although in mouse MAIT cells Bcl11b did not appear to regulate MAIT1 program, BCL11B did bind at essential MAIT1 genes in human MAIT cells in regions associated with putative active enhancers, suggesting a different role of BCL11B in the control of MAIT1 program in humans and mice.
Discussion
The TFs PLZF and RORγt have been demonstrated to be essential in MAIT cells (Okada et al., 2015; Koay et al. 2016, 2019; Godfrey et al., 2019). In this study, we established that BCL11B has a fundamental role in MAIT cells and positioned BCL11B upstream of PLZF and RORγt. Absence of Bcl11b in mice affected MAIT cell numbers and impacted particularly MAIT17 program. MAIT17 cells were not only reduced in the thymus and lung in the absence of Bcl11b, but they completely fail to produce IL17A even in conditions of bacterial infection. Transcriptomics analysis shows that Bcl11b controls the MAIT17 cell program both at steady state and in infection conditions. In addition, in human MAIT cells BCL11B did not only bind at RORC locus, but also at essential genes of the MAIT17 cell program, including at ZBTB16, MAF, IL23R, CCR6, and IL17A. Importantly binding at MAIT17 program genes was in locations enriched in H3K27Ac, some of which possibly novel enhancers. It will be important to compare the binding and H3K27Ac activity at these genes in MAIT cells versus iNKT cells, given the opposite role of BCL11B in these two lineages, namely sustaining the expression of MAIT17 program genes in MAIT cells, but blocking the expression of iNKT17 program in iNKT cells (Uddin et al., 2016). Conversely, it possible that BCL11B binds in a similar manner at ZBTB16 locus both in MAIT cells and iNKT cells, given that BCL11B positively controlled the expression of this gene both in iNKT cells (Uddin et al., 2016) and MAIT cells (this study). This is different from DN2 thymocytes, where Bcl11b represses Zbtb16 expression, likely associated with the repression of the innate-like programs in these cells (Hosokawa et al. 2018, 2020). Thus, Bcl11b plays divergent roles in regulation of Zbtb16 gene expression in DN2 thymocytes versus MAIT cells and iNKT cells, repressing its expression in DN2 thymocytes, but positively controlling its expression in the innate-like T cells, MAIT and iNKT. Binding of BCL11B in human MAIT cells at ZBTB16 locus seems to occur at similar location to its binding in murine DN3 cells (Hosokawa et al., 2020), despite the different outcomes of its ablation in the two cell types. It will be of interest to establish what switches BCL11B from suppressing ZBTB16 expressing in DN2 thymocytes to promoting its expression in the innate-like cells T cells such as iNKT and MAIT cells, and determine how BCL11B regulates specific enhancer activity and chromatin accessibility.
Further comparisons are important as well, given that in Th2 cells, Rorc and Th17 program was derepressed in the absence of Bcl11b (Lorentsen et al., 2018), again supporting the idea of a complex subset/lineage specific behavior of Bcl11b in regulation of these programs and TFs, rather than a common generalized role. This is surprising, given that in MAIT cells, CD4+ T cells and Treg cells, the top two binding motifs for Bcl11b are ETS and RUNX (Lorentsen et al., 2018; Drashansky et al., 2019). In ILC2s, similarly to Th2 cells and iNKT cells, Rorc and type 3 program were derepressed (Califano et al., 2015), however top Bcl11b binding motifs seem to be divergent in these cells (Hosokawa et al., 2020). Molecular details on how Bcl11b restricts Rorc expression and type 3 program in primary ILC2s, Th2 effector cells and iNKT cells, but supports its expression in MAIT cells, are under investigation.
Binding of BCL11B at MAIT17 program genes was specific for MAIT cells and did not occur in the divergent Treg cells, in which instead BCL11B bound at Treg specific genes ((Drashansky et al., 2019) and data not shown), further supporting the notion that in divergent T cells, BCL11B specifically controls cell specific programs in addition to T cell-broad programs, such as TCR signaling. Indeed, BCL11B also plays a generalized common role in T cells, including in MAIT cells, in controlling TCR signaling program. BCL11B bound at the CD3 complex, CD28, ICOS, ITK, LCK, and PIK3CD loci. Binding at these locations in MAIT cells was associated with H3K27Ac peaks, indicative of putative active enhancers. Along this line, TCR signaling program genes were reduced in murine Bcl11b KO MAIT cells both at steady state and in infection. Thus, BCL11B not only initiates expression of these genes at commitment stage, but further it is needed to sustain their expression.
BCL11B bound in human MAIT cells at MAIT1 program genes, including at TBX21, IFNγ, GZMB and GZMA, at regions associated with putative active enhancers, even though these genes were not differentially expressed in mouse MAIT cells at steady state or during infection. One possibility may stand in the more pronounced MAIT1 nature of human MAIT cells as compared to the mouse MAIT cells, also reflected in the scarcity of MAIT1 cells in mice, or differences in BCL11B-mediated regulation in the two species. Though mRNAs for Gzmb and Gzma were not different in mouse BCL11B-deficient MAIT cells, protein levels were elevated. Related to this, numerous genes belonging to cytotoxic granule exocytosis pathway, were downregulated, suggesting that increased granzyme levels in Bcl11b-deficient MAIT cells are likely due to their accumulation related to the reduction in this pathway. However in human MAIT cells BCL11B bound at GZMB and GZMA loci, as well as at genes of the CGE pathway, suggesting a complex role for BCL11B in regulation of pathways involved in Granzyme expression and production in human MAIT cells.
In summary, our findings demonstrate that BCL11B is an essential MAIT cell TF and regulates fundamental programs of these cells, including MAIT17 and TCR signaling programs, and potentially MAIT1 program in human MAIT cells. In addition, our study positions BCL11B in the network of TFs that regulate MAIT cells, specifically upstream of PLZF and RORγt. Importantly, BCL11B does not only control MAIT17 program through RORγt, but also directly, by binding to genes of this pathway including at c-MAF, IL17A, CCR6 and IL23R. Understanding how programs and responses of these cells are regulated will help in designing future therapies for bacterial infections and anti-tumor immune responses.
Limitations of the study
One limitation is the reduced number of MAIT cells in common laboratory mice and thus the impossibility to conduct Bcl11b ChIP-seq in murine MAIT cells, which would have provided a more clear picture on the programs commonly and differentially regulated by BCL11B in humans versus mice.
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Dr. Dorina Avram, Dorina.avram@moffitt.org.
Material availability
No new or unique reagents were generated by this study.
Data and code availability
RNA-seq and ChIP-seq data shown in this study have been deposited in the Gene Expression Omnibus database with accession GEO: GSE145779. All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplemental information. Additional data and code related to this paper may be requested from the authors.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We thank Dr. Stephen J. McSorley of UC- Davis for Salmonella enterica ser. Typhimurium BRD509. We gratefully acknowledge the National Institutes of Health Tetramer Core Facility (NIH TCF) for providing the MR1 monomers. We gratefully acknowledge C. Tao and P. Kumar for technical assistance and Xiaoping Luo for maintaining the mouse colony. Funding: This work was supported by NIH grants R01AI067846, R01AI33623 (to D.A.) and UF Health Cancer Center (to D.A.), R01AI33623 (to B.K.), R01DK105562 and R01AI132391 (to L.Z.) and 2T32DK074367 (to T.T.D.).
Author contributions
Conceptualization: T.T.D., E.H., and D.A. Experimental design: T.T.D., E.H. L.M., Z.H., and D.A. Methodology: T.T.D., N.C., E.H., J.C., and D.A. Performed experiments: T.T.D., N.C., E.H., P.C., X.C., and J.C. Software: T.T.D., E.H., L.M., X.H. Formal analysis: T.T.D., N.C., E.H., J.C. L.M., Z.H., and D.A. Resources: D.A., B.K, L.Z., and W.Z. Data curation: T.T.D. and D.A. Writing and editing: T.T.D., E.H., N.G., and D.A.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102307.
Supplemental information
(Koay et al., 2019); NC, not changed
(compiled from Koay et al., 2019)
References
- Abboud G., Stanfield J., Tahiliani V., Desai P., Hutchinson T.E., Lorentsen K.J., Cho J.J., Avram D., Salek-Ardakani S. Transcription factor Bcl11b controls effector and memory CD8 T cell fate decision and function during poxvirus infection. Front. Immunol. 2016;7:425. doi: 10.3389/fimmu.2016.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albu D.I., Feng D., Bhattacharya D., Jenkins N.A., Copeland N.G., Liu P., Avram D. BCL11B is required for positive selection and survival of double-positive thymocytes. J. Exp. Med. 2007;204:3003–3015. doi: 10.1084/jem.20070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albu D.I., Vanvalkenburgh J., Morin N., Califano D., Jenkins N.A., Copeland N.G., Liu P., Avram D. Transcription factor Bcl11b controls selection of invariant natural killer T-cells by regulating glycolipid presentation in double-positive thymocytes. Proc. Natl. Acad. Sci. U S A. 2011;108:6211–6216. doi: 10.1073/pnas.1014304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram D., Califano D. The multifaceted roles of Bcl11b in thymic and peripheral T cells: impact on immune diseases. J. Immunol. 2014;193:2059–2065. doi: 10.4049/jimmunol.1400930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram D., Fields A., Pretty on Top K., Nevrivy D.J., Ishmael J.E., Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J. Biol. Chem. 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram D., Fields A., Senawong T., Topark-Ngarm A., Leid M. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem. J. 2002;368:555–563. doi: 10.1042/BJ20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano D., Cho J.J., Uddin M.N., Lorentsen K.J., Yang Q., Bhandoola A., Li H., Avram D. Transcription factor Bcl11b controls identity and function of mature type 2 innate lymphoid cells. Immunity. 2015;43:354–368. doi: 10.1016/j.immuni.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano D., Sweeney K.J., Le H., VanValkenburgh J., Yager E., O'Connor W., Kennedy J.S., Jones D.M., Avram D. Diverting T helper cell trafficking through increased plasticity attenuates autoimmune encephalomyelitis. J. Clin. Invest. 2014;124:174–187. doi: 10.1172/JCI70103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wang H., D'Souza C., Sun S., Kostenko L., Eckle S.B., Meehan B.S., Jackson D.C., Strugnell R.A., Cao H. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 2017;10:58–68. doi: 10.1038/mi.2016.39. [DOI] [PubMed] [Google Scholar]
- Ciofani M., Madar A., Galan C., Sellars M., Mace K., Pauli F., Agarwal A., Huang W., Parkurst C.N., Muratet M. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides M.G., Link V.M., Tamoutounour S., Wong A.C., Perez-Chaparro P.J., Han S.J., Chen Y.E., Li K., Farhat S., Weckel A. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019;366:eaax6624. doi: 10.1126/science.aax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett A.J., Eckle S.B., Birkinshaw R.W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- Cui Y., Franciszkiewicz K., Mburu Y.K., Mondot S., Le Bourhis L., Premel V., Martin E., Kachaner A., Duban L., Ingersoll M.A. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J. Clin. Invest. 2015;125:4171–4185. doi: 10.1172/JCI82424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J., Boulouis C., Gorin J.B., van den Biggelaar R., Lal K.G., Gibbs A., Loh L., Gulam M.Y., Sia W.R., Bari S. The CD4(-)CD8(-) MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8(+) MAIT cell pool. Proc. Natl. Acad. Sci. U S A. 2018;115:E11513–E11522. doi: 10.1073/pnas.1812273115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolens A.C., Durinck K., Lavaert M., Van der Meulen J., Velghe I., De Medts J., Weening K., Roels J., De Mulder K., Volders P.J. Distinct Notch1 and BCL11B requirements mediate human gammadelta/alphabeta T cell development. EMBO Rep. 2020;21:e49006. doi: 10.15252/embr.201949006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drashansky T.T., Helm E., Huo Z., Curkovic N., Kumar P., Luo X., Parthasarathy U., Zuniga A., Cho J.J., Lorentsen K.J. Bcl11b prevents fatal autoimmunity by promoting Treg cell program and constraining innate lineages in Treg cells. Sci. Adv. 2019;5:eaaw0480. doi: 10.1126/sciadv.aaw0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusseaux M., Martin E., Serriari N., Peguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- Fang D., Cui K., Hu G., Gurram R.K., Zhong C., Oler A.J., Yagi R., Zhao M., Sharma S., Liu P. Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation. J. Exp. Med. 2018;215:1449–1462. doi: 10.1084/jem.20171127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez L., Famili F., Cordes M., Brugman M., van Eggermond M., Wu H., Chouaref J., Granado D.S.L., Tiemessen M.M., Pike-Overzet K. Functional definition of a transcription factor hierarchy regulating T cell lineage commitment. Sci. Adv. 2020;6:eaaw7313. doi: 10.1126/sciadv.aaw7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardin N.A., Loh L., Admojo L., Davenport A.J., Richardson K., Rogers A., Darcy P.K., Jenkins M.R., Prince H.M., Harrison S.J. Enumeration, functional responses and cytotoxic capacity of MAIT cells in newly diagnosed and relapsed multiple myeloma. Sci. Rep. 2018;8:4159. doi: 10.1038/s41598-018-22130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioulbasani M., Galaras A., Grammenoudi S., Moulos P., Dent A.L., Sigvardsson M., Hatzis P., Kee B.L., Verykokakis M. The transcription factor BCL-6 controls early development of innate-like T cells. Nat. Immunol. 2020;21:1058–1069. doi: 10.1038/s41590-020-0737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Koay H.F., McCluskey J., Gherardin N.A. The biology and functional importance of MAIT cells. Nat. Immunol. 2019;20:1110–1128. doi: 10.1038/s41590-019-0444-8. [DOI] [PubMed] [Google Scholar]
- Gold M.C., Cerri S., Smyk-Pearson S., Cansler M.E., Vogt T.M., Delepine J., Winata E., Swarbrick G.M., Chua W.J., Yu Y.Y. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad E.K., Wu X., Hammer J.A., 3rd, Henkart P.A. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J. Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.N., Sharma A., Ghosh S., Hong S.W., Roy-Chowdhuri S., Im S.H., Kang K., Rudra D. Bcl11b prevents catastrophic autoimmunity by controlling multiple aspects of a regulatory T cell gene expression program. Sci. Adv. 2019;5:eaaw0706. doi: 10.1126/sciadv.aaw0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes T.D., Vinay Pandey R., Helm E.Y., Schlums H., Han H., Campbell T.M., Drashansky T.T., Chiang S., Wu C.-Y., Tao C., Shoukier M. The transcription factor Bcl11b promotes both canonical and adaptive NK cell differentiation. Science Immunology. 2021;6(57) doi: 10.1126/sciimmunol.abc9801. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt O., Kanno E., Bossi G., Booth S., Daniele T., Santoro A., Arico M., Saegusa C., Fukuda M., Griffiths G.M. Slp1 and Slp2-a localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic. 2008;9:446–457. doi: 10.1111/j.1600-0854.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa H., Romero-Wolf M., Yang Q., Motomura Y., Levanon D., Groner Y., Moro K., Tanaka T., Rothenberg E.V. Cell type-specific actions of Bcl11b in early T-lineage and group 2 innate lymphoid cells. J. Exp. Med. 2020;217:e20190972. doi: 10.1084/jem.20190972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa H., Romero-Wolf M., Yui M.A., Ungerback J., Quiloan M.L.G., Matsumoto M., Nakayama K.I., Tanaka T., Rothenberg E.V. Bcl11b sets pro-T cell fate by site-specific cofactor recruitment and by repressing Id2 and Zbtb16. Nat. Immunol. 2018;19:1427–1440. doi: 10.1038/s41590-018-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P., Chan S., Vogel W.K., Zhang L.J., Topark-Ngarm A., Golonzhka O., Jost B., Le Gras S., Gross M.K., Leid M. Bcl11b represses a mature T-cell gene expression program in immature CD4(+)CD8(+) thymocytes. Eur. J. Immunol. 2010;40:2143–2154. doi: 10.1002/eji.200940258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- Koay H.F., Gherardin N.A., Enders A., Loh L., Mackay L.K., Almeida C.F., Russ B.E., Nold-Petry C.A., Nold M.F., Bedoui S. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- Koay H.F., Su S., Amann-Zalcenstein D., Daley S.R., Comerford I., Miosge L., Whyte C.E., Konstantinov I.E., d'Udekem Y., Baldwin T. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol. 2019;4:eaay6039. doi: 10.1126/sciimmunol.aay6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo S., Tanaka H., Endo T.A., Muroi S., Liu Y., Seo W., Tenno M., Kakugawa K., Naoe Y., Nair K. Priming of lineage-specifying genes by Bcl11b is required for lineage choice in post-selection thymocytes. Nat. Commun. 2017;8:702. doi: 10.1038/s41467-017-00768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D., Uche O.U., Eladad S., Hobbs R.M., Yi W., Alonzo E., Chua K., Eidson M., Kim H.J., Im J.S. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka A., Ussher J.E., Cosgrove C., Clough C., Fergusson J.R., Smith K., Kang Y.H., Walker L.J., Hansen T.H., Willberg C.B., Klenerman P. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J., Roundabush F.L., Hutton Moore P., Montague M., Oldham W., Li Y., Chin L.S., Li L. Hrs interacts with SNAP-25 and regulates Ca(2+)-dependent exocytosis. J. Cell Sci. 2000;113:2273–2284. doi: 10.1242/jcs.113.12.2273. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L., Dusseaux M., Bohineust A., Bessoles S., Martin E., Premel V., Core M., Sleurs D., Serriari N.E., Treiner E. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourhis L., Martin E., Peguillet I., Guihot A., Froux N., Core M., Levy E., Dusseaux M., Meyssonnier V., Premel V. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Holzapfel K.L., Zhu J., Jameson S.C., Hogquist K.A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoux F., Bellet D., Daviaud C., El Morr Y., Darbois A., Niort K., Procopio E., Salou M., Gilet J., Ryffel B. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366:494–499. doi: 10.1126/science.aaw2719. [DOI] [PubMed] [Google Scholar]
- Legoux F., Gilet J., Procopio E., Echasserieau K., Bernardeau K., Lantz O. Molecular mechanisms of lineage decisions in metabolite-specific T cells. Nat. Immunol. 2019;20:1244–1255. doi: 10.1038/s41590-019-0465-3. [DOI] [PubMed] [Google Scholar]
- Li L., Leid M., Rothenberg E.V. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Burke S., Wang J., Chen X., Ortiz M., Lee S.C., Lu D., Campos L., Goulding D., Ng B.L. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentsen K.J., Cho J.J., Luo X., Zuniga A.N., Urban J.F., Jr., Zhou L., Gharaibeh R., Jobin C., Kladde M.P., Avram D. Bcl11b is essential for licensing Th2 differentiation during helminth infection and allergic asthma. Nat. Commun. 2018;9:1679. doi: 10.1038/s41467-018-04111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Treiner E., Duban L., Guerri L., Laude H., Toly C., Premel V., Devys A., Moura I.C., Tilloy F. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménasché G., Ménager M., Lefebvre J., Deutsch E., Athman R., Lambert N., Mahlaoui N., Court M., Garin J., Fischer A., de Saint Basile G. A newly identified isoform of Slp2a associates with Rab27a in cytotoxic T cells and participates to cytotoxic granule secretion. Blood. 2008;112:5052–5062. doi: 10.1182/blood-2008-02-141069. [DOI] [PubMed] [Google Scholar]
- Mielke L.A., Liao Y., Clemens E.B., Firth M.A., Duckworth B., Huang Q., Almeida F.F., Chopin M., Koay H.F., Bell C.A. TCF-1 limits the formation of Tc17 cells via repression of the MAF-RORgammat axis. J. Exp. Med. 2019;216:1682–1699. doi: 10.1084/jem.20181778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Markle J.G., Deenick E.K., Mele F., Averbuch D., Lagos M., Alzahrani M., Al-Muhsen S., Halwani R., Ma C.S. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349:606–613. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimpour A., Koay H.F., Enders A., Clanchy R., Eckle S.B., Meehan B., Chen Z., Whittle B., Liu L., Fairlie D.P. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels J., Kuchmiy A., De Decker M., Strubbe S., Lavaert M., Liang K.L., Leclercq G., Vandekerckhove B., Van Nieuwerburgh F., Van Vlierberghe P., Taghon T. Distinct and temporary-restricted epigenetic mechanisms regulate human alphabeta and gammadelta T cell development. Nat. Immunol. 2020;21:1280–1292. doi: 10.1038/s41590-020-0747-9. [DOI] [PubMed] [Google Scholar]
- Salou M., Legoux F., Gilet J., Darbois A., du Halgouet A., Alonso R., Richer W., Goubet A.G., Daviaud C., Menger L. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 2019;216:133–151. doi: 10.1084/jem.20181483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler A., Dang-Heine C., Reinke P., Babel N. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur. J. Immunol. 2015;45:2286–2298. doi: 10.1002/eji.201445313. [DOI] [PubMed] [Google Scholar]
- Savage A.K., Constantinides M.G., Han J., Picard D., Martin E., Li B., Lantz O., Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichter C.K., McDavid A., Miller H.W., Finak G., Seymour B.J., McNevin J.P., Diaz G., Czartoski J.L., McElrath M.J., Gottardo R., Prlic M. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight. 2016;1:e86292. doi: 10.1172/jci.insight.86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe J.C., Barral D.C., Mules E.H., Booth S., Hume A.N., Machesky L.M., Seabra M.C., Griffiths G.M. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilloy F., Treiner E., Park S.H., Garcia C., Lemonnier F., de la Salle H., Bendelac A., Bonneville M., Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Uddin M.N., Sultana D.A., Lorentsen K.J., Cho J.J., Kirst M.E., Brantly M.L., Califano D., Sant'Angelo D.B., Avram D. Transcription factor Bcl11b sustains iNKT1 and iNKT2 cell programs, restricts iNKT17 cell program, and governs iNKT cell survival. Proc. Natl. Acad. Sci. U S A. 2016;113:7608–7613. doi: 10.1073/pnas.1521846113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher J.E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T.H., Klenerman P., Willberg C.B. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchini A., Chancellor A., Spagnuolo J., Mori L., De Libero G. MR1-Restricted T cells are unprecedented cancer fighters. Front. Immunol. 2020;11:751. doi: 10.3389/fimmu.2020.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B., Loh L., Chen Z., Pediongco T.J., Wang H., Shi M., Zhao Z., Koutsakos M., Nussing S., Sant S. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat. Commun. 2018;9:4706. doi: 10.1038/s41467-018-07207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B., Scherwitzl I., Hutchinson E.C., Leng T., Kurioka A., Kulicke C., de Lara C., Cole S., Vasanawathana S., Limpitikul W. MAIT cells are activated during human viral infections. Nat. Commun. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanvalkenburgh J., Albu D.I., Bapanpally C., Casanova S., Califano D., Jones D.M., Ignatowicz L., Kawamoto S., Fagarasan S., Jenkins N.A. Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J. Exp. Med. 2011;208:2069–2081. doi: 10.1084/jem.20102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y., Watanabe H., Inoue J., Takeda N., Sakata J., Mishima Y., Hitomi J., Yamamoto T., Utsuyama M., Niwa O. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat. Immunol. 2003;4:533–539. doi: 10.1038/ni927. [DOI] [PubMed] [Google Scholar]
- Walker J.A., Oliphant C.J., Englezakis A., Yu Y., Clare S., Rodewald H.R., Belz G., Liu P., Fallon P.G., McKenzie A.N. Bcl11b is essential for group 2 innate lymphoid cell development. J. Exp. Med. 2015;212:875–882. doi: 10.1084/jem.20142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kjer-Nielsen L., Shi M., D'Souza C., Pediongco T.J., Cao H., Kostenko L., Lim X.Y., Eckle S.B.G., Meehan B.S. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci. Immunol. 2019;4:eaaw0402. doi: 10.1126/sciimmunol.aaw0402. [DOI] [PubMed] [Google Scholar]
- Yu Y., Wang C., Clare S., Wang J., Lee S.C., Brandt C., Burke S., Lu L., He D., Jenkins N.A. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J. Exp. Med. 2015;212:865–874. doi: 10.1084/jem.20142318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Laouar A., Denzin L.K., Sant'Angelo D.B. Zbtb16 (PLZF) is stably suppressed and not inducible in non-innate T cells via T cell receptor-mediated signaling. Sci. Rep. 2015;5:12113. doi: 10.1038/srep12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Rozell M., Verma R.K., Albu D.I., Califano D., Vanvalkenburgh J., Merchant A., Rangel-Moreno J., Randall T.D., Jenkins N.A. Antigen-specific clonal expansion and cytolytic effector function of CD8+ T lymphocytes depend on the transcription factor Bcl11b. J. Exp. Med. 2010;207:1687–1699. doi: 10.1084/jem.20092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Koay et al., 2019); NC, not changed
(compiled from Koay et al., 2019)
Data Availability Statement
RNA-seq and ChIP-seq data shown in this study have been deposited in the Gene Expression Omnibus database with accession GEO: GSE145779. All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplemental information. Additional data and code related to this paper may be requested from the authors.