Abstract
TRAF3 has diverse signaling functions, which vary by cell type. Uniquely in B lymphocytes, TRAF3 inhibits homeostatic survival. Highlighting the role of TRAF3 as a tumor suppressor, loss-of-function TRAF3 mutations are associated with human B-cell malignancies, while B-cell-specific deletion of TRAF3 in mice leads to autoimmunity and lymphoma development. The role of TRAF3 in inhibiting noncanonical NF-κB activation, CD40 and BAFF-R signaling to B cells is well documented. In contrast, TRAF3 enhances many T-cell effector functions, through associating with and enhancing signaling by the T-cell receptor (TCR)-CD28 complex. The present study was designed to determine the role of TRAF3 in signaling via the B-cell antigen receptor (BCR). The BCR is crucial for antigen recognition, survival, proliferation, and antibody production, and defects in BCR signaling can promote abnormal survival of malignant B cells. Here, we show that TRAF3 is associated with both CD79B and the BCR-activated kinases Syk and Btk following BCR stimulation. BCR-induced phosphorylation of Syk and additional downstream kinases was increased in TRAF3−/− B cells, with regulation observed in both follicular and marginal zone B-cell subsets. BCR stimulation of TRAF3−/− B cells resulted in increased surface expression of MHC-II, CD80, and CD86 molecules. Interestingly, increased survival of TRAF3−/− primary B cells was resistant to inhibition of Btk, while TRAF3-deficient malignant B-cell lines showed enhanced sensitivity. TRAF3 serves to restrain normal and malignant BCR signaling, with important implications for its role in normal B-cell biology and abnormal survival of malignant B cells.
Keywords: TNF receptor-associated factor (TRAF), B-cell receptor (BCR), inhibition mechanism, immunology, lymphocyte, lymphoma, spleen tyrosine kinase (Syk), Btk, MAPK, cell signaling
Abbreviations: BAFF, B-cell activating factor; BCM, B-cell medium; BCR, B-cell antigen receptor; Btk, Bruton's tyrosine kinase; DLBCL, diffuse large B-cell lymphoma; Erk, extracellular signal-regulated kinase; FO, follicular; IκBα, NF-κB inhibitor alpha; ITAM, immunoreceptor tyrosine-based activation motif; LMP1, latent membrane protein 1; Lyn, Lck/yes novel tyrosine kinase; MAPK, mitogen-activated protein kinase; MZ, marginal zone; NIK, NF-κB-inducing kinase; PI3K, phosphatidyl inositol-3 kinase; PLCγ, phospholipase C gamma; SEM, standard error of the mean; SHIP, SH2 domain-containing inositol polyphosphate 5-phosphatase; SHP, Src homology region 2 domain-containing phosphatase; STAT, signal transducer and activator of transcription; Syk, spleen-associated tyrosine kinase; TCR, T-cell receptor; TLR, Toll-like receptor; TNF, tumor necrosis factor; TRAF, TNF receptor-associated factor; Ub, ubiquitin; WB, western blot; WCL, whole-cell lysate
Tumor necrosis factor receptor (TNFR)-associated factor 3 (TRAF3) is an intracellular adaptor protein that regulates many signaling pathways in a cell-type and context-specific manner (1). Whole-body deletion of Traf3 in mice results in early postnatal death, highlighting the importance of this protein in many different cell types and processes (2). B-cell-specific Traf3 deletion in mice (B-Traf3−/−) leads to enhanced B-cell survival in vitro and in vivo that manifests as pronounced B-cell accumulation in the spleen, lymph nodes, and liver, spontaneous germinal center development, and autoimmune manifestations (3, 4). Aged B-Traf3−/− mice frequently develop spontaneous B-cell lymphoma (BCL) (5), and TRAF3 gene loss may also be an important factor in the development of human B-cell tumors (6). TRAF3 deletions or mutations resulting in loss of function have been identified in several B-cell malignancies, including chronic lymphocytic leukemia, multiple myeloma, Waldenström’s macroglobulinemia, Hodgkin lymphoma, diffuse large B-cell lymphoma, and splenic and gastric marginal zone (MZ) lymphoma (7, 8, 9, 10). Avid binding and sequestration of TRAF3 protein by the Epstein–Barr virus (EBV)-encoded oncogenic protein latent membrane protein 1 (LMP1) also produces a TRAF3-deficient phenotype in mouse and human BCL cell lines, showing that a TRAF3-deficient state can occur even in the absence of alterations to the TRAF3 gene (11). Hence, by restricting survival, TRAF3 functions as an important tumor suppressor in B cells.
The BCR is crucial for many important B-cell functions including antigen recognition, survival, proliferation, and antibody production (12). After binding of antigen, BCR clustering occurs, with formation of a multiprotein complex containing a heterodimer of CD79 A and CD79 B (13). Phosphorylation of CD79A/B at the immunoreceptor tyrosine-based activation motifs (ITAM) by Src family kinases creates a docking site for the tyrosine kinase Syk (14). Activated Syk in turn phosphorylates several targets, including phosphatidyl inositol 3-kinase (PI3K) and phospholipase C-γ2 (PLCγ2), resulting in activation of several downstream effector pathways. BCR signaling is tightly regulated in order to prevent aberrant B-cell activation and autoimmunity.
Dysregulated BCR signaling can promote malignant B-cell survival and contribute to the development of B-cell cancers (15). Due to the importance of BCR signaling in the survival of malignant B cells, several BCR pathway inhibitors are in current use or in clinical trials to treat B-cell malignancies (16, 17, 18). The Btk inhibitor ibrutinib is FDA approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma, MZ lymphoma, and Waldenström’s macroglobulinemia (19, 20, 21). Ibrutinib has also shown promise in several clinical trials for other types of B-cell malignancies, including diffuse large BCL (22). However, the response to ibrutinib is variable and several different mechanisms of ibrutinib resistance have been described (23). It is important to understand the mechanism and pathways involved in resistance or sensitivity to BCR pathway inhibitors in order to most effectively treat B-cell malignancies (24).
TRAF3 regulates several signaling cascades in B cells that interact with the BCR signaling pathway, including those mediated by CD40 and Toll-Like receptors (TLRs) (25, 26, 27, 28). In the BCL-derived cell line CH12.LX, TRAF3 plays a negative role in regulating CD40-BCR synergy (29, 30, 31). TRAF3 directly associates with Syk after TLR (32) and BAFF stimulation (33). A recent report showed that TRAF3 plays a role in BCR-induced regulation of Ig class-switch recombination (34). In T lymphocytes, TRAF3 associates with the TCR complex and enhances TCR signaling by restraining negative regulators of this complex (35, 36, 37). However, the role that TRAF3 plays in regulating BCR signaling is not well understood.
Here, we show that after BCR stimulation, phosphorylation of Syk was increased in TRAF3−/− B cells, resulting in increased phosphorylation of downstream kinases. Both follicular and MZ TRAF3−/− B cells showed increased activation after BCR stimulation, indicating that TRAF3 regulates signaling in both mature B cell subsets. BCR stimulation of TRAF3−/− B cells resulted in increased surface expression of MHC-II, CD80, and CD86 molecules, all of which are important for antigen presentation. TRAF3 is associated with Syk and Btk after BCR stimulation in both primary mouse B cells and the BJAB human B BCL-derived cell line. TRAF3−/− mouse primary B cells were resistant, while TRAF3-deficient human malignant B-cell lines were sensitive to Btk and Syk inhibitor-induced cell death. Thus, TRAF3 restrains BCR signaling, with important consequences for the biology of both normal and malignant B cells.
Results
TRAF3 inhibits early BCR-induced kinase activation
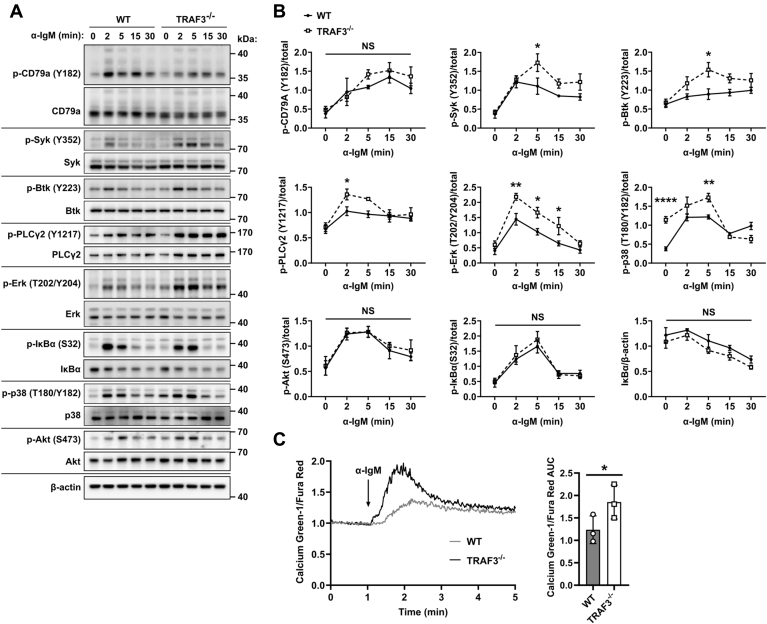
To determine the impact of TRAF3 status on early BCR-mediated signaling, we stimulated mouse splenic B cells isolated from B-cell-specific Traf3 deficient mice (CD19-Cre; B-Traf3−/−) (3) or Traf3flox/flox littermate controls (referred to here as WT for simplicity) with stimulatory anti-IgM Ab. BCR-induced phosphorylation of the early-activated tyrosine kinases Syk (Y352) and Btk (Y223) was increased and prolonged in TRAF3−/− B cells compared with WT controls (Fig. 1, A and B). This difference in phosphorylation was apparent as early as 2 min after activation. Increased BCR-induced phosphorylation was also seen in the mitogen-activated protein kinases (MAPK) Erk1/2 (T202/Y204) and p38 (T108/Y182). We did not detect a difference in phosphorylation of AKT (S473), IκBα (S32), or IκBα degradation in the TRAF3−/− B cells, possibly due to the moderate dose of anti-IgM used in our experiments and/or to the limitations of sensitivity of the western blot (WB) as a detection method. We also found that calcium flux after BCR stimulation was increased in TRAF3−/− B cells (Fig. 1C). These results are consistent with a recent report that also found increased phosphorylation of Syk and Btk and elevated calcium flux in TRAF3−/− B cells after BCR stimulation (34).
Figure 1.
TRAF3 is a negative regulator of BCR signaling.A, representative WB and (B) graphs (mean ± SEM) of densitometry of protein lysates from WT and TRAF3−/− mouse splenic B cells stimulated with 10 μg/ml anti-IgM Ab for indicated times (N = 3–4 mice). A two-way ANOVA (# p < 0.05, NS = not significant) followed up with an unpaired t-test with Sidak correction for multiple comparisons (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗∗ p < 0.0001, NS = not significant) was used to determine statistical significance. C, representative tracing of the ratio of Calcium-Green-1/Fura fluorescent in WT and TRAF3−/− mouse splenic B cells after stimulation with 5 μg/ml anti-IgM Ab. The graph represents the mean area under the curve (AUC) ± SEM (N = 3 mice). An unpaired t-test was used to evaluate differences for statistical significance (∗ p < 0.05).
The increased BCR signaling seen in TRAF3−/− B cells was not due to differential IgM surface expression (Fig. S1, A and B). TRAF3−/− B cells had no difference in total protein expression of BCR pathway proteins (Fig. S1, C and D), showing that differences in protein expression were not responsible for the signaling differences observed in Figure 1. There was no detectable difference in the Traf3flox/flox controls and CD19-Cre B cells, indicating that expression of Cre or CD19 haploinsufficiency was not responsible for the observed signaling differences (Fig. S2A). TRAF3 protein expression displayed a gene dosage effect (Fig. S2B). TRAF3 haploinsufficient B cells had an intermediate level of BCR-induced Syk activation compared with homozygous WT or TRAF3−/− B cells (Fig. S2C).
TRAF3 regulation of BCR signaling in marginal zone and follicular B cells
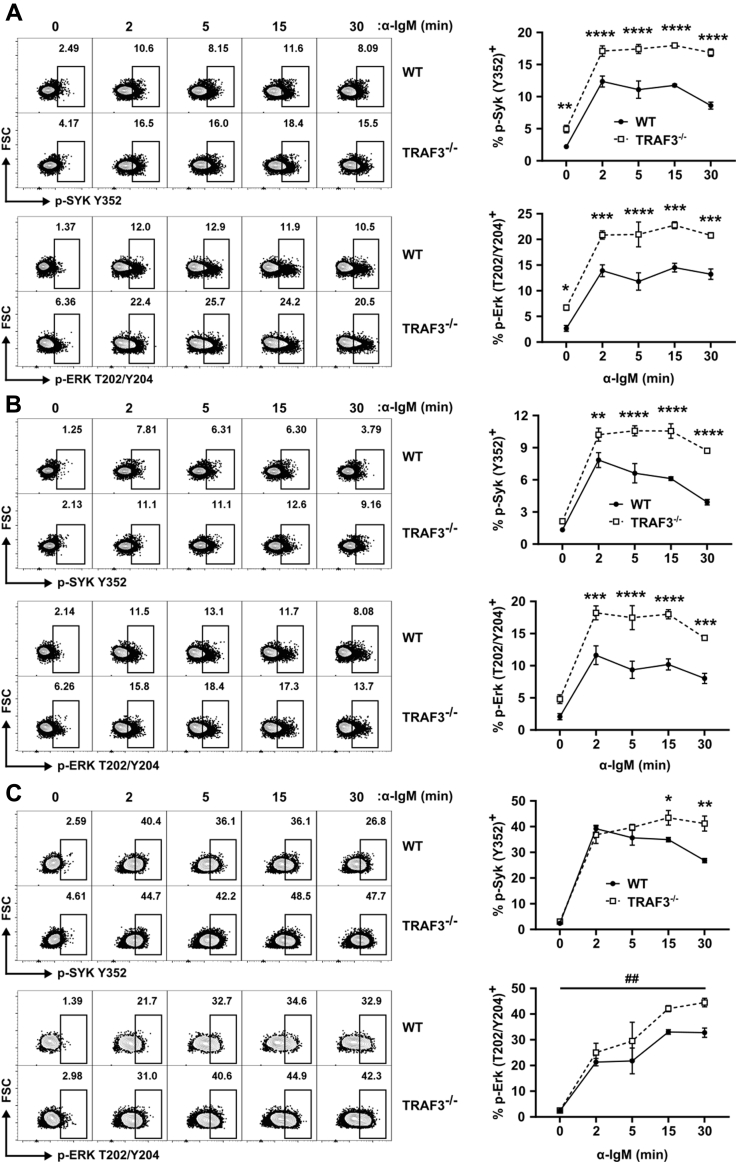
B-Traf3−/− mice have an increased proportion of MZ B cells (3), raising the possibility that their increased BCR signaling results from this enhanced subpopulation of B cells. MZ B cells show increased calcium signaling and kinase activation after anti-IgM Ab stimulation compared with follicular (FO) B cells (38, 39) so we compared BCR signaling in TRAF3−/− MZ and FO B cells (gating strategy shown in Fig. S3A). Consistent with the results presented in Figure 1, TRAF3−/− B220+ splenocytes had increased BCR-induced Syk and Erk activation (Fig. 2A). Both TRAF3−/− MZ (CD21hi, CD23-) and FO B cells (CD21lo, CD23-) showed increased BCR-induced Erk activation compared with WT controls, although the effect was larger in the TRAF3−/− FO B cells (Fig. 2, B and C). The p-Erk (T202/Y204) gMFI of the responding WT and TRAF3−/− B cells was similar (Fig. S3B). This result suggests that the increased response of the TRAF3−/− B cells was due to the lowering of the activation threshold, resulting in a greater number of responding cells, rather than increased Erk phosphorylation per cell. TRAF3−/− B220+ and FO B cells also showed increased basal Erk phosphorylation (Fig. 3C). Basal Syk phosphorylation showed a trending increase in the TRAF3−/− total and FO B cells. Thus, TRAF3 inhibited BCR signaling in both the MZ and, to a larger extent, FO B cell subsets, so the overall increase in BCR signaling resulting from TRAF3 deficiency was not restricted to MZ B cells, nor due to their increased numbers.
Figure 2.
TRAF3-mediated regulation of BCR signaling in FO and MZ B cells. Mouse splenocytes were stimulated with 10 μg/ml anti-IgM Ab for indicated times. Representative plots and graphs of p-Erk (T202/Y204) and p-Syk (Y352) for (A) total B220+, (B) FO (CD21lo CD23+), and (C) MZ (CD21hi CD23-) B cells. Data were analyzed using FlowJo software, and population frequencies are expressed as percent of the parent population. Graphs represent mean ± SEM (N = 4 mice). A two-way ANOVA (## p < 0.01) followed up with an unpaired t-test with Holm–Sidak correction for multiple comparisons (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001, ∗∗∗∗ p < 0.0001) was used to determine statistical significance.
Importance of TRAF3 interactions with Syk and Btk in regulation of BCR signaling
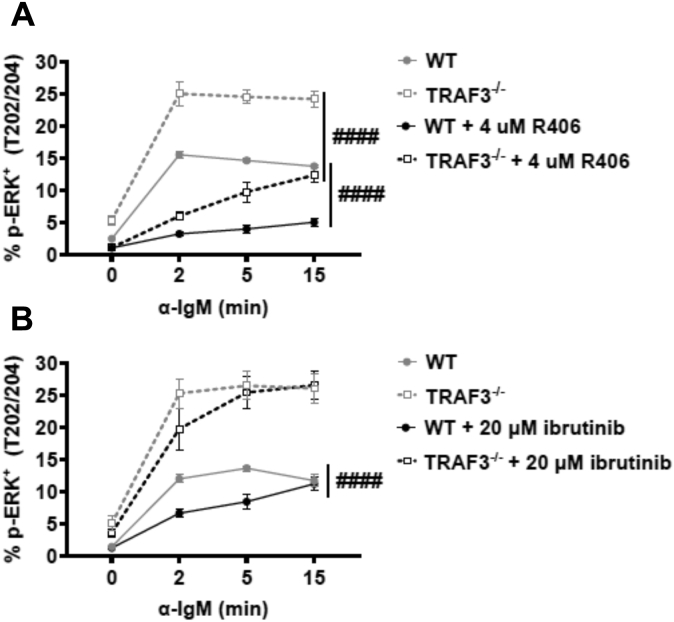
We wished to determine if the increased activation of Erk seen in TRAF3−/− B cells was due to the enhanced upstream Syk and/or Btk activity. Primary mouse B cells from B-Traf3−/− or WT controls were treated with the Btk inhibitor ibrutinib (40) or the Syk inhibitor R406 (41, 42) and subsequent BCR-mediated Erk activation was measured (Fig. 3A). TRAF3−/− B cells still showed increased BCR-induced Erk activation in the presence of Btk inhibition, while Syk inhibition lowered Erk activation levels in TRAF3-deficient B cells to those similar to WT B cells (Fig. 3B). These results suggest that TRAF3 is inhibiting signaling at the level of Syk activation, resulting in increased signaling in the absence of TRAF3. These data are consistent with previous reports that Erk activation is highly dependent upon Syk and partially dependent upon Btk (43).
Figure 3.
Impact of inhibition of Syk and Btk on BCR-mediated Erk activation in WT and TRAF3−/−mouse B cells. Splenocytes from WT or B-Traf3−/− mice were pretreated with (A) 4 μM of R406 or (B) 20 μM of ibrutinib for 1 h prior to stimulation with 10 μg/ml anti-IgM Ab. Graphs represent % p-Erk (T202/Y204) positive (mean ± SEM). A two-way ANOVA (#### p < 0.0001) was used to determine statistical significance (N = 3 mice).
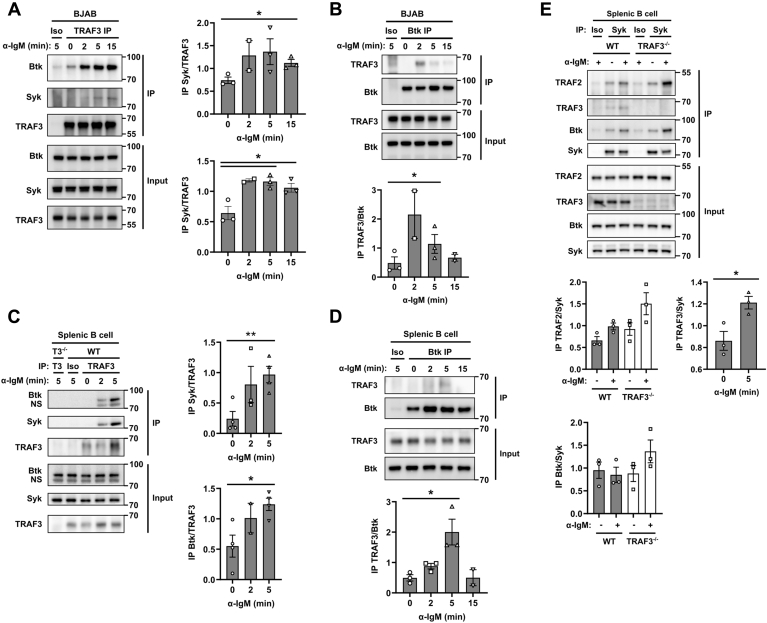
Both the human and mouse forms of Btk and Syk contain a putative TRAF2/3-binding site (44). TRAF3 associates with Syk after TLR stimulation in macrophages and when TRAF3 and Syk are overexpressed in HEK293 epithelial cells (32). To determine whether BCR stimulation induced TRAF3-Syk association in B cells, we performed an immunoprecipitation of TRAF3 from the human BCL-derived BAJB cell line. Both the TRAF3 IP and the reciprocal IP of Btk revealed BCR-induced Btk-TRAF3 protein association after BCR stimulation (Fig. 4, A and B). There was also a similar BCR-induced Btk-TRAF3 association in mouse splenic B cells (Fig. 4, C and D). In both the BJAB and mouse splenic B cells, there was a BCR-induced association of TRAF3 and Syk (Fig. 4, A, C and E). We also found that as in macrophages (32), TLR4 stimulation of B cells induced TRAF3-Syk association. But unlike stimulation through the BCR, no TRAF3-Btk association was seen after TLR4 stimulation (Fig. S4). These data show that TRAF3 associates with multiple BCR-complex-associated proteins, either directly or indirectly.
Figure 4.
BCR-induced association of TRAF3 with Syk and Btk.A) TRAF3 or B) Btk was immunoprecipitated from cell lysates of BJAB cells following 20 μg/ml anti-human IgM Ab stimulation. C) TRAF3, D) Btk, or E) Syk was immunoprecipitated from cell lysates of mouse splenic B cells following 20 μg/ml anti-mouse IgM Ab stimulation. Co-immunoprecipitation was assayed with WB. Images are representative of three independent experiments. Graphs represent densitometry of protein of interest normalized to the IP protein (mean ± SEM). An unpaired t-test was used to determine statistical significance (∗ p < 0.05).
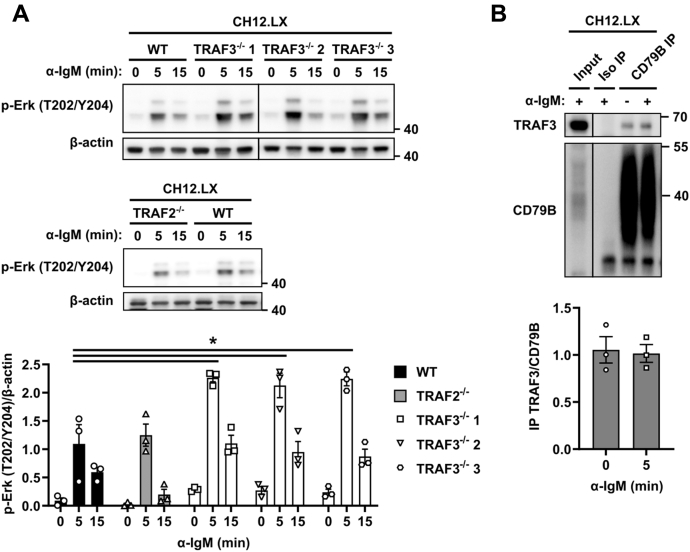
Although TRAFs 2 and 3 have been suggested to serve redundant roles in signaling that regulates noncanonical NF-κB activation (4), TRAF2 plays a nonredundant role to that of TRAF3 in restraining BCR-induced class-switch recombination (34). To determine the role that TRAF2 plays in BCR signaling, we utilized TRAF2−/− and TRAF3−/− subclones of CH12.LX mouse BCL cells (45, 46). While the TRAF3−/− cells showed increased BCR-induced Erk activation similar to TRAF3−/− mouse primary B cells, Erk activation in the TRAF2−/− cells was unchanged (Fig. 5A). BCR-mediated signaling is enhanced despite increased Syk-TRAF2 association, indicating that TRAF2 is not able to compensate for TRAF3 in restraining BCR signaling. We thus investigated whether TRAF2 associated with Syk after BCR stimulation and whether this association was altered by TRAF3 deficiency. B cells isolated from TRAF3−/− mice have increased expression of TRAF2 (3). TRAF2 was associated with Syk and the association increased after BCR stimulation (Fig. 5B). In TRAF3−/− B cells, TRAF2-Syk association was moderately increased, consistent with its increased expression in these B cells. In the mouse BCL line CH12.LX, TRAF3 was associated with CD79B of the BCR complex even in the absence of stimulation (Fig. 5B). In the absence of TRAF3, association of Btk and Syk was modestly increased, suggesting that TRAF3 may regulate the localization of these tyrosine kinases, and TRAF2 may actually enhance this.
Figure 5.
Association of TRAF3 with CD79 B in the mouse lymphoma CH12.LX cell line.A, representative WB and graphs (mean ± SEM) of densitometry of protein lysates from TRAF3−/−, TRAF2−/−, and WT CH12.LX cells stimulated with 10 μg/ml anti-IgM Ab for the indicated times (N = 3 independent experiments). An unpaired t-test was used to determine statistical significance (∗ p < 0.05). B, CD79B was immunoprecipitated from cell lysates of CH12.LX cells with or without 5 min of 20 μg/ml anti-mouse IgM Ab stimulation. Images are representative of three independent experiments. Graphs represent densitometry of protein of interest normalized to the IP protein (mean ± SEM). An unpaired t-test was used to determine statistical significance (∗ p < 0.05).
Impact of TRAF3 upon BCR-mediated effector functions
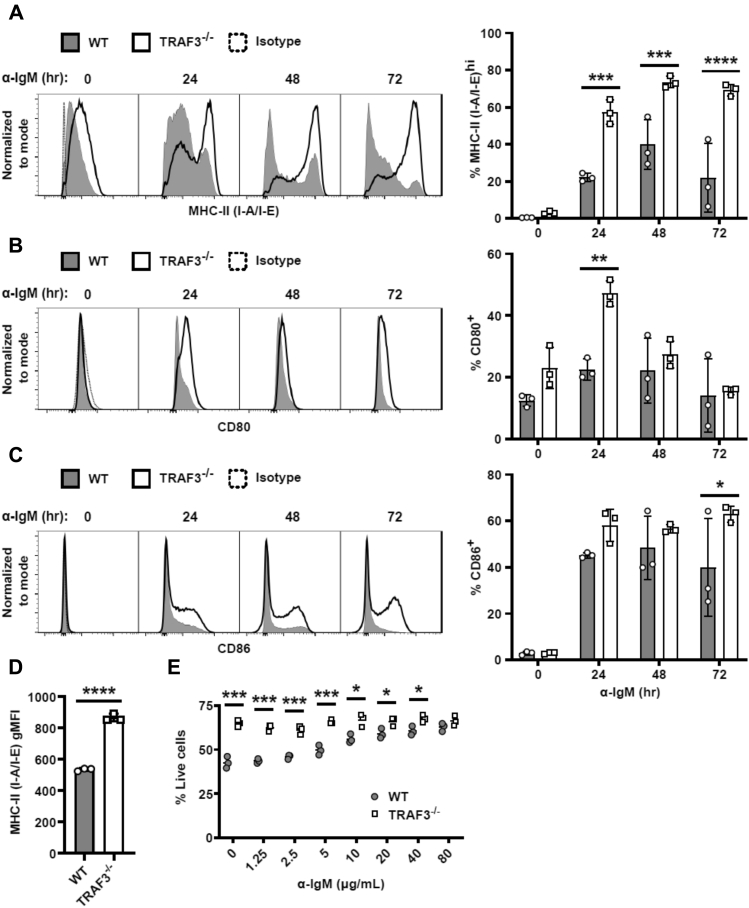
BCR stimulation induces upregulation of surface expression of CD80, CD86, and MHC-II molecules, which contribute to enhanced antigen presentation capability of B cells (47). We found that expression of these surface molecules was increased in BCR-stimulated TRAF3−/− B cells compared with WT controls (Fig. 6, A–C). Additionally, TRAF3−/− B cells had increased surface expression of MHC-II prior to any stimulation (Fig. 6D). These data are consistent with a role for TRAF3 as a regulator of BCR signaling pathways that control important BCR effector functions.
Figure 6.
BCR-induced activation marker expression and survival in TRAF3−/−mouse splenic B cells. Mouse splenic B cells were stimulated with 5 μg/ml anti-mouse IgM Ab for the indicated times. (A–C, Left) Representative histograms of B cell expression of the indicated surface receptors, measured by flow cytometry. (A–C, Right), graphs of % positive (mean ± SEM) of B cells (N = 3 mice). D, graph of gMFI (mean ± SEM) of MHC-II (I-A/I-E) expression of unstimulated mouse splenic B cells (N = 3 mice). E, mouse splenic B cells were treated with indicated doses of anti-IgM Ab for 24 h (N = 3 mice). A two-way ANOVA followed up with an unpaired t-test with Sidak correction for multiple comparisons (A–C, E) or an unpaired t-test (D) was used to determine statistical significance (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001, ∗∗∗∗ p < 0.0001).
Depending on the signal strength, BCR stimulation can increase B-cell survival or lead to activation-induced cell death (13, 48). TRAF3−/− B cells have greatly increased homeostatic survival (3), but their response to BCR-mediated signals that regulate survival was unknown. WT B cells displayed increased viability when treated with increasing amounts of an agonistic anti-IgM Ab stimulus, (Fig. 6E). In contrast, TRAF3−/− B cells showed their typical increased basal survival, but this did not change after BCR stimulation, showing that TRAF3 deficiency renders B cells independent of BCR-mediated survival signals.
Impact of TRAF3 status upon sensitivity of normal and malignant B cells to inhibitors of BCR signaling
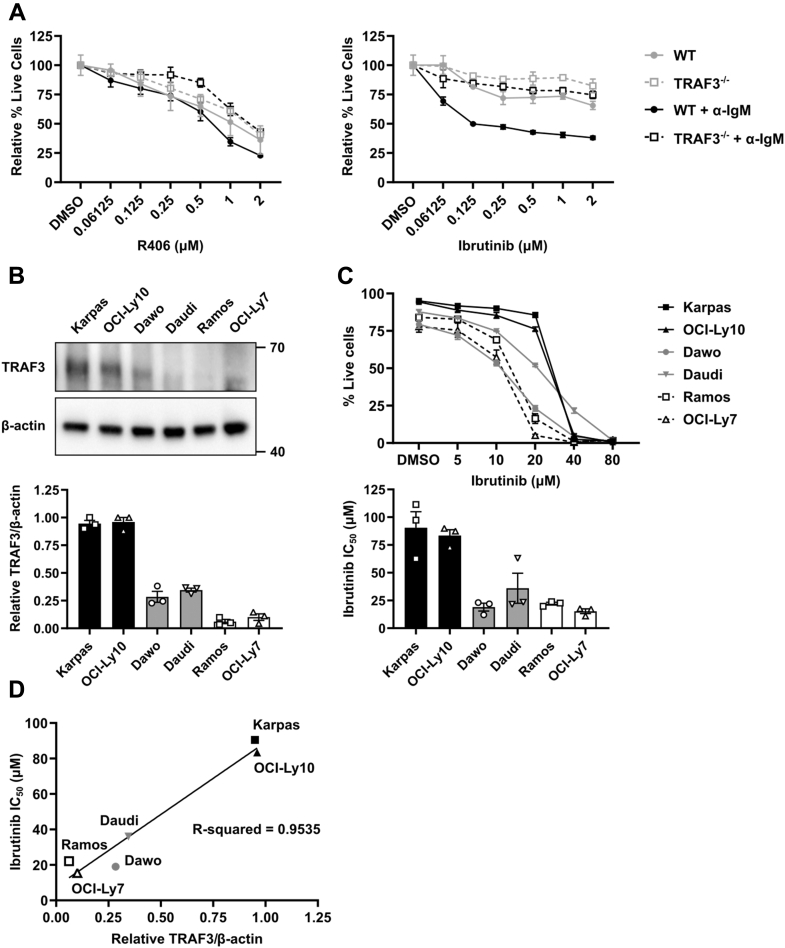
Even in the absence of antigen, tonic signaling from the BCR is required for mature B-cell survival (49, 50). Several types of human B-cell malignancies display enhanced survival resulting from tonic or antigen-induced BCR signaling (17, 18). As TRAF3 deficiency (both genetic and posttranslational) is regularly seen in these malignancies (6, 11), we examined how inhibition of BCR-induced kinase activation impacted survival of primary and malignant B cells. The Btk inhibitor ibrutinib is FDA approved to treat several types of B-cell malignancies (19, 20, 21). Primary WT mouse B cells showed decreased viability when stimulated through the BCR in the presence of ibrutinib, but TRAF3−/− primary B cells were ibrutinib-resistant (Fig. 7A). However, both WT and TRAF3−/− primary B cells displayed similar sensitivity to R406-(Syk-inhibitor)-induced cell death. This suggests that although both Btk activation and Syk activation are enhanced by B-cell TRAF3 deficiency, Btk activation plays a larger role in BCR-induced cell survival. It may also be the case that R406 has greater nonspecific effects than ibrutinib.
Figure 7.
Impact of TRAF3 expression on survival of mouse splenic B cells and human B-cell lymphoma cell lines following BCR pathway inhibition.A, mouse splenic B cells were treated with indicated doses of R406 (left) or ibrutinib (right) with (black) or without (gray) 5 μg/ml anti-IgM Ab for 24 h. Cell viability was determined by exclusion of live/dead stain by flow cytometry. The percent viability was calculated as the mean viability of inhibitor-treated samples normalized to DMSO vehicle-treated sample for each genotype. Graphs indicate mean values ± SEM of percent viable cells (N = 3 mice). A two-way ANOVA followed up with unpaired t-tests with Holm–Sidak correction for multiple comparisons was used to evaluate differences for statistical significance (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001). B, TRAF3 expression in the indicated human lymphoma cell lines was measured by Western blot (N = 3 independent experiments). Graphs represent the mean normalized TRAF3 expression ± SEM. C, the indicated human B lymphoma cell lines were treated with indicated doses of ibrutinib for 48 h. Cell viability was determined by exclusion of live/dead stain by flow cytometry. Line graph indicates mean values ± SEM of percent viable cells. The half maximal inhibitory concentration (IC50) was calculated using Graphpad Prism (N = 3 independent experiments). D, line graph of mean relative TRAF3 protein expression versus IC50. Trendline and R-squared value was calculated with Graphpad Prism.
Interestingly, BCL-derived cell lines showed an inverse relationship between TRAF3 expression and susceptibility to BCR pathway inhibitors, with cell lines expressing low levels of TRAF3 protein more susceptible to death induced by lower concentrations of ibrutinib (Fig. 7, B–D). This may be an example of so-called “oncogene addiction” (51, 52). We previously saw this phenomenon with inhibitors of several other survival pathways that are constitutively dysregulated in TRAF3−/− B cells (53). TRAF3 thus modulated survival of BCR-stimulated survival pathways, as well as homeostatic survival pathways constitutively upregulated in TRAF3−/− B cells.
Discussion
Here, we show that TRAF3 is a newly appreciated inhibitor of BCR signaling. TRAF3 inhibited proximal BCR signaling, detectable at the level of Syk and Btk kinase activation, subsequently resulting in increased activation of PLCγ2, Erk, and p38 MAPK. The increased BCR signaling observed in TRAF3−/− B cells resulted in increased downstream BCR effector functions. Interestingly, B-cell TRAF3 thus plays an opposite role in BCR signaling to the role it plays in T cells, where TCR-associated TRAF3 is required for full TCR-mediated activation and T-cell effector functions (35, 36). These differential roles of antigen receptor-associated TRAF3 in B and T cells underline the dependence of TRAF3 functions upon cellular context and distinct protein interactions.
Prior to stimulation, TRAF3−/− B cells already showed increased activation of Erk and p38 MAPK, suggestive of increased tonic BCR signaling. The increased Erk activation observed may be due in part to an increase in the proportion of MZ B cells in TRAF3−/− mice, as MZ B cells are reported to display increased tonic Erk activation (54). However, TRAF3−/− FO B cells also showed increased basal Syk and Erk activation, indicating that this feature of TRAF3-deficient B cells is not confined to the MZ subset (Fig. S3C). In the BCL-derived cell line CH12.LX, TRAF3 associated constitutively with the BCR complex, consistent with constitutive Src kinase activation and tonic signaling in these cells (55). There was also association of TRAF3 with Syk and Btk prior to BCR stimulation. TRAF3 may be playing a role in tonic as well as stimulation-induced BCR signaling.
TRAF3 regulated BCR signaling in both MZ and FO B cell subsets but to a greater extent in FO B cells. This shows that the increased signaling seen in the bulk TRAF3−/− B cell population isn’t due to an increased proportion of MZ B cells, which are reported to show increased activation after BCR stimulation (3, 38, 39). These results are an interesting complement to TRAF3 inhibition of TLR signaling. TRAF3 inhibits TLR signaling in both CD1dhi (MZ) and CD1dlo (non-MZ) B-cell populations (28). In the context of both TLR and BCR signaling, loss of TRAF3 made FO B cells function in a more MZ B cell-like manner (56, 57). TRAF3 mRNA expression increases over the course of B-cell maturation but is the highest in FO B cells (58).
Following BCR stimulation, both TRAF3 and TRAF2 associate with Syk in both mouse splenic B cells and the human BCL cell line BJAB. TRAF3 associates with Syk after TLR stimulation in macrophages (32), and we show here that this is also the case in B cells. Both TRAF2 and TRAF3 associate with Syk in chronic lymphocytic leukemia (CLL) and Raji BCL-derived B cells after BAFF stimulation (33). Exogenous overexpression analysis of the protein domains required for the interaction showed that the interdomain B of Syk and the coiled-coil domain of TRAF3 were necessary for the interaction (32). Both human and mouse Syk proteins have putative TRAF2/3 sites, although they are located in the N-terminal SH2 domains (44). There may be a cryptic TRAF2/3-binding site in Syk, or the interaction may be mediated by another protein that has yet to be identified. TRAF2 plays a similar role to TRAF3 in restraining BCR-induced Ig class-switch recombination (34). Although TRAF6 also associates with Syk in macrophages after TLR4 stimulation, we were unable to detect association of TRAF6 with Syk after BCR stimulation (A. Whillock and G. Bishop, Unpublished results).
To our knowledge, the present findings are the first to show that Btk and TRAF3 can associate in B cells. Btk has two putative TRAF2/3-binding sites; one is within the pleckstrin homology (PH) domain and the second in the TEC homology domain (44). It is unknown if the interaction between TRAF3 and Btk is direct or is mediated through another protein and which, if any, of these sites is required for the association. In the absence of TRAF3, we observed increased association of Btk and Syk. The activity of Syk is tightly regulated by the phosphatase SHP-1 (59, 60) and the ubiquitin ligases CBL-B and c-CBL (61, 62, 63). We did not detect decreased association of negative regulators such as SHP-1, CBL-B, and c-CBL with Syk in the absence of TRAF3, nor did we detect association of TRAF3 with any of these negative regulators (A. Whillock and G. Bishop, Unpublished results). We also did not detect a difference in Syk or CD79B ubiquitination in the absence of TRAF3. It thus appears more likely that TRAF3 restrains recruitment of positive regulators to the BCR complex, rather than mediating the recruitment of negative regulators to inhibit BCR signaling.
BCR-induced expression of the costimulatory molecules CD80, CD86, and MHC-II was increased in TRAF3−/− B cells. Both CD80 and CD86 are transcriptional targets of NF-κB (64, 65). We did not detect differences in canonical NF-κB activation after BCR stimulation in TRAF3−/− B cells, but the constitutive noncanonical NF-κB activation seen in TRAF3−/− B cells (3, 4) may contribute to this phenotypic feature. Depletion of RelB in dendritic cells results in decreased CD80 and CD86 expression. Basal expression of MHC-II was also increased in TRAF3−/− B cells, possibly due to the increased nuclear CREB in TRAF3−/− B cells (66), which also promotes transcription of MHC-II genes (67, 68).
TRAF3−/− B cells have remarkably increased basal survival compared with WT B cells (3, 4). WT B cells received a survival advantage with increasing BCR signal strength, but enhanced survival of TRAF3−/− B cells was independent of BCR-mediated stimulation (Fig. 5). As discussed above, both NF-κB2 and CREB-mediated survival signals contribute to the enhanced basal viability of TRAF3-deficient B cells.
Results presented here demonstrate that expression of TRAF3 can impact susceptibility to cell death induced by Syk and Btk inhibitors. We previously found that TRAF3−/− primary B cells display enhanced expression of the prosurvival proteins Pim2 and c-myc and are resistant to cell death induced by drug inhibitors of these proteins. However, in contrast, malignant B cells deficient in TRAF3 display enhanced sensitivity to killing by Pim 2 or c-myc inhibition (53), consistent with oncogene addiction (51). This contrast between resistance of TRAF3-deficient primary cells and enhanced sensitivity of TRAF3-deficient malignant B cells was also seen in the present study for inhibitors of the kinases Syk and Btk, whose activation by BCR signals is elevated in the absence of TRAF3. Thus, defining TRAF3-regulated pathways contributing to survival and activation of B cells is valuable in understanding both normal B cell biology and informing choice of promising pathway inhibitors for B cell malignancies, as TRAF3 deficiency is seen in a significant proportion of these tumors.
Experimental procedures
Mice
The Traf3flox/flox Cd19-Cre (B-Traf3−/−) mouse strain, bred extensively to C57BL/6 mice, was described previously (3). Mice of 2–6 months of age and similar numbers of males and females were used for all experiments. Sex and age-matched Traf3flox/flox mice were used as controls unless otherwise indicated. All mice were housed in specific pathogen-free conditions and in accordance with National Institute of Health guidelines under an animal protocol approved by the Animal Care and Use Committees at the University of Iowa and the Iowa City Veteran’s Affairs Medical Center.
Mouse primary B-cell isolation and cell culture
Splenic B cells were purified by negative selection using a mouse B-cell isolation kit according to manufacturer’s protocols (STEMCELL Technologies, Vancouver, Canada). Isolated B cells were maintained in RPMI 1640 medium (Life Technologies, Carlsbad, CA) containing 10 μM 2-β-mercaptoethanol (Sigma Aldrich, St Louis, MO), 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA, USA), 2 mM L-glutamine (Life Technologies), and 100 U/ml penicillin-streptomycin antibiotics (Life Technologies), referred to as B-cell medium-10 (BCM10).
Cell lines
Cell lines used in the experiments reported here included: BJAB (69), Karpas 422 (70), OCI-Ly10 (71), Daudi (72), Dawo (73), Ramos (74), and OCI-Ly7 (71), and CH12.LX (29). TRAF2−/− and TRAF3−/− CH12.LX cell lines were described previously (45, 46). All cell lines were maintained in BCM10.
Antibodies and reagents
Antibodies (Abs) used in immunoblotting were purchased from Cell Signaling Technologies (Danvers, MA). They are specific for: p-CD79 A (Y182), p-Syk (Y352), p-Btk (Y223), p-Erk (T202/Y204), p-Akt (S473), p-p38 MAPK (T180/Y182), p-IκBα (S32), p-PLCγ2 (Y1217), Syk, Btk, Erk, p38 MAPK, IκBα, PLCγ2, K48 polyubiquitin, MyD88, and Akt. Abs against CD79A and CD79B were purchased from R&D systems (Minneapolis, MN). The Abs recognizing mouse TRAF3 were purchased from Santa Cruz Biotechnology (H-122; Dallas, TX) or Cell Signaling Technologies. The Ab recognizing human TRAF3 was purchased from Proteintech (Rosemont, IL). The anti-TRAF2 and anti-TRAF6 Abs were purchased from MBL International (Woburn, MA). The anti-β-actin Ab and Protein A-HRP conjugate were purchased from Thermo Fisher (Waltham, MA). HRP-conjugated goat-anti-mouse IgG and goat-anti-rabbit Ig were from Jackson ImmunoResearch Laboratories (West Grove, PA), and HRP-conjugated goat anti-sheep Abs were from R&D systems. Flow cytometry Abs used included those specific for: B220-APC (eBioscience; San Diego, CA), CD21/35-PE-Cy7 (Invitrogen), CD23-PE (Invitrogen), p-Erk T202/Y204-A488 (Invitrogen), p-Syk Y352-A488 (Invitrogen), MHC-II (I-A/I-E)-PE-Cy5 (eBioscience), CD80-FITC (Biolegend; San Diego, CA), and CD86-PE (BD Biosciences; San Jose, CA). The pharmacologic inhibitors ibrutinib and R406 were purchased from Selleckchem (Houston, TX). LPS from E. coli O111:B4 was purchased from Sigma Aldrich (St Louis, MO).
Western blotting
Protein lysates were resolved by SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes for immunoblotting. Densitometry of the band of interest was quantified with Multigauge (FujiFilm, Minato City, Tokyo, Japan) or ImageJ software (75). The intensity of the band was normalized across the blot and then to the intensity of the control band (total protein of interest or β-actin).
BCR stimulation
Goat anti-mouse IgM F(ab’)2 (Jackson ImmunoResearch Laboratories) used for BCR stimulation in Figure 1 had endotoxin removed using EndoToxin Eraser (Thermo Fisher) until the endotoxin levels were <0.5 U (measured with Endotoxin chromo, Thermo Fisher). 1 x107 B cells per mL were rested for 1 h at 37 °C prior to stimulation. After stimulation, the cells were then rested on ice for 2 min before pelleting. For Western blotting, cell lysates were prepared with 2X SDS-PAGE loading buffer (1% SDS, 2% β-mercaptoethanol, 62.5 mM Tris, pH 6.8). Lysates were heated for 10 min at 95 °C prior to gel electrophoresis.
Immunoprecipitation
After stimulation with 20 μg/ml of anti-IgM stimulatory antibody, 20–30 x 106 cells/sample were lysed in IP Lite lysis buffer [0.5% Triton X, 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 100 mM MgCl2, 100 mM CaCl2, EDTA-free protease inhibitors (Roche), DNAse I (5 mg/ml; Roche)]. The buffer was supplemented with 20 mM N-ethylmaleimide (Sigma) for ubiquitin analysis. In total, 5% of the lysate was used for input. IP primary abs were first preincubated with protein G Dynabeads (Life Technologies) for 1 h at 25 °C, washed, and then incubated with the lysate for 2 h at 4 °C with agitation. The immune complex was isolated with a magnet, washed, resuspended in SDS-PAGE loading buffer, and then heated to 95 °C for 10 min prior to WB analysis. For densitometry quantification, the intensity of the band of interest was normalized to the intensity of the IP protein band.
Flow cytometry
For intracellular staining, mouse splenocytes were resuspended at 1 x107 cells/ml in BCM10 in wells of a 96-well plate and rested for 30 min at 37 °C. Then, the splenocytes were stimulated with 10 μg/ml stimulatory antibody for the indicated times and rested on ice for 2 min. After pelleting, the cells were fixed with Lyse/fix buffer (BD Bioscience) for 15 min at 37 °C. Cells were permeabilized with Perm III buffer (BD Bioscience) for 30 min on ice. Cells were washed twice with FACS buffer prior to incubation with anti-CD16/CD32 Fc receptor blocking Ab (BD Bioscience) and rat serum for 15 min on ice. Phospho-specific antibodies were added to the cells and incubated for 30 min on ice. After washing, the cells were analyzed with an Accuri C6 Flow Cytometer or LSR II flow cytometer (BD Bioscience). Results were analyzed with FlowJo software (TreeStar). The gating strategy for MZ and FO B cells is shown in Figure S4A.
Cell viability
For viability assays, cells were plated at 1 x 106 cells/ml for primary B cells and 1 x 105 cells/ml for cell lines and then treated with the indicated inhibitor. After 24 h (primary B cells) or 48 h (cell lines), the cells were collected, stained with Fixable Far Red Dead Cell stain (Thermo), and then fixed with fixation buffer (Biolegend) prior to analysis by flow cytometry.
Calcium signaling
B cells were stained in a buffer containing 145 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, 2 mM glutamine, and 2% fetal bovine serum (48). Cells were stained with Calcium-Green-1 (Thermo) and Fura Red (Thermo) for 10 min at 37 °C, washed, and then stored on ice until analysis. Prior to analysis, the cells were warmed to 37 °C for 5 min. A baseline measurement was collected for 2 min prior to stimulation with 5 μg/ml anti-IgM Ab. In total, 1 μM ionomycin was used as a positive control for dye loading.
Statistical analysis
In most experiments, statistical significance was first evaluated with a two-way ANOVA (NS = not significant, # p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001). If significant, a multiple unpaired two-tailed Student’s t-test with the Holm–Sidak correction was used to evaluate statistical significance for each timepoint or concentration (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001, ∗∗∗∗ p < 0.0001, NS = not significant). Other statistical details are listed in the caption of each figure. Statistical analysis and graphs were prepared using GraphPad Prism (GraphPad Software, San Diego, CA).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare no competing financial or nonfinancial interests.
Acknowledgments
The authors acknowledge the services of the Flow Cytometry Core, which receives support from NIH P30 award CA086862.
Author contributions
A. L. W. designed and performed the experiments, analyzed the data, and wrote the article. T. K. Y. designed the experiments, performed the experiments, and analyzed the data. G. A. B. contributed to the conception and design of the experiments, interpreted the data, and edited the article.
Funding and additional information
This work was supported by the Holden Comprehensive Cancer Center at The University of Iowa and its National Cancer Institute Award P30CA086862, NIH P50 grant CA97274, NIH R21 grant AG065532, VA Merit Review I01 BX001702, and a Senior Research Career Scientist award from the Department of Veterans Affairs to G. A. B. A. L. W. received support from T32 awards GM007337, AI007485, and AI007533 and Department of Defense Congressionally Directed Medical Research Program Horizon Award W81XWH-19-1-0442. T. K. Y. was supported by NIH T32 AI07485. This material is based upon work supported in part by facilities and equipment provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
Edited by Peter Cresswell
Supporting information
References
- 1.Bishop G.A., Stunz L.L., Hostager B.S. TRAF3 as a Multifaceted regulator of B lymphocyte survival and activation. Front Immunol. 2018;9:2161. doi: 10.3389/fimmu.2018.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y., Cheng G., Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 3.Xie P., Stunz L.L., Larison K.D., Yang B., Bishop G.A. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardam S., Sierro F., Basten A., Mackay F., Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Moore C.R., Liu Y., Shao C., Covey L.R., Morse H.C., 3rd, Xie P. Specific deletion of TRAF3 in B lymphocytes leads to B-lymphoma development in mice. Leukemia. 2012;26:1122–1127. doi: 10.1038/leu.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu S., Jin J., Gokhale S., Lu A.M., Shan H., Feng J., Xie P. Genetic alterations of TRAF proteins in human cancers. Front Immunol. 2018;9:2111. doi: 10.3389/fimmu.2018.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klintman J., Appleby N., Stamatopoulos B., Ridout K., Eyre T.A., Robbe P., Lopez Pascua L., Knight S.J., Dreau H.M., Cabes M., Popitsch N., Ehinger M., Martin-Subero I., Campo E., Mansson R. Genomic and transcriptomic correlates of Richter's transformation in chronic lymphocytic leukemia. Blood. 2020 doi: 10.1182/blood.2020005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushell K.R., Kim Y., Chan F.C., Ben-Neriah S., Jenks A., Alcaide M., Fornika D., Grande B.M., Arthur S., Gascoyne R.D., Steidl C., Morin R.D. Genetic inactivation of TRAF3 in canine and human B-cell lymphoma. Blood. 2015;125:999–1005. doi: 10.1182/blood-2014-10-602714. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B., Calado D.P., Wang Z., Frohler S., Kochert K., Qian Y., Koralov S.B., Schmidt-Supprian M., Sasaki Y., Unitt C., Rodig S., Chen W., Dalla-Favera R., Alt F.W., Pasqualucci L. An oncogenic role for alternative NF-kappaB signaling in DLBCL revealed upon deregulated BCL6 expression. Cell Rep. 2015;11:715–726. doi: 10.1016/j.celrep.2015.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.San Miguel J.F. Introduction to a series of reviews on multiple myeloma. Blood. 2015;125:3039–3040. doi: 10.1182/blood-2015-01-613596. [DOI] [PubMed] [Google Scholar]
- 11.Bangalore-Prakash P., Stunz L.L., Mambetsariev N., Whillock A.L., Hostager B.S., Bishop G.A. The oncogenic membrane protein LMP1 sequesters TRAF3 in B-cell lymphoma cells to produce functional TRAF3 deficiency. Blood Adv. 2017;1:2712–2723. doi: 10.1182/bloodadvances.2017009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurosaki T., Shinohara H., Baba Y. B cell signaling and fate decision. Annu. Rev. Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 13.Kurosaki T. Regulation of BCR signaling. Mol. Immunol. 2011;48:1287–1291. doi: 10.1016/j.molimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mocsai A., Ruland J., Tybulewicz V.L. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger J.A., Wiestner A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer. 2018;18:148–167. doi: 10.1038/nrc.2017.121. [DOI] [PubMed] [Google Scholar]
- 16.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 17.Davis R.E., Ngo V.N., Lenz G., Tolar P., Young R.M., Romesser P.B., Kohlhammer H., Lamy L., Zhao H., Yang Y., Xu W., Shaffer A.L., Wright G., Xiao W., Powell J. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunleavy K., Erdmann T., Lenz G. Targeting the B-cell receptor pathway in diffuse large B-cell lymphoma. Cancer Treat Rev. 2018;65:41–46. doi: 10.1016/j.ctrv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 19.de Claro R.A., McGinn K.M., Verdun N., Lee S.L., Chiu H.J., Saber H., Brower M.E., Chang C.J., Pfuma E., Habtemariam B., Bullock J., Wang Y., Nie L., Chen X.H., Lu D.R. FDA approval: Ibrutinib for Patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin. Cancer Res. 2015;21:3586–3590. doi: 10.1158/1078-0432.CCR-14-2225. [DOI] [PubMed] [Google Scholar]
- 20.Denlinger N.M., Epperla N., William B.M. Management of relapsed/refractory marginal zone lymphoma: Focus on ibrutinib. Cancer Manag. Res. 2018;10:615–624. doi: 10.2147/CMAR.S133291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papanota A.M., Ntanasis-Stathopoulos I., Kastritis E., Dimopoulos M.A., Gavriatopoulou M. Evaluating ibrutinib in the treatment of symptomatic Waldenstrom's macroglobulinemia. J. Blood Med. 2019;10:291–300. doi: 10.2147/JBM.S183997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou K., Yu Z., Jia Y., Fang H., Shao S., Huang L., Feng Y. Efficacy and safety of ibrutinib in diffuse large B-cell lymphoma: A single-arm meta-analysis. Crit. Rev. Oncol. Hematol. 2020;152:103010. doi: 10.1016/j.critrevonc.2020.103010. [DOI] [PubMed] [Google Scholar]
- 23.George B., Chowdhury S.M., Hart A., Sircar A., Singh S.K., Nath U.K., Mamgain M., Singhal N.K., Sehgal L., Jain N. Ibrutinib resistance mechanisms and treatment Strategies for B-cell lymphomas. Cancers (Basel) 2020;12:1328. doi: 10.3390/cancers12051328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ondrisova L., Mraz M. Genetic and non-genetic mechanisms of resistance to BCR signaling inhibitors in B cell malignancies. Front Oncol. 2020;10:591577. doi: 10.3389/fonc.2020.591577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hostager B.S., Bishop G.A. Cutting edge: Contrasting roles of TNF receptor-associated factor 2 (TRAF2) and TRAF3 in CD40-activated B lymphocyte differentiation. J. Immunol. 1999;162:6307–6311. [PubMed] [Google Scholar]
- 26.Hostager B.S., Catlett I.M., Bishop G.A. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- 27.Bishop G.A., Hostager B.S. Signaling by CD40 and its mimics in B cell activation. Immunol. Res. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- 28.Xie P., Poovassery J., Stunz L.L., Smith S.M., Schultz M.L., Carlin L.E., Bishop G.A. Enhanced Toll-like receptor (TLR) responses of TNFR-associated factor 3 (TRAF3)-deficient B lymphocytes. J. Leukoc. Biol. 2011;90:1149–1157. doi: 10.1189/jlb.0111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishop G.A., Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc. Natl. Acad. Sci. U. S. A. 1986;83:7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haxhinasto S.A., Bishop G.A. Synergistic B cell activation by CD40 and the B cell antigen receptor: role of B lymphocyte antigen receptor-mediated kinase activation and tumor necrosis factor receptor-associated factor regulation. J. Biol. Chem. 2004;279:2575–2582. doi: 10.1074/jbc.M310628200. [DOI] [PubMed] [Google Scholar]
- 31.Haxhinasto S.A., Hostager B.S., Bishop G.A. Cutting edge: Molecular mechanisms of synergy between CD40 and the B cell antigen receptor: Role for TNF receptor-associated factor 2 in receptor interaction. J. Immunol. 2002;169:1145–1149. doi: 10.4049/jimmunol.169.3.1145. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y.C., Huang D.Y., Chu C.L., Lin Y.L., Lin W.W. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci. Signal. 2013;6:ra71. doi: 10.1126/scisignal.2003973. [DOI] [PubMed] [Google Scholar]
- 33.Paiva C., Rowland T.A., Sreekantham B., Godbersen C., Best S.R., Kaur P., Loriaux M.M., Spurgeon S.E.F., Danilova O.V., Danilov A.V. SYK inhibition thwarts the BAFF - B-cell receptor crosstalk and thereby antagonizes Mcl-1 in chronic lymphocytic leukemia. Haematologica. 2017;102:1890–1900. doi: 10.3324/haematol.2017.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z., Krinsky A., Woolaver R.A., Wang X., Chen S.M.Y., Popolizio V., Xie P., Wang J.H. TRAF3 Acts as a Checkpoint of B cell receptor signaling to control antibody class switch recombination and Anergy. J. Immunol. 2020;205:830–841. doi: 10.4049/jimmunol.2000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie P., Kraus Z.J., Stunz L.L., Liu Y., Bishop G.A. TNF receptor-associated factor 3 is required for T cell-mediated immunity and TCR/CD28 signaling. J. Immunol. 2011;186:143–155. doi: 10.4049/jimmunol.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis A.M., Wallace E.C., Hostager B.S., Yi Z., Houtman J.C.D., Bishop G.A. TRAF3 enhances TCR signaling by regulating the inhibitors Csk and PTPN22. Sci. Rep. 2017;7:2081. doi: 10.1038/s41598-017-02280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arkee T., Bishop G.A. TRAF family molecules in T cells: Multiple receptors and functions. J. Leukoc. Biol. 2020;107:907–915. doi: 10.1002/JLB.2MR1119-397R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver A.M., Martin F., Gartland G.L., Carter R.H., Kearney J.F. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 39.Oliver A.M., Martin F., Kearney J.F. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 40.Honigberg L.A., Smith A.M., Sirisawad M., Verner E., Loury D., Chang B., Li S., Pan Z., Thamm D.H., Miller R.A., Buggy J.J. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braselmann S., Taylor V., Zhao H., Wang S., Sylvain C., Baluom M., Qu K., Herlaar E., Lau A., Young C., Wong B.R., Lovell S., Sun T., Park G., Argade A. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 42.Suljagic M., Longo P.G., Bennardo S., Perlas E., Leone G., Laurenti L., Efremov D.G. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eμ- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116:4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 43.Jiang A., Craxton A., Kurosaki T., Clark E.A. Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J. Exp. Med. 1998;188:1297–1306. doi: 10.1084/jem.188.7.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar M., Gouw M., Michael S., Samano-Sanchez H., Pancsa R., Glavina J., Diakogianni A., Valverde J.A., Bukirova D., Calyseva J., Palopoli N., Davey N.E., Chemes L.B., Gibson T.J. ELM-the eukaryotic linear motif resource in 2020. Nucleic Acids Res. 2020;48:D296–D306. doi: 10.1093/nar/gkz1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hostager B.S., Haxhinasto S.A., Rowland S.L., Bishop G.A. Tumor necrosis factor receptor-associated factor 2 (TRAF2)-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J. Biol. Chem. 2003;278:45382–45390. doi: 10.1074/jbc.M306708200. [DOI] [PubMed] [Google Scholar]
- 46.Xie P., Hostager B.S., Bishop G.A. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 2004;199:661–671. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X., Jensen P.E. The role of B lymphocytes as antigen-presenting cells. Arch. Immunol. Ther. Exp. (Warsz) 2008;56:77–83. doi: 10.1007/s00005-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 48.Berry C.T., Liu X., Myles A., Nandi S., Chen Y.H., Hershberg U., Brodsky I.E., Cancro M.P., Lengner C.J., May M.J., Freedman B.D. BCR-induced Ca(2+) signals Dynamically Tune survival, Metabolic Reprogramming, and proliferation of Naive B cells. Cell Rep. 2020;31:107474. doi: 10.1016/j.celrep.2020.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam K.P., Kühn R., Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 50.Kraus M., Alimzhanov M.B., Rajewsky N., Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein I.B., Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. discussion: 3080. [DOI] [PubMed] [Google Scholar]
- 52.Pagliarini R., Shao W., Sellers W.R. Oncogene addiction: Pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015;16:280–296. doi: 10.15252/embr.201439949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whillock A.L., Mambetsariev N., Lin W.W., Stunz L.L., Bishop G.A. TRAF3 regulates the oncogenic proteins Pim2 and c-Myc to restrain survival in normal and malignant B cells. Sci. Rep. 2019;9:12884. doi: 10.1038/s41598-019-49390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampel F., Ehrenberg S., Hojer C., Draeseke A., Marschall-Schroter G., Kuhn R., Mack B., Gires O., Vahl C.J., Schmidt-Supprian M., Strobl L.J., Zimber-Strobl U. CD19-independent instruction of murine marginal zone B-cell development by constitutive Notch2 signaling. Blood. 2011;118:6321–6331. doi: 10.1182/blood-2010-12-325944. [DOI] [PubMed] [Google Scholar]
- 55.Ke J., Chelvarajan R.L., Sindhava V., Robertson D.A., Lekakis L., Jennings C.D., Bondada S. Anomalous constitutive Src kinase activity promotes B lymphoma survival and growth. Mol. Cancer. 2009;8:132. doi: 10.1186/1476-4598-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cerutti A., Cols M., Puga I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin F., Kearney J.F. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 58.Project I.G. ImmGen at 15. Nat. Immunol. 2020;21:700–703. doi: 10.1038/s41590-020-0687-4. [DOI] [PubMed] [Google Scholar]
- 59.Cyster J.G., Goodnow C.C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 60.Pao L.I., Lam K.P., Henderson J.M., Kutok J.L., Alimzhanov M., Nitschke L., Thomas M.L., Neel B.G., Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Sohn H.W., Gu H., Pierce S.K. Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J. Exp. Med. 2003;197:1511–1524. doi: 10.1084/jem.20021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitaura Y., Jang I.K., Wang Y., Han Y.C., Inazu T., Cadera E.J., Schlissel M., Hardy R.R., Gu H. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26:567–578. doi: 10.1016/j.immuni.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katkere B., Rosa S., Drake J.R. The Syk-binding ubiquitin ligase c-Cbl mediates signaling-dependent B cell receptor ubiquitination and B cell receptor-mediated antigen processing and presentation. J. Biol. Chem. 2012;287:16636–16644. doi: 10.1074/jbc.M112.357640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.George A.A., Sharma M., Singh B.N., Sahoo N.C., Rao K.V. Transcription regulation from a TATA and INR-less promoter: Spatial segregation of promoter function. EMBO J. 2006;25:811–821. doi: 10.1038/sj.emboj.7600966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Liu Z., Jiang S., Cortesini R., Lederman S., Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J. Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- 66.Mambetsariev N., Lin W.W., Stunz L.L., Hanson B.M., Hildebrand J.M., Bishop G.A. Nuclear TRAF3 is a negative regulator of CREB in B cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1032–1037. doi: 10.1073/pnas.1514586113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreno C.S., Beresford G.W., Louis-Plence P., Morris A.C., Boss J.M. CREB regulates MHC class II expression in a CIITA-dependent manner. Immunity. 1999;10:143–151. doi: 10.1016/s1074-7613(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 68.Choi N.M., Majumder P., Boss J.M. Regulation of major histocompatibility complex class II genes. Curr. Opin. Immunol. 2011;23:81–87. doi: 10.1016/j.coi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975;22:276–284. [PubMed] [Google Scholar]
- 70.Dyer M.J., Fischer P., Nacheva E., Labastide W., Karpas A. A new human B-cell non-Hodgkin's lymphoma cell line (Karpas 422) exhibiting both t (14;18) and t(4;11) chromosomal translocations. Blood. 1990;75:709–714. [PubMed] [Google Scholar]
- 71.Tweeddale M.E., Lim B., Jamal N., Robinson J., Zalcberg J., Lockwood G., Minden M.D., Messner H.A. The presence of clonogenic cells in high-grade malignant lymphoma: A prognostic factor. Blood. 1987;69:1307–1314. [PubMed] [Google Scholar]
- 72.Klein E., Klein G., Nadkarni J.S., Nadkarni J.J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968;28:1300–1310. [PubMed] [Google Scholar]
- 73.Tompkins V.S., Han S.-S., Olivier A., Syrbu S., Bair T., Button A., Jacobus L., Wang Z., Lifton S., Raychaudhuri P., Morse H.C., III, Weiner G., Link B., Smith B.J., Janz S. Identification of Candidate B-lymphoma genes by Cross-Species gene expression Profiling. Plos One. 2013;8:e76889. doi: 10.1371/journal.pone.0076889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein G., Lindahl T., Jondal M., Leibold W., Menezes J., Nilsson K., Sundstrom C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc. Natl. Acad. Sci. U. S. A. 1974;71:3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials.