Highlights
-
•
Programmed death 1 (PD-1) inhibitors have been proved to be effective in advanced esophageal cancer.
-
•
PD-1 inhibitors significantly prolonged the OS when compared with chemotherapy.
-
•
There was no significant improvement in terms of PFS and ORR for PD-1 inhibitors when compared with chemotherapy.
-
•
PD-1 inhibitors lowered grade 3 - 5 treatment-related adverse effects versus chemotherapy.
-
•
A subgroup analysis showed that esophageal squamous cell carcinoma was more effective than adenocarcinoma for PD-1 inhibitors.
Keywords: Immunotherapy, Meta-analysis, Esophageal cancer, PD-1, Chemotherapy
Abstract
Background
A novel therapy based on programmed death 1 (PD-1) inhibitors has been proved to be effective in advanced esophageal cancer. This article is a meta-analysis that aims to compare the efficacy and safety of anti-PD-1 therapy with chemotherapy in esophageal cancer.
Patients and methods
Data were collected from eligible studies searched from PubMed, Web of Science, Cochrane Library, and Embase. Pooled hazard ratio (HR) for overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) was estimated to assess the efficacy of PD-1 inhibitors versus chemotherapy. The subgroup analysis was also performed to evaluate the OS benefits. The OR for the occurrence of treatment-related adverse effects was calculated to assess the safety of anti-PD-1 therapy.
Results
A total of 4 studies were analyzed. Compared with patients with chemotherapy, patients with anti-PD-1 therapy had a significant improvement in OS (HR = 0.79, 95% CI: 0.71–0.88, and P<0.001), but no significant relationship was observed in PFS (HR = 0.96, 95% CI: 0.76–1.20, and P = 0.69) and ORR (OR = 1.92, 95% CI: 0.98–3.72, and P = 0.06). A similar result was observed in esophageal squamous cell carcinoma. The significant predictor for treatment benefit alone was histology (P = 0.009). The incidence of grade 3 - 5 treatment-related adverse effects in anti-PD-1 therapy was distinctly lower than that in chemotherapy, but there is no statistical difference in all treatment-related adverse effects.
Conclusion
Anti-PD-1 therapy significantly prolonged the OS, simultaneously lowered grade 3 - 5 treatment-related adverse effects versus chemotherapy.
1. Introduction
Esophageal cancer is one of the most common malignancies globally, ranking the 8th in morbidity and the 6th in mortality among all malignancies [1,2]. There were 572,034 cases of newly diagnosed esophageal cancer worldwide and 508,585 deaths were reported in 2018 [3]. Generally, esophageal cancer can be filed into two categories: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) [4,5]. Because the clinical symptoms of early esophageal cancer are obscure, more than half of the patients are in the advanced stage when detected [6]. For patients with unresectable or metastatic esophageal cancer, systemic chemotherapy is the first choice. The National Comprehensive Cancer Network (NCCN) guidelines recommended cisplatin (or oxaliplatin) together with fluorouracil (or capecitabine) as the first-line of chemotherapy regimen for esophageal cancer [7]. However, due to the resistance and dose-limiting toxicity of chemotherapy, there are still many patients who have not been satisfied with treatment [8,9]. As mentioned above, it is highly necessary to optimize the existing treatment measures and find novel measures to increase the survival rates.
In recent years, immunotherapy has provided new treatment options for patients with various tumors [10,11]. Programmed death-1 (PD-1), a member of the CD28 superfamily, is an essential immunosuppressive molecule [12,13]. Usually, the interaction between PD-1 and programmed death ligand 1 (PD-L1) can suppress T-cell migration, proliferation, secretion of cytotoxic mediators, and restrict cancer cell death [14]. Blocking the interaction between PD-1 and PD-L1 can restore the activity of T cells and enhance the immune response. It can also help to reduce the metastasis of tumor cells and the volume of tumor [15]. Several experiments have confirmed that the high expression of PD-1 and PD-L1 in esophageal cancer is closely related to the depth of tumor infiltration and poor prognosis [16], [17], [18]. Therefore, blocking the PD-1 pathway by PD-1 or PD-L1 inhibitors could be a practical approach for treating esophageal cancer.
The monoclonal antibodies of (PD-1) and its ligand (PD-L1) have made breakthroughs in the treatment of malignant melanoma, non-small cell lung cancer, kidney cancer, and other tumors. Initial achievements were gradually obtained by clinical trials, which focused on the mechanism and efficacy of anti-PD-1 therapy in esophageal cancer [19], [20], [21]. To date, several monoclonal antibodies that target PD-1 have already been advanced. Pembrolizumab is the first PD-1 inhibitor to enter clinical trials and is also the most widely approved [22], [23], [24]. In 2018, the FDA approved pembrolizumab to treat recurrent locally, unresectable, or metastatic gastric and esophagogastric junction adenocarcinoma. Nivolumab is another representative PD-1 monoclonal antibody. Some studies suggested that nivolumab alone was effective and safe in patients with esophageal cancer [25,26]. There are still many PD-1 inhibitors that made initial achievements in esophageal cancer, including SHR-1210, Sintilimab, etc. [27], [28], [29], [30]. Based on these study results, the anti-PD-1 therapy exerts a highly promising treatment paradigm in patients with advanced esophageal cancer. However, the adverse effects of PD-1 inhibitors cannot be ignored, which has been reported previously in several studies [18].
Meta-analysis is generally considered a powerful statistical tool to overcome the limitations of different sample sizes from individual studies to generate the best overall estimation. Thus, it is necessary to perform a meta-analysis to explore the efficacy and safety of immunotherapy for patients with esophageal cancer. This article is a meta-analysis of currently available trials that compare PD-1 inhibitor with chemotherapy, which will provide essential and useful information.
2. Material and methods
2.1. Search strategy
Comprehensive searches published in English were carried out in PubMed, Web of Science, Cochrane Library, and Embase to collect all relevant citations. The date of the latest search was Oct 1, 2020. Meeting abstracts were also searched from the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO). Keywords were used for the search: “Esophageal Neoplasms,” “Esophageal Neoplasm,” “Neoplasm, Esophageal,” “Esophagus Neoplasm,” “Esophagus Neoplasms,” “Neoplasm, Esophagus,” “Neoplasms, Esophagus,” “Neoplasms, Esophageal,” “Cancer of Esophagus,” “Cancer of the Esophagus,” “Esophagus Cancer,” “Cancer, Esophagus,” “Cancers, Esophagus,” “Esophagus Cancers,” “Esophageal Cancer,” “Cancer, Esophageal,” “Cancers, Esophageal,” “Esophageal Cancers,” “Nivolumab,” “Opdivo,” “Pembrolizumab,” “Lambrolizumab,” “Atezolizumab,” “Camrelizumab,” “SHR-1210,” “Tislelizumab,” “Toripalimab,” “JS001,” “Sintilimab,” and “PD-1.”
All searched results are evaluated according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The publication language was limited to English.
2.2. Selection criteria
Inclusion criteria are the following: randomized clinical Phase II or III trials in patients with advanced esophageal cancer; random assignment of single anti-PD-1 therapy or chemotherapy alone; studies, including one or all of the following information: objective response rate (ORR), overall survival (OS), progression-free survival (PFS), and the frequency of adverse events (AEs). Observational studies, editorials, commentaries, reviews, case reports, and duplicate publications were excluded. If datasets were duplicated or overlapped, only the most recent information was included. Two authors (Guan LL and Lu Y) independently selected included studies in the systematic review by searching the databases. The full texts of relevant articles were retrieved for eligibility.
2.3. Data extraction
Data were extracted independently by two authors (Lu Y and Wang F) from eligible studies, and all disagreements were resolved by consensus of all investigators. Study characteristics were extracted from each eligible study as follows: authors, treatment strategy, ORR, PFS, OS, duration of response, 12-month survival rate, the frequency of AEs, number of patients, age, sex, region, Eastern Cooperative Oncology Group performance status (ECOG PS), histological type, lymph node metastasis, and PD-L1 status. When we needed additional information that was not provided, we contacted the corresponding authors to request it. Two authors (Xu ML and Wang F) assessed the quality of included trials independently in accordance with the five-point Jadad scoring system [31]. Two authors (Lu Y and Guan LL) used the Cochrane Collaboration tool to evaluate the risk of bias.
2.4. Statistical analyses
We derived the HRs and 95% confidence intervals (CI) for OS and PFS from each study of advanced esophageal cancer. For ORR, the odds ratio (OR) and corresponding 95% CI were the principal summary measures. Relevant data were extracted from each study, and the pooled ORs and HRs were estimated through a meta-analysis. We performed several subgroup analyses to explore the variables on immunotherapy efficacy for esophageal cancer. Statistical heterogeneity between studies was evaluated by using Cochran's Q test and Higgins I2 statistic. The random effects model was chosen if apparent heterogeneity was present (I2 >50%). Otherwise, the choice would be the fixed effects model. All analyses were carried out by Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and Stata 12.0 (Stata Corporation). All reported P-values were two-sided, and P<0.05 was considered statistically significant. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISM) guidelines for this meta-analysis.
3. Results
3.1. Literature selection process and characteristics of the selection studies
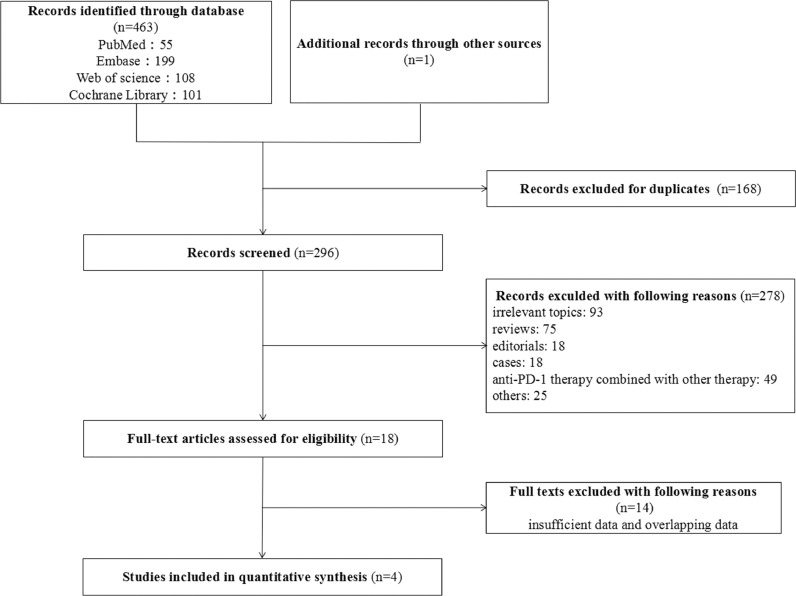
The PRISMA diagram for the study selection is summarized in Fig. 1. In total, our search strategy identified 464 potentially relevant records from databases and conferences. A total of 168 studies were excluded for the duplication, and 278 studies were not meeting the eligibility criteria in the selection. A total of 4 studies were considered eligible for the current meta-analysis. The characteristics of these 4 studies are summarized in Table 1. Four studies involving 1685 patients with advanced esophageal cancer were included in the following analysis [24,25,28,30]. All trials were Phase III randomized controlled clinical trials and compared the efficacy and safety of PD-1 inhibitor with chemotherapy.
Fig. 1.
The flowchart of the study selection process for the meta-analysis.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Reference | Clinal Trials | Phase | Line | Treatment Regimen | Patient Number | Median follow-up (month) | mOS (month) (95% CI) | mPFS (month) (95% CI) | ORR(%) (95% CI) | 12-month survival rate(%) | mDOR (months) (95% CI) | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kato et al. 2019 | ATTRACTION-03 | III | ≥2 | armA: nivolumab | 210 | 10.5 | 10.9 (9.2–13.3) | 1.7(1.5–2.7) | 19.0 (14.0–26.0) | 47.0 | 6.9 (5.4–11.1) | 4 |
| armB: paclitaxel or docetaxel | 209 | 8.0 | 8.4 (7.2–9.9) | 3.4 (3.0–4.2) | 22.0 (15.0–29.0) | 34.0 | 3.9 (2.8–4.2) | |||||
| Kojima et al. 2020 | KEYNOTE-181 | III | ≥2 | armA: pembrolizumab | 314 | 7.1 | 7.1 (6.2–8.1) | 2.1 (2.1–2.2) | 13.1 (9.5–17.3) | 32.4 | 8.5 (2.1+ - 25.8+) | 4 |
| armB: paclitaxel or docetaxel or irinotecan | 314 | 6.9 | 7.1 (6.3–8.0) | 3.4 (2.8–3.9) | 6.7 (4.2–10.0) | 24.2 | 10.7 (1.8+ - 16.8+) | |||||
| Huang et al. 2020 | ESCORT | III | ≥2 | armA: SHR-1210 | 228 | 8.3 | 8.3 (6.8–9.7) | 1.9 (1.9–2.4) | 20.2 (15.2–26.0) | 34 | 7.4 (3.8–10.8) | 4 |
| armB: docetaxel or irinotecan | 220 | 6.2 | 6.2 (5.7–6.9) | 1.9 (1.9–2.1) | 6.4 (3.5–10.5) | 22 | 3.4 (0.9-not reached) | |||||
| Xu et al. 2020 | ORIENT-2 | III | ≥2 | armA: sintilimab | 95 | 7.2 | 7.2 (5.8–9.7) | 1.6 (1.5–2.8) | 12.6 | NA | 8.3 (2.9–20.9) | 4 |
| armB: docetaxel or irinotecan | 95 | 62 | 6.2 (5.4–7.9) | 2.9 (2.6–3.6) | 6.3 | NA | 6.2 (4.6–8.4) |
Abbreviations:CI = confidence interval, ORR = objective response rate, mOS = median overall survival, mPFS = median progression-free survival, mDOR = median duration of Response, and NA = not available.
3.2. Efficacy outcomes of PD-1 inhibitor versus chemotherapy in all patients
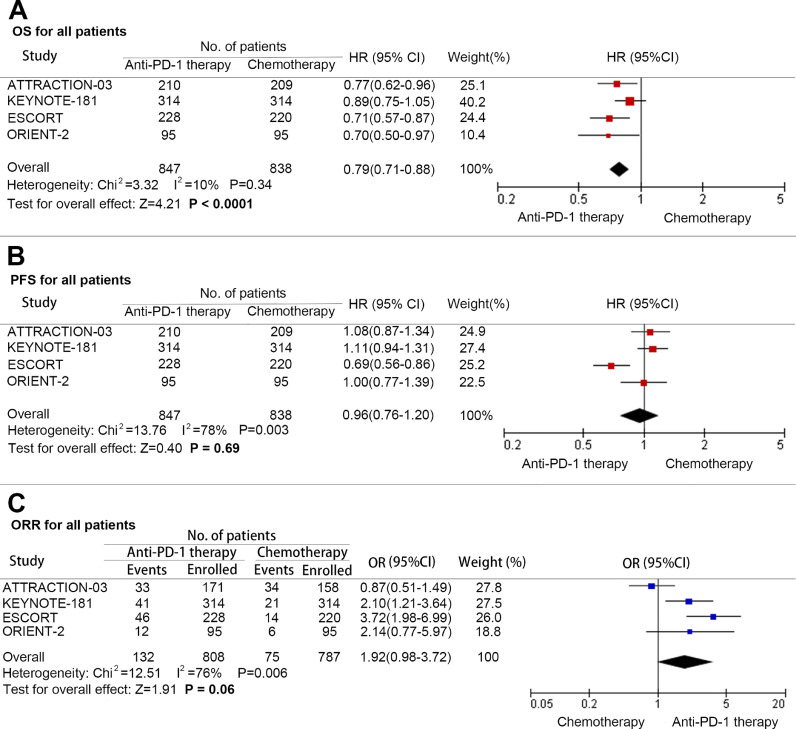
At final analysis, a significant improvement in OS was found among patients with advanced esophageal cancer treated with PD-1 inhibitors when compared with those treated with chemotherapy (HR: 0.79; 95% CI: 0.71–0.88; P<0.001; and heterogeneity: P = 0.34) (Fig. 2A). However, there was limited benefit in terms of PFS (HR: 0.96; 95% CI: 0.76–1.20; and P = 0.69; heterogeneity: P = 0.003) (Fig. 2B). In addition, the difference of ORR benefit obtained a near significant trend (OR = 1.92; 95% CI: 0.98–3.72; P = 0.06; and heterogeneity: P = 0.006) (Fig. 2C).
Fig. 2.
Pooled hazard ratio for overall survival (A), progression-free survival (B), and pooled odds ratio for objective response rate (C) in advanced esophageal cancer treated with anti-PD-1 versus chemotherapy. (HR: Hazard ratio; OR: Odds ratio; CI: Confidence interval; and PD-1: Programmed cell death 1).
3.3. Efficacy outcomes of PD-1 inhibitor versus chemotherapy in ESCC
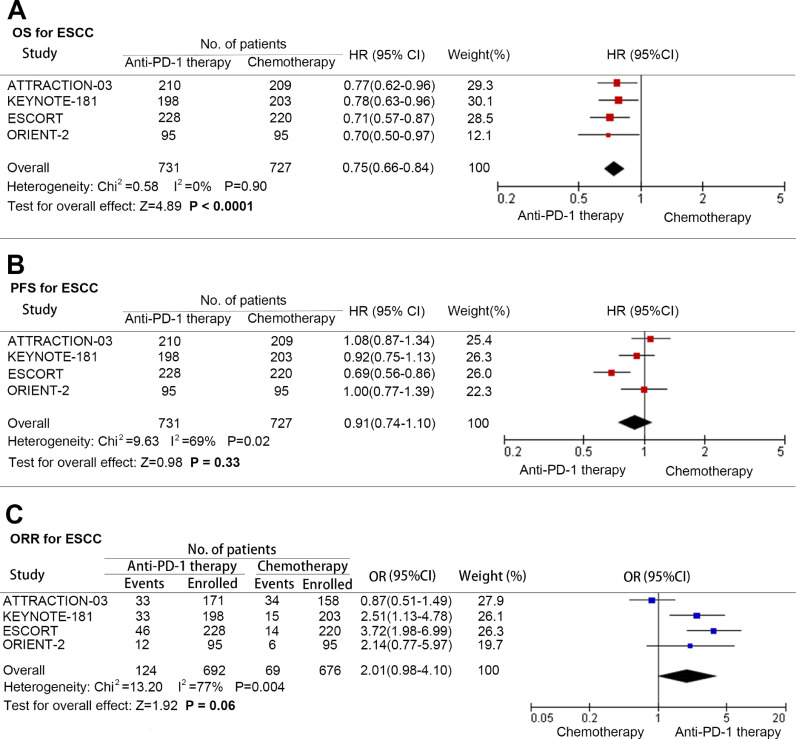
Meanwhile, we analyzed the patients with ESCC to figure out the benefit of anti-PD-1 therapy. Same as before, patients with ESCC treated with PD-1 inhibitors received superior OS to chemotherapy (HR: 0.75; 95% CI: 0.66–0.84; P<0.001; and heterogeneity: P = 0.90)(Fig. 3A), and there was no significant improvement in PFS (HR: 0.91; 95% CI: 0.74–1.10; P = 0.33; and heterogeneity: 0.02)(Fig. 3B) and ORR (OR: 2.01; 95% CI: 0.98–4.10; P = 0.06; and heterogeneity: P = 0.004)(Fig. 3C).
Fig. 3.
Pooled hazard ratio for overall survival (A), progression-free survival (B), and pooled odds ratio for objective response rate (C) in advanced esophageal squamous cell carcinoma treated with anti-PD-1 versus chemotherapy. (HR: Hazard ratio; OR: Odds ratio; CI: Confidence interval; and PD-1: Programmed cell death 1).
3.4. Subgroup analysis
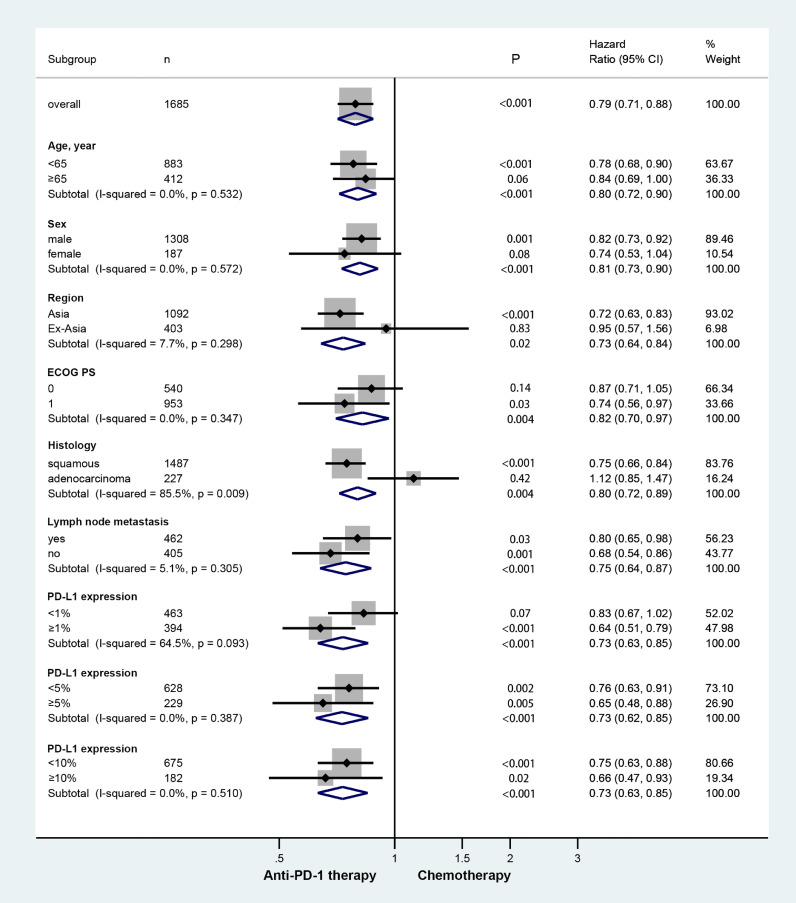
Regarding OS, we performed subgroup analyses according to some basic information, histological type, lymph node metastasis, and PD-L1 status (Fig. 4). PD-L1 tumor proportion score (TPS) was assessed by a central laboratory using immunohistochemistry. In all subgroup analyses, only histology could predict OS benefit from anti-PD-1 therapy over chemotherapy (squamous HR = 0.75 vs adenocarcinoma HR = 1.12; and P = 0.009). None of the other factors predicted OS benefit with the anti-PD-1 therapy versus chemotherapy, which includes age (P = 0.532), sex (P = 0.572), region (P = 0.298), ECOG PS (P = 0.298), lymph node metastasis (P = 0.350), PD-L1 expression (<1% vs ≥5% P = 0.093; <5% vs ≥5% P = 0.387; and <10% vs ≥10% P = 0.510).
Fig. 4.
Forest plot of hazard ratio in subgroup analysis comparing overall survival in patients who received anti-PD-1 therapy versus chemotherapy. (HR: Hazard ratio; CI: Confidence interval; and PD-1: Programmed cell death 1).
3.5. The analysis on safety of anti-PD-1 therapy
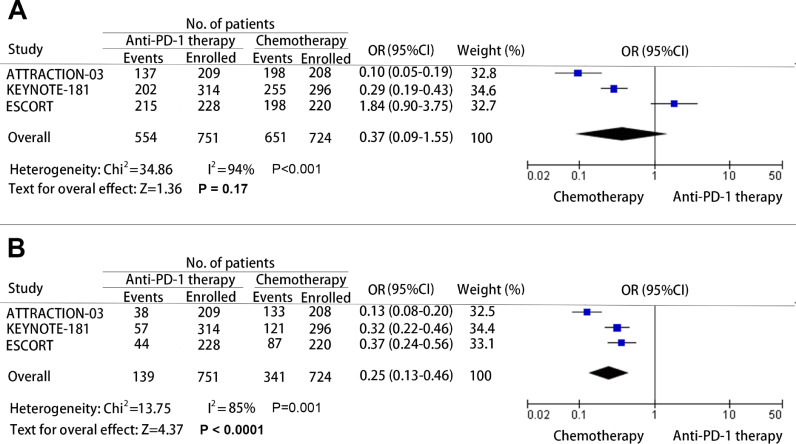
The treatment-related adverse effects are an important evaluation index for any antitumor therapies. Many treatments have to be discounted for the severe adverse effects caused by the treatment agents. To determine whether PD-1 inhibitors are safe in patients with advanced esophageal cancer, data of the total treatment-related adverse effects, and grade 3 - 5 treatment-related adverse effects were collected and analyzed. The OR of the total adverse effects for patients who receive anti-PD-1 therapy versus chemotherapy was 0.37 (95% CI: 0.09–1.55; P = 0.17; and heterogeneity: P<0.001) (Fig. 5A), and the OR of grade 3 - 5 adverse effects was 0.25 (95%: CI 0.13–0.46; P<0.001; and heterogeneity: P = 0.001) (Fig. 5B). Based on the observed results, no significant difference was found in the incidence of all treatment-related adverse effects. However, the results revealed that the incidence of grade 3 - 5 treatment-related adverse effects caused by PD-1 inhibitors was vitally lower than that caused by chemotherapy.
Fig. 5.
Pooled odds ratio (OR) for the incidence of any grade treatment-related adverse effect (A) and grade 3 - 5 treatment-related adverse effect (B). (OR: Odds ratio; CI: Confidence interval; and PD-1: Programmed cell death 1).
3.6. Study quality and sensitivity analysis
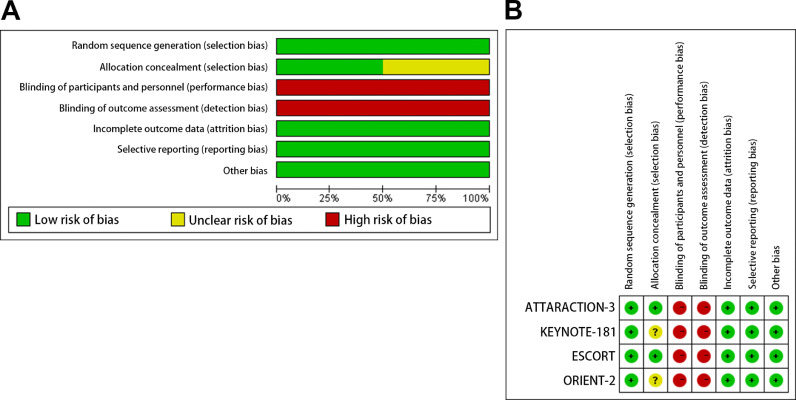
All trials included in this study were multicenter and open-label. The Jadad score was all 4, indicating that the quality was high (Table 1). The bias risk assessment was strictly carried out according to the guidelines outlined in the Cochrane handbook. The bias risk of the included studies was shown in Fig. 6. All trials included random sequence generation. The risk of bias for allocation in two studies were unclear. All studies were open-label, and their main bias was the lack of blinding. Nevertheless, all studies were felt to be low risk for attrition and reporting bias.
Fig. 6.
Risk of bias. We evaluated the risk of bias by using the Review Manager 5.3. Risk of bias graph (A): summary of risk of bias is presented as percentage across all included studies. Risk of bias summary (B): individual studies were determined using the Cochrane tool for the assessment of risk of bias.
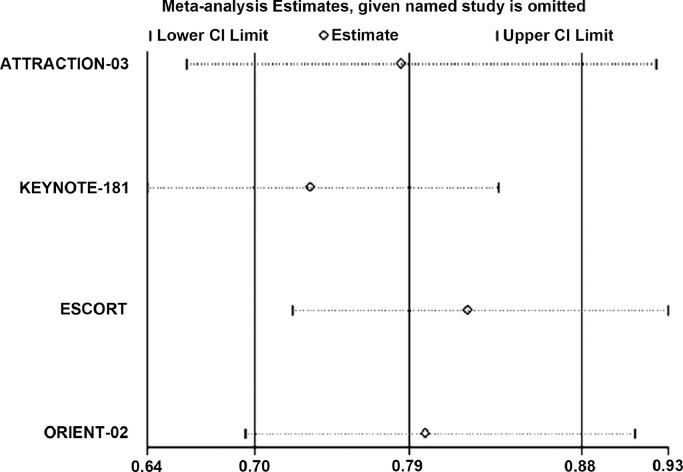
To evaluate the robustness of the combined outcomes, we carried out sensitivity analyses by omitting specific studies or altering statistical models. The results showed that the pooled HRs for OS were stable in our study and no significant deviation from the overall results was detected (Fig. 7).
Fig. 7.
Sensitivity analysis of the hazard ratios of overall survival. (CI: Confidence interval and PD-1: Programmed cell death 1).
4. Discussion
A novel effective treatment diagram is thirsty for patients with advanced esophageal cancer who are in rapid progression during or after standard chemotherapy [32]. Recently, it is worth noting that the successive discovery and further study of immune checkpoints such as PD-1 make immunotherapy to serve as the fourth antitumor strategy following surgery, radiotherapy, and chemotherapy [33]. Single agent PD-1 inhibitors have been explored as treatment strategies for patients with advanced esophageal cancer. We conducted this meta-analysis to investigate the efficacy and safety of anti-PD-1 therapy for patients with advanced esophageal cancer.
In this study, we first compared the efficacy of PD-1 inhibitors with chemotherapy in patients with advanced esophageal cancer. OS, PFS, and ORR were selected as the primary endpoints. We observed that PD-1 inhibitors significantly improved OS in advanced esophageal cancer when compared with chemotherapy. However, no significant improvement in PFS was found. Nevertheless, the ORR benefit difference obtained a near-significant trend (P = 0.06), which needs to be compared in more randomized studies. Similar results were found in patients with ESCC. In the subgroup analysis, squamous cell carcinoma was more effective than adenocarcinoma for anti-PD-1 therapy. Hence, it was concluded that the anti-PD-1 therapy significantly improved the OS rather than the PFS and ORR when compared with chemotherapy, particularly in ESCC. In addition to histology, overall survival assessed consistently favored PD-1 inhibitors versus chemotherapy in all subgroups. Although significant interactions were observed for age ≥65 years, female and ECOG PS = 0, the HRs were less than 1. The result suggested that there was no change in the direction of the treatment effect. Then, we compared the safety of PD-1 inhibitors with chemotherapy in patients with esophageal cancer. Based on the observed results, no significant difference was found in the incidence of all treatment-related adverse effects. However, our results show that PD-1 inhibitors had a lower incidence of grade 3 - 5 treatment-related adverse effects than chemotherapy, which demonstrated patients receiving PD-1 inhibitors had a significant overall improvement in the quality of life.
Several recent trials demonstrated that PD-L1 expression had a significant correlation with OS, PFS, and ORR. However, the significance of PD-L1 expression level for anti-PD-1 therapy is still controversial [34]. Here, we conducted a subgroup analysis to clarify the OS benefit of PD-1 inhibitors in patients with esophageal cancer with different PD-L1 expression. The OS benefit with PD-1 inhibitors occurred for patients in whom the PD-L1 TPS was at least 1% (OR = 0.83; 95% CI: 0.67–1.02; and P = 0.07), and when the PD-L1 TPS was less than 1%, the OS benefit with PD-1 inhibitors was not statistically different (OR = 0.64; 95% CI: 0.51–0.79; and P<0.001). However, the magnitude of OS benefit was not significantly different among subgroups of PD-L1 TPS, which indicate the survival benefit with PD-1 inhibitors regardless of patients’ level of tumor PD-L1 expression. Therefore, exploratory clinical trials and extended follow-up are needed to fully evaluate the role of PD-L1 expression in immunotherapy.
The results of our study are consistent with previous studies. KEYNOTE 180 showed that pembrolizumab had long-term clinical benefits with controlled adverse events, which provided treatment options for patients with esophageal cancer who had previously failed treatment [23]. KEYNOTE 181 achieved the main-OS endpoint, which has demonstrated a survival benefit in esophageal cancer immunotherapy [24]. In the ATTRACTION-03 trial, the nivolumab group showed a statistically significant improvement in OS when compared with the chemotherapy group. The survival benefit of nivolumab was observed regardless of the expression level of PD-L1 in tumors. In terms of PFS and ORR, there was no significant improvement between the two groups [25]. The subgroup analyses in Huang et al. showed that PD-L1 was not significantly associated with ORR in the clinical trial of SHR-1210 for esophageal cancer [27].
There were some exploratory biomarker analyses to evaluate the role of PD-1 inhibitors in patients with esophageal cancer, in whom treatment options have been minimal for decades, and the prognosis remains poor. Some patients with esophageal cancer were reported to carry frequent amplification of chromosome 11q13 and those patients without 11q13 amplification, had significantly better ORR and PFS when compared with 11q13-amplified individuals [35]. Several studies have shown that both PFS and OS are prolonged with the increase of tumor mutation burden (TMB) in immunotherapy. TMB has the potential to be a biomarker to evaluate the efficacy of immunotherapy [36], [37], [38]. Greally et al. analyzed the relationship between TMB and survival in 62 patients with immunotherapeutic esophageal cancer. This clinical study found that patients in the high TMB group obtained significant survival benefits [39]. Microsatellite instability (MSI) was usually caused by deficiencies mismatch repair (dMMR) [40]. The microenvironment of dMMR made it more likely to express PD-L1, which influenced the efficacy of PD-1 inhibitors, and dMMR tumors were associated with prolonged PFS when compared with mismatch repair-proficient tumors, regardless of the original tissue of cancer [41]. Although the incidence of MSI-H in ESCC is rare and only accounted for about 8%, this biomarker is critical and may affect the efficacy of immune checkpoint inhibitors [42]. This may explain why patients with advanced esophageal cancer might benefit from the OS when treated with PD-1 inhibitors and the difference of PFS and ORR in these studies.
This is the initial meta-analysis to explore the efficacy and safety of PD-1 inhibitors on patients with advanced esophageal cancer. All studies were multicenter, randomized, open-label, phase 3 trials. After the comprehensive evaluation, researchers found that the results were less likely to have been affected by the absence of blindness. Therefore, the results of this meta-analysis are reliable and helpful for clinical treatment. We observed several limitations in this research. The number of included studies was limited. At the same time, one study was limited from the ASCO meeting abstract.
Anti-PD-1 therapy has shown initial efficacy in treating the patients with advanced esophageal cancer. Moreover, it has become one of the main research directions in the treatment of advanced esophageal cancer. With the increase of biomarker analysis and clinical experience, anti-PD-1 therapy will have a broader application prospect in esophageal cancer. It is necessary to comprehensively carry out more randomized controlled studies to further investigate the immune mechanism of esophageal cancer. In addition, it is also imperative to screen out potential biomarkers to identify the beneficial population.
5. Conclusion
In conclusion, our analysis revealed that PD-1 inhibitors significantly prolonged the OS when compared with chemotherapy, while no significant difference in PFS and ORR for the population of esophageal cancer. Patients with ESCC might receive more OS benefit from the anti-PD-L1 therapy than esophageal adenocarcinoma. Based on the analysis of grade 3 - 5 treatment-related adverse effects, a lower risk was associated with the anti-PD-1 therapy versus chemotherapy. As a result, anti-PD-1 therapy may be an optional treatment for patients with esophageal cancer.
Author Contributions Statement
Lu Yao: Conceptualization; Software; Writing-Original Draft; Visualization; and Project administration. Guan Lulu: Validation; Investigation; and Resources. Xu Mengli: Formal analysis and Data Curation. Wang Feng: Methodology; Writing-Review & Editing; Supervision; and Funding acquisition.
Funding
This work was supported by the National Natural Science Funds of China (No. 81672442); and the Scientific and technological projects in Henan Province (No. 182102310379).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mattiuzzi C., Lippi G. Current cancer epidemiology [J] J. Epidemiol. Glob. Health. 2019;9(4):217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I.,et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods [J]. 2019, 144(8): 1941–1953. [DOI] [PubMed]
- 3.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J] CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Short M.W., Burgers K.G., Fry V.T. Esophageal cancer [J] Am. Fam. Phys. 2017;95(1):22–28. [PubMed] [Google Scholar]
- 5.Zhang H.Z., Jin G.F., Shen H.B. Epidemiologic differences in esophageal cancer between Asian and Western populations [J] Chin. J. Cancer. 2012;31(6):281–286. doi: 10.5732/cjc.011.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domper Arnal M.J. Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries [J] World J. Gastroenterol. 2015;21(26):7933–7943. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajani J.A., D'Amico T.A., Bentrem D.J., al et. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology [J] J. Natl. Compr. Canc. Netw. 2019;17(7):855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 8.Chang S., Kohrt H., Maecker H.T. Monitoring the immune competence of cancer patients to predict outcome [J] Cancer Immunol. Immunother. 2014;63(7):713–719. doi: 10.1007/s00262-014-1521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi S., Miyamoto S., Kikuchi O. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma [J] Gastroenterology. 2015;149(7):1700–1715. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 10.Gotwals P., Cameron S., Cipolletta D. Prospects for combining targeted and conventional cancer therapy with immunotherapy [J] Nat. Rev. Cancer. 2017;17(5):286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 11.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy [J] Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman G.J., Long A.J., Iwai Y. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation [J] J. Exp. Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian S.L., Drake C.G., Pardoll D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity [J] Curr. Opin. Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory pathways in immunotherapy for cancer [J] Annu. Rev. Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 15.Guan J., Lim K.S., Mekhail T., Chang C.C. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers [J] Arch. Pathol. Lab. Med. 2017;141(6):851–861. doi: 10.5858/arpa.2016-0361-RA. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh C.C., Hsu H.S., Li A.F., Chen Y.J. Clinical relevance of PD-L1 and PD-L2 overexpression in patients with esophageal squamous cell carcinoma [J] J Thorac Dis. 2018;10(7):4433–4444. doi: 10.21037/jtd.2018.06.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., Deng H., Lu M. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer [J] Int. J. Clin. Exp. Pathol. 2014;7(9):6015–6023. [PMC free article] [PubMed] [Google Scholar]
- 18.Barsouk A., Rawla P. Targeted therapies and immunotherapies in the treatment of esophageal cancers [J]. 2019, 7(10): [DOI] [PMC free article] [PubMed]
- 19.Wang X., Teng F., Kong L., Yu J. PD-L1 expression in human cancers and its association with clinical outcomes [J] Onco Targets Ther. 2016;9:5023–5039. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer J.R., Tykodi S.S., Chow L.Q. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer [J] N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moujaess E., Haddad F.G. The emerging use of immune checkpoint blockade in the adjuvant setting for solid tumors: a review [J]. 2019, 11(16): 1409–1422. [DOI] [PubMed]
- 22.Doi T., Piha-Paul S.A., Jalal S.I. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma [J] J. Clin. Oncol. 2018;36(1):61–67. doi: 10.1200/JCO.2017.74.9846. [DOI] [PubMed] [Google Scholar]
- 23.Shah M.A., Kojima T., Enzinger P.C. Pembrolizumab for patients with previously treated metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: phase 2 KEYNOTE-180 study [J] J. Clin. Oncol. 2018;36(15) doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima T., Shah M.A. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer [J] J. Clin. Oncol. 2020 doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 25.Kato K., Cho B.C., Takahashi M. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial [J] Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 26.Kudo T., Hamamoto Y., Kato K. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial [J] Lancet Oncol. 2017;18(5):631–639. doi: 10.1016/S1470-2045(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 27.Huang J., Xu B., Mo H. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma [J] Clin. Cancer Res. 2018;24(6):1296–1304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 28.Huang J., Xu J., Chen Y. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study [J] Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 29.Xu R.H., Wang F., Shi J. Recombinant humanized anti-PD-1 monoclonal antibody (JS001) as salvage treatment for advanced esophageal squamous cell carcinoma: preliminary results of an open-label, multi-cohort, phase Ib/II clinical study [J] J. Clin. Oncol. 2018;36(4) [Google Scholar]
- 30.Xu J., Li Y., Fan Q. Sintilimab in patients with advanced esophageal squamous cell carcinoma refractory to previous chemotherapy: a randomized, open-label phase II trial (ORIENT-2) [J] J. Clin. Oncol. 2020;38(15) [Google Scholar]
- 31.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? [J] Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Shah M.A. Update on metastatic gastric and esophageal cancers [J] J. Clin. Oncol. 2015;33(16):1760–1769. doi: 10.1200/JCO.2014.60.1799. [DOI] [PubMed] [Google Scholar]
- 33.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer [J] N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo W., Wang P., Li N. Prognostic value of PD-L1 in esophageal squamous cell carcinoma: a meta-analysis [J] Oncotarget. 2018;9(17):13920–13933. doi: 10.18632/oncotarget.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F., Ren C., Zhao Q. Association of frequent amplification of chromosome 11q13 in esophageal squamous cell cancer with clinical benefit to immune check point blockade [J] J. Clin. Oncol. 2019;37 [Google Scholar]
- 36.Samstein R.M., Lee C.H., Shoushtari A.N. Tumor mutational load predicts survival after immunotherapy across multiple cancer types [J]. 2019, 51(2): 202–206. [DOI] [PMC free article] [PubMed]
- 37.Chan T.A., Yarchoan M., Jaffee E. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic [J] Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 2019;30(1):44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ott P.A., Bang Y.J., Piha-Paul S.A. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028 [J] J. Clin. Oncol. 2019;37(4):318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 39.Greally M., Ku G.Y. Immune checkpoint inhibitors in esophagogastric adenocarcinoma: do the results justify the hype? [J] J. Thorac. Dis. 2018;10(12):6407–6411. doi: 10.21037/jtd.2018.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabrizio D.A., George T.J., Jr., Dunne R.F. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition [J] J. Gastrointest. Oncol. 2018;9(4):610–617. doi: 10.21037/jgo.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency [J] N. Engl. J. Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mocellin S., Wang E., Marincola F.M. Cytokines and immune response in the tumor microenvironment [J] J. Immunother. (Hagerstown, Md: 1997) 2001;24(5):392–407. [PubMed] [Google Scholar]