Abstract
Cell culture typically employs inexpensive, disposable plasticware, and standard humidified CO2/room air incubators (5% CO2, ∼20% oxygen). These methods have historically proven adequate for the maintenance of viability, function, and proliferation of many cell types, but with broad variation in culture practices. With technological advances it is becoming increasingly clear that cell culture is not a “one size fits all” procedure. Recently, there is a shift toward comprehension of the individual physiological niches of cultured cells. As scale-up production of single cell and 3D aggregates for therapeutic applications has expanded, researchers have focused on understanding the role of many environmental metabolites/forces on cell function and viability. Oxygen, due to its role in cell processes and the requirement for adequate supply to maintain critical energy generation, is one such metabolite gaining increased focus. With the advent of improved sensing technologies and computational predictive modeling, it is becoming evident that parameters such as cell seeding density, culture media height, cellular oxygen consumption rate, and aggregate dimensions should be considered for experimental reproducibility. In this review, we will examine the role of oxygen in 3D cell culture with particular emphasis on primary islets of Langerhans and stem cell-derived insulin-producing SC-β cells, both known for their high metabolic demands. We will implement finite element modeling (FEM) to simulate historical and current culture methods in referenced manuscripts and innovations focusing on oxygen distribution. Our group and others have shown that oxygen plays a key role in proliferation, differentiation, and function of these 3D aggregates. Their culture in plastic consistently results in core regions of hypoxia/anoxia exacerbated by increased media height, aggregate dimensions, and oxygen consumption rates. Static gas permeable systems ameliorate this problem. The use of rotational culture and other dynamic culture systems also have advantages in terms of oxygen supply but come with the caveat that these endocrine aggregates are also exquisitely sensitive to mechanical perturbation. As recent work demonstrates, there is a strong rationale for the use of alternate in vitro systems to maintain physio-normal environments for cell growth and function for better phenotypic approximation of in vivo counterparts.
Keywords: 3D culture, spheroids, oxygenation, gas-permeable, hypoxia, hyperoxia (oxygen)
Introduction
Biomedical research of 3D cell culture has been ongoing for decades, increasing dramatically with the recent boom in stem cell research and associated cellular therapies (Moscona A., 1961; Knisely et al., 1969; Bruland et al., 1985; Li et al., 1992; De Moor et al., 2018; Gargotti et al., 2018; Jokinen et al., 2020; Qadir et al., 2020). Early seminal research focused on tumor spheroids and the generation of in vitro models to test therapies against the malignant cells (Inch et al., 1970; Bruland et al., 1985; Vescio et al., 1987). These early works spawned expansive research into basic biological mechanisms such as proliferation, differentiation, cell death, and nutrient/metabolite requirements in culture (Moscona A.A., 1961; Knisely et al., 1969; Carlsson et al., 1979; Gille and Joenje, 1992; Matta et al., 1994; Singhvi et al., 1994; Bader et al., 1996; Gassmann et al., 1996; Groebe, 1996).
Resoundingly, the majority of early research found dramatic improvements with 3D culture compared with 2D culture of the same cells. Simultaneous molecular and functional analysis of 2D and 3D cultures led to the discovery that 3D cultures tend to more accurately recapitulate the in vivo environment in cell shape/structure and environment. This, in turn, influences gene/protein expression and cell function. As a result, 3D cultures traditionally behave more like their in vivo counterparts. Despite dramatic improvements in expression and function, a substantial gap exists between 3D aggregates/spheroids and the in vivo organoids they are meant to model. We posit that one potential explanation for these observed differences is the variability in employed culture methods, particularly related to tissue oxygenation. Through years of foundational research and technological advances, the limitations of tissue oxygenation in culture methods have been described and the critical importance of variables such as cell seeding density, oxygen consumption rate (OCR), growth rate, and media height have been described (Carlsson et al., 1979; Mueller-Klieser and Sutherland, 1982; Heacock and Sutherland, 1986; Mueller-Klieser et al., 1986; Freyer et al., 1991; Gassmann et al., 1996; Groebe and Mueller-Klieser, 1996; Papas et al., 2005; Grayson et al., 2006; Fehrer et al., 2007). Due to the urgency with which all living cells require oxygen for the maintenance of metabolic processes, much focus has shifted to addressing insufficiencies in cell culture either through the design of culture systems based on convective flow and improved oxygen transfer through culture surfaces or by tissue engineering approaches to improve vasculogenesis.
For years, static culture has been performed in gas impermeable systems where steep oxygen and nutrient gradients form, shifting cells/aggregates down alternate pathways of metabolism, growth, and differentiation. Recent work from many groups, including our own, demonstrates that proper oxygenation can dramatically alter the viability and function of cell cultures, particularly of 3D tissues, resulting in an even more physio-normal approximation of their in vivo counterparts (Carlsson et al., 1979; Tuncay et al., 1994; Saini and Wick, 2004; Wang et al., 2005; Fraker et al., 2007, 2009, 2013; Powers et al., 2008, 2010; Simon and Keith, 2008; Anada et al., 2012; Cechin et al., 2014). This article will examine the role of oxygen in the growing area of 3D culture for therapeutic cell-based applications as it pertains to both primary endocrine cell aggregates (islets of Langerhans) and endocrine stem cell aggregates (SC-β). The utility of finite element modeling (FEM) as a way of optimizing culture settings to target physiologically relevant tissue oxygen concentration/partial pressures (pO2 in millimeters of mercury, relative to atmospheric pressure, 760 mmHg) will be highlighted. Particularly, we will examine alternate culture methods utilized historically, prior to the advent of efficient computational modeling, and will examine by retrospective FEM the oxygen profiles in these systems to determine the role of tissue oxygenation in some of the experimental outcomes. FEM can also dramatically reduce experimental waste in terms of materials and labor hours making it an efficient method for designing and validating experiments.
Importantly, tissue oxygenation in both 2D and 3D culture in various devices has been well-studied but the majority of these studies have been focused on conventional oxygen concentrations (95% RA) or extremely hypoxic conditions <4% O2 and primarily in gas impermeable plastic systems (Knisely et al., 1969; Mueller-Klieser and Sutherland, 1982, 1984; Mueller-Klieser, 1984; Mueller-Klieser et al., 1986; Acker et al., 1987; Deshpande and Heinzle, 2009; Murphy et al., 2017). The conclusions of many such studies in 3D culture demonstrate the steep gradient formation and tissue volume anoxia that is expected in plastic systems. The advent of membrane-based culture systems and methods for improving oxygen delivery to the cell culture surface has created a paradigm shift where historical studies are being repeated under more physiological oxygenation with pronounced differences in cellular viability and function.
Cellular Oxygen Demand
Mammalian development occurs at a partial pressure of oxygen (pO2) governed by mass transfer prior to tissue vascularization (Simon and Keith, 2008). Mass transfer limitations dictate that ∼ 0.5–1 mm3 is the maximal volume that can be adequately oxygenated by simple diffusive permeability, dependent on the tissue specific OCR and the local niche pO2 (Colen et al., 1999; McGrath et al., 2003). Therefore, much tissue development occurs at a pO2 approximating anoxia and this is the time period when much of the characteristic tissue proliferation is observed. With the advent of blood flow, pO2 increases to tissue specific ranges from 3.8 to 100 mmHg with little variation across much larger, perfused regions.
Cellular OCR in the literature ranges between <1 and 350 × 10–18 mol/s. This is in line with the oxygen requirements needed for the metabolism of 2,500 kcal per day which requires approximately 22 mols of oxygen or 2.5 × 10–4 mol/s. Assuming the commonly accepted number of cells in the adult human body of 37.2 trillion, this equates to an average 6.72 × 10–18 mol/cell s–1 with variation due to metabolic demand and cell function. Based on an average human cell diameter of 20 μm, this translates to an average rate of 1.6 × 10–3 mol/m3 of tissue s–1 (Wagner et al., 2011).
Historically, both 2D and 3D cell culture protocols have utilized gas-impermeable plastics kept within humidified incubators containing 95% room air (RA) and 5% carbon dioxide (CO2). Accounting for vapor pressure differences, the standard incubator oxygen partial pressure is approximately 142 mmHg. However, there is much variability in utilized methods related to cell seeding density and media height, further exacerbated by the range of OCR associated with mammalian cells. Particularly, different culture platform geometries necessitate variations in media height to prevent evaporation and provide adequate nutrients. These variations, exhibited in Table 1, can be detrimental to cultured cells, especially those with higher OCR.
TABLE 1.
Inherent variations in oxygen diffusion distances (culture medium height) due to the dimensions of typically utilized plastic cell culture systems.
| Culture system | Culture surface area (cm2) | Typical culture medium volume range (mL) | Culture medium height range (mm) |
| 96-well | 0.32 | 0.1–0.2 | 3.12–6.25 |
| 24-well | 1.9 | 0.5–1 | 2.63–5.26 |
| 6-well | 9.6 | 1–3 | 1.04–3.13 |
| T-75 flask | 75 | 8–15 | 1.07–2 |
| T-175 flask | 175 | 35–53 | 2–3.03 |
1D oxygen diffusion from the air/liquid interface varies from 1.04 to 6.25 mm depending on dimensions and culture medium volume.
2D vs. 3D Culture: Endocrine Cells and Clusters as an Example
The above variables can affect both 2D monolayer cultures and, more so, 3D aggregates resulting in prolonged exposure to elevated oxygen levels that can serve as a substrate for the formation of toxic free radical species on one extreme and pronounced hypoxia/anoxia, necrosis and impaired function on the other extreme (Kazzaz et al., 1999; Martens et al., 2005). Particularly, the high OCR and 3D geometry of endocrine aggregates (islets of Langerhans, stem cell-derived beta cells) restrict them to a low seeding density and culture medium height of ∼1.3 mm. These cells and clusters have reported OCR most often ranging from 1 to 5.0 × 10–2 mol/m3 s–1, more than an order of magnitude higher than the cellular average. This is not surprising given the critical role they play in the production and storage of multiple hormones and the tight regulation of glucose homeostasis (Papas et al., 2000; Murray et al., 2005; Pi et al., 2007; Buchwald, 2009, 2011; Fraker et al., 2013; Suszynski et al., 2014b; Qadir et al., 2020).
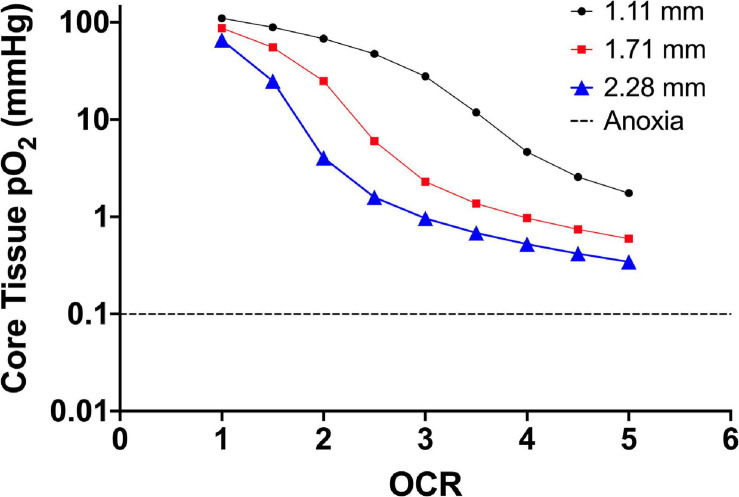
Figure 1 is finite element modeling (FEM) of 2D monolayer cultures showing the pO2 at the core region across a parametric sweep of OCR relevant to endocrine cells and clusters examining the effect of culture media height on tissue pO2.
FIGURE 1.
Core tissue oxygen profile of 2D monolayer (20 μm cell diameter) as a factor of culture media height (three different line plots) and increasing OCR (along the x-axis). The decrease in core pO2 corresponds to an increase in OCR along the x-axis and the downward shift in core pO2 is a result of increased media height. The OCR are representative of the published range of most primary endocrine somatic cells and cell lines. The dashed line shows the accepted theoretical cutoff for tissue anoxia and eventual necrotic death in endocrine aggregates.
From the model, 2D monolayer culture with 100% confluence is not prone to anoxia. In fact, at oxygen consumption rates at the lower end of the presented range, the pO2 experienced by the cells is “supra-physiological.” This could explain reports with culture at reduced environmental pO2 that results in improved proliferation and viability, as the lower external pO2 better represents the physiological niche of the cells (Fraker et al., 2007; Powers et al., 2008, 2010; Millman et al., 2009; Cechin et al., 2014). Using an oxygen consumption rate of 3.0 × 10–2 mol/m3 s–1 as a reference value, simply changing the media height by ∼1 mm shifts the core tissue pO2 nearly 30 mmHg (27 mmHg at 1.11 mm height to 0.9 mmHg at 2.28 mm height). As diffusion distances in vascularized tissues are typically not more than 5–10 μm, there are limited gradients that form in vivo related to not only oxygen but also most metabolites and waste products. The extent to which the gradients in culture affects cell function and viability, on the other hand, is not fully known.
In 3D cultures, core anoxia is common due to increased diffusion distances. This is evident in islets and islet-like clusters with sizes typically ranging from 50 to 400 μm. Islets above 100 μm cultured on plasticware experience increasing anoxia and loss of secretory capacity dependent primarily on oxygen consumption rate and media height and less so, related to seeding density. With a typical oxygen consumption range of 1.0–5.0 × 10–2 mol/m3 s–1, islets are limited to a seeding density that is well-established in the literature of about 3% of the culture surface area and a media depth not more than 1.1–1.3 mm (Papas et al., 2005; Avgoustiniatos et al., 2008; Kitzmann et al., 2014). As islet/endocrine spheroid viability and function are exquisitely dependent on tissue pO2, deviations from the culture recommendations can result in pronounced islet/endocrine cluster loss during culture. In fact, it has been demonstrated in the literature that apoptosis in cultured islets directly correlates with expression of HIF-1a (Moritz et al., 2002). This results in increased costs related to islet culture as a typical human islet preparation of approximately 250,000 islet equivalents (islet size scale correction to mean diameter of 150 μm; IEQ) requires 10–12 T175 flasks. Early work by the group of and Colton, Papas, Avgoustiniatos, and Dionne utilized computational modeling to investigate the oxygenation limitations in conventional culture methods for islets of Langerhans (Dionne et al., 1989, 1991, 1993, 1996; Papas et al., 2005). Through their detailed study of OCR in endocrine cells and spheroids, they identified the Km value associated with islet OCR (0.4 mmHg), and pO2 values associated with anoxia (∼0.1 mmHg and below) and impaired insulin secretion (∼2.5 mmHg and below). They continued this important work with further in-depth study of (a) the use of OCR as a metric of islet potency, (b) limitations in encapsulation devices and in vivo cellular replacement therapies in Type 1 Diabetes Mellitus (T1D), and (c) the development of gas permeable culture systems to address oxygen limitations (Avgoustiniatos et al., 2008; Kitzmann et al., 2014; Suszynski et al., 2014a, 2016; Papas et al., 2016; Cao et al., 2020). In one seminal paper by the group, FEM was performed assuming the standard IEQ diameter of 150 μm cultured in a square array and varying the culture density and culture medium volume to maintain the 1,000 IEQ/mL culture standard. Three different oxygen consumption rates were evaluated. In addition to plastic, culture was also simulated on 275 μm silicone membranes. They observed precipitous increases in anoxia on plastic culture platforms relative to OCR and the combined effect of culture density/medium depth (Papas et al., 2005).
The equivalent scaling originated from hand-counts of numerous islet preparations (rodent, dog, non-human primate, porcine, and human) demonstrating that the size bin with the largest tissue volume percentage was ∼150 μm. Our group confirmed this in studies comparing automated counters with hand counting protocols (Buchwald et al., 2009). Table 2 shows representative tissue volume distributions across size bins from 184 human islet preparation hand-counts.
TABLE 2.
Tissue volume percentages from 184 human islet preparations broken into IEQ counting size bins.
| Size bin | 50–100 | 100–150 | 150–200 | 200–250 | 250–300 | 300–350 | 350–400 | >400 |
| Mean | 7.34% | 15.23% | 21.1% | 16.27% | 14.47% | 12.40% | 9.1% | 4.07% |
| SEM | 0.50% | 0.70% | 0.70% | 0.70% | 0.90% | 0.90% | 0.90% | 0.70% |
The bins centered around 150 μm account for nearly 37% of the total preparation volume, the rationale behind the 150 μm IEQ size.
The rationale behind the IEQ standardization is clear from the ∼37% of the tissue volume represented by the bins centered around 150 μm. However, over 60% of the tissue volume is above 150 μm which leads to underestimation in anoxic volume in the tissue preparations. To better represent this, our group generated FEM models to represent anoxia in the full islet preparation volume. After solving, anoxic volume percentages were calculated for each size range and then total anoxic tissue volume percentage was determined by the following equation:
where, %Atotal is total volume percentage of anoxic tissue, %Asize is the percentage of anoxic tissue volume determined by FEM for each islet size bin (Table 2) and %Vsize is the mean volume percentage of tissue for each islet size bin (Table 2).
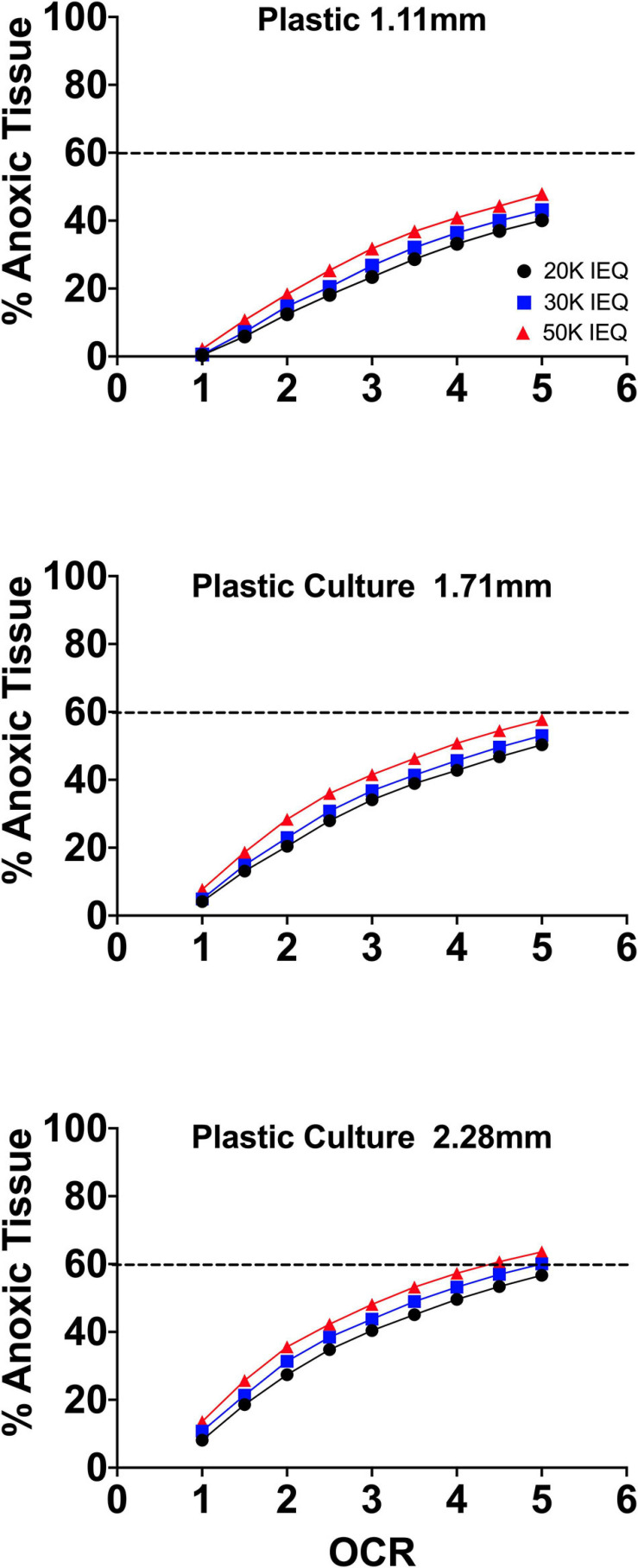
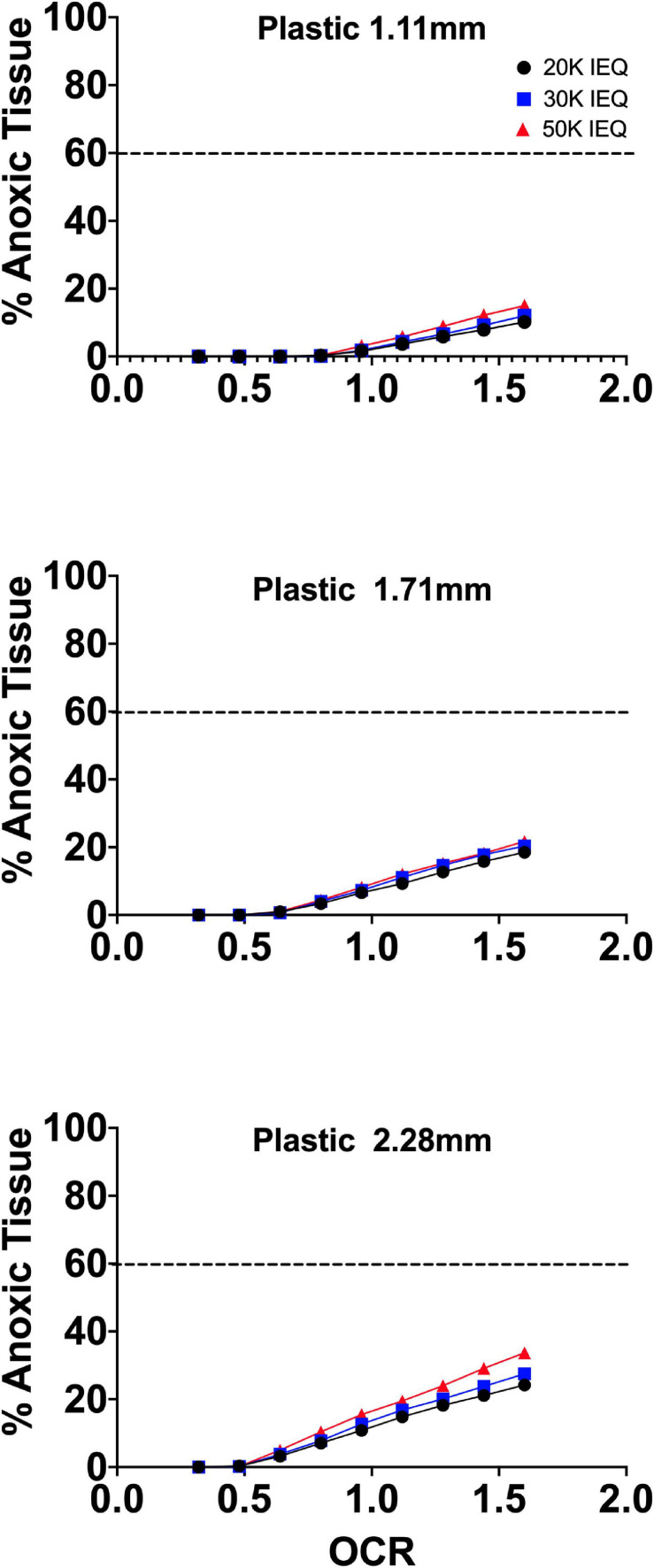
Figure 2 summarizes the effects of the oxygen consumption rate range, three conventional medium height variations (20, 30, 50 mL in a T-175 flask; 1.11, 1.71, and 2.28 mm, respectively) and three seeding densities (20, 30, and 50,000 IEQ in a T-175 flask; 2.76%, 3.67%, and 5.05%, respectively) on islet/islet-like cluster anoxia on standard plastic culture devices.
FIGURE 2.
FEM results of islet spheroids in standard culture (95% RA/5% CO2; plastic surface; 37°C) with varied seeding density and culture medium height across the range of reported islet oxygen consumption rate. Tissue anoxia is observed, primarily in IEQ sizes >100 μm, even at low oxygen consumption rates and is significantly affected by culture medium height (∼Δ20% min to max) and increased OCR (∼Δ50% min to max) and less so by seeding density (∼Δ7% min to max).
The models confirm the observed and previously modeled deficiencies of islet/endocrine spheroid culture on plastic systems. Tissue anoxia is observed in IEQ > 100 μm heavily dependent on OCR and culture medium height and less so on seeding density, albeit still a factor.
Despite well-documented culture suggestions and supportive FEM studies from multiple groups, there are still broad deviations in the literature in both islet/endocrine spheroid seeding density and culture medium height that could explain reported variations in pre/post-transplant function and viability. There is a clear need for culture standardization and cost-effective methods for improving culture oxygenation. Recent work has focused on addressing these challenges for the scale-up and implementation of clinical cell-based therapies.
Methods to Improve Oxygenation in 3D Culture: Endocrine Clusters as an Example
Early islet research groups including those of Colton, Kevin Lafferty and islet isolation pioneer, Paul Lacy, understood the importance of oxygen limitations in culture from early modeling and observed loss of function and viability (Bowen et al., 1980; Dionne et al., 1989, 1991). They explored various methods to improve oxygenation ranging from increasing incubator oxygen concentration to levels as high as 95%, low temperature culture, perfusion systems, and gyroscopic/rotational culture (Scharp et al., 1978; Ono et al., 1979; Lacy et al., 1982). All of these methods ameliorated tissue hypoxia/anoxia but had associated challenges and beneficial unexpected findings.
Increasing External Oxygen Concentration
In studies first by the group of Lafferty and then by Lacy et al. (1982) culture in elevated incubator pO2 resulted in a stark depletion of tissue-resident immune cells present in islets. These cells surveil and relay information about islet well-being to immune response elements. Additionally, the extended culture resulted in aggregation of individual IEQ into “mega-islets.” The depletion of the tissue-resident immune cells in elevated oxygen resulted in significantly prolonged islet engraftment in allogeneic and concordant xenogeneic transplant settings (Bowen et al., 1980).
In one paper by Bowen et al. (1980), they cultured 50 IEQ in 35 mm dishes with 2 mL of culture medium, initially, moving to 0.75 mL after the first of three culture medium changes in the 4–7 days culture period. Incubator gas concentration was 95% O2 and 5% CO2. After the first change of culture medium, they also began to observe aggregation of the IEQ into one large “mega-islet.” Interestingly, IEQ that failed to aggregate did not survive the culture at elevated oxygen concentrations suggesting that the aggregation may result in a “mega-islet” pO2 that better approximates standard culture or in vivo levels.
In another paper by Lacy et al. (1982), they modified the 2-stage protocol of Lafferty by first culturing 50 IEQ/well in U-bottom 96-well plates with 200 μL of culture medium. This significantly increased the culture medium height from ∼2 mm in the Lafferty protocol to ∼6.2 mm for the first stage of the culture progressing to culture on a 35 mm dish with 0.75 mL of culture medium, as in the Lafferty protocol.
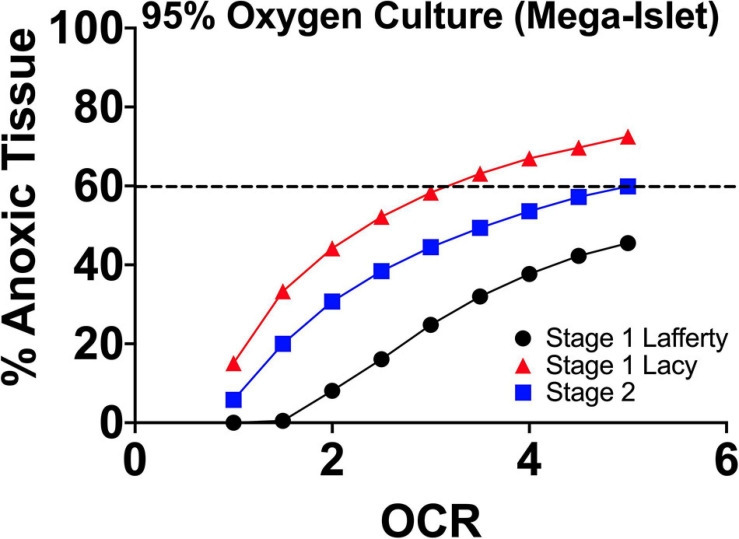
In Figure 3, using the culture parameters described in the papers of Lafferty and Lacy, we performed FEM of the estimated anoxia during 2-stage mega-islet culture in order to better understand the effects of culture oxygenation on the experimental observations.
FIGURE 3.
FEM anoxia results of two stage mega-islet cultures (95% O2/5% CO2; plastic surface; 37°C) from the group of Lafferty and Lacy, respectively. Prior to aggregation (Stage 1), the tissue anoxia is lower at all oxygen consumption rates but with aggregation, anoxia become significantly higher throughout the tissue given the increase in tissue geometry. The elevated pO2 maintains non-anoxic tissue at levels approximating culture of IEQs of standard size range (50–400 μm) and seeding density. At the same time, the supra-physiological pO2 depletes the tissue resident immune cells.
The increased pO2 sustains the larger tissue dimensions at a level of hypoxia/anoxia approximating the culture of IEQ of standard size range and seeding density. There is no apparent oxygenation benefit but the elevated pO2 allows for IEQ aggregation and reduction of tissue-resident immune cells. The thought of both groups was that increased oxygen was responsible for the loss of tissue-resident cells, but the presence, indicated by FEM, of similar anoxic tissue volumes to standard 95% RA/5% CO2 cultures suggests that hypoxia/anoxia may also play a role. In both research efforts, when these mega-islets were utilized in sub-renal capsule transplants in chemically induced diabetic recipients, they improved allogeneic/concordant xenogeneic graft longevity without concurrent immunosuppression. This suggests that potentially (1) the tissue-resident cells play a critical role in graft rejection signaling recipient immune responses and (2) extended culture reduces antigen shedding and thereby, proinflammatory immune responses.
Our group also implemented increased oxygen levels in standard islet culture examining islet function and viability. FEM of this approach is shown in Figure 4. The modeled reduction in tissue anoxia was 30–50% compared to conventional 20% O2. This did not translate to significant in vitro functional improvements. This could be the result of supra-physiological concentrations in smaller islets (100, 150 μm) resulting in increased oxidant concentration and potentially, free-radical mediated damage to secretory capacity. These high oxygen levels were not experienced by seeded IEQ in the prior mega-islet experiments due either to increased media depth or closer IEQ seeding proximity. Clearly, there is a balance of proper oxygenation where both too much and too little are detrimental, and the multiple variables involved further complicate efforts to adequately maintain endocrine clusters.
FIGURE 4.
FEM of standard islet culture on plastic with an external oxygen concentration of 35%. The modeled anoxia was ∼50–70% relative to culture at 20% O2.
Culture on Liquid Permeable Transwell Devices
Another approach that has been increasingly implemented in the culture of 3D aggregates, including islets and endocrine precursors/pancreatic progenitors is the use of transwell devices. These come ready to use in most plate well geometries. 3D aggregates are suspended on a liquid permeable membrane raised off of the basal plastic surface of the culture well ∼1 mm. Simply elevating the culture above the gas impermeable surface and allowing for mass transfer on both the basal and apical surface of the tissue improves the oxygen profiles. The apical surface is covered by a minimal height of culture medium reducing oxygen mass transfer distances but requiring frequent changes to prevent tissue drying due to evaporative loss (Bigas et al., 1995; Uroic et al., 2010).
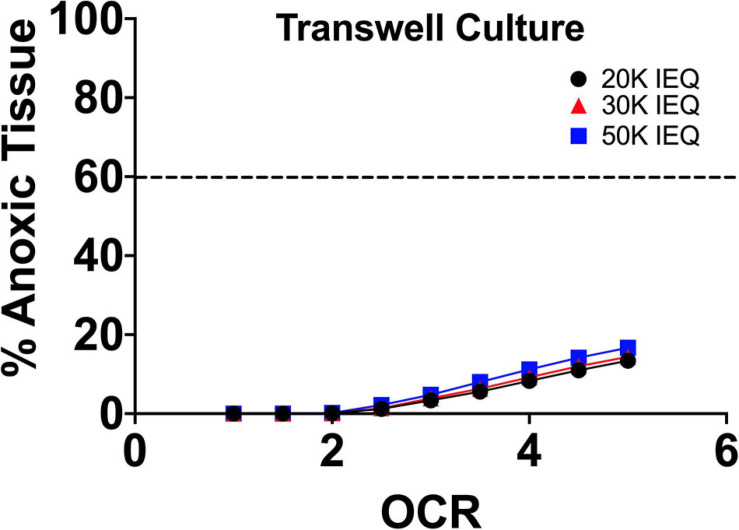
As can be seen in FEM (Figure 5), the elevation of the tissue above the gas impermeable plastic and closer proximity to the liquid/gas interface causes a significant reduction in tissue hypoxia. Limited device geometries, however, evaporative losses/tissue dehydration at the apical surface and improved culture alternatives have made this approach recently less utilized.
FIGURE 5.
FEM of islet culture on transwell surfaces. The reduction of culture medium height due to closer proximity of the islets to the liquid/gas interface and the imposed distance from the gas impermeable basal surface (plastic) results in significant drops in tissue hypoxia/anoxia at all oxygen consumption rates.
Low Temperature Culture
Another early and continued approach to prevent post-isolation IEQ loss and anoxia is low temperature culture (20–25°C). Started in the late 1970s by the research groups of Lacy, Talmage and others, low temperature culture was observed to reduce antigenicity and immune response, improve function and viability and prevent anoxia and culture losses (Chase et al., 1979; Ono et al., 1979). The benefit of the low temperature culture comes from the reduction in oxygen consumption and metabolic activity of the endocrine clusters, which follow an Arrhenius behavior. These reaction rates display an exponential relationship to temperature described by the equation:
where, Rate0 is a pre-exponential reaction factor, EA is the activation energy for the metabolic reaction, R, the universal gas constant (8.314 mol⋅kJ–1 K–1) and T, the temperature in degrees Kelvin. For reactions such as oxygen diffusion in the system, this also results in an exponential decrease in diffusivity. This drop is countered by an increase in dissolved oxygen content (solubility) resulting in a minimal change in oxygen transfer, overall. The exponential drop in oxygen consumption rate (60–70%), however, has a pronounced effect on the tissue oxygen distribution and this significantly reduces the volume of tissue affected by hypoxia/anoxia. This can be seen in the FEM analysis in Figure 6.
FIGURE 6.
FEM of low temperature islet culture on conventional plastic culture systems. The reduction of temperature significantly reduces tissue anoxia due to the relative Arrhenius drop in oxygen consumption rate (68%) indicated along the x-axis (0.32 × standard islet OCR values, 1.0–5.0 × 10−2 mol/m3 s−1). Tissue anoxia was 20–35% less in low temperature culture relative to standard culture settings and dependent on seeding density and culture medium height.
Overall, tissue anoxia was reduced by 20–35% in low temperature culture relative to standard culture settings and dependent on seeding density and culture medium height. It is not surprising that this reduction in tissue loss would translate to improved function and viability upon return to physiological temperature and would result in reduced antigen shedding and recipient immune response, as was observed when transplanted.
Bioreactors, Gyrotational Culture and Convective-Flow Culture Systems
It is clear from the FEM of various static culture settings that oxygen consumption rate, plating density and tissue diameter are critical variables that are often disregarded in culture design. Additionally, pO2 gradients range from surface to core values that are non-physiological and this can adversely affect tissue function and viability. This is more prevalent in 3D cell aggregates and presents a substantial obstacle to scale-up and implementation for therapeutic cell replacement applications. Given that most historical research has been performed using plastic culture systems and using immortalized cell lines, oxygen limitations have been frequently overlooked. Recently, as culture is moving toward physiologically relevant 3D aggregates, the limitations of oxygenation in plasticware have become apparent and there is a growing body of engineering work in the development of alternative culture systems for enhanced oxygenation.
Initially used in scale-up production of fermentation processes and the production/synthesis of medicinal substances from biological reactions (e.g., insulin production from recombinant DNA), bioreactors have been further engineered for a multitude of applications. Newer systems have varied device geometries and real-time controllers for atmospheric gas concentrations, fluidics, and temperature. The advantage of these systems is that they have the potential to eliminate pO2 gradients within the culture medium either by continued supply of fresh medium or through constant mixing in contact with the liquid/air interface.
In the context of islets and endocrine aggregates/SC-β, flow systems have long been in use. Early work in the laboratory of Paul Lacy utilized gyrotational culture devices to preserve isolated islets in collaborations with NASA and McDonnell Douglas examining the effects of rotation and microgravity on islet isolation, function, and viability (Buitrago et al., 1977; Scharp et al., 1978; Britt et al., 1981). These devices employed rotation along multiple axes to prevent islet settling onto basal, gas-impermeable culture surfaces and to continuously bathe the islets in well-mixed culture medium. These and further studies in microgravity culture systems with isolated islets demonstrated reduced immunogenicity and improved viability and function over long-term culture periods (Rutzky et al., 2001, 2002; Tobin et al., 2001; Luca et al., 2006).
Spinner flasks aid in the formation of 3D aggregates from single cells by maintaining rotational flow driven by magnetic stirrers. Many systems come with ports for easy gas exchange and media sampling/replacement. Dependent on cell density, rotational speed and time of culture, uniform clusters of a desired spherical geometry can be readily formed. These systems have been utilized in the generation of 3D islet-like spheroids from immortalized beta-cell insulinoma lines (MIN-6) exhibiting improved viability, proliferation and insulin secretion (Lock et al., 2011).
As SC-β production has scaled for pre-clinical and clinical work, high-throughput and large volume culture has dictated the transition from inefficient static culture systems to flow systems, primarily spinner flasks/bioreactors. The groups of Doug Melton and Semma Therapeutics along with former lab members, such as Jeffrey Millman, have established differentiation culture protocols implementing rotational devices to successfully generate reproducible large-scale batches of SC-β (Hrvatin et al., 2014; Pagliuca et al., 2014; Millman et al., 2016; Vegas et al., 2016; Peterson et al., 2020; Helman and Melton, 2021). Of note, until the recent paper by the group of Yoshihara et al. (2020) these aggregates relied on steps of in vivo terminal differentiation and a delayed function greater than the 5 days reported in the recent paper (Yoshihara et al., 2020). This delayed response has been attributed by Davis and Melton to a lack of anaplerotic cycling resulting in inefficient glucose responsiveness. This was reversed by exposing the SC-β to metabolites from late glycolysis and intermediate stages of the citric acid cycle (Davis et al., 2020). The SC-β generated by Yoshihara et al. (2020) displayed increased oxidative metabolism with oxygen consumption rates similar to healthy primary islets indicating, perhaps, that the early in vivo function was due to proper anaplerotic cycling not observed in the work of Davis and Melton. It should be noted that the entire differentiation procedure of the Evans group was performed on gas-impermeable plastic systems with a basal methylcellulose layer while the Davis and Melton paper utilized planar followed by suspension rotational culture, per their standard protocols.
Culture bags on mechanical rockers can also be used for 3D aggregates, but typically of pre-formed aggregates. In this case, the cells are bathed in culture media that is gently agitated in a rocking motion across the cells (Singh, 1999; Tsai et al., 2017). Their utilization with islets and endocrine spheroids has been limited, however (Schmied et al., 2000). As with spinner flasks, the more advanced systems come with controllers for gas concentration, temperature, fluidics, and contain ports for easy addition and sampling of culture media. Additionally, more advanced spinner flask and rocker systems come with a variety of sensors measuring not only dissolved gas concentration, but also glucose consumption, lactate production and pH of the culture medium.
As would be expected, these systems are ideal for oxygenation throughout the culture environment external to cell clusters/tissue maintaining a uniform tissue surface pO2. This system completely prevents anoxia in the relevant size range of islets (50–400 μm) across the range of typical oxygen consumption rates (1.0–5.0 mol/m3 s–1). In fact, even at tissue geometries approaching 750–1000 μm, anoxia is less than experienced by islets of standard size range (50–450 μm) in standard plastic culture.
While ideal for preventing hypoxia/anoxia, bioreactor systems are often cumbersome, require additional equipment, such as magnetic stir plates and interfaces for sensors and gas lines and can increase reagent utilization. Importantly, bioreactors and their associated convective-flow devices can increase the chance for contamination due to needed manipulation and additional component interfaces. The systems have also been shown to generate undesirable shear forces and bubbles that can have adverse effects on cells/tissues. The critical scalar factor related to adverse effects of shear is energy dissipation rate (EDR) expressed in units of W/m3. In a paper by Shenkman et al. (2009) shear forces on islets of Langerhans in flow culture systems appeared to have minimal effect on viability and function as measured by Caspase activity and oxygen consumption rate (Shenkman et al., 2009). This was observed up to a volumetric flow rate of 50 mL/min translating to an EDR of 9,600 W/m3. As reference, a spinner flask at a maximal rotation of 200 rpm has an EDR of about 1,500 W/m3 while bubble rupture or passage through a 200 μL pipette tip in 0.2 s have an EDR of approximately 1 × 105 W/m3. This paper did not examine secretory function, however, which could be more informative in understanding the subtleties of shear effects as most papers in the field examine endpoints like flow induced necrosis or lysis and not functional effects.
More recent publications utilizing microfluidic systems have demonstrated that shear forces with EDR well below the levels of spinner flasks impair calcium channel mediated insulin secretion in surface cells of islets (Sankar et al., 2011; Silva et al., 2013). The lack of definitive understanding of the effects of fluidic shear on islet/endocrine spheroid function and viability could explain some of the unexpected outcomes in large-scale SC-β culture protocols, such as delays in terminal differentiation and function. Given the near-clinical stage of this cellular replacement research, there is a need for further detailed study into the range of effects of shear forces on endocrine spheroid viability and function.
Microfluidic Culture Platforms
A recent trend in islet/SC-β culture is the use of microfluidic or lab-on-a-chip culture systems. These platforms assimilate multiple functions into a single unit utilizing flow-based culture. They typically have geometries on the order of square millimeters to centimeters and due to reduced reagent consumption, they can dramatically shrink experimental costs. They are also typically designed with an array of sensors for longitudinal monitoring of temperature, fluid flow/pressure, gas concentration and other pertinent parameters to a specific cell type. They can be used to monitor cellular feedback, for imaging analysis and for biochemical assays, like glucose-stimulated insulin release with islets. The fluidic inputs allow for the introduction of an unlimited array of compounds making the platform useful for pharmaceutical studies and the development of differentiation/culture protocols. Importantly, these platforms allow for the study of compartmentalized cells and tissues to better understand the physiological interactions of, for example, vascular or immune cells with other specific somatic tissues, like islets.
In the context of islet/SC-β research, lab-on-a-chip platforms have been used in select studies examining the prevention of endothelial cell loss in islets post-isolation, dynamic imaging studies, insulin/glucagon/somatostatin secretion studies, differentiation of human embryonic stem cells into SC-β and endocrine spheroid microencapsulation (Sankar et al., 2011; Silva et al., 2013; McMillan et al., 2016; Nourmohammadzadeh et al., 2016; Lenguito et al., 2017; Sharma et al., 2017; Lee G. et al., 2018; Jun et al., 2019). These novel systems are remarkable for small-scale discovery research but one stark omission in most studies has been a comparison to conventional culture methods to see if these systems improve endocrine spheroid viability or function. Additionally, scale-up of these fluidic systems to manage whole islet preparations or batches of SC-β would likely be costly and difficult relative to existing large-scale systems unless merited by significant metabolic improvement in the endocrine spheroids.
Gas-Permeable Culture Platforms: Preventing Anoxia in Endocrine Spheroid Static Culture
A simple and cost-effective solution to providing adequate oxygen in the culture of endocrine and other 3D spheroids while eliminating any effects of shear forces is the use of polydimethylsiloxane culture systems (PDMS). Utilized previously in cell culture, primarily as a hydrophobic and non-adherent substrate, PDMS has recently gained favor due to its improved permeability to gasses, including oxygen and carbon dioxide relevant to cell culture (Harris, 1984; Rosdy et al., 1991; Singhvi et al., 1994; Wang and Deen, 2003). Our group and others have implemented gas permeable static culture systems using PDMS as the basal culture surface for the culture of primary islets of Langerhans and endocrine spheroids derived from stem cells (Papas et al., 2005; Fraker et al., 2007, 2013; Avgoustiniatos et al., 2008; Cechin et al., 2014; Kitzmann et al., 2014). PDMS has reported oxygen permeabilities 100–500 times that of polystyrene (Papas et al., 2005; Zhang, 2006). It is also an inert and biocompatible material that is FDA approved for numerous in vivo applications and devices making it ideal for in vitro cell culture use.
Early PDMS culture work related to islets was done by the group of Papas, Avgoustiniatos, and Colton. They utilized FEM to interrogate oxygen limitations in conventional islet culture methods for islets of Langerhans (Papas et al., 2005). Their results, confirmed by OCR as a measure of islet viability post-culture, demonstrated that culture on silicone rubber devices completely abrogated islet anoxia at culture densities up to ∼4,400 IEQ/cm2, nearly 25 times the standard culture density. One hundred cm2 prototypes from Wilson Wolf Manufacturing were implemented with a membrane thickness of 275 μm filled with 500 mL of culture medium. It should be noted that these studies were performed at an incubator pO2 corresponding to a fully humidified 95% RA/5% CO2 system (142 mmHg) and that the models assumed an IEQ diameter of 150 μm and did not examine the size range distribution of IEQ. This early prototype was the basis for the G-RexTM technology now broadly implemented in therapeutic immune and stem cell expansion (Bajgain et al., 2014).
One added benefit of the gas permeable basal surface is that the majority of the oxygen is delivered in close proximity to the cells and this eliminates reliance on oxygen diffusion from the apical liquid/air interface. This dependence on diffusion in plastic culture systems limits the amount of culture medium and therefore, the nutrient supply, that can be added without limiting oxygen supply. In PDMS systems, nutrient supply is not limited by this height restriction. While the devices have a higher per unit cost, culture using the PDMS systems is more efficient and cost-effective by reducing the number of needed flasks, the amount of culture medium and labor involved with maintaining cell cultures.
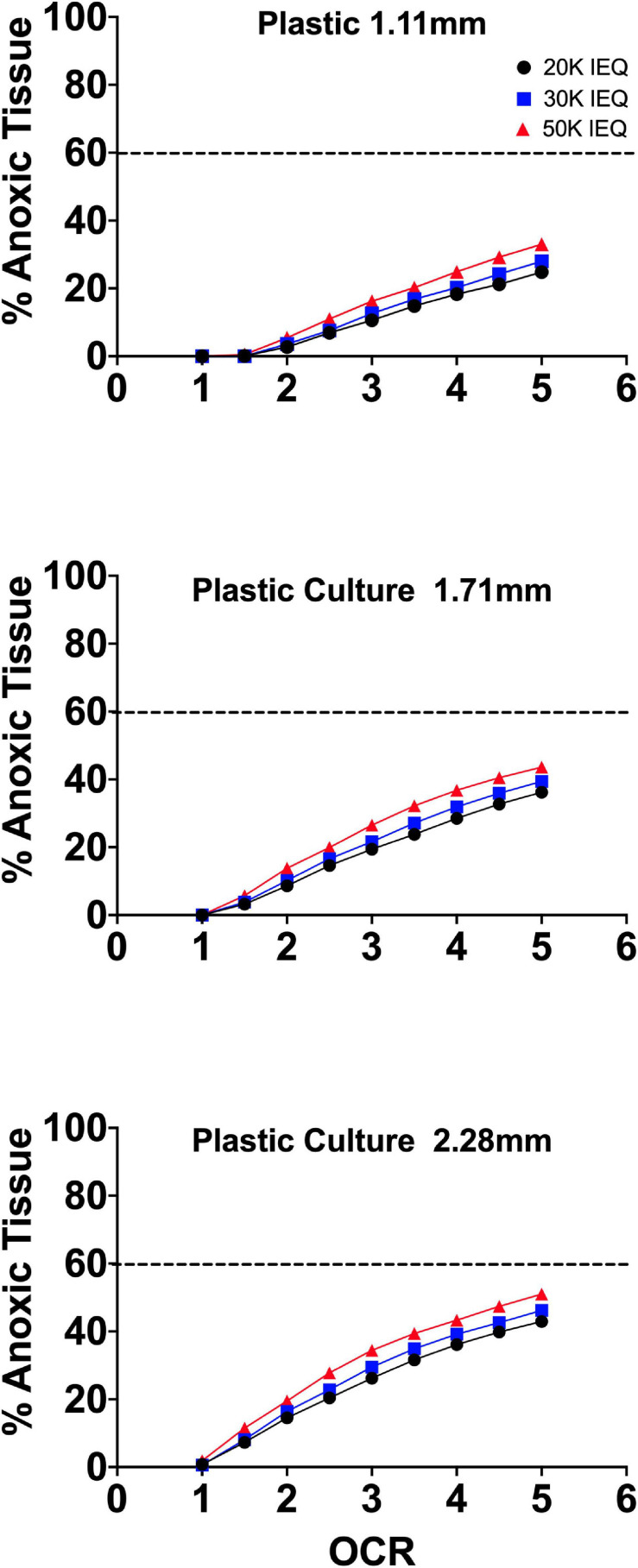
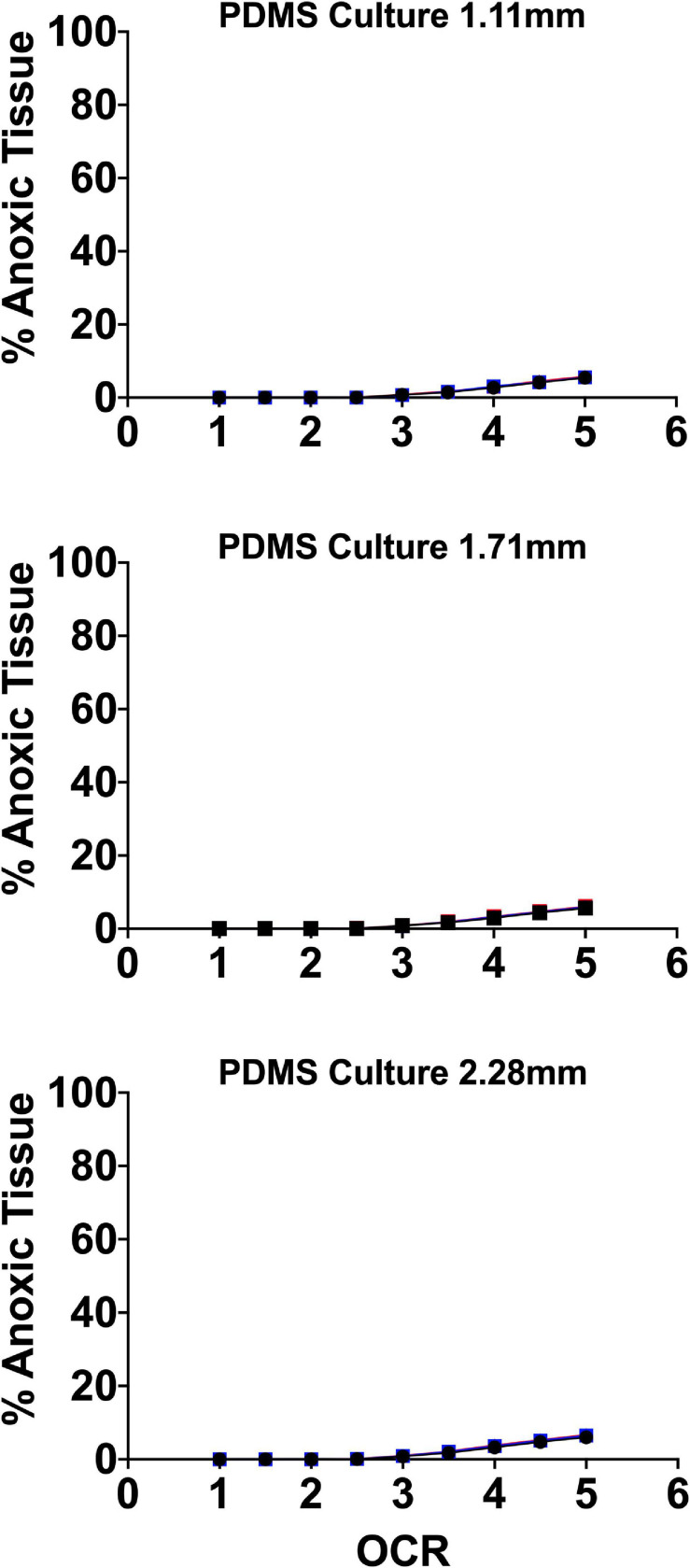
Our group developed a perfluorosilane-impregnated PDMS 275 μm membrane with a honeycomb support structure designed to maximize surface area for gas transport with support sufficient to prevent membrane damage. Given the high affinity of perfluoro-compounds for oxygen, the inclusion of perfluorosilane further improved oxygen mass transfer. Figure 7 shows FEM of the anoxic tissue volume percentage of endocrine clusters of representative size range and OCR cultured on the perfluorosilane/PDMS constructs. FEM was performed, as above, using three different seeding densities and three relevant culture medium heights.
FIGURE 7.
Anoxic tissue volume percentages of endocrine spheroids (representative size range and OCR) cultured on perfluorosilane/PDMS constructs. Anoxia is eliminated in all but the largest aggregate size at the highest oxygen consumption rates. FEM was performed using three seeding densities (2.76–5.05%) and three culture medium heights (1.11, 1.71, and 2.28 mm).
Of note, culture on these platforms all but eliminates anoxia in all spheroid geometries except at elevated OCR. In agreement with the earlier findings of Avgoustiniatos et al. (2008), there is virtually no effect from either seeding density or culture medium height at these settings typically utilized in culture on plastic, where both variables have a significant effect.
Recent advances using PDMS surfaces in the culture of endocrine spheroids include the development of printed microwells for controlled seeding of aggregates in a 96 and 384 well format. These devices have the added benefit of providing oxygen from an increased surface area surrounding the 3D clusters in the wells. These concave wells have been successfully used in the formation of pancreatic clusters from purified beta cells (Lee S.H. et al., 2018).
Gas-Permeable Platforms Improve Culture and Terminal Differentiation of Murine Pancreatic Buds
Studies performed in the laboratory of Clark Colton using culture of mouse embryonic stem cells on plastic examined the effect of external oxygen concentrations from 0 to 285 mmHg on proliferative capacity and differentiation either +/− the differentiation inhibiting factor, LIF. They observed that oxygen had minimal effect on undifferentiated cell growth and phenotype (+LIF) and posited that it was likely to have a more pronounced effect on cells undergoing differentiation, as we observed (Powers et al., 2008).
In another paper by the same group, they studied the effect of low oxygen culture on murine ES cells, again demonstrating the reduction of spontaneous differentiation but also the loss of pluripotent gene expression. Importantly, they noted that the oxygen level in the gas phase is often quite different from the oxygen level in the microenvironment of the cells and this is neglected in the vast majority of the literature, until recently, making interpretation of results difficult. This can be seen in the FEM models presented above for 2D and 3D culture. They emphasize the importance of tools like FEM to better understand culture conditions (Millman et al., 2009).
Our group examined the role of oxygen in pancreatic development using pancreatic buds from mouse embryoid bodies (Fraker et al., 2007). In our comparison of pancreatic buds cultured on plastic, both at standard (20%) and elevated concentration (35%) to buds cultured on the perfluoro/PDMS composites, the data supported our models. There was both improved proliferation and differentiation in the buds cultured on the perfluoro/PDMS, as well as absence of tissue hypoxia, in contrast with our immunohistochemical observations in samples cultured at either 20% and 35% incubator oxygen concentration. Gene and protein expression of important pancreatic/endocrine/β-cell developmental markers were significantly elevated in the buds cultured on the perfluoro/PDMS constructs as opposed to both normoxic (20%) and hyperoxic (35%) culture on plastic. Most notably, when compared to the in vivo endpoint of terminal differentiation in the pancreatic buds, e16.5, gene and protein expression of key pancreatic markers was non-significantly different between the perfluoro/PDMS culture and the in vivo counterparts, further confirming the importance of oxygenation in the final stages of pancreatic development, as hypothesized. Also, in examining the expression of pancreatic exocrine markers in this setting, oxygenation promoted endocrine over exocrine differentiation. It should be noted that the size of the buds cultured in higher oxygen levels reached 1 mm and there was no evidence of tissue hypoxia by immune staining indicating that their oxygen consumption rate was likely lower than that of conventional islets of Langerhans.
Too Much or Too Little: The Goldilocks Paradox of Oxygen Supply
The supply of oxygen to cultured cells/tissues is a delicate balance between anoxia/hypoxia (too little) and oxidant/radical formation from culture medium substrates (too much). While conventional culture occurs at pO2 of ∼142 mmHg, vascularized cells in the human body do not typically experience a pO2 greater than 100 mmHg and are supplied with sufficient oxygen to avoid sustained hypoxia due to short vascular diffusion distances. With 3D islets/endocrine spheroids, it becomes immediately clear from the above FEM why culture of these aggregates is difficult. Their high metabolic rate and large geometries make it difficult to maintain adequate oxygen supply and impossible to do so without non-physiological pO2 gradients across the path of oxygen transfer (surface to core). In vivo, islets are thought to experience a nearly uniform tissue pO2 of approximately 20–40 mmHg. Therefore, even culture at standard incubator pO2 results in apical endocrine spheroid surface exposure to supra-physiological levels and basal surface/core anoxia except in the smallest endocrine spheroid dimensions (50–100 μm). In gas permeable culture platforms in standard incubator settings (142 mmHg), the tissue exposure to supra-physiological levels is even greater.
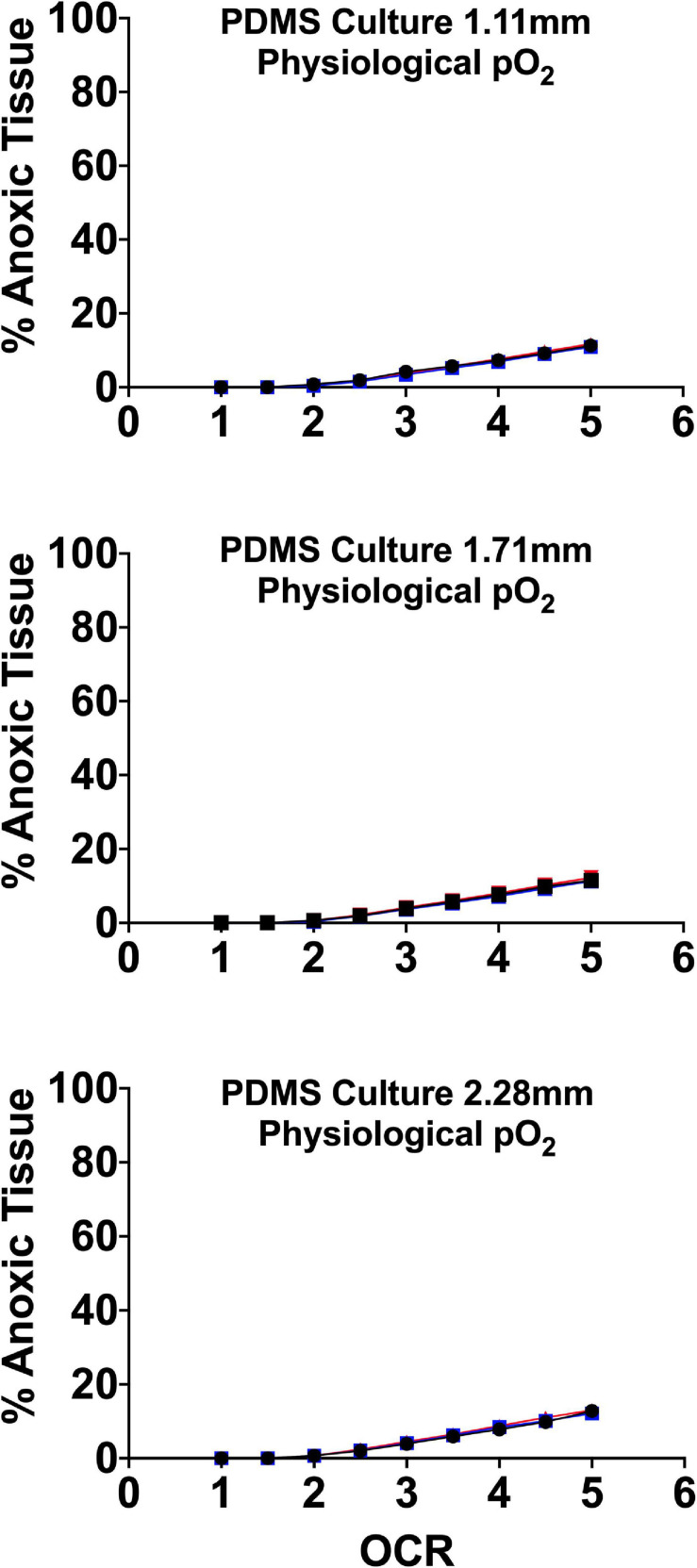
There is evidence in the literature that islets are uniquely susceptible to free-radical/oxidant damage due to glucose sensing suppression of superoxide dismutase and therefore prolonged exposure to elevated pO2 could be sub-optimal (Gille and Joenje, 1992; Kazzaz et al., 1999; Martens et al., 2005; Pi et al., 2007; Maddalena et al., 2017). For all these reasons, optimizing endocrine spheroid culture requires devices that allow for the maintenance of tissue specific oxygen profiles where core pO2 is (1) above the anoxic and functional cutoff noted by Avgoustiniatos and others and (2) below arterial concentrations of ∼100 mmHg (Papas et al., 2005; Buchwald, 2009, 2011). To that end, our group and others have examined the effect of physiologically relevant culture pO2 on endocrine spheroid function and viability utilizing gas permeable platforms demonstrating that culturing at more physiologically relevant pO2 improves in vitro and in vivo islet function and viability (Fraker et al., 2013). Culture incubator pO2 was set guided by FEM to target a maximum tissue volume maintained within a physiological range of 5–95 mmHg, minimizing “hyperoxia” and anoxia. Figure 8 details the FEM and the subsequent anoxic tissue volume percentage. The models used three seeding densities and culture medium heights, as in prior models, above.
FIGURE 8.
Anoxic tissue volume percentages of endocrine spheroids (representative size range and OCR) cultured on perfluorosilane/PDMS constructs in the presence of physiological pO2 (95 mmHg). Anoxia is minimal in all but the largest aggregate size at the highest oxygen consumption rates. FEM was performed using three seeding densities (2.76–5.05%) and three culture medium heights (1.11, 1.71, and 2.28 mm).
Gas-Permeable Platforms Improve Human Embryonic Stem (hES) Cell Specification Into Endocrine Fates
As shown in an increasing number of studies, oxygen in culture is recognized as a factor driving both stem cell proliferation and terminal differentiation into endocrine clusters. In 3D aggregates where oxygen gradients are prevalent, lower pO2 is associated with proliferative capacity and higher pO2 drives terminal differentiation through Notch and HIF-1. As shown from the FEM models, above, it is not feasible to successfully modulate oxygen supply to 3D tissues in plastic culture systems, so gas permeable platforms present a useful tool for the tight control of pO2 experienced by culture spheroids.
A paper by Powers et al. (2010) from 2010 described the historical disconnect between ambient incubator pO2, pO2gas, and the pO2 experienced by cells in culture, pO2cell, using FEM and mouse embryonic stem cell differentiation into cardiomyocytes on plastic and PDMS culture surfaces to demonstrate how critical cellular oxygen supply is to desired outcome. They used monolayer cultures on polystyrene and functionalized PDMS surfaces implementing FEM to target specific ranges of pO2cell to determine the effect of standard (142 mmHg), physiological (37 mmHg), and hypoxic (7 mmHg) culture on function and viability. Only on PDMS membranes, where pO2cell is equivalent to pO2gas due to the proximity of the gas supply at the basal surface, were functional cardiomyocytes observed. As reported in other cell culture studies on PDMS, including our own, they frequently observed spontaneous aggregation of single cells into larger spheroids. They noted that this resulted in the formation of pO2 gradients that had minimal effect on the tissue anoxia of the PDMS group but would have resulted in ∼80% anoxia in spheroids cultured on polystyrene at the highest pO2 of 142 mmHg. Unfortunately, the only functional assessment was the observation of spontaneous contraction of the cells, which was observed only on PDMS and polystyrene with a PDMS membrane. The largest number of spontaneously contracting cells were observed on the PDMS surface at a pO2gas of 36 mmHg. This demonstrates the importance of physio-normal pO2 (36 vs. 142 mmHg) in terminal differentiation.
During our work with an early group in the SC-β field, BetaLogics, we postulated that the use of gas permeable culture systems to tailor core tissue pO2 to that of the physiological niche of pancreatic endocrine tissue (∼20–40 mmHg core tissue pO2; 80–100 mmHg incubator pO2) would significantly improve terminal differentiation of hES spheroid progenitor clusters into endocrine fates (Cechin et al., 2014). As these clusters were typically in the size range of IEQ (50–400 μm), the culture on PDMS was designed to maintain maximal tissue volume percentage within a physiological range (∼2.7–100 mmHg). Introducing the PDMS culture systems at the last stage of standard differentiation protocols, after they were formed into spheroids, resulted in significantly improved differentiation into insulin and other endocrine hormone positive cells. Analysis by qRT-PCR showed significantly elevated pancreatic gene expression in hES clusters cultured in gas permeable systems relative to controls. In vitro production of insulin in response to glucose, improved engraftment and reversal of diabetes in vivo, were also observed. Notably, culture in these systems at physiological pO2 resulted in dramatically improved segregation of α and β cells, eliminating the challenge of single cell bi-hormonal expression that had plagued other protocols up to that point. This data again confirmed the role of oxygen in endocrine development and function. Given the low oxygen levels (relative to standard oxygen pO2) present in the in vivo niches of most cell types, it is not surprising that mechanisms such as proliferation, terminal differentiation, and pleiotropic function are increasingly being tied to bioenergetic shifts dependent on oxygen.
Conclusion and Future Directions
As 3D culture increasingly becomes a preferred mode of physio-mimetic culture, historical culture methods on plastics need to be re-evaluated. Particularly related to tissue oxygenation, the steep gradients that develop from apical to basal surface, both in the culture milieu and in the 3D organoids, lead to hypoxic and anoxic regions, inefficient nutrient metabolism and potentially, shifts in viability, function and gene expression that may deviate in comparison to the same tissue in vivo. There is a critical need and a recent surge in device/method research to improve metabolite/nutrient delivery and waste removal in 3D cell cultures. With the emergent data coming from this area of research, it is clear that other variables besides oxygen, such as mechanical forces, growth factors and supportive matrices also play an important role in the culture of organoid systems but metabolic gas exchange has been one of the most challenging obstacles in 3D cell/spheroid research. Given the growing number of therapeutic applications for 3D spheroids, there is a critical need to revisit expansion and differentiation protocols in order to optimize them for their desired end product and application. Much like personalized medicine, individual cell/tissue types have different metabolic and physiological characteristics that are difficult to recapitulate in general culture practices. Like oxygen in the data presented in this work and others, tailoring environmental factors to address the physiological demands of the cultured spheroids can result in a much better in vitro model/approximation of the in vivo organoid counterpart.
Related to the clinical application of endocrine spheroids, there is much ongoing work to improve/maintain function and viability prior to transplant in an attempt to minimize immune response and accelerate engraftment. Strategies focused on revascularization and co-cultures with supportive mesenchymal stem cells (MSCs) and endothelial precursors (ECs) are making great strides in minimizing post-transplant loss and time to reversal of hyperglycemia. In terms of culture, there is still work to be done to fully understand the role of oxygen in differentiation and function/viability, in vitro. Building on past work, minimizing tissue anoxia has a clear effect on transplant immunogenicity and function. With that in mind, combinatorial approaches that slow cellular metabolism (OCR) and maintain tight control of pO2cell could move endocrine spheroid research one-step closer to clinical impact. This could be achieved, as an example, by the use of low temperature culture on gas permeable systems.
Clearly, oxygen is only one of many culture metabolites that plays a role in the viability and function of 2D and 3D cultures. It could be argued that it is the most important given that it has an immediate effect on cell metabolism and the lack of oxygen can rapidly progress to cellular dysfunction and death. A lack of oxygen in cell systems also results in a build-up of toxic waste products that while largely unnoticeable in the larger culture milieu, might have a pronounced detrimental effect in the cell microenvironment. For example, a shift to anaerobic metabolism and glycolysis increases production of acidic byproducts to compensate for loss of the efficiency of energy production using aerobic respiration. This, in turn, can affect both cellular and environmental pH leading to damage beyond the immediate apoptosis/necrosis caused by anoxia. While not the only participant in the viability of cultured cell/tissue biology, oxygen has an undeniably important role in culture approximation of in vivo cell/tissue counterparts.
Author Contributions
CF and GG contributed to the conception of the manuscript. CF, GG, HT, and JD-B reviewed the manuscript and wrote the original draft. GG, HT, and JD-B contributed to the manuscript revision and editing. All authors read and approved the submitted version.
Conflict of Interest
CF and JD-B have intellectual property/potential financial interests related to the gas permeable culture devices (Oxygen Sandwich) described in the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the ongoing support of the Diabetes Research Institute Foundation and donors who generously fund the cure-motivated research of the DRI.
Footnotes
Funding. Research reported in this manuscript was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK116875 and the Diabetes Research Institute Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Acker H., Carlsson J., Mueller-Klieser W., Sutherland R. M. (1987). Comparative pO2 measurements in cell spheroids cultured with different techniques. Br. J. Cancer 56 325–327. 10.1038/bjc.1987.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anada T., Fukuda J., Sai Y., Suzuki O. (2012). An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 33 8430–8441. 10.1016/j.biomaterials.2012.08.040 [DOI] [PubMed] [Google Scholar]
- Avgoustiniatos E. S., Hering B. J., Rozak P. R., Wilson J. R., Tempelman L. A., Balamurugan A. N., et al. (2008). Commercially available gas-permeable cell culture bags may not prevent anoxia in cultured or shipped islets. Transplant. Proc. 40 395–400. 10.1016/j.transproceed.2008.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader A., Knop E., Kern A., Boker K., Fruhauf N., Crome O., et al. (1996). 3-D coculture of hepatic sinusoidal cells with primary hepatocytes-design of an organotypical model. Exp. Cell Res. 226 223–233. 10.1006/excr.1996.0222 [DOI] [PubMed] [Google Scholar]
- Bajgain P., Mucharla R., Wilson J., Welch D., Anurathapan U., Liang B., et al. (2014). Optimizing the production of suspension cells using the G-Rex “M” series. Mol. Ther. Methods Clin. Dev. 1:14015. 10.1038/mtm.2014.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigas A., Martin D. I., Bernstein I. D. (1995). Generation of hematopoietic colony-forming cells from embryonic stem cells: synergy between a soluble factor from NIH-3T3 cells and hematopoietic growth factors. Blood 85 3127–3133. 10.1182/blood.v85.11.3127.bloodjournal85113127 [DOI] [PubMed] [Google Scholar]
- Bowen K. M., Andrus L., Lafferty K. J. (1980). Successful allotransplantation of mouse pancreatic islets to nonimmunosuppressed recipients. Diabetes 29(Suppl. 1) 98–104. 10.2337/diab.29.1.s98 [DOI] [PubMed] [Google Scholar]
- Britt L. D., Stojeba P. C., Scharp C. R., Greider M. H., Scharp D. W. (1981). Neonatal pig pseudo-islets. A product of selective aggregation. Diabetes 30 580–583. 10.2337/diab.30.7.580 [DOI] [PubMed] [Google Scholar]
- Bruland O., Fodstad O., Pihl A. (1985). The use of multicellular spheroids in establishing human sarcoma cell lines in vitro. Int. J. Cancer 35 793–798. 10.1002/ijc.2910350616 [DOI] [PubMed] [Google Scholar]
- Buchwald P. (2009). FEM-based oxygen consumption. Theor. Biol. Med. Model. 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald P. (2011). A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor. Biol. Med. Model. 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald P., Wang X., Khan A., Bernal A., Fraker C., Inverardi L., et al. (2009). Quantitative assessment of islet cell products: estimating the accuracy of the existing protocol and accounting for islet size distribution. Cell Transplant. 18 1223–1235. 10.3727/096368909x476968 [DOI] [PubMed] [Google Scholar]
- Buitrago A., Gylfe E., Henriksson C., Pertoft H. (1977). Rapid isolation of pancreatic islets from collagenase digested pancreas by sedimentation through Percol at unit gravity. Biochem. Biophys. Res. Commun. 79 823–828. 10.1016/0006-291x(77)91185-8 [DOI] [PubMed] [Google Scholar]
- Cao R., Avgoustiniatos E., Papas K., de Vos P., Lakey J. R. T. (2020). Mathematical predictions of oxygen availability in micro- and macro-encapsulated human and porcine pancreatic islets. J. Biomed. Mater. Res. B Appl. Biomater. 108 343–352. 10.1002/jbm.b.34393 [DOI] [PubMed] [Google Scholar]
- Carlsson J., Stalnacke C. G., Acker H., Haji-Karim M., Nilsson S., Larsson B. (1979). The influence of oxygen on viability and proliferation in cellular spheroids. Int. J. Radiat. Oncol. Biol. Phys. 5 2011–2020. 10.1016/0360-3016(79)90953-2 [DOI] [PubMed] [Google Scholar]
- Cechin S., Alvarez-Cubela S., Giraldo J. A., Molano R. D., Villate S., Ricordi C., et al. (2014). Influence of in vitro and in vivo oxygen modulation on beta cell differentiation from human embryonic stem cells. Stem Cells Transl. Med. 3 277–289. 10.5966/sctm.2013-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H. P., Ocrant I., Talmage D. W. (1979). The effects of different conditions of organ culture on the survival of the mouse pancreas. Diabetes 28 990–993. 10.2337/diabetes.28.11.990 [DOI] [PubMed] [Google Scholar]
- Colen K. L., Crisera C. A., Rose M. I., Connelly P. R., Longaker M. T., Gittes G. K. (1999). Vascular development in the mouse embryonic pancreas and lung. J. Pediatr. Surg. 34 781–785. 10.1016/s0022-3468(99)90373-1 [DOI] [PubMed] [Google Scholar]
- Davis J. C., Alves T. C., Helman A., Chen J. C., Kenty J. H., Cardone R. L., et al. (2020). Glucose response by stem cell-derived beta cells in vitro is inhibited by a bottleneck in glycolysis. Cell Rep. 31:107623. 10.1016/j.celrep.2020.107623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor L., Merovci I., Baetens S., Verstraeten J., Kowalska P., Krysko D. V., et al. (2018). High-throughput fabrication of vascularized spheroids for bioprinting. Biofabrication 10:035009. 10.1088/1758-5090/aac7e6 [DOI] [PubMed] [Google Scholar]
- Deshpande R. R., Heinzle E. (2009). Online monitoring of oxygen in spinner flasks. Biotechnol. Lett. 31 665–669. 10.1007/s10529-009-9919-2 [DOI] [PubMed] [Google Scholar]
- Dionne K. E., Cain B. M., Li R. H., Bell W. J., Doherty E. J., Rein D. H., et al. (1996). Transport characterization of membranes for immunoisolation. Biomaterials 17 257–266. 10.1016/0142-9612(96)85563-3 [DOI] [PubMed] [Google Scholar]
- Dionne K. E., Colton C. K., Yarmush M. L. (1989). Effect of oxygen on isolated pancreatic tissue. ASAIO Trans. 35 739–741. 10.1097/00002216-198907000-00185 [DOI] [PubMed] [Google Scholar]
- Dionne K. E., Colton C. K., Yarmush M. L. (1991). A microperifusion system with environmental control for studying insulin secretion by pancreatic tissue. Biotechnol. Prog. 7 359–368. 10.1021/bp00010a011 [DOI] [PubMed] [Google Scholar]
- Dionne K. E., Colton C. K., Yarmush M. L. (1993). Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42 12–21. 10.2337/diab.42.1.12 [DOI] [PubMed] [Google Scholar]
- Fehrer C., Brunauer R., Laschober G., Unterluggauer H., Reitinger S., Kloss F., et al. (2007). Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6 745–757. 10.1111/j.1474-9726.2007.00336.x [DOI] [PubMed] [Google Scholar]
- Fraker C. A., Alvarez S., Papadopoulos P., Giraldo J., Gu W., Ricordi C., et al. (2007). Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem Cells 25 3155–3164. 10.1634/stemcells.2007-0445 [DOI] [PubMed] [Google Scholar]
- Fraker C. A., Cechin S., Alvarez-Cubela S., Echeverri F., Bernal A., Poo R., et al. (2013). A physiological pattern of oxygenation using perfluorocarbon-based culture devices maximizes pancreatic islet viability and enhances beta-cell function. Cell Transplant. 22 1723–1733. 10.3727/096368912x657873 [DOI] [PubMed] [Google Scholar]
- Fraker C. A., Ricordi C., Inverardi L., Dominguez-Bendala J. (2009). Oxygen: a master regulator of pancreatic development? Biol. Cell 101 431–440. 10.1042/bc20080178 [DOI] [PubMed] [Google Scholar]
- Freyer J. P., Schor P. L., Jarrett K. A., Neeman M., Sillerud L. O. (1991). Cellular energetics measured by phosphorous nuclear magnetic resonance spectroscopy are not correlated with chronic nutrient deficiency in multicellular tumor spheroids. Cancer Res. 51 3831–3837. [PubMed] [Google Scholar]
- Gargotti M., Lopez-Gonzalez U., Byrne H. J., Casey A. (2018). Comparative studies of cellular viability levels on 2D and 3D in vitro culture matrices. Cytotechnology 70 261–273. 10.1007/s10616-017-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M., Fandrey J., Bichet S., Wartenberg M., Marti H. H., Bauer C., et al. (1996). Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 93 2867–2872. 10.1073/pnas.93.7.2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille J. J., Joenje H. (1992). Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat. Res. 275 405–414. 10.1016/0921-8734(92)90043-o [DOI] [PubMed] [Google Scholar]
- Grayson W. L., Zhao F., Izadpanah R., Bunnell B., Ma T. (2006). Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J. Cell. Physiol. 207 331–339. 10.1002/jcp.20571 [DOI] [PubMed] [Google Scholar]
- Groebe K. (1996). Practical applications of models of oxygen supply, diffusion, and consumption: past, perspectives, and problems. Adv. Exp. Med. Biol. 388 161–175. 10.1007/978-1-4613-0333-6_20 [DOI] [PubMed] [Google Scholar]
- Groebe K., Mueller-Klieser W. (1996). On the relation between size of necrosis and diameter of tumor spheroids. Int. J. Radiat. Oncol. Biol. Phys. 34 395–401. 10.1016/0360-3016(95)02065-9 [DOI] [PubMed] [Google Scholar]
- Harris A. K., Jr. (1984). Tissue culture cells on deformable substrata: biomechanical implications. J. Biomech. Eng. 106 19–24. 10.1115/1.3138449 [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. (1986). Induction characteristics of oxygen regulated proteins. Int. J. Radiat. Oncol. Biol. Phys. 12 1287–1290. 10.1016/0360-3016(86)90155-0 [DOI] [PubMed] [Google Scholar]
- Helman A., Melton D. A. (2021). A stem cell approach to cure type 1 diabetes. Cold Spring Harb. Perspect. Biol. 13:a035741. 10.1101/cshperspect.a035741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvatin S., O’Donnell C. W., Deng F., Millman J. R., Pagliuca F. W., DiIorio P., et al. (2014). Differentiated human stem cells resemble fetal, not adult, beta cells. Proc. Natl. Acad. Sci. U.S.A. 111 3038–3043. 10.1073/pnas.1400709111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inch W. R., McCredie J. A., Sutherland R. M. (1970). Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth 34 271–282. [PubMed] [Google Scholar]
- Jokinen M., Pittois K., van den Akker S., Gutschoven I., Assmuth T., Metz T., Lehtila H., et al. (2020). Multiphase matrix of silica, culture medium and air for 3D mammalian cell culture. Cytotechnology 72 271–282. 10.1007/s10616-020-00376-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Y., Lee J., Choi S., Yang J. H., Sander M., Chung S., et al. (2019). In vivo-mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci. Adv. 5:eaax4520. 10.1126/sciadv.aax4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazzaz J. A., Horowitz S., Li Y., Mantell L. L. (1999). Hyperoxia in cell culture. A non-apoptotic programmed cell death. Ann. N. Y. Acad. Sci. 887 164–170. 10.1111/j.1749-6632.1999.tb07930.x [DOI] [PubMed] [Google Scholar]
- Kitzmann J. P., Pepper A. R., Gala-Lopez B., Pawlick R., Kin T., O’Gorman D., et al. (2014). Human islet viability and function is maintained during high-density shipment in silicone rubber membrane vessels. Transplant. Proc. 46 1989–1991. 10.1016/j.transproceed.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely M. H., Reneau D. D., Jr., Bruley D. F. (1969). The development and use of equations for predicting the limits of the rates of oxygen supply to the cells of living tissues and organs. A contribution to the biophysics of health and disease. Angiology 20(Suppl.) 1–56. 10.1177/0003319769020011s01 [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Finke E. H., Janney C. G., Davie J. M. (1982). Prolongation of islet xenograft survival by in vitro culture of rat megaislets in 95% O2. Transplantation 33 588–592. 10.1097/00007890-198206000-00004 [DOI] [PubMed] [Google Scholar]
- Lee G., Jun Y., Jang H., Yoon J., Lee J., Hong M., et al. (2018). Enhanced oxygen permeability in membrane-bottomed concave microwells for the formation of pancreatic islet spheroids. Acta Biomater. 65 185–196. 10.1016/j.actbio.2017.10.045 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Hong S., Song J., Cho B., Han E. J., Kondapavulur S., et al. (2018). Microphysiological analysis platform of pancreatic islet beta-cell spheroids. Adv. Healthc. Mater. 7:1701111. 10.1002/adhm.201701111 [DOI] [PubMed] [Google Scholar]
- Lenguito G., Chaimov D., Weitz J. R., Rodriguez-Diaz R., Rawal S. A., Tamayo-Garcia A., et al. (2017). Resealable, optically accessible, PDMS-free fluidic platform for ex vivo interrogation of pancreatic islets. Lab Chip 17 772–781. 10.1039/c6lc01504b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. P., Colburn S. M., Beck D. J. (1992). A simplified method for the culturing of primary adult rat and human hepatocytes as multicellular spheroids. In Vitro Cell. Dev. Biol. 28A 673–677. 10.1007/bf02631045 [DOI] [PubMed] [Google Scholar]
- Lock L. T., Laychock S. G., Tzanakakis E. S. (2011). Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J. Biotechnol. 151 278–286. 10.1016/j.jbiotec.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Luca G., Calvitti M., Nastruzzi C., Macchiarulo G., Becchetti E., Neri L. M., et al. (2006). Effects of simulated microgravity on the morphology and function of neonatal porcine cell clusters cultured with and without Sertoli cells. Cell Transplant. 15 55–65. 10.3727/000000006783982223 [DOI] [PubMed] [Google Scholar]
- Maddalena L. A., Selim S. M., Fonseca J., Messner H., McGowan S., Stuart J. A. (2017). Hydrogen peroxide production is affected by oxygen levels in mammalian cell culture. Biochem. Biophys. Res. Commun. 493 246–251. 10.1016/j.bbrc.2017.09.037 [DOI] [PubMed] [Google Scholar]
- Martens G., Cai Y., Hinke S., Stange G., Van de Casteele M., Pipeleers D. (2005). Nutrient sensing in pancreatic beta cells suppresses mitochondrial superoxide generation and its contribution to apoptosis. Biochem. Soc. Trans. 33(Pt 1) 300–301. 10.1042/bst0330300 [DOI] [PubMed] [Google Scholar]
- Matta S. G., Wobken J. D., Williams F. G., Bauer G. E. (1994). Pancreatic islet cell reaggregation systems: efficiency of cell reassociation and endocrine cell topography of rat islet-like aggregates. Pancreas 9 439–449. 10.1097/00006676-199407000-00005 [DOI] [PubMed] [Google Scholar]
- McGrath K. E., Koniski A. D., Malik J., Palis J. (2003). Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101 1669–1676. 10.1182/blood-2002-08-2531 [DOI] [PubMed] [Google Scholar]
- McMillan K. S., Boyd M., Zagnoni M. (2016). Transitioning from multi-phase to single-phase microfluidics for long-term culture and treatment of multicellular spheroids. Lab Chip 16 3548–3557. 10.1039/c6lc00884d [DOI] [PubMed] [Google Scholar]
- Millman J. R., Tan J. H., Colton C. K. (2009). The effects of low oxygen on self-renewal and differentiation of embryonic stem cells. Curr. Opin. Organ. Transplant. 14 694–700. 10.1097/mot.0b013e3283329d53 [DOI] [PubMed] [Google Scholar]
- Millman J. R., Xie C., Van Dervort A., Gurtler M., Pagliuca F. W., Melton D. A. (2016). Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat. Commun. 7:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz W., Meier F., Stroka D. M., Giuliani M., Kugelmeier P., Nett P. C., et al. (2002). Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB J. 16 745–747. 10.1096/fj.01-0403fje [DOI] [PubMed] [Google Scholar]
- Moscona A. (1961). Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp. Cell Res. 22 455–475. 10.1016/0014-4827(61)90122-7 [DOI] [PubMed] [Google Scholar]
- Moscona A. A. (1961). How cells associate. Sci. Am. 205 142–162. 10.1038/scientificamerican0961-142 [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W. (1984). Microelectrode measurement of oxygen tension distributions in multicellular spheroids cultured in spinner flasks. Recent Results Cancer Res. 95 134–149. 10.1007/978-3-642-82340-4_8 [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W., Freyer J. P., Sutherland R. M. (1986). Influence of glucose and oxygen supply conditions on the oxygenation of multicellular spheroids. Br. J. Cancer 53 345–353. 10.1038/bjc.1986.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Klieser W. F., Sutherland R. M. (1982). Oxygen tensions in multicell spheroids of two cell lines. Br. J. Cancer 45 256–264. 10.1038/bjc.1982.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Klieser W. F., Sutherland R. M. (1984). Oxygen consumption and oxygen diffusion properties of multicellular spheroids from two different cell lines. Adv. Exp. Med. Biol. 180 311–321. 10.1007/978-1-4684-4895-5_30 [DOI] [PubMed] [Google Scholar]
- Murphy K. C., Hung B. P., Browne-Bourne S., Zhou D., Yeung J., Genetos D. C., et al. (2017). Measurement of oxygen tension within mesenchymal stem cell spheroids. J. R. Soc. Interface 14:20160851. 10.1098/rsif.2016.0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. E., Paget M. B., Downing R. (2005). Preservation of glucose responsiveness in human islets maintained in a rotational cell culture system. Mol. Cell. Endocrinol. 238 39–49. [DOI] [PubMed] [Google Scholar]
- Nourmohammadzadeh M., Xing Y., Lee J. W., Bochenek M. A., Mendoza-Elias J. E., McGarrigle J. J., et al. (2016). A microfluidic. Lab Chip 16 1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono J., Lacy P. E., Michael H. E., Greider M. H. (1979). Studies of the functional and morphologic status of islets maintained at 24 C for four weeks in vitro. Am. J. Pathol. 97 489–503. [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F. W., Millman J. R., Gurtler M., Segel M., Van Dervort A., Ryu J. H., et al. (2014). Generation of functional human pancreatic beta cells in vitro. Cell 159 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas K. K., Avgoustiniatos E. S., Suszynski T. M. (2016). Effect of oxygen supply on the size of implantable islet-containing encapsulation devices. Panminerva Med. 58 72–77. [PubMed] [Google Scholar]
- Papas K. K., Avgoustiniatos E. S., Tempelman L. A., Weir G. C., Colton C. K., Pisania A., et al. (2005). High-density culture of human islets on top of silicone rubber membranes. Transplant. Proc. 37 3412–3414. [DOI] [PubMed] [Google Scholar]
- Papas K. K., Long R. C., Jr., Constantinidis I., Sambanis A. (2000). Effects of short-term hypoxia on a transformed cell-based bioartificial pancreatic construct. Cell Transplant. 9 415–422. [DOI] [PubMed] [Google Scholar]
- Peterson Q. P., Veres A., Chen L., Slama M. Q., Kenty J. H. R., Hassoun S., et al. (2020). A method for the generation of human stem cell-derived alpha cells. Nat. Commun. 11:2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J., Bai Y., Zhang Q., Wong V., Floering L. M., Daniel K., et al. (2007). Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56 1783–1791. [DOI] [PubMed] [Google Scholar]
- Powers D. E., Millman J. R., Bonner-Weir S., Rappel M. J., Colton C. K. (2010). Accurate control of oxygen level in cells during culture on silicone rubber membranes with application to stem cell differentiation. Biotechnol. Prog. 26 805–818. [DOI] [PubMed] [Google Scholar]
- Powers D. E., Millman J. R., Huang R. B., Colton C. K. (2008). Effects of oxygen on mouse embryonic stem cell growth, phenotype retention, and cellular energetics. Biotechnol. Bioeng. 101 241–254. [DOI] [PubMed] [Google Scholar]
- Qadir M. M. F., Alvarez-Cubela S., Weitz J., Panzer J. K., Klein D., Moreno-Hernandez Y., et al. (2020). Dominguez-Bendala, long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nat. Commun. 11:3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosdy M., Grisoni B., Clauss L. C. (1991). Proliferation of normal human keratinocytes on silicone substrates. Biomaterials 12 511–517. [DOI] [PubMed] [Google Scholar]
- Rutzky L., Kloc M., Bilinski S., Phan T., Zhang H., Stepkowski S. M., et al. (2001). Microgravity culture conditions decrease immunogenicity but maintain excellent morphology of pancreatic islets. Transplant. Proc. 33:388. [DOI] [PubMed] [Google Scholar]
- Rutzky L. P., Bilinski S., Kloc M., Phan T., Zhang H., Katz S. M., et al. (2002). Microgravity culture condition reduces immunogenicity and improves function of pancreatic islets1. Transplantation 74 13–21. [DOI] [PubMed] [Google Scholar]
- Saini S., Wick T. M. (2004). Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 10 825–832. [DOI] [PubMed] [Google Scholar]
- Sankar K. S., Green B. J., Crocker A. R., Verity J. E., Altamentova S. M., Rocheleau J. V. (2011). Culturing pancreatic islets in microfluidic flow enhances morphology of the associated endothelial cells. PLoS One 6:e24904. 10.1371/journal.pone.0024904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharp D. W., Merrell R. C., Feldman S. D., Downing R., Lacy P. E., Ballinger W. F. (1978). The use of gyrotation culture for the preservation of isolated islets. Surg. Forum 29 100–102. [PubMed] [Google Scholar]
- Schmied B. M., Ulrich A., Matsuzaki H., Ding X., Ricordi C., Moyer M. P., et al. (2000). Maintenance of human islets in long-term culture. Differentiation 66 173–180. [DOI] [PubMed] [Google Scholar]
- Sharma V., Hunckler M., Ramasubramanian M. K., Opara E. C., Katuri K. C. (2017). Microfluidic approach to cell microencapsulation. Methods Mol. Biol. 1479 71–76. [DOI] [PubMed] [Google Scholar]
- Shenkman R. M., Godoy-Silva R., Papas K. K., Chalmers J. J. (2009). Effects of energy dissipation rate on islets of Langerhans: implications for isolation and transplantation. Biotechnol. Bioeng. 103 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. N., Green B. J., Altamentova S. M., Rocheleau J. V. (2013). A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab Chip 13 4374–4384. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Keith B. (2008). The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 9 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. (1999). Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology 30 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi R., Kumar A., Lopez G. P., Stephanopoulos G. N., Wang D. I., Whitesides G. M., et al. (1994). Engineering cell shape and function. Science 264 696–698. [DOI] [PubMed] [Google Scholar]
- Suszynski T. M., Avgoustiniatos E. S., Papas K. K. (2014a). Intraportal islet oxygenation. J. Diabetes Sci. Technol. 8 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszynski T. M., Avgoustiniatos E. S., Papas K. K. (2016). Oxygenation of the intraportally transplanted pancreatic islet. J. Diabetes Res. 2016:7625947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszynski T. M., Wilhelm J. J., Radosevich D. M., Balamurugan A. N., Sutherland D. E., Beilman G. J., et al. (2014b). Islet size index as a predictor of outcomes in clinical islet autotransplantation. Transplantation 97 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin B. W., Leeper-Woodford S. K., Hashemi B. B., Smith S. M., Sams C. F. (2001). Altered TNF-alpha, glucose, insulin, and amino acids in islets of Langerhans cultured in a microgravity model system. Am. J. Physiol. Endocrinol. Metab. 280 E92–E102. [DOI] [PubMed] [Google Scholar]
- Tsai A. C., Liu Y., Yuan X., Chella R., Ma T. (2017). Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnol. J. 12:1600448. [DOI] [PubMed] [Google Scholar]
- Tuncay O. C., Ho D., Barker M. K. (1994). Oxygen tension regulates osteoblast function. Am. J. Orthod. Dentofacial Orthop. 105 457–463. [DOI] [PubMed] [Google Scholar]
- Uroic D. S., Baudouin G., Ferguson L. A., Docherty H. M., Vallier L., Docherty K. (2010). A factor(s) secreted from MIN-6 beta-cells stimulates differentiation of definitive endoderm enriched embryonic stem cells towards a pancreatic lineage. Mol. Cell. Endocrinol. 328 80–86. [DOI] [PubMed] [Google Scholar]
- Vegas A. J., Veiseh O., Gurtler M., Millman J. R., Pagliuca F. W., Bader A. R., et al. (2016). Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescio R. A., Redfern C. H., Nelson T. J., Ugoretz S., Stern P. H., Hoffman R. M. (1987). In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc. Natl. Acad. Sci. U.S.A. 84 5029–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B. A., Venkataraman S., Buettner G. R. (2011). The rate of oxygen utilization by cells. Free Radic. Biol. Med. 51 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Deen W. M. (2003). Nitric oxide delivery system for cell culture studies. Ann. Biomed. Eng. 31 65–79. [DOI] [PubMed] [Google Scholar]
- Wang D. W., Fermor B., Gimble J. M., Awad H. A., Guilak F. (2005). Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J. Cell. Physiol. 204 184–191. [DOI] [PubMed] [Google Scholar]
- Yoshihara E., O’Connor C., Gasser E., Wei Z., Oh T. G., Tseng T. W., et al. (2020). Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. (2006). “The permeability characteristics of silicone rubber,” in Proceedings of the Society for the Advancement of Material and Process Engineering Fall Technical Conference, Dallas, TX. [Google Scholar]