Abstract
Objectives
Despite reports highlighting citrate association with different diseases, serum citrate is scarcely used for diagnosis. Existing methods to quantify citrate are limited by their complexity and practicality of implementation. A simple and rapid NMR-based method to measure circulating citrate is described here, and its analytical performance evaluated.
Design
and Methods: Citrate was quantified from NMR spectra using a non-negative linear least squares deconvolution algorithm. The analytical characteristics of the assay were evaluated using CLSI guidelines. To determine if the assay has adequate sensitivity to measure clinically relevant concentrations of citrate, the assay was used to quantify citrate in apparently healthy adults (n = 553), and in the general population (n = 133,576).
Results
The LOQ for the assay was determined to be 1.48 mg/dL. Linearity was demonstrated over a wide range of concentrations (1.40–4.46 mg/dL). Coefficients of variation (%CV) for intra- and inter-assay precision ranged from 5.8–9.3 and 5.2–9.6%, respectively. Substances tested did not elicit interference with assay results. Specimen type comparison revealed <1% bias between serum and plasma samples, except for heparin plasma (3% bias). Stability was demonstrated up to 8 days at room temperature and longer at lower temperatures. In a cohort of apparently healthy adults, the reference interval was <1.48–2.97 mg/dL. Slightly higher values were observed in the general population.
Conclusions
The newly developed NMR-based assay exhibits analytical characteristics that allow the accurate quantification of clinically relevant citrate concentrations. The assay provides a simple and fast means to analyze samples for research and clinical studies.
Keywords: Citrate, Mortality, Nuclear magnetic resonance spectroscopy
Abbreviations: 1D, one dimensional; CLSI, Clinical and Laboratory Standards Institute; CV, coefficient of variation; 1H, proton; LOB, limit of blank; LOD, limit of detection; LOQ, limit of quantitation; NMR, Nuclear magnetic resonance spectroscopy; MS, Mass Spectrometry; NAFLD, non-alcoholic fatty liver disease
1. Introduction
In mammalian cells, citrate is an important substrate in intermediary metabolism because it is a key regulator of energy production by inducing/inhibiting enzymes in glycolysis, gluconeogenesis, fatty acid synthesis and tricarboxylic acid (TCA) cycle [1]. Recent studies show that citrate is involved in other biological processes such as inflammation, cancer, insulin secretion, acetylation of histones, neurological development and hydroxylglutaric aciduria [2], indicating that it has functions beyond energy regulation. Furthermore, it is not surprising that citrate associations with glaucoma [3], non-alcoholic fatty liver disease (NAFLD) [4], bone disease [5,6] and mortality [7,8] have been observed. Thus, monitoring circulating citrate could potentially be a diagnostic tool. While at present, urinary citrate is commonly used as a risk factor in kidney stone formation, serum/plasma citrate is scarcely utilized for disease diagnosis or prognosis.
Historically, citrate in serum was measured using colorimetric techniques and involved tedious sample preparation [9,10], even with commercially available kits [10], which could limit testing throughput. Other techniques that are increasingly being used in the clinical laboratory such as mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy [11], can also quantify citrate in biological samples. Recently, mass spectrometry (MS)-based methodologies to measure circulating citrate have been reported [12,13]. These methods involve removal of serum/plasma proteins by precipitation prior to sample analysis and throughput of several minutes (sample preparation plus run time) per sample. NMR spectroscopy has been shown to provide an automated, high-throughput platform to interrogate serum/plasma samples with minimal sample preparation (i.e., only mixing phosphate buffer with serum/plasma) [[14], [15], [16], [17], [18]]. Here, we report a simple and rapid (1.5 min per sample) NMR-based method to quantify circulating citrate, and extensively validate its analytical performance.
2. Methods
2.1. Buffer and specimens
The aqueous phosphate buffer was composed of Na2HPO4 and CaEDTA (Sigma-Aldrich, St. Louis, MO) at pH 7.4. De-identified residual clinical serum samples were pooled at Labcorp (Morrisville, NC) for analytical validation studies. All volunteers signed informed consent forms and procedures were carried out in accordance with the Declaration of Helsinki and cleared by a local Institutional Review Board. Specimens drawn in Greiner tubes (Part # 456293P) were allowed to clot (30 min) in an upright position and centrifuged (3000 rpm, 10–15 min) immediately after clotting. Samples collected into plain red-top tubes and BD Gel Barrier serum tube were held upright (red-top tubes for 45 min; BD Gel Barrier tubes for 30 min) at room temperature to clot and were promptly centrifuged according to manufacturer’s directions. Ethylenediaminetetraacetic acid (EDTA)- and sodium heparin tubes specimens were processed per manufacturer’s instructions.
2.2. Sample preparation and NMR data collection
Sample preparation (i.e., 1:1 (v/v) dilution of serum or plasma with phosphate buffer) was performed automatically on the Vantera® Clinical Analyzer as previously described for the NMR LipoProfile® test [17,18]. One-dimensional 1H NMR spectra were collected on a 400 MHz spectrometers at 47 °C. WET was used to suppress the water signal. The total acquisition time for each spectrum was 48 s. Spectral acquisition and processing parameters are described in detail elsewhere [17,18]. The NMR instruments are calibrated using 15 mM trimethyl acetic acid as a calibrator and reference standard to verify instrument performance on a daily basis. This is followed by the analysis of two levels of serum control materials to verify assay performance.
2.3. Quantification of citrate by peak deconvolution
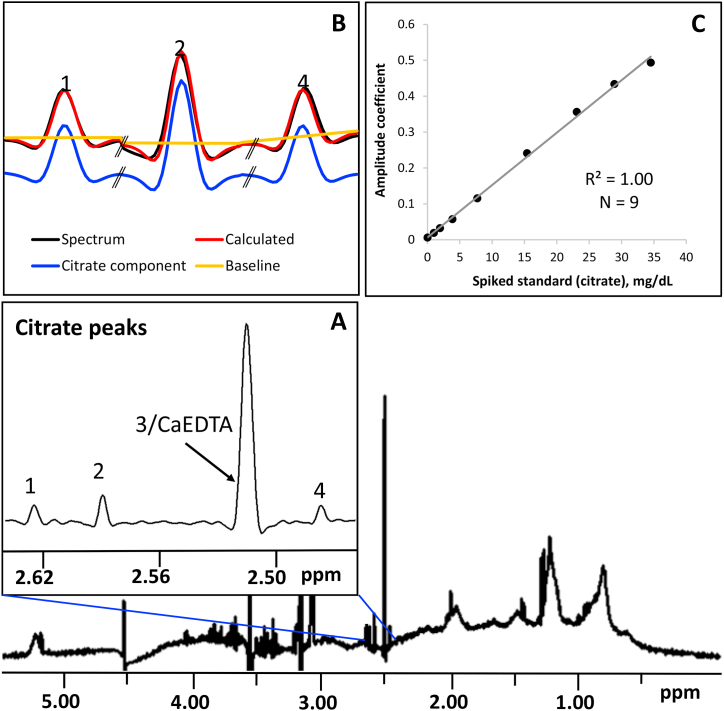
A restricted region of the collected spectrum, where the four citrate resonances appear, was used for quantification (Fig. 1). Due to overlap between one of these resonance peaks with that of CaEDTA, only three were used for quantification. A non-negative linear least squares deconvolution algorithm using QR decomposition [19] was implemented to resolve each citrate peak into its constituent peak components and baseline. A spectrum for the pure analyte collected under the same experimental conditions described above was used as citrate peak component while the baseline was modeled using a linear function. After deconvolution, the calculated amplitude coefficients for each peak were added and converted to concentration units by multiplying their sum with a conversion factor that was obtained by relating the calculated coefficients to the amount of analyte spiked at various known concentrations into dialyzed serum. In brief, dialyzed serum spiked with known amounts of citrate (Fisher Scientific, Pittsburg, PA) was used to establish the standard curve. The serum pool was dialyzed overnight using a Slide-A-Lyzer 10 kDa cutoff cassette (Thermo Scientific, Rockford, IL) in phosphate buffer (pH 7.4) at 4 °C to elute citrate and other small metabolites. A total of nine samples containing different citrate concentrations were prepared and tested in quadruplicate to produce the standard curve.
Fig. 1.
Quantification of circulating citrate using a 1D 1H NMR spectrum of serum/plasma. (A) Expansion of spectrum region showing the citrate peaks (1, 2 and 4) used by the deconvolution software. (B) For each peak, the experimental spectrum (black) is resolved into its peak components (blue) and baseline (yellow). The deconvolution coefficients for the calculated spectrum (red) is then converted to concentration unit using a standard curve (C). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.4. Analytical characteristics of the citrate assay
For the limit of blank (LOB), five serum protein pools were isolated by ultracentrifugation from de-identified residual clinical samples as previously described [17]. Five serum pools containing low levels of citrate were prepared to determine the limit of detection (LOD) and the limit of quantification (LOQ). All samples were tested in quadruplicate for 3 days and the LOB, LOD and LOQ were calculated as previously described [17]. These studies were carried out according to Clinical and Laboratory Standards (CLSI) guidelines [20].
Spiking and recovery studies were conducted to assess accuracy of the NMR assay. Accuracy, expressed as % recovery, was calculated as the ratio of the citrate recovered from serum to the added citrate concentration multiplied by 100. The first study involved spiking a low (0.68 mg/dL) and a high (3.17 mg/dL) citrate concentration in serum pools with values as measured by the NMR assay prior to spiking. The recovered citrate was calculated as the difference between the measured citrate before and after spiking. Measurements were done in triplicate. A second study involved spiking the analyte at various concentrations (0.51, 1.92, 2.87 and 3.83 mg/dL) into serum samples, and were compared to samples that were not spiked. All samples were randomly selected. The average concentration of naturally occurring citrate in the non-spiked sera were used as the basal value. The recovered citrate was calculated as the difference between the measured (in spiked samples) and the basal value. Measurements were done in singlicate.
Assay linearity was evaluated according to CLSI guidelines [21] using serially mixed source pools with low and high levels of citrate. A total of 11 mixtures were generated. The source pools and mixtures were tested in quadruplicate on a single instrument in one day. Linearity was evaluated by comparing linear regression to higher order polynomial regression of results for the prepared mixtures (measured against expected concentration).
The assay imprecision was determined in accordance with CLSI guidelines [22]. Two serum pools with low and high citrate were prepared by mixing selected de-identified residual clinical specimens. Within-run imprecision was determined by testing replicates of each pool in a single day (n = 20), while within-laboratory imprecision involved testing the same pools in duplicate twice per day over 20 days (n = 80). Mean, standard deviation (SD) and % coefficients of variation (%CV) were calculated.
2.5. Test for interfering substances
A total of 22 substances (8 endogenous and 14 common drugs/metabolites) were tested in vitro for potential analytical interference with citrate test results according to CLSI guidelines [23]. Stock solutions and samples were prepared as previously described [17]. Substances eliciting >10% change in test results are deemed interferents.
2.6. Specimen collection tube comparison
Blood was drawn into Greiner tube (serum separator manufactured by Greiner Bio-One, Inc. Part # 456293P also known as LipoTubes), BD Vacutainer serum tube (red-top, plain serum, no gel barrier), BD Gel Barrier serum tube, K2EDTA plasma tube and Na-heparin plasma tubes from 20 volunteers. Samples were tested in duplicate. A bias of >10% between the Greiner tube and the other collection tubes was considered significant. This study was conducted in accordance with CLSI guidelines [24,25].
2.7. Specimen stability at different temperature and number of freeze-thaws
Samples were collected from 10 donors for stability assessed at room (20–25 °C), refrigerated (2–8 °C) and frozen (−20 and <-70 °C) temperatures, as well as several cycles of freezing and thawing. Blood samples were obtained from four separate studies corresponding to each collection tube type (i.e., Greiner tube, BD Vacutainer serum, K2EDTA plasma, Na-heparin plasma tubes). Citrate was deemed stable in the sample over time and freeze-thaw cycles if the differences in the results were within ±10% of the baseline value (draw day/day 0).
2.8. Citrate interval in apparently healthy adults and in the general population
A study was conducted to determine the reference interval for citrate in serum from 567 apparently healthy adult men and women between the ages 18 and 84. Samples were drawn from fasting/non-fasting volunteers into Greiner tubes. Donors were recruited by Labcorp and all signed informed consents. Citrate was measured using the NMR assay, as described above, in singlicate. Lipids and apoB were derived from NMR spectra as previously described [26]. Samples with missing information were excluded for further analysis leaving a total of 553 participants. The reference interval for citrate was determined from the 2.5th and 97.5th percentiles (statistical software: JMP version 13.1.0, SAS, Cary, NC).
In order to determine the circulating citrate values in the general population, digitally stored NMR spectra (n = 133,576) of samples received in the clinical laboratory for the NMR LipoProfile test were analyzed using the newly developed assay. The distribution of citrate was calculated using JMP version 13.1.0.
3. Results
3.1. Measurement of citrate using a non-negative deconvolution algorithm
The concentration of citrate was determined by modeling its NMR signals on a 1D 1H NMR spectrum of serum (Fig. 1) and converting the signal amplitudes into concentration units (mg/dL). Each of the well isolated citrate peaks (Fig. 1, inset A) was resolved into its component lineshape and baseline. The spectral deconvolution was achieved using a QR decomposition algorithm. The fit (or calculated spectrum; Fig. 1, inset B) to the observed spectrum yields amplitudes of the peak components used for modeling the citrate signal. As the peak amplitudes relate linearly to analyte concentrations [27], they can be transformed into concentration units (mg/dL) using a standard curve. The standard curve used by the citrate software is shown in Fig. 1 (inset C).
3.2. Assay performance
3.2.1. Sensitivity, accuracy, linearity and imprecision
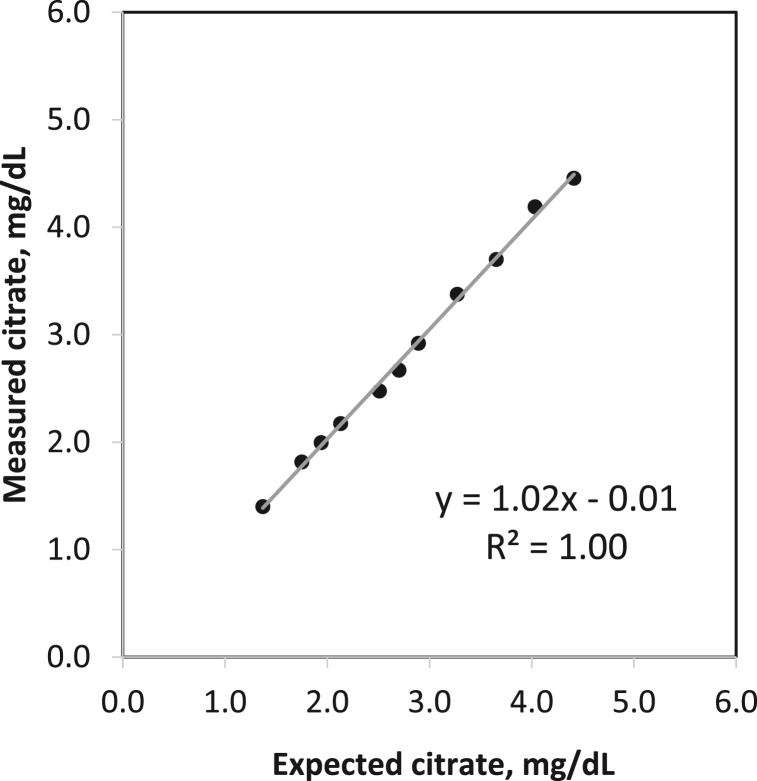
The limit of blank (LOB) was calculated to be 0.50 mg/dL and the analytical sensitivity or limit of detection (LOD) was 0.76 mg/dL. Repeated testing of samples with different citrate levels gave a functional sensitivity or limit of quantitation (LOQ) of 1.48 mg/dL. The imprecision (expressed as %CV) at the LOD and LOQ are 20% and 10%, respectively. The spectra for a blank sample and serum containing citrate at the LOQ are shown in Fig. S1. Recovery studies demonstrated acceptable accuracy of 91.2% at low and 94.5% at high citrate concentration (Table 1) in pooled serum. A complementary dataset using serum samples showed a recovery of 93–99.7% (Supplementary Table S1). Linearity was demonstrated over a range of citrate concentrations (1.40–4.46 mg/dL) with a correlation coefficient (R2) of 1.00 (Fig. 2). Intra-assay (within-run) and inter-assay (within-lab) precision were evaluated using serum pools with low, intermediate and high citrate concentrations. The results are summarized in Table 2. The %CV for intra-assay precision ranged from 5.8 to 9.3 while the %CV for inter-assay precision ranged from 5.2 to 9.6.
Table 1.
Accuracy of the citrate assay.
| Spiked citrate (mg/dL) | Recovered (mg/dL) | % Recovery | |
|---|---|---|---|
| Low | 0.68 | 0.62 | 91.2 |
| High | 3.17 | 3.00 | 94.5 |
Recovered = measured citrate before spiking – measured citrate after spiking in pooled serum; measurements were done in triplicate. A complementary accuracy dataset is provided in Supplementary Table S1.
Fig. 2.
Results of linearity testing for the citrate assay.
Table 2.
Within-run and within-lab precision of the citrate assay.
| Citrate (mg/dL) |
|||
|---|---|---|---|
| Within-Run Precisiona | Within-Laboratoryb | ||
| Low | Mean | 2.1 | 2.1 |
| SD | 0.2 | 0.2 | |
| %CV | 9.3 | 9.6 | |
| High | Mean | 3.3 | 3.2 |
| SD | 0.2 | 0.2 | |
| %CV | 5.8 | 5.2 | |
a Serum pools tested in 1 run of 20 replicates. b Serum pools tested in 2 runs of duplicates per day for 20 days (n = 80 per analyte). %CV, coefficient of variation expressed as percent.
3.2.2. In vitro testing of substances for potential interference
A total of 22 endogenous (e.g. bilirubin, hemoglobin) and exogenous (over-the-counter and prescription drugs) substances were tested for potential interference with generation of accurate citrate assay results. Tested substance concentrations were those prescribed by CLSI guidelines. Table 3 shows for each substance the highest concentration tested that did not elicit interference with assay results. None of the substances mediated interferance at the levels they naturally occur (endogenous) or at their therapeutic concentrations (exogenous).
Table 3.
Interference testing results showing highest concentration (mg/dL) of substances eliciting no interference.
| Substance | Drug name | Concentration (mg/dL) |
|---|---|---|
| Bilirubin, unconj. | ─ | 21 |
| Bilirubin, conj. | ─ | 34 |
| Creatinine | ─ | 5.7 |
| Hemoglobin | ─ | 219 |
| Urea | ─ | 264 |
| Uric acid | ─ | 24 |
| Protein (albumin) | ─ | 10,528 |
| Triglycerides (lipemic) | ─ | 10,276 |
| Acetaminophen | Tylenol | 22 |
| Acetylsalicylic acid | Aspirin | 66 |
| Atorvastatin | Lipitor | 4.9 |
| Enalaprilat dihydrate | Vasotec | 0.04 |
| Ethanol | ─ | 398 |
| Fenofibrate | Tricor | 4.6 |
| Hydralazine hydrochloride | Apresoline | 19 |
| Hydrochlorothiazide | HCT | 0.75 |
| Ibuprofen sodium salt | Advil | 59 |
| Metformin hydrochloride | Glucophage | 66 |
| Metoprolol tartrate | Lopressor | 1.5 |
| Naproxen sodium | Aleve | 56 |
| Nifedipine | Adalat | 0.04 |
| Salicylic acid | ─ | 61 |
3.2.3. Evaluation of different specimen collection tube types
The suitability of other sample types on citrate measurements was assessed by comparing results obtained for BD Vacutainer serum tubes (red-top, plain serum, no gel barrier), BD Gel Barrier serum tube, K2EDTA plasma tubes and Na-heparin plasma tubes with Greiner tubes. No significant bias was observed with the use of other sample collection tube types (Table 4). Thus, the assay is not limited to a particular sample matrix.
Table 4.
%Bias in citrate for different type of collection tubes compared to serum collected in Greiner tubes.
| Plain Serum | BD Gel Barrier serum tube | EDTA Plasma | Sodium Heparin Plasma |
|---|---|---|---|
| Avg. %Bias | Avg. %Bias | Avg. %Bias | Avg. %Bias |
| −0.6 | 0.5 | 0.3 | 3 |
3.2.4. Stability of citrate at various temperatures and multiple freeze-thaw cycles
The stability of citrate as reported by the assay at different storage temperatures and freeze-thaw cycles was evaluated in serum and plasma samples. Table 5 summarizes the results of the stability study. Citrate is stable up to 8 days at room temperature and up to 15 days at refrigerated and frozen temperatures. As expected, longer stability was observed at lower temperatures (<-70C°). In addition, no significant change in results was observed after 5 freeze-thaw cycles. Stability may extend beyond the time points tested in the current study.
Table 5.
Summary of citrate stability in different types of sample collection tubes.
| Storage Condition | LipoTube Serum | Plain Red Top Serum | EDTA Plasma | Heparin Plasma |
|---|---|---|---|---|
| Controlled Room Temperature, 20–25 °C |
Up to 8 days | Up to 8 days | Up to 8 days | Up to 8 days |
| Refrigerated, 2–8 °C | Up to 15 days | Up to 15 days | Up to 15 days | Up to 15 days |
| Frozen, −25 to −10 °C | Up to 15 days | Up to 15 days | Up to 15 days | Up to 15 days |
| Frozen, < −70 °C | 3 years | a | a | a |
| Number of Freeze-Thaw cycles | 5x | 5x | 5x | 5x |
No data available.
3.2.5. Expected values in apparently healthy individuals and in the general population
The assay to measure circulating citrate by NMR described herein was applied to apparently healthy individuals (n = 553) with a median age of 39 and comprised of 60.9% women. Demographics and clinical measures for this cohort are shown in Table 6. The median citrate was 1.85 mg/dL, and the 95% reference interval was <1.48–2.97 mg/dL (Table 7). Wilcoxon rank sums test indicated that there was a statistically significant difference in citrate distribution between women (median[IQR]: 1.92 [<1.48–3.01]) and men (1.72 [<1.48–2.92]).
Table 6.
Population characteristics of healthy controls (n = 553).
| Clinical characteristics | Values |
|---|---|
| Age (years) | 39 (29–49) |
| BMI | 24.4 (21.9–27.2) |
| Male (%) | 39.1 |
| Race (%) | |
| African American | 16.5 |
| Asian | 16.1 |
| Caucasian | 63.8 |
| Other | 3.6 |
| Blood pressure > 140/90 mm Hg (%) | 4.0 |
| Smoker (%) | 8.9 |
| TC, mg/dL | 182 (159–205) |
| HDL-C, mg/dL | 55 (45–67) |
| TG, mg/dL | 97 (69–140) |
| apoB, mg/dL | 86 (74–101) |
| LDL-C, mg/dL | 103 (85–125) |
Values are expressed as median (IQR), unless otherwise indicated.
Table 7.
Citrate distribution and population mean in generally healthy adults.
| Percentile | Overall (n = 553) | Women (n = 337) | Men (n = 216) |
|---|---|---|---|
| 0th | <1.48a | <1.48a | <1.48a |
| 2.5th | <1.48a | <1.48a | <1.48a |
| 25.0th | 1.57 | 1.68 | 1.43 |
| 50.0th | 1.85 | 1.92 | 1.72 |
| 75.0th | 2.18 | 2.24 | 2.08 |
| 97.5th | 2.97 | 3.01 | 2.92 |
| 100th | 3.63 | 3.63 | 3.48 |
| Mean (SD) | 1.89 (0.46) | 1.96 (0.44) | 1.79 (0.48) |
Values in mg/dL. SD, standard deviation. aLowest reportable value is limited by the LOQ of this assay.
Based on interrogation of samples collected in the clinical laboratory, citrate was slightly increased (median = 2.19 mg/dL) in the general population compared to the cohort used to determine the reference interval (Table 8). A wider range was also observed (2.5th – 97.5th percentile: <1.48–3.62 mg/dL) with the highest value reaching 10.7 mg/dL.
Table 8.
Citrate distribution in the general population (n = 133,576).
| Percentile | Value |
|---|---|
| 0th | <1.48a |
| 2.5th | <1.48a |
| 25.0th | 1.85 |
| 50.0th | 2.19 |
| 75.0th | 2.59 |
| 97.5th | 3.62 |
| 100th | 10.7 |
| Mean (SD) | 2.26 (0.60) |
Values in mg/dL. SD, standard deviation. aLowest reportable value is limited by the LOQ of this assay.
4. Discussion
This study is the first to report performance characteristics of an NMR-based assay that measures concentrations of citrate in serum/plasma on high-throughput Vantera® Clinical Analyzers. While NMR has been applied to quantify citrate in serum/plasma, previous reports involved multi-step sample preparation, use of more than one software application and longer NMR acquisition times [8,28]. In addition, while used extensively for epidemiologic research studies, no comprehensive analytical performance characteristics have been reported.
There are several advantages offered by the NMR assay described here over existing methods. Aside from minimal sample preparation (addition of phosphate buffer) and rapid data collection, the assay does not require separate assay-based calibration between instruments and use of the expensive isotope-labeled standards required by MS-based methods. Despite being less sensitive than MS, the NMR assay exhibits sufficient sensitivity to measure the levels of circulating citrate observed in apparently healthy individuals (Table 7), in the general population (Table 8) as well as in observational studies [7,8]. While the sensitivity of the assay described here is adequate for routine use, higher sensitivity can be achieved by using a higher magnetic field strength. With higher field magnets, a lower LOQ could be obtained, therefore expanding the measurement range. Alternatively, extending the total acquisition time and/or using a cryogenic probe can improve sensitivity, and are expected to have similar effect. Furthermore, the newly developed assay is insensitive to interfering effects of substances intrinsic to serum/plasma as in the case of lipemic and icteric samples commonly affecting colorimetric methods. While the highest hemoglobin concentration tested in the current study prescribed by CLSI guideline does not represent gross hemolysis, the NMR assay tolerance of hemolysis is acceptable for routine use.
While at present circulating citrate is barely utilized for disease diagnostics, recent reports highlight its potential clinical utility. For example, circulating citrate was predictive of the risk for all-cause mortality (HR per 1SD increment = 1.33, 95% CI: 1.21–1.45, p = 5 × 10−10) in an Estonian cohort (n = 9842) even after adjusting for conventional risk factors [8]. In another study (n = 122), citrate was associated with increased 3-month mortality in patients with acute heart failure (odds ratio = 11.74, 95% CI: 1.44–113.20, p = 0.026) in a logistic regression analysis adjusted for clinical covariates [7]. Citrate was also found significantly increased (2-fold, p < 0.0001) in serum of patients with NAFLD compared to reference values [4]. While these studies relate citrate to clinical outcomes, it is likely that its utility may be enhanced by a panel of biomarkers given the pathophysiological complexity of a disease. Multiplexing the citrate assay with additional analytes into a panel would be readily feasible because it uses the same acquisition and processing parameters as that of the NMR LipoProfile® test. The spectrum used by the NMR LipoProfile® test provides clinically relevant analytes/markers such as lipoprotein (LDL and HDL) particle numbers, lipoprotein subclasses, the Lipoprotein Insulin Resistance Index (LP-IR), the GlycA marker of systemic inflammation, and concentrations of ketone bodies and branched chain amino acids [15,16,18,27,[29], [30], [31]]. Citrate, in addition to these analytes/markers, could help improve risk assessment for a disease such as NAFLD or mortality.
5. Conclusions
The newly developed NMR assay has analytical characteristics that reliably quantify citrate in serum and plasma. The assay provides a simple and fast method to scrutinize samples for research and clinical studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author statement
Erwin Garcia: Conceptualization, Formal analysis, Validation, Original draft writing, Writing - review & editing. Margery A. Connelly: Conceptualization, Formal analysis, Validation, Writing - review & editing. Steven P. Matyus: Software, Formal analysis, Validation, Writing - review & editing. James D. Otvos: Conceptualization, Methodology, Software, Writing - review & editing. Irina Shalaurova: Conceptualization, Methodology, Software, Writing - review & editing. All authors approved the final manuscript.
Declaration of competing interest
EG, MAC, SPM, JDO and IS are employees of Labcorp.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2021.e00213.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
1D 1H NMR spectrum for a blank (blue) sample (serum proteins isolated by ultracentrifugation) and a serum sample (black) containing citrate concentration (1.44 mg/dL) very close to the LOQ (1.48 mg/dL). Citrate peaks are indicated for serum.
References
- 1.Lehninger A.L., Nelson D.L., Cox M.M. second ed. Worth Publishers, Inc; New York, NY: 1993. Principles of Biochemistry. [Google Scholar]
- 2.Iacobazzi V., Infantino V. Citrate--new functions for an old metabolite. Biol. Chem. 2014;395(4):387–399. doi: 10.1515/hsz-2013-0271. [DOI] [PubMed] [Google Scholar]
- 3.Plasma citrate levels as a potential biomarker for glaucoma. J. Ocul. Pharmacol. Therapeut. 2011;27(6):577–580. doi: 10.1089/jop.2011.0062. [DOI] [PubMed] [Google Scholar]
- 4.van de Wier B. Elevated citrate levels in non-alcoholic fatty liver disease: the potential of citrate to promote radical production. FEBS Lett. 2013;587(15):2461–2466. doi: 10.1016/j.febslet.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Granchi D. Role of citrate in pathophysiology and medical management of bone diseases. Nutrients. 2019;11(11) doi: 10.3390/nu11112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H. Bone and plasma citrate is reduced in osteoporosis. Bone. 2018;114:189–197. doi: 10.1016/j.bone.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Stryeck S. Serum concentrations of citrate, tyrosine, 2- and 3- hydroxybutyrate are associated with increased 3-month mortality in acute heart failure patients. Sci. Rep. 2019;9(1):6743. doi: 10.1038/s41598-019-42937-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer K. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. 2014;11(2) doi: 10.1371/journal.pmed.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello L.C., O’Neill J.J. A simplified and sensitive method for citrate determination in biological samples. J. Appl. Physiol. 1969;27(1):120–122. doi: 10.1152/jappl.1969.27.1.120. [DOI] [PubMed] [Google Scholar]
- 10.Borland W.W., Fergusson J.C., Dryburgh F.J. A fast automated method for measuring serum and urine citrate. Ann. Clin. Biochem. 1989;26(Pt 3):286–288. doi: 10.1177/000456328902600315. [DOI] [PubMed] [Google Scholar]
- 11.Clarke W., Marzinke M.A. fourth ed. Academic Press; 2020. Contemporary Practice in Clinical Chemistry. [Google Scholar]
- 12.Al Kadhi O. Development of a LC-MS/MS method for the simultaneous detection of tricarboxylic acid cycle intermediates in a range of biological matrices. Journal of Analytical Methods in Chemistry. 2017;2017:5391832. doi: 10.1155/2017/5391832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathod R. Simultaneous measurement of tricarboxylic acid cycle intermediates in different biological matrices using liquid chromatography-tandem mass spectrometry; quantitation and comparison of TCA cycle intermediates in human serum, plasma, kasumi-1 cell and murine liver tissue. Metabolites. 2020;10(3) doi: 10.3390/metabo10030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connelly M.A. GlycA, a marker of acute phase glycoproteins, and the risk of incident type 2 diabetes mellitus: PREVEND study. Clin. Chim. Acta. 2016;452:10–17. doi: 10.1016/j.cca.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Garcia E. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J. Clin. Med. 2020;9(2):321. doi: 10.3390/jcm9020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolak-Dinsmore J. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin. Biochem. 2018;54:92–99. doi: 10.1016/j.clinbiochem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Matyus S.P. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin. Biochem. 2014;47(16–17):203–210. doi: 10.1016/j.clinbiochem.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Matyus S.P. HDL particle number measured on the Vantera(R), the first clinical NMR analyzer. Clin. Biochem. 2015;48(3):148–155. doi: 10.1016/j.clinbiochem.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Van Loan C.F., Golub G.H. Johns Hopkins University Press; Baltimore: 1983. Matrix Computations. [Google Scholar]
- 20.CLSI Document EP17-A: Protocols for Determination of Limits of Detection and Limits of Quantitation; Approved Guideline. Clinical and Laboratory Standards Institute; Wayne, PA USA: 2004. [Google Scholar]
- 21.CLSI Document EP6-A: Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach. Clinical and Laboratory Standards Institute; Wayne, PA: 2003. [Google Scholar]
- 22.CLSI Document EP5-A2: Evaluation of Precision Performance of Quantitative Measurements Methods; Approved Guideline. second ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2004. [Google Scholar]
- 23.CLSI Document EP7-A2: Interference Testing in Clinical Chemistry; Approved Guideline. second ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. [Google Scholar]
- 24.CLSI Document EP14-A2: Evaluation of Matrix Effects; Approved Guideline. second ed. 2005. Wayne, PA. [Google Scholar]
- 25.CLSI Document EP14-A3: Evaluation of Cummutability of Processed Samples. Approved Guideline-Third Edition; Wayne, PA: 2014. [Google Scholar]
- 26.Garcia E. The extended lipid panel assay: a clinically-deployed high-throughput nuclear magnetic resonance method for the simultaneous measurement of lipids and Apolipoprotein B. Lipids Health Dis. 2020;19(1):247. doi: 10.1186/s12944-020-01424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyarajah E.J., Cromwell W.C., Otvos J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006;26(4):847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Alkan H.F. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metabol. 2018;28(5):706–720.e6. doi: 10.1016/j.cmet.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matyus S.P., Braun P.J., Wolak-Dinsmore J., Jeyarajah E.J., Shalaurova I., Xu Y., Warner S.M., Clement T.S., Connelly M.A., Fischer T.J. Clinical Biochemistry; 2014. NMR Measurement of LDL Particle Number Using the Vantera Clinical Analyzer. [DOI] [PubMed] [Google Scholar]
- 30.Shalaurova I. Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab. Syndr. Relat. Disord. 2014;12(8):422–429. doi: 10.1089/met.2014.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otvos J.D. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin. Chem. 2015;61(5):714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1D 1H NMR spectrum for a blank (blue) sample (serum proteins isolated by ultracentrifugation) and a serum sample (black) containing citrate concentration (1.44 mg/dL) very close to the LOQ (1.48 mg/dL). Citrate peaks are indicated for serum.