Abstract
Purpose
To present two cases of neonatal endophthalmitis with poor prognosis that were managed with primary evisceration.
Observations
Case 1 is a 27-weeks’ gestation neonate who developed Pseudomonas aeruginosa endophthalmitis complicated by globe rupture. Case 2 describes a 34-weeks’ gestation neonate with Serratia marcescens endophthalmitis. Both patients had poor prognosis and thus underwent primary evisceration with good long-term cosmetic outcomes at 15 years and 17 months, respectively.
Conclusions and Importance
Primary evisceration should be considered in neonates with endophthalmitis with a poor prognosis and can result in good long-term cosmesis.
Keywords: Endophthalmitis, Retina, Oculoplastics, Evisceration
1. Introduction
Neonatal endogenous endophthalmitis is one of the feared complications of neonatal sepsis that can result in loss of vision and the eye.1 This results from the hematogenous spread of microorganisms across the blood-ocular barrier which infect the vitreous or aqueous humor of the eye.2 Despite advances in neonatal care and antimicrobial therapy, systemic infections remain common among premature infants. Nearly one-third of extremely premature infants, defined as birth before 28 weeks of pregnancy, develop sepsis3,4 with associated high mortality and high risk of long-term complications, including blindness from neonatal endophthalmitis.5 A premature infant suffering from bacteremia, perinatal infections, or respiratory disorders is twice as likely to get endophthalmitis.6 In one series examining 4323 infants in the neonatal intensive care unit over five years, among the six patients that developed endophthalmitis all had constitutional signs of infection.7
Treatment of endogenous endophthalmitis in the neonate includes, as with adults, control of the systemic infection by intravenous antimicrobials with adjuvant intravitreal injections and pars plana vitrectomy in select patients. However, despite maximal therapy, long term visual outcomes are typically poor with worse outcomes associated with more virulent organisms.7 In such cases, primary evisceration can be utilized both as a definitive therapy to control the infection locally and to preserve a good cosmetic outcome.
Herein, we report two cases of neonatal endophthalmitis associated with virulent causative organisms managed with primary evisceration with good cosmesis.
2. Findings
2.1. Case 1
The ophthalmology service was consulted to evaluate a 27-weeks’ gestation neonate born to a 23-year-old female via emergent Caesarean section for premature rupture of membranes. The patient's complicated perinatal course was marked by the development of intraventricular hemorrhages, hydrocephalus, respiratory distress, intussusception, and sepsis. Blood cultures were positive for Pseudomonas aeruginosa and Candida albicans. The patient was started on intravenous vancomycin, gentamicin, ceftazidime, imipenem, and amphotericin B.
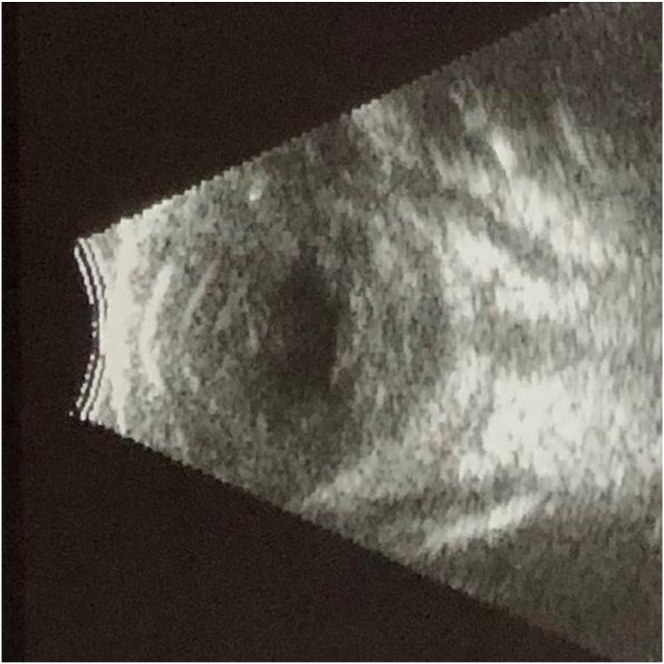
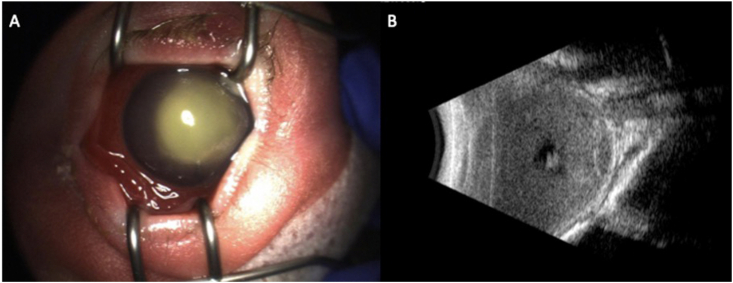
On the 35th day of life the patient was transferred to our care due to concern for endogenous endophthalmitis. Ophthalmic examination of the right eye was notable for diffuse injection, a corneal epithelial defect with a large infiltrate, a shallow anterior chamber with a mixed hypopyon and hyphema, and no view to the iris or posterior pole. Ultrasound of the eye (Fig. 1) identified dense infiltrates in the vitreous, choroid, and retina without evidence of shadowing to indicate calcification. Examination of the left eye disclosed no evidence of infection.
Fig. 1.
B-scan ultrasound of the right eye demonstrating dense infiltrates in the vitreous, choroid, and retina without evidence of shadowing.
A corneal scrape of the right eye was sent for culture. An intravitreal paracentesis was performed but minimal fluid was aspirated. Vancomycin (1mg/0.1ml) and amikacin (0.2g/0.05ml) were injected into the vitreous cavity. Subsequently, the eye developed a marked rise in intraocular pressure, so an intravitreal ceftazidime injection was deferred. Subconjunctival depots of vancomycin and ceftazidime were placed.
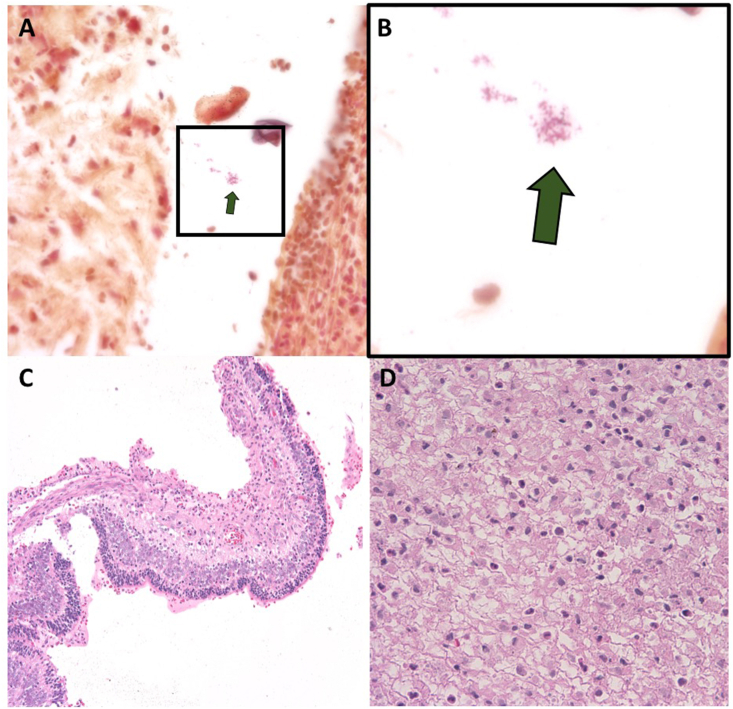
The corneal culture later grew Pseudomonas aeruginosa resistant to cefazolin. No organisms were identified in the vitreous sample. Four days later, the cornea had perforated with expulsion of intraocular material. Once the patient was deemed medically stable, the right eye was eviscerated at the bedside and a 16mm acrylic implant placed (Fig. 2). The patient remained admitted to the neonatal intensive care unit for 16 weeks, during which the left eye was treated with laser for Stage 2 retinopathy of prematurity in Zone II (Fig. 3).
Fig. 2.
A-D: Gram stain of the evisceration specimen from the right eye demonstrated gram negative rods (box in A, enlarged in B). The retina demonstrated necrosis with inflammatory infiltrate (C). The vitreous also demonstrated inflammatory cell infiltrate (D).
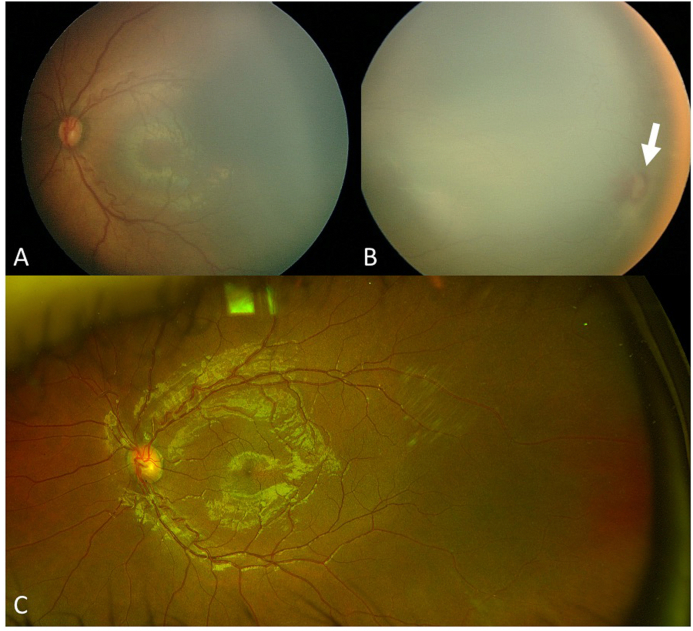
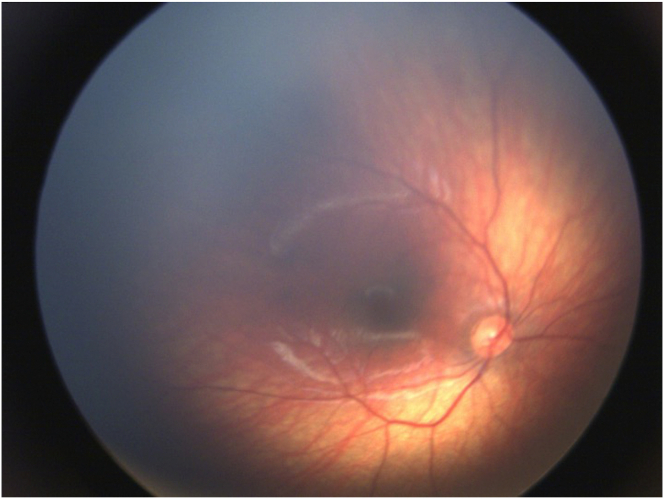
Fig. 3.
A-C: Fundus photos taken at baseline of the left eye (A: macula and B: temporal retina) demonstrate stage 2 retinopathy of prematurity with a temporal ridge in zone II (arrow in B). C: Fundus photography of the left eye taken 15 years later after laser treatment demonstrates regression of the retinopathy of prematurity including resolution of the temporal ridge and vascularization of the peripheral retina.
The patient underwent acrylic implant exchange at 24 months and again at 9 years of age with placement of a larger 20mm scleral-wrapped MEDPOR porous polyethylene implant (Stryker Corporation, Kalamazoo, Michigan). At the most recent follow up, 15 years later, examination showed a well-formed post-surgical socket and a visual acuity of 20/40 in the seeing eye (Fig. 4).
Fig. 4.
A,B: External photograph of the right eye showing a well formed post-surgical socket 15 years after evisceration with implant removed (A) and with ocular prosthesis placed (B).
2.2. Case 2
The second patient was a male born at 34-weeks’ gestation to a healthy mother via Caesarean section for preeclampsia and premature rupture of membranes. The mother was not tested for Group B streptococcus prior to delivery but received five doses of ampicillin intrapartum. Blood cultures obtained at birth were negative for organisms. However, on day 4 of life, the patient developed episodes of fever and apnea and was promptly started on ampicillin and gentamicin. Two days later, the left eye was noted to be diffusely chemotic with corneal clouding. Cefepime was added to the patient's regimen and the patient was transferred to our institution for management of presumed endogenous endophthalmitis.
Blood cultures obtained at the onset of symptoms were positive for Serratia marcescens sensitive to cefepime and gentamicin. Ophthalmic examination on day 7 of life revealed periorbital edema surrounding the left eye, diffuse chemosis, hyperemia, and a dense yellow hypopyon filling the anterior chamber (Fig. 5A). Ultrasound revealed a dense vitreous opacity without posterior shadowing (Fig. 5B). Examination of right eye revealed no evidence of infection or retinopathy of prematurity (Fig. 6).
Fig. 5.
A,B: External photograph of the left eye (A) of Case 2 on day 7 of life demonstrates periorbital edema, diffuse chemosis, hyperemia, and a dense yellow hypopyon filling the anterior chamber. B-scan ultrasound (B) reveals a dense vitreous opacity without shadowing. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Fundus photography of the right eye demonstrates a normal fundus without retinopathy of prematurity or infection.
Given the poor prognosis, the patient underwent primary evisceration in the operating room on day 13 of life with placement of a 14mm silicone implant. All cultures of blood, urine, cerebrospinal fluid, and conjunctival secretions subsequent to the initiation of antimicrobial therapy revealed no growth. At 17months follow-up, the patient maintained good symmetry and brow and cheek projection (Fig. 7). There was no evidence of infection. A prosthesis refitting was planned to promote continue growth of the orbit.
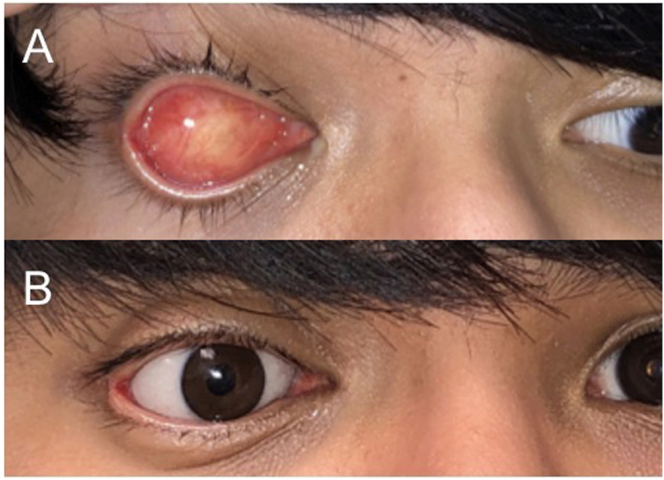
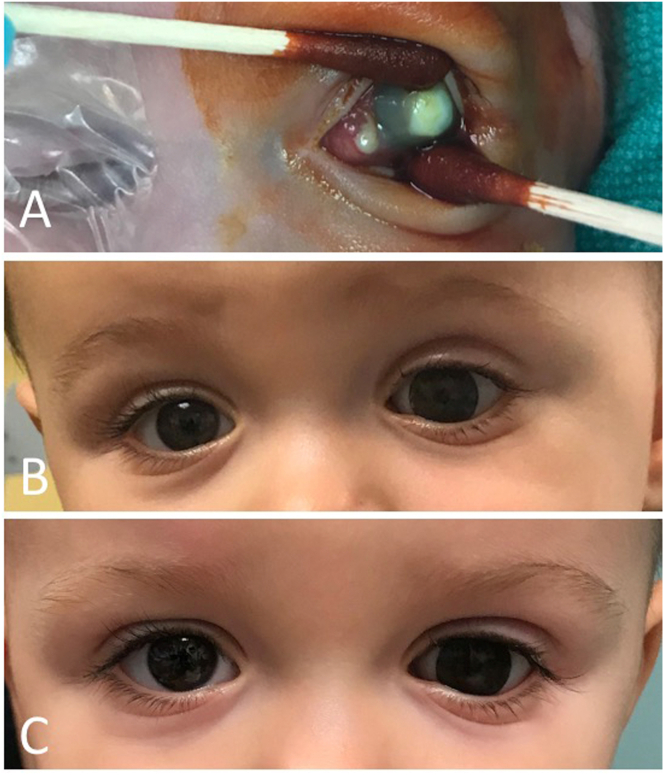
Fig. 7.
A-C: Intraoperative photo at baseline demonstrates central corneal infiltration with surrounding corneal clouding of the left eye with suppuration (A). Follow up at 10 months (B) and 17 months (C) after evisceration of the left eye demonstrated good symmetry and brow and cheek projection.
3. Discussion
Neonatal endogenous endophthalmitis is a debilitating complication of neonatal sepsis that frequently results in blindness. A large series from India found that vision was only salvageable in eyes where the presenting sign was focal retinitis, which was noted during routine ROP screening.1 All cases that had visible involvement of the cornea and inflammation of adnexal tissues resulted in blindness. The outcome in neonatal endophthalmitis is heavily influenced by the virulence of the infectious organism.
Pseudomonas aeruginosa, the causative organism in Case 1, has been implicated in 80% of invasive bacterial eye infections in neonates, including penetrating keratitis and endophthalmitis,8,9 and has a particularly poor prognosis. Endophthalmitis due to Pseudomonas is typically fulminant and usually results in blindness.9 In a series of 17 eyes from 16 neonates with endophthalmitis, all 7 surviving infants who were infected by Pseudomonas became blind in the affected eye.8
Endophthalmitis due to Serratia marcescens, the causative organism in Case 2, is less commonly encountered than Pseudomonas aeruginosa but is similarly associated with poor outcomes. The first case of Serratia marcescens endophthalmitis in a neonate was described by De Courten in 1988 and resulted in loss of the eye.10 Latorre described a second case of Serratia marcescens endophthalmitis in an infant born at 29-weeks gestation.11 This patient developed sepsis on day 11 of life and was noted to have corneal clouding of her right eye on day 20. This patient was treated with systemic and topical ciprofloxacin and similarly underwent enucleation. Lastly, Al Hazzaa and colleagues reported a case of Serratia marcescens endophthalmitis in a full-term infant who in addition to systemic antibiotics underwent intravitreal paracentesis and injection of gentamicin and cefazolin with clearance of the infection.12 Nevertheless, three months later, the eye became phthisical.
Given the likely result of blindness and phthisis with particularly virulent causative organisms in neonatal endophthalmitis, primary evisceration should be considered and can result in good cosmetic outcomes. While pars plana vitrectomy can lead to successful outcomes in endophthalmitis in adults and older children, vitrectomy is much more difficult in neonates whose anatomy is comprised of smaller ocular structures.13 For this reason as well as the advanced presentation of our cases and the virulence of the causative organisms, vitrectomy was not performed.13 Primary evisceration or enucleation are nevertheless not frequently performed likely due to concerns over challenges in the management of the pediatric anophthalmic socket. However, the present cases demonstrate that excellent cosmetic outcomes can be achieved with appropriate follow-up care, including resizing of ocular implants.
Long-term follow up of the patient presented in Case 1 demonstrates that adequate growth of the orbital socket can be sustained well into young adulthood. While the patient did undergo two implant exchanges to maximize the final cosmetic outcome, prior studies suggest that leaving in the original 16mm implant would not have significantly affected orbital growth had the patient been deemed a poor surgical candidate or elected to avoid further surgery.14 Despite his young age, the patient adjusted well to the use of an ocular prosthesis. Infrequent episodes of papillary conjunctivitis were managed with topical antibiotic and steroids, as well as polishing of the prosthesis by an ocularist.
If the patient had not undergone evisceration, he likely would have developed phthisis bulbi given the severity of his infection. Phthisical eyes, particularly in children, may require therapeutic interventions to manage corneal sensitivity, enophthalmos, and orbital fat atrophy.15 While phthisical eyes can sometimes be managed cosmetically using cosmetic lenses, scleral shells, and other rehabilitative treatments, the success of these interventions depends on the integrity of the underlying ocular tissue.15 Particularly in children, enophthalmos and fat atrophy related to phthisis can lead to asymmetry between eyes. Therefore, even if these patients had responded to treatment, the resultant phthisis would likely have led to a poor cosmetic outcome given the virulence of the organisms and disease course.
Complications of eviscerations include implant migration, exposure, infection, proptosis, enophthalmos, superior sulcus deformity, socket contraction, ptosis and ectropion. However, these complications can be mitigated with appropriate sizing and technique.16 Typical sizes for pediatric implants range from 16 to 20 mm in diameter. Implants between 15 and 19 mm stimulate sufficient bony growth of the orbit to the extent that differences between the operated and fellow eye are difficult to appreciate on external exam.14 Sizing implants too small is more problematic than sizing too large and can lead to poor development of the orbit. Consequently, we recommend sizing implants as large as possible. A study measuring ocular volume by A-scan ultrasound in infants found that at 5 months age the globe occupies a volume of 4.3 ml, which can accommodate implants of up to 18 mm.16
4. Conclusions
Primary evisceration should be considered as a treatment option in neonatal endophthalmitis in the setting of virulent organisms and poor prognosis. Evisceration can achieve control of the infection while also maintaining good cosmesis. Consistent follow-up care is critical to avoiding complications and ensuring a good cosmetic outcome.
Patient consent
Consent for treatment and for the use of clinical photos in educational material is obtained from all patients treated at our institution. Consent to publish the case report was not obtained, but the present report does not contain any personal information that could lead to the identification of the patient. All photos have been cropped to prevent identification of the patients and do not contain unique patient identifying features.
Funding
No research funding was used.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
The authors have no relevant financial disclosures to report.
Acknowledgements
The research was supported in part by the National Eye Institute Center Core Grant (P30EY014801).
References
- 1.Jalali S., Pehere N., Rani P.K. Treatment outcomes and clinicomicrobiological characteristics of a protocol-based approach for neonatal endogenous endophthalmitis. Eur J Ophthalmol. 2014;24:424–436. doi: 10.5301/ejo.5000395. [DOI] [PubMed] [Google Scholar]
- 2.Jackson T.L., Eykyn S.J., Graham E.M., Stanford M.R. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 3.Stoll B.J., Hansen N.I., Bell E.F. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stensvold H.J., Klingenberg C., Stoen R. Neonatal morbidity and 1-year survival of extremely preterm infants. Pediatrics. 2017;139 doi: 10.1542/peds.2016-1821. [DOI] [PubMed] [Google Scholar]
- 5.Younge N., Goldstein R.F., Bann C.M. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376:617–628. doi: 10.1056/NEJMoa1605566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moshfeghi A.A., Charalel R.A., Hernandez-Boussard T., Morton J.M., Moshfeghi D.M. Declining incidence of neonatal endophthalmitis in the United States. Am J Ophthalmol. 2011;151:59–65.e1. doi: 10.1016/j.ajo.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Aziz H.A., Berrocal A.M., Sisk R.A. Intraocular infections in the neonatal intensive care unit. Clin Ophthalmol. 2012;6:733–737. doi: 10.2147/OPTH.S26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohrer R., Belohradsky B.H. Bacterial endophthalmitis in neonates. Eur J Pediatr. 1987;146:354–359. doi: 10.1007/BF00444936. [DOI] [PubMed] [Google Scholar]
- 9.Boyle E.M., Ainsworth J.R., Levin A.V., Campbell A.N., Watkinson M. Ophthalmic Pseudomonas infection in infancy. Arch Dis Child Fetal Neonatal Ed. 2001;85:F139–F140. doi: 10.1136/fn.85.2.F139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Courten C., Sancho P., BenEzra D. Metastatic Serratia marcescens endophthalmitis. J Pediatr Ophthalmol Strabismus. 1988;25:45–47. doi: 10.3928/0191-3913-19880101-12. [DOI] [PubMed] [Google Scholar]
- 11.Latorre G. Endogenous Serratia marcescens endophthalmitis in a preterm infant. Indian J Pediatr. 2008;75:410. [PubMed] [Google Scholar]
- 12.al Hazzaa S.A., Tabbara K.F., Gammon J.A. Pink hypopyon: a sign of Serratia marcescens endophthalmitis. Br J Ophthalmol. 1992;76:764–765. doi: 10.1136/bjo.76.12.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Results of the endophthalmitis vitrectomy study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- 14.Fountain T.R., Goldberger S., Murphree A.L. Orbital development after enucleation in early childhood. Ophthalmic Plast Reconstr Surg. 1999;15:32–36. doi: 10.1097/00002341-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal H., Singh R.D., Kumar P., Gupta S.K., Alvi H.A. Prosthetic guidelines for ocular rehabilitation in patients with phthisis bulbi: a treatment-based classification system. J Prosthet Dent. 2014;111:525–528. doi: 10.1016/j.prosdent.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Kaltreider S.A., Peake L.R., Carter B.T. Pediatric enucleation: analysis of volume replacement. Arch Ophthalmol. 2001;119:379–384. doi: 10.1001/archopht.119.3.379. [DOI] [PubMed] [Google Scholar]