Graphical abstract
Keywords: Reuterin, Antimicrobial activity, Cytotoxicity, In vitro digestion, Eukaryotic cells
Highlights
-
•
Reuterin (3-hyrdoxypropionaldehyde (3-HPA)) is a highly potent metabolite of Lactobacillus reuteri.
-
•
Reuterin is highly stable in gastrointestinal condition.
-
•
Human colorectal adenocarcinoma cells’ viability and membrane integrity remained unaltered by reuterin.
-
•
No significant hemolytic activity was detected.
-
•
Reuterin is a promising therapeutic and/or food preservative.
Abstract
Reuterin (3-hyrdoxypropionaldehyde (3-HPA)) is a highly potent metabolite of L. reuteri, which has applications in food, health, and veterinary sectors. Similar to other natural antimicrobial compounds, the approval of reuterin as a bio-preservative or therapeutic agent by regulatory agencies relies on sufficient data on its cytotoxicity and behavior in the gastrointestinal environment. Although the antimicrobial activity of reuterin has been broadly studied, its safety and toxicity are yet to be explored in detail. In this study, the stability and activity of reuterin were investigated in the gastrointestinal tract using in vitro models simulating gastrointestinal conditions. In addition, hemolytic activity and in vitro cytotoxicity of reuterin were evaluated by neutral red assay and lactate dehydrogenase (LDH) colorimetric assay using the same cell line. Activity of reuterin was observed to be stable during gastrointestinal transit. Viability and membrane integrity of cells remained unaltered by reuterin up to 1080 mM concentration. Furthermore, no hemolysis was observed in blood cells exposed to 270 mM reuterin. This study provides unique and highly relevant in vitro data regarding gastrointestinal behavior and toxicity of reuterin. In conclusion, the current study indicates that within a certain concentration range, reuterin can be safely used in bio-preservation and therapeutics applications. However, further in vivo studies are required to confirm these findings.
1. Introduction
Research on bio-preservatives has recently increased greatly in response to the growing consumer demand for natural and “chemical-free” food products. Protective cultures, including lactic acid bacteria (LAB) and Lactobacillus spp. in particular are considered as promising candidates for food safety and bio-preservation strategies [1]. They are known to produce a variety of antimicrobial metabolites, such as bacteriocins, organic acids, hydrogen peroxide, and reuterin [2]. Protective cultures have attracted a lot of interest in the food industry because they are biodegradable, recognized as Generally Regarded As Safe (GRAS) by regulatory agencies, and have a safe history of use as a starter culture in food fermentation. Among bacterial strains, L. reuterin has greatly drawn attention as a protective culture due to its ability to produce reuterin, which is an aldehyde with broad inhibitory activity.
L. reuterin is an obligate hetero-fermentative lactobacilli and a natural inhabitant of the gastrointestinal (GI) tract of animals and humans [3,4]. They can survive the digestive enzymes and grow and colonize in the GI tract. Significant therapeutic potential of L. reuterin in animals and humans has been documented based on beneficial activities, such as immune-modulatory effect [5], cholesterol-lowering effect [6], improvement of gut barrier function [7,8], anti-inflammatory effect [9], producing inhibitory substances, etc. [10]. L. reuterin strains are commonly used as probiotic supplements to improve GI health in humans and animals [11,12]. Certain strains of L. reuterin can produce reuterin (3-hydroxypropionaldehyde) during anaerobic fermentation of glycerol [13]. Therefore, it is suggested that reuterin-producing strains of L. reuteri are potential candidates for additional applications in medicine or animal and human health.
Reuterin is a low molecular weight aldehyde complex, consisting of monomers, dimers, trimers and tetramers obtained by either biotransformation of glycerol or chemical synthesis [14]. Reuterin has been shown to be water-soluble, stable at a wide range of pH values, and resistant to lipolytic and proteolytic enzymes [15]. It exhibits a broad-spectrum of antimicrobial activity against Gram-positive and Gram-negative bacteria, fungi, and protozoa [16]. Reuterin exerts its antimicrobial activity through reacting with the thiol groups in microorganisms, inhibiting DNA synthesis [17] and inducing oxidative stress. Interestingly, reuterin shows synergistic interaction in combination with other antimicrobial peptides, and therefore, it could be a potential alternative to antibiotics in order to combat antibiotic-resistant pathogens [18]. The potential use of reuterin as a preservative against food spoilage and pathogenic microorganisms has been well studied in food products, such as milk and dairy products [19], cooked ham [20], meat and chicken [15]. In addition, it has been shown to be effective in the decontamination of vegetables [21].
As a food preservative, reuterin can be either directly added as a pure compound or produced through reuterin-producing strains, such as L. reuterin, added as a food supplement. Some studies have shown that reuterin could be sufficiently produced in situ in dairy products supplemented with producing strain and glycerol. However, in situ production of reuterin has been shown to be greatly dependant on pH, temperature, oxygen and biomass concentration in the medium [22]. Moreover, the addition of glycerol in foods is not well accepted. In addition, although in situ production of reuterin has been reported to significantly reduce the total viable count of the starter culture in cheese [23], it was shown to effectively preserved the fermented milk products without any adverse effects on the odor and quality of the products [23,24]. Adding purified reuterin is a more straightforward approach, offering several advantages. However, this approach requires purification of the molecule, better knowledge and rigorous scientific data on the stability of reuterin in complex media, its inhibitory activity (MIC, a spectrum of action) and toxicity.
In previous studies in our lab, minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of reuterin was determined against a large panel of mold and yeast isolates. Besides, the antifungal effect of reuterin was evaluated for bio-preservation of yogurt [25]. These data confirmed that reuterin possesses a high potential as a bio-preservative in the food industry.
While the application of reuterin in the food sector has been a central topic for research, its therapeutic applications are yet to be investigated in details. Since reuterin has been shown to improve gut health by modulating the microbiota composition and suppressing specific infections via the inhibition of colonization of pathogenic microorganisms [17,26], it is suggested to be a potential therapeutic agent which can be employed in the treatment and management of related diseases. Indeed, the broad-spectrum inhibitory action of reuterin may be effective in controlling infectious diseases. For example, it can be used as a potential alternative to antibiotics for the treatment of Helicobacter pylori infection [26]. Moreover, it can prevent biofilm formation associated with dental cavities [27]. Interestingly, it was reported that the implant loaded with a therapeutically amount of reuterin was able to treat the target tissue [28].
Despite its significant potential in both food and medical sectors, reuterin is not yet commercially available, which might be due to legislative and regulatory requirements. Although reuterin is produced as a metabolite of Lactobicillus spp. possess GRAS status of safety in research; it requires to meet specific safety regulations for food, medical, and veterinary applications [2]. Unlike the antimicrobial activity of reuterin, which has been extensively studied, very little work has been done to evaluate its toxicity in vitro [29,30] and in vivo [31]. In addition, GI stability of reuterin across GI epithelial cells has not been completely investigated.
Taking into consideration the great potential of reuterin and the need for more data on its safety and toxicity for regulatory approval, a study to investigate the behaviour of reuterin in the GI tract and cytotoxicity is of great interest and an essential step towards its use in the food industry and veterinary and human medicine. The aim of this study is to produce, purify, and characterize reuterin obtained through bioconversion of glycerol by L. reuteri and to conduct in vitro studies using Caco-2 cell line in order to determine the cytotoxicity of reuterin and its stability during GI transit.
2. Materials and methods
2.1. Bacterial strains and culture conditions
Reuterin was produced by Lactobacillus reuteri ATCC 53608, cultured at 37 °C overnight in Man-regosa-sharpes (MRS) (Oxoid, Nepean, ON, Canada), under anaerobic condition (10 % H2, 10 % CO2, 80 % N2) in Forma Anaerobic Chamber (Thermo Scientific, Walthman, MA, USA). For the antibacterial activity of digestion mixtures, L. ivanovii HPB28 (Public Health Agency of Canada) and S. newport (STELA Collection, University Laval) were used as indicator Gram-positive and Gram-negative bacteria, respectively. L. ivenovii HPB28 was cultured overnight at 30 °C in Tryptic Soy Broth (Difco Laboratories, Spark, MD, USA) supplemented with 0.6 % yeast extract (TSBY). S. newport was cultured overnight at 37 °C in Luria-Bertani (LB) (Difco Laboratories, Spark, MD, USA). All strains were reactivated from glycerol stock stored at -80 °C, sub-cultured two times, streaked on an agar plate and finally a single colony was used for inoculations, while the rest was stored at 4 °C.
2.2. Production and quantification of reuterin
Reuterin was produced according to a method by Vimont et al. [25]. Briefly, a fresh bacterial culture of L. reuteri was inoculated at 1 L MRS broth (1 % v/v) supplemented with 20 mM glycerol and incubated at 37 °C for 16 h in an anaerobic condition in Forma anaerobic chamber (Thermo Scientific Waltman, Ma, USA). Bacterial cells were centrifuged at 1500 ×g at 20 °C for 10 min, washed with 0.1 M potassium phosphate buffer (pH 7), suspended in different concentrations of glycerol solution (100, 200, 300 and 400 mM) and incubated anaerobically for 45, 60, 90 or 120 min at room temperature. Cells were then removed by centrifugation at 8000 x g at 4 °C for 10 min, and the supernatant was filter-sterilized and stored at -20 °C for further experiments.
Purity and quantity of reuterin in the supernatant was verified using an analytical HPLC system (Waters, Milford, MA) equipped with ICsep-ion-300 column (Transgenomic, San Jose, CA), using mobile phase of 10 mM H2SO4, and refractive index detector (Hitachi L-7490 model, Tokyo, Japan) as previously described [25]. The area of the peak corresponding to reuterin was calculated by integration using HPLC software, and the standard curve was obtained from the area of the known concentrations of pure reference reuterin. Optimum production conditions allowing a high yield of pure reuterin and lowest residual glycerol and acrolein were maintained for further reuterin production. Reuterin quantification was confirmed by the colorimetric assay as described previously [32], 1 mL sample was added to a mixture of 3 mL HCl (37 %) and 750 μL tryptophane (10 mmol/L, dissolved in 0.05 mmol/L HCl) and incubated at 37 °C for 20 min, and then absorbance was measured at 560 nm. The standard curve was obtained with acrolein ranging from 0.05 to 6 mM in an aqueous solution using the same procedure.
2.3. In vitro simulation of gastrointestinal digestion
Simulated oral, gastric and small intestinal digestion process was carried out according to the INFOGEST method [33] in three independent replicates. The initial concentration of reuterin was selected in order to have sufficient reuterin in digestion products for further activity test by agar well diffusion assay and quantification by reversed phase high performance liquid chromatography (RP-HPLC). The entire digestion procedure was performed at 37 °C in pre-warmed solutions. For oral phase, 5 mL of 2700 mM reuterin was mixed with simulated salivary fluid (SSF) in the 1:1 (v/v) ratio, and the final 10 mL volume was incubated for 2 min. For the gastric phase, the pH of the oral solution was adjusted to 3 using HCl and was diluted in 1:1 (v/v) ratio with simulated gastric fluid and pepsin (2000 U/mL in the gastric mixture) to the final volume of 20 mL, which was incubated under shaking for 60 min. For small intestinal phase, the pH of small intestine solution was adjusted to 7 using NaOH, mixed with 20 mL of simulated intestinal fluid (SIF) in 1:1 (v/v) ratio, and added with bile salt (10 mmol/L in the final gastric solution) and pancreatin (100 U/mL in final digestion mixture) to obtain the final volume of 40 mL which was further incubated for a further 2 h. The digestion products were heat-treated at 80 °C for 10 min and centrifugated at 8000 x g for 10 min at 4 °C for further analysis. Samples were analyzed using analytical HPLC for reuterin detection and quantification. In addition, the antimicrobial activity of samples was estimated using agar diffusion assay and microtitration method. Acrolein concentration was also measured in samples after GI digestion.
2.4. Antimicrobial activity assays
Antimicrobial activity was estimated qualitatively by agar well diffusion assay according to the method described by Tagg et al. [34]. Briefly, 80 μl of digestive samples were transferred in wells of LB, and TSBY agar plates (0.75 % w/v agar) for the detection of antimicrobial activity against S. newport and L. ivanovii seeded as indicator strains, respectively. Plates were then incubated at 30 °C and 37 °C, respectively, for 18 h. The activity of reuterin was evaluated by measuring the diameter of the zones that appeared as a result of reuterin inhibition in bacterial lawn formed around the wells. Pictures were taken by ChemiDoc XRS (Bio-Rad, Hercules, CA, USA).
Minimal inhibitory concentration (MIC) of different reuterin samples were determined using serial microdilution assay according to Turcotte et al. [35]. Flat bottom 96-well polypropylene plate was used to prepare two-fold serial dilution (to 2−10) made in a media seeded with the bacterial strain (105 CFU/mL). The microplate was then incubated at 30 °C for 18 h, and OD of the medium was measured at 595 nm using a microplate reader (Infinite, F200 PRO, Tecan Inc, Durham, NC USA).
2.5. Cell culture
Caco-2 cell monolayer is a human colon epithelial cancer cell line, commonly used as a model for drug transport and permeability studies across intestinal epithelium (Knipp et al. 1997). Therefore, exploiting this cell line can assist in evaluating oral bioavailability of reuterin and its interaction with the intestinal epithelium during GI transit to determine the toxicity of reuterin in vitro. Caco-2 cells were obtained from ATCC, and passage 4 cells were cultured in DMEM medium supplemented with 10 % inactivated FBS (PAA Laboratories; A15-70), 1X Pen/Strep (Hyclone), 1 % non-essential amino acids, 100 mM sodium pyruvate (Lonza). Cells were maintained in a humidified atmosphere containing 5 % CO2 at 37 °C and the medium was changed every two-three days.
2.6. Viability and cytotoxicity assays
2.6.1. Neutral red assay
The cell viability was assessed by NRU (Neutral red dye uptake) assay according to [36]. Cells were seeded at 2 × 105 cells/mL in a 96 well plate for 72 h. The growing cells were treated with 100 μL of reuterin at various concentrations (1.05, 2.109, 4.21, 8.43, 16.87, 33.75, 67.5, 135, 270, 540, 1080 mM) and incubated at 37 °C for 48 h with 5 % CO2. After aspirating the solution from the plates, cells were rinsed with 200 μL of media containing NR solution (final concentration of 33 μg/mL) and incubated for 3 h at 37 °C with 5 % CO2. Following incubation time, media was aspirated, cells were rinsed with E-MEM solution and 100 μL of NR fixative (50 % ethanol and acetic acid) was added to solubilize the incorporated dye. After shaking for 10 min, the absorbance was measured at 540 nm by spectrophotometry (SPARK 20 M, Tecan). The viability was calculated by reporting absorbance for each condition as a ratio with PBS treated cell values (% cytotoxicity).
2.6.2. LDH release assay
LDH release was evaluated using the CytoTox-ONE™ cytotoxicity assay kit (Promega, USA). Briefly, cell preparation, seeding density and treatment steps were same as those mentioned in the NRU assay. However, prior to medium collection, 2 μL of lysis solution was added to selected control wells in order to induce the maximum release of LDH. After 5 min, 100 μL of the medium was then transferred to another 96-well plate for LDH release analysis. A volume of CytoTox-ONE™ reagent was added to each well, mixed and incubated at room temperature for 10 min. Subsequently, 50 μL of stop solution was added to each well, and the plate was read using a fluorescence spectrophotometer with an excitation wavelength of 560 nm and an emission wavelength of 590 nm. Each compound treated value was blanked with the reading obtained from control-treated cells and calculated in terms of percent maximal LDH release (Lysis solution treated cells) according to the following formula:
| % Cytotoxicity = (Compound – treated LDH activity) – (Spontaneous LDH activity)/(Maximum LDH activity – Spontaneous LDH activity) |
2.7. Hemolytic activity
Freshly collected rat blood was added in a heparin-treated tube from which 100 μL was added in a 1:1 ratio (v/v) to various concentrations of reuterin (1.05–1080 mM in PBS; 100 μL) in 1 mL conical 96-well plate. 10 % Triton X-100 served as a maximal hemolysis control. The plate was then sealed and incubated for 45 min at 37 °C, which was followed by centrifugation for 10 min at 2000 g in order to pellet down red blood cells. The supernatant was then transferred into a clear 96-well plate and absorbance was read at 540 nm (hemoglobin). Each treated value was blanked with the control-treated blood values and calculated in terms of percent maximal hemolysis control.
2.8. Statistical analysis
In the current study, data are expressed as mean ± standard deviation (SD) from three independent experiments. Dose-response curves were fitted using GraphPad Prism version 8.2 GraphPad Software (San Diego, CA).
3. Results
3.1. Reuterin production and characterization
Reuterin was produced by L. reuteri ATCC 53608 via bioconversion of glycerol. The effect of different glycerol concentrations (ranging from 200 mM to 400 mM) and incubation times (45, 60, 90, and 120 min) was evaluated on the rate of glycerol bioconversion. Residual glycerol concentration was also assessed. According to the results obtained by the agar diffusion assay, there was no significant difference in the inhibition activity between treatments which was 22 mm for all of the samples (Fig. 1). However, Fig. 2 shows HPLC profile for reuterin before (A) and after (B) optimization, and in non-optimized conditions, a high amount of residual glycerol was observed (Fig. 2A). It was observed that glycerol concentrations higher than 300 mM had no significant effect on the bioconversion of glycerol and subsequently, reuterin concentration. The highest rate of bioconversion with no residual glycerol was observed to be 89 %, which was obtained from 300 mM glycerol with an incubation period of 60 and 90 min (Fig. 2B). These conditions were selected for further reuterin production.
Fig. 1.
Inhibitory activity of reuterin produced by bioconversion of 300 mM glycerol after incubation for (A) 45 min, (B) 60 min, (C) 90 min, (D) 120 min.
Fig. 2.
RP-HPLC profile of reuterin (A) before optimization (B) after optimization of reuterin production.
The inhibitory activity of reuterin obtained using optimized condition was confirmed further against both gram-positive and gram-negative indicator strains, L. ivanovii HPB28 and S. newport ATCC 6362, respectively. The MIC value of reuterin against L. ivanovii (2 mM) was approximately two times higher than S. newport (1.05 mM).
3.2. Stability and activity of reuterin in the gastrointestinal tract
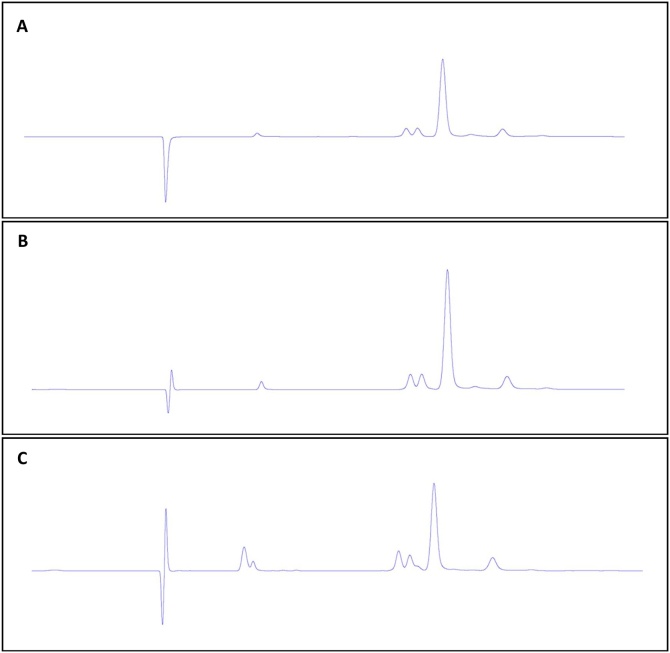
Stability of Reuterin in the GI tract was determined by RP-HPLC, and its antibacterial activity was evaluated by agar well diffusion and microdilution assays. As shown in Fig. 3, HPLC profiles of reuterin after oral (A), gastric (B), and small intestinal (C) phases were identical, indicating high resistance to stressful GI conditions without degradation and bioconversion. Furthermore, according to tryptophane colorimetric assay recovery rate of reuterin during gastrointestinal digestion was >93 % which confirms that there was no degradation or bioconversion of reuterin. The recovery rate of reuterin during gastrointestinal digestion was >90 %. This is an indication that gastrointestinal condition did not induce any conversion of reuterin to acrolein
Fig. 3.
RP-HPLC chromatogram of (A) reuterin after oral digestion (diluted to 40.5 mM), (B) reuterin after gastric digestion (diluted to 135 mM), and (C) reuterin after small intestinal digestion (diluted to 67.5 mM).
Fig. 4 presents reuterin activity by agar diffusion assay after oral, gastric (60 min) and small intestinal digestion (120 min). After oral digestion, the inhibition zones of reuterin were measured to be 30 mm and 29 mm against S. newport and L. ivanovii strains, respectively, while after gastric digestion, these inhibition zones were reduced to 25 mm and 22 mm. In case of the small intestinal digestion, the inhibition zones were further reduced to 18 mm and 15 mm for S. newport and L. ivanovii strains, respectively. Considering the fact of reuterin being diluted two times after each phase of digestion, these results clearly indicate that the antibacterial activity of reuterin remained stable and was unaffected by GI conditions. These results were further confirmed by microtitration assay, and determination of MICs of reuterin against S. newport and L. ivanovii strains after each digestion phase (Table 1).
Fig. 4.
Agar well diffusion assay showing inhibitory activity of reuterin against L. ivanovii (A) and S. newport (B) after Oral, Gastric, and Small intestinal digestion.
Table 1.
Minimal Inhibitory concentrations of reuterin against S. newport and L. ivanovii HPB28.
| Testing solution | L. ivanovii HPB28 | S. newport ATCC6962 |
|---|---|---|
| Oral digested reuterin | 2mM | 1.05 mM |
| Gastric digested reuterin | 5.2mM | 2.6 mM |
| Small intestinal digested reuterin | 10.5 mM | 5.2mM |
3.3. Cytotoxicity of reuterin
The viability of Caco-2 cells exposed to different concentrations of reuterin for 24 h was investigated (up to 1080 mM) using a neutral red uptake assay (Fig. 5A). Upon exposure to reuterin, cells remained viable even up to 10.8 mM concentration. Moreover, treatment with a high concentration of reuterin (1080 mM) showed significantly increased cell viability up to 340 %, compared to control cells.
Fig. 5.
Effect of increasing concentration of reuterin (1.05, 2.109, 4.21, 8.43, 16.87, 33.75, 67.5, 135, 270, 540, 1080 mM) on Caco-2 cells after 48 h exposure. A: cellular viability by Neutral red assay and B: membrane integrity by LDH release assay. The values represent a mean ± SEM of three independent experiments.
Similarly, the effect of increasing concentration of reuterin was determined on membrane integrity (LDH leakage) of Caco-2 cells after 24 h exposure by measuring LDH leakage (Fig. 5B). Since extracellular LDH activity did not change in cells treated with increasing concentrations of reuterin, it is indicated that membrane integrity remains unaltered even in cells exposed to 1080 mM reuterin. However, it should be noted that at the highest concentrations of reuterin (540 mM and 1080 mM), cytotoxicity assay is not reliable due to reuterin interference with readout.
3.4. Hemolysis
The sensitivity of rat erythrocytes to reuterin was evaluated by exposing the cells to increasing concentration of reuterin (up to 180 mM) for 2 h (Fig. 6). Hemolytic activity was calculated and expressed as a percentage of hemolysis. No hemolytic activity was observed at reuterin concentrations up to 270 mM. However, at concentrations above 270 mM, reuterin showed significantly increased hemolytic activity, with maximum hemolysis observed to reach 100 % at 1080 mM concentration.
Fig. 6.
Concentration-dependent hemolytic activity of reuterin (1.05, 2.109, 4.21, 8.43, 16.87, 33.75, 67.5, 135, 270, 540, 1080 mM) on rat blood. The values are mean ± SEM of three independent experiments.
4. Discussion
Reuterin has gained considerable interest as a potential bio-preservative in both the food industry and animal husbandry. Production of reuterin is a critical step for its use in food preservation or other applications. Reuterin is produced via in vitro biotransformation of glycerol by L. reuteri strains. It consists of monomeric, hydrated monomeric and cyclic dimeric forms of 3 hydroxypropionaldehyde (3-HPA) [13]. In this study, a highly purified reuterin was produced. Production of highly purified solutions of reuterin is essential to evaluate its antimicrobial activity (MIC, MBC, a spectrum of inhibition) and safety. In this study, glycerol concentration and biotransformation time were optimized in order to achieve the highest biotransformation yield and the lowest concentration of residual glycerol without the need for a time consuming purification step. A biotransformation yield of 89 % was achieved with 300 mM glycerol and 60 min bioconversion time. HPLC analysis of the samples shown a major pick, which confirmed the high purity of the reuterin solution. Moreover, our reuterin preparation did not seem to contain acrolein, an undesirable toxic compound. These results were consistent with those obtained in a study by [25] in terms of yield and purity after purification [37]. It is important to mention that, production of pure reuterin in large quantities is not a costly process, allowing it be a more potential candidate for application in food preservation.
In order to be used as a food preservative, reuterin has to pass through strict efficacy and safety evaluation to be legally approved for human consumption. Recently, a Guideline for evaluation and approval of new food additives has been proposed [2], which includes different steps, especially a precise identification of the active compound, proof of its efficacy under the actual conditions of use, and the scientific data verifying its safety for the consumer. One of the aims of this study was to evaluate the toxicity of pure reuterin using different well approved in vitro assays. Different aspects were taken into account, including GI stability, and in vitro cytotoxicity and hemolytic potential. The toxic effect of reuterin was evaluated on Caco-2 cells and erythrocytes. Reuterin stability is shown to depend on various parameters, such as temperature, pH, etc. [22]. However, in a recent study, it was shown that reuterin concentration remains stable after 24 h exposure to Caco2 cells under mentioned cell culture condition [30]. In order to evaluate the toxicity of reuterin on mammalian cells, two different cytotoxicity assays were performed to assess both cell viability (NRU assay) and membrane integrity (LDH release assay). Exposure to reuterin at concentrations of up to 1080 mM did not change the cell viability significantly, which indicates that cell membrane integrity remains unaltered in the presence of reuterin. Indeed, at higher concentrations, there was a significant increase in cell viability, which might be associated with the cellular proliferation, mediated by exposure to the high concentrations of reuterin (>33.7 mM). Nevertheless, the proliferative activity of reuterin and its mechanism of action need to be investigated in future studies. However, it should be noted that these concentrations are significantly higher than those observed to be effective against different strains. To the best of our knowledge, there are few studies regarding the toxicity of reuterin in vitro and in vivo. In 1993, [31] provided the only available data on the acute toxicity of reuterin in mice administered through the intraperitoneal route. As a result of this study, LD50 (lethal dose for 50 % of animal) of reuterin was estimated to be about 3374 mM /kg bw of mice. In another study by [29], IC50 (50 % reduced cell viability concentration) value of reuterin was reported to be 0.27 mM in 3T3 fibroblast cells. In a similar study by [30], reuterin was reported to be cytotoxic against HepG2 cells with an IC50 value of 0.41 mM. However, Urrutia-Baca et al. [26] reported that reuterin did not cause cytotoxicity in normal human gastric cells at concentrations of up to 6.06 mg/mL Hs738.St/Int. The difference in effects of reuterin in different studies could be related to the type of eukaryotic cells used, purity of reuterin, or changes in the reuterin activity after purification. Additionally, different cytotoxicity assays measure different endpoints with different exposure time; they can give different results. Therefore, all these parameters need to be considered in order to estimate the cytotoxicity of reuterin. In addition to cytotoxic effect, reuterin was investigated for its hemolytic activity, and it was observed that at concentrations of up to 270 mM, it did not exert any lytic activity on erythrocytes, whereas at higher concentrations, hemolysis was observed. It should be considered that it is higher than the effective concentration which will be used in applications. In a recent study it was reported that some antimicrobial peptides may cause hemolysis at concentration of 150 μM [38]. Similarly some antibiotics such as Amphothericin B nano-aggregates showed hemolytic potential at concentration higher than 200 μg/mL [39]. The reason for the erythrocytes and Caco-2 cells to behave differently in response to reuterin (in hemolytic activity test and LDH assay) might be due to their distinct membrane lipid composition resulting in higher sensitivity of erythrocytes to reuterin compared to Caco-2 cells. However, further studies will be required to investigate the exact mechanism.
Another important factor which is needed to be assessed in different types of applications is GI stability and absorption of reuterin. To our knowledge, this study is the first to provide a complete portrait and solid in vitro scientific data on the GI behavior and cytotoxicity of reuterin. In the current study, reuterin has been observed to be stable during GI passage. This important finding should be analysed with great caution, depending on the intended use of reuterin. For food applications, degradation of reuterin during GI transit is in favor for safety approval, while for therapeutic application, its stability in the digestive system is in favor. In the latter, encapsulation strategy can be considered for protection and controlled release of compounds lacking full stability throughout the GI tract. As a therapeutic agent, the compound requires to resist to digestive enzymes throughout the GI tract and pass across the intestinal barrier to reach the target site; therefore, its interaction with mammalian cells and cytotoxicity are of great interest. Intestinal barrier acts as a selective filter and prevents the penetration of toxic substances or microorganisms, while nutrients can be absorbed [40]. According to Spadoni et al. [41], this selectivity depends on the size and physicochemical properties of compounds. Reuterin is produced by probiotic strains, residents in the GI tract, and because of its very low molecular weight (74.079 g/mol), it may be able to pass through the intestinal barrier and enter the circulatory system and exert its anti-infective action; however, it is required to be investigated.
Altogether, this study provides a framework for future research, including in vivo studies to estimate the toxicity exerted by acute, subacute and long term exposures of reuterin, determine its stability in the GI tract under more complex conditions. In addition, more complex toxicology studies such as effect on mucus layer, loss of barrier integrity are needed for GI safety assessment.
5. Conclusion
In this study, a global portrait of reuterin behavior, its stability and toxicity in the GI tract was generated in vitro. These results are of paramount importance and are necessary for the approval of the use of reuterin in various applications. Due to its chemical nature, reuterin has shown good stability in the GI tract. Cytotoxicity results showed that reuterin does not exert any toxic effect on eukaryotic cells at effective antimicrobial concentrations (MIC and up to 50xMIC). Although this in vitro study provides reliable data regarding stability and toxicity of reuterin in the GI tract, more sophisticated in vitro models (such as 3D culture and multi-tissue organ-on-a-chip) platforms, and in vivo experiments are required to be conducted in order to confirm these findings.
Conflict of Interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
This work is supported by Natural Sciences and Engineering Research (NSERC) and International Development Research Center (CRDI). Grant number: IRCPJ 499946-15.
Edited by Dr. A.M. Tsatsaka
References
- 1.Shehata M., El Sohaimy S., El-Sahn M.A., Youssef M. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016;61:65–75. [Google Scholar]
- 2.Soltani S., Hammami R., Cotter P.D., Rebuffat S., Said L.B., Gaudreau H., Bédard F., Biron E., Drider D., Fliss I. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol. Rev. 2020;45(1):fuaa039. doi: 10.1093/femsre/fuaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh P.L., Benson A.K., Peterson D.A., Patil P.B., Moriyama E.N., Roos S., Walter J. Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution. ISME J. 2010;4:377–387. doi: 10.1038/ismej.2009.123. [DOI] [PubMed] [Google Scholar]
- 4.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greifová G., Májeková H., Greif G., Body P., Greifová M., Dubničková M. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol. 2017;62:515–524. doi: 10.1007/s12223-017-0524-9. [DOI] [PubMed] [Google Scholar]
- 6.Jones M.L., Martoni C.J., Parent M., Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012;107:1505–1513. doi: 10.1017/S0007114511004703. [DOI] [PubMed] [Google Scholar]
- 7.Li S., Qi C., Zhu H., Yu R., Xie C., Peng Y., Yin S.-W., Fan J., Zhao S., Sun J. Lactobacillus reuteri improves gut barrier function and affects diurnal variation of the gut microbiota in mice fed a high-fat diet. Food Funct. 2019;10:4705–4715. doi: 10.1039/c9fo00417c. [DOI] [PubMed] [Google Scholar]
- 8.Yang J., Chen W., Xia P., Zhang W. Dynamic comparison of gut microbiota of mice infected with Shigella flexneri via two different infective routes. Exp. Ther. Med. 2020;19:2273–2281. doi: 10.3892/etm.2020.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh P.-S., Chen C.-W., Kuo Y.-W., Ho H.-H. Lactobacillus spp. reduces ethanol‑induced liver oxidative stress and inflammation in a mouse model of alcoholic steatohepatitis. Exp. Ther. Med. 2021;21:1. doi: 10.3892/etm.2021.9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsson L., Chung T., Dobrogosz W., Lindgren S. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989;2:131–136. [Google Scholar]
- 11.Savino F., Pelle E., Palumeri E., Oggero R., Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics. 2007;119:e124–e130. doi: 10.1542/peds.2006-1222. [DOI] [PubMed] [Google Scholar]
- 12.Schepper J.D., Collins F.L., Rios‐Arce N.D., Raehtz S., Schaefer L., Gardinier J.D., Britton R.A., Parameswaran N., McCabe L.R. Probiotic Lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. J. Bone Miner. Res. 2019;34:681–698. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vollenweider S., Lacroix C. 3-Hydroxypropionaldehyde: applications and perspectives of biotechnological production. Appl. Microbiol. Biotechnol. 2004;64:16–27. doi: 10.1007/s00253-003-1497-y. [DOI] [PubMed] [Google Scholar]
- 14.Vollenweider S., Grassi G., König I., Puhan Z. Purification and structural characterization of 3-hydroxypropionaldehyde and its derivatives. J. Agric. Food. Chem. 2003;51:3287–3293. doi: 10.1021/jf021086d. [DOI] [PubMed] [Google Scholar]
- 15.El-Ziney M., Van Den Tempel T., Debevere J., Jakobsen M. Application of reuterin produced by Lactobacillus reuteri 12002 for meat decontamination and preservation. J. Food Prot. 1999;62:257–261. doi: 10.4315/0362-028x-62.3.257. [DOI] [PubMed] [Google Scholar]
- 16.Ávila M., Gómez-Torres N., Hernández M., Garde S. Inhibitory activity of reuterin, nisin, lysozyme and nitrite against vegetative cells and spores of dairy-related Clostridium species. Int. J. Food Microbiol. 2014;172:70–75. doi: 10.1016/j.ijfoodmicro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Cleusix V., Lacroix C., Vollenweider S., Duboux M., Le Blay G. Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 2007;7:101. doi: 10.1186/1471-2180-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanchi H., Hammami R., Gingras H., Kourda R., Bergeron M.G., Ben Hamida J., Ouellette M., Fliss I. Inhibition of MRSA and of Clostridium difficile by durancin 61A: synergy with bacteriocins and antibiotics. Future Microbiol. 2017;12:205–212. doi: 10.2217/fmb-2016-0113. [DOI] [PubMed] [Google Scholar]
- 19.Arqués J.L., Rodríguez E., Nuñez M., Medina M. Inactivation of Gram-negative pathogens in refrigerated milk by reuterin in combination with nisin or the lactoperoxidase system. Eur. Food Res. Technol. 2008;227:77–82. [Google Scholar]
- 20.Montiel R., Martín-Cabrejas I., Medina M. Reuterin, lactoperoxidase, lactoferrin and high hydrostatic pressure on the inactivation of food-borne pathogens in cooked ham. Food Control. 2015;51:122–128. [Google Scholar]
- 21.Asare P.T., Greppi A., Stettler M., Schwab C., Stevens M.J., Lacroix C. Decontamination of minimally-processed fresh lettuce using reuterin produced by Lactobacillus reuteri. Front. Microbiol. 2018;9:1421. doi: 10.3389/fmicb.2018.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lüthi-Peng Q., Schärer S., Puhan Z. Production and stability of 3-hydroxypropionaldehyde in Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2002;60:73–80. doi: 10.1007/s00253-002-1099-0. [DOI] [PubMed] [Google Scholar]
- 23.Langa S., Landete J.M., Martín-Cabrejas I., Rodríguez E., Arqués J.L., Medina M. In situ reuterin production by Lactobacillus reuteri in dairy products. Food Control. 2013;33:200–206. [Google Scholar]
- 24.Ortiz-Rivera Y., Sánchez-Vega R., Gutiérrez-Méndez N., León-Félix J., Acosta-Muñiz C., Sepulveda D. Production of reuterin in a fermented milk product by Lactobacillus reuteri: inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017;100:4258–4268. doi: 10.3168/jds.2016-11534. [DOI] [PubMed] [Google Scholar]
- 25.Vimont A., Fernandez B., Ahmed G., Fortin H.-P., Fliss I. Quantitative antifungal activity of reuterin against food isolates of yeasts and moulds and its potential application in yogurt. Int. J. Food Microbiol. 2019;289:182–188. doi: 10.1016/j.ijfoodmicro.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Urrutia-Baca V.H., Escamilla-García E., de la Garza-Ramos M.A., Tamez-Guerra P., Gomez-Flores R., Urbina-Ríos C.S. In vitro antimicrobial activity and downregulation of virulence gene expression on Helicobacter pylori by reuterin. Probiotics Antimicrob. Proteins. 2018;10:168–175. doi: 10.1007/s12602-017-9342-2. [DOI] [PubMed] [Google Scholar]
- 27.Widyarman A.S., Theodorea C.F. Effect of reuterin on dual-species biofilm in vitro of Streptococcus mutans and Veillonella parvula. J. Int. Dent. Med. 2019;12:77–83. [Google Scholar]
- 28.H.-W. Sung, C.-N. Chen, H.-F. Liang, H. Tu, Medical use of reuterin, (2005) Google Patents.
- 29.Chen C.N., Sung H.W., Liang H.F., Chang W.H. Feasibility study using a natural compound (reuterin) produced by Lactobacillus reuteri in sterilizing and crosslinking biological tissues. J. Biomed. Mater. Res. 2002;61:360–369. doi: 10.1002/jbm.10153. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Cruz M.L., Martín-Cabrejas I., Pérez-del Palacio J., Gaya P., Díaz-Navarro C., Navas J.M., Medina M., Arqués J.L. In vitro toxicity of reuterin, a potential food biopreservative. Food Chem. Toxicol. 2016;96:155–159. doi: 10.1016/j.fct.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Yunmbam M.K., Roberts J.F. In vivo evaluation of reuterin and its combinations with suramin, melarsoprol, DL-alpha-difluoromethylornithine and bleomycin in mice infected with Trypanosoma brucei brucei. Comp. Biochem. Phys. C. 1993;105:521–524. doi: 10.1016/0742-8413(93)90095-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu F., Yu B. Efficient production of reuterin from glycerol by magnetically immobilized Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2015;99:4659–4666. doi: 10.1007/s00253-015-6530-4. [DOI] [PubMed] [Google Scholar]
- 33.Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carrière F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14:991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 34.Tagg J.R., Dajani A.S., Wannamaker L.W. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 1976;40:722. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turcotte C., Lacroix C., Kheadr E., Grignon L., Fliss I.l. A rapid turbidometric microplate bioassay for accurate quantification of lactic acid bacteria bacteriocins. Int. J. Food Microbiol. 2004;90:283–293. doi: 10.1016/s0168-1605(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 36.Repetto G., Del Peso A., Zurita J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008;3:1125. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 37.Talarico T.L., Dobrogosz W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989;33:674–679. doi: 10.1128/aac.33.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greco I., Molchanova N., Holmedal E., Jenssen H., Hummel B.D., Watts J.L., Håkansson J., Hansen P.R., Svenson J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-69995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zia Q., Mohammad O., Rauf M.A., Khan W., Zubair S. Biomimetically engineered Amphotericin B nano-aggregates circumvent toxicity constraints and treat systemic fungal infection in experimental animals. Sci. Rep. 2017;7:1–19. doi: 10.1038/s41598-017-11847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farhadi A., Banan A., Fields J., Keshavarzian A. Intestinal barrier: an interface between health and disease. J. Gastroentrol. Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 41.Spadoni I., Zagato E., Bertocchi A., Paolinelli R., Hot E., Di Sabatino A., Caprioli F., Bottiglieri L., Oldani A., Viale G. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]