Abstract
Although positive effects of oxytocin (OT) on social functioning are well-demonstrated, little is known about the mechanisms through which OT may drive early social development, or its therapeutic efficacy in infancy. To address these critical issues, we investigated the effects of exogenous OT on neural (EEG) and behavioral responses during observation of live facial gestures in infant macaques with limited social exposure (i.e. nursery-reared). Three key findings were revealed. First, OT increased alpha suppression over posterior scalp regions during observation of facial gestures but not non-biological movement, suggesting that OT targets self-other matching and attentional cortical networks involved in social perception from very early infancy. Second, OT increased infant production of matching facial gestures and attention towards the most socially-relevant facial stimuli, both behaviors typically silenced by early social deprivation. Third, infants with higher cortisol levels appeared to benefit the most from OT, displaying greater improvements in prosocial behaviors after OT administration. Altogether, these findings suggest that OT promotes prosocial behaviors and associated neural responses likely impacted by early social adversity, and demonstrate the potential of OT administration to ameliorate social difficulties in the context of neurodevelopmental and early-emerging psychiatric disorders, at a developmental stage when brain plasticity is greatest.
Keywords: Infancy, EEG mu/alpha suppression, Self-other matching, Social attunement, Oxytocin
1. Introduction
Although first recognized as a regulator of parturition and lactation, the hormone and neuropeptide oxytocin (OT) has now been implicated in a wide range of social behaviors in diverse mammalian species (Chang and Platt, 2014; Guastella and MacLeod, 2012; Lukas et al., 2011). Critically, the oxytocinergic system is functional very soon after birth, and is modulated via early social interactions and other components of early parenting (Clark et al., 2013; Feldman et al., 2010; Hammock, 2015; Weisman et al., 2012). OT signaling could therefore represent a main driver of early social development (Hammock, 2015; Miller and Caldwell, 2015), with perturbations in the oxytocinergic system likely to result in the emergence of impaired socio-emotional functioning (Meyer-Lindenberg et al., 2011; Rajamani et al., 2018).
Intranasally administered OT enhances various socio-emotional processes, including emotion recognition (Lischke et al., 2012), social attention (Dal Monte et al., 2014; Guastella et al., 2008), and empathy (Domes et al., 2007b). Neuroimaging studies in adult populations (e.g., Domes et al., 2007a; Gamer et al., 2010; Labuschagne et al., 2010) suggest that these effects are likely mediated by the amygdala, a subcortical structure strictly involved in emotion processing and social perception. The amygdala is OT-receptor dense in rodents (e.g. Bale et al., 2001; Insel et al., 1993), with one study providing immunohistochemical evidence for OT receptors (OXTRs) in the human amygdala as well (Boccia et al., 2013); though see Freeman et al. (2014) for discussion regarding the specificty of such findings. In nonhuman primates (NHPs), OXTRs are largely expressed in the nucleus basalis of Meynert (NBM) (Freeman et al., 2014; Putnam et al., 2018), a source of cholinergic innervation to the amygdala and cortical mantle, and a major regulator of visual attention. Therefore, this cholinergic input could represent a core neural mechanism through which OT mediates visual attention in response to socially relevant cues (Freeman et al., 2014; Putnam et al., 2018). Research with rhesus macaque monkeys also indicates that OT modulates serotonergic communication between the raphe nucleus and amygdala via 5HT1A receptors (Lefevre et al., 2017). Interestingly, some prosocial consequences of OT have been linked to reduction in anxiety and increased stress coping (Campbell, 2010; Heinrichs et al., 2003), with such anxiolytic effects proposed to rely on the amygdala and other affective brain structures (Bethlehem et al., 2013; Labuschagne et al., 2010). OXTRs are also expressed in the superior colliculus (SC) of NHPs, an area of the brain involved in gaze control (Freeman et al., 2014). As such, another possibility is that OT effects on social behavior are mediated by an increase in visual orienting responses to social relevant stimuli, and thereby modulating amygdala activity in relation to social perception and social-decision making (Forcelli et al., 2016; Gangopadhyay et al., 2021).
Adult human electroencephalography (EEG) and magnetoencephalography (MEG) studies indicate that OT also influences widespread cortical activity during social perception tasks, particularly in the alpha frequency band (8−13 Hz in adults; 5−9 Hz in infants) (Festante et al., 2020; Levy et al., 2016; Perry et al., 2010). Oscillations in this frequency band are maximally expressed in amplitude (they are ‘synchronized’) during periods of rest, becoming suppressed (or desynchronized) during tasks requiring cortical engagement. Alpha synchronization is classically related to a cortical idling state that results from the synchronous neural firing of wide cortical areas (Nunez et al., 2001), while more recent theories posit that high amplitude oscillations in this band also reflect a selective cortical inhibition of thalamo-cortical and cortico-cortical information transfer associated with task-irrelevant or task-competing processes (Klimesch, 2012). Conversely, a suppression of alpha oscillations typically represents task-dependent cortical activation (Klimesch, 2012). In particular, suppression of alpha band activity over centro-parietal cortical regions (i.e., the mu rhythm or sensorimotor alpha) has been linked to self-other mapping (Arnstein et al., 2011; Fox et al., 2016), and over parieto-occipital regions to attentional processes (i.e., visual/attentional alpha) (Pfurtscheller et al., 1994). Altogether, this indicates that OT modulates cortical network activity underlying both these cognitive processes.
Through activation of socio-emotional brain networks, OT signaling has been proposed to play a critical role in the emergence of social behavior during early development (Hammock, 2015; Miller and Caldwell, 2015). However, this proposal remains largely unexplored, and very few studies thus far have investigated the relationship between OT and social behavior in infants and young children. One study showed that OT administration increases affiliative behavior in infant monkeys (Simpson et al., 2014), and a recent fNIRS study has linked oxytocin receptor gene methylation (OXTRm) in 5-month-old infants to later neural responses to emotional faces (Krol et al., 2019).
In older children and adolescents, plasma OT levels positively predict socio-cognitive performance in both typically developing (TD) and autism spectrum disorders groups (Parker et al., 2014). Recently, it has also been reported that children exposed to early life adversity have lower levels of endogenous oxytocin, and show reduced social attention towards face stimuli compared to TD children (Suzuki et al., 2020). These findings in pediatric populations are also in line with adult research where a dysfunctional oxytocinergic system has been linked to maladaptive socio-emotional processing in the context of various psychiatric and neurodevelopmental disorders (Bakermans-Kranenburg and van IJzendoorn, 2013; Meyer-Lindenberg et al., 2011; Rajamani et al., 2018).
From a therapeutic perspective, although there is mixed evidence concerning the efficacy of OT administration (Erdozain and Peñagarikano, 2020), several clinical trials have demonstrated beneficial OT effects on social functioning (e.g., Guastella et al., 2010; Parker et al., 2017). Additionally, in some cases where no social improvement was reported, specific OT-related effects in other symptom domains were found (e.g. decrease in repetitive behaviors) (Bernaerts et al., 2020).
It is therefore of particular importance to better clarify the role of oxytocin in early social development, especially in the context of early-emerging socio-emotional deficits, and elucidating whether neuromodulatory dysregulation and negative behavior associated with impaired oxytocin signaling can be reversed via early interventions in infants and young children is vital to achieve this goal. Accordingly, the current study was designed to address these critical questions by investigating the effects of exogenous OT administration on infant cortical activity and behavioral responses to live facial gestures, in a group of three-month-old nursery-reared rhesus macaques (Macaca mulatta). Nursery-reared macaques (i.e. raised in a nursery since birth) have limited early social experience and are at increased risk for maladaptive social outcomes, including increased stress reactivity (Dettmer et al., 2012) and socio-emotional difficulties from the earliest months of life (e.g., Paukner et al., 2020; Simpson et al., 2019, 2016; Vanderwert et al., 2015). Such deficits can be predictive of longer-term negative outcomes, such as an increased risk for anxiety (Conti et al., 2012; Dettmer and Suomi, 2014). These NHP findings parallel those from human studies concerning the negative consequences of early social deprivation (Nelson, 2017; Sonuga-Barke et al., 2017; Wade et al., 2019). In addition, and of particular relevance here, nursery-rearing in macaques has been associated with dysregulation of the OT system in previous research (Baker et al., 2017; Winslow et al., 2003).
Here we used a blind, placebo-controlled, within-subjects design and infants were nebulized with oxytocin or saline (one per day) on two different days, before undergoing EEG testing. EEG assessments were performed while infants observed an experimenter producing dynamic facial gestures, a paradigm that has been found to elicit EEG alpha/mu suppression previously in human infants (Rayson et al., 2017, 2016) and macaque neonates (Ferrari et al., 2012). Infant prosocial behaviors displayed throughout the assessments, namely attention to the stimulus and production of facial communicative gestures, were also assessed. Finally, we measured infant cortisol levels prior to EEG recordings in order to evaluate the relationship between infant stress-responsivity or anxiety and OT effects.
2. Materials and methods
2.1. Subjects and housing conditions
The sample consisted of 22 (12 females) three-month-old rhesus macaques (Macaca mulatta) from two cohorts of healthy infants, born in 2014 (n = 10) and 2015 (n = 12) respectively. All infants were born and raised at the Laboratory of Comparative Ethology at the National Institutes of Health. All animal care and testing procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the University of Maryland Institutional Animal Care and Use Committee. Infants were separated from their mothers on the day of birth, and subsequently raised in a nursery following the protocol reported by Simpson et al. (2016). Further details concerning rearing procedures are provided in Supplementary Information (SI) Methods. Three infants were excluded from the original sample due to technical issues during EEG data acquisition, therefore the final sample comprised 19 infants (11 females).
2.2. Oxytocin administration for EEG experiment
Infants were tested on two separate days in their third month of life (Mdays = 96.10, SDdays = 3.94) using a blind, placebo-controlled design. During these test sessions, either OT (25 IU/mL; Bimeda- MTC Animal Health) or a sterile saline solution was administered using a Pari Baby Nebulizer (established protocol from previous research; Simpson et al. (2014). The order of administration (oxytocin/saline) was counterbalanced across subjects. Further details concerning solution administration and analysis are provided in SI Methods.
2.3. Saliva collection procedure
To evaluate OT and cortisol levels after oxytocin/saline administration, saliva sample were collected one hour after the end of nebulization and prior to the EEG assessment on both testing days. See SI Methods for more details concerning saliva collection procedure, processing and analysis.
2.4. Experimental procedure
At the beginning of each treatment session, infants were brought to a testing room. During EEG acquisition, one experimenter held the infant while a second served as the stimuli-presentation model. The procedure was based on an imitation task previously developed for infant macaques (Ferrari et al., 2006), and comprised three conditions: 1) lip-smacking (LS), an affiliative gesture in macaques; 2) tongue protrusion (TP), a non-communicative facial gesture; and 3) a nonsocial control comprised of a white plastic rotating disk with black/red orthogonal stripes (Disk). Condition order was randomized across infants and treatments.
Each condition (LS/TP/Disk) started with a 40-second static period, in which the model either displayed a neutral facial expression in the LS and TP conditions, or held the disk still in the Disk condition. The model then displayed a LS or TP gesture for 20 s, or rotated the disk for the same period of time. The model then displayed either a neutral expression/still disk again for 20 s. This movement-still face sequence was repeated three times in total. A schematic representation of the experimental procedure is illustrated in Fig. 1.
Fig. 1.
Experimental procedure. Schematic representation of the experimental procedure during EEG acquisition. A. Experimental setting. A familiar caretaker (holder) held the capped infant monkey in front of a second experimenter who served as the stimulus model (presenter). A camera, positioned behind the presenter, recorded the infant monkey. B. Experimental conditions: TP, tongue protrusion; LS, lip smacking; Disk, rotating disk. C. Design and timings for each experimental condition.
All the three conditions were presented during the same EEG recording session. Each session lasted approximately 15 min, which included the time to place the EEG cap and short breaks (∼ 1−2 min) between each condition. All experimenters were familiar caretakers, blind to the treatment (i.e., oxytocin or saline) at the time of testing. Stimuli were presented to all infants by the same presenter, who wore the same lab scrubs consistently across subjects, sessions and treatments.
2.5. Behavioral coding
All testing sessions were video recorded using a 30 Hz video camera (Sony Digital Video Camcorder ZR600, USA), positioned behind the human model. The video was time-stamped with a vertical integrated time code, synchronized online with the EEG acquisition system (James Long Company, NY, USA). Videos were then coded off-line frame-by-frame using the Video Coding System (James Long Company, NY, USA). The following infant behaviors were coded: (a) attention to the experimental stimuli (i.e. gaze towards the stimulus or away from it); (b) LS (i.e., rapid opening and closing of the mouth); (c) TP (i.e. extension of the tongue that crosses the inner edge of the lower lip, then retraction of tongue); (d) arm and hand movements; and (e) gross body movements. Inter-observer agreement was calculated for a random 20 % of all videos using percent agreement, with a minimum of 75 % achieved for each behavior scored.
2.6. EEG acquisition
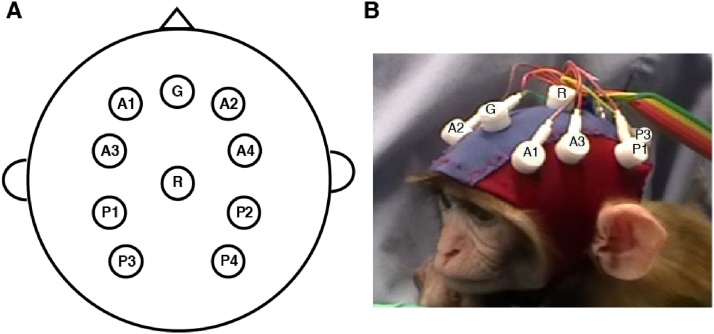
All EEG recordings were obtained using a custom lycra cap (Electro-Cap International, OH, USA) fitted with ten tin electrodes (see Fig. 2). Four anterior electrodes were placed approximately over the frontal/motor cortex (A1, A3: anterior left; A2, A4: anterior right) and four posterior electrodes were placed approximately over the parietal cortex (P1, P3: posterior left; P2, P4: posterior right). The vertex served as the reference, while an electrode located on the forehead served as the ground. The subject’s head was shaved and a mild abrading gel was applied to clean the scalp and improve impedances, which were kept below 20 kΩ throughout data acquisition. The EEG signal was band-pass filtered online from 0.1–100 Hz, digitized with a 16-bit A/D converter (±5 V input range) at 1 KHz. Data acquisition was performed using the James Long recording system (James Long Company, NY, USA). EEG preprocessing details can be found in SI.
Fig. 2.
Custom lycra EEG cap fitted with 10 tin electrodes. A. Schematic representation of electrode positions on the scalp. Four anterior electrodes were placed approximately over the frontal/motor cortex and four posterior electrodes were placed approximately over the parietal cortex. Anterior left electrodes: A1 and A3; Anterior right electrodes: A2 and A4; Posterior left electrodes: P1 and P3; Posterior right electrodes: P2 and P4; Reference electrode: R; Ground electrode: G. B. Close-up view of the EEG cap fitted on a 3-month-old macaque.
EEG suppression was computed in the alpha frequency band: 5−7 Hz in infant macaques (Ferrari et al., 2012; Vanderwert et al., 2012). This was calculated as the percentage change in average absolute power (μV2) during the stimulus presentation (i.e., LS, TP or disk rotation) from baseline (i.e., still face or still disk), with condition-specific (averaged across epochs in that condition) baselines utilized. As in previous EEG studies of alpha activity in macaques (Ferrari et al., 2012; Festante et al., 2018; Vanderwert et al., 2015), suppression was calculated for two clusters of electrodes: one anterior (4 electrodes) and one posterior (4 electrodes). For each cluster (anterior/posterior), in each experimental condition (LS/TP/Disk) and treatment (oxytocin/saline), suppression values were calculated for each subject.
EEG suppression was also computed in the beta band (15−17 Hz in infant macaques (Festante et al., 2018)), another sub-component of the mu rhythm. However, no effects of OT were revealed in this frequency band (See results of this analysis in SI Results).
2.7. Statistical analyses
A mixed model framework was used for statistical analysis using R v3.6.3 (R Development Core Team, 2020). Further details on the R packages utilized and model checks are provided in SI Methods. For analysis of the EEG, linear mixed models were utilized with alpha suppression during observation treated as the dependent measure. For each condition (LS/TP/Disk), a model was run that included electrode cluster (anterior/posterior), treatment (saline/oxytocin), and their interaction as fixed effects, and subject-specific intercepts and by-treatment subject-specific slopes as random effects.
Linear mixed models were used to explore infants’ own behavior during the different experimental conditions (LS/TP/Disk). For each condition, models were run to investigate the proportion of time infants spent gazing at the stimulus (i.e. time attending to the stimulus divided by total time the stimulus was presented), log-transformed to avoid issues with analyzing raw proportions using linear models (Jaeger, 2008). Treatment (oxytocin/saline) was included as a fixed effect and subject-specific intercepts as a random effect. To examine infants’ own gesture production (frequency of lip-smacks or tongue protrusions; i.e. count data), a Poisson generalized linear model with a logit link function was run for each condition (LS/TP/Disk), with treatment included as a fixed effect (saline/oxytocin) and subject-specific intercepts as a random effect. The same models were also run with infants’ cortisol level added as a fixed effect.
Comparable models to all those described above were run with sex and cortisol level as an additional factor. No significant effects were revealed (all p > 0.05) apart from those concerning cortisol level and attention/facial gestures described in Section 3.3. Cortisol level and infant behavior. Similarly, no relationships were found between EEG suppression and infant behavioral responses.
P-values for fixed effects and their interactions were obtained using type III Wald chi-square or F tests, and all post-hoc tests (least-square means) were corrected for multiple comparisons using Tukey-Kramer contrasts.
3. Results
To ensure the administration of oxytocin was successful, oxytocin level was assessed from saliva samples collected prior to EEG assessments. Analysis of these samples confirmed that oxytocin levels were higher in the oxytocin than the saline treatment. Detailed results are reported in SI (see section 2. SI Results and Fig. S1).
3.1. Infant alpha suppression during observation
To be included in the following analyses, subjects were required to have a minimum of five epochs per condition (disk/lip-smacking/tongue protrusion) and treatment (oxytocin/saline) after pre-processing of the EEG data, in keeping with similar EEG studies (Rayson et al., 2017, 2016). The number of remaining subjects and average epoch numbers can be found in SI (Table S1). Exploratory mixed model analyses revealed no significant differences between the number of usable EEG epochs in the different conditions or treatments (all p > 0.05).
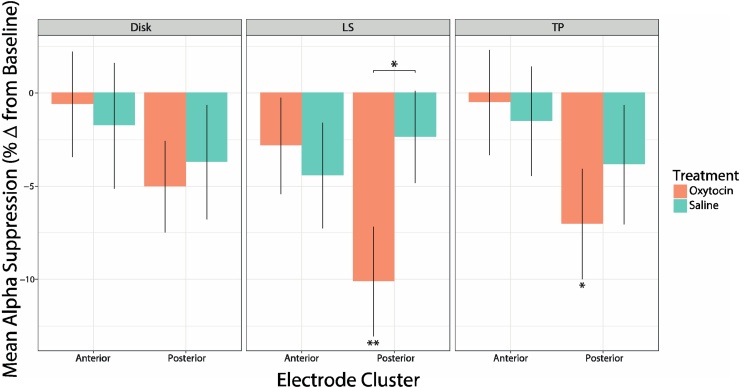
During observation of lip-smacking (LS), a significant main effect of electrode cluster (anterior/posterior) was revealed [F1, 34) = 6.49, p = 0.016], as well as a significant electrode cluster by treatment (oxytocin/saline) interaction [F(1, 34) = 5.35, p = 0.027]. Follow-up analyses revealed that in the OT treatment, more alpha suppression occurred in the posterior compared to anterior electrode cluster [t(34) = 2.55, p = 0.016]; and in the posterior cluster, more suppression occurred in the OT treatment compared to saline treatment [t(31.8) = -2.08, p = 0.046]. During observation of tongue protrusion (TP), there was a significant main effect of electrode cluster (more suppression in the posterior compared to anterior electrode cluster [F(1, 33) = 5.31, p = 0.028]), but no significant interaction between cluster and treatment. A significant main effect of electrode cluster was also revealed in the disk condition, with more decrease in power in the posterior compared to anterior cluster [F(1, 52) = 5.25, p = 0.026] but not significant alpha suppression revealed in either clusters. These results are illustrated in Fig. 3. See Table S2 in SI for results concerning power differences from baseline.
Fig. 3.
Alpha suppression for each condition in anterior and posterior electrode clusters. Mean percentage of alpha power change from baseline during observation of the Disk, lip-smacking (LS), and tongue protrusion (TP) conditions, in the saline and oxytocin (OT) treatments. Error bars represent ±1 standard error. Significant differences from baseline and between treatments are denoted by asterisks (* p < 0.05, ** p < 0.01).
3.2. Infants’ own facial gestures and gaze towards experimental stimuli
In the LS condition, there was a significant main effect of treatment on the frequency of infants’ own lip-smacks [z = -1.98, p = 0.003], with more gestures produced in the OT treatment (n = 18; M = 3.00, SD = 5.33) than in the saline (n = 19; M = 0.947, SD = 2.738) treatment. No significant effect of treatment on infant lips-smacks was found in the disk or TP condition. In the TP condition, there was a significant main effect of treatment on the frequency of infants’ own tongue protrusions [z = -1.98, p = 0.048], with more gestures produced in the OT (n = 18; M = 2.06, SD = 3.02) compared to saline (n = 19; M = 0.95, SD = 2.17) treatment. In the disk condition, a significant main effect of treatment on the frequency of infant tongue protrusions was also revealed [χ2(1) = 8.08, p < 0.01], however in this condition, more tongue protrusions were produced in the saline (n = 19; M = 1.21, SD = 2.59) compared to the OT (n = 18; M = 0.33, SD = 0.69) treatment. Results concerning infants’ own facial gesture production are outlined in Fig. 4.
Fig. 4.
Frequency of infant lip-smacking and tongue protrusion. Infant production of lip-smacks (LS) and tongue protrusions (TP) during observation of the Disk, LS, and TP conditions, in the saline and oxytocin (OT) treatments. Error bars represent ±1 standard error. Significant differences between treatments are denoted by asterisks (** p < 0.01, *** p < 0.001).
In the LS condition only, a significant main effect of treatment was revealed on the proportion of time infants spent looking at the experimenter presenting the stimulus [F(1, 17.48) = 10.32, p = 0.005], with more gaze to the experimenter in the OT treatment (n = 18; M = 0.44, SD = 0.16) versus saline (n = 19; M = 0.32, SD = 0.11) treatment. No significant differences between OT and saline were found in the TP or disk condition (all p> 0.05). Results concerning the proportion of infant attention during stimuli presentation are illustrated in Fig. S2.
3.3. Cortisol level and infant behavior
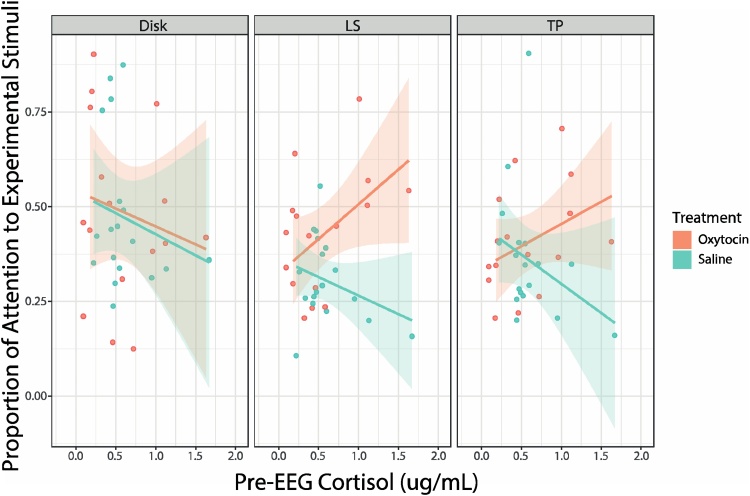
In the LS condition, a significant cortisol by treatment interaction was revealed [F(1, 15.96) = 9.66, p = 0.007]. Specifically, higher levels of cortisol were related to more gaze towards the stimulus in the OT treatment (n = 16; M = 0.43, SD = 0.16), and less gaze towards the stimulus in the saline treatment (n = 18; M = 0.31, SD = 0.11). Similarly, a significant cortisol by treatment interaction was revealed in the TP condition [F(1, 22.53) = 5.98, p = 0.023], with higher levels of cortisol again related to more attention towards the experimenter presenting the stimulus in the OT treatment (n = 16; M = 0.41, SD = 0.14), but less gaze towards the experimenter in the saline treatment (n = 18; M = 0.36, SD = 0.17). No significant effects of cortisol were found in the disk condition (all p> 0.05). See Fig. 5 for illustration of these results.
Fig. 5.
Relationship between cortisol and attention to stimuli. Scatter plots reflect the relationship between cortisol level and infants’ attention toward the stimuli during the Disk, LS, and TP conditions, in the saline and oxytocin (OT) treatments. Shaded regions around each line represent ±1 standard error.
In the LS condition, a relationship between cortisol levels and infants’ own facial gestures was also found. Specifically, cortisol was linked to infants’ own LS, but not own TP, with a significant interaction between treatment and cortisol revealed [χ2(1) = 6.362, p = 0.012]. That is, higher levels of cortisol were related to more infant LS in the oxytocin treatment (n = 16; M = 3.38, SD = 5.55), but lower levels of infant LS in the saline treatment (n = 18; M = 0.33, SD = 0.59). No effects of cortisol on infant facial gestures were revealed in the TP or disk condition (all p> 0.05). These results are illustrated in Fig S3.
4. Discussion
Three key effects of OT administration on infant macaque responses to live facial gestures were revealed here: i) increased cortical activation in the alpha frequency band; ii) more frequent production of infant own facial gestures; and iii) modulation of a relationship between cortisol levels and prosocial behavior. Altogether, this suggests that OT has an active role in the early emergence of social competences, targeting neural circuits associated with social perception and facilitating prosocial behavior.
Our EEG analysis revealed an effect of acute exogenous OT on activity in the alpha frequency band during observation of an experimenter performing lip-smacking (LS) and tongue protrusion (TP) gestures. In the OT treatment, alpha suppression occurred over the posterior electrode cluster in both conditions, but not in the control disk condition. We also found an increase in alpha suppression in the OT compared to saline treatment during LS observation. Significant suppression did not occur in any condition following saline administration. These results represent the first neurophysiological evidence for exogenous OT targeting cortical regions involved in social processing and socially-driven visuomotor development early in infancy, and substantiates the idea that the oxytocinergic system modulates activity in brain networks that support face perception (Dal Monte et al., 2014; Liu et al., 2015; Taubert et al., 2019; Tillman et al., 2019) and other socio-cognitive processes (Festante et al., 2020; Levy et al., 2016; Perry et al., 2010).
Increased alpha suppression after OT administration is in keeping with adult action observation studies (Festante et al., 2020; Levy et al., 2016; Perry et al., 2010) pointing to the mirror system as a neural target of OT. Classically, this system comprises premotor regions, the inferior frontal gyrus, and more posterior regions within the parietal cortex, and is involved in self-other social mapping (Rizzolatti and Sinigaglia, 2016). Alpha suppression over sensorimotor regions is considered a reliable proxy measure of the mirror system activity (Arnstein et al., 2011; Fox et al., 2016), and occurs in both adults and infants during observation and execution of facial gestures (Ferrari et al., 2012; Moore et al., 2012; Rayson et al., 2017, 2016; Vanderwert et al., 2015). As such, effects on activity in the 5−7 Hz band in our study could reflect the recruitment of this system, suggesting it could be an OT target in macaques from early infancy. This interpretation is supported by a recent study with human infants, where methylation of oxytocin receptor (OXTRm) was associated specifically with inferior frontal cortex activity in response to faces later on in development (Krol et al., 2019). Not only is this a key region of the human mirror system (Rizzolatti and Sinigaglia, 2016), but it is also linked consistently to various forms of social dysfunction (Dapretto et al., 2006; Patriquin et al., 2016). Notably, putative mirror system activity during facial gesture observation has already been associated with early social deprivation in macaques (Vanderwert et al., 2015) and the quality of mother-infant interactions in humans (Rayson et al., 2017), which may explain the lack of suppression observed in our nursery-reared animals during the saline session.
In the infant EEG research outlined above (Ferrari et al., 2012; Rayson et al., 2017, 2016; Vanderwert et al., 2015), cortical activity corresponded closely to central/anterior scalp regions where sensorimotor versus visual alpha is classically recorded (Fox et al., 2016). However, in our study, alpha suppression during the OT session was more posteriorly located, indicating that it may not, or may not exclusively, reflect sensorimotor activity, but instead could represent visual/attentional alpha modulation. In human adults, OT does increase alpha suppression over much of the scalp during action observation (Festante et al., 2020; Perry et al., 2010), not just in central regions. An interesting possibility is that suppression in parietal locations found in our study reflects increased functional connectivity between occipital and central brain regions, and thus greater coupling of mirroring and attentional processes, which are likely to occur concurrently (Debnath et al., 2019; Festante et al., 2020).
A possible mechanism through which OT influences alpha suppression involves OT-sensitive cholinergic innervations from NBM to the amygdala and the cerebral cortex (Freeman et al., 2014). Across NHP species, NBM is a key regulator of visual attention, especially in response to social stimulation, and its activity has been linked to alpha band reactivity in humans (Wan et al., 2019). It is therefore plausible that OT–OXTR binding in NBM activates this cholinergic circuitry, thereby facilitating alpha suppression linked to social attention and facial gesture coupling. It is also possible that mechanisms of visual attention mediated by the SC modulate visual orienting responses, and therefore influence how a face stimulus is explored and processed, both in terms of gesture recognition and its reward value. Here, it is important to note that the effects of OT on social perception and social responsiveness could result from several mechanisms that act synergistically at different levels. For example, this could occur via modulation of visual attention mechanisms involving the NBM or SC (Freeman et al., 2014; Putnam et al., 2018); while at the same time, through activation of mechanisms related to stress /anxiety inhibition which involve the amygdala and other affective brain regions (Gangopadhyay et al., 2021).
In keeping with our neurophysiological findings, behavioral results here also suggest that OT affected both attentional and self-other matching mechanisms. In fact, infants increased their attention toward the most socially-relevant stimulus (LS) following OT administration, which is consistent with previous research showing that OT modulates social attention and gaze toward faces (Dal Monte et al., 2014; Liu et al., 2015; Nishizato et al., 2017; Parr et al., 2013). Moreover, OT administration affected infant production of facial gestures. Intriguingly, not only did own facial gestures increase after OT administration, but infant gesture production was also very well attuned to the gestures produced by the experimenter. In fact, increases in TP occurred specifically in the TP condition, and increases in LS in the LS condition. This result is somewhat surprising as macaques do not typically express such synchronous, matched responses at three months. Newborn macaques do tend to respond to their mother’s LS with LS themselves (Ferrari et al., 2009), but this kind of response becomes increasingly scarce over the first month of life. In infants with limited early social experience, the frequency of matched responses drops even more dramatically, almost disappearing within a week (Ferrari et al., 2006). This indicates that, without adequate social stimulation from the mother, infant’s capacity to respond appropriately to social stimuli can be impaired. Results from the current study suggest that in similarly limited social conditions, OT is capable of ‘promoting’ matching responses to facial stimuli, and thus that OT administration can positively impact impaired socio-emotional behavior often linked to early social adversity. As implied by our EEG results, and in keeping with adult findings, this could involve a self-other matching mechanism. For example, in adults, intranasal OT enhances motor facilitation during manual action observation (Prinsen et al., 2018), and increases both facial and finger movement mimicry (De Coster et al., 2014; Korb et al., 2016). Our study demonstrates similar motor facilitation effects of OT in early infancy.
One idea is that OT is involved in experience-dependent plasticity processes whereby OT mediates the effects of therapeutic agents or social inputs linked to the reopening of sensitive developmental periods for social reward learning (Feldman, 2015; Nardou et al., 2019). Our results are in line with this proposition to some extent, with OT administration increasing prosocial behaviors likely impacted early on by a lack of typical parenting input. Other evidence that early adversity, in the form of social deprivation, affects functioning of the oxytocinergic system in macaques (Baker et al., 2017; Winslow et al., 2003) further supports the idea that this system was compromised in our nursery-reared sample, though this should be explored more explicitly in future research.
Finally, we found a relationship between infant cortisol and prosocial behaviors, with higher cortisol levels related to more time spent gazing towards facial gestures, and increased production of LS gestures in the LS observation condition only. This could indicate that more stressed or anxious infants benefited most from OT due to its anxiolytic effects, in accordance with studies suggesting that prosocial effects of OT, at least in part, are linked to reductions in anxiety (Campbell, 2010). More anxious or stressed individuals might be more inhibited in their social behavior, as suggested by previous infant NHP research (Dettmer et al., 2012; Dettmer and Suomi, 2014), and consequently, may demonstrate a greater magnitude of positive OT-induced effects on social responsiveness. This idea would also be consistent with research showing that OT promotes prosocial behavior and related neural responses to a greater extent in individuals with lower social processing capacities at baseline (Hecht et al., 2017).
There are some limitations of our study that must be considered. First, effects of OT can differ depending on dosage and whether it is chronically versus acutely delivered (Parr et al., 2016; Rault et al., 2013), so the use of only acute administration here limits the extendibility of our findings. Second, we only assessed infants at one-time point, so possible long-term rescue effects of OT remain unknown. Finally, it is possible that our sample size limited our ability to detect relationships between our different variables. Therefore, although these findings further our understanding and are extremely promising in terms of potential therapeutic application, further investigations into the effects of OT in infancy and its usefulness in terms of early therapeutic treatments is necessary.
To conclude, findings from the current study constitute the first evidence for exogenous OT enhancing prosocial responsiveness and related cortical activity in infant macaques at a developmental stage when brain plasticity is greatest. This adds to our knowledge concerning the role of OT in early socio-emotional development, and can guide future research with human infants. Our results also have important translational and clinical implications, suggesting that OT administration can promote social responses that were potentially impacted by early social adversity. Ultimately, such knowledge could be used to inform the design of early OT interventions aimed at manipulating specific brain mechanisms underlying social dysfunction, in the context of neurodevelopmental disorders and early-emerging psychopathology.
Data statement
The data that support the findings of this study are available from the corresponding author P.F.F. upon request.
Author contributions
P.F.F., N.A.F., F.F. and A.P. designed the study. F.F., S.S.K.K., A.P. and G.T. collected data lead by F.F. F.F., S.S.K.K. and G.T. carried out behavioral scoring. H.R. analyzed data. F.F., H.R. and P.F.F. drafted the manuscript. P.F.F. provided resources for the study. All authors edited and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Institutes of Health (NICHD Grant P01HD064653 to P.F.F.) and the NICHD Division of Intramural Research. This work was performed within the framework of the LABEX CORTEX (ANR-11-LABX-0042) of Université de Lyon operated by the French National Research Agency (ANR). Data were collected at the Laboratory of Comparative Ethology, Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. A special thanks to Dr. Stephen J. Suomi and Dr. Elizabeth A. Simpson. We are grateful to Amanda F. Hamel and Jerrold S. Meyer at University of Massachusetts Amherst for assaying the saliva samples. Thank you to the staff and researchers at the Laboratory of Comparative Ethology for assistance with data collection and to the animal care and veterinary staff at the NIH Animal Center.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100950.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Arnstein D., Cui F., Keysers C., Maurits N.M., Gazzola V. μ-Suppression during action observation and execution correlates with BOLD in dorsal Premotor, inferior parietal, and SI cortices. J. Neurosci. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Lindell S.G., Driscoll C.A., Zhou Z., Yuan Q., Schwandt M.L., Miller-Crews I., Simpson E.A., Paukner A., Ferrari P.F., Sindhu R.K., Razaqyar M., Sommer W.H., Lopez J.F., Thompson R.C., Goldman D., Heilig M., Higley J.D., Suomi S.J., Barr C.S. Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proc. Natl. Acad. Sci. 2017;114:11769–11774. doi: 10.1073/pnas.1706206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., van IJzendoorn M.H. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Davis A.M., Auger A.P., Dorsa D.M., McCarthy M.M. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci. 2001;21:2546–2552. doi: 10.1523/jneurosci.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaerts S., Boets B., Bosmans G., Steyaert J., Alaerts K. Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up. Mol. Autism. 2020 doi: 10.1186/s13229-020-0313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem R.A.I., van Honk J., Auyeung B., Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Boccia M.L., Petrusz P., Suzuki K., Marson L., Pedersen C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Pers. Soc. Psychol. Rev. 2010;14:281–295. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- Chang S.W.C., Platt M.L. Oxytocin and social cognition in rhesus macaques: implications for understanding and treating human psychopathology. Brain Res. 2014;1580:57–68. doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.L., St. John N., Pasca A.M., Hyde S.A., Hornbeak K., Abramova M., Feldman H., Parker K.J., Penn A.A. Neonatal CSF oxytocin levels are associated with parent report of infant soothability and sociability. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G., Hansman C., Heckman J.J., Novak M.F.X., Ruggiero A., Suomi S.J. Primate evidence on the late health effects of early-life adversity. Proc. Natl. Acad. Sci. 2012;109:8866–8871. doi: 10.1073/pnas.1205340109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O., Noble P.L., Costa V.D., Averbeck B.B. Oxytocin enhances attention to the eye region in rhesus monkeys. Front. Neurosci. 2014;8:41. doi: 10.3389/fnins.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006 doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster L., Mueller S.C., T’Sjoen G., De Saedeleer L., Brass M. The influence of Oxytocin on automatic motor simulation. Psychoneuroendocrinology. 2014;50:220–226. doi: 10.1016/j.psyneuen.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Debnath R., Salo V.C., Buzzell G.A., Yoo K.H., Fox N.A. Mu rhythm desynchronization is specific to action execution and observation: evidence from time-frequency and connectivity analysis. Neuroimage. 2019 doi: 10.1016/j.neuroimage.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer A.M., Suomi S.J. Nonhuman primate models of neuropsychiatric disorders: influences of early rearing, genetics, and epigenetics. ILAR J. 2014 doi: 10.1093/ilar/ilu025. [DOI] [PubMed] [Google Scholar]
- Dettmer A.M., Novak M.A., Suomi S.J., Meyer J.S. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Gläscher J., Büchel C., Braus D.F., Herpertz S.C. Oxytocin attenuates amygdala responses to emotional faces regardless of Valence. Biol. Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Erdozain A.M., Peñagarikano O. Oxytocin as treatment for social cognition, not there yet. Front. Psychiatry. 2020 doi: 10.3389/fpsyt.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high-risk parenting. Dev. Psychopathol. 2015;27:369–395. doi: 10.1017/S0954579415000048. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm. Behav. 2010 doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Ferrari P.F., Visalberghi E., Paukner A., Fogassi L., Ruggiero A., Suomi S.J. Neonatal imitation in Rhesus macaques. PLoS Biol. 2006;4:e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P.F., Paukner A., Ionica C., Suomi S.J. Reciprocal face-to-Face communication between Rhesus macaque mothers and their newborn infants. Curr. Biol. 2009;19:1768–1772. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P.F., Vanderwert R.E., Paukner A., Bower S., Suomi S.J., Fox Na. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J. Cogn. Neurosci. 2012;24:1165–1172. doi: 10.1162/jocn_a_00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festante F., Vanderwert R.E., Sclafani V., Paukner A., Simpson E.A., Suomi S.J., Fox N.A., Ferrari P.F. EEG beta desynchronization during hand goal-directed action observation in newborn monkeys and its relation to the emergence of hand motor skills. Dev. Cogn. Neurosci. 2018;30:142–149. doi: 10.1016/j.dcn.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festante F., Ferrari P.F., Thorpe S.G., Buchanan R.W., Fox N.A. Intranasal oxytocin enhances EEG mu rhythm desynchronization during execution and observation of social action: an exploratory study. Psychoneuroendocrinology. 2020 doi: 10.1016/j.psyneuen.2019.104467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli P.A., DesJardin J.T., West E.A., Holmes A.L., Elorette C., Wellman L.L., Malkova L. Amygdala selectively modulates defensive responses evoked from the superior colliculus in non-human primates. Soc. Cogn. Affect. Neurosci. 2016;11:2009–2019. doi: 10.1093/scan/nsw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Bakermans-Kranenburg M.J., Yoo K.H., Bowman L.C., Cannon E.N., Vanderwert R.E., Ferrari P.F., Van Ijzendoorn M.H. Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychol. Bull. 2016;142:291–313. doi: 10.1037/bul0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M., Inoue K., Smith A.L., Goodman M.M., Young L.J. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangopadhyay P., Chawla M., Dal Monte O., Chang S.W.C. Prefrontal–amygdala circuits in social decision-making. Nat. Neurosci. 2021;24:5–18. doi: 10.1038/s41593-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A.J., MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm. Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Mitchell P.B., Dadds M.R. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Einfeld S.L., Gray K.M., Rinehart N.J., Tonge B.J., Lambert T.J., Hickie I.B. Intranasal oxytocin improves emotion recognition for youth with autism Spectrum disorders. Biol. Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hammock E.A.D. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht E.E., Robins D.L., Gautam P., King T.Z. Intranasal oxytocin reduces social perception in women: neural activation and individual variation. Neuroimage. 2017;147:314–329. doi: 10.1016/j.neuroimage.2016.12.046. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003 doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Insel T.R., Young L., Witt D.M., Crews D. Gonadal steroids have paradoxical effects on brain oxytocin receptors. J. Neuroendocrinol. 1993;5:619–628. doi: 10.1111/j.1365-2826.1993.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Jaeger T.F. Categorical data analysis: away from ANOVAs (transformation or not) and towards logit mixed models. J. Mem. Lang. 2008;59:434–446. doi: 10.1016/j.jml.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. (Regul. Ed.) 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb S., Malsert J., Strathearn L., Vuilleumier P., Niedenthal P. Sniff and mimic - Intranasal oxytocin increases facial mimicry in a sample of men. Horm. Behav. 2016 doi: 10.1016/j.yhbeh.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Krol K.M., Puglia M.H., Morris J.P., Connelly J.J., Grossmann T. Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev. Cogn. Neurosci. 2019;37 doi: 10.1016/j.dcn.2019.100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., Angstadt M., Chua P., Heinrichs M., Stout J.C., Nathan P.J. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A., Richard N., Jazayeri M., Beuriat P.-A., Fieux S., Zimmer L., Duhamel J.-R., Sirigu A. Oxytocin and serotonin brain mechanisms in the nonhuman primate. J. Neurosci. 2017;37:6741–6750. doi: 10.1523/JNEUROSCI.0659-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Goldstein A., Zagoory-Sharon O., Weisman O., Schneiderman I., Eidelman-Rothman M., Feldman R. Oxytocin selectively modulates brain response to stimuli probing social synchrony. Neuroimage. 2016;124:923–930. doi: 10.1016/j.neuroimage.2015.09.066. [DOI] [PubMed] [Google Scholar]
- Lischke A., Berger C., Prehn K., Heinrichs M., Herpertz S.C., Domes G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology. 2012;37:475–481. doi: 10.1016/j.psyneuen.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Liu N., Hadj-Bouziane F., Jones K.B., Turchi J.N., Averbeck B.B., Ungerleider L.G. Oxytocin modulates fMRI responses to facial expression in macaques. Proc. Natl. Acad. Sci. 2015 doi: 10.1073/pnas.1508097112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M., Toth I., Reber S.O., Slattery D.A., Veenema A.H., Neumann I.D. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Miller T.V., Caldwell H.K. Oxytocin during development: possible organizational effects on behavior. Front. Endocrinol. (Lausanne) 2015;6 doi: 10.3389/fendo.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A., Gorodnitsky I., Pineda J. EEG mu component responses to viewing emotional faces. Behav. Brain Res. 2012;226:309–316. doi: 10.1016/j.bbr.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Nardou R., Lewis E.M., Rothhaas R., Xu R., Yang A., Boyden E., Dölen G. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature. 2019 doi: 10.1038/s41586-019-1075-9. [DOI] [PubMed] [Google Scholar]
- Nelson C.A. Hazards to early development: the biological embedding of early life adversity. Neuron. 2017 doi: 10.1016/j.neuron.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Nishizato M., Fujisawa T.X., Kosaka H., Tomoda A. Developmental changes in social attention and oxytocin levels in infants and children. Sci. Rep. 2017;7:2540. doi: 10.1038/s41598-017-02368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Wingeier B.M., Silberstein R.B. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum. Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K.J., Garner J.P., Libove R.A., Hyde S.A., Hornbeak K.B., Carson D.S., Liao C.-P., Phillips J.M., Hallmayer J.F., Hardan A.Y. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc. Natl. Acad. Sci. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K.J., Oztan O., Libove R.A., Sumiyoshi R.D., Jackson L.P., Karhson D.S., Summers J.E., Hinman K.E., Motonaga K.S., Phillips J.M., Carson D.S., Garner J.P., Hardan A.Y. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. 2017;114:8119–8124. doi: 10.1073/pnas.1705521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr L.A., Modi M., Siebert E., Young L.J. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38:1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr L.A., Brooks J.M., Jonesteller T., Moss S., Jordano J.O., Heitz T.R. Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology. 2016 doi: 10.1016/j.psyneuen.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin M.A., DeRamus T., Libero L.E., Laird A., Kana R.K. Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum. Brain Mapp. 2016;37:3957–3978. doi: 10.1002/hbm.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A., Capitanio J.P., Blozis S.A. A new look at neurobehavioral development in rhesus monkey neonates (Macaca mulatta) Am. J. Primatol. 2020 doi: 10.1002/ajp.23122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Bentin S., Shalev I., Israel S., Uzefovsky F., Bar-On D., Ebstein R.P. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–1453. doi: 10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Neuper C., Mohl W. Event-related desynchronization (ERD) during visual processing. Int. J. Psychophysiol. 1994;16:147–153. doi: 10.1016/0167-8760(89)90041-X. [DOI] [PubMed] [Google Scholar]
- Prinsen J., Brams S., Alaerts K. To mirror or not to mirror upon mutual gaze, oxytocin can pave the way: a cross-over randomized placebo-controlled trial. Psychoneuroendocrinology. 2018;90:148–156. doi: 10.1016/j.psyneuen.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Putnam P.T., Young L.J., Gothard K.M. Bridging the gap between rodents and humans: the role of non-human primates in oxytocin research. Am. J. Primatol. 2018 doi: 10.1002/ajp.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R Found. Stat. Comput.; 2020. A Language and Envirorment for Statistical Computing. [Google Scholar]
- Rajamani K.T., Wagner S., Grinevich V., Harony-Nicolas H. Oxytocin as a modulator of synaptic plasticity: implications for neurodevelopmental disorders. Front. Synaptic Neurosci. 2018;10 doi: 10.3389/fnsyn.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rault J.L., Carter C.S., Garner J.P., Marchant-Forde J.N., Richert B.T., Lay D.C. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 2013 doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Rayson H., Bonaiuto J.J., Ferrari P.F., Murray L. Mu desynchronization during observation and execution of facial expressions in 30-month-old children. Dev. Cogn. Neurosci. 2016;19:279–287. doi: 10.1016/j.dcn.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayson H., Bonaiuto J.J., Ferrari P.F., Murray L. Early maternal mirroring predicts infant motor system activation during facial expression observation. Sci. Rep. 2017;7:11738. doi: 10.1038/s41598-017-12097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat. Rev. Neurosci. 2016 doi: 10.1038/nrn.2016.135. [DOI] [PubMed] [Google Scholar]
- Simpson E.A., Sclafani V., Paukner A., Hamel A.F., Novak M.A., Meyer J.S., Suomi S.J., Ferrari P.F. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc. Natl. Acad. Sci. 2014;111:6922–6927. doi: 10.1073/pnas.1402471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.A., Miller G.M., Ferrari P.F., Suomi S.J., Paukner A. Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Sci. Rep. 2016;6:20233. doi: 10.1038/srep20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.A., Paukner A., Pedersen E.J., Ferrari P.F., Parr L.A. Visual preferences for direct-gaze faces in infant macaques (Macaca mulatta) with limited face exposure. Dev. Psychobiol. 2019;61:228–238. doi: 10.1002/dev.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Kennedy M., Kumsta R., Knights N., Golm D., Rutter M., Maughan B., Schlotz W., Kreppner J. Child-to-adult neurodevelopmental and mental health trajectories after early life deprivation: the young adult follow-up of the longitudinal English and Romanian Adoptees study. Lancet. 2017 doi: 10.1016/S0140-6736(17)30045-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Fujisawa T.X., Sakakibara N., Fujioka T., Takiguchi S., Tomoda A. Development of social attention and oxytocin levels in maltreated children. Sci. Rep. 2020;10:7407. doi: 10.1038/s41598-020-64297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert J., Flessert M., Liu N., Ungerleider L.G. Intranasal oxytocin selectively modulates the behavior of rhesus monkeys in an expression matching task. Sci. Rep. 2019;9:15187. doi: 10.1038/s41598-019-51422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman R., Gordon I., Naples A., Rolison M., Leckman J.F., Feldman R., Pelphrey K.A., McPartland J.C. Oxytocin enhances the neural efficiency of social perception. Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert R.E., Ferrari P.F., Paukner A., Bower S.B., Fox N.A., Suomi S.J. Spectral characteristics of the newborn rhesus macaque EEG reflect functional cortical activity. Physiol. Behav. 2012 doi: 10.1016/j.physbeh.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert R.E., Simpson E.A., Paukner A., Suomi S.J., Fox N.A., Ferrari P.F. Early social experience affects neural activity to affiliative facial gestures in newborn nonhuman Primates. Dev. Neurosci. 2015;37:243–252. doi: 10.1159/000381538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M., Fox N.A., Zeanah C.H., Nelson C.A. Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proc. Natl. Acad. Sci. 2019;116:1808–1813. doi: 10.1073/pnas.1809145116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Huang H., Schwab N., Tanner J., Rajan A., Lam N.B., Zaborszky L., Li C.R., Price C.C., Ding M. From eyes-closed to eyes-open: role of cholinergic projections in EC-to-EO alpha reactivity revealed by combining EEG and MRI. Hum. Brain Mapp. 2019;40:566–577. doi: 10.1002/hbm.24395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O., Zagoory-Sharon O., Feldman R. Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol. Psychiatry. 2012;72:982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Winslow J.T., Noble P.L., Lyons C.K., Sterk S.M., Insel T.R. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003 doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.