Abstract
Availability of clean water is of concern due to pollution and diminishing supply pollution. However, purification is possible depending on the incorporated contaminants. Domestic wastewater contains dissolved organic matter and its remediation can be done by oxidation. The best oxidation can be achieved by electron transfer the same way metabolic processes occur. This study exploited the use of a film of iron (III) doped titanium dioxide applied on an electrode which was found to be effective. Natural light conditions generated electrons that migrated through the electrode leaving behind holes which oxidized the contaminants as the excess electrons were discharged at the cathode after passing through the casted proton exchange membrane (PEM) separating the two half cells of the prepared reactor. This electrochemical method has the advantage in that the organic pollutants are oxidized to carbon dioxide with no secondary pollutants and the inorganic pollutants into insoluble matter. The assembled cell was applied to purify both synthetic and real water samples of green leafy vegetable solution from the kitchen by clarification. The clarification process was monitored by UV-Vis using distilled water as a reference to compare the light that transmitted through a sample. It was observed that the electro-oxidation process took place showing a high potential 105 mV within the first 150 min followed by degradation at a high rate. The oxidation of the organic matter was confirmed by UV-Vis analysis as well as by cyclic voltametric analysis of iron released into the solution of the synthetic samples. The electro chemical treatment of the water was then applied to purify real water samples made from a sample of 4.5 g minced of green vegetables dispersed in one liter of water (4.5 g/l). The green leafy coloured solution was clarified after 154 h of continuous oxidation. The degradation process was confirmed to be independent of intermediates or other species present in solution as it was of first order reaction kinetics. The electrochemical oxidation of organic matter in water using iron (III) doped titanium dioxide coated graphite electrode has potential application on the purification of water.
Keywords: Electrochemical cell, Proton exchange membrane, Oxidation, Pollution, Domestic, Wastewater
Electrochemical cell, Proton exchange membrane, Oxidation, Pollution, Domestic; Wastewater
1. Introduction
Water shortage has increasingly become a global concern especially due to its dwindling natural resources and widespread environmental pollution. Consumption of such water can be a threat to the wellbeing of man and animals. This is because of the lopsided structure of water that gives it a unique property to stably disperse uniformly ionic or polar species, some that could be harmful after it interacts with them as a solvent. Despite the dissolving power of water being very important for life as it evenly distributes chemicals, minerals, and nutrients that support living organisms its appreciation may be compromised by its appearance and dispersed ions. That strong dissolving power of water enables it to be a cleansing agent leading to generation of wastewater. This means that within a household, a lot of wastewater is generated both black and grey. Domestic wastewaters are characterized by their main contaminants. Thus wastewater from the toilet is termed “black” while that from the kitchen and bathroom is termed “grey” and disposal of such wastewater is a public concern. This is because, water upon interaction with matter becomes impure making availability of pure water a challenge and remedial measures needs be taken for the wellbeing of humans, plants and animals (Soteris, 2005).
To mitigate the negative effects of pollution, and reduce the pollutant load, alternative approaches should be considered and applied for remediation of this vital commodity. Since the polluted water is more than 90% pure, and we cannot create new water on the planet, the alternative is purification. This can be achieved to by establishing a wastewater reuse system within any entity such as a household. This is because water recycling presents itself as one of the available alternatives to curb the increasing water shortage. For wastewater to be recycled, the dissolved contaminants; organic and inorganic need to be removed (Naidoo and Olaniran 2013). Some of the methods that are used to purify wastewater include chemical, biological/enzymatic, physiochemical, advanced oxidation processes which require addition of other substances into water contributing to secondary pollution.
In recent times, the electrochemical method has become popular because it is easy to construct and operate. Furthermore, electrochemical treatment of wastewater does not require the use of chemical reagents unlike in the chemical treatment method because the oxidation of the organics takes place on the surface of the electrode (anodes)-which are the fixed active sites (Martínez-Huitle et al., 2011). This study exploited the exchange property of electrons at the interface of an anode to degrade dissolved organic matter and any other oxidizable species dispersed in domestic wastewater. This was done by considering the fact that graphite electrodes do not interact with water to introduce harmful species and are suitable for long-term deployment as the anode of the half cell (Reimers et al., 2006; Shao et al., 2018). The electrons in the graphite electrode can be delocalized in a certain resonance speed, when in contact with organic matter in water, making their kinetic energy to be boosted by the potential energy of constituents in the water due to the oxidation reaction that occurs (Utomo et al., 2019). Other electrodes such as iron and lead are usually degraded in an electrolysis process (Martı'nez-Huitle and Ferro 2006). This implies that graphite serves as an electron acceptor as reported by Dumas et al. (2007); Richter et al. (2008); Yi et al. (2009) and is inexpensive. In this work, a graphite electrode was coated with iron (III) doped titanium dioxide to trigger the photo oxidation of organic species in the wastewater to harmless products (Mwangi et al., 2013).
Titanium dioxide has been used previously in wastewater treatment as a photo catalyst because of its inert nature both biologically and chemically (Al-Mamun et al., 2019). It is also relatively cheap and non-toxic when dissolved in water low in contaminants concentration (Duby, 1993; Beer 1980). Wang et al. doped titanium dioxide with Fe (III) and formed a uniform solid solution of iron-titanium oxide at a pH above 6. By doping TiO2, its conductivity is significantly improved by lowering the band gap such that it is activated at the visible region (Wang et al., 2004). When TiO2 is illuminated by sunlight, electrons are ejected from its surface leaving behind electron deficient holes as shown in Eq. (1) (Trasatti 2000; Chen et al., 2001; Pathiraja et al., 2014).
| (1) |
This is a promising ways by using solar energy for converting aerated water into species that have oxidative properties (Fan et al., 2009). This happens because that, when titanium dioxide is doped with nitrogen ions or some metal oxides exhibits excitation also under visible light without being poisoned (Kurtoglu et al., 2011). Doping ensures that there is no decrease in the photoactivity as it reduces the ability of recombination of the photogenerated charges improving titania's photocatalytic activity. As a result, the electron deficient holes that processes strong oxidative potential oxidize to generate hydroxyl radicles capable to oxidize organic matter directly (Hanaor and Sorrell, 2013).
In this study, the photogenerated holes oxidized the organic matter dispersed in water and the electrons migrated to the surface of graphite. This leaves a H+ enriched environment in water which then migrate to the cathode environment through the proton exchange membrane (PEM) separating the two half cells of the reactor (Yan et al., 2017). The PEM in this study was made from a conducting polymeric material inert in aqueous media thus will not take part in the reaction. When the electrodes are connected externally, the circuit is complete and the degradation process at the anode of the half-cell treatment setup continues (Bond et al., 2002; Lovley, 2006; Huang et al., 2009). The cathode chamber is filled with an electrolyte to enable the conduction of electrons to improve the efficiency of the degradation process. The key component of the electrochemical system is the electrode (anode), where the oxidation of the organic contaminants to carbon dioxide and water takes place. The material making up the anode has a high influence on the efficiency and the selectivity of the electrochemical process (Sequeira and Santos 2009), making it a right choice of a successful anode of an electrochemical process. This work presents the preparation and application of iron (III) doped Titanium dioxide dip-coated onto graphite anode for use in the electrochemical treatment of wastewater.
2. Materials and methods
All reagents used in this study were of analytical grade and all solutions unless otherwise stated were dispersed in distilled water. Titanium oxide, potassium iodide, iron (III) chloride, iodine, hydrochloric acid, iron (III) nitrate and calcium silicate were all from Sigma-Aldrich supplied by Kobian which has an outlet in Nairobi, Kenya. The graphite electrode 5.0 mm diameter and 5 cm length was also supplied by Sigma-Aldrich. A Rocker pump model Chemker 410 was used to circulate the anode water during the experiment.
2.1. Instrumentation
The electromotive force across the cell was monitored using a Fluke 115 True-RMS digital multimeter (Singapore) while the UV-VIS spectra of the green leaves solutions were determined using a Shimadzu UV-2450 (Tokyo, Japan) UV–Vis spectrophotometer at its normal wavelength range (190–1,100 nm). The same instrument coupled with an integrating spheres assembly was used to confirm doping of titanium dioxide. Voltammogram measurements were done using Basi Epsilon potentiostat and the turbidity meter model LP 2000 from HANNA Instruments (USA) was used to determine the extent of turbidity (Wijnen et al., 2014).
2.2. Preparation of the titanium coated graphite rods
The titanium oxide powder was mixed with calcium silicates powder to the ration of 5:1 respectively in a 250 ml beaker. To this mixture, 1 mL of 0.1 M Fe (NO3)3 solution was added as a dopant so as to activate the conductivity of the titanium oxide through sunlight. Distilled water (100mL) was then added to the mixture and the mixture sonicated for 10 min to form a paste.
The effect of the iron (III) doping on the gel was carried out using UV–Vis spectrophotometer coupled with an integrating spheres assembly to measure the total radiation reflectance and transmittance from the sample and confirm the shift in frequency of excitement.
Then graphite rods were then (immersed) dipped into this paste for 30 min and then sundried for 2 h at first and then oven dried for 24 h at a temperature of 80 °C. The dried rods were stored under water for 24 h before use.
2.3. Preparation of a biomass-hydrogel
This procedure for the preparation of the hydrogel was adopted as reported by Mwangi and co-workers (2019) who modified cow dung to hydrogels, a compound with high water retention capacity. This was done by weighing 20.0 g of starch into a reactor vessel containing 100 mL of distilled water and stirred for 30 min. To that mixture, 50 mL glycerol were added and the resultant heated in a water bath at 60 °C for 2 h and then allowed to settle at temperature for 72 h. The final material was filtered, dried at 60 °C and 1.5 g of the dry material mixed with the conducting polymer for the purpose of sealing all the pores on the final PEM material and also hydrate the membrane to enhance its constant conduction property (Wan et al., 1984).
2.4. Preparation of the conducting polyaniline
This was done by mixing freshly distilled phenylamine (200 mL) with 300 mL of 2M HCl in a reactor vessel and the mixture stirred for 30 min. To that mixture, (36.0 g) potassium persulphate solution previously dissolved in 200 mL of 0.2M HCl were added followed by 1.5 g of the biomass hydrogel and the resulting mixture thoroughly stirred to form the constant hydrated conducting polyaniline polymer.
2.5. Preparation of the proton exchange membrane material
The method for the preparation of the proton exchange membrane was done as reported by Huang et al. (2016). This was done by mixing 6 g of PVA with 100 mL of distilled water under heating for 2 h. The solution was then cooled to room temperature. To that solution, 10 g the previously prepared conducting polyaniline and 5 g of calcium silicate were added with constant stirring. The mixture was frozen for 24 h, and then thawed for 24 h at room temperature. It was then immersed in distilled water for 24 h to remove inorganic impurities. This membrane was then characterized by Fourier transform infrared spectroscopy.
2.6. Preparation of the anodic and the cathodic solutions
The anodic wastewater to be purified was prepared by weighing 9 g of green leafy vegetables, chopping them and then placed in 200mL of boiling water. They were allowed to boil for 15 min, and then allowed to cool to room temperature. This solution was filtered to remove any suspended particles. It was then diluted with distilled water to make 2 L of the anodic solution. The cathodic solution was prepared by dissolving 0.755 g of KCl in a litre of distilled water to improve the conductivity of the distilled water. The temperature of the solution was 27 °C, while the anodic water had a pH of 6.6.
2.7. Preparation of iodine solution
The method for the standard (0.05M) iodine solution was as found in the Vogel's Textbook of Quantitative chemical Analysis (Vogel, 1989). This was done by dissolving 7.0 g of iodine in a solution of 18.0 g of potassium iodide dispersed in 100 mL of distilled water. To that solution, 3 drops of 2 M HCL were added and the solution diluted to a litre. This solution was then stored in a dark bottle.
2.8. Voltage measurements
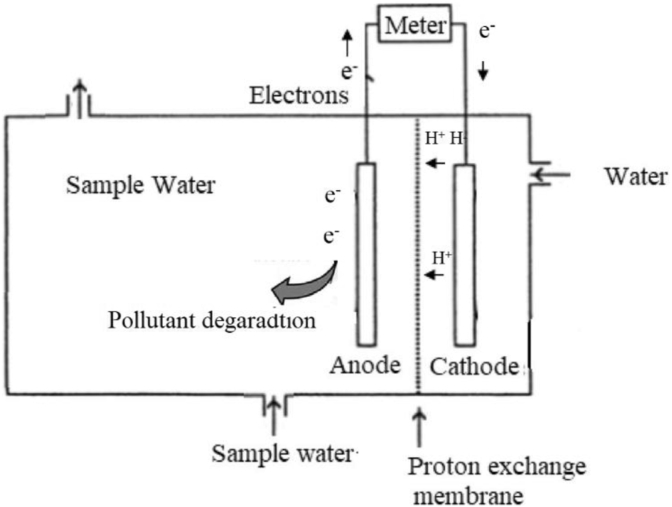
The electrochemical cell was assembled and setup as shown by Figure 1 using the prepared iron (III) titanium oxide on graphite as the anode and a graphite rod as a cathode. An earlier prepared proton exchange membrane from a conducting polymer and cast onto a sintered glass disc was used in the assembled electrochemical cell. The anode wastewater (2 L) solution was allowed to circulate using a water pump at a rate of 18 L per minute. The electromotive force (EMF) between the electrodes of the electrochemical cell was monitored using a digital multimeter and the variation of potential (volts) with time recorded.
Figure 1.
The experimental working of the electrochemical cell setup assembled.
2.9. Optical analysis of transparency of water
The degradation of dissolved organic matter in the water sample was analyzed optically by drawing 5 mL of the water samples for the anode compartment at regular time intervals and the extent of degradation monitored using UV-VIS instrument at a wavelength range between 200 nm to 600 nm.
2.10. Optical analysis of degradation of dissolved organic starch species
The extent of degradation was also investigated by using the starch iodine complex method. This was done by drawing 5 ml of the water samples at regular time intervals, and to each sample, one drop of iodine solution was added and the mixture activated in a water bath at 70 °C for 2 h to form starch iodine complexes. The samples were allowed to cool to 25 °C then analyzed on a UV-VIS spectrophotometer.
2.11. Voltammetric (CV) analysis of the waste water
Samples (5 mL) from the anode compartment were drawn within 30 min from one another for a period of three hours and analyzed using the potentiostat by carrying out cyclic voltammetry mode on a platinum electrode as a working electrode and Ag/AgCl reference electrode. The scan rate was 100mV and a maximum current of 100 mA. A drop of the sample solution was dissolved in 25mL of 1M KNO3 solution as a supporting electrolyte. These experiments were done at room temperature of about 25 °C.
3. Results and discussion
3.1. Analysis of the photo active material
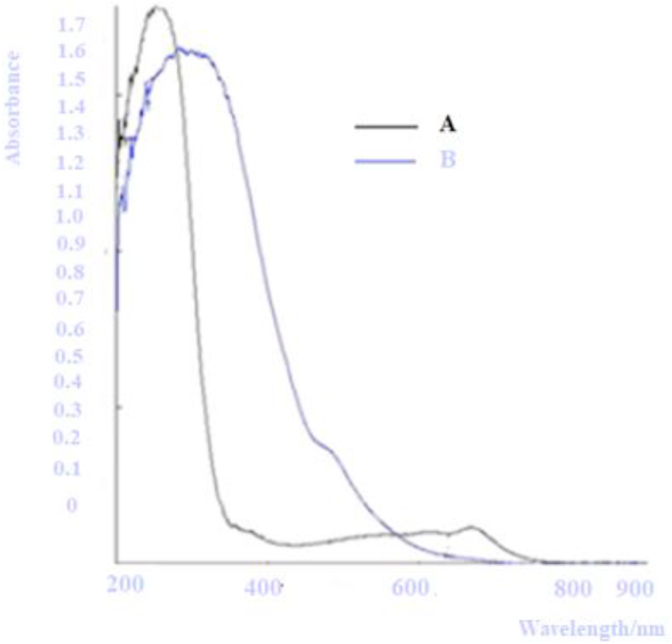
To enable the degradation process to be carried under normal day light conditions, the band gap of the phot active material had to be lowered. This was done by doping with iron (III) nitrate. The material was able to provide advanced oxidation processes as a heterogeneous oxidant of organic pollutants in wastewater hence an efficient agent the degradation of organic pollutants in water. This doping was confirmed by analyzing the sample with UV-Vis coupled with an integrating spheres assembly to measure the total, diffuse reflectance and diffuse transmittance. The results obtained on the analysis of the photoactive material before and after doping are as presented in Figure 2.
Figure 2.
UV–Vis characterization of A and B pure and Fe (III)-doped TiO2 respectively POTENTIAL/mv.
The results show a variation of photo activity from the UV region to the visible region after doping. It is evident that by pure TiO2 material (curve A) starts at 200 nm (UV region) with maximum activation at 261 nm and significantly falling at around 340 nm while the Fe (III)-doped TiO2 material showed that the absorption to start at 200 nm but the maximum activation at 340 nm. The activation range of the doped material is extended to 570 nm (visible region). The wavelengths of maximum absorption for the two materials were found to be 261 and 340 nm respectively which shows a red shift effect on the spectra (Shandrikov et al., 2018).
3.2. Degradation of dissolved organic matter
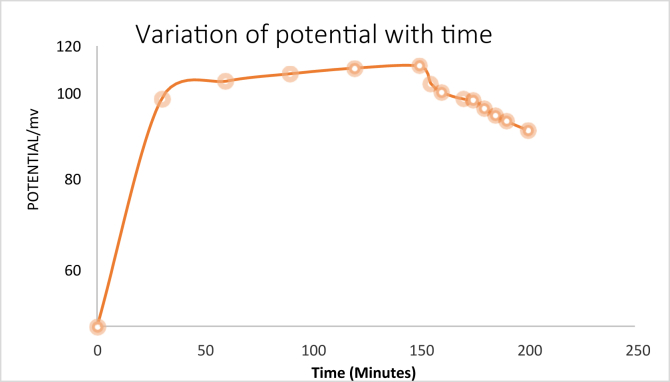
The organic matter degraded in this study was a heterogeneous class of water-soluble multi-component dissolved compounds that contain reducible (organic) carbon from biomass (vegetable washings) with differing chemical reactivity. The degradation process done in natural light illuminated environment was monitored by recording the potential difference across the electrodes of electrochemical cell using a digital voltmeter and the results recorded as presented in Figure 3.
Figure 3.
The variation of potential difference of the electrochemical H+ H+.
The results in Figure 3 show that cell recorded a high voltage of about 105 mV within the first 150 min of the oxidation process after which the potential starts to decrease with time at a relatively high rate. This could be attributed to the decrease in the oxidizable species in the redox reaction or the deactivation of the anode. The two processes show that the oxidation reactions taking place on the titanium oxide coated rod are time dependent. The concentration changes of the species undergoing oxidation are proportional to the adsorption rate and the catalytic reactions taking place at the anode, hence the deactivation of the electrode.
3.3. Time dependent clarification
The degradation rate of dissolved organic matter in the water sample was done by drawing 5 mL of the anode solution at a regular time interval of 30 min for three hours. These samples were analyzed using UV-Vis spectrophotometer at a wavelength range between 270nm to 300nm. This is because UV–VIS spectroscopy is regarded as a signature and tracer of DOM composition, source, and reactivity (Li and Hur, 2017).
The results are presented in Figure 4.
Figure 4.
The results of electro-oxidation of dissolved organic matter with time.
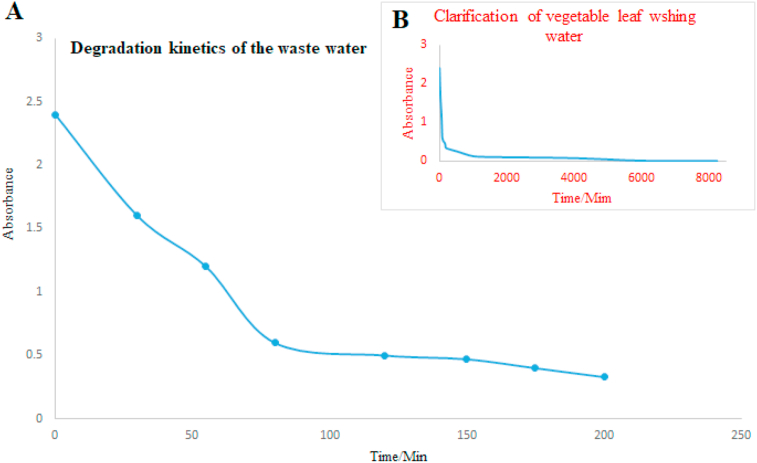
The results obtained show a peak of maximum absorption at 300 nm wavelength. This was attributed to the presence of dissolved small molecular organic matter after the oxidation of the complex organic matrix of the green leafy sample wastewater (Lei et al., 2019). It was observed that the concentration of the oxidized products from the complex organic content decreased with the oxidation time. The optical analysis of transparency of water was monitored with time using a UV-Vis spectrophotometer at 300 nm. The change in clarity was recorded as time absorbance variation data presented as shown in Figure 5.
Figure 5.
The results of UV-Vis absorbance data plotted against time-(A) Degradation kinetics and (B) Time dependent clarification of the wastewater.
The results showed a decrease in absorbance with time, indicating that the electrochemical process for the dissolved organic matter decayed exponentially following first-order kinetics. The green colourating material from the vegetable leaves leached water is optically active thus absorbing the radiation. But the generated oxidizing species interacted with the dissolved material lowering its concentration in water contributing to the observed phenomenon (Yoo and Kim, 2019). The colour change was clearly observable as it happened. It was observed that the green leafy coloured solution was clarified after 154 h of continuous oxidation even though half that time was at night with no solar radiation.
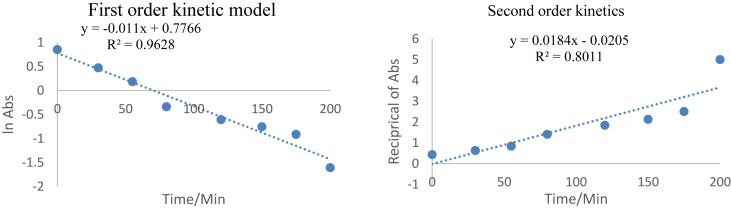
The kinetic data in Figure 5 was treated with the Lagergreg (1898) and Ho et al. (2000) kinetic models. The first and second order kinetic models are expressed as is given by Eqs. (2) and (3) respectively
| (2) |
| (3) |
This was to establish the molecularity of the degradation process with a view to establish the number of species that affect the oxidation process (Woo et al., 2020). The results of the first order model was as presented in Figure 6.
Figure 6.
First and second order degradation kinetics.
The respective plots gave straight lines with a correlation coefficient R2 = 0.9628 for first order kinetics and R2 = 0.8011 for second order kinetics. This confirmed that the electrochemical process for the degradation of organic substances was unimolecular and other species in solution did not affect the electro-oxidation process of the dissolved organic matter. This confirms that the degradation process was a time dependent reaction implying that with a continuation of the redox process in the cell, there is a depletion of the dissolved organic matter content in the wastewater.
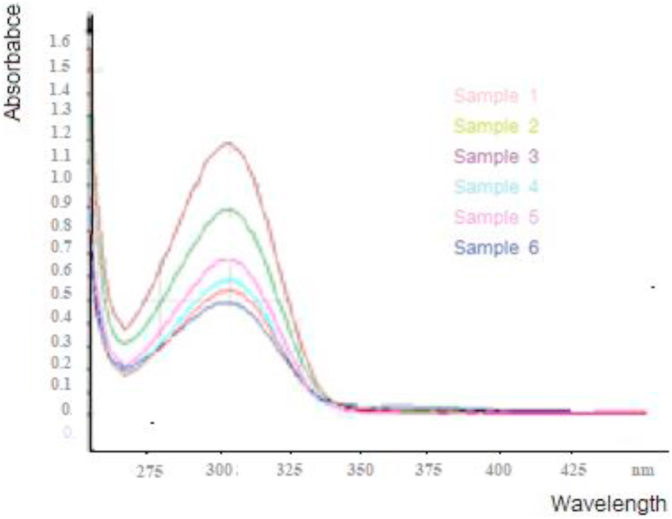
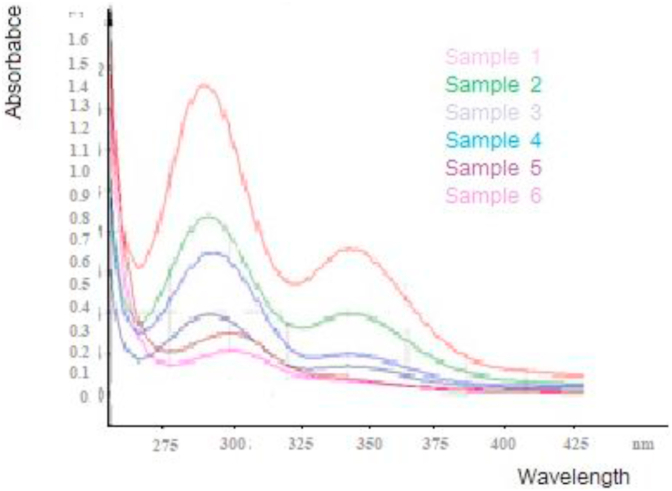
The dissolved organic matter was also determined using the starch iodine complex method is based on the formation of coloured starch iodine complex (Nwokocha and Ogunmola, 2014; Shi et al., 2009)). This was done by addition of iodine and the samples analyzed using UV-Vis spectrophotometer at a wavelength range between 200 nm to 600 nm and the results presented in Figure 7.
Figure 7.
Results of starch iodine complex of the dissolved organic matter.
The results show peaks between 275-300 nm and at between 325-375nm. The maximum absorption of radiation found at 290 nm was attributed to the chromophore complex (Mwangi et al., 2013: Moulay, 2013). This led to the suggestion that the oxidation process of the dissolved organic matter in the green leafy vegetable water sample resulted to smaller molecules of starch which complexed with the iodine solution (Hansen et al., 2006). The peak profile showed that the samples that had undergone oxidation for the longest time had a high absorbance value than the others. This indicates that the more the oxidation time, the higher the concentration of starch compounds from the oxidation process of the organic matter in the green leafy solution. It was also observed that as the reaction time increased, the sample water changed colour from light green to dark brown solution which was likely due to oxidation of the organic starch material to carbon dioxide and water the result to the release of iodine from the starch-iodine complex. This is similar to what was reported by Redel et al. (2009) as they studied a starch-iodine model in inorganic–organic hybrid compounds. They observed that the deep blue color of starch–iodine also disappears when air is bubbled through its bubbled through its colloidal solutions due to the oxidation of the iodate ion to iodine (Redel et al., 2009).
3.4. Cyclic voltammetry (CV) analysis of the waste water
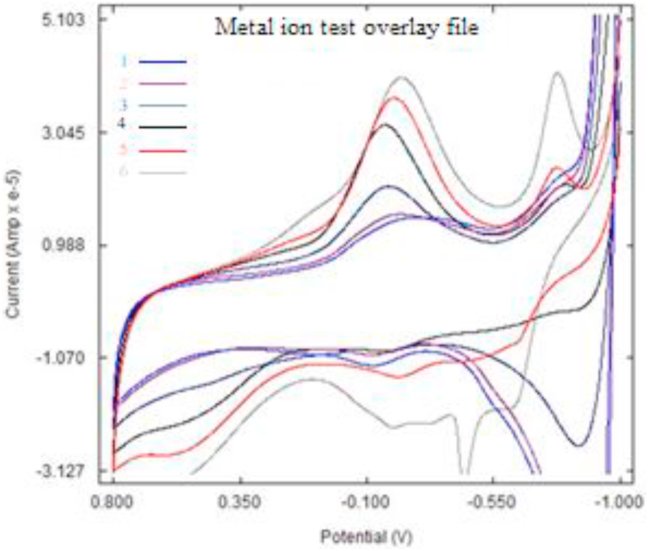
The electro-oxidation process was also studied by cyclic voltametric method. As in the previous spectrophotometric experiments, the samples were drawn from the reactor at different intervals and introduced to the cell of the electro-analyser. The analyser used for the cyclic voltammetry (CV) measurement was a Basi Epsilon potentiostat version 2.13.77. The measurements were done when the samples were dispersed in potassium nitrate solution (1M) which was continuously stirred by bubbling in white spot nitrogen gas. The potential sweep range was set between −1.0 to 0.8 V (vs. SCE) with a scan rate range of 1–100 mV s−1. These experiments were done at a temperature of 25 °C and the results obtained are as presented in Figure 8.
Figure 8.
The cyclic voltamograms of the wastewater under oxidation in the electrochemical treatment.
The results show different voltamograms of well-defined reduction peaks confirming time dependent electro oxidation of the dissolved organic matter. This is evidence of the degradation of the oxidizable species on surface of the titanium dioxide coated dimensionally stable electrode (Pipi et al., 2013). The signals recorded as -0.180 V at the physiological pH of water was considered and were assigned to iron as suggested by Toh et al. (2014) when they studied haemoglobin electrochemically. The results show an increase in the content of iron with time at that potential. The reason for that observation was that iron bound complex form by the organic matter of the leaves was being released into the solution as a free ion from the and thus its concentration increased with degradation time. This was due to the degradation of the dissolved organic matter as shown in the voltamograms. Sample 1 had the least reduction (contact) time resulting to the least concentration of iron while sample 6 had the highest due to high reaction time hence more iron leached in the solution that the other samples. The observation was due to the fact that iron and manganese are the two main metals in the soil and organic matter that exist in oxidized and reduced forms capable form organic complexes with biomass (Doane1 et al., 2019). The results show that the oxidation of dissolved organic matter releases labile form of iron from the bio material (Doane1 et al., 2019). This suggests that the different samples analyzed by the CV had different concentrations of dissolved (leached) iron due to the difference in the concentrations of the dissolved matter (complexing agent) was being depleted with time. Thus the titanium dioxide coated graphite electrode degraded the dissolved organic matter in the solution at a rate that is time dependent making it an effective water purification method.
4. Conclusion
In conclusion, an electrode whose diameter was 5 mm and a length of 5 cm was prepared by dip coating graphite with iron (III) doped TiO2 which was activated by natural light. The prepared electrode was used as an anode for the assembled electrochemical cell to purify wastewater sample of green leafy vegetable solution in the laboratory. Results of the pollutants degradation process demonstrated that the electro-oxidation of the organic matter in water decreased with time, as confirmed by the potential measurements of the cell recording a high voltage of about 105 mV within the first 150 min of the oxidation process. The electrochemical process would take place even for other inorganic substances dissolved in the wastewater as confirmed by the CV measurements. This method clarified 4.5 g/l of green leafy solution in 154 h of continuous oxidation even though half that time was at night with no solar radiation. The rate of the degradation process was not dependent on any intermediate or other species present in solution as shown by the first order and second order reaction kinetics, meaning that the prepared electrode could degrade various materials in wastewater and therefore it is an effective method of water purification. The iron (iii) doped titanium dioxide coated dimensionally electrode has potential application achieving wastewater reuse system by electro oxidation of grey water within a household.
Declarations
Author contribution statement
Kinyua, E. M.: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mwangi, I. W.: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nthumbi, R., Ngila, J.C.: Conceived and designed the experiments.
Wanjau, R.N., Swaleh, S.: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
EM Kinyua is grateful to Kenyatta University (KU) for her registration as a postgraduate student and Dr. Mwangi’s research group for helpful discussion.
References
- Al-Mamun M.R., Kader S., IslamM S., KhanM Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: a review. J. Environ. Sci. Chem. Eng. 2019;7(5):103248. [Google Scholar]
- Beer H.B. The invention and industrial development of metal anodes. J. Electrochem. Soc. 1980;127(8):303C. [Google Scholar]
- Bond D.R., Holmes D.E., Tender L.M., Lovley D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295(5554):483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- Chen X., Chen G., Yue P. Stable Ti/IrOx −Sb2O5 −SnO2 anode for O2 evolution with low Ir content. J. Phys. Chem. 2001;105:4623–4628. [Google Scholar]
- Doane1 T.A., Silva L.C.R., Horwath W.R. Exposure to light elicits a spectrum of chemical changes in soil. J. Geophys. Res.: Earth Surf. 2019:2288–2310. [Google Scholar]
- Duby P. The history of progress in dimensionally stable anodes. J. Mine. 1993;45:41–43. [Google Scholar]
- Dumas C., Mollica A., Féron D., Basséguy R., Etcheverry L., Bergel A. Marine microbial fuel cell: use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta. 2007;53:468–473. [Google Scholar]
- Fan Y., Li D., Deng Mi., Luo Y., Meng Q. An overview on water splitting photocatalysts. Front. Chem. China. 2009;4(4):343–351DOI. [Google Scholar]
- Hanaor D.A.H., Sorrell C.C. Sand supported mixed-phase TiO2Photocatalysts for water decontamination applications. Adv. Eng. Mater. 2013;16(2):248–254. [Google Scholar]
- Hansen A.M., Kraus T.E.C., Pellerin B.A., Fleck J.A., Bryan D., Downing B.D., Bergamaschi B.A. Optical properties of dissolved organic matter (DOM): effects of biological and photolytic degradation. Limnol. Oceanogr. Limnol. Oceanogr. 2006;61(2016):1015–1032. [Google Scholar]
- Ho Y.S., McKay G., Wase D.J., Foster C.F. Study of the sorption of divalent metal ions on to peat. Adsorp. Sci. Technol. 2000;18:639–665. [Google Scholar]
- Huang H., Yao J., Li L., Zhu F., Liu Z., Zeng X., Huang Z. Reinforced polyaniline/polyvinyl alcohol conducting hydrogel from a freezing–thawing method. J. Mater. Sci. 2016 [Google Scholar]
- Huang L., Cheng S., Rezaei F., Logan B.E. Reducing organic loads in industrial effluents using microbial fuel cells. Environ. Technol. 2009;30:499–504. doi: 10.1080/09593330902788244. [DOI] [PubMed] [Google Scholar]
- Kurtoglu M.E., Longenbach T., Gogotsi Y. Preventing sodium poisoning of photocatalytic TiO2 films on glass by metal doping. Int. J. Appl. Glass Sci. 2011;2(2):108–116. [Google Scholar]
- Lagergreg S. The theory of so-called adsorption of soluble substances. Handlingar. 1898;24(4):1–3. [Google Scholar]
- Lei X., Pan J., Devlin A.T. Characteristics of absorption spectra of chromophoric dissolved organic matter in the pearl river estuary in spring. Rem. Sens. 2019;11(2019):1533–1548. [Google Scholar]
- Li P., Hur J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: a review. Crit. Rev. Environ. Sci. Technol. 2017;47:131–154. [Google Scholar]
- Lovley D.R. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- Martı´nez-Huitle C.A., Ferro S. Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem. Soc. Rev. 2006;35:1324–1340. doi: 10.1039/b517632h. [DOI] [PubMed] [Google Scholar]
- Martínez-Huitle, Carlos A., Andrade, Leonardo S. Electro catalysis in wastewater treatment: recent mechanism advances. Quím. Nova. 2011;34(5):850–858. [Google Scholar]
- Moulay S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013;33(5):389–443. [Google Scholar]
- Mwangi I.W., Ngila J.C., Ndungu P., Msagati T.A.M., Kamau J.N. Immobilized Fe (III)- doped titanium dioxide for photodegradation of dissolved organic compounds in water. Environ. Sci. Poll. Resour. 2013:6028–6038. doi: 10.1007/s11356-013-1600-6. [DOI] [PubMed] [Google Scholar]
- Mwangi I., Kiriro G., Swaleh S., Wanjau R., Mbugua P., Ngila J.C. 2019. Int J Recycling of Organic Waste Agricult. [Google Scholar]
- Naidoo S., Olaniran A.O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Publ. Health. 2013;11(1):249–270. doi: 10.3390/ijerph110100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwokocha L.M., Ogunmola G.B. Colour of starch-iodine complex as index of retrogradability of starch pastes. Afr. J. Pure Appl. Chem. 2014;8:89–93. [Google Scholar]
- Pathiraja Gayani Chathurika, Jayathilaka Pavithra Bhakthil, Weerakkody Chandima, co-workers Comparison study of dimensionally stable anodes for degradation of chlorpyrifos in water. Curr. Sci. 2014;107:2. [Google Scholar]
- Pipi A.R.F., Neto S.A., De Andrade A.R. Electrochemical degradation of diuron in chloride medium using DSA® based anodes. J. Brazil. Chem. Soc. 2013;24(8):1259–1266. [Google Scholar]
- Redel E., Rohr C., Janiak C. An inorganic starch–iodine model: the inorganic–organic hybrid compound {(C4H12N2)2[CuII4](I2)}nw. Royal. Soc. Chem. 2009;16:2103–2105. doi: 10.1039/b820151j. [DOI] [PubMed] [Google Scholar]
- Reimers C.E., Girguis P., Stecher H.A., Tender L.M., Ryckelynck N., Whaling P. Microbial fuel cell energy from an ocean cold seep. Geobiology. 2006;4:123–136. [Google Scholar]
- Richter H., McCarthy K., Nevin K.P., Johnson J.P., Rotello V.M., Lovley D.R. Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir. 2008;24:4376–4379. doi: 10.1021/la703469y. [DOI] [PubMed] [Google Scholar]
- Sequeira C.A.C., Santos D.M.F. Electrochemical routes for industrial synthesis. J. Braz. Chem. Soc. 2009;20(3):387–406. [Google Scholar]
- Shao Y., Kady M.F., El-Sun J., co-workers Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018;118(18):9233–9280. doi: 10.1021/acs.chemrev.8b00252. [DOI] [PubMed] [Google Scholar]
- Shi L., Li W., Wang F. Experimental study of a closed system in the chlorine dioxide-iodine-malonic acid-sulfuric acid oscillation reaction by UV–vis spectrophotometric method. J. Solut. Chem. 2009;38:571–588. doi: 10.1155/2011/130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteris A. Seawater desalination using renewable energy sources. Prog. Energy Combust. Sci. 2005;31:242–281. [Google Scholar]
- Shandrikov M.V., Bugaev A.S., Vizir A.V., Savkin K.P., Oks E.M. Red shift of absorption spectra of metal-doped TiO2 coatings, international congress “energy fluxes and radiation effects” IOP conference series. J. Phys. Conf. 2018;1115 [Google Scholar]
- Toh R.J., Peng W.K., Han J., Pumera M. Direct in vivo electrochemical detection of haemoglobin in red blood cells. Sci. Rep. 2014;4(6209):1–7. doi: 10.1038/srep06209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasatti S. Electrocatalysis: understanding the success of DSA. Electrochim. Acta. 2000;45:2377–2385. [Google Scholar]
- Utomo S.B., Widodo A.S., Wardana I.N.G. The role of mineral sea water bonding process with graphite-aluminum Electrodes as Electric Generator. Sci. World J. 2019:1–12. doi: 10.1155/2019/7028316. 7028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Arthur Israel. QD101.2.V63 3.28 Preparation and Storage of Standard Solutions 107. fifth ed. John Wiley & Sons, Inc.; New York: 1989. Vogel's Textbook of Quantitative Inorganic Analysis; p. 389. [Google Scholar]
- Wan G., Shingera K., Tsuchida E., Anson F.C. Virtues of a copolymer containing pyrrolidone and iron porphyrin groups in the catalysis of the reduction of dioxygen at graphite electrodes. J. Am. Chem. Soc. 1984;179(1–2):239–250. [Google Scholar]
- Wang W., Varghese O.K., Paulose M., Grimes C.A. A study on the growth and structure of titania nanotubes. J. Mat. Res. 2004;19(2):417–422. [Google Scholar]
- Wijnen B., Anzalone G.C., Pearce J.M. Open-source mobile water quality testing platform. J. Water, Sanit. Hyg. Dev. 2014;4(3):532–537. [Google Scholar]
- Woo M., Maier L., Tischer S., Deutschmann O., Wörner M. A qualitative numerical study on catalytic hydrogenation of nitrobenzene in gas-liquid taylor flow with detailed reaction mechanism. Fluids. 2020;5:234–254. [Google Scholar]
- Yan M., Kawamata Y., Baran P.S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017;117(21):13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Nevin K.P., Kim B.C., Franks A.E., Klimes A., Tender L.M., Lovley D.R. Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens. Bioelectron. 2009;24:3498–3503. doi: 10.1016/j.bios.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Yoo H., Kim H.S. Real-time colorimetric water content monitoring of organic solvents by an azo dye incorporated into AlPO4-5 nanochannel, Journal of Materials Chemistry C 24. J. Mater. Chem. C. 2019;7:7336–7343DOI. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.