Highlights
-
•
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies, which is usually diagnosed at an advanced stage.
-
•
Non-coding RNAs (ncRNAs) have been recognized to play a central role in PDAC pathogenesis and could be used as biomarkers for PDAC.
-
•
microRNAs (miRs) can act either as oncogenes or tumour suppressors in PDAC.
-
•
Long non-coding RNAs (lncRNAs) are around 25% of the total RNA distribution in the human cell and their deregulation is widely implicated in PDAC carcinogenesis.
-
•
Circular RNAs (circRNAs) can be potential novel biomarkers and therapeutic targets for PDAC diagnosis and treatment via their function as miR molecular "sponges", RNA-binding protein (RBP) sponges, protein translators and gene transcription regulators.
Keywords: Non-coding RNAs, MicroRNA, Long non-coding RNA, Circular RNA, Pancreatic ductal adenocarcinoma
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies with a 5-year survival rate less than 8%, which has remained unchanged over the last 50 years. Early detection is particularly difficult due to the lack of disease-specific symptoms and a reliable biomarker. Multimodality treatment including chemotherapy, radiotherapy (used sparingly) and surgery has become the standard of care for patients with PDAC. Carbohydrate antigen 19–9 (CA 19–9) is the most common diagnostic biomarker; however, it is not specific enough especially for asymptomatic patients. Non-coding RNAs are often deregulated in human malignancies and shown to be involved in cancer-related mechanisms such as cell growth, differentiation, and cell death. Several micro, long non-coding and circular RNAs have been reported to date which are involved in PDAC. Aim of this review is to discuss the roles and functions of non-coding RNAs in diagnosis and treatments of PDAC.
Graphical abstract
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a major cause of cancer-associated deaths in Western countries and is the eighth main source of cancer-related deaths globally, with the median survival rate of 3–10 months [1,2]. The complex biology and aggressive features of PDAC have been associated with the limited efficiency of treatments, especially in advanced stages of PDAC [3]. PDAC cases present significant heterogeneity of somatic DNA modifications, and alterations in genetic pathways [4]. Therefore, a substantial number of complex genetic networks and transcriptomics have been correlated with the development of PDAC [5]. The progression of PDAC can be divided into three major PDAC precursor lesions: pancreatic intraepithelial neoplasia (PanIN I, II, III); mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN) [6]. Each of these PDAC precursor lesions presents unique clinical, pathological and molecular features [6]. The most frequent genetic alterations that are associated with PDAC development are mutations of KRAS and overexpression of human epidermal growth factor receptor (HER-2/neu) [7]. Furthermore, at later stages, inactivation of tumour suppressor genes (TS) such as cyclin-dependant kinase inhibitor 2A (CDKN2A), tumour protein 53 (TP53) and SMAD family number 4 (SMAD4) have been closely linked to pathogenesis and metastasis of PDAC [7]. Carbohydrate antigen 19–9 (CA 19–9), carcinoembryonic antigen (CEA) and duke pancreatic monoclonal antigen type 2 (DUPAN2) are the most commonly used biomarkers for PDAC [8,9]. However, even CA 19–9, the best biomarker available, cannot be characterized as a PDAC-specific biomarker, especially for asymptomatic patients [10], as the false-positive rate of CA19–9 is relatively high at 20–30% [11]. Hence, these biomarkers are most commonly utilized to monitor during and post-treatment, and assess prognosis, but are not robust enough [12]. There is an urgent need for novel non-invasive biomarkers for the early diagnosis and prognosis [13], which could be used to ascertain follow-up strategies for PDAC. Molecular modifications, genetic and epigenetic factors could be a crucial for the early diagnosis of PDAC [14]. Technological advances have prompted a new era of “omics” based research for the establishment of circulating biomarkers, including proteins, cell-free DNAs, non-coding RNAs, circulating tumour cells (CTCs), and exosomes’ molecular content [15]. In terms of the treatment, currently, combination chemotherapies such as FOLFIRINOX (5-FU, irinotecan, oxaliplatin and leucovorin) and gemcitabine-nab-paclitaxel are considered superior to gemcitabine monotherapy for advanced pancreatic cancer [16].
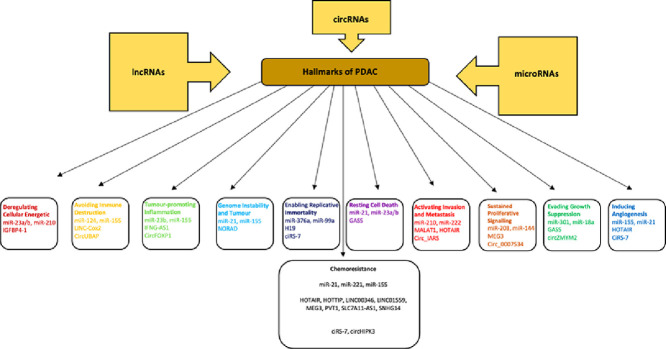
Non-coding RNAs (ncRNAs) are a class of RNA molecules that cannot be translated into protein and are classified into subtypes based on their length [17]. Most commonly studied ncRNAs are long non-coding RNA (lncRNAs: longer than 200 nucleotides) and microRNAs (miRs: 18–24 nucleotides long) however, small interfering RNAs (siRNAs), tRNA-derived stress-induced RNAs (tiRNAs), enhancer non-coding RNAs (eRNAs) and circular RNAs (circRNAs) are also classified as ncRNAs [18]. ncRNAs are key regulators in several physiological cellular and biological processes, which involve the modulation of gene expressions both transcriptionally and post-transcriptionally [19]. Recent studies showed that ncRNAs play a crucial role in cancer prognosis by controlling both cellular and biological processes including chromatin remodelling, transcription, post-transcriptional modifications and signal transduction [20, 21]. ncRNAs can act both as tumour suppressors and oncogenic drivers in numerous malignancies including PDAC, and correlation between aberrant expression levels of several ncRNAs in PDAC has been reported [22,23]. Therefore, the aim of this review is to evaluate the expression profiles of ncRNAs to determine the most significant miR, lncRNA and circRNA signatures, which could be critical both for the early diagnosis and effective therapy strategies of PDAC.
miRNAs as Diagnostic and Prognostic Biomarkers for PDAC
miRs, are small (18 to 24 nucleotides in length), endogenous, non-coding, evolutionary conserved, single-stranded RNA molecules that can moderate gene expression at the post-transcriptional level through binding to the complementary sequences of their target mRNAs at 3′ untranslated regions (UTRs) [24]. Epigenetic alterations, which are regulated by miRs have been suggested to be implicated in the prognosis of PDAC may explain its complexity [25]. The aberrant expression of miRs plays a significant role in initiation, proliferation, induction of the epithelial to mesenchymal transition (EMT), metastasis, and chemo-resistance of PDAC cells [26]. Stable miR expression was detected in tissues, blood plasma and several body fluids such as serum, urine, and breast milk [27]. Thus, miRs can be characterized as “circulating microRNAs”, which can be found encapsulated in cell-secreted vesicles or vesicle-free [27]. miRs can act as oncogenes (oncomiR) or tumour suppressor genes (tsmiRNA) in PDAC [28,29]. The aberrant expression of miRs can be the result of amplification or deletion in a genomic region where miRs genes are expressed [30]. Even though the biological functions of the identified miRs present considerable ambiguity, the examination of the expression profiles of these biomarkers can provide useful information regarding their regulation and function [31]. Despite the narrow knowledge of these molecules, a plethora of miRs can be characterized as vital biomarkers not only for the early prognosis and diagnosis of PDAC but also for better management of therapeutics [32].
Oncogenic miRs in PDAC
miR-376a and miR-301 are upregulated in tissues while miR-23a and miR-23b over expressions were detected in saliva of PDAC patients [33,34]. Moreover, miR-21, is one of the most studied miRs, along with miR-20a, miR-24, miR-25, miR-99a, miR-185 and miR-191 and, due to their increased expression levels in PDAC, were suggested as potential diagnostic markers [34], [35], [36]. Interestingly, miRs that identified as biomarkers showed an accuracy of 83.6% compared to CA 19–9, which is only 56.4% [37], [38], [39]. Another study reported increased expressions of miR-1246, miR-4644, miR-3976 and miR-4306 in serum derived exosomes of PDAC patients [40], whereas miR-221 and miR-18a were also found to be upregulated in plasma of PDAC patients [41], [42], [43]. miR-221 is associated with distant metastasis, interestingly, miR-221 and miR-18a expression levels were reduced after surgery [41], [42], [43]. Therefore, both miR-221 and miR-18a could be effective diagnostic and prognostic biomarkers [41], [42], [43]. Furthermore, miR-194 expression levels were significantly elevated and linked with the poor prognosis of PDAC [44], while Bloomston et al. (2007) suggested the overexpression of miR-155, miR-181a, b, c, d and miR-196a in PDAC cases [45]. Moreover, miR-10a, miR-17–5p and miR-92 expressions were significantly upregulated in PDAC cases [46]. Lin et al. (2014) also showed the overexpression of miR-1238, miR-4290 and miR-483–5p in PDAC patients [47]. miR-486–5p and miR-938 have been proposed as potential diagnostic serum biomarkers for PDAC [48]. Further studies have suggested the upregulation of miR-203, miR-210, miR-222 [49], miR-196b and miR-196a [50]. Wang et al. (2013) also showed that the upregulation of miR-27a-3p effectively discriminated PDAC cases from benign pancreatic/peri-pancreatic diseases (BPD) [51]. Conclusively, miR-135b, which is also overexpressed in PDAC, can be characterized as a potential diagnostic biomarker for PDAC due to the fact that it presented high sensitivity and specificity for the discrimination of PDAC cases [52] (Table 1, Fig. 1).
Table 1.
Oncogenic miRs associated with PDAC.
| miRs | Expression in PDAC | Clinical Values | Functional Involvement in PDAC | Detected | Biology Tested | Control | Number of Patients | Group Tested | References(s) |
|---|---|---|---|---|---|---|---|---|---|
| miR-376a | Up | D | Increase cell proliferation, invasion, migration | Tissue + Panc-1, HS766T, MIA PaCa-2, BxPC3, Panc10.05 cell lines | Cells, Patients | 6 Normal pancreatic tissue | 28 | Stage II, III | [33] |
| miR-301 | Up | D | Increase cell growth | Tissue +Panc-1, HS766T, MIA PaCa-2, BxPC3, Panc10.05 cell lines | Cells, Patients | 6 Normal pancreatic tissue | 28 | Stage II, III | [33] |
| miR-21 | Up | D, P, T | Inhibition of apoptosis, increase gemcitabine resistance, aggressiveness | Saliva, Blood, Tissue | Patients, Animals | 4 Healthy controls | 7 | Locally advanced and unresectable PDAC | [34,36,41,45,53,54,55] |
| miR-23a | Up | D, T | Inhibition of apoptosis | Saliva | Patients, Animals | 4 Healthy controls | 7 | Locally advanced and unresectable PDAC | [34,36,56] |
| miR-23b | Up | D, T | Inhibition of apoptosis, Radioresistance | Saliva | Patients, Animals | 4 Healthy controls | 7 | Locally advanced and unresectable PDAC | [34,57] |
| miR-24 | Up | D, P | Inhibition of apoptosis | Blood-Serum | Patients | 158 Healthy controls | 197 | Stages I, II, III, IV | [35,37,58] |
| miR-25 | Up | D, P | Inhibition of apoptosis | Blood-serum | Patients | 158 Healthy controls | 197 | Stages I, II, III, IV | [35,37,58] |
| miR-99a | Up | D, P | Increase cell proliferation, invasion, migration | Blood-serum | Patients | 158 Healthy controls | 197 | Stages I, II, III, IV | [35,37,58] |
| miR-185 | Up | D, P | Increase invasion, migration | Blood-serum | Patients | 158 Healthy controls | 197 | Stages I, II, III, IV | [35, 37, 58] |
| miR-191 | Up | D, P | Inhibition of cell differentiation | Blood-serum | Patients | 158 Healthy controls | 197 | Stages I, II, III, IV | [35,37,58] |
| miR-1246 | Up | D, T | Increase chemoresistance, cell invasion and migration | Serum | Patients | 12 Healthy controls | 131 | Stages I, II, III, IV | [40,59] |
| miR-4644 | Up | D | Increase cell invasion and migration | Serum | Patients | 12 Healthy controls | 131 | Stages I, II, III, IV | [40] |
| miR-3976 | Up | D | Increase cell invasion and migration | Serum | Patients | 12 Healthy controls | 131 | Stages I, II, III, IV | [40] |
| miR-4306 | Up | D | Increase cell invasion and migration | Serum | Patients | 12 Healthy controls | 131 | Stages I, II, III, IV | [40] |
| miR-221 | Up | D, P, T | Increase cell proliferation, migration, EMT | Plasma, Tissue +Panc-1 cell line | Patients, Cell lines | 30 Healthy volunteers | 47 | Stages I, II, III, IV | [42,45] |
| miR-18a | Up | D, P, T | Inhibition of apoptosis, increase cell growth | Plasma, Tissue + Panc-1 | Patients, Cell lines | 30 Healthy controls | 36 | Stages I, II, IV | [41] |
| miR-194 | Up | D, P | Increase tumour growth and invasion | Tissue + Panc-1, BxPC3, AsPC-1, Capan-1, MIA Paca-2, SW1990 cell line | Patients, Cell lines | 3 Adjacent non-cancerous tissues | 9 | PDAC patients with surgical resection | [44] |
| miR-155 | Up | D, P, T | Decrease apoptosis, Increase cell invasion, migration, metastasis, generation of reactive oxygen species | Plasma, Tissue | Patients | Adjacent non-cancerous tissues | 65 | PDAC patients with surgical resection | [45,36,60,61] |
| miR-181a, b, c, d | Up | D, T | Increase migration and metastasis | Plasma, Tissue | Patients | Adjacent non-cancerous tissues | 65 | PDAC patients with surgical resection | [45,62,58] |
| miR-196a | Up | D, P | Increase invasion and migration | Plasma, Tissue | Patients | Adjacent non-cancerous tissues | 65 | PDAC patients with surgical resection | [45,50,58] |
| miR-10a | Up | D, T | Increase chemoresistance and metastasis | Tissue +Panc-1, BxPC3, AsPC-1, Capan-1, MIA Paca-2, SW1990, HDPE cell line | Cell lines | HDPE | 15 Cell lines | Primary tumours | [63,64] |
| miR-17–5p | Up | D, P, T | Increase cell growth apoptosis, decreased chemosensitivity to gemcitabine | Tissue +Panc-1, BxPC3, AsPC-1, Capan-1, MIA Paca-2, SW1990, HDPE cell line | Cell lines | HDPE | 15 Cell lines | Primary tumours | [36,63,65] |
| miR-92 | Up | D | Increase cell growth, inhibition of cell differentiation | Tissue +Panc-1, BxPC3, AsPC-1, Capan-1, MIA Paca-2, SW1990, HDPE cell line | Cell lines | HDPE | 15 Cell lines | Primary tumours | [63] |
| miR-1238 | Up | D | Inhibition of apoptosis | Serum | Patients | 27 Matched Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-4290 | Up | D | Inhibition of cell differentiation | Serum | Patients | 27 Matched Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-483–5p | Up | D | Increase proliferation and colony formation in vitro | Serum | Patients | 27Matched Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-486–5p | Up | D | Increase cell proliferation, migration and invasion | Plasma | Patients | 5 Healthy controls | 7 | Pre-operative PDAC | [48] |
| miR-938 | Up | D | Increase cell proliferation, migration and invasion | Plasma | Patients | 5 Healthy controls | 7 | Pre-operative PDAC | [48] |
| miR-203 | Up | D, P | Increase cell proliferation, migration, invasion, decrease apoptosis | Tissue | Patients | 7 Normal pancreatic tissue | 10 | Stage III, IV | [49,36,60] |
| miR-210 | Up | D, P | Promotes invasion and EMT | Plasma, Tissue | Patients | 7 Normal pancreatic tissue | 10 | Stage III, IV | [49,36,58,60] |
| miR-222 | Up | D, P | Increase cell proliferation, migration, invasion, decrease apoptosis | Tissue | Patients | 7 Normal pancreatic tissue | 10 | Stage III, IV | [36,49,60] |
| miR-196b | Up | D, P | Increase invasion and migration | Tissue | Patients | 35 Normal pancreatic tissue | 165 | Stage IA, IB, IIA, IIB | [66] |
| miR-27a-3p | Up | D, T | Increase growth, migration, and colony formation in vitro | Blood | Patients | 20 Healthy controls | 20 | Stage IA, IB, IIA, IIB, III, IV | [51,56] |
| miR-135b | Up | D, P | Increase tumour growth, promote cell adaptation to metabolic stress, suppress glycolysis | Tissue | Patients | Normal pancreatic tissue | 52 | - | [52] |
| miR-212 | Up | D, P | Increase proliferation | Tissue + MIA Paca-2, AsPC1 cell line | Patients, Cell lines | Normal pancreatic tissue | 41 | PDAC patients with surgical resection | [67] |
| miR-182 | Up | D, P | Increase tumour growth, invasion and migration | Plasma | Patients | Healthy controls | 109 | Stages I, II, III, IV | [68] |
D: diagnostic biomarker, P: prognostic biomarker, T: therapeutic target
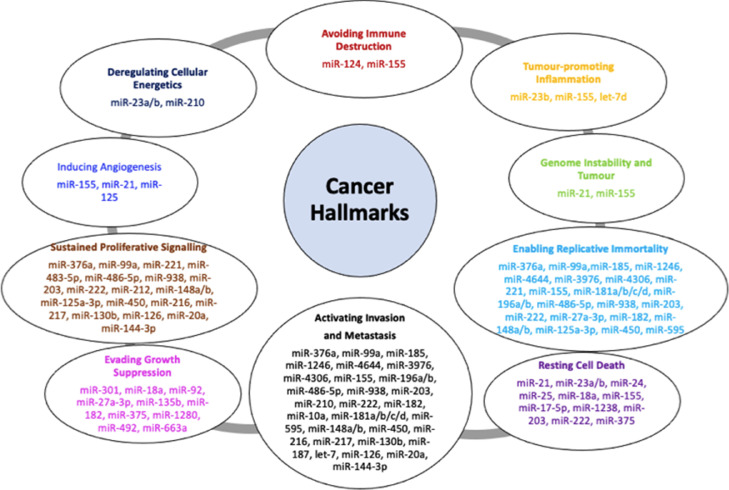
Fig. 1.
Involvement of miRs in PDAC progression. miRs are classified both as oncogenes and tumour suppressors by moderating several key downstream gene targets which control different cellular and biological processes involved in cancer progression.
Tumour suppressor miRs and PDAC
Downregulation of miR-148a, b, and miR-375 expression could be used to differentiate PDAC, normal pancreatic and pancreatitis tissues [45]. Reduced expression of miR-125a-3p was correlated with EMT and gemcitabine resistance in PDAC [69,70]. Another study reported that both miR-450 and miR-205 aberrantly expressed in PDAC [63], while Lin et al. (2014) suggested that the downregulation of miR-1280, miR-492, miR-595 and miR-663a in PDAC patients [47]. Specifically, miR-663a is found to be closely correlated with the tumour-node-metastasis (TNM) stages in PDAC. Thus, these novel non-invasive biomarkers could be utilized for the prognosis of PDAC [47], while a combined miR panel could be a valuable diagnostic and prognostic strategy for PDAC. miR-216 and miR-217 were down regulated in PDAC samples and hence could be used as diagnostic biomarkers for PDAC patients [49]. Moreover, the expression levels of three miRs, which are transcribed from the miR-216/-217 miR family, were decreased in the P48+/Cre;LSL-KRASG12D, PDX-1-Cre;LSL-KRASG12D, and ELa-KrasG12D mice [71]. Similarly, reduced expression of miR-216a, miR-216b and miR-217 were detected in the pancreas of P48+/Cre;LSL-KRASG12D mice [72]. Further studies indicate that miR knockout mouse models increased the lethality in mice [73,74]. miR-130b also considerably downregulated in PDAC cases and was closely related to poor prognosis, elevated tumour size, late TNM stage, lymphatic invasion and distant metastasis [75] (Table 2, Fig. 1).
Table 2.
Tumour suppressor miRs are identified in PDAC.
| miRs | Expression in PDAC | Clinical Values | Biological processes involved | Detected | Biology Tested | Control | Number of patients | Group Tested | References |
|---|---|---|---|---|---|---|---|---|---|
| miR-148a | Down | D, P, T | Cell proliferation, invasion, migration | Plasma, Tissue | Patients | Matched Adjacent non-cancerous tissues | 65 | PDAC patients with surgical resection | [45,50,76,77] |
| miR-148b | Down | D, T | Cell proliferation, invasion, migration, inhibition of chemo-sensitization | Plasma, Tissue | Patients | Matched Adjacent non-cancerous tissues | 65 | PDAC patients with surgical resection | [45,78] |
| miR-375 | Down | D, P | Tumour growth and apoptosis | Plasma, Tissue | Patients | Matched Adjacent non-cancerous tissues | 65 | PDAC patients with surgical resection | [45,49] |
| miR-125a-3p | Down | D, T, P | Cell proliferation and migration, chemosensitivity, EMT | Blood | Patients, Panc-1, BxPC3, AsPC-1, Capan-2, MIA-PaCa-2 cell lines | Adjacent non-cancerous tissues | 421 | Advanced PDAC cases | [69,70,79] |
| miR-450 | Down | D | Cell differentiation, proliferation, migration and invasion | Panc-1, BxPC3, AsPC-1, Capan-2, MIA-PaCa-2 cell lines | 15 PDAC cell lines | Primary tumours | [63] | ||
| miR-1280 | Down | D, P | Tumour growth | Blood | Patients | 27 Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-492 | Down | D, P | Tumour growth and stage | Blood | Patients | 27 Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-595 | Down | D, P | Migration, metastasis | Blood | Patients | 27 Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-663a | Down | D, P | Tumour growth and stage | Blood | Patients | 27 Healthy controls | 49 | Stages I, II, III, IV | [47] |
| miR-216 | Down | D, T | Increase cell proliferation, invasion | Tissue | Patients, +3 Cell Lines | Normal Pancreatic tissue | 10 | Stage III, IV | [49,80] |
| miR-217 | Down | D, T | Increase cell proliferation, migration, invasion, DNA damage, stress responses, genome stability and cell survival | Tissue | Patients, +3 Cell Lines | Normal Pancreatic tissue | 10 | Stage III, IV | [49,80] |
| miR-130b | Down | D, P, T | Cell proliferation, invasion | Tissue | Patients, Panc-1, BxPC3, AsPC-1, SW1990, MIA-PaCa-2 cell lines | Matched Normal Pancreatic tissue | 52 | Stage I, II III, IV | [75] |
| miR-187 | Down | D, P | Invasion, migration | Tissue | Patients | Normal Pancreatic tissue | 170 | Stages IA, IB, IIA, IIB | [76] |
| let-7 | Down | D, P, T | EMT, invasion | Tissue | Patients | Normal Pancreatic tissue | 170 | Stages IA, IB, IIA, IIB | [76] |
| miR-205 | Down | D, T | Chemoresistance | Pancreatic Juice | Patients | 19 Non-healthy (NPNH) controls | 50 | Advanced PDAC cases | [50,79,81,82] |
| miR-126 | Down | D, P | Cell proliferation, migration, invasion | Tissue | Patients | Normal Pancreatic tissue | 455 | Stages O, I, II, III, IV | [36] |
| miR-20a | Down | D, P | Proliferation, invasion | Blood-plasma | Patients | Healthy individuals | 197 | Stages I, II, III, IV | [35,37,58] |
| miR-144–3p | Down | D, P | Cell cycle arrest, migration, invasion, metastasis, cell proliferation | Tissue | Patients + Panc-1 cell line | Paired adjacent non-tumour tissues | 10 | Stages IA, IB, II, III | [83] |
D: diagnostic biomarker, P: prognostic biomarker, T: therapeutic target.
Early stage of PDAC associated miRs
It was suggested that miR-1290 could be used to determine low-stage PDAC with high sensitivity and specificity [84]. Besides, it was demonstrated that in addition to miR-16, miR-155 and miR-181a, miR-181b and miR-210 were overexpressed in the plasma of PDAC patients compared to normal controls [58]. Therefore, miR combination panels presented high specificity and sensitivity for the diagnosis of PanIN-I PDAC patients [58]. miR-16, especially, presented a sensitivity of 92.0% and a specificity of 95.6% for the determination of PDAC cases between normal samples [85]. miR-19a-3p was shown to be overexpressed in PDAC and could be utilized not only as an early non-invasive diagnostic biomarker for PDAC but also as a prognostic biomarker for patients with poor overall survival rate [86]. Another study identified the upregulation of miR-29a, miR-29b, miR-103 and miR-320 as early diagnostic predictors of PDAC and therefore it can be proposed that these miRs could be also utilized for the early diagnosis of PDAC [35] (Table 3, Fig. 2).
Table 3.
Deregulated miRs in PDAC lesions.
| PDAC Lesion | Overexpressed miRs | Down Regulated miRs | References |
|---|---|---|---|
| PanIN-I | miR-21, miR-155, miR-182, miR-200a, miR-200b, miR-221, miR-1290, miR-181a, miR-181b, miR-210, miR-103, miR-145, miR-193b, miR-320 | miR-296–5p, miR-107, miR-181c | [33,58,84,85,86] |
| PanIN-II | miR-21, let-7, miR-155, miR-200, miR-205, miR-222, miR-10b, miR-196a, miR-196b, miR-29b/a, miR-486–3p, miR-425, miR-708, miR-874, miR-145, miR-200a, miR-200b, miR-200c, miR-193a-3p | miR-296–5p, miR-148, miR-217 | [23,68,85,87,88,89,90] |
| PanIN-III | let-7, miR-18a, miR-21, miR-155, miR-145, miR-196b, miR-200, miR-222, miR-338–3p, miR-486–3p, miR-29b, miR-425, miR-708, miR-874, miR-10b, miR-196a, miR-182, miR-205, miR-221 | miR-125b, miR-126, miR-218, miR-296–5p, miR-452, miR-148, miR-217 | [68,85,87,89,91,92,93,94] |
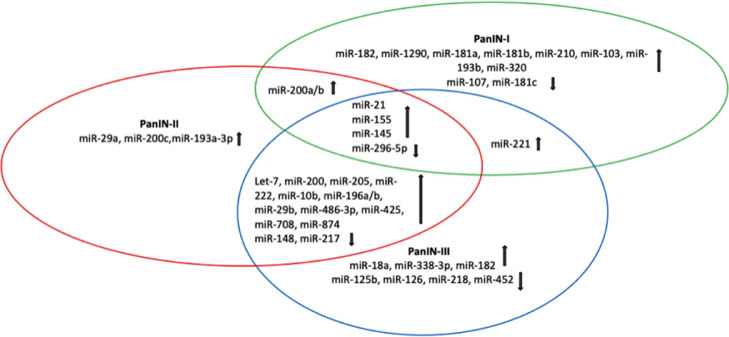
Fig. 2.
Venn diagram shows the overlap between differentially expressed miRs in different lesions of PDAC. It can be noted that miR-21, miR-155, miR-145 and miR-296–5p are presented in all PanIN lesions, while miR-200a/b in both PanIN-I and II. miR-221 are detected only in PanIN-I and III.
Late stage of PDAC associated miRs
Both miR-196a and miR-196b were found to be overexpressed in PDAC patients with PanIN-II & III lesions and correlated with poor survival [87]. In particular, miR-196a and miR-196b presented 100% sensitivity and specificity in the discrimination of PDAC cases compared to healthy controls [87]. Significantly, miR-196a is an effective prognostic biomarker, due to the fact that patients with unresectable PDAC (stages III and IV) had considerable higher expression levels of miR-196a in comparison with patients in the early stages of the disease (stages I and II), which presented lower levels [85]. miR-182 was overexpressed in PDAC patients in correlation to healthy controls and was linked to advanced clinical stages and lymph node metastasis [68]. Furthermore, overexpressed miR-21 has been associated with advanced stages of PDAC metastasis to lymph nodes and liver, increased gemcitabine-resistant and poor survival in PDAC patients [88], [89], [90]. Previous studies indicated that miR-21, miR-29b, miR-146a, miR-182, miR-193a-3p, miR-193b, miR-200a, miR-200b, miR-425, miR-486–3p, miR-708 and miR-874 were significantly dysregulated in PanIN-II & III lesions in comparison to PanIN-I lesions and normal pancreatic tissues [91,92]. Besides, Ryu and colleagues (2010) noted that miR-155 was upregulated in PanIN-II and III in comparison to PanIN-I and healthy controls [88]. Thus, a large body of evidence strongly suggests the utility of specific miRs profiles as potential effective early diagnostic and prognostic tools for PDAC subtypes (Table 3, Fig. 2).
Prognostic miRs in PDAC
Upregulated miR-132 [95], miR-21 [54] and downregulated miR-96 [96], miR-34a [97] are aberrantly expressed in PDAC tissues, in relation to normal adjacent tissue, and are associated with poor overall survival [98]. A study by Giovannetti et al. (2010) demonstrated that miR-21 expression could identify shorter overall survival rates in PDAC patients, who have undergone gemcitabine therapy [99]; miR-21 upregulation is also associated with poor survival rate in 79% of PDAC cases [54,100]. Another study indicated that PDAC tissues containing hypermethylated miR-124–1, miR-124–2 and miR-124–3, were related to poor survival rate in PDAC [101].
Further examples of miRs with considerable prognostic impact in PDAC are miR-451a and miR-1290 and tissue miR-10b, miR-17–5p, miR-29c, miR-126, miR-155, miR-203, miR-218, miR-221 and miR-222 [36]. In particular, miR-21, miR-451a, miR-23a, miR-155 and miR-218 presented the highest prognostic impact in PDAC patients and linked to poor survival [36]. Frampton et al. (2014) confirmed the prognostic impact of miR-23a and miR-27a in PDAC patients, who have undergone surgical resection [56]. In addition, upregulation of miR-200c was also linked with limited survival in PDAC patients through the reduction in the levels of mucin 4 (MUC4) and mucin 16 (MUC16) [102]. Similarly, the overexpression of miR-210 is associated with poor survival rate in PDAC patients [60]. miR-4521 down regulation is linked with uncontrolled proliferation of PDAC cells and poor overall survival rate [103]. Matrix metallopeptidase 7 (MMP7) is highly expressed in PDAC tissues and associated with metastasis, while it can be targeted with the upregulation of miR-144–3p, which leads to longer survival rates [83]. Upregulation in miR-182 presented shorter disease-free survival and overall survival compared to patients with low expression levels [68]. Similarly, Schultz et al. (2012) designated that the upregulation of miR-212 and miR-675 and down-regulation of miR-148a, miR-187 and let-7 g were linked to a worse prognosis in PDAC cases [104]. On the contrary, oncogenic miR-30a-3p, miR-105, miR-127, miR-187, miR-452 and miR-518a-2 are associated with a better prognosis of PDAC patients [45], whereas Greither et al. (2010) noted that miR-200c and miR-302 are related to PDAC patients’ outcomes and poor survival [60]. Besides, it has been suggested that downregulated miR-506 is associated with poor prognosis in PDAC [105, 106]. A further study revealed that miR-6075, miR-4294, miR-6880–5p, miR-6799–5p, miR-125a-3p, miR-4530, miR-6836–3p and miR-4476 presented a higher accuracy for the diagnosis of PDAC in correlation with CA19–9 and CEA [78]. Further examples of miRs with better survival rates are the upregulated miR-142 and miR-204 [46], while downregulated miR-19a-3p corresponds to higher survival impact [86]. Hence, miRs could act as effective prognostic biomarkers.
LncRNAs in PDAC
Long non-coding RNAs (lncRNAs) have emerged as a class of factors that are of importance in regulating normal development and cancer pathology [107,108]. Although less functionally characterised than miRs, lncRNAs accounts for 25% of the total RNA distribution in the human cell, are more abundant than miRs and are accepted as an important class of pervasive ncRNAs [109]. The interplay between lncRNAs and their target molecules are involved in a wide range of fundamental and critical biological processes, deregulation of which is widely implicated in carcinogenesis [110], [111], [112], [113]. Notably, lncRNAs are confirmed to play important roles including transcriptional and post-transcriptional regulation, epigenetic regulation, chromatin remodelling, organ or tissue development, cell differentiation and apoptosis, cell cycle control, cellular transport, metabolic processes and chromosome dynamics [110,114]. The intrinsic ability of lncRNAs to interact with DNA, RNA and proteins allows them to regulate gene expression at almost every stage by taking the role as guides, scaffolds, signals, and decoys [112,115]. A mounting number of studies have demonstrated that deregulation of lncRNAs is a critical factor in malignant transformation performing in either oncogenic or tumour-suppressive capacities and have potential to contribute to precise diagnosis and individualised therapy for cancers including PDAC [116]. Based on their different features and functional mechanisms, lncRNAs could be classed as: (1) long intergenic ncRNAs (lincRNAs); (2) long intronic ncRNAs [117]; (3) transcribed ultra-conserved regions; (4) transcribed pseudogenes [118]; (5) natural antisense transcripts (NATs); (6) promoter-associated long RNAs; (7) promoter upstream transcripts; (8) repetitive element-associated ncRNAs, and (9) enhancer-like ncRNAs [114]. lncRNAs can either act as “miR sponges” or competitive endogenous RNAs (ceRNAs) that regulate miR activity and consequently gene expression [111]. There are several widely recognised dysregulated lncRNAs in other cancers apart from PDAC, which have been functionally characterised and are shown to be promising diagnostic and prognostic biomarkers as well as novel therapeutic targets (e.g., H19, HULC, HOTAIR, HOTTIP & MALAT-1) [119]. However, functional elucidation of the lncRNAs have only recently been identified as being linked specifically to PDAC tumorigenesis, including the identification of their target molecules (e.g., miRs), and may have the potential to serve as early diagnostic tools for cancer risk, sub-typing and prognosis as well as targeted therapeutics [120].
Oncogenic lncRNAs mediated in PDAC
LncRNA H19 was not only found to be up-regulated in PDAC, compared to adjacent normal pancreatic tissues, but also promoted PDAC cell invasion and migration by increasing high mobility group AT-hook 2 (HMGA2)-mediated EMT through antagonising let-7 [121]. Additionally, H19 contributes to metastasis by promoting PDAC stem-like cell adhesion by up-regulating the expression of integrin and CD24 [122]. H19 may act as a sponge of miR-675 to promote PDAC cell proliferation by enhancing E2F-1 expression and is crucial for regulating the growth and progression of PDAC [123]. H19 may be a good prognostic tool for the overall survival of PDAC patients [124] as well as sensitivity diagnostic marker for chemotherapy response [114]. Feng and colleagues (2018) showed that the over-expression of HULC activated PI3K/AKT pathway in Panc-1 cells by downregulating miR-15a while the suppression of HULC had opposite effects and dramatically induced Panc-1 cell apoptosis [125]. Another recent study revealed that miR-133b targets HULC directly and attenuates PDAC cell invasion and migration by inhibiting HULC expression highlighting its value as a potential therapeutic target for PDAC [126]. Moreover, overexpression of miR-622, which was significantly downregulated by transforming growth factor beta (TGF-β) in a panel of PDAC cells, significantly reduced cell invasion and migration whereas inhibition of miR-622 increased HULC expression and promoted EMT signalling, invasion, and migration of PDAC cells [127]. Upregulation of HULC was significantly correlated with large tumour size, advanced lymph node metastasis, and vascular invasion; it may serve as an independent predictor for overall survival in PDAC [128]. Overexpression of HOX Antisense Intergenic RNA (HOTAIR) has been linked to susceptibility, metastasis and/or poor prognosis of pancreatic cancers [129]. Li and associates (2016) demonstrated that lncRNA HOTAIR can physically interact with the miR-34a promoter and use its enhancer of zeste homologue 2 (EZH2)-interacting regions to guide EZH2 in targeting miR-34a gene and its consequent silencing [130]. HOTAIR epigenetically regulates the expression of miR-663b via the histone modification [131], while Cai et al. (2019) showed that stable over-expression of miR-613 or knock-down of HOTAIR suppressed tumour growth and also reduced the expression of Notch3 suggesting that HOTAIR functions as a ceRNA to regulate Notch3 expression via sponging miR-613 in PDAC [132]. In addition, HOTAIR may be induced by gemcitabine and acts as a tumour promoter by inhibiting the chemosensitivity and promoting the self-renewal capacity, proliferation and migration of Panc-1 cancer stemness [133]. Yang et al. (2017) showed that epigenetic modulation of the death receptor 5 (DR5) gene by HOTAIR regulates the resistance of PDAC cells to TRA-8-induced apoptosis, which may contribute to TNF-related apoptosis-inducing ligand (TRAIL) resistance [134]. It has also been indicated that HOTAIR knockdown promotes radiosensitivity of PDAC by regulating autophagy via autophagy related 7 (Atg7) gene expression [135]. The growing interest in HOTAIR as an important pro-oncogenic lncRNA is justified since it has been shown to be detectable in serum [136] as well as in saliva, making it a suitable candidate for a non-invasive biomarker [137].
HOXA transcript at the distal tip (HOTTIP) is another HOX-associated lncRNA that shows oncogenic-like activity in PDAC [120]. Overexpression of HOTTIP is significantly correlated with lymph node metastasis and overall survival, which can be detected through plasma-based HOTTIP-005, one of the most stable spice variants of HOTTIP in PDAC tissues [138]. Additionally, HOTTIP promotes gemcitabine resistance by regulating HOXA13 in PDAC, which suggests the HOTTIP/HOXA13 axis as a potential therapeutic target for ‘chemo gene’ therapy [136,139]. Recently, it has also been shown that silencing HOTTIP reverses cisplatin resistance of PDAC cells by promoting miR-137 expression [140]. The oncogenic property of widely expressed lncRNA (MALAT-1) has also been found in PDAC [141], [142], [143], [144] and its up-regulation significantly associated with tumour size, advanced stages, deeper invasion [145] and poor overall survival [146]. Zhou and associates (2018) demonstrated that downregulation of MALAT1 to suppress proliferation, migration and invasion, and induce apoptosis of pancreatic cancer cells by regulating the expression of LATS1 and YAP1 in the Hippo-YAP signalling [147]. They also showed that downregulation of MALAT1 inhibited the tumour growth of PDAC in vivo highlighting the crucial role MALAT1 played in PDAC progression [147]. MALAT1 acts as a ceRNA to regulate K-RAS protein expression by sponging miR-217 [148]. MALAT1 knockdown does not directly affect cellular miR-217 expression but decreases the miR-217 nucleus/cytoplasm ratio, suggesting that MALAT1 inhibits the translocation of miR-217 from the nucleus to the cytoplasm [148,149]. In addition, overexpressed PVT1 is also a negative regulator of gemcitabine sensitivity in PDAC [124] and can be used to predict patient outcomes [150]. PVT1 functions as a ceRNA for miR-448 binding to regulate the miRNA target serpine1 mRNA binding protein 1 (SERBP1) and therefore promote the proliferation and migration of PDAC cells [151]. The expression level of PVT1 was positively correlated with SERBP1 in PDAC tissues [151]. SERBP1 is a known transcription factor of lipogenic genes, ACC, FASN and SCD1, which plays important roles in regulating de novo lipogenesis, and is crucial for PDAC tumorigenesis [152]. It has also been reported that PVT1 up-regulated the expression of both Pygo2 and ATG14 and thus regulated Wnt/β-catenin signalling and autophagic activity to overcome gemcitabine resistance through sponging miR-619–5p [153]. PVT1 has also been shown to be readily detectable in patient saliva samples making it a versatile and non-invasive biomarker for early diagnosis and gemcitabine response [137]. Furthermore, high expression of lncRNA AFAP1 antisense RNA 1 (AFAP1-AS1) may act as a negative prognostic factor in PDAC patients with surgical resection [154]. Ye and associates (2015) indicated that the overall survival and progression-free survival was significantly worse in PDAC patients with higher AFAP1-AS1 expression in their tumour tissues as their levels correlated strongly with lymph node metastasis and perineural invasion [154]. AFAP1-AS1 could regulate the progression of pancreatic cancer by acting as a ceRNA for miR-133a [155]. The insulin like growth factor 1 receptor (IGF1R) oncogene, which is an important regulator of MEK/ERK signalling pathway, was positively regulated by AFAP1-AS1 through ameliorating miR-133a-mediated IGF1R repression in PDAC [155]. IGF1R has been shown to coordinate the regulation of multiple cellular pathways involved in survival, proliferation, metastasis, EMT, apoptosis and cell cycle signalling [156]. Interestingly, miR-133a has also been shown to down-regulate the expression of XIST, another long non-coding RNA implicated in PDAC [157]. Over-expression of X inactive-specific transcript (XIST) has been shown to significantly promote proliferation, migration and invasion, and suppressed cell apoptosis in PDAC cells [158]. Wei and associates (2017) showed that XIST and miR-133a reciprocally inhibited each other in PDAC cells [157]. Moreover, they showed that miR-133a bound to XIST and the 3′UTR of epidermal growth factor receptor (EGFR) by direct targeting, and that XIST expression was positively correlated with EGFR expression [157]. EGFR overexpression is thought to confer poor survival, correlating with a more advanced stage and the presence of metastases in PDAC [157]. XIST has also been demonstrated to act as a sponge for miR-429 to modulate zinc finger E-box binding homeobox 1 (ZEB1) expression, promoting migration, invasion and EMT [159]. Additionally, it has also been demonstrated that overexpression of XIST accelerates cell migration and invasion in PDAC by directly targeting and suppressing tumour-suppressor miR-34a-5p, and that miR-34a-5p mimics inhibited this acceleration induced by XIST [160]. Zou and colleagues (2020) demonstrated that XIST promotes TGF-β1-induced EMT by regulating the miR-34a/YAP/EGFR axis in PDAC [161]. Moreover, XIST also directly interacts with miR-141–3p, which negatively regulates TGF-β2 expression [160]. LincRNA 152 (LINC00152) has recently been identified to be upregulated in PDAC [162] not only as a potential biomarker but also a novel therapeutic target [163]. Specifically, Yuan and colleagues (2020), showed that LINC00152 promotes PDAC cell proliferation, migration and invasion via targeting miR-150 which in turn directly targets ZEB1 [164]. ZEB1 is a transcriptional repressor that has been identified as an inducer of EMT and has been shown to be associated with drug resistance of pancreatic cancer cells [165]. Further examples of oncogenic lncRNAs in PDAC are described in Table 4 and Fig. 3.
Table 4.
Oncogenic LncRNAs in PDAC listed with their functions and miR interactions.
| lncRNA | Expression in PDAC | miR Interactions in PDAC | Clinical Values | Functional Involvement | References |
|---|---|---|---|---|---|
| AF339813 | up | - | D, T | cell cycle regulation and apoptotic escape | [166] |
| AFAP1-AS1 | up | miR-133a | P | cell proliferation, lymph node metastasis and perineural invasion | [154,155,162,167] |
| ANRIL | up | - | P | EMT | [168] |
| BX111 (lncRNA-BX111887) | up | - | T | hypoxia and cancer metastasis | [169] |
| CASC9 | up | - | P | cell migration and invasion | [170] |
| CCAT1 | up | - | P, T | cell proliferation, cell cycle at G0/G1 stage, migration and EMT | [171] |
| CCAT2 | up | - | D, T | cell proliferation, tumourigenesis and invasion | [172] |
| DUXAP8 | up | - | P, T | epigenetic regulation of cell proliferation, cell cycle and apoptosis | [173] |
| H19 | up | miR-675, miR-194, let 7 | P, T | cell invasion and migration | [114,121,122,123,124] |
| HCP5 | up | miR-214–3p, miR-29b-3p, miR-29c-3p, miR-140–5p | P, T | cell proliferation, invasion, migration, apoptosis and autophagy | [174], [175], [176] |
| HOTAIRM1 | up | - | T | cell cycle regulation at G0/G1 phase, apoptosis and migration | [177] |
| HULC | up | miR-372, miR-15a, miR-133b, miR-622 | D, P | cell proliferation, metastasis and invasion | [126], [127], [128] |
| IRAIN | up | - | T | cell proliferation and apoptotic escape | [178] |
| Linc-ROR | up | miR-145, miR-124, Let 7 family | D, P, T | cell proliferation, migration, invasion, EMT and autophagy | [179,180,181] |
| LINC00152 | up | miR-150 | D, P, T | cell migration and invasion | [162,163,164,167] |
| LINC00976 | up | miR-137 | P | cell proliferation, migration and invasion | [182] |
| LINC01638 | up | - | D, T | migration and invasion | [183] |
| LOC389641 | up | - | D, P | cell proliferation, invasion and apoptotic escape | [184] |
| MEG8 | up | miR-34a, miR-203 | P, T | EMT | [185] |
| MIR31HG | up | miR-193b | P, T | cell proliferation, cell cycle progression, invasion and apoptotic escape | [186] |
| NEAT1 | up | miR-506–3p, miR-335–5p | D, P, T | cell proliferation, cell cycle progression, migration, invasion, metastasis and apoptotic escape | [187], [188], [189] |
| NORAD | up | miR-125a-3p | D, P, T | hypoxia induced EMT | [190] |
| NUTF2P3–001 | up | miR-3923 | P, T | hypoxia, cell proliferation and invasion | [191] |
| POU6F2-AS2 | up | - | D | unknown; implicated in other cancers | [192][193] |
| SNHG16 | up | miR-218–5p, miR-195, miR-200a-3p, miR-302b-3p | D, P, T | cell proliferation, migration, invasion and lipogenesis | [194], [195], [196], [197] |
| SNHG7 | up | miR-342–3p | T | cell proliferation, migration and invasion | [198] |
| TP53TG1 | up | miR-96 | T | cell proliferation, migration and invasion and apoptotic escape | [199] |
| TP73-AS1 | up | miR-141–3p | P, T | cell migration, invasion and metastasis | [200] |
| XIST | up | miR-133a, miR-429, miR-34a-5p, miR-141–3p, miR-137 | P, T | cell proliferation, migration, invasion, EMT and apoptotic escape | [[157], [158], [159], [160],201] |
| XLOC_006390 | up | - | P, T | glutamate metabolism and tumour progression | [202] |
| JHDM1D-AS1 | up | - | T | angiogenesis in response to nutrient starvation | [203] |
| LINC00511 | up | miR-29b-3p, miR-29c-3p | P, T | cell proliferation, invasion and tumour angiogenesis | [204], [205] |
| MALAT-1 | up | miR-216a, miR-217 | D, P, T | cell proliferation, angiogenesis, migration, invasion and EMT | [141], [142], [143], [144], [145] |
| UCA1 | up | miR-509–3p, miR-135a, miR-96–5p, miR-107, | P, T | cell proliferation, invasion, migration, metastasis, hypoxia, apoptotic escape, stemness and angiogenesis | [167,206] |
D: diagnostic biomarker, P: prognostic biomarker, T: therapeutic target.
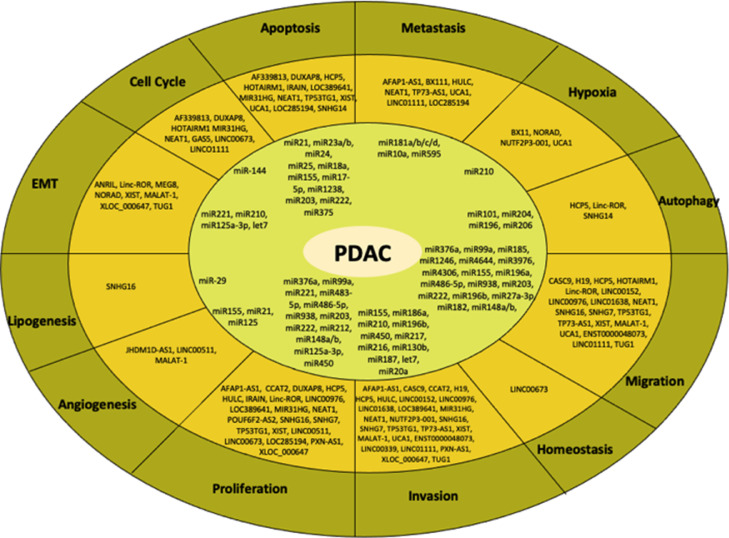
Fig. 3.
Biological processes and their related ncRNAs involved in PDAC progression. Both lncRNAs and miRs are aberrantly expressed in several biological processes during PDAC development including metastasis, cell proliferation, invasion, homoeostasis, migration, autophagy, hypoxia, apoptosis, cell cycle arrest, EMT, lipogenesis and angiogenesis. miR-21 is the most known oncogenic miR and is involved in both cell proliferation and apoptosis, while a well-described lncRNA known as HOTAIRM1 contributes to migration, apoptosis and cell cycle arrest.
Tumour suppressive lncRNAs in PDAC
Downregulation of growth arrest-specific 5 (GAS5) in PDAC has been shown to affect cell proliferation by negatively regulating cyclin dependant kinase 6 (CDK6) expression in vitro and in vivo [207]. GAS5 has also been shown to antagonise the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p, consequently regulating the Hippo signalling pathway [208]. Another study demonstrated that GAS5 reverses EMT and tumour stem cell-mediated gemcitabine resistance and metastasis in PDAC by functioning as a ceRNA for miR-221 in the miR-221/SOCS3 pathway [209]. In addition, GAS5 acts as a molecular switch for regulating quiescence and growth arrest in CD133+ population, a typical representation of the tumour initiating cells that is responsible for tumour recurrence suggesting that GAS5 is a key tumour suppressive lncRNA in PDAC that has clinical value [210]. Moreover, downregulation of long intergenic non-protein coding RNA, p53 induced transcript, which is known as Linc-pint is involved in tumour cell viability and proliferation. Specifically, Linc-pint expression levels in plasma could be used for monitoring and predicting tumour recurrence, whereas tissue Linc-pint levels could be used for predicting patient prognosis [211]. LINC01111 has negatively correlated with the TNM stage but positively correlated with the survival of PDAC patients [212]. It has been reported that overexpression of LINC01111 upregulated dual specificity phosphatase 1 (DUSP1) levels by sequestering miR-3924, resulting in the blockage of SAPK phosphorylation and the inactivation of the SAPK/JNK signalling pathway in PDAC cells and inhibiting aggressiveness [212]. ENST00000480739 expression level was remarkably decreased in PDAC in response to hypoxia and is negatively associated with lymph node metastasis and may serve as an independent prognostic factor of PDAC patient survival following surgery [206,213] (Table 5, Fig. 3).
Table 5.
Tumour suppressive LncRNAs in PDAC.
| lncRNA | Expression in PDAC | miR Interactions in PDAC | Clinical Values | Functional Involvement | References |
|---|---|---|---|---|---|
| BC008363 | down | - | P, T | tumour growth and drug resistance | [214] |
| ENST00000480739 | down | - | P, T | cell proliferation, migration and invasion | [213] |
| GAS5 | down | miR-181c-5p, miR-221, miR-32–5p | D, P, T | cell cycle progression and cell proliferation | [[207], [208], [209], [210],215] |
| HNF1A-AS1 | down | - | D, P, T | unknown; implicated in other cancers and shown to be detectable in exosomes | [162,216,217,218,219,220,221,222,223] |
| Linc-pint | down | - | D, P | tumour cell viability and proliferation | [211] |
| LINC00339 | down | miR-497–5p | - | cell proliferation and invasion | [224] |
| LINC00673 | down | miR-504, miR-23 | D | cell homoeostasis, proliferation and cell cycle progression | [162,206,225] |
| LINC01111 | down | miR-3924 | D, P, T | cell proliferation, cell cycle, invasion, migration, tumourigenesis and metastasis | [212] |
| LOC285194 | down | miR-34a | P, T | cell proliferation, lymph node metastasis, liver metastasis and apoptotic regulation in vascular smooth muscle cells | [226], [227], [228] |
| PXN-AS1 | down | miR-3064 | D, T | cell proliferation, invasion and sphere formation | [229] |
| XLOC_000647 | down | - | P, T | cell proliferation, invasion, and EMT | [230] |
D: diagnostic biomarker, P: prognostic biomarker, T: therapeutic target.
Role of lncRNAs in metastasis, proliferation, angiogenesis and apoptosis
Small Nucleolar RNA Host Gene (SNHG16) is another lncRNA that is upregulated in PDAC and was linked to the TNM stage, distant metastasis, tumour differentiation, and poor overall survival [194]. High levels of oncogenic LncRNA TP73 antisense RNA 1T (TP73-AS1) correlated with poor clinicopathological characteristics and shorter overall survival [200]. Targeting the miR-141/BDH2 axis, TP73-AS1 knockdown significantly inhibited the migration and invasion of PDAC cells, while the miR-141 inhibitor significantly restored the migration and invasion making this a potential biomarker and therapeutic target for PDAC [200]. Markedly up regulated in PDAC, the MIR31 host gene (MIR31HG) functions as an oncogenic lncRNA that promotes tumour progression with strong involvement and inverse correlation with miR-193b [186]. LINC00339 has been shown to be markedly overexpressed in PDAC tissues and cells and promoted cell proliferation, invasion, and migration via sponging miR-497–5p, thereby increasing the expression of its target gene IGF1R [224]. Lei et al. (2019) demonstrated that LINC00976 enhances the proliferation and invasion ability of PDAC cells by interacting with miR-137 and consequently upregulating its target OTUD7B, a mediator of the EGFR and mitogen-activated protein kinase (MAPK) signalling pathways [182]. The small nucleolar RNA host gene 14 (SNHG14) was significantly overexpressed in PDAC tissues compared to normal tissues and these levels negatively correlated with miR-101 [231]. Moreover, SNHG14 knockdown and miR-101 mimics both led to attenuation of gemcitabine resistance-PDAC cell viability and promoted cell apoptosis rate, as well as the reduction of autophagy-related proteins RAB5A and ATG4D [231]. SNHG14 has been demonstrated to modulate annexin A2 (ANXA2) expression by acting as a ceRNA for miR-613 [232] and to affect E-cadherin expression via an interaction with EZH2 [232]. High expression of SNHG14 has been associated with poor tumour differentiation, advanced TNM stage and nodal metastasis in pancreatic cancer patients and can be utilised as a prognostic tool [232,233]. High lncRNA taurine up-regulated gene 1 (TUG1) expression was associated with high TNM staging, lymphatic invasion, unfavourable prognosis, and distal metastasis of PDAC and can promote the migration, invasion, EMT and apoptotic escape in PDAC cells and xenograft models [234]. Mechanistically, TUG1 has been shown to function as an oncogenic lncRNA promoting tumour progression through its function as an endogenous sponge competing for miR-382, miR-299–3p and miR-29c, consequently regulating their targets: Notch1 pathway [234]; EZH2 [235]; and ITGB1, MMP2 and MMP9 [236] respectively. Microvascular invasion in hepatocellular carcinoma (MVIH) lncRNA also serves as a potential therapeutic target, which regulates multiple oncogenic pathways in PDAC including Hippo signalling pathway, FoxO signalling pathway and AGE-RAGE signalling pathway by activating MMP2 and MMP9, downregulating miR-199a, or binding to AT-rich interaction domain 1A (ARID1A). Consequently, MVIH can accelerate tumour growth and metastasis [237]. Specifically, MVIH decreased the secretion of phosphoglycerate kinase 1 (PGK1) to enhance microvessel density and accelerated angiogenesis in PDAC cells [237] (Fig. 3).
CircRNAs as diagnostic and prognostic biomarkers for PDAC
circRNAs are a new, novel type of endogenous ncRNAs, which act as miR sponges that suppress the ability of miRs to bind to their target mRNAs [238], [239], [240]. circRNAs are a class of stable, diverse and conserved RNA molecules [241], [242], that have been considered to be involved in the development of several malignancies such as bladder cancer [243], oesophageal squamous cell carcinoma [244], basal cell carcinoma [245], colorectal cancer [246] and PDAC [247]. Moreover, numerous circRNAs have presented tissue/developmental stage-specific expression [241,242,248,249], whereas previous studies have shown that circRNAs are linked to neuronal differentiation, synaptogenesis, neurological disorders, angiogenesis, prion disease and cancer [250], [251], [252], [253], [254], [255]. circRNAs are aberrantly expressed in PDAC. Specifically, circ-LDLRAD3 was not only significantly upregulated in PDAC tissues, plasma, and PDAC cell lines but also related to venous and lymphatic invasion [256]. circ-LDLRAD3 directly targets miR-137–3p, which further controls proliferation, migration and invasion of PDAC cells through the miR-137−3p/pleiotrophin (PTN) axis [257]. circ_0030235 is linked to advanced tumour stage and positive lymph node metastasis in PDAC [247,258], while it also acts as a miR-sponge of miR-1253 and miR-1294 [258]. circ_0007534 is overexpressed in PDAC tissues and is associated with aggressiveness of PDAC [259], while their miRs-targets, miR-625 and miR-892b, promote cell proliferation, migration, and invasion in PDAC cell lines [259]. circ-PDE8A, is correlated with poor prognosis and acts as a sponge of miR-338 [260]. circ-IARS is also highly expressed in PDAC patients and is related with vessel invasion, liver and tumour-node metastasis [261] through the absorption of miR-122 [261]. ciRS-7 is another oncogenic circRNA in PDAC, which acts as miR-7 sponge, while ciRS-7 knockdown resulted to the reduction in both EGFR and STAT3 expression, which led to the suppression of proliferation and decrease invasion of PDAC cells [262]. It has also been reported that circRHOT1 regulates proliferation, invasion and metastasis through the binding to miR-26b, miR-125a, miR-330, and miR-382 in PDAC [263]; circZMYM2 promote PDAC progression through the inhibition of JMJD2C expression levels via acting as miR-355–5p sponges [264]. Furthermore, has_circ_0001649 is not only associated with tumour stage and differentiation grade, but also with prognosis of PDAC patients who had undergone surgery [265]. Conclusively, Qu et al. (2015) demonstrated that the tumour suppressors circRNAs including hsa_circ_0006913, hsa_circ_0000257, hsa_circ_0005785, hsa_circ_0041150 and hsa_circ_0008719 are contributed to PDAC initiation and progression [266]. Therefore, it can be noted that numerous studies have shown the role of circRNAs in PDAC progression and hence these molecules could be used as potential novel diagnostic biomarkers in PDAC. However, the biological functions of circRNAs in PDAC, remain to be clarified (Fig. 4).
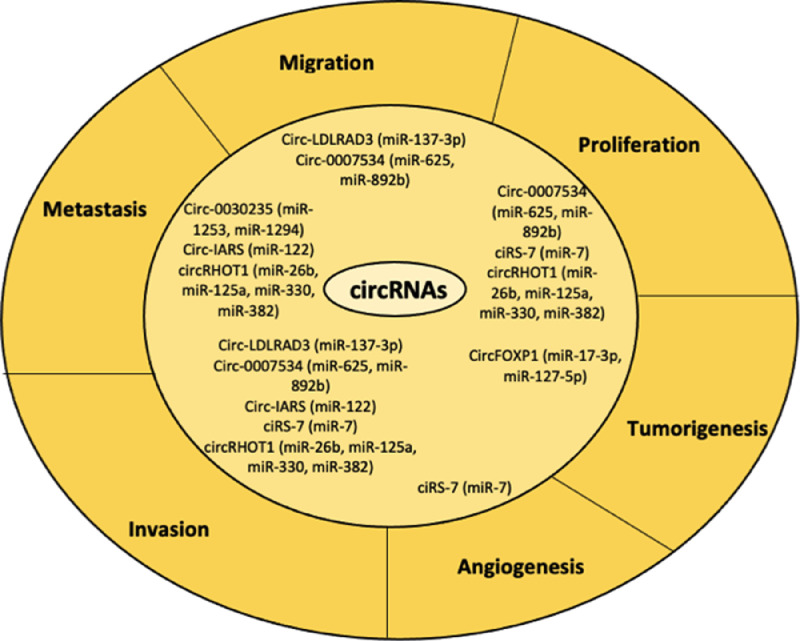
Fig. 4.
Biological processes and their related circRNAs involved in PDAC development. circRNAs are also linked to several biological processes, which are related to PDAC progression. Especially, aberrant expression of these ncRNAs subtype is associated with invasion, metastasis, angiogenesis, tumorigenesis, migration, proliferation. ciRS-7 is a well-known circRNA, which is highly correlated to angiogenesis, invasion and proliferation, while circRHOT1 with invasion, metastasis and proliferation.
Current & potential therapy approaches by using non-coding RNAs in PDAC
miRNA therapeutic targets
Several researchers have examined the regulation of miR activity for the development of beneficial therapeutics strategies for PDAC [100]; current therapies have limited impact on median overall survival of PDAC patients [267]. miRs can promote the generation of tailored treatment strategies for individual PDAC cases [268]. Consequently, miR-targeting approaches can induce alterations in both chemosensitivity and radiosensitivity of PDAC cells [100]. A handful of studies have indicated that antisense targeting of both miR-21 and miR-221 can promote an improvement in the chemosensitivity of gemcitabine and inhibition of cell proliferation [55]. Moreover, transfection of miR-21 could elevate the activity of phosphatase and tensin homologue (PTEN) [269], which further results in the enhancement of gemcitabine-induced cell apoptosis. Another study suggested that the inhibition of miR-21 using a lentivirus vector can inhibit cell proliferation [270]. Additionally, the inhibition of miR-21 led to apoptosis and reduced PDAC cell proliferation [271], while low miR-21 expression levels correlated with higher chemosensitivity to 5-fluorouracil (5-FU) [272]. It has also been suggested that miR-181b inhibition resulted in a high sensitivity to gemcitabine and higher levels of apoptosis [62]. miR-17–5p inhibition in PDAC cells causes reduced cell growth, elevated caspase-3 activation and a higher chemosensitivity to gemcitabine via the overexpression of Bim [65]. miR-205 mimics could promote the restoration of chemosensitivity to gemcitabine and a minimized expression of stem cell markers OCT3/4 and CD44 [81]. Furthermore, expression of tumour suppressor miR-205 decreased PDAC cell invasion and the restoration of gemcitabine chemosensitivity [82]. An elevated expression level of miR-23b leads to the inhibition of radiation-induced autophagy and sensitization of PDAC cells to radiation [57]. The restoration of let-7 in PDAC cells showed an inhibition of cell proliferation, KRAS expression and MAPK activation [273], while Zhao et al. (2010) denoted that the transfection of PDAC cells with miR-217 resulted in a significant suppression of cell growth through the reduction in K-RAS protein levels and decreased downstream activation of the AKT pathway [80]. Moreover, upregulated miR-148b resulted in the suppression of PDAC cell growth, which further led to the induction of apoptosis and cell-cycle arrest at S phase, inhibition of invasion and enhancement of chemosensitivity of PDAC cells [77]. In addition, miR-137 mimic resulted in reduced cell invasion, tumour growth in vivo and an elevated sensitivity to fluorouracil in PDAC cells [274]. Similarly, miR-216a upregulation can have as an outcome the targeting of the JAK2/STAT3 signalling pathway and xenograft tumour growth in vivo in PDAC cells [275]. Another study showed that when miR-218 expression is restored, cell migration and invasion reduced in PDAC [276]. miR-34a and miR-143/145 expressions showed a therapeutic efficacy, inhibition of tumour growth and induction of apoptosis in PDAC subcutaneous and orthotopic xenograft models, through the downregulation of sirtuin 1 (SIRT1), CD44, aldehyde dehydrogenase (ALDH), KRAS, and Ras responsive element binding protein 1 (RREB1) [277]. Particularly, miR-34a nanocomplexes can considerably result in the suppression of PDAC cell growth and in the elevation of apoptosis through the downregulation of E2F3, Bcl-2, c-myc and cyclin D1 [278]. Therefore, miR-34a nanocomplexes could be nominated as novel therapeutic strategies for PDAC [278]. Further studies revealed that inhibition of miR-155 expression led re-expression of TP53INP1 and enhanced apoptosis [61]. Moreover, when miR-27a was targeted, reduced cell growth, colony formation and migration was seen in PDAC [279]. Anti-miR-371–5p treatment can result in the inhibition of cell proliferation [280], whereas combined inhibition of miR-21, miR-23a and miR-27a can have as a synergistic result in decreased cell proliferation, which could be utilized for the development of effective PDAC therapies [56]. Additionally, ectopic expression of miR-96 could inhibit KRAS, which promotes apoptosis, reduces tumour growth in vivo, invasion and migration in vitro [96]. It has been also suggested that miR-506 can result in the enhancement of chemosensitivity in PDAC through the targeting of cell proliferation and the induction of cell cycle arrest at the G1/S transition [281]. The knockdown of miR-1246 caused a gemcitabine sensitivity in gemcitabine-resistant PDAC cell lines [56]; upregulation of transcription factor–activating protein 2γ, which is negatively controlled by miR-10a-5p, can lead to the re-sensitization of PDAC cells to gemcitabine [64]. miR-34b could be used as an effective therapeutic agent, due to the fact that it negatively regulates oncogenic SMAD3 [282]. miR-101 expression can promote E-cadherin and thus decrease PDAC tumour growth [283]. miR-204 expression leads to downregulation of myeloid cell leukaemia-1 (Mcl-1) as well as apoptosis [284]. Furthermore, targeting miR-31 in vitro reduces cell proliferation, migration and invasion [285]. Besides, targeting both miR-132 and miR-212 by antisense miRNA oligonucleotides led to the inhibition of PDAC tumour growth through the action on the retinoblastoma tumour suppressor (Rb1) [95]. The restoration of miR-150 could inhibit cell growth in PDAC cells [286]. Conclusively, it can be suggested that miR-based therapeutics could be a pioneer and effective therapeutic approach for PDAC [287]. However, specific issues, including the specificity in the delivery to certain cells of interest and safety, should be also addressed [288] (Fig. 5).
Fig. 5.
Role of lncRNAs with their target miRs in PDAC therapy. Interactions between miRs and lncRNAs have prompted novel therapeutic strategies for PDAC. Specifically, MALAT1, PVT1 and HOTAIR together with their target miRs could be used for the prediction of gemcitabine-based chemotherapy efficacy as first-line treatment of PDAC patients.
LncRNA therapeutic strategies
In a similar approach to the therapies based on miRNA modulation, lncRNAs could also potentially be custom applied specifically to regulate gene expression based on the genomic profile of the patient [289]. Due to their restricted spatio-temporal expression and capability of modulating the function and/or expression of key genes or their interacting partners, lncRNAs represent an ideal therapeutic target for PDAC [119]. There are a few therapeutic approaches for regulating lncRNAs either by transcript targeting or functional inhibition including (1) small interfering RNA/short hairpin RNA, (2) CRISPR-Cas9, (3) antisense oligonucleotides, (4) morpholino oligonucleotides, (5) Locked Nucleic Acid Gapmers, (6) small molecule inhibitors, (7) exploiting lncRNA promoters [119]. In a recent study, Takahashi and associates (2020) showed that overexpression of miR-622 via miR mimics, as a miR downregulated by TGF-β, could downregulate HULC and suppress invasion and migration by inhibiting EMT signalling via extracellular vesicle transfer [127]. In contrast, inhibition of miR-622 increased HULC expression and promoted epithelial-mesenchymal transition signalling, invasion, and migration of PDAC cells [127]. The potential of using the aforementioned strategies that would induce gain/loss-of-function to target lncRNAs is promising and enormous although modifying the expression levels of functional lncRNAs in vivo may present another level of difficulty due to their secondary structures [127]. Nevertheless, the most appealing therapeutic strategy when it comes to immediate clinical translatability is chemogene therapy [290].
There are several lncRNAs demonstrated to be involved in chemoresistance, and especially gemcitabine resistance, including those detailed in Table 6. LncRNA SLC7A11-AS1 promotes chemoresistance through reducing intracellular reactive oxygen species (ROS) by stabilizing nuclear factor erythroid-2-related factor 2 (NRF2), the key regulator in antioxidant defence [291]. SLC7A11-AS1 knockdown has been shown to weaken PDAC stemness and sensitises resistant PDAC cells toward gemcitabine in vitro and in vivo [291]. Similarly, downregulating the levels of oncogenic lncRNAs including HOTAIR [292], PVT1 [153,292], Linc-ROR [293], MALAT-1 [144], TUG1 [294] may revert the gemcitabine resistance in PDAC. It has been demonstrated that levels of MALAT1, HOTTIP and PVT1 in PDAC patient serum can be utilised to predict the efficacy of gemcitabine-based chemotherapy as first-line treatment of pancreatic cancer patients [133]. In addition, it has been shown that miR-216a can silence MALAT1 expression consequently inducing apoptosis both in the presence and absence of gemcitabine in PDAC cells [144]. Interestingly, curcumin has been shown to sensitise pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression [295]. On the other hand, upregulating the expression of tumour suppressive lncRNAs such as GAS5 [209] and MEG3 [296] may also have the same effect. Ma and associates (2018) demonstrated that forced expression of MEG3 in PDAC cells attenuated cell proliferation, cell migration and invasion, EMT, decreased the sphere-forming ability and cancer stem cell properties, and increased the chemosensitivity to gemcitabine in vitro [296]. Moreover, in experiments conducted in PDAC animal models, the sequential administration of the H19-promoter-targeted vector BC-819 (also known as DTA-H19) and gemcitabine has been shown to have better antitumour activity as compared to the effect of each of them alone [297]. These suggest that the lncRNAs involved in conferring chemoresistance to PDAC and their functional involvements warrant further investigation to be able to be utilised as therapeutic targets in PDAC (Fig. 5).
Table 6.
LncRNAs implicated in chemoresistance in PDAC.
| lncRNA | Expressionin PDAC | Detected | miR Interactions in PDAC | Clinical Value(s) | Functional Involvement | References |
|---|---|---|---|---|---|---|
| HOTAIR | up | Plasma, Tissue, Saliva | miR-34a, miR-663b, miR-613 | D, P, T | cell proliferation, cell cycle progression, apoptotic escape and gemcitabine resistance | [[129], [130], [131],[133], [134], [135],137] |
| HOTTIP | up | Tissue, Serum | miR-137 | P, T | cell proliferation, migration, apoptotic escape, cisplatin resistance and PCSC stemness | [120,136,[138], [139], [140],167] |
| LINC00346 | up | Tissue | miR-188–3p | P, T | gemcitabine resistance, cell proliferation and cell cycle regulation at the G2/M-phase | [298], [299], [300] |
| LINC01559 | up | Tissue | miR-607, miR-1343–3p | D, T | cell proliferation, migration, invasion, metastasis, autophagy and gemcitabine resistance | [301], [302], [303] |
| MEG3 | down | Tissue | - | P, T | cell proliferation, cell migration and invasion, EMT, cancer stem cell properties and chemosensitivity | [296] |
| PVT1 | up | Saliva | miR-619–5p, miR-448, miR-20–5p | D, P, T | EMT, cell proliferation, migration and gemcitabine resistance | [137,151,153,162,167,295] |
| SLC7A11-AS1 | up | Tissue | - | P, T | PDAC stemness and gemcitabine resistance | [291] |
| SNHG14 | up | Tissue | miR-613, miR-101 | T | cell proliferation, invasion, apoptosis escape, gemcitabine resistance and autophagy | [231], [232], [233] |
D: diagnostic biomarker, P: prognostic biomarker, T: therapeutic target.
CircRNAs as therapeutic biomarkers for PDAC
Recent studies have suggested the role of specific circRNAs in chemotherapy-resistant PDAC including ciRS-7 and circHIPK3 [262,304]. Specifically, ciRS-7 increased gemcitabine resistance as an outcome of impaired moderation of EGFR/STAT3 signalling pathway [262]. Furthermore, the upregulation of circHIPK3 has been linked to gemcitabine resistance in PDAC cell lines through the negative regulation of RASSF1 through sponging miR-330–5p [304].
Conclusion
Despite the latest efforts and breakthroughs to develop better, more effective therapeutic strategies for PDAC, it still remains one of the most fatal cancers with high mortality rates. ncRNAs expressions found to be significant and specific to cancer in general and miRs, lncRNAs and circRNAs found to be differentially expressed in PDAC. ncRNAs involve crucial regulation on cell proliferation, invasion and apoptosis and hence the strategy of altering their expression and activity in order to prevent cancer development and progression is promising. Several approaches to date have been used to mimic or inhibit ncRNAs expressions in vitro and in vivo. However, further studies are needed to understand their precise role in PDAC with regards to diagnosis, prognosis and therapeutics.
KRAS mutations and the overexpression of HER-2/neu [305] are common mutations in PDAC, while in more advanced stages, CDKN2A, TP53 and SMAD4 [305,306] are key regulators of PDAC pathogenesis [307]. Specifically, KRAS mutations have been detected in more than 90% [308,309], whereas HER2 upregulation in 4–50% of PDAC cases [310]. SMAD4 mutations are found in 60% of PDAC cases [311] and loss of TP53 in more than 70% of PDAC patients [312]. CDKN2A tumour suppressor gene alterations have been found in 95% of PDAC cases [313]. Furthermore, previous studies have indicated that 12 main signalling pathways including KRAS signalling, Hedgehog signalling, apoptosis, control of G1/S phase transition and TGF-β signalling are altered in more than 80% of PDAC patients [312,314,315]. The recent discovery of ncRNAs has procured further insights regarding not only pathophysiology but also a better diagnosis and intervention of PDAC [316]. More recent evidence has shown that the aberrant expressions of ncRNAs play a considerable role in numerous human tumours and especially in initiation, proliferation and chemoresistance of PDAC [26]. ncRNAs are aberrantly expressed in numerous malignancies, while they can act as oncogenes or tumour suppressors [28]. ncRNAs research has emerged a significant interest, which is developed in all the fields of biological science for the examination of ncRNAs for human applications. Particularly, new advances in precision medicine have transpired the ability of the differentiation of distinct ncRNAs expression profiles, which could further discriminate between subtypes of normal and malignant tissues [317]. Especially, progressions in technology such as the use of next generation sequencing (NGS) have procured advances in ncRNAs expression profiles for PDAC diagnosis [318]. This could minimize the number of PDAC-associated deaths through the development of an improved understanding of PDAC biology. Despite the narrow knowledge of these molecules, ncRNAs can be characterized as vital biomarkers not only for the early prognosis and diagnosis of PDAC but also for a better management of therapeutics specimens [32]. These new approaches have remarked both cell and tissue specific ncRNAs expression models and thus not only clinical but also translational research have suggested the use of ncRNAs as early diagnostic, prognostic and therapeutic tools. However, there is a long way to go before the establishment of ncRNAs in the early diagnosis and treatment of PDAC and subsequently further evaluation of all the ncRNAs targets, silencing mechanisms and networks is required [319]. In this review, we elucidated the roles of ncRNAs in PDAC prognosis and metastasis and evaluated the recent developments in ncRNAs-based therapies in PDAC, which have shown substantial promise in controlling tumour progression and metastasis. However, more advanced preclinical and clinical trials are needed to overcome certain challenges such as adverse effects of ncRNA-based therapies.
Author contributions
Conceptualization, MM, ZT, EDA, HK and PUO; writing—original draft preparation, MM, ZT, EDA, HK and PUO; writing—review and editing, MM, ZT, EDA, HK and PUO supervision, PUO. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
Authors declare that there is no financial/personal interest or belief that could affect the results, discussions or conclusions, which are reported in this work.
Funding
MM is supported by a SLS PhD scholarship by the University of Westminster.
Contributor Information
Maria Mortoglou, Email: w1754188@my.westminster.ac.uk.
Zoey Kathleen Tabin, Email: zoey.tabin@icloud.com.
E. Damla Arisan, Email: damlaarisan@gmail.com.
Hemant M Kocher, Email: h.kocher@qmul.ac.uk.
Pinar Uysal-Onganer, Email: p.onganer@westminster.ac.uk.
References
- 1.Von Hoff D.D., Korn R., Mousses S. Pancreatic cancer–could it be that simple? A different context of vulnerability. Cancer Cell. 2009;16(1):7–8. doi: 10.1016/j.ccr.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan D., Saied A., Kocher H.M. Analysis of mortality rates for pancreatic cancer across the world. HPB. 2008;10(1):58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocher H. Pancreatic cancer - symptoms, diagnosis and treatment. BMJ Best Practice. 2020 https://bestpractice.bmj.com/topics/en-gb/265 [Google Scholar]
- 4.Jemal A. Global cancer statistics. CA Cancer J. Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Marzec J., Dayem Ullah A.Z., Pirrò S., Gadaleta E., Crnogorac-Jurcevic T., Lemoine N.R., Kocher H.M, Chelala C. The pancreatic expression database: 2018 update. Nucleic Acids Res. 2018;46(D1):D1107–D1110. doi: 10.1093/nar/gkx955. 10.1093/nar/gkx955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitra A., Hruban R.H. Pancreatic cancer. Ann. Rev. Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014;371(22):2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 8.Scara S., Bottoni P., Scatena R. Ca 19-9: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015;867:247–260. doi: 10.1007/978-94-017-7215-0_15. [DOI] [PubMed] [Google Scholar]
- 9.Nazli O., Bozdag A.D., Tansug T., Kir R., Kaymak E. The diagnostic importance of CEA and CA 19-9 for the early diagnosis of pancreatic carcinoma. Hepatogastroenterology. 2000;47(36):1750–1752. PMID: 11149048. [PubMed] [Google Scholar]
- 10.Chan A. Validation of biomarkers that complement ca19.9 in detecting early pancreatic cancer. Clin. Cancer Res. 2014;20(22):5787–5795. doi: 10.1158/1078-0432.CCR-14-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawabu N., Watanabe H., Yamaguchi Y., Ohtsubo K., Motoo Y. Serum tumour markers and molecular biological diagnosis in pancreatic cancer. Pancreas. 2004;28(3):263–267. doi: 10.1097/00006676-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Chang J.C., Kundranda M. Novel diagnostic and predictive biomarkers in pancreatic adenocarcinoma. Int. J. Mol. Sci. 2017;18(3):667. doi: 10.3390/ijms18030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel R. Cancer Statis. ACS. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [Google Scholar]
- 14.Costello E., Greenhalf W., Neoptolemos J.P. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat. Rev. Gastroenterol. Hepatol. 2012;9(8):435–444. doi: 10.1038/nrgastro.2012.119. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J.D. Combined circulating tumour DNA and protein biomarker-based liquid. biopsy for the earlier detection of pancreatic cancers. PNAS. 2017;114(38):10202–10207. doi: 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 17.Matsui M., Corey D. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16(3):167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Y., Li J., Zhu L. Cancer and non-coding RNAs. Nutritional Epigenom. 2019;14:119–132. [Google Scholar]
- 19.Goodall G.J., Wickramasinghe V.O. RNA in cancer. Nat. Rev. Cancer. 2021;21(1):22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 20.De Almeida R.A., Fraczek M.G., Parker S., Delneri D., O'Keefe R.T. Non-coding RNAs and disease: the classical ncRNAs make a comeback. Biochem. Soc. Trans. 2016;44:1073–1078. doi: 10.1042/BST20160089. 10.1042/BST20160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavet V., Portal M.M., Moulin J.C., Herbrecht R., Gronemeyer H. Towards novel paradigms for cancer therapy. Oncogene. 2011;30(1):1–20. doi: 10.1038/onc.2010.460. [DOI] [PubMed] [Google Scholar]
- 23.Yu J. MicroRNA alterations of pancreatic intraepithelial neoplasia. Clin. Cancer Res. 2012;18(4):981–992. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 25.Piletič K., Kunej T. MicroRNA epigenetic signatures in human disease. Arch. Toxicol. 2016;90(10):2405–2419. doi: 10.1007/s00204-016-1815-7. [DOI] [PubMed] [Google Scholar]
- 26.Gilles M. Personalized RNA medicine for pancreatic cancer. Clin. Cancer Res. 2018;24(7):1734–1747. doi: 10.1158/1078-0432.CCR-17-2733. [DOI] [PubMed] [Google Scholar]
- 27.Turchinovich A. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galasso M., Sandhu S.K., Volinia S. MicroRNA expression signatures in solid malignancies. Cancer J. 2012;18(3):238–243. doi: 10.1097/PPO.0b013e318258b5f4. [DOI] [PubMed] [Google Scholar]
- 29.Hussain S.P. Pancreatic cancer: current progress and future challenges. Int. J. Biol. Sci. 2016;12(3):270. doi: 10.7150/ijbs.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calin G.A. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA, 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garzon R. MicroRNA expression and function in cancer. Trends Mol. Med. 2006;12(12):580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Jay C. miRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol. 2007;26(5):293–300. doi: 10.1089/dna.2006.0554. [DOI] [PubMed] [Google Scholar]
- 33.Lee E.J., Gusev Y., Jiang J., Nuovo G.J. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer. 2007;120(5):1046–1054. doi: 10.1002/ijc.22394. 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humeau M., Vignolle-Vidoni A., Sicard F., Martins F. Salivary MicroRNA in pancreatic cancer patients. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R., Chen X., Du Y. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin. Chem. 2012;58(3):610–618. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F., Wei C., Cui M.Y., Xia Q.Q., Wang S.B., Zhang Y. Prognostic value of microRNAs in pancreatic cancer: a meta-analysis. Aging. 2020;12(10):9380–9404. doi: 10.18632/aging.103214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Gao J., Du Y., Li Z., Ren Y., Gu J. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int. J. Cancer. 2012;131(3):683–691. doi: 10.1002/ijc.26422. [DOI] [PubMed] [Google Scholar]
- 38.Kunovsky L., Tesarikova P., Kala Z., Kroupa R., Kysela P., Dolina J. The use of biomarkers in early diagnostics of pancreatic cancer. Can. J. Gastroenterol. Hepatol. 2018;(2018) doi: 10.1155/2018/5389820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Yiwen B.S. Identification of Serum microRNA-25 as a novel biomarker for pancreatic cancer. Medicine (Baltimore). 2020;99(52):e23863. doi: 10.1097/MD.0000000000023863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madhavan B. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer. 2015;136(11) doi: 10.1002/ijc.29324. [DOI] [PubMed] [Google Scholar]
- 41.Morimura R., Komatsu S., Ichikawa D. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br. J. Cancer. 2011;105(11):1733–1740. doi: 10.1038/bjc.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawaguchi T., Komatsu S., Ichikawa D., Morimura R., Tsujiura M., Konishi H. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br. J. Cancer. 2013;108(2):361–369. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komatsu S., Ichikawa D., Takeshita H., Morimura R., Hirajima S., Tsujiura M., Kawaguchi T. Circulating miR-18a: a sensitive cancer screening biomarker in human cancer. In Vivo. 2014;28(3):293–297. https://pubmed.ncbi.nlm.nih.gov/24815829/ [PubMed] [Google Scholar]
- 44.Zhang J., Zhao C., Zhang S. Upregulation of miR-194 contributes to tumour growth and progression in pancreatic ductal adenocarcinoma. Oncol. Rep. 2014;31:1157–1164. doi: 10.3892/or.2013.2960. [DOI] [PubMed] [Google Scholar]
- 45.Bloomston M., Frankel W.L., Petrocca F. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297(17):1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 46.Ohuchida K., Mizumoto K., Kayashima T. MicroRNA expression as a predictive marker for gemcitabine response after surgical resection of pancreatic cancer. Ann. Surg. Oncol. 2011;18(8):2381–2387. doi: 10.1245/s10434-011-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin M.S., Chen W.C., Huang J.X., Gao H.J., Sheng H.H. Aberrant expression of microRNAs in serum may identify individuals with pancreatic cancer. Int. J. Clin. Exp. Med. 2014;7(12):5226–5234. PMID: 25664025. [PMC free article] [PubMed] [Google Scholar]
- 48.Le Large Y.S. Circulating microRNAs as diagnostic biomarkers for pancreatic cancer. Expert Rev. Mol. Diagn. 2015;15(12):1525–1529. doi: 10.1586/14737159.2015.1112273. [DOI] [PubMed] [Google Scholar]
- 49.Szafrańska A.E., Davison T.S., John J., Cannon T., Sipos B., Maghnouj A., Labourier E., Hahn S.A. MicroRNA expression alterations are linked to tumourigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26(30):4442–4452. doi: 10.1038/sj.onc.1210228. 10.1038/sj.onc.1210228 [DOI] [PubMed] [Google Scholar]
- 50.Schultz 50.N.A. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311(4):392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 51.Wang W.S., Liu L.X., Li G.P. Combined serum CA19-9 and miR-27a-3p in peripheral blood mononuclear cells to diagnose pancreatic cancer. Cancer Prev. Res. 2013;6(4):331–338. doi: 10.1158/1940-6207.CAPR-12-0307. [DOI] [PubMed] [Google Scholar]
- 52.Munding B., Adai A.T., Maghnouj A., Urbanik A., Zollner H., Liffers S.T., Chromik A.M., Uhl W., Szafranska-Schwarzbach A.E., Tannapfel A., Hahn S.A. Global microRNA expression profiling of microdissected tissues identifies miR-135b as a novel biomarker for pancreatic ductal adenocarcinoma. Int. J. Cancer. 2012;131:E86–E95. doi: 10.1002/ijc.26466. [DOI] [PubMed] [Google Scholar]
- 53.Wang J. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev. Res. 2009;2(9):807–813. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dillhoff M., Liu J., Frankel W., Croce C., Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J. Gastrointest. Surg. 2008;12(12):2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J.K. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38(7):e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 56.Frampton A.E., Castellano L., Colombo T. MicroRNAs cooperatively inhibit a network of tumour suppressor genes to promote pancreatic tumour growth and progression. Gastroenterology. 2014;146(1):268–277. doi: 10.1053/j.gastro.2013.10.010. e18. [DOI] [PubMed] [Google Scholar]
- 57.Wang P. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology. 2013;145:1133–1143. doi: 10.1053/j.gastro.2013.07.048. e1112. [DOI] [PubMed] [Google Scholar]
- 58.Liu R. Serum MicroRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin. Chem. 2012;58(3):610–618. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa S. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer. 2014;111(8):1572–1580. doi: 10.1038/bjc.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greither T. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumours is associated with poorer survival. Int. J. Cancer. 2012;126(1):73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 61.Gironella M. Tumour protein 53- induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumour development. Proc. Natl. Acad. Sci. U. S. A. 2007;104(41):16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai B. miRNA-181b increases the sensitivity of pancreatic ductal adenocarcinoma cells to gemcitabine in vitro and in nude mice by targeting BCL-2. Oncol. Rep, 2013;29(5):1769–1776. doi: 10.3892/or.2013.2297. [DOI] [PubMed] [Google Scholar]
- 63.Ohuchida K. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene. Ann. Surg. Oncol. 2012;19:2394–2402. doi: 10.1245/s10434-012-2252-3. [DOI] [PubMed] [Google Scholar]
- 64.Xiong G. MiR-10a-5p targets TFAP2C to promote gemcitabine resistance in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2018;37(76):76. doi: 10.1186/s13046-018-0739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan H.J. miR-17-5p inhibitor enhances chemosensitivity to gemcitabine via upregulating Bim expression in pancreatic cancer cells Dig. Dis. Sci. 2012;57:3160–3167. doi: 10.1007/s10620-012-2400-4. [DOI] [PubMed] [Google Scholar]
- 66.Calatayud D. Tissue MicroRNA profiles as diagnostic and prognostic biomarkers in patients with resectable pancreatic ductal adenocarcinoma and periampullary cancers. Biomark. Res. 2017;5(8):8. doi: 10.1186/s40364-017-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue H., Liu L., Song Z. miR‑212 regulated by HIF‑1α promotes the progression of pancreatic cancer. Exper. Therapeut. Med. 2019;17:2359–2365. doi: 10.3892/etm.2019.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Q. Circulating microRNA-182 in plasma and its potential diagnostic and prognostic value for pancreatic cancer. Med. Oncol. 2014;31(225):225. doi: 10.1007/s12032-014-0225-z. [DOI] [PubMed] [Google Scholar]
- 69.Moriya C. Inhibition of PRDM14 expression in pancreatic cancer suppresses cancer stem-like properties and liver metastasis in mice. Carcinogenesis. 2017;38(6):638–648. doi: 10.1093/carcin/bgx040. [DOI] [PubMed] [Google Scholar]
- 70.Liu G. miR-125a-3p is responsible for chemosensitivity in PDAC by inhibiting epithelial-mesenchymal transition via. Fyn. Biomed. Pharmacother. 2018;106:523–531. doi: 10.1016/j.biopha.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 71.Azevedo-Pouly A.C., Sutaria D.S., Jiang J., Elgamal O.A., Amari F., Allard D., Grippo P.J., Coppola V., Schmittgen T.D. miR-216 and miR-217 expression is reduced in transgenic mouse models of pancreatic adenocarcinoma, knockout of miR-216/miR-217 host gene is embryonic lethal. Funct. Integr. Genomics. 2017;17(2-3):203–212. doi: 10.1007/s10142-016-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rachagani S., Macha M.A., Menning M.S., Dey P., Pai P., Smith L.M., Mo Y.Y., Batra S.K. Changes in microRNA (miRNA) expression during pancreatic cancer development and progression in a genetically engineered KrasG12D;Pdx1-Cre mouse (KC) model. Oncotarget. 2015;6:40295–40309. doi: 10.18632/oncotarget.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farmer D.T., Shariat N., Park C.Y., Liu H.J., Mavropoulos A., McManus M.T. Partiall penetrant postnatal lethality of an epithelial specific microRNA in a mouse knockout. PLoS One. 2013;8:e76634. doi: 10.1371/journal.pone.0076634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuhnert F., Mancuso M.R., Hampton J., Stankunas K., Asano T., Chen C.Z., Kuo C.J. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 75.Zhao G. MiR-130b is a prognostic marker and inhibits cell proliferation and invasion in pancreatic cancer through targeting STAT3. PLoS One. 2013;8(9):e73803. doi: 10.1371/journal.pone.0073803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schultz N.A. Prognostic MicroRNAs in cancer tissue from patients operated for pancreatic cancer—five MicroRNAs in a prognostic index. World J. Surg. 2012;36(11):2699–2707. doi: 10.1007/s00268-012-1705-y. [DOI] [PubMed] [Google Scholar]
- 77.Zhao G. miR-148b functions as a tumour suppressor in pancreatic cancer by targeting AMPKalpha1. Mol. Cancer Ther. 2013;12(1):83–93. doi: 10.1158/1535-7163.MCT-12-0534-T. [DOI] [PubMed] [Google Scholar]
- 78.Kojima M. MicroRNA markers for the diagnosis of pancreatic and biliary-tract cancers. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J. Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic cancer. J. Cancer. 2014;5(8):696–705. doi: 10.7150/jca.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao W.G. The miR-217 microRNA functions as a potential tumour suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31(10):1726–1733. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
- 81.Singh S. miRNA profiling in pancreatic cancer and restoration of chemosensitivity. Cancer Lett. 2013;334(2):211–220. doi: 10.1016/j.canlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 82.Mittal A., Chitkara D., Behrman S.W., Mahato R.I. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35(25):7077–7087. doi: 10.1016/j.biomaterials.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 83.Yang J. Circular RNA hsa_circRNA_0007334 is predicted to promote MMP7 and COL1A1 expression by functioning as a miRNA sponge in pancreatic ductal adenocarcinoma. J. Oncol. 2019;2019 doi: 10.1155/2019/7630894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li A. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin. Cancer Res. 2013;19(13):3600–3610. doi: 10.1158/1078-0432.CCR-12-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kong X. Detection of differentially expressed microRNAs in serum of pancreatic ductal adenocarcinoma patients: MiR-196a could be a potential marker for poor prognosis. Dig. Dis. Sci. 2011;56:602–609. doi: 10.1007/s10620-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 86.Zou X. Identification of a six-miRNA panel in serum benefiting pancreatic cancer diagnosis. Cancer Med. 2019;8(6):2810–2822. doi: 10.1002/cam4.2145. 10.1002/cam4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slater E.P. MicroRNA-196a and -196b as potential biomarkers for the early detection of familial pancreatic cancer. Transl. Oncol. 2014;7(4):464–471. doi: 10.1016/j.tranon.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryu J.K., Hong S.M., Karikari C.A., Hruban R.H., Goggins M.G., Maitra A. Aberrant MicroRNA-155 expression is an early event in the multistep progression of pancreatic adenocarcinoma. Pancreatology. 2010;10(1):66–73. doi: 10.1159/000231984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song W.F., Wang L., Huang W.Y., Cai X., Cui J.J., Wang L.W. MiR-21 upregulation induced by promoter zone histone acetylation is associated with chemoresistance to gemcitabine and enhanced malignancy of pancreatic cancer cells. Asian Pac. J. Cancer Prev. 2013;14(12):7529–7536. doi: 10.7314/apjcp.2013.14.12.7529. [DOI] [PubMed] [Google Scholar]
- 90.Abue M. Circulating miR-483-3p and miR-21 is highly expressed in plasma of pancreatic cancer. Int. J. Oncol. 2015;46(2):539–547. doi: 10.3892/ijo.2014.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mees S.T. Involvement of CD40 Targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann. Surg. Oncol. 2009;16:2339–2350. doi: 10.1245/s10434-009-0531-4. [DOI] [PubMed] [Google Scholar]
- 92.Yu J., Li A., Hong S.M., Hruban R.H., Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias human. Cancer Biol. 2011;23 doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alemar B., Gregório C., Ashton-Prolla P. miRNAs as diagnostic and prognostic biomarkers in pancreatic ductal adenocarcinoma and its precursor lesions: a review. Biomarker Insight. 2015;10:113–124. doi: 10.4137/BMI.S27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernandez Y.G., Lucas A.L. MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions. World. J. Gastrointest. Oncol. 2016;8(1):18–29. doi: 10.4251/wjgo.v8.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park J.K. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumour suppressor. Biochem. Biophys. Res. Commun. 2011;406(4):518–523. doi: 10.1016/j.bbrc.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu S. miRNA-96 suppresses KRAS and functions as a tumour suppressor gene in pancreatic cancer. Cancer Res. 2010;70(14):6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 97.Ji Q. MicroRNA miR-34 inhibits human pancreatic cancer tumour-initiating cells. PLoS One. 2009;4(8):e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jamieson N.B. MicroRNA molecular profiles associated with diagnosis, clinicopathologic criteria, and overall survival in patients with resectable pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2012;18(2):534–545. doi: 10.1158/1078-0432.CCR-11-0679. [DOI] [PubMed] [Google Scholar]
- 99.Giovannetti E. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 100.Vorvis C., Koutsioumpa M., Iliopoulos D. Developments in miRNA gene signaling pathways in pancreatic cancer. Future Oncol. (London, England) 2016;12(9):1135–1150. doi: 10.2217/fon-2015-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang P. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene. 2014;33:514–524. doi: 10.1038/onc.2012.598. [DOI] [PubMed] [Google Scholar]
- 102.Radhakrishnan P. MicroRNA-200c modulates the expression of MUC4 and MUC16 by directly targeting their coding sequences in human pancreatic cancer. PLoS One. 2013;8(10):e73356. doi: 10.1371/journal.pone.0073356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liao X. Genome-scale analysis to identify prognostic microRNA biomarkers in patients with early-stage pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Cancer Manag. Res. 2018;10:2537–2551. doi: 10.2147/CMAR.S168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schultz N.A. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod. Pathol. 2012;25(12):1609–1622. doi: 10.1038/modpathol.2012.122. [DOI] [PubMed] [Google Scholar]
- 105.Guo S. miR-15a inhibits cell proliferation and epithelial to mesenchymal transition in pancreatic ductal adenocarcinoma by down-regulating Bmi-1 expression. Cancer Lett. 2014;344:40–46. doi: 10.1016/j.canlet.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Li J. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-kappaB signalling. Oncogene. 2016;35:5501–5514. doi: 10.1038/onc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bao Z., Zhang W., Dong D. A potential prognostic lncRNA signature for predicting survival in patients with bladder urothelial carcinoma. Oncotarget. 2017;8(6):10485–10497. doi: 10.18632/oncotarget.14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gong Y., Huang H.T., Liang Y., Trimarchi T., Aifantis I., Tsirigos A. lncRNA-screen: an interactive platform for computationally screening long non-coding RNAs in large genomics datasets. BMC Genomics. 2017;18(1):434. doi: 10.1186/s12864-017-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flippot R., Malouf G.G., Su X., Mouawad R., Spano J.P., Khayat D. Cancer subtypes classification using long non-coding RNA. Oncotarget. 2016;7(33):54082–54093. doi: 10.18632/oncotarget.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang C., Liu S., Wang H., Zhang Z., Yang Q., Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am. J. Transl. Res. 2016;8(11):5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 111.Liz J., Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim. Biophys. Acta. 2016;1859(1):169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 112.Chen Q.N., Chen X., Chen Z.Y., Nie F.Q., Wei C.C., Ma H.W., Wan L., Yan S., Ren S.N., Wang Z.X. Long intergenic non-coding RNA 00152 promotes lung adenocarcinoma proliferation via interacting with EZH2 and repressing IL24 expression. Mol. Cancer. 2017;16(1):17. doi: 10.1186/s12943-017-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan J.Q., Zhang Y.Q., Wang J.H., Xu P., Wang W. lncRNA co-expression network model for the prognostic analysis of acute myeloid leukemia. Int. J. Mol. Med. 2017;39(3):663–671. doi: 10.3892/ijmm.2017.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen X., Yan C.C., Zhang X., You Z.H. Long non-coding RNAs and complex diseases: from experimental results to computational models. Brief. Bioinform. 2017;18(4):558–576. doi: 10.1093/bib/bbw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhat S.A., Ahmad S.M., Mumtaz P.T., Malik A.A., Dar M.A., Urwat U., Shah R.A., Ganai N.A. Long non-coding RNAs: Mechanism of action and functional utility. Noncoding RNA Res. 2016;1(1):43–50. doi: 10.1016/j.ncrna.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang X., Zhi X., Gao Y., Ta N., Jiang H., Zheng J. LncRNAs in pancreatic cancer. Oncotarget. 2016;7(35):57379–57390. doi: 10.18632/oncotarget.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tahira A.C., Kubrusly M.S., Faria M.F., Dazzani B., Fonseca R.S., Maracaja-Coutinho V., Verjovski-Almeida S., Machado M.C., Reis E.M. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol. Cancer. 2011;10:141. doi: 10.1186/1476-4598-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kung J.T., Colognori D., Lee J.T. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pandya G., Kirtonia A., Sethi G., Pandey A.K., Garg M. The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim. Biophys. Acta Rev. Cancer. 2020;1874(2) doi: 10.1016/j.bbcan.2020.188423. [DOI] [PubMed] [Google Scholar]
- 120.Nie L., Wu H.J., Hsu J.M., Chang S.S., Labaff A.M., Li C.W., Wang Y., Hsu J.L., Hung M.C. Long non-coding RNAs: versatile master regulators of gene expression and crucial players in cancer. Am. J. Transl. Res. 2012;4(2):127–150. [PMC free article] [PubMed] [Google Scholar]
- 121.Ma C., Nong K., Zhu H., Wang W., Huang X., Yuan Z., Ai K. H19 promotes pancreatic cancer metastasis by derepressing let-7′s suppression on its target HMGA2-mediated. EMT Tumour Biol. 2014;35(9):9163–9169. doi: 10.1007/s13277-014-2185-5. [DOI] [PubMed] [Google Scholar]
- 122.Wang F., Rong L., Zhang Z., Li M., Ma L., Ma Y., Xie X., Tian X., Yang Y. LncRNA H19-derived miR-675-3p promotes epithelial-mesenchymal transition and stemness in human pancreatic cancer cells by targeting the STAT3. Pathway J. Cancer. 2020;11(16):4771–4782. doi: 10.7150/jca.44833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma L., Tian X., Guo H., Zhang Z., Du C., Wang F., Xie X., Gao H., Zhuang Y., Kornmann M., Gao H., Yang Y. Long noncoding RNA H19 derived miR-675 regulates cell proliferation by down-regulating E2F-1 in human pancreatic ductal adenocarcinoma. J. Cancer. 2018;9(2):389–399. doi: 10.7150/jca.21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng J.F., Zhuang Y.Y., Huang F.T., Zhang S.N. Noncoding RNAs and pancreatic cancer. World J. Gastroenterol. 2016;22(2):801–814. doi: 10.3748/wjg.v22.i2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng H., Wei B., Zhang Y. Long non-coding RNA HULC promotes proliferation, migration and invasion of pancreatic cancer cells by down-regulating microRNA-15a. Int. J. Biol. Macromol. 2019;126:891–898. doi: 10.1016/j.ijbiomac.2018.12.238. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi K., Ota Y., Kogure T., Suzuki Y., Iwamoto H., Yamakita K., Kitano Y., Fujii S., Haneda M., Patel T., Otan T. Circulating extracellular vesicle encapsulated HULC is a potential biomarker for human pancreatic cancer. Cancer Sci. 2020;111(1):98–111. doi: 10.1111/cas.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takahashi K., Koyama K., Ota Y., Iwamoto H., Yamakita K., Fujii S., Kitano Y. The interaction between long non-coding RNA HULC and MicroRNA-622 via transfer by extracellular vesicles regulates cell invasion and migration in human pancreatic. Cancer Front. Oncol. 2020;10:1013. doi: 10.3389/fonc.2020.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peng W., Gao W., Feng J. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Med. Oncol. 2014;31(12):346. doi: 10.1007/s12032-014-0346-4. [DOI] [PubMed] [Google Scholar]
- 129.Jiang D., Xu L., Ni J., Zhang J., Cai M., Shen L. Functional polymorphisms in LncRNA HOTAIR contribute to susceptibility of pancreatic cancer. Cancer Cell Int. 2019;19:47. doi: 10.1186/s12935-019-0761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li C.H., Xiao Z., Tong J.H., To K.F., Fang X., Cheng A.S., Chen Y. EZH2 coupled with HOTAIR to silence MicroRNA-34a by the induction of heterochromatin formation in human pancreatic ductal adenocarcinoma. Int. J. Cancer. 2017;140(1):120–129. doi: 10.1002/ijc.30414. [DOI] [PubMed] [Google Scholar]
- 131.Cai H., An Y., Chen X., Sun D., Chen T., Peng Y., Zhu F., Jiang Y., He X. Epigenetic inhibition of miR-663b by long non-coding RNA HOTAIR promotes pancreatic cancer cell proliferation via up-regulation of insulin-like growth factor 2. Oncotarget. 2016;7(52):86857–86870. doi: 10.18632/oncotarget.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cai H., Yao J., An Y., Chen X., Chen W., Wu D., Luo B., Yang Y., Jiang Y., Sun D., He X. LncRNA HOTAIR acts a competing endogenous RNA to control the expression of notch3 via sponging miR-613 in pancreatic cancer. Oncotarget. 2017;8(20):32905–32917. doi: 10.18632/oncotarget.16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang L., Dong P., Wang W., Huang M., Tian B. Gemcitabine treatment causes resistance and malignancy of pancreatic cancer stem-like cells via induction of lncRNA HOTAIR. Exp. Ther. Med. 2017;14(5):4773–4780. doi: 10.3892/etm.2017.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang S.Z., Xu F., Zhou T., Zhao X., McDonald J.M., Chen Y. The long non-coding RNA HOTAIR enhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand. J. Biol. Chem. 2017;292(25):10390–10397. doi: 10.1074/jbc.M117.786830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu C., Yang L., Qi X., Wang T., Li M., Xu K. Inhibition of long non-coding RNA HOTAIR enhances radiosensitivity via regulating autophagy in pancreatic cancer. Cancer Manag. Res. 2018;10:5261–5271. doi: 10.2147/CMAR.S174066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cheng Y., Jutooru I., Chadalapaka G., Corton J.C., Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6(13):10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie Z., Chen X., Li J., Guo Y., Li H., Pan X., Jiang J., Liu H., Wu B. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget. 2016;7(18):25408–25419. doi: 10.18632/oncotarget.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., Li Z., Zheng S., Zhou Y., Zhao L., Ye H., Zhao X., Gao W., Fu Z., Zhou Q., Liu Y., Chen R. Expression profile of long non-coding RNAs in pancreatic cancer and their clinical significance as biomarkers. Oncotarget. 2015;6(34):35684–35698. doi: 10.18632/oncotarget.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z., Zhao X., Zhou Y., Liu Y., Zhou Q., Ye H., Wang Y., Zeng J., Song Y., Gao W., Zheng S., Zhuang B., Chen H., Li W., Li H., Li H., Fu Z., Chen R. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J. Transl. Med. 2015;13:84. doi: 10.1186/s12967-015-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin F., Zhang Q., Dong Z., Hu J., Ma Z. LncRNA HOTTIP participates in cisplatin resistance of tumour cells by regulating miR-137 expression in pancreatic cancer. Onco. Targets Ther. 2020;13:2689–2699. doi: 10.2147/OTT.S234924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiao F., Hu H., Han T., Yuan C., Wang L., Jin Z., Guo Z., Wang L. Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int. J. Mol. Sci. 2015;16(4):6677–6693. doi: 10.3390/ijms16046677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiao F., Hu H., Yuan C., Wang L., Jiang W., Jin Z., Guo Z., Wang L. Elevated expression level of long noncoding RNA MALAT-1 facilitates cell growth, migration and invasion in pancreatic cancer. Oncol. Rep. 2014;32(6):2485–2492. doi: 10.3892/or.2014.3518. [DOI] [PubMed] [Google Scholar]
- 143.Li L., Chen H., Gao Y., Wang Y.W., Zhang G.Q., Pan S.H., Ji L., Kong R., Wang G., Jia Y.H., Bai X.W., Sun B. Long noncoding RNA MALAT1 promotes aggressive pancreatic cancer proliferation and metastasis via the stimulation of autophagy. Mol. Cancer Ther. 2016;15(9):2232–2243. doi: 10.1158/1535-7163.MCT-16-0008. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y., Tang X., Shi M., Wen C., Shen B. MiR-216a decreases MALAT1 expression, induces G2/M arrest and apoptosis in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2017;483(2):816–822. doi: 10.1016/j.bbrc.2016.12.167. [DOI] [PubMed] [Google Scholar]
- 145.Liu J.H., Chen G., Dang Y.W., Li C.J., Luo D.Z. Expression and prognostic significance of lncRNA MALAT1 in pancreatic cancer tissues. Asian Pac. J. Cancer Prev. 2014;15(7):2971–2977. doi: 10.7314/apjcp.2014.15.7.2971. [DOI] [PubMed] [Google Scholar]
- 146.Tian X., Xu G. Clinical value of lncRNA MALAT1 as a prognostic marker in human cancer: systematic review and meta-analysis. BMJ Open. 2015;5(9) doi: 10.1136/bmjopen-2015-008653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Y., Shan T., Ding W., Hua Z., Shen Y., Lu Z., Chen B., Dai T. Study on mechanism about long noncoding RNA MALAT1 affecting pancreatic cancer by regulating Hippo-YAP signaling. J. Cell. Physiol. 2018;233(8):5805–5814. doi: 10.1002/jcp.26357. [DOI] [PubMed] [Google Scholar]
- 148.Liu P., Yang H., Zhang J., Peng X., Lu Z., Tong W., Chen J. The lncRNA MALAT1 acts as a competing endogenous RNA to regulate KRAS expression by sponging miR-217 in pancreatic ductal adenocarcinoma. Sci. Rep. 2017;7(1):5186. doi: 10.1038/s41598-017-05274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu S.N., Ma Y.H., Zhao W.G., Jin X.L., Yang H.Y., Liu P.P., Chen J. KRAS-related noncoding RNAs in pancreatic ductal adenocarcinoma. Chronic Dis. Transl. Med. 2016;2:215–222. doi: 10.1016/j.cdtm.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang C., Yu W., Wang Q., Cui H., Wang Y., Zhang L., Han F., Huang T. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med. 2015;106(3):143–149. [PubMed] [Google Scholar]
- 151.Zhao L., Kong H., Sun H., Chen Z., Chen B., Zhou M. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J. Cell. Physiol. 2018;233(5):4044–4055. doi: 10.1002/jcp.26072. [DOI] [PubMed] [Google Scholar]
- 152.Sun Y., He W., Luo M., Zhou Y., Chang G., Ren W., Wu K., Li X., Shen J., Zhao X., Hu Y. SREBP1 regulates tumourigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. 2015;36(6):4133–4141. doi: 10.1007/s13277-015-3047-5. [DOI] [PubMed] [Google Scholar]
- 153.Zhou C., Yi C., Yi Y., Qin W., Yan Y., Dong X., Zhang X., Huang Y., Zhang R., Wei J., Ali D.W., Michalak M., Chen X.Z., Tang J. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/beta-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol. Cancer. 2020;19(1):118. doi: 10.1186/s12943-020-01237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ye Y., Chen J., Zhou Y., Fu Z., Zhou Q., Wang Y., Gao W., Zheng S., Zhao X., Chen T., Chen R. High expression of AFAP1-AS1 is associated with poor survival and short-term recurrence in pancreatic ductal adenocarcinoma. J. Transl. Med. 2015;13:137. doi: 10.1186/s12967-015-0490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen B., Li Q., Zhou Y., Wang X., Zhang Q., Wang Y., Zhuang H., Jiang X., Xiong W. The long coding RNA AFAP1-AS1 promotes tumour cell growth and invasion in pancreatic cancer through upregulating the IGF1R oncogene via sequestration of miR-133a. Cell Cycle. 2018;17(16):1949–1966. doi: 10.1080/15384101.2018.1496741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Subramani R., Lopez-Valdez R., Arumugam A., Nandy S., Boopalan T., Lakshmanaswamy R. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS One. 2014;9(5):e97016. doi: 10.1371/journal.pone.0097016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei W., Liu Y., Lu Y., Yang B., Tang L. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J. Cell. Biochem. 2017;118(10):3349–3358. doi: 10.1002/jcb.25988. [DOI] [PubMed] [Google Scholar]
- 158.Sun Z., Zhang B., Cui T. Long non-coding RNA XIST exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncol. Rep. 2018;39(4):1591–1600. doi: 10.3892/or.2018.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen J., Hong L., Yu D., Cao T., Zhou Z., He S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int. J. Biochem. Cell Biol. 2019;113:17–26. doi: 10.1016/j.biocel.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 160.Sun J., Zhang Y. LncRNA XIST enhanced TGF-beta2 expression by targeting miR-141-3p to promote pancreatic cancer cells invasion. Biosci. Rep. 2019;39(7) doi: 10.1042/BSR20190332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zou L., Chen F.R., Xia R.P., Wang H.W., Xie Z.R., Xu Y., Yu J.H., Wang K.H. Long noncoding RNA XIST regulates the EGF receptor to promote TGF-beta1-induced epithelial-mesenchymal transition in pancreatic cancer. Biochem. Cell. Biol. 2020;98(2):267–276. doi: 10.1139/bcb-2018-0274. [DOI] [PubMed] [Google Scholar]
- 162.Muller S., Raulefs S., Bruns P., Afonso-Grunz F., Plotner A., Thermann R., Jager C., Schlitter A.M., Kong B., Regel I., Roth W.K., Rotter B., Hoffmeier K., Kahl G., Koch I., Theis F.J., Kleeff J., Winter P., Michalski C.W. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol. Cancer. 2015;14:94. doi: 10.1186/s12943-015-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu Y., Yang J., Li Q., Xu B., Lian Y., Miao L. LINC00152: a pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif. 2017;50(4) doi: 10.1111/cpr.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yuan Z.J., Yu C., Hu X.F., He Y., Chen P., Ouyang S.X. LINC00152 promotes pancreatic cancer cell proliferation, migration and invasion via targeting miR-150. Am. J. Transl. Res. 2020;12(5):2241–2256. [PMC free article] [PubMed] [Google Scholar]
- 165.Wellner U., Brabletz T., Keck T. ZEB1. Pancreatic Cancer Cancers (Basel) 2010;2(3):1617–1628. doi: 10.3390/cancers2031617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu P., Shangguan J., Zhang L. Downregulation of NUF2 inhibits tumour growth and induces apoptosis by regulating lncRNA AF339813. Int. J. Clin. Exp. Pathol. 2015;8(3):2638–2648. [PMC free article] [PubMed] [Google Scholar]
- 167.Fu X.L., Liu D.J., Yan T.T., Yang J.Y., Yang M.W., Li J., Huo Y.M., Liu W., Zhang J.F., Hong J., Hua R., Chen H.Y., Sun Y.W. Analysis of long non-coding RNA expression profiles in pancreatic ductal adenocarcinoma. Sci. Rep. 2016;6:33535. doi: 10.1038/srep33535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen S., Zhang J.Q., Chen J.Z., Chen H.X., Qiu F.N., Yan M.L., Chen Y.L., Peng C.H., Tian Y.F., Wang Y.D. The over expression of long non-coding RNA ANRIL promotes epithelial-mesenchymal transition by activating the ATM-E2F1 signaling pathway in pancreatic cancer: an in vivo and in vitro study. Int. J. Biol. Macromol. 2017;102:718–728. doi: 10.1016/j.ijbiomac.2017.03.123. [DOI] [PubMed] [Google Scholar]
- 169.Deng S.J., Chen H.Y., Ye Z., Deng S.C., Zhu S., Zeng Z., He C., Liu M.L., Huang K., Zhong J.X., Xu F.Y., Li Q., Liu Y., Wang C.Y., Zhao G. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene. 2018;37(44):5811–5828. doi: 10.1038/s41388-018-0382-1. [DOI] [PubMed] [Google Scholar]
- 170.Yu X., Lin Y., Sui W., Zou Y., Lv Z. Analysis of distinct long noncoding RNA transcriptional fingerprints in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6(3):673–680. doi: 10.1002/cam4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu Q., Zhou X., Xia Q., Shen J., Yan J., Zhu J., Li X., Shu M. Long non-coding RNA CCAT1 that can be activated by c-Myc promotes pancreatic cancer cell proliferation and migration. Am. J. Transl. Res. 2016;8(12):5444–5454. [PMC free article] [PubMed] [Google Scholar]
- 172.Cai Y., Li X., Shen P., Zhang D. CCAT2 is an oncogenic long non-coding RNA in pancreatic ductal adenocarcinoma. Biol. Res. 2018;51(1):1. doi: 10.1186/s40659-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lian Y., Yang J., Lian Y., Xiao C., Hu X., Xu H. DUXAP8, a pseudogene derived lncRNA, promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2. Cancer Commun. (Lond) 2018;38(1):64. doi: 10.1186/s40880-018-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Y., Wang J., Dong L., Xia L., Zhu H., Li Z., Yu X. Long noncoding RNA HCP5 regulates pancreatic cancer gemcitabine (GEM) resistance by sponging Hsa-miR-214-3p To Target HDGF. Onco. Targets Ther. 2019;12:8207–8216. doi: 10.2147/OTT.S222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang W., Lou W., Ding B., Yang B., Lu H., Kong Q., Fan W. A novel mRNA-miRNA-lncRNA competing endogenous RNA triple sub-network associated with prognosis of pancreatic cancer. Aging (Albany NY) 2019;11(9):2610–2627. doi: 10.18632/aging.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yuan B., Guan Q., Yan T., Zhang X., Xu W., Li J. LncRNA HCP5 regulates pancreatic cancer progression by miR-140-5p/CDK8. Axis Cancer Biother. Radiopharm. 2020;35(9):711–719. doi: 10.1089/cbr.2019.3294. [DOI] [PubMed] [Google Scholar]
- 177.Luo Y., He Y., Ye X., Song J., Wang Q., Li Y., Xie X. High expression of long noncoding RNA HOTAIRM1 is associated with the proliferation and migration in pancreatic ductal adenocarcinoma. Pathol. Oncol. Res. 2019;25(4):1567–1577. doi: 10.1007/s12253-018-00570-4. [DOI] [PubMed] [Google Scholar]
- 178.Lian Y., Wang J., Feng J., Ding J., Ma Z., Li J., Peng P., De W., Wang K. Long non-coding RNA IRAIN suppresses apoptosis and promotes proliferation by binding to LSD1 and EZH2 in pancreatic cancer. Tumour Biol. 2016;37(11):14929–14937. doi: 10.1007/s13277-016-5380-8. [DOI] [PubMed] [Google Scholar]
- 179.Chen W., Wang H., Liu Y., Xu W., Ling C., Li Y., Liu J., Chen M., Zhang Y., Chen B., Gong A., Xu M. Linc-RoR promotes proliferation, migration, and invasion via the Hippo/YAP pathway in pancreatic cancer cells. J. Cell. Biochem. 2020;121(1):632–641. doi: 10.1002/jcb.29308. [DOI] [PubMed] [Google Scholar]
- 180.Fu Z., Li G., Li Z., Wang Y., Zhao Y., Zheng S., Ye H., Luo Y., Zhao X., Wei L., Liu Y., Lin Q., Zhou Q., Chen R. Endogenous miRNA Sponge LincRNA-ROR promotes proliferation, invasion and stem cell-like phenotype of pancreatic cancer cells. Cell Death Discov. 2017;3:17004. doi: 10.1038/cddiscovery.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gao S., Wang P., Hua Y., Xi H., Meng Z., Liu T., Chen Z., Liu L. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget. 2016;7(2):1608–1618. doi: 10.18632/oncotarget.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lei S., He Z., Chen T., Guo X., Zeng Z., Shen Y., Jiang J. Long noncoding RNA 00976 promotes pancreatic cancer progression through OTUD7B by sponging miR-137 involving EGFR/MAPK pathway. J. Exp. Clin. Cancer Res. 2019;38(1):470. doi: 10.1186/s13046-019-1388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu H., Ye J., Zhang L., Li M., Lu S., Yang D., Hu W. Downregulation of LINC01638 lncRNA inhibits migration and invasion of pancreatic ductal adenocarcinoma cells by reducing TGFbeta signaling. Mol. Med. Rep. 2019;20(5):4533–4539. doi: 10.3892/mmr.2019.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zheng S., Chen H., Wang Y., Gao W., Fu Z., Zhou Q., Jiang Y., Lin Q., Tan L., Ye H., Zhao X., Luo Y., Li G., Ye L., Liu Y., Li W., Li Z., Chen R. Long non-coding RNA LOC389641 promotes progression of pancreatic ductal adenocarcinoma and increases cell invasion by regulating E-cadherin in a TNFRSF10A-related manner. Cancer Lett. 2016;371(2):354–365. doi: 10.1016/j.canlet.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 185.Terashima M., Ishimura A., Wanna-Udom S., Suzuki T. MEG8 long noncoding RNA contributes to epigenetic progression of the epithelial-mesenchymal transition of lung and pancreatic cancer cells. J. Biol. Chem. 2018;293(47):18016–18030. doi: 10.1074/jbc.RA118.004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang H., Liu P., Zhang J., Peng X., Lu Z., Yu S., Meng Y., Tong W.M., Chen J. Long noncoding RNA MIR31HG exhibits oncogenic property in pancreatic ductal adenocarcinoma and is negatively regulated by miR-193b. Oncogene. 2016;35(28):3647–3657. doi: 10.1038/onc.2015.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang B., Liu C., Wu Q., Zhang J., Min Q., Sheng T., Wang X., Zou Y. Long non-coding RNA NEAT1 facilitates pancreatic cancer progression through negative modulation of miR-506-3p. Biochem. Biophys. Res. Commun. 2017;482(4):828–834. doi: 10.1016/j.bbrc.2016.11.120. [DOI] [PubMed] [Google Scholar]
- 188.Cao J., Zhang Y., Yang J., He S., Li M., Yan S., Chen Y., Qu C., Xu L. NEAT1 regulates pancreatic cancer cell growth, invasion and migration though mircroRNA-335-5p/c-met axis. Am. J. Cancer Res. 2016;6(10):2361–2374. [PMC free article] [PubMed] [Google Scholar]
- 189.Feng Y., Gao L., Cui G., Cao Y. LncRNA NEAT1 facilitates pancreatic cancer growth and metastasis through stabilizing ELF3 mRNA. Am. J. Cancer Res. 2020;10(1):237–248. [PMC free article] [PubMed] [Google Scholar]
- 190.Li H., Wang X., Wen C., Huo Z., Wang W., Zhan Q., Cheng D., Chen H., Deng X., Peng C., Shen B. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol. Cancer. 2017;16(1):169. doi: 10.1186/s12943-017-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li X., Deng S.J., Zhu S., Jin Y., Cui S.P., Chen J.Y., Xiang C., Li Q.Y., He C., Zhao S.F., Chen H.Y., Niu Y., Liu Y., Deng S.C., Wang C.Y., Zhao G. Hypoxia-induced lncRNA-NUTF2P3-001 contributes to tumourigenesis of pancreatic cancer by derepressing the miR-3923/KRAS pathway. Oncotarget. 2016;7(5):6000–6014. doi: 10.18632/oncotarget.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Metzenmacher M., Varaljai R., Hegedus B., Cima I., Forster J., Schramm A., Scheffler B., Horn P.A., Klein C.A., Szarvas T., Reis H., Bielefeld N., Roesch A., Aigner C., Kunzmann V., Wiesweg M., Siveke J.T., Schuler M., Lueong S.S. Plasma next generation sequencing and droplet digital-qPCR-based quantification of circulating Cell-Free RNA for noninvasive early detection of cancer. Cancers (Basel) 2020;12(2) doi: 10.3390/cancers12020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu J., Sun X., Zhu H., Qin Q., Yang X., Sun X. Long noncoding RNA POU6F2-AS2 is associated with oesophageal squamous cell carcinoma. J. Biochem. 2016;160(4):195–204. doi: 10.1093/jb/mvw025. [DOI] [PubMed] [Google Scholar]
- 194.Liu S., Zhang W., Liu K., Liu Y. LncRNA SNHG16 promotes tumour growth of pancreatic cancer by targeting miR-218-5p. Biomed. Pharmacother. 2019;114 doi: 10.1016/j.biopha.2019.108862. [DOI] [PubMed] [Google Scholar]
- 195.Yu Y., Dong J.T., He B., Zou Y.F., Li X.S., Xi C.H., Yu Y. LncRNA SNHG16 induces the SREBP2 to promote lipogenesis and enhance the progression of pancreatic cancer. Future Oncol. 2019;15(33):3831–3844. doi: 10.2217/fon-2019-0321. [DOI] [PubMed] [Google Scholar]
- 196.Guo J.Q., Yang Z.J., Wang S., Wu Z.Z., Yin L.L., Wang D.C. LncRNA SNHG16 functions as an oncogene by sponging miR-200a-3p in pancreatic cancer. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4):1718–1724. doi: 10.26355/eurrev_202002_20347. [DOI] [PubMed] [Google Scholar]
- 197.Xu H., Miao X., Li X., Chen H., Zhang B., Zhou W. LncRNA SNHG16 contributes to tumour progression via the miR-302b-3p/SLC2A4 axis in pancreatic adenocarcinoma. Cancer Cell Int. 2021;21(1):51. doi: 10.1186/s12935-020-01715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cheng D., Fan J., Ma Y., Zhou Y., Qin K., Shi M., Yang J. LncRNA SNHG7 promotes pancreatic cancer proliferation through ID4 by sponging miR-342-3p. Cell Biosc. 2019;9:28. doi: 10.1186/s13578-019-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199.Zhang Y., Yang H., Du Y., Liu P., Zhang J., Li Y., Shen H., Xing L., Xue X., Chen J., Zhang X. Long noncoding RNA TP53TG1 promotes pancreatic ductal adenocarcinoma development by acting as a molecular sponge of microRNA-96. Cancer Sci. 2019;110(9):2760–2772. doi: 10.1111/cas.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cui X.P., Wang C.X., Wang Z.Y., Li J., Tan Y.W., Gu S.T., Qin C.K. LncRNA TP73-AS1 sponges miR-141-3p to promote the migration and invasion of pancreatic cancer cells through the up-regulation of BDH2. Biosci. Re. 2019;39(3) doi: 10.1042/BSR20181937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu P.J., Pan Y.H., Wang D.W., You D. Long noncoding RNA XIST promotes cell proliferation of pancreatic cancer through miR137 and Notch1 pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24(23):12161–12170. doi: 10.26355/eurrev_202012_24005. [DOI] [PubMed] [Google Scholar]
- 202.He J., Li F., Zhou Y., Hou X., Liu S., Li X., Zhang Y., Jing X., Yang L. LncRNA XLOC_006390 promotes pancreatic carcinogenesis and glutamate metabolism by stabilizing c-Myc. Cancer Lett. 2020;469:419–428. doi: 10.1016/j.canlet.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 203.Kondo A., Nonaka A., Shimamura T., Yamamoto S., Yoshida T., Kodama T., Aburatani H., Osawa T. Long noncoding RNA JHDM1D-AS1 promotes tumour growth by regulating angiogenesis in response to nutrient starvation. Mol. Cell. Biol. 2017;37(18):e00125. doi: 10.1128/MCB.00125-17. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang K., Zhu G., Bao S., Chen S. Long non-coding RNA LINC00511 mediates the effects of ESR1 on proliferation and invasion of ovarian cancer through miR-424-5p and miR-370-5p. Cancer Manag. Res. 2019;11:10807–10819. doi: 10.2147/CMAR.S232140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao X., Liu Y., Li Z., Zheng S., Wang Z., Li W., Bi Z., Li L., Jiang Y., Luo Y., Lin Q., Fu Z., Rufu C., Lin C. c00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J. Cell. Mol. Med. 2018;22(1):655–667. doi: 10.1111/jcmm.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gong R., Jiang Y. Non-coding RNAs in pancreatic ductal adenocarcinoma. Front. Oncol. 2020;10:309. doi: 10.3389/fonc.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu X., Fang Y., Wang Z., Xie J., Zhan Q., Deng X., Chen H., Jin J., Peng C., Li H., Shen B. Downregulation of gas5 increases pancreatic cancer cell proliferation by regulating CDK6. Cell Tissue Res. 2013;354(3):891–896. doi: 10.1007/s00441-013-1711-x. [DOI] [PubMed] [Google Scholar]
- 208.Gao Z.Q., Wang J.F., Chen D.H., Ma X.S., Yang W., Zhe T., Dang X.W. Long non-coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p. Biomed. Pharmacother. 2018;97:809–817. doi: 10.1016/j.biopha.2017.10.157. [DOI] [PubMed] [Google Scholar]
- 209.Liu B., Wu S., Ma J., Yan S., Xiao Z., Wan L., Zhang F., Shang M., Mao A. lncRNA GAS5 reverses EMT and tumour stem cell-mediated gemcitabine resistance and metastasis by targeting miR-221/SOCS3 in pancreatic cancer. Mol. Ther. Nucleic Acids. 2018;13:472–482. doi: 10.1016/j.omtn.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sharma N.S., Gnamlin P., Durden B., Gupta V.K., Kesh K., Garrido V.T., Dudeja V., Saluja A., Banerjee S. Long non-coding RNA GAS5 acts as proliferation "brakes" in CD133+ cells responsible for tumour recurrence. Oncogenesis. 2019;8(12):68. doi: 10.1038/s41389-019-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li L., Zhang G.Q., Chen H., Zhao Z.J., Chen H.Z., Liu H., Wang G., Jia Y.H., Pan S.H., Kong R., Wang Y.W., Sun B. Plasma and tumour levels of Linc-pint are diagnostic and prognostic biomarkers for pancreatic cancer. Oncotarge. 2016;7(44):71773–71781. doi: 10.18632/oncotarget.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pan S., Shen M., Zhou M., Shi X., He R., Yin T., Wang M., Guo X., Qin R. Long noncoding RNA LINC01111 suppresses pancreatic cancer aggressiveness by regulating DUSP1 expression via microRNA-3924. Cell Death. Dis. 2019;10(12):883. doi: 10.1038/s41419-019-2123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sun Y.W., Chen Y.F., Li J., Huo Y.M., Liu D.J., Hua R., Zhang J.F., Liu W., Yang J.Y., Fu X.L., Yan T., Hong J., Cao H. A novel long non-coding RNA ENST00000480739 suppresses tumour cell invasion by regulating OS-9 and HIF-1alpha in pancreatic ductal adenocarcinoma. Br. J. Cancer. 2014;111(11):2131–2141. doi: 10.1038/bjc.2014.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li J., Liu D., Hua R., Zhang J., Liu W., Huo Y., Cheng Y., Hong J., Sun Y. Long non-coding RNAs expressed in pancreatic ductal adenocarcinoma and lncRNA BC008363 an independent prognostic factor in PDAC. Pancreatology. 2014;14(5):385–390. doi: 10.1016/j.pan.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 215.Koldemir O., Ozgur E., Gezer U. Accumulation of GAS5 in exosomes is a marker of apoptosis induction. Biomed. Rep. 2017;6(3):358–362. doi: 10.3892/br.2017.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fang C., Qiu S., Sun F., Li W., Wang Z., Yue B., Wu X., Yan D. Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA. Cancer Lett. 2017;410:50–62. doi: 10.1016/j.canlet.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 217.Liu Z., Wei X., Zhang A., Li C., Bai J., Dong J. Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p. Biochem. Biophys. Res. Commun. 2016;473(4):1268–1275. doi: 10.1016/j.bbrc.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 218.Luo X., Wei J., Yang F.L., Pang X.X., Shi F., Wei Y.X., Liao B.Y., Wang J.L. Exosomal lncRNA HNF1A-AS1 affects cisplatin resistance in cervical cancer cells through regulating microRNA-34b/TUFT1 axis. Cancer Cell Int. 2019;19:323. doi: 10.1186/s12935-019-1042-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 219.Zhang G., An X., Zhao H., Zhang Q., Zhao H. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed. Pharmacother. 2018;98:594–599. doi: 10.1016/j.biopha.2017.12.080. [DOI] [PubMed] [Google Scholar]
- 220.Guo X., Zhang Y., Liu L., Yang W., Zhang Q. HNF1A-AS1 regulates cell migration, invasion and glycolysis via modulating miR-124/MYO6 in colorectal cancer. Cells Onco. Targets Ther. 2020;13:1507–1518. doi: 10.2147/OTT.S231249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Z., Li H., Fan S., Lin H., Lian W. STAT3-induced upregulation of long noncoding RNA HNF1A-AS1 promotes the progression of oral squamous cell carcinoma via activating Notch signaling pathway. Cancer Biol. Ther. 2019;20(4):444–453. doi: 10.1080/15384047.2018.1529119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu L., Chen Y., Li Q., Duan P. lncRNA HNF1A-AS1 modulates non-small cell lung cancer progression by targeting miR-149-5p/Cdk6. J. Cell. Biochem. 2019;120(11):18736–18750. doi: 10.1002/jcb.29186. [DOI] [PubMed] [Google Scholar]
- 223.Liu H.T., Ma R.R., Lv B.B., Zhang H., Shi D.B., Guo X.Y., Zhang G.H., Gao P. LncRNA-HNF1A-AS1 functions as a competing endogenous RNA to activate PI3K/AKT signalling pathway by sponging miR-30b-3p in gastric cancer. Br. J. Cancer. 2020;122(12):1825–1836. doi: 10.1038/s41416-020-0836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang R., Hao S., Yang L., Xie J., Chen S., Gu G. LINC00339 promotes cell proliferation and metastasis in pancreatic cancer via miR-497-5p/IGF1R axis. J. BUON. 2019;24(2):729–738. [PubMed] [Google Scholar]
- 225.Childs E.J., Mocci E., Campa D., Bracci P.M., Gallinger S., Goggins M., Li D., Neale R.E., Olson S.H., Scelo G., Amundadottir L.T., Bamlet W.R., Bijlsma M.F., Blackford A., Borges M., Brennan P., Brenner H., Bueno-de-Mesquita H.B., Canzian F., Capurso G., Cavestro G.M., Chaffee K.G., Chanock S.J., Cleary S.P., Cotterchio M., Foretova L., Fuchs C., Funel N., Gazouli M., Hassan M., Herman J.M., Holcatova I., Holly E.A., Hoover R.N., Hung R.J., Janout V., Key T.J., Kupcinskas J., Kurtz R.C., Landi S., Lu L., Malecka-Panas E., Mambrini A., Mohelnikova-Duchonova B., Neoptolemos J.P., Oberg A.L., Orlow I., Pasquali C., Pezzilli R., Rizzato C., Saldia A., Scarpa A., Stolzenberg-Solomon R.Z., Strobel O., Tavano F., Vashist Y.K., Vodicka P., Wolpin B.M., Yu H., Petersen G.M., Risch H.A., Klein A.P. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 2015;47(8):911–916. doi: 10.1038/ng.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ding Y.C., Yu W., Ma C., Wang Q., Huang C.S., Huang T. Expression of long non-coding RNA LOC285194 and its prognostic significance in human pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2014;7(11):8065–8070. [PMC free article] [PubMed] [Google Scholar]
- 227.Wang H., Jiao H., Jiang Z., Chen R. Propofol inhibits migration and induces apoptosis of pancreatic cancer PANC-1 cells through miR-34a-mediated E-cadherin and LOC285194 signals. Bioengineered. 2020;11(1):510–521. doi: 10.1080/21655979.2020.1754038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 228.Mehrad-Majd H., Ravanshad S., Moradi A., Khansalar N., Sheikhi M., Akhtari J. Decreased expression of lncRNA loc285194 as an independent prognostic marker in cancer: a systematic review and meta-analysis. Pathol. Res. Pract. 2019;215(6) doi: 10.1016/j.prp.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 229.Yan J., Jia Y., Chen H. Long non-coding RNA PXN-AS1 suppresses pancreatic cancer progression by acting as a competing endogenous RNA of miR-3064 to upregulate PIP4K2B expression. J. Exp. Clin. Cancer Res. 2019;38:390. doi: 10.1186/s13046-019-1379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hu H., Wang Y., Ding X. Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3. Mol. Cancer. 2018;17:18. doi: 10.1186/s12943-018-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang X., Zhao P., Wang C., Xin B. SNHG14 enhances gemcitabine resistance by sponging miR-101 to stimulate cell autophagy in pancreatic cancer. Biochem. Biophys. Res. Commun. 2019;510(4):508–514. doi: 10.1016/j.bbrc.2019.01.109. [DOI] [PubMed] [Google Scholar]
- 232.Deng P.C., Chen W.B., Cai H.H., An Y., Wu X.Q., Chen X.M., Sun D.L., Yang Y., Shi L.Q., Yang Y. LncRNA SNHG14 potentiates pancreatic cancer progression via modulation of annexin A2 expression by acting as a competing endogenous RNA for miR-613. J. Cell. Mol. Med. 2019;23(11):7222–7232. doi: 10.1111/jcmm.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xie F., Huang Q., Wang C., Chen S., Liu C., Lin X., Lv X., Wang C. Downregulation of long noncoding RNA SNHG14 suppresses cell proliferation and invasion by regulating EZH2 in pancreatic ductal adenocarcinoma (PDAC) Cancer Biomark. 2020;27(3):357–364. doi: 10.3233/CBM-190908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu K., Zhang L. Inhibition of TUG1/miRNA-299-3p Axis Represses Pancreatic Cancer Malignant Progression via Suppression of the Notch1 Pathway. Dig. Dis. Sci. 2020;65:1748–1760. doi: 10.1007/s10620-019-05911-0. [DOI] [PubMed] [Google Scholar]
- 235.Zhao L., Sun H., Kong H., Chen Z., Chen B., Zhou M. The Lncrna-TUG1/EZH2 axis promotes pancreatic cancer cell proliferation, migration and EMT phenotype formation through sponging Mir-382. Cell. Physiol. Biochem. 2017;42:2145–2158. doi: 10.1159/000479990. [DOI] [PubMed] [Google Scholar]
- 236.Lu Y., Tang L., Zhang Z. Long noncoding RNA TUG1/miR-29c axis affects cell proliferation, invasion, and migration in human pancreatic cancer. Dis. Markers. 2018;2018 doi: 10.1155/2018/6857042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hu S., Zheng Q., Xiong J., Wu H., Wang W., Zhou W. Long non-coding RNA MVIH promotes cell proliferation, migration, invasion through regulating multiple cancer-related pathways, and correlates with worse prognosis in pancreatic ductal adenocarcinomas. Am. J. Transl. Res. 2020;12(5):2118–2135. [PMC free article] [PubMed] [Google Scholar]
- 238.Li J., Yang J., Zhou P., Le Y., Zhou C., Wang S., Xu D., Lin H.K., Gong Z. Circular RNAs in cancer: novel insights into origins, properties, functions and implicationsAm. J. Cancer Res. 2015;5(2):472–480. [PMC free article] [PubMed] [Google Scholar]
- 239.Qu S., Yang X., Li X., Wang J., Gao Y., Shang R., Sun W., Dou K., Li H. Circular RNA: a new star of noncoding. RNAs Cancer Lett. 2015;365(2):141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 240.Gong R., Jiang Y. Non-coding RNAs in pancreatic ductal adenocarcinoma. Front. Oncol. 2020;10:309. doi: 10.3389/fonc.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., Loewer A., Ziebold U., Landthaler M., Kocks C., le Noble F., Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;21:333–338. doi: 10.1038/nature11928. 495. [DOI] [PubMed] [Google Scholar]
- 242.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhong Z., Lv M., Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci. Rep. 2016;6(30919) doi: 10.1038/srep30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xia W., Qiu M., Chen R., Wang S., Leng X., Wang J., Xu Y., Hu J., Dong G., Xu P.L., Yin R. Circular RNA has_circ_0067934 is upregulated in oesophageal squamous cell carcinoma and promoted proliferation. Sci. Rep. 2016;6(35576) doi: 10.1038/srep35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sand M., Bechara F.G., Sand D., Gambichler T., Hahn S.A., Bromba M., Stockfleth E., Hessam S. Circular RNA expres- sion in basal cell carcinoma. Epigenomics. 2016;8:619–632. doi: 10.2217/epi-2015-0019. [DOI] [PubMed] [Google Scholar]
- 246.Xie H., Ren X., Xin S., Lan X., Lu G., Lin Y., Yang S., Zeng Z., Liao W., Ding Y.Q., Liang L. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7:26680–26691. doi: 10.18632/oncotarget.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li H., Hao X., Wang H., Liu Z., He Y., Pu M., Zhang H., Yu H., Duan J., Qu S. Circular RNA expression profile of pancreatic ductal adenocarcinoma revealed by microarray. Cell. Physiol. Biochem. 2016;40:1334–1344. doi: 10.1159/000453186. [DOI] [PubMed] [Google Scholar]
- 248.Suzuki H., Tsukahara T. A view of pre-mRNA splicing from RNase R resistant. RNAs Int J Mol Sci. 2014;15:9331–9342. doi: 10.3390/ijms15069331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Salzman J., Chen R.E., Olsen M.N, Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLos Genet. 2013;9 doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., Herzog M., Schreyer L., Papavasileiou P., Ivanov A., Öhman M., Refojo D., Kadener S., Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 251.You X., Vlatkovic I., Babic A., Will T., Epstein I., Tushev G., Akbalik G., Wang M., Glock C., Quedenau C., Wang X., Hou J., Liu H., Sun W., Sambandan S., Chen T., Schuman E.M., Chen W. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 2015;18:603–610. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hansen T.B., Kjems J., Damgaard C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 253.Boeckel J.N., Jaé N., Heumüller A.W., Chen W., Boon R.A., Stellos K., Zeiher A.M., John D., Uchida S., Dimmeler S. Identification and characterization of hypoxia-regulated endothelial circular. RNA Circ. Res. 2015;117:884–890. doi: 10.1161/CIRCRESAHA.115.306319. [DOI] [PubMed] [Google Scholar]
- 254.Satoh J., Yamamura T. Gene expression profile following stable expression of the cellular prion protein. Cell. Mol. Neurobiol. 2004;24:793–814. doi: 10.1007/s10571-004-6920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li F., Zhang L., Li W., Deng J., Zheng J., An M., Lu J., Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yang F., Liu D.Y., Guo J.T., Ge N., Zhu P., Liu X., Wang S., Wang G.X., Sun S.Y. Circular RNA circ-LDLRAD3 as a biomarker in diagnosis of pancreatic cancer. World J. Gastroenterol. 2017;23(47):8345–8354. doi: 10.3748/wjg.v23.i47.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yao J., Zhang C., Chen Y., Gao S. Downregulation of circular RNA circ-LDLRAD3 suppresses pancreatic cancer progression through miR-137-3p/PTN axis. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.116871. [DOI] [PubMed] [Google Scholar]
- 258.Xu Y., Yao Y., Gao P., Cui Y. Upregulated circular RNA circ_0030235 predicts unfavourable prognosis in pancreatic ductal adenocarcinoma and facilitates cell progression by sponging miR-1253 and miR-1294Biochem. Biophys. Res. Commun. 2019;509(1):138–142. doi: 10.1016/j.bbrc.2018.12.088. [DOI] [PubMed] [Google Scholar]
- 259.Hao L., Rong W., Bai L., Cui H., Zhang S., Li Y., Chen D., Meng X. Upregulated circular RNA circ_0007534 indicates an unfavourable prognosis in pancreatic ductal adenocarcinoma and regulates cell proliferation, apoptosis, and invasion by sponging miR-625 and miR-892b. J. Cell. Biochem. 2019;120(3):3780–3789. doi: 10.1002/jcb.27658. [DOI] [PubMed] [Google Scholar]
- 260.Li Z., Yanfang W., Li J., Jiang P., Peng T., Chen K., Zhao X., Zhang Y., Zhen P., Zhu J., Li X. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi: 10.1016/j.canlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 261.Li J., Li Z., Jiang P., Peng M., Zhang X., Chen K., Liu H., Bi H., Liu X., Li X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018;37(1):177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu L., Liu F.B., Huang M., Xie K., Xie Q.S., Liu C.H., Shen M.J., Huang V. Circular RNA ciRS-7 promotes the proliferation and metastasis of pancreatic cancer by regulating miR-7-mediated EGFR/STAT3 signaling pathway. HBPD INT. 2019;18(6):580–586. doi: 10.1016/j.hbpd.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 263.Qu S., Hao X., Song W., Niu K., Yang X., Zhang X. Circular RNA circRHOT1 is upregulated and promotes cell proliferation and invasion in pancreatic cancer. Epigenomics. 2019;11:53–63. doi: 10.2217/epi-2018-0051. [DOI] [PubMed] [Google Scholar]
- 264.An Y., Cai H., Zhang Y., Liu S., Duan Y., Sun D. circZMYM2 competed endogenously with miR-335–5p to regulate JMJD2C in pancreatic cancer. Cell. Physiol. Biochem. 2018;51:2224–2236. doi: 10.1159/000495868. [DOI] [PubMed] [Google Scholar]
- 265.Jiang Y., Wang T., Yan L., Qu L. A novel prognostic biomarker for pancreatic ductal adenocarcinoma: hsa_circ_0001649. Gene. 2018;675:88–93. doi: 10.1016/j.gene.2018.06.099. [DOI] [PubMed] [Google Scholar]
- 266.Qu S., Song W., Yang X., Wang J., Zhang R., Zhang Z. Microarray expression profile of circular RNAs in human pancreatic ductal adenocarcinoma. Genome. Data. 2015;5:385–387. doi: 10.1016/j.gdata.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Renouf D., Moore M. Evolution of systemic therapy for advanced pancreatic cancer. Expert Rev. Anticancer Ther. 2010;10(4):529–540. doi: 10.1586/era.10.21. [DOI] [PubMed] [Google Scholar]
- 268.Subramani R. Emerging roles of microRNAs in pancreatic cancer diagnosis, therapy and prognosis (Review) Int. J. Oncol. 2015;47:1203–1210. doi: 10.3892/ijo.2015.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhu S., Wu H., Wu F., Nie D., Sheng S., Mo Y.Y. MicroRNA-21 targets tumour suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 270.Sicard F. Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. 2013;21(5):986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Moriyama T. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol. Cancer Ther. 2009;8(5):1067–1074. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 272.Hwang J-H. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010;5(5):e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Torrisani J. let-7 microRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumour progression. Hum. Gene Ther. 2009;20(8):831–844. doi: 10.1089/hum.2008.134. [DOI] [PubMed] [Google Scholar]
- 274.Xiao J. microRNA-137 modulates pancreatic cancer cells tumour growth, invasion and sensitivity to chemotherapy. Int. J. Clin. Exp. Pathol. 2014;7(11):7442–7450. PMC4270551. [PMC free article] [PubMed] [Google Scholar]
- 275.Wang S., Chen X., Tang M. MicroRNA-216a inhibits pancreatic cancer by directly targeting Janus kinase 2. Oncol. Rep. 2014;32(6):2824–2830. doi: 10.3892/or.2014.3478. [DOI] [PubMed] [Google Scholar]
- 276.He H. MicroRNA-218 inhibits cell invasion and migration of pancreatic cancer via regulating ROBO1. Cancer Biol. Ther. 2014;15(10):1333–1339. doi: 10.4161/cbt.29706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pramanik D. Restitution of tumour suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. Cancer. Ther. 2011;10(8):1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hu Q.L. Cationic microRNA-delivering nanovectors with bifunctional peptides for efficient treatment of PANC-1 xenograft model. Biomaterials. 2013;34:2265–2276. doi: 10.1016/j.biomaterials.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 279.Ma Y., Yu S., Zhao W., Lu Z., Chen J. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298(2):150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 280.He D. MiR-371-5p facilitates pancreatic cancer cell proliferation and decreases patient survival. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jung H., Ertl L., Janson C., Schall T., Charo I. Abstract A107: inhibition of CCR2 potentiates the checkpoint inhibitor immunotherapy in pancreatic cancer. Cancer Immunol. Res. 2016;4:A107. doi: 10.1158/2326-6066.IMM2016-A107. -A. [DOI] [Google Scholar]
- 282.Liu C. MicroRNA-34b inhibits pancreatic cancer metastasis through repressing Smad3. Curr. Mol. Med. 2013;13(4):467–478. doi: 10.2174/1566524011313040001. [DOI] [PubMed] [Google Scholar]
- 283.Qazi A.M. Restoration of E-cadherin expression in pancreatic ductal adenocarcinoma treated with microRNA-101. Surgery. 2012;152:704–711. doi: 10.1016/j.surg.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 284.Chen Z. miR-204 mediated loss of Myeloid cell leukemia-1 results in pancreatic cancer cell death. Mol. Cancer. 2013;12(1):105. doi: 10.1186/1476-4598-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Laurila E.M. Both inhibition and enhanced expression of miR-31 lead to reduced migration and invasion of pancreatic cancer cells. Genes Chromosomes Cancer. 2012;51(6):557–568. doi: 10.1002/gcc.21941. [DOI] [PubMed] [Google Scholar]
- 286.Srivastava S.K. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Li Y., Sarkar F.H. MicroRNA targeted therapeutic approach for pancreatic cancer. Int. J. Biol. Sci. 2016;12(3):326–337. doi: 10.7150/ijbs.15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sun T., Kong X., Du Y., Li Z. Aberrant MicroRNAs in pancreatic cancer: researches and clinical implications. Gastroenterol. Res. Practise. 2014;2014 doi: 10.1155/2014/386561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Galasso M., Sana M.E., Volinia S. Non-coding RNAs: a key to future personalized molecular therapy? Genome Med. 2010;2(2):12. doi: 10.1186/gm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rejiba S., Bigand C., Parmentier C., Hajri A. Gemcitabine-based chemogene therapy for pancreatic cancer using Ad-dCK: UMK GDEPT and TS/RR siRNA strategies. Neoplasia. 2009;11(7):637–650. doi: 10.1593/neo.81686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang Q., Li K., Huang X., Zhao C., Mei Y., Li X., Jiao L., Yang H. lncRNA SLC7A11-AS1 promotes chemoresistance by blocking SCF (beta-TRCP)-mediated degradation of NRF2 in pancreatic cancer. Mol. Ther. Nucleic Acids. 2020;19:974–985. doi: 10.1016/j.omtn.2019.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang C.J., Shi S.B., Tian J., Xu J., Niu Z.X. lncRNA MALAT1, HOTTIP and PVT1 as predictors for predicting the efficacy of GEM based chemotherapy in first-line treatment of pancreatic cancer patients. Oncotarget. 2017;8(56):95108–95115. doi: 10.18632/oncotarget.19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li C., Zhao Z., Zhou Z., Liu R. Linc-ROR confers gemcitabine resistance to pancreatic cancer cells via inducing autophagy and modulating the miR-124/PTBP1/PKM2 axis. Cancer Chemother. Pharmacol. 2016;78(6):1199–1207. doi: 10.1007/s00280-016-3178-4. [DOI] [PubMed] [Google Scholar]
- 294.Yang F., Li X., Zhang L., Cheng L., Li X. LncRNA TUG1 promoted viability and associated with gemcitabine resistant in pancreatic ductal adenocarcinoma. J. Pharmacol. Sci. 2018;137(2):116–121. doi: 10.1016/j.jphs.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 295.Yoshida K., Toden S., Ravindranathan P., Han H., Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38(10):1036–1046. doi: 10.1093/carcin/bgx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ma L., Wang F., Du C., Zhang Z., Guo H., Xie X., Gao H., Zhuang Y., Kornmann M., Gao H., Tian X., Yang Y. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol. Rep. 2018;39(3):1132–1140. doi: 10.3892/or.2018.6178. [DOI] [PubMed] [Google Scholar]
- 297.Sorin V., Ohana P., Gallula J., Birman T., Matouk I., Hubert A., Gilon M., Hochberg A., Czerniak A. H19-promoter-targeted therapy combined with gemcitabine in the treatment of pancreatic cancer. ISRN Oncol. 2012;2012 doi: 10.5402/2012/351750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Peng W.X., He R.Z., Zhang Z., Yang L., Mo Y.Y. LINC00346 promotes pancreatic cancer progression through the CTCF-mediated Myc transcription. Oncogene. 2019;38:6770–6780. doi: 10.1038/s41388-019-0918-z. [DOI] [PubMed] [Google Scholar]
- 299.Zhang B., Li C., Sun Z. Long non-coding RNA LINC00346, LINC00578, LINC00673, LINC00671, LINC00261, and SNHG9 are novel prognostic markers for pancreatic cancer. Am. J. Transl. Res. 2018;10(8):2648–2658. [PMC free article] [PubMed] [Google Scholar]
- 300.Shi W., Zhang C., Ning Z., Hua Y., Li Y., Chen L., Liu L., Chen Z., Meng Z. Long non-coding RNA LINC00346 promotes pancreatic cancer growth and gemcitabine resistance by sponging miR-188-3p to derepress BRD4 expression. J. Exp. Clin. Cancer Res. 2019;38:60. doi: 10.1186/s13046-019-1055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lou C., Zhao J., Gu Y., Li Q., Tang S., Wu Y., Tang J., Zhang C., Li Z., Zhang Y. LINC01559 accelerates pancreatic cancer cell proliferation and migration through YAP-mediated pathway. J. Cell. Physiol. 2020;235(4):3928–3938. doi: 10.1002/jcp.29288. [DOI] [PubMed] [Google Scholar]
- 302.Chen X., Wang J., Xie F., Mou T., Zhong P., Hua H., Liu P., Yang Q. Long noncoding RNA LINC01559 promotes pancreatic cancer progression by acting as a competing endogenous RNA of miR-1343-3p to upregulate RAF1 expression. Aging. 2020;12:14452–14466. doi: 10.18632/aging.103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Deng Z., Li X., Shi Y., Lu Y., Yao W., Wang J. A novel autophagy-related IncRNAs signature for prognostic prediction and clinical value in patients with pancreatic cancer front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.606817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Liu Y., Xia L., Dong L., Wang J., Xiao Q., Yu X., Zhu H. CircHIPK3 promotes Gemcitabine (GEM) resistance in pancreatic cancer cells by sponging miR-330-5p and targets RASSF1. Cancer Manag. Res. 2020;12:921–929. doi: 10.2147/CMAR.S239326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Iacobuzio-Donahue C.A., Velculescu V.E., Wolfgang C.L., Hruban R.H. Genetic basis of pancreas cancer development and progression: insights from whole-exome and whole-genome sequencing. Clin. Cancer Res. 2012;18:4257–4265. doi: 10.1158/1078-0432.ccr-12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hezel A.F., Kimmelman A.C., Stanger B.Z., Bardeesy N., Depinho R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 307.Bükki J. Pancreatic adenocarcinoma. New Engl. J. Med. 2014;371:2139–2141. doi: 10.1056/nejmc1412266. [DOI] [PubMed] [Google Scholar]
- 308.Kanda M., Matthaei H., Wu J., Hong S., Yu J., Borges M., Hruban R.H., Maitra A., Kinzler K., Vogelstein B. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733. doi: 10.1053/j.gastro.2011.12.042. e978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Timar J., Kashofer K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020;39:1029–1038. doi: 10.1007/s10555-020-09915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Aumayr K., Soleiman A., Sahora K., Schindl M., Werba G., Schoppmann S.F., Birner P. HER2 gene amplification and pro- tein expression in pancreatic ductal adenocarcinomas. Appl. Immunohistochem. Mol. Morphol. 2014;22:146–152. doi: 10.1097/pai.0b013e31828dc392. [DOI] [PubMed] [Google Scholar]
- 311.Jones S., Zhang X., Parsons D.W., Lin J.C.-H., Leary R.J., Angenendt P., Mankoo P., Carter H., Kamiyama H., Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.McCleary-Wheeler A.L., McWilliams R., Fernandez-Zapico M.E. Aberrant signaling pathways in pancreatic cancer: a two compartment view. Mol. Carcinog. 2011;51:25–39. doi: 10.1002/mc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sharpless N.E., DePinho R.A. Cancer: crime and punishment. Nature. 2005;436:636–637. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 314.Sun C., Sang M., Li S., Sun X., Yang C., Xi Y., Wang L., Zhang F., Bi Y., Fu Y. Hsa-miR-139-5p inhibits proliferation and causes apoptosis associated with down-regulation of c-Met. Oncotarget. 2015;6:39756–39792. doi: 10.18632/oncotarget.5476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 315.Mortoglou M., Wallace D., Buha Djordjevic A., Djordjevic V., Arisan E.D., Uysal-Onganer P. MicroRNA-regulated signaling pathways: potential biomarkers for pancreatic. Ductal Adenocarcinoma Stresses. 2021;1(1):30–47. doi: 10.3390/stresses1010004. [DOI] [Google Scholar]
- 316.Slack F.J., Weidhaas J.B. MicroRNA in cancer prognosis. N. Engl. J. Med. 2008;359(25):2720–2722. doi: 10.1056/NEJMe0808667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Iorio M.V. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. https://www.ncbi.nlm.nih.gov/pubmed/16103053 [DOI] [PubMed] [Google Scholar]
- 318.Morin R.D. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18(4):610–621. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rawat M. MicroRNA in pancreatic cancer: from biology to therapeutic. Potential Genes. 2019;10(10):752. doi: 10.3390/genes10100752. [DOI] [PMC free article] [PubMed] [Google Scholar]