Abstract
We evaluated the Standard Q COVID-19 Ag test for the diagnosis of coronavirus disease 2019 (COVID-19) compared to the reverse transcription-polymerase chain reaction (RT-PCR) test. We applied both tests to patients who were about to be hospitalized, had visited an emergency room, or had been admitted due to COVID-19 confirmed by RT-PCR. Two nasopharyngeal swabs were obtained; one was tested by RT-PCR and the other by the Standard Q COVID-19 Ag test. A total of 118 pairs of tests from 98 patients were performed between January 5 and 11, 2021. The overall sensitivity and specificity for detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for the Standard Q COVID-19 Ag test compared to RT-PCR were 17.5% (95% confidence interval [CI], 8.8–32.0%) and 100% (95% CI, 95.3–100.0%). Analysis of the results using RT-PCR cycle thresholds of ≤ 30 or ≤ 25 increased the sensitivity to 26.9% (95% CI, 13.7–46.1%), and 41.1% (95% CI, 21.6–64.0%), respectively.
Keywords: SARS-CoV-2, COVID-19, Antigen Test
Graphical Abstract
Reverse transcription-polymerase chain reaction (RT-PCR) assay of nucleic acids in respiratory specimens is the diagnostic standard for coronavirus disease 2019 (COVID-19).1 The limitations of the RT-PCR tests include their high costs, longer turn-around time, and required equipment for testing.1 Rapid antigen tests, diagnostic methods based on lateral immunochromatography, could be used as point-of-care detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens. Although rapid antigen tests have the advantages of lower cost, short turn-around time, and lack of requirement for professional skill or instruments, their sensitivity in detecting SARS-CoV-2 virus is low compared to RT-PCR, especially for clinical specimens with low viral loads.2,3,4,5 Several rapid antigen tests are currently available in clinical practice. Among these, the Standard Q COVID-19 Ag Test (SD Biosensor, Inc., Suwon, Korea) employs a lateral flow assay in a cassette-based format with a visual read-out. This test showed higher sensitivity and a lower limit of detection compared to other rapid antigen tests.6,7 The present study evaluated the performance of the Standard Q COVID-19 Ag test for the detection of SARS CoV-2 compared to RT-PCR in clinical applications.
In a study hospital, the performance of the Standard Q COVID-19 Ag test was assessed between January 5 and 11 2021. We applied both RT-PCR and Standard Q COVID-19 Ag tests to patients who were about to be hospitalized, visited an emergency room, or were admitted due to COVID-19 confirmed by RT-PCR. We applied both tests in the same patient at the same time. Skilled nurses or medical doctors acquired two nasopharyngeal swabs from each patient; one for RT-PCR and the other for the Standard Q COVID-19 Ag test. RT-PCR was performed using the Standard M nCoV Real-Time Detection kit (SD Biosensor, Inc.). The Standard Q COVID-19 Ag tests were performed according to the manufacturer's instructions.
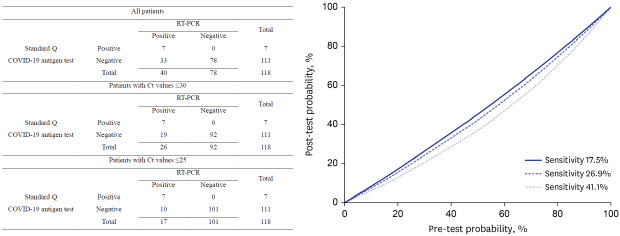
A total of 118 pairs of RT-PCR and Standard Q COVID-19 Ag tests were performed for 98 patients between January 5 and 11, 2021. Of these, 14 patients underwent tests for screening before admission and 52 had undergone tests when they visited an emergency room regardless of COVID-19 symptoms. In addition, 32 patients with COVID-19 confirmed by RT-PCR who were admitted to the study hospital during the study period underwent tests. Out of 32 patients, 26 have received high flow oxygen therapy or mechanical ventilation. Repeated tests were performed at several time intervals during the hospital stays of 20 patients who were admitted due to COVID-19. The overall results of both tests are summarized in Table 1. The overall sensitivity and specificity of the Standard Q COVID-19 Ag test for detecting SARS-CoV-2 compared to RT-PCR were 17.5% (95% confidence interval [CI], 8.8–32.0%) and 100% (95% CI, 95.3–100.0%), respectively. Analysis according to cycle threshold (Ct) value showed sensitivity and specificity of the Standard Q COVID-19 Ag test for detecting SARS-CoV-2 with Ct ≤ 30 of 26.9% (95% CI, 13.7–46.1%) and 100% (95% CI, 96.0–100.0%), respectively. In contrast, the sensitivity and specificity of detecting SARS-CoV-2 virus for Ct ≤ 25 were 41.1% (95% CI, 21.6–64.0%) and 100% (95% CI, 96.3–100.0%), respectively.
Table 1. Results of RT-PCR and Standard Q COVID-Ag tests.
| Variables | RT-PCR | Total | ||
|---|---|---|---|---|
| Positive | Negative | |||
| All patients | ||||
| Positive | 7 | 0 | 7 | |
| Negative | 33 | 78 | 111 | |
| Total | 40 | 78 | 118 | |
| Patients with Ct values ≤ 30 | ||||
| Positive | 7 | 0 | 7 | |
| Negative | 19 | 92 | 111 | |
| Total | 26 | 92 | 118 | |
| Patients with Ct values ≤ 25 | ||||
| Positive | 7 | 0 | 7 | |
| Negative | 10 | 101 | 111 | |
| Total | 17 | 101 | 118 | |
RT-PCR = reverse transcription-polymerase chain reaction, Ct = cycle threshold.
The standard Q COVID-19 Ag test had lower sensitivity compared to RT-PCR, especially in patients with low viral loads, consistent with the findings of previous studies.6,7,8 The Food and Drug Administration (FDA) prefers the use of natural clinical specimens to contrived specimens to evaluate the performance of diagnostic tests. The results of this study which used clinical specimens at point of care did not differ from those of previous studies evaluating the performance of rapid antigen tests using stored respiratory samples in viral transport media.6,9 The sensitivity in this study was a little lower compared to previous studies which also evaluated the Standard Q COVID-19 Ag test.3,7,8,10 Several studies evaluated the Standard Q COVID-19 Ag test compared with RT-PCR. They applied both tests to people who visited an emergency room or acute care hospitals with or without symptoms of COVID-19. Sensitivity ranged from 50.0% to 89.9%, and specificity from 93.1% to 100%. In this study, nasopharyngeal swabs were done by skilled nurses or medical doctors, and two samples, one for RT-PCR and the other for the Standard Q COVID-19 Ag test, were acquired at the same time. Therefore, we thought performance of nasopharyngeal swabs was not a reason of lower sensitivity. Although there could be the possibilities of errors in performing Ag tests, we thought that the differences were originated mainly from different methods of RT-PCR. It has been well known that Ct values were not comparable between RT-PCR tests.1
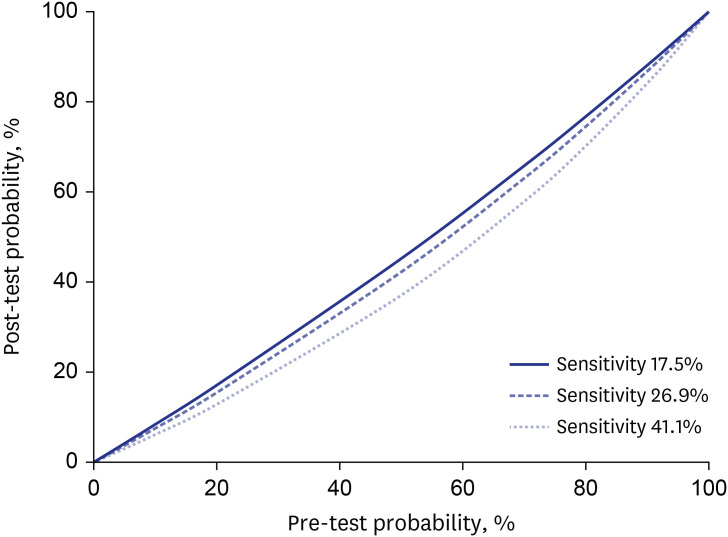
In many hospitals, RT-PCR tests for COVID-19 were required in patients visiting an emergency room before further diagnostic or therapeutic approach to prevent the hospital outbreak. Considering that the turnaround time of conventional RT-PCR is at least 8 hours, delaying procedures while awaiting the results of COVID-19 tests could negatively affect patients with urgent conditions. In this context, rapid antigen testing could be considered as a diagnostic test to obtain results within 30 minutes. The post-test probability is dependent on the pre-test probability, which itself depends on COVID-19 prevalence, exposure history, and symptoms.11 We plotted the pre-test and post-test probabilities of infection based on sensitivities ranging from 17.5% to 41.1% and a specificity of 100% for the Standard Q COVID-19 Ag tests (Fig. 1). When the pre-test probability was 10% and the sensitivity and specificity were 17.5% and 100%, respectively, the post-test probability of a negative result was 8.40%, which was too high to safely assume that someone was uninfected. If we assumed the Standard Q COVID-19 Ag test sensitivity of 41.1% based on a clinically significant Ct value of ≤ 25, the post-test probability of a negative result was 6.14%, which is still too high to safely rule out COVID-19 infection. We could not safely rule out a COVID-19 infection if the pre-test probability was ≥ 10%. False-negative rapid antigen test results could undermine efforts at containment and potentially lead to outbreaks in vulnerable patients. Thus, despite its high specificity (100%), the Standard Q COVID-19 Ag test might not be an optimal clinical test due to its low sensitivity (17.5–41.1%). We evaluated a total of 118 samples using the Standard Q COVID-19 Ag test and the samples were not enough to get a solid conclusion. There have been many reports which evaluated various rapid antigen tests including the Standard Q COVID-19 Ag test,3,7,8 Sofia SARS Antigen FIA,2 Panbio COVID-19CMI,5 etc. The sensitivity varied between studies and ranged from 35.8% to 89.9%. The clinical applicability of rapid antigen test for diagnosis of COVID-19 could be higher if tests with higher performance were applied.
Fig. 1. Post-test probability of severe acute respiratory syndrome coronavirus 2 infection, given a negative test result, according to variable sensitivities.
Diagnostic tests for COVID-19 could be used as clinical tests to detect individual patients or as screening tests. Clinical tests aiming to detect individual patients with COVID-19 require high analytic sensitivity. In contrast, tests used for screening to reduce the population spread should be inexpensive and easy to execute to allow frequent testing.12 Serial testing of asymptomatic and symptomatic persons have been proposed for the prevention of SARS-CoV-2 transmission in congregate settings and rapid antigen tests could be suitable for this purpose.12 The value of the Standard Q COVID-19 Ag test for screening requires further evaluation.
In conclusion, the sensitivity of the Standard Q COVID-19 Ag test was 17.5–41.1% according to the Ct value of RT-PCR, with a 100% specificity. The Standard Q COVID-19 Ag test was not an optimal clinical test due to its low sensitivity.
Ethics statement
This study was approved by the Institutional Review Board of Seoul National University Hospital and the requirement for informed consent was waived (IRB 2101-089-1189).
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim NJ.
- Data curation: Oh SM, Jeong H, Chang E, Choe PG, Kang CK, Park WB, Kim TS, Kwon WY, Oh MD.
- Formal analysis: Oh SM, Jeong H, Chang E, Kim NJ.
- Investigation: Oh SM, Jeong H, Chang E, Choe PG, Kang CK, Park WB, Kim TS, Kwon WY, Oh MD, Kim NJ.
- Methodology: Choe PG, Kang CK, Park WB, Kim TS, Kwon WY, Oh MD, Kim NJ.
- Supervision: Kim NJ.
- Validation: Oh SM, Jeong H, Chang E, Choe PG, Kang CK, Park WB, Kim TS, Kwon WY, Oh MD, Kim NJ.
- Writing - original draft: Oh SM, Kim NJ.
- Writing - review & editing: Oh SM, Jeong H, Chang E, Choe PG, Kang CK, Park WB, Kim TS, Kwon WY, Oh MD, Kim NJ.
References
- 1.CDC's diagnostic test for COVID-19 only and supplies. [Updated December 9, 2020]. [Accessed January 27, 2021]. https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html.
- 2.Pray IW, Ford L, Cole D, Lee C, Bigouette JP, Abedi GR, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turcato G, Zaboli A, Pfeifer N, Ciccariello L, Sibilio S, Tezza G, et al. Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: a preliminary report. J Infect. 2021;82(3):e14–6. doi: 10.1016/j.jinf.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Li J. Immunologic testing for SARS-CoV-2 infection from the antigen perspective. J Clin Microbiol. doi: 10.1128/JCM.02160-20. Forthcoming 2020. DOI: 10.1128/JCM.02160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes MÁ, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27(3):472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamayoshi S, Sakai-Tagawa Y, Koga M, Akasaka O, Nakachi I, Koh H, et al. Comparison of rapid antigen tests for COVID-19. Viruses. 2020;12(12):1420. doi: 10.3390/v12121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak GC, Lau SS, Wong KK, Chow NL, Lau CS, Lam ET, et al. Analytical sensitivity and clinical sensitivity of the three rapid antigen detection kits for detection of SARS-CoV-2 virus. J Clin Virol. 2020;133:104684. doi: 10.1016/j.jcv.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner AK, Nikolai O, Kausch F, Wintel M, Hommes F, Gertler M, et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J. doi: 10.1183/13993003.03961-2020. Forthcoming 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toptan T, Eckermann L, Pfeiffer AE, Hoehl S, Ciesek S, Drosten C, et al. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J Clin Virol. 2021;135:104713. doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv; [Updated January 1, 2020]. [Accessed January 27, 2021]. https://www.medrxiv.org/content/10.1101/2020.10.01.20203836v1. [Google Scholar]
- 11.Candel FJ, Barreiro P, San Román J, Abanades JC, Barba R, Barberán J, et al. Recommendations for use of antigenic tests in the diagnosis of acute SARS-CoV-2 infection in the second pandemic wave: attitude in different clinical settings. Rev Esp Quimioter. 2020;33(6):466–484. doi: 10.37201/req/120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity - a strategy for containment. N Engl J Med. 2020;383(22):e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]