Abstract
Background
Critical congenital heart diseases (CCHDs), 10% to 25% of all CHD, are duct-dependent defects that are life threatening without intervention in the neonatal period or infancy. One third of neonates with CCHDs are discharged home undetected and have a poorer outcome. Pulse oximetry screening before discharge is increasingly being used to diagnose CCHDs in developed countries.
Methods
This prospective observational study conducted at a tertiary care hospital from September 2016 to March 2019 screened all asymptomatic intramural neonates after 24 hours of life using a Masimo pulse oximeter with signal extraction technology using the standard American Academy of Pediatrics algorithm. A positive screen was followed by a confirmatory echocardiography (gold standard) and a negative screen by clinical examination at 6, 10 and 14 weeks and identification of readmissions during the study period.
Results
A total of 1855 neonates (82.99% of the eligible 2235 neonates) underwent screening at a mean (SD) age at screening of 32.4 (6.8) hours and took a mean (SD) time of 3.5 (1.2) minutes. The sensitivity, specificity, positive and negative predictive value of pulse oximetry screening for detection of CCHDs in asymptomatic neonates was 75% (95% CI: 28.91% to 96.59%), 99.29% (95% CI: 98.79% to 99.60%), 18.75% (95% CI: 5.80% to 43.80%) and 99.94% (95% CI: 99.66 to 99.99%), respectively.
Conclusion
Pulse oximetry screening of asymptomatic neonates between 24 and 48 hours of life improved the detection of CCHDs with high specificity and negative predictive value, moderate sensitivity and a reasonably low false positivity rate.
Keywords: Critical congenital heart diseases, Pulse oximetry screening, Asymptomatic neonates, Diagnostic accuracy
Introduction
Congenital heart diseases (CHDs) are among one of the commonest congenital malformations encountered in clinical practice and remain an important cause of morbidity and mortality in infants and children. The prevalence of CHD ranges from 8 to 12 per 1000 live births.1,2 With 27 million live births every year, India has a huge burden of children with CHD. However, there are significant problems in the form of poor parental awareness, delayed diagnosis and late referrals to limited and unequally distributed paediatric cardiac care facilities in the public and private sector.3
Critical CHD (CCHD) have been defined in literature as duct-dependent CHD that are life-threatening without treatment in the neonatal period or infancy. They include duct-dependent pulmonary defects such as pulmonary atresia with intact ventricular septum, pulmonary stenosis, tetralogy of fallot, total anomalous pulmonary venous return, transposition of the great arteries (TGA), tricuspid atresia, truncus arteriosus or duct-dependent systemic defects such as coarctation of aorta, interrupted aortic arch, hypoplastic left heart syndrome and aortic stenosis.4, 5, 6 Life-threatening CCHD form around 10%–25% of all CHD in the newborn and one third of these neonates are discharged home without a diagnosis.7
The routine antenatal anomaly scan at 18–20 weeks is able to detect only around 50% of CHD.8, 9, 10The discharge neonatal clinical examination also misses many of these CCHD.4,11,12 Delayed diagnosis leads to clinical recognition of CCHD when these neonates present with cardiovascular collapse in the postnatal ward or at home as the ductus arteriosus closes in the first few days after birth. This late detection of CCHD in almost 30% of neonates and subsequent late referral is associated with a poorer outcome and mortality.13, 14, 15
The majority of the CCHD are asymptomatic at birth and the degree of cyanosis is not clinically recognizable. Cyanosis is apparent clinically only when there is at least 5 g/dl of deoxygenated haemoglobin or an SpO2 of <80%.16,17
Pulse oximetry screening to detect CCHD was first studied in the beginning of the 21st century. The underlying principle is the ability of pulse oximetry in detecting clinically inapparent cyanosis. Pulse oximetry screening studies have come from the bench to bedside in the last few years with a moderate sensitivity of 76.3%, high specificity of 99.9% and low false positive rate of 0.14% in the detection of CCHD among asymptomatic newborns in hospitals before discharge.18 Today, US, UK, Canada, Norway, Sweden and many other European countries use universal pulse oximetry screening among asymptomatic neonates for detection of CCHD before discharge from hospital.5,19,20
Although there is enough evidence for the routine use of pulse oximetry screening of CCHD in many parts of the world, the situation in India regarding universal implementation of pulse oximetry screening is complex and needs deliberation. The existing paediatric cardiac care facilities (including paediatric cardiologists and cardiothoracic surgeons) are unequally distributed and insufficient to handle the burden of delayed diagnosis and late referrals of CCHD. There is also the possibility of increased referrals for a confirmatory echocardiography after a positive pulse oximetry screen which will stretch the existing facilities.21,22 The cost of treatment in many cases is also prohibitively expensive for many families.
The scenario in the Armed Forces Medical Services deserves special considerations. With a wide geographic spread and regional paediatric cardiac care centres located in major zonal or command hospitals, an early diagnosis of CCHD at peripheral hospitals is essential and practically feasible before cardiovascular compromise and enables safe transport to the appropriate centre for further care.
Materials and methods
Study design
Prospective observational study.
Study setting
The study was conducted at the postnatal ward of a tertiary care, multi-specialty referral hospital from September 2016 to March 2019 (31 months).
Inclusion criteria
All stable and asymptomatic intramural neonates delivered by normal vaginal delivery/ caesarean section during the study period were included in the study.
Exclusion criteria
The neonates with the following conditions were excluded from the study:
-
1.
Neonates with antenatal ultrasound/ echocardiographic diagnosis of CHD
-
2.
Any neonate requiring Neonatal Intensive Care Unit (NICU) admission
-
3.
Any major congenital malformations
Sample size
Being a feasibility study of implementation of pulse oximetry screening, all asymptomatic neonates born during the study period were screened (n = 1855) using a pulse oximeter before discharge as per the protocol of the study.
Ethical clearance
The study was approved by the Institutional Ethics Committee. Informed parental consent was taken from parents of all neonates enrolled in the study.
Equipment
Two Masimo Radical-7 pulse oximeters with Signal Extraction Technology (SET) and ability to read SpO2 with a pulse waveform even in states of low perfusion and motion tolerant were used along with reusable probes, which were cleaned between use.The pulse oximeters were calibrated at regular intervals by the service engineer from the Original Equipment Manufacturer.
Pulse oximetry screening algorithm
The algorithm used for pulse oximetry screening was the American Academy of Pediatrics (AAP) and American Heart Association (AHA) endorsed US algorithm.The algorithm was placed as charts and also given as a laminated handout to the nursing staff in the postnatal ward.
A positive pulse oximetry screen was defined as an SpO2 < 90% in either the right hand or foot or an SpO2 between 90% and 94% in either site or a >3% difference between the two sites (repeated twice at 1-h intervals).
A negative pulse oximetry screen was defined as an SpO2 ≥ 95% in the right hand or foot and ≤3% difference between the two sites.
Methods
As part of the screening, all asymptomatic neonates roomed-in with the mother were screened with a pulse oximeter in a designated warm and quiet room in the postnatal ward (which was also used for the mandatory otoacoustic emission hearing screening before discharge) after 24 h of life. The neonates were awake and calm or breastfeeding during the screening.
The nursing staff in the postnatal ward, labour room and NICU complex was trained on using the pulse oximeter correctly by demonstration and dry runs for a week before the study began. The pulse oximetry screening was carried out twice a day in the morning and evening shifts by the nursing staff, who were supervised by the principal investigator at regular intervals weekly for the correct technique, interpretation and recording the raw data. Every three months, a fresh demonstration of the correct technique of pulse oximetry was carried out for the new nursing staff on duty in the postnatal ward.
Two separate sites, the right hand (preductal) and either foot (postductal) were tested consecutively by the nursing staff by application of the reusable SpO2 sensor after ensuring that the hands and feet of the neonates were warm to touch. The SpO2 reading was recorded once a stable waveform was displayed on the monitor.
The neonate who had a positive screen underwent a thorough clinical examination and a confirmatory 2D echocardiography by the cardiologist (Reference Standard) in the Department of Cardiology using a Siemens Echocardiography Machine for diagnosis or exclusion of CCHD. The neonates who had a negative screen were followed up clinically at 6-, 10- and 14-weeks during immunization visits and evaluated for symptoms of poor feeding, sweating, respiratory distress, praecordial pulsations, femoral pulses and any cardiac murmur. The hospital admission and discharge register was also analysed for the duration of the study for any readmissions of these neonates.
Statistical analysis
Descriptive statistics were represented as number and percentage, mean and standard deviation (SD) or median and interquartile range as appropriate. Test accuracy using 2D echocardiography as the gold standard was studied using sensitivity, specificity, positive and negative predictive value. All the statistical analyses were carried out using Graph Pad Prism software (Graph Pad Software Inc. USA). A p value of <0.05 was considered statistically significant.
Results
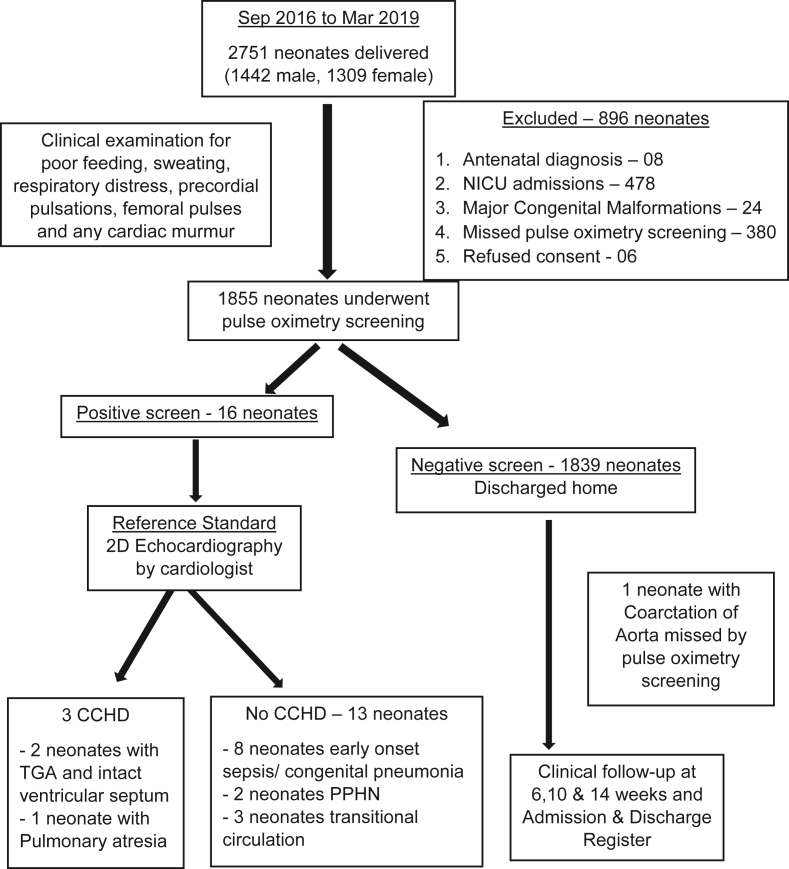
A total of 2751 neonates (1442 male, 1309 female) were born during the period of study from September 2016 to March 2019. A total of 896 neonates were excluded - 8 pregnancies had an antenatal diagnosis of CHD, 24 neonates had major congenital malformations identified at birth, 478 neonates were admitted to the NICU, 380 neonates missed pulse oximetry screening due to oversight by the nursing staff in the postnatal ward and equipment non-availability (equipment under use in the NICU) and 6 neonates whose parents refused consent (Fig. 1).
Fig. 1.
Study flow diagram.
A total of 1855 neonates (82.99% of the eligible 2235 neonates) underwent pulse oximetry screening, with a mean (SD) age at screening of 32.4 (6.8) hours. Pulse oximetry screening took a mean (SD) time of 3.5 (1.2) minutes (Table 1).
Table 1.
Baseline characteristics of neonates.
| Baseline characteristics | Neonates screened (n = 1855) | Neonates missed (n = 380) | P value |
|---|---|---|---|
| Gestational age (wks); mean (SD) | 38.9 (1.9) | 38.8 (1.8) | 0.34 |
| Birth weight (g); mean (SD) | 2889 (261) | 2862 (243) | 0.06 |
| Gender, male; n (%) | 958 (51.64) | 183 (48.15) | 0.23 |
| Age at pulse oximetry screening (hours); mean (SD) | 32.4 (6.8) | – | – |
| Time taken for pulse oximetry screening (min); mean (SD) | 3.5 (1.2) | – | – |
SD, standard deviation.
The prevalence of CCHD in our study was 3.63 per 1000 live births. The eight pregnancies with an antenatal diagnosis of CHD had two foetuses with tetralogy of fallot, two with ventricular septal defect, one each with Ebstein's anomaly, pulmonary atresia, hypoplastic left heart syndrome and TGA and intact ventricular septum.
The sensitivity, specificity, positive predictive value, and negative predictive value with 95% Confidence Interval (CI) of pulse oximetry screening in the detection of CCHD in asymptomatic neonates was 75% (95% CI: 28.91%–96.59%), 99.29% (95% CI: 98.79%–99.60%), 18.75% (95% CI: 5.80%–43.80%) and 99.94% (95% CI: 99.66–99.99%), respectively (Table 2). The false positive rate of pulse oximetry screening in the detection of CCHD was 0.81% (95% CI: 0.56%–0.94%). The other conditions which were detected among the screen positive neonates included 8 neonates with early onset sepsis/congenital pneumonia, 2 with persistent pulmonary hypertension of newborn (PPHN) and 3 neonates with transitional circulation.
Table 2.
Accuracy of pulse oximetry screening in detection of CCHD.
| Diagnostic accuracy | Pulse oximetry screening | CCHD | |
| CCHD present | CCHD absent | ||
| Screen positive | 3 | 13 | |
| Screen negative | 1 | 1838 | |
| Sensitivity |
TP TP + FN |
75% (95% CI: 28.91% to 96.59%) | |
| Specificity |
TN FP + TN |
99.29% (95% CI: 98.79% to 99.60%) | |
| Positive Predictive Value |
TP TP + FP |
18.75% (95% CI: 5.80% to 43.80%) | |
| Negative Predictive Value |
TN FN + TN |
99.94% (95% CI: 99.66% to 99.99%) | |
CCHD: Critical congenital heart disease; TP: True Positive; FP: False Positive; TN: True Negative; FN: False Negative.
The neonates with the antenatal diagnosis of CHD (excluding the CCHD) were delivered under supervision of a paediatrician and reviewed by 2D echocardiography after birth and managed as per standard guidelines. The four pregnancies with antenatal diagnosis of CCHD were referred in-utero to a higher centre with cardiothoracic surgical facilities. The three neonates with true positive pulse oximetry screening (2 neonates with TGA and intact ventricular septum and 1 neonate with pulmonary atresia) were stabilized in the NICU on prostaglandin E 1 infusion and transferred by ambulance to a higher centre with cardiothoracic surgical facilities. The neonate with coarctation of aorta which was missed by pulse oximetry screening presented with cardiovascular collapse to a peripheral hospital and could not be salvaged.
The false positive pulse oximetry screening results picked up neonates with early onset sepsis/congenital pneumonia, PPHN and transitional circulation which were managed as per standard guidelines.
Discussion
Pulse oximetry screening for the detection of CCHD among asymptomatic neonates at a tertiary care referral hospital of the Armed Forces was feasible and implemented safely with a total of 82.99% of the eligible 2235 neonates screened in accordance with the protocol, similar to most of the reported studies.23, 24, 25, 26
The mean (SD) time taken for the screening was 3.5 (1.2) minutes, and the nursing staff were able to complete the pulse oximetry screening during their routine shifts with no extra time required to complete the screening. The mothers were counselled before and after the pulse oximetry screening.
Pulse oximetry screening for detection of CCHD among asymptomatic neonates showed a moderate sensitivity, high specificity and negative predictive value and a reasonably low false positive rate similar to the reported test accuracy across most of the studies that were part of the recent meta-analysis by Plana et al.18 and in the previous meta-analysis by Thangaratinam et al.7
The prevalence of CCHD in our study was 3.63 per 1000 live births. This is similar to many of the previous studies from India.1,27, 28, 29 This may, however, not represent the true prevalence in this diverse, multiethnic population seeking care at a tertiary referral hospital. The reference standard for a positive pulse oximetry screen was 2D echocardiography, and for a negative screen, it was clinical follow-up at 6-, 10- and 14-week immunization visits and analysis of the hospital admission and discharge register for the duration of the study for any readmissions of these neonates.
The false positive rate of pulse oximetry screening in our study was 0.81% (95% CI: 0.56%–0.94%). This is at variance from the rate of 0.14% reported by the recent meta-analysis of 19 studies with 436,758 neonates. This was despite screening after 24 h of life, which was shown by Plana et al.18 to decrease the false positive rate by tenfold. This may be due to the higher incidence of early onset sepsis/congenital pneumonia, PPHN and transitional circulation observed in our study. These were in the true sense not ʽasymptomaticʼ neonates (they had varying symptoms of silent tachypnoea, poor feeding, feed intolerance, and temperature instability, which came to attention at the time of the pulse oximetry screening). They were considered false positive as bedside 2D echo ruled out any CCHD. These findings are similar to results reported in literature and may be seen as the secondary targets of pulse oximetry screening and are equally important from the management perspective.30
The strengths of our study include a prospective study of healthy neonates using the Masimo motion-tolerant pulse oximeter with SET and ability to read the pulse waveform in low perfusion states. The standard algorithm for detection as endorsed by AAP and AHA was used and screening was carried out between 24 and 48 h after birth as recommended. The reference standard for positive pulse oximetry screen results was a 2D echocardiography performed by an experienced cardiologist, and for negative screen results, it was clinical follow-up at 6-, 10- and 14-week visits for immunization and an analysis of the hospital admission and discharge register.
The limitations of our study include a relatively small sample size in comparison to other larger studies. This was, however, due to our inclusion criteria of intramural neonates. The pulse oximetry screening was missed in 380 neonates over the study period due to oversight by the nursing staff in the postnatal ward (busy with other clinical duties, new staff who were unaware of the screening protocol and register, forgetfulness) and non-availability of the Masimo pulse oximeters (being used inside the busy NICU for monitoring of sick neonates). These neonates were however comparable in their baseline characteristics to the neonates who were screened, thus reducing the chances of any sampling/selection bias.
Conclusion
Pulse oximetry screening among healthy, asymptomatic neonates using a Masimo pulse oximeter between 24 and 48 h of life was feasible, easy to perform, acceptable to care providers and parents.
Combined with a thorough clinical examination (including palpation of all peripheral pulses), pulse oximetry screening before discharge with moderate sensitivity, high specificity and negative predictive value and a reasonably low false positive rate can help improve detection of CCHD.
Disclosure of competing interest
The authors have none to declare.
Acknowledgements
(a) This article is based on Armed Forces Medical Research Committee Project No 4804/2016, granted and funded by the Office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
(b) The authors are grateful to the administration and staff of Command Hospital (Western Command), Chandimandir for their support during conduct of this study.
References
- 1.Saxena A., Mehta A., Sharma M. Birth prevalence of congenital heart disease: a cross-sectional observational study from North India. Ann Pediatr Cardiol. 2016;9(3):205–209. doi: 10.4103/0974-2069.189122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxena A. Congenital heart disease in India: a status report. Indian Pediatr. 2018;55(12):1075–1082. [PubMed] [Google Scholar]
- 3.Saxena A. Pediatric cardiac care in India: current status and the way forward. Future Cardiol. 2018;14(1):1–4. doi: 10.2217/fca-2017-0084. [DOI] [PubMed] [Google Scholar]
- 4.Wren C., Reinhardt Z., Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F33–F35. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 5.Mahle W.T., Newburger J.W., Matherne G.P. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics. 2009;124(2):823–836. doi: 10.1542/peds.2009-1397. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q.M., Ma X.J., Ge X.L. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014;384(9945):747–754. doi: 10.1016/S0140-6736(14)60198-7. [DOI] [PubMed] [Google Scholar]
- 7.Thangaratinam S., Brown K., Zamora J., Khan K.S., Ewer A.K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379(9835):2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 8.Chew C., Halliday J.L., Riley M.M., Penny D.J. Population-based study of antenatal detection of congenital heart disease by ultrasound examination. Ultrasound Obstet Gynecol. 2007;29(6):619–624. doi: 10.1002/uog.4023. [DOI] [PubMed] [Google Scholar]
- 9.Westin M., Saltvedt S., Bergman G. Routine ultrasound examination at 12 or 18 gestational weeks for prenatal detection of major congenital heart malformations? A randomised controlled trial comprising 36,299 fetuses. BJOG. 2006;113(6):675–682. doi: 10.1111/j.1471-0528.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- 10.Tegnander E., Williams W., Johansen O.J., Blaas H.G., Eik-Nes S.H. Prenatal detection of heart defects in a non-selected population of 30,149 fetuses--detection rates and outcome. Ultrasound Obstet Gynecol. 2006;27(3):252–265. doi: 10.1002/uog.2710. [DOI] [PubMed] [Google Scholar]
- 11.Meberg A., Andreassen A., Brunvand L. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr. 2009;98(4):682–686. doi: 10.1111/j.1651-2227.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 12.Hokanson J.S. Pulse oximetry is beneficial in screening newborns for critical congenital heart disease. J Pediatr. 2012;160(3):529. doi: 10.1016/j.jpeds.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Fixler D.E., Xu P., Nembhard W.N., Ethen M.K., Canfield M.A. Age at referral and mortality from critical congenital heart disease. Pediatrics. 2014;134(1):e98–e105. doi: 10.1542/peds.2013-2895. [DOI] [PubMed] [Google Scholar]
- 14.Peterson C., Ailes E., Riehle-Colarusso T. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168(4):361–370. doi: 10.1001/jamapediatrics.2013.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang R.K., Gurvitz M., Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 2008;162(10):969–974. doi: 10.1001/archpedi.162.10.969. [DOI] [PubMed] [Google Scholar]
- 16.Lees M.H. Cyanosis of the newborn infant. Recognition and clinical evaluation. J Pediatr. 1970;77(3):484–498. doi: 10.1016/s0022-3476(70)80024-5. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell C.P., Kamlin C.O., Davis P.G., Carlin J.B., Morley C.J. Clinical assessment of infant colour at delivery. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F465–F467. doi: 10.1136/adc.2007.120634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plana M.N., Zamora J., Suresh G., Fernandez-Pineda L., Thangaratinam S., Ewer A.K. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst Rev. 2018;3(3) doi: 10.1002/14651858.CD011912.pub2. CD011912. Published 2018 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong K.K., Fournier A., Fruitman D.S. Canadian cardiovascular society/Canadian pediatric Cardiology association position statement on pulse oximetry screening in newborns to enhance detection of critical congenital heart disease. Can J Cardiol. 2017;33(2):199–208. doi: 10.1016/j.cjca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 20.de-Wahl Granelli A., Meberg A., Ojala T., Steensberg J., Oskarsson G., Mellander M. Nordic pulse oximetry screening--implementation status and proposal for uniform guidelines. Acta Paediatr. 2014;103(11):1136–1142. doi: 10.1111/apa.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R.K. Congenital heart disease profile: four perspectives. Ann Pediatr Cardiol. 2016;9(3):203–204. doi: 10.4103/0974-2069.189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R.K. Screening for congenital heart disease in India: rationale, practical challenges, and pragmatic strategies. Ann Pediatr Cardiol. 2016;9(2):111–114. doi: 10.4103/0974-2069.181499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewer A.K., Furmston A.T., Middleton L.J. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess. 2012;16(2):v–v184. doi: 10.3310/hta16020. [DOI] [PubMed] [Google Scholar]
- 24.Riede F.T., Wörner C., Dähnert I., Möckel A., Kostelka M., Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine--results from a prospective multicenter study. Eur J Pediatr. 2010;169(8):975–981. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meberg A., Brügmann-Pieper S., Due R., Jr. First day of life pulse oximetry screening to detect congenital heart defects. J Pediatr. 2008;152(6):761–765. doi: 10.1016/j.jpeds.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 26.Oakley J.L., Soni N.B., Wilson D., Sen S. Effectiveness of pulse-oximetry in addition to routine neonatal examination in detection of congenital heart disease in asymptomatic newborns. J Matern Fetal Neonatal Med. 2015;28(14):1736–1739. doi: 10.3109/14767058.2014.967674. [DOI] [PubMed] [Google Scholar]
- 27.Dixit R., Rai S.K., Yadav A.K. Epidemiology of congenital heart disease in India. Congenit Heart Dis. 2015;10(5):437–446. doi: 10.1111/chd.12220. [DOI] [PubMed] [Google Scholar]
- 28.Thakur J.S., Negi P.C., Ahluwalia S.K., Sharma R., Bhardwaj R. Congenital heart disease among school children in Shimla hills. Indian Heart J. 1995;47(3):232–235. [PubMed] [Google Scholar]
- 29.Bhat N.K., Dhar M., Kumar R., Patel A., Rawat A., Kalra B.P. Prevalence and pattern of congenital heart disease in Uttarakhand, India. Indian J Pediatr. 2013;80(4):281–285. doi: 10.1007/s12098-012-0738-4. [DOI] [PubMed] [Google Scholar]
- 30.Mahle W.T., Newburger J.W., Matherne G.P. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]