Abstract
Background
Endometrial cancer (EC) is treated by comprehensive surgical staging that includes a systematic lymphadenectomy. The low rates of lymph node metastasis (LNM) in early stages question the benefit of routine lymphadenectomy in low-risk disease, but the absence of a reliable method to identify these patients in whom lymphadenectomy could be omitted makes complete staging the standard of care. This study evaluated a method of preoperative staging in EC to identify patients at low risk of LNM and adjuvant treatment.
Methods
This prospective observational study compared the presurgical staging and risk triage based on endometrial biopsy (EB) and imaging (magnetic resonance imaging [MRI], Positron Emission Tomography [PET] scan) in 94 cases of EC with the final surgicopathological staging and evaluated the role of each modality in presurgical evaluation and triage.
Results
Ninety-four cases were triaged into 42 low-risk and 52 non–low-risk cases preoperatively. EB showed a sensitivity, specificity, and accuracy of 51.55%, 89.83%, and 75.53%, respectively, in identifying high-risk grade and histology. MRI was effective for local staging and identified tumor size, myometrial invasion, and cervical involvement with accuracy ranging from 82.20% to 97.78% for these parameters. MRI detected LNM with an accuracy of 85.11%, whereas PET exhibited an accuracy of 86.17%. The combined presurgical staging could identify low-risk disease with a sensitivity, specificity, and accuracy of 85.37%, 86.79%, and 86.17%, respectively.
Conclusion
Preoperative staging may triage patients into low-risk and non–low-risk cases, thereby facilitating a conscious decision to omit lymphadenectomy in low-risk cases, thus avoiding unnecessary morbidity without compromising oncological safety.
Keywords: Endometrial carcinoma, Surgical staging, Systematic lymphadenectomy, Lymph nodal metastasis, Presurgical triage
Introduction
Endometrial cancer (EC) is the 7th most frequent cause of cancer in women worldwide and comprises two distinct histological types: type I/low-risk disease (80%) with good prognosis and type II/high-risk disease (20%) that behaves aggressively and portends poor prognosis.1 EC is a surgically staged disease, and the final surgicopathological staging prognosticates the disease and individualizes adjuvant therapy where indicated. Hence, a preoperative metastatic workup is not routinely considered except in poor surgical risks.2
Although current International Federation of Gynecology and Obstetrics (FIGO) guidelines advocate a systematic lymphadenectomy to identify those with positive nodes with a 20% overall node positivity rate, evidence favors a lymphadenectomy only in the high-risk group because nodal disease in low-risk cases is rare. However, despite the morbidity being associated with lymphadenectomy, surgical staging continues to be the standard of care because 20% of presumed low-risk disease gets upstaged after a complete staging, thus warranting adjuvant therapy to prevent recurrence.3,4 A reliable preoperative triage algorithm to identify patients with low risk of lymph node metastasis (LNM) and recurrence in whom lymphadenectomy could be omitted without risk of undertreatment could potentially avoid considerable morbidity in this group.5 This led to trials of various risk stratification models based on preoperative biopsy and imaging (Gynecological Oncology Group (GOG) 33, European Society of Medical Oncology (ESMO)-European Society of Radiotherapy & Oncology (ESTRO)-European Society of Gynecological Oncolog (ESGO) guidelines, and Korean Gynecologic oncology group [KGOG] model) and “Mayo protocol” using intraoperative examination (IOE) and frozen section (FS) to triage patients based on the risk of LNM and recurrence3,6, 7, 8 (Table 1). All these models have been validated in various studies and currently being used in original or modified forms at many centers.9
Table 1.
Risk stratification models for endometrial cancers.
| ESMO-ESGO-ESTRO consensus guidelines (Colombo et al)6 | ||||
|---|---|---|---|---|
| Method of evaluation | High-risk factors | Low-risk factors | Non–low-risk factors | Purpose of triage |
| EB, preoperative MRI |
EB: Non-endometrioid histology, FIGO G3, LVSI Imaging: MI >50%, cervical involvement |
Stage I, endometrioid, G1–2 LVSI –ve, <50% MI |
Anything more than low risk |
Omitting lymphadenectomy and adjuvant therapy |
|
Korean Gynecologic oncology group (KGOG) guidelines (Kang et al)8 | ||||
| EB, serum Ca 125, preoperative MRI |
Non-endometrioid histology, Ca 125 > 35 mIU/mL, MI, high-volume index of tumor, extrauterine disease |
Stage I, endometrioid, Ca 125 < 35, low-volume index |
Anything more than low risk |
Selective lymphadenectomy |
|
Mayo Clinic protocol (Mariani et al)3 | ||||
| Intraoperative examination (IOE), peritoneal cytology, frozen section (FS) |
Non-endometrioid histology, grade, MI, cervical involvement, extrauterine disease |
Endometrioid histology, G1–2, stage I, MI <50%, PTD <2 cm OR endometrioid, stage I, no MI (irrespective of grade/PTD) |
Anything more than low risk |
Omission of lymphadenectomy |
|
Modified Mayo Clinic criteria (used in this study) | ||||
|
Endometrial biopsy: Histology grade Imaging: Preoperative MRI and 18F-FDG PET–CT scan |
Non-endometrioid histology, G3, MI >50%, tumor diameter (TD) >2 cm, cervical involvement, LN disease, extrauterine disease | Endometrioid histology, G1–2, stage I, TD< 2 cm, MI <50%, no cervical or extrauterine spread, no LN disease | Anything more than low risk | Presurgical triage into low-risk and non–low-risk groups |
EB, endometrial biopsy; MRI, magnetic resonance imaging; MI, myometrial invasion; LN, lymph node.; ESMO, European Society of Medical Oncology; ESGO, European society of Gynecological Oncology; ESTRO, European Society of Radiotherapy and Oncology; FIGO, International Federation of Gynecology and Obstetrics; LVSI, Lymph Vascular Space Invasion; PTD, Primary Tumor Diameter; 18 F FDG - PET-CT , 18 F Fluorodeoxyglucose Positron Emission Tomography - Computerised Tomography.
Our prospective observational study used a modification of Mayo protocol for presurgical staging and risk stratification using findings of preoperative biopsy and imaging to identify patients at low risk of LNM and recurrence, hence unlikely to require lymphadenectomy and adjuvant treatment. This would enable triage before surgery and thus help in preoperative counseling and better planning of surgery.
Materials and methods
The aim of this prospective observational study was to assess the feasibility of presurgical staging to triage EC into low-risk and non–low-risk groups (to collectively include all intermediate- and high-risk EC in this study) for LNM and recurrence based on modified Mayo criteria based on endometrial biopsy (EB) and imaging (Table 1). The risk factors included were non-endometrioid histology, FIGO grade 3 tumor (G3), tumor diameter (TD) >2 cm, myometrial invasion (MI) >50%, cervical involvement, LNM, and disease outside the pelvis. The patients were triaged into the low-risk group in the absence of risk factors and the non–low-risk group when risk factors were present. The final histopathology report (HPR) was taken as the gold standard and compared with the presurgical stage and risk category. The study was ratified by the tumor board, and ethical clearance was obtained from the institutional ethical committee. All cases of EC—endometrioid and non-endometrioid (papillary serous, adenosquamous, and clear cell)—diagnosed or referred for treatment and planned for surgical staging were eligible for the study. Patients who consented were enrolled after counseling and obtaining written informed consent. Patients with uterine sarcoma, those with cervical cancers (endocervical) on biopsy, and those unfit for surgical treatment were excluded from the study.
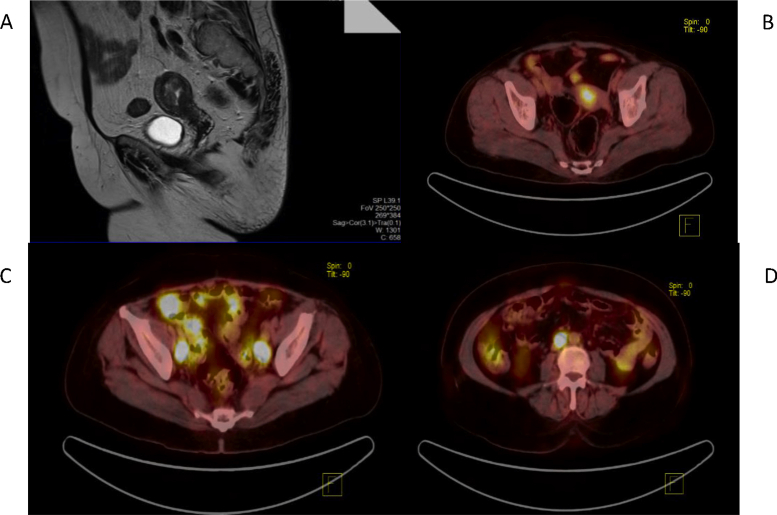
The diagnosis of EC was made via office EB, Dilatation and Curettage (D&C), or hysteroscopic biopsy, which was reported for histology and grade. The presurgical workup included a clinical examination; conventional magnetic resonance imaging (MRI) of the abdomen and pelvis (T1-weighted and T2-weighted images with intravenous contrast) for tumor size (TS), depth of MI, cervical involvement, LNM, and extrauterine disease (Fig. 1A); and a 18 F Fluoro-deoxyglucose PET-CT Scan : Positron Emission Tomography - Computerised Tomograhy (18F-FDG PET–CT) Scan from the skull base to mid-thigh for nodal and extrauterine metastasis (Fig. 1B, C, D). A presurgical stage was assigned, and the patient was triaged into the low-risk/non–low-risk category as per the study criteria.
Fig. 1.
(A) MRI showing a 20 × 25 × 25mm mass lesion in the endometrial cavity arising posterosuperiorly in the region of the fundus violating the junctional zone and infiltrating >50% of the myometrium. (B) PET–CT scan showing FDG avid uterine mass. (C) PET–CT scan showing FDG avid pelvic lymph nodes. (D) PET–CT scan showing FDG avid para-aortic LN. CT, Computerised tomography; FDG, Fluorodeoxyglucose; MRI, magnetic resonance imaging; LN, lymph node; PET: positron emission tomography.
All patients thereafter underwent a complete surgical staging through a midline laparotomy (peritoneal cytology, type I radical hysterectomy + bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy, and omentectomy/omental biopsy), and the specimen was grossed and processed by standard techniques.10 Final surgicopathological staging and risk category were reassigned based on the HPR as the gold standard. The presurgical risk factors studied were individually compared with the final HPR, and the combined presurgical risk assigned was compared with the final risk category and statistically analyzed using IBM SPSS version 25.
Results
One hundred four patients who fulfilled the criteria were enrolled in the study with a diagnosis of EC on EB. Two patients who were unfit for surgery were excluded. Six patients were excluded from the study after the final HPR—two with synchronous tumors of the endometrium and ovary, one with endocervical adenocarcinoma, and 5 with sarcoma of the uterus. Data were finally available for 94 patients, whose characteristics are tabulated in (Table 2).
Table 2.
Characteristics of the study population (n = 94).
| Age, median (range), years | 63 (42–79) |
| Parity, median (range) yrs | 2 (0–6) |
| Comorbidities | |
| Diabetes | 24 |
| Hypertension | 14 |
| Obesity (BMI >30) | 06 |
| Hypothyroidism | 03 |
| IHD on drug-eluting stent | 01 |
| Multiple myeloma | 01 |
| Carcinoma breast on tamoxifen | 03 |
| Symptomatology | |
| Postmenopausal bleeding | 76 |
| Postmenopausal discharge PV | 04 |
| Carcinoma breast post treatment follow up with abnormal ET | 02 |
| Heavy menstrual bleeding | 06 |
| Irregular bleeding | 02 |
| Abdominal distension | 01 |
| Abnormal cells on PAP | 01 |
| Method of biopsy | |
| Pipelle biopsy | 22 |
| OPD EB | 54 |
| Hysteroscopy + biopsy | 14 |
| D&C/FC | 04 |
EB, endometrial biopsy; BMI, body mass index. IHD, Ischemic Heart disease; PV, Per vaginum; ET, Endometrial Thickness; PAP, Papanicoloau Smear; OPD, Out Patient Department; D&C, Dilatation & Curettage; FC, Fractional Curettage.
Endometrial biopsy
EB was compared with the final HPR for concordance of histology and grade. Of the 94 cases, 76 (80.85%) were correctly reported for histotype and 74 (78.72%) were correctly reported for grade. Among the 70 cases diagnosed as G1/2, 56 (80%) were correctly reported or did not show any residual tumor (4 cases) on the final HPR, whereas 14 were upgraded to a higher grade. EB thus showed a sensitivity of 54.55%, specificity of 97.59%, and accuracy of 92.55% in identifying histotype and a sensitivity of 56.25%, specificity of 90.72%, and accuracy of 78.72% in identifying high-grade (G3) disease, with an overall accuracy of 75.53% (Table 3).
Table 3.
Surgicopathological characteristics (n = 94).
| Characteristics | Presurgical EB/imaging | Final HPR | Correctly reported presurgical | Percentage |
|---|---|---|---|---|
| Histology | ||||
| Endometrioid adenocarcinoma | 82 | 76 | 68/76 | |
| Adenosquamous carcinoma | 04 | 03 | 02/03 | |
| Papillary serous carcinoma | 04 | 08 | 03/08 | |
| Clear cell carcinoma | 04 | 03 | 03/03 | |
| Endometrial hyperplasia | 00 | 02 | 0/02 | |
| Proliferative endometrium | 00 | 02 | 0/02 | |
| (no malignancy/hyperplasia) | ||||
| Total | 76/94 | 80.85% | ||
| Tumor grade | ||||
| High grade | 24 | 32 | 18/32 | 56.25% |
| Grade1/2 endometrioid | 70 | 62 | 56/62 | 90.32% |
| Total | 74/94 | 78.72% | ||
| Cervical extension (stroma) | 04 | 06 | 4/6 | 66.66% |
| Tumor diameter on MRI | ||||
| <2 cm | 38 | 46 | 36/46 | 78.2% |
| >2 cm | 56 | 48 | 44/48 | 91.66% |
| Myometrial invasion | ||||
| <50% | 42 | 38 | 34/42 | 80.90 |
| >50% | 52 | 56 | 48/56 | 85.71 |
| Nodal metastasis (MRI+PET) | 18 | 22 | 12/22 | 55.54 |
| Pelvic only | 20 | 16 | – | |
| Para-aortic only | 2 | 2 | – | |
| Pelvic and para-aortic | 6 | 4 | – | |
| Abdominal disease | 02 | 03 | 2/3 | 66.66 |
| Gross | 02 | 02 | 2/2 | 100 |
| Microscopic | 00 | 01 | 0/1 | 0 |
| Cytology positive | – | 05 | – | – |
| Staging | ||||
| Stage 1A | 24 | 32 | – | |
| Stage 1B | 34 | 29 | – | |
| Stage II | 4 | 4 | – | |
| Stage IIIA | 2 | 5 | – | |
| Stage IIIB | 2 | 3 | – | |
| Stage IIIC1 | 20 | 16 | – | |
| Stage IIIC2 | 6 | 4 | – | |
| Stage IV | 2 | 1 | – | |
EB, endometrial biopsy; HPR, final histopathology report; MRI, magnetic resonance imaging; PET: Positron emission tomography.
MRI and 18F-FDG PET–CT scan
MRI was used to study local factors and LNM and showed a sensitivity of 91.67% in identifying TS >2 cm, with a specificity of 73.91% and accuracy of 82.98%, and was also found to effectively recognize MI >50%, with an accuracy of 87.20% and negative predictive value (NPV) of 92.30% (see Table 4). MRI successfully detected 4 of 6 cases of cervical involvement, with 100% specificity and an accuracy of 97.78%. However, MRI could detect only 12 of the 22 cases of LNM (pelvic and para-aortic), with a low sensitivity (54.55%) but high specificity (94.44%); MRI failed to identify most metastatic nodes less than 10 mm in size (Table 5). PET successfully identified 16 of these 22 patients, with a better sensitivity and specificity of 72.72% and 97.22%, but missed out on metastatic nodes that were less than 5 mm in size (Table 6). PET also successfully identified the extrauterine disease in 2 of the 3 cases.
Table 4.
Endometrial biopsy for histology and grade.
|
Final HPR: FIGO grade |
Total |
Sensitivity: 56.25%, specificity: 90.72%, NPV: 80.00%, PPV: 75.00%, accuracy: 78.72% |
|||
| High-grade |
Low-risk (G1–2) |
||||
| Preoperative grade |
High-grade (G3) | 18 | 6 | 24 | |
| Low-grade (G1–2) | 14 | 56 | 70 | ||
| Total |
32 |
62 |
94 |
||
|
Final HPR: histology |
Total |
Sensitivity: 54.55%, specificity: 97.59%, NPV: 94.19%, PPV: 75.00%, accuracy: 92.55% |
|||
| High-risk | Low-risk | ||||
| Preoperative histology |
High-risk | 6 | 2 | 8 | |
| Low-risk | 5 | 81 | 86 | ||
| Total |
11 |
83 |
94 |
||
|
Final HPR |
Total |
Sensitivity: 51.43%, specificity: 89.83%, NPV: 75.71%, PPV: 75.00%, accuracy: 75.72% |
|||
| High-risk |
Low-risk |
||||
| Preoperative HPR |
High-risk | 18 | 6 | 24 | |
| Low-risk | 17 | 53 | 70 | ||
| Total | 35 | 59 | 94 | ||
FIGO, International federation of gynecology and obstetrics; HPR, final histopathology report; NPV, negative predictive value; PPV, positive predictive value.
Table 5.
MRI in presurgical evaluation.
| Tumor diameter (TD) | TD on the HPR | Total | Sensitivity: 91.67%, specificity: 73.91% NPV: 89.47% PPV: 78.57%, accuracy: 82.98% | |
| TD>2 cm | TD <2 cm | |||
| TD>2 cm on MRI | 44 | 12 | 56 | |
| TD<2 cm on MRI | 04 | 34 | 38 | |
| Total |
48 |
46 |
94 |
|
| Myometrial invasion (MI) | MI on the HPR | Total | Sensitivity: 85.71%, specificity: 89.47%, NPV: 92.30%, PPV: 40.42%, accuracy: 87.20% | |
| MI >50% | MI <50% | |||
| MI >50% on MRI | 48 | 04 | 52 | |
| MI <50% on MRI | 08 | 34 | 42 | |
| Total |
56 |
38 |
94 |
|
| Cervical disease | Cervical involvement on the HPR | Total | Sensitivity: 66.67%, specificity: 100%, NPV: 97.78%, PPV: 100%, accuracy: 97.87% | |
| + | – | |||
| Cx Inv on MRI, + | 4 | 0 | 04 | |
| Cx Inv on MRI, – | 2 | 88 | 90 | |
| Total |
06 |
88 |
94 |
|
| LN metastasis | LN metastasis on the HPR | Sensitivity: 54.55%, specificity: 94.44%, NPV: 87.18%, PPV: 75.00%, accuracy: 85.11% | ||
| + | – | |||
| LN on MRI, + | 12 | 04 | 16 | |
| LN on MRI, – | 10 | 68 | 78 | |
| Total | 22 | 72 | 94 | |
Cx Inv, Cervical involvement; MRI, magnetic resonance imaging; HPR, final histopathology report; LN, lymph node; NPV, negative predictive value; PPV, positive predictive value.
Table 6.
PET–CT scan in detection of nodal metastasis.
| Nodal metastasis on the HPR | Sensitivity: 85.37%, specificity: 86.79% NPV: 88.46% PPV: 83.33%, accuracy: 86.17% | ||||
| Nodal metastasis | No nodal metastasis | ||||
| PET scan | Node +ve on PET | 16 | 02 | 18 | |
| Node -ve on PET | 06 | 70 | 76 | ||
| Total | 22 | 72 | 94 | ||
CT, Computed tomography; HPR, final histopathology report; NPV, negative predictive value; PET, Positron emission tomography; PPV, positive predictive value.
Combined presurgical triage
All the 94 cases of EC underwent a presurgical risk triage based on the aforementioned biopsy and imaging criteria and were stratified into 42 low-risk and 52 non–low-risk cases. The results of combined presurgical risk assignment were compared with the final risk assigned after complete surgicopathological staging. Thirteen cases of 94 had to be reassigned the risk group after staging—7 of 42 in the low-risk group were reassigned to the non–low-risk group, and 6 of 52 in the non–low-risk group were reassigned to the low-risk group after complete staging. The combined presurgical triage thus showed a sensitivity of 85.37% and specificity of 86.79%, NPV of 88.46%, and positive predictive value (PPV) of 83.33%, with a reasonable accuracy of 86.17% (Table 7).
Table 7.
Combined presurgical triage (EB +MRI + imaging).
| Staging | Surgicopathologic staging | Total | Sensitivity: 85.37%, specificity: 86.79% NPV: 88.46% PPV: 83.33%, accuracy: 86.17% | ||
| Risk | Low-risk | High-risk | |||
| Presurgical staging | Low-risk | 35 | 07 | 42 | |
| High-risk | 06 | 46 | 52 | ||
| Total | 41 | 53 | 94 | ||
EB, endometrial biopsy; MRI, magnetic resonance imaging; NPV, negative predictive value; PPV, positive predictive value.
Discussion
The standard diagnostic evaluation for EC does not mandatorily include a metastatic workup because the standard treatment involves a comprehensive surgical staging to remove the disease, detect spread, and direct adjuvant treatment.2 Evidence suggests that a comprehensive surgical staging is justified only in the non–low-risk group owing to the high morbidity associated with lymphadenectomy with no definite survival benefit.4,5,11 However, owing to lack of reliable presurgical prediction models, identification of low-risk patients for omission of lymphadenectomy is complicated and not failsafe because about 20% of the presumed low-risk patients may get upstaged after surgical staging.12
Various risk stratification models such as GOG 33, ESMO-ESTRO-ESGO, KGOG, and Mayo criteria (Table 1) have been proposed to stratify disease into risk groups preoperatively.6,8 Tsikouras et al.9 retrospectively evaluated the ESMO-ESTRO-ESGO criteria for preoperative assessment and found a sensitivity and specificity of 96.1% and 73.6%, respectively, to discriminate low-risk and non–low-risk EC and concluded that 50% of patients with endomeroid EC can be excluded from a full staging procedure based on this preoperative triage. The KGOG model too underwent studies for external and internal validation and underwent a prospective study on a Japanese cohort wherein it could identify 5% of low-risk patients with a false NPV of 1.4%.13 Mayo Clinic devised an alternative strategy of intraoperative triage by examining the uterus after hysterectomy for TS and the FS to determine the grade and histology, depth of invasion, and cervical involvement.3 This algorithm has been prospectively validated by Lefringhouse et al.14 in 2017, and they concluded that an intraoperative triage can help in deciding the extent of surgery. However, Mayo Clinic protocol, being an intraoperative triage, had the inherent flaw of subjectivity, with decision-making based on inconclusive and often inaccurate FS reports.8 Korkmaz et al.15 in a comparative study on the various risk stratification models (GOG 33, ESMO, and Mayo Clinic models) found that ESMO-modified classification most accurately predicted lymph node (LN) involvement in early-stage EC and the Mayo-modified classification was an effective alternative for intraoperative triage, both with acceptable oncological safety.
This study used preoperative biopsy and imaging to finally triage patients into low-risk and non–low-risk groups. Preoperative EB was an important part of the study and a significant component of the triage. Studies have shown that risk of LNM is <10% in stage I grade 1 endometrioid EC, 18% in stage I G3 disease, and up to 40% in non-endometrioid disease.5,8,11 Preoperative biopsy was however only a modest predictor of the pathology and hence always underestimated the risk of recurrence when considered in isolation.16 Non-endometrioid histology had the highest accuracy in preoperative biopsies, and most of the non-concordant reports were in the endometrioid group.17 The grade shifts were also common in the latter group, that too mainly from G1 to G2 and vice versa and fortunately very rarely from G1 to G3, and hence did not significantly influence treatment decisions. The non-concordance was attributed to the volume of tissue available for examination, with the concordance being better with D&C than with pipelle biopsy.10 EB in our study showed an accuracy of 92.55% for histology and 78.72% for grade, with an overall accuracy of 75.53% in identifying non–low-risk patients. We feel the overall accuracy in our study was low mainly owing to a large number of samples being low-volume pipelle biopsies, wherein the amount of tissue was small. The histological subtype and grade of the tumor are important predictors of disease spread, nodal involvement, and final outcome but only when considered along with other uterine histopathological features such as TS, MI, cervical disease, and Lymph vascular space invasion (LVSI), which are usually available only after surgical staging. Alternatively, this information could be obtained from a good preoperative imaging as was carried out in this study.3,7
Preoperative MRI with a paramagnetic contrast is the imaging of choice for locoregional staging in EC because tumor issue, endometrium, myometrium, and cervix show different MR signals especially on T2-weighted images and can evaluate TS, MI, and cervical and parametrial extension. It can establish the origin of a tumor (endometrial or endocervical) and confirm early-stage disease by excluding MI, LNM, and adnexal disease.6,18 MRI in this study was used for measuring TS, measuring the depth of MI, detection of cervical extension, detection of parametrial invasion, and detection of LNM. TS has been considered an important risk factor for MI and LNM, both being indicators of poor prognosis and requiring adjuvant treatment.19 Canlorbe et al.20 in a French multicentric study found TS to be an independent prognostic factor of LNM in women with low-risk EC and was valuable in identifying women with increased risk of LNM and risk of recurrence to better adapt surgical staging. The depth of MI is the single most important risk factor for LNM even in G3 tumors and can be measured accurately by MRI. Deep MI (DMI) is a >50% ratio between maximum tumor depth and total myometrial thickness, is a high risk factor for LNM, and indicates requirement of adjuvant Radio Therapy (RT).21 MRI for assessment of depth of MI has shown a sensitivity and specificity of 69–94% and 64–100%, respectively, in various studies.22 MRI showed a sensitivity of 85.71%, a specificity of 89.47%, and an accuracy of 87.20% for detection of MI in our study too. Cervical extension of endometrial disease presents a 2-fold risk of LNM and parametrial metastasis, warranting a complete surgical staging, and can be accurately identified by MRI.2,7,8 Various studies have demonstrated a sensitivity and specificity up to 100% and accuracy up to 98% for Contrast Enhanced Magnetic Resonance Imaging (CEMRI) in diagnosing cervical invasion,21 which is in agreement with the findings of our study. MRI also helps in the detection of extrauterine pelvic disease in the parametrium and in the adnexa and early abdominal and omental disease although inferior to PET–CT scan.23 LNM in EC is the commonest site of extrapelvic metastasis and is a strong predictor of recurrence, thus warranting adjuvant treatment. MRI although commonly used for metastatic node detection has low sensitivity and specificity, especially to detect nodes smaller than 10 mm. However, MRI can effectively identify three surrogate markers of LNM—TD >2 cm, DMI, and cervical involvement, thus helping in triage.24 Our study showed a low sensitivity of 54.55% for MRI in the detection of nodes although the specificity was 94.44%, with a reasonable accuracy of 85.11%, missing out on nodes that were <10 mm in size.
The 18F-FDG PET-CT scan provides functional imaging of metastasis and hence was thought to be superior to MRI in metastatic evaluation. Recently, a meta-analysis reported that the overall pooled sensitivity, specificity, and accuracy of using PET–CT for detection of LNM in EC were 72.0%, 94.0%, and 88.0%, respectively.24,25 The sensitivity of PET–CT in our study was 72.72% for metastatic node detection, with a specificity of 97.22% and accuracy of 91.48%. PET–CT presently is an excellent modality for detection of extrauterine disease and recurrences, but its role in detection of nodal metastasis is nodal size dependent and is possibly not good enough to replace lymphadenectomy.25,26 PET–CT hence cannot be an alternative to a thorough preoperative risk assessment and intraoperative evaluation in deciding the extent of surgery. Hence, the routine use of PET in the evaluation and staging of EC is routinely not recommended in view of the low sensitivity especially for sub-5-mm nodes, high cost, and limited availability.26
The sensitivity and specificity of the combined presurgical triage to differentiate low-risk and non–low-risk patients for LNM as per the study were 85.37% and 86.79%, respectively, with an overall accuracy of 86.17%. The study by Korkmaz et al,15 which is a prospective evaluation of ESMO criteria, showed a sensitivity and specificity of 91.4% and 63.9%, respectively, with an NPV of 98.3% and PPV of 24.2%, and avoided lymphadenectomy in 57% patients but with risk of missing out on 8.6% positive nodes. Sala et al27 compared the GOG 33 with Mayo criteria and found that the sensitivity, specificity, PPV, and NPV for the GOG-33 were 92%, 94%, 92%, and 93%, whereas with the Mayo algorithm, these were 98%, 91%, 77%, and 99%, respectively. However, both studies were dependent on IOE and the high level of pathological expertise in a high-volume oncocenter. This study used a modification of the Mayo criteria without the use of FS and IOE and obtained the staging information from preoperative biopsy and imaging. This modification was carried out for three reasons—(1) the modification permitted a presurgical staging based on the FIGO 2009, (2) the identification of the non–low-risk group was possible before surgery, thus enabling preoperative counseling of the patient and planning of surgery well in advance—something not possible by intraoperative triage in Mayo protocol, and (3) the triage was not based on the subjectivity of IOE and FS.
Limitations
The limitations of our study were as follows: Histo-pathological examination (HPE) and MRI being reported by different pathologists and radiologists although with adequate experience in oncology, the non-availability of LVSI for all small biopsies, and routine FS examination not being performed for all cases of EC. The accuracy of this triage could be improved by incorporation of the other variables described earlier—LVSI, Ca-125, and routine IOE and FS during surgery for confirmation of histology, MI, and cervical disease. This protocol would need further validation after suitable modification in a larger study before it can be considered for clinical application. This method of triage would be extremely useful for presurgical decision-making in non-oncological centers, where advanced infrastructure and surgical expertise may not be available, thus facilitating timely transfer of high-risk cases to an appropriate higher center for optimal management.
The intraoperative Sentinel Lymph Node (SLN) algorithm is a less morbid alternative for targeted removal of LNs to avoid the morbidity of lymphadenectomy in centers where the infrastructure and facilities are available.28 Based on experience gained from melanomas and malignancies of the breast and vulva, SLN mapping has now been extensively studied in EC. Use of colorimetric mapping using blue dye, nuclear imaging using radiocolloid TC99, and near-infrared imaging using Indo Cyanine Green (ICG) for SLN mapping incorporated into a SLN algorithm coupled with pathological ultrastaging appears to be a promising alternative to lymphadenectomy in detection of LNM.28 Although SLN mapping now figures in the consensus recommendation of Society of Gynecological Oncology (SGO) as an alternative to lymphadenectomy in EC, studies are yet to provide a robust level I evidence with regard to long-term survival outcomes so that they can be made the standard of care.29
Conclusion
Preoperative staging using EB and imaging may triage patients into the low-risk and non–low-risk groups to portend the chance of LNM and requirement of adjuvant therapy. An effective preoperative triage has the potential to identify cases at high risk of LNM, thus ensuring their optimal management and facilitating a conscious decision to omit or modify lymphadenectomy in low-risk cases, thereby reducing surgical morbidity without compromising oncological safety.
Acknowledgements
This article is based on Armed Forces Medical Reaserch Committee Project No 4331/2012 granted and funded by office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
Disclosure of competing interest
The authors have none to declare.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Amant F., Mirza M.R., Koskas M., Creutzberg C.L. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2018;143(suppl 2):37–50. doi: 10.1002/ijgo.12612. [DOI] [PubMed] [Google Scholar]
- 3.Mariani A., Dowdy S.C., Cliby W.A. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109(1):11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creasman W.T., Morrow C.P., Bundy B.N., Homesley H.D., Graham J.E., Heller P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(suppl 8):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035:aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti Panici P., Basile S., Maneschi F. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 6.Colombo N., Creutzberg C., Amant F. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2017 Jul 1;28(suppl_4):iv167-iv168] Ann Oncol. 2016;27(1):16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- 7.Dowdy S.C., Borah B.J., Bakkum-Gamez J.N. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127(1):5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Kang S., Kang W.D., Chung H.H. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean gynecologic oncology group study. J Clin Oncol. 2012;30(12):1329–1334. doi: 10.1200/JCO.2011.38.2416. [DOI] [PubMed] [Google Scholar]
- 9.Tsikouras P., Koukouli Z., Bothou A. Preoperative assessment in endometrial cancer. Is triage for lymphadenectomy possible? J BUON. 2017;22(1):34–43. [PubMed] [Google Scholar]
- 10.Malpicia A., Euscher D.E., Hecht J.L. Endometrial carcinoma grossing and processing issues: recommendations of the international society of gynecological pathologists. Int J Gynecol Pathol. 2019;38(suppl 1):S9–S24. doi: 10.1097/PGP.0000000000000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ASTEC study group. Kitchener H., Swart A.M., Qian Q., Amos C., Parmar M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136. doi: 10.1016/S0140-6736(08)61766-3. [published correction appears in Lancet. 2009 May 23;373(9677):1764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todo Y., Kato H., Kaneuchi M., Watari H., Takeda M., Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375(9721):1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [published correction appears in Lancet. 2010 Aug 21;376(9741):594] [DOI] [PubMed] [Google Scholar]
- 13.Kang S., Todo Y., Odagiri T. A low-risk group for lymph node metastasis is accurately identified by Korean gynecologic oncology group criteria in two Japanese cohorts with endometrial cancer. Gynecol Oncol. 2013;129:33–37. doi: 10.1016/j.ygyno.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Lefringhouse J.R., Elder J.W., Baldwin L.A. Prospective validation of an intraoperative algorithm to guide surgical staging in early endometrial cancer. Gynecol Oncol. 2017;145(1):50–54. doi: 10.1016/j.ygyno.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Korkmaz V., Meydanli M.M., Yalçın I. Comparison of three different risk-stratification models for predicting lymph node involvement in endometrioid endometrial cancer clinically confined to the uterus. J Gynecol Oncol. 2017;28(6):e78. doi: 10.3802/jgo.2017.28.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koskas M., Bassot K., Graesslin O. Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol Oncol. 2013;129(2):292–297. doi: 10.1016/j.ygyno.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Helpman L., Kupets R., Covens A. Assessment of endometrial sampling as a predictor of final surgical pathology in endometrial cancer. Br J Cancer. 2014;110(3):609–615. doi: 10.1038/bjc.2013.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch G.M., Kaur H., Choi H. Optimization of MR imaging for pretreatment evaluation of patients with endometrial and cervical cancer. Radiographics. 2014;34(4):1082–1098. doi: 10.1148/rg.344140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schink J.C., Rademaker A.W., Miller D.S., Lurain J.R. Tumor size in endometrial cancer. Cancer. 1991;67(11):2791–2794. doi: 10.1002/1097-0142(19910601)67:11<2791::aid-cncr2820671113>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Canlorbe G., Bendifallah S., Laas E. Tumor size, an additional prognostic factor to include in low-risk endometrial cancer: results of a French multicenter study. Ann Surg Oncol. 2016;23(1):171–177. doi: 10.1245/s10434-015-4583-3. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo S., Femia M., Buscarino V. Endometrial cancer: an overview of novelties in treatment and related imaging keypoints for local staging. Canc Imag. 2018 Dec 4;18(1):45. doi: 10.1186/s40644-018-0180-6. PMID: 30514387; PMCID: PMC6280395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S.K., Niu X.K., Wang J.L. Usefulness of DWI in preoperative assessment of deep myometrial invasion in patients with endometrial carcinoma: a systematic review and meta-analysis. Canc Imag. 2014;14:32. doi: 10.1186/s40644-014-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith B.Q., Boone J.D., Thomas E.D. The reliability of intraoperative assessment on predicting tumour size, myometrial invasion, and cervical involvement in patients with a preoperative diagnosis of complex atypical hyperplasia or (clinical stage I) endometrial cancer: a prospective cohort study. Am J Clin Oncol. 2020;43(2):122–127. doi: 10.1097/COC.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin M.Y., Dobrotwir A., McNally O., Abu-Rustum N.R., Narayan K. Role of imaging in the routine management of endometrial cancer. Int J Gynaecol Obstet. 2018;143(suppl 2):109–117. doi: 10.1002/ijgo.12618. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollineni V.R., Ytre-Hauge S., Bollineni-Balabay O., Salvesen H.B., Haldorsen I.S. High diagnostic value of 18F-FDG PET/CT in endometrial cancer: systematic review and meta-analysis of the literature. J Nucl Med. 2016;57:879–885. doi: 10.2967/jnumed.115.170597. [DOI] [PubMed] [Google Scholar]
- 26.Konuralp Atakul B., Taşkın S., Soydal C. Preoperative 18F-fluorodeoxyglucose positron emission tomography/CT in prediction of uterine risk factors and lymph node metastasis: an analysis of 111 endometroid endometrial cancer patients. Gynecol Obstet Invest. 2017;82:340–348. doi: 10.1159/000452100. [DOI] [PubMed] [Google Scholar]
- 27.Sala P., Morotti M., Menada M.V. Intraoperative frozen section risk assessment accurately tailors the surgical staging in patients affected by early-stage endometrial cancer. Int J Gynecol Cancer. 2014;24:1021–1026. doi: 10.1097/IGC.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 28.Holloway R.W., Abu-Rustum N.R., Backes F.J. Sentinel lymph node mapping and staging in endometrial cancer: a Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol. 2017;146(2):405–415. doi: 10.1016/j.ygyno.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darai E., Dubernard G., Bats A.S. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136:54–59. doi: 10.1016/j.ygyno.2014.09.011. 25450151. [DOI] [PubMed] [Google Scholar]