Abstract
Background
Childhood immunization against hepatitis B is one of the most effective strategies for reducing the global burden of chronic hepatitis B infection and its sequelae. There are limited data from India on both the anti-Hep B antibody titres in children after vaccination and the age-related decline in the titres. This study was planned to estimate the proportion of children in the age group of 1–10 years who develop protective levels of anti-hepatitis B antibodies after childhood vaccination and to examine the change in antibody titres with age in these children.
Methods
A hospital-based cross-sectional study was carried out in children admitted to the hospital for various ailments. Basic demographic data, vaccination history and HBsAg status of the mother were recorded. All the enrolled children were evaluated for HBsAg and anti hepatitis B surface antibody (anti-HBS) titres. Institutional ethical clearance was obtained, and informed consent from the parents of the children was taken before drawing samples.
Results
We found that 68.86% Confidence Interval ((CI): 59.8–76.8%) of the children showed protective antibody titres after vaccination, while 31.14% (CI: 23.1–40.2%) of the children had titres less than 10 IU/L. Although 100% of children in the age group from birth to three years had titres more than 10 IU/L, this percentage showed a decline across the age groups, and 60% of children aged 9–10 years had titres less than 10 IU/L.
Conclusion
Childhood vaccination against hepatitis B is effective in 68% children, and the antibody levels showed a steady decline with increasing age.
Keywords: Hepatitis B, Childhood vaccination, Immunization, Anti-Hbs titres, Protective anti-Hbs antibody
Introduction
Chronic hepatitis B (CHB) infection continues to remain a global public health problem. The World Health Organization estimates that as of 2017, there are around 257 million people who are living with CHB infection.1 Prevalence wise, India falls in the intermediate group with a population prevalence rate of 2–4%.2,3 In India, about 50 million people are carriers of Chronic Hepatitis B infection (HBV), and each year, around 115,000 people die due to CHB and its complications. Around 1 million children of a total of 26 million born every year in India are at risk of developing CHB during their lifetime.4, 5, 6 Horizontal transmission is established as the commonest mode of transmission among children in India, while the perinatal transmission may be responsible for around one-third of all the adult carriers of the infections.3,7 There has been no significant breakthrough in the treatment of CHB; hence, preventive strategies are extremely important to control the infection and consequent disease burden of chronic liver disease and hepatocellular carcinoma. One such important preventive strategy is vaccination against hepatitis B. With an initial rollout of the Hepatitis B vaccine in certain cities and districts of India in the year 2002–2003, the Government of India finally included Chronic Hepatitis B infection (HBV) vaccine in the childhood immunization programme in the year 2011–12. Presently, all institutional deliveries are given four doses of the vaccine starting at birth followed by 6,10 and 14 weeks while home deliveries are given three doses of the vaccine at 6, 10 and 14 weeks.6,8,9 While the birth dose is required to prevent perinatal transmission, subsequent doses prevent the horizontal transmission, which is an important source of infection in India. Recently, a multicentric study by Puliyel et al. 7 concluded that a three-dose schedule had the same benefit as a four-dose schedule when compared with no vaccination. Effectiveness of this vaccine in children is extremely important because the majority of infections acquired in this age group go on to become a chronic infection.
Immunogenicity and protective efficacy of HBV vaccine are well established in the adult population and are found to be more than 90% in multiple studies.10,11 Data regarding the efficacy of childhood immunization from various countries have shown variable results. In addition, certain authors have also reported an age-dependent decline of the antibody titres in children. There are limited data regarding HBV vaccine efficacy in our country.7,12 Age-dependent decline in protective antibody titres after childhood vaccination has been reported by certain authors.13,14There are also some controversies over the long-term persistence of postvaccination immunity against HBV and the need for booster doses of the vaccine15, 16, 17 However, there is limited literature from India on the seroprevalence and decline in antibody titres after childhood vaccination. This study was planned to estimate the proportion of children in the age group of 1–10 years who develop protective levels of anti-HB antibodies after childhood vaccination and to examine the change in antibody titres with age in these children.
Material and methods
We performed a cross-sectional observational study at a tertiary care teaching hospital in the state of Maharashtra in western India. Ethical clearance was obtained from the institutional ethics committee before the commencement of the study. Children aged 1–10 years admitted in the paediatric ward of our hospital who had documented evidence of complete vaccination against HBV were included in our study. All patients admitted from September 2018 to March 2019 were included in the study after obtaining written informed consent from the parents of the child and verbal assent from the child where possible. Children born to HBsAg-positive mother and children admitted with jaundice or any other liver disease were excluded from the study. The minimum sample size calculated assuming the following was 113: proportion of children with protective antibody levels as 75%, precision at 8% and alpha error as 5%. However, a total of 122 children were included in the study. Basic demographic data, vaccination history and HBsAg status of the mother were recorded. All the enrolled children were evaluated for HBsAg and anti-HBS titres. Three ml blood sample was obtained simultaneously whenever the child was subjected to any laboratory investigation, and a separate needle prick to the participant was not given for this study. Sera were separated and stored at −80 °C till analysis. Serum specimens were tested for HBsAg and anti-HBs using automated enzyme-linked immunoassays. The assay principle combined an enzyme immunoassay sandwich method with a final fluorescent detection. The solid-phase receptacle (SPR) in VIDAS (VITEK Immunodiagnostic Assay System) had ad and ay HB surface antigens coated on the interior of the SPR. Antibodies in the serum bind to the antigen coated on the SPR leading to antigen–antibody complex formation which in turn will bind to biotinylated antigens in the diluent. Later, biotin is bound to the antibiotin–alkaline phosphatase conjugate. The alkaline phosphatase catalyses the hydrolysis of the substrate (4-methylumbelliferyl phosphate) into a fluorescent product (4-methyl umbelliferone), the fluorescence of which was measured at 450 nm. The intensity of fluorescence is proportional to the quantity of anti-HB antibody in the sample. At the end of the assay, results are automatically calculated by the instrument with the calibration curve stored in the memory and then printed out. The coefficient of variance of the assay was 10%. HBsAg status was evaluated by an ELISA-based rapid test.
Data record and statistical analysis
The Chi-square test and Fisher exact test were used with the SPSS 16 Package program (Chicago, IL, USA). Data were presented as mean ± standard deviation or, when indicated, as an absolute number and percentage with confidence intervals. Analysis of Variance (ANOVA) was used for statistical analysis to compare the means between the different age groups.
Results
A total of 122 children participated in this study. The baseline characteristics of the children are described in Table 1. The 77 boys and 45 girls included in our study did not differ significantly in their mean age (68.64 months and 73.31 months, respectively), mean weight (18.21 and 17.97 kg, respectively) or their mean antibody titres (134.46 IU/L and 111.98 IU/L, respectively). A significantly (p = 0.036) higher percentage of boys (76%) showed protective antibody titres as compared with the girls (55.6%). Most children had 4 doses of Hep B vaccine. Only 03 boys and a single girl had been administered 03 doses of the vaccine. None of the mothers were found to be positive for Hep B surface antigen. None of the children included in the study were HBsAg positive.
Table 1.
Baseline characteristics of the study population.
| Characteristics | Male (N = 77) | Female (N = 45) | P value |
|---|---|---|---|
| Age (months) | 68.64 (7.2) | 73.31 (35.8) | 0.86 |
| Weight (kgs) | 18.21 (7.25) | 17.97 (7.3) | 0.48 |
| Mean titres (IU/L) | 134.46 (181) | 111.98 (170.9) | 0.51 |
| Antibody titres | |||
| <10 IU/L | 18 (23.4%) | 20 (44.4%) | p |
| >10 IU/L | 59 (76.6%) | 25 (55.6%) | |
| Immunization schedule | |||
| 4 doses | 74 (96.1%) | 44 (97.8%) | p |
| 3 doses | 3 (3.9%) | 1 (2.2%) | |
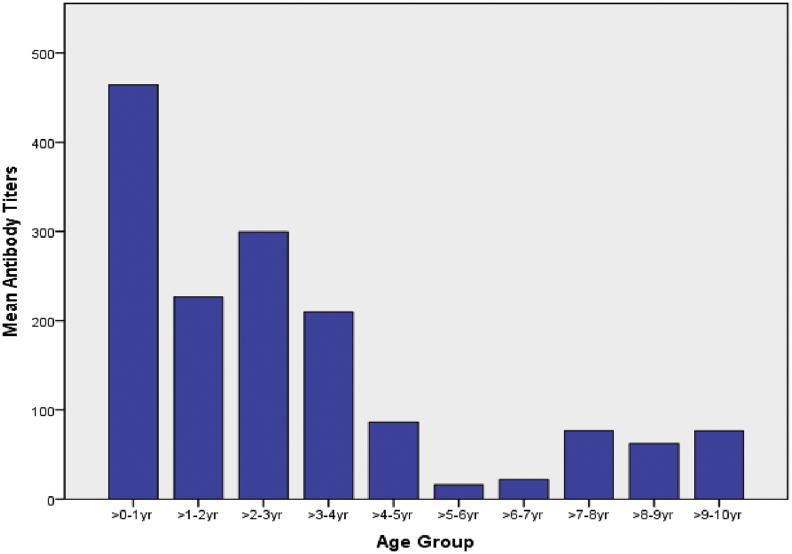
In our study, 68.86% (95% CI: 59.8–76.8%) of the children showed protective antibody titres after vaccination, while 31.14% (95% CI: 23.1–40.2%) of the children had titres less than 10 IU/L (Table 2). In the 04 children who received three doses of the vaccine, 50% of them had titres less than 10IU/L and the remaining had protective antibody levels(not in table). The mean antibody titres reduced with age, and children aged younger than one year had mean titres around 464 IU/L and the titres kept reducing, to the lowest level at 5–6 years of age (Fig. 1).This difference in mean antibody titres was found to be statistically significant (ANOVA for difference between means, p = 0.00). The proportion of children with protective antibody titres kept decreasing with age consistently till 5–6 years of age. While 100% of children between birth to three years of age had titres >10 IU/L, this proportion kept declining with age, and at 09–10 years of age, 60% of the children had titres less than 10 IU/L (chi square for trends, p = 0.00013). A simple linear regression was calculated to estimate change in antibody titres with increase in age (Fig. 2). A significant regression equation was found ((F1,115) = 32.88, p < 0.000), with R2 of 0.22. Participants predicted antibody titres were equal to 291.53–2.36 (age) IU/L, when age is measured in months. Average antibody titres decreased 2.364 IU/L for each month of age.
Table 2.
Immune response to hepatitis B vaccine in different age groups.
| Age | N | Mean Titres (IU/L) | P valuea | <10 IU/L | >10 IU/L | P valueb |
|---|---|---|---|---|---|---|
| 0–1 yr | 3 | 464.33 | 0.000 | 0 | 3 (100%) | 0.000013 |
| >1–2yr | 17 | 226.76 | 0 | 17 (100%) | ||
| >2–3yr | 10 | 299.33 | 0 | 10 (100%) | ||
| >3–4yr | 11 | 209.73 | 2 (18.2%) | 9 (81.8%) | ||
| >4–5yr | 14 | 86.23 | 6 (42.8%) | 8 (57.2%) | ||
| >5–6 yr | 7 | 16.00 | 3 (42.8%) | 4 (57.2%) | ||
| >6–7 yr | 16 | 22.00 | 6 (37.5%) | 10 (62.5%) | ||
| >7–8 yr | 13 | 76.50 | 5 (38.4%) | 8 (62.6%) | ||
| >8-9yr | 16 | 62.14 | 7 (43.2%) | 9 (56.2%) | ||
| >9–10yr | 15 | 76.20 | 9 (60%) | 6 (40%) | ||
| Total | 122 | 126.20 | 38 (31.14%) (CI = 59.8–76.8%) |
84 (68.86%) (CI = 23.1–40.2%) |
a = ANOVA for difference between means, b = Extended Mantel-Haenszel chi square for linear trend.
CI, Confidence interval; ANOVA, Analysis of variance.
Fig. 1.
Mean antibody titres in various age groups.
Fig. 2.
Simple linear regression for antibody titres with age.
Discussion
We studied the protective antibody titres in 122 children who were vaccinated during infancy against hepatitis B. In our study, 68.86% (95% CI: 59.8–76.8%) of the children showed protective antibody titres after vaccination, while 31.14% (95% CI: 23.1–40.2%) of the children had titres less than 10 IU/L. The percentage of children showing protective titres fell with age, while 100% had protective levels till 03 years of age; this percentage fell to 40% for children aged between 09 and 10 years. A recent multicentric study by Puliyel et al. 7 in India carried out in 2671 children reported protective antibody titres in 70% of the vaccinated children; the authors also reported that the levels waned from 82% in the first year to 47% by the age of 5 years. In a study carried out by Agarwal et al.12, in 2014, in rural Andhra Pradesh, 53% of the immunized children in the age group of 5–11 years were detected to have protective levels; however, the proportion of children with protective levels decreased with age.
We report an average decline in antibody titres of 2.36 IU/L for each advancing month of age. In a similar study carried out in South Korea, the authors observed an average decline of around 18 IU/L for each year of age.14
A study from China reported protective titres in 45.29–63.33% of the children aged between 7 and 14 years; however, similar to our findings, the authors also described a reducing level of protection with increasing age.18 In a similar study from Iran, authors reported protective antibody titres in 88% of the children aged younger than 5 years, which declined to 74% at 10 years of age.19
In our study, the mean antibody titre was 123.28 IU/L. The titres reduced with age, and children aged younger than 01 years had the highest titres, and a steadily declining trend was observed thereafter. In a study from China, He et al. 20 reported the mean antibody titres of 23 mIU/L in a sample of 1526 children aged 15 years and younger who had undergone Hep B immunization during infancy. The authors also documented similar declining antibody levels with children aged one year having 147.8 IU/L and those at 15 years showing levels as low as 30.7 IU/L.20 A study from Palestine reported 60% of children aged 1–15 years showing protective antibody levels, and similar to our study, while 92% of children aged one year had protective titres, this percentage fell to 39% for children aged between 7 and 19 years.21 The mean titres also declined from 257 IU/L at age one year to 24 IU/L at age 5 years. Similar results were also reported from other low endemic countries such as the USA, Italy and Canada.22, 23, 24, 25
Our study showed that a significantly higher percentage of boys had protective antibody titres as compared with the girls; this is similar to the finding reported by Lee et al. 14 from South Korea. However in contrast, Alssamei et al. 26 reported a slightly higher protective rate of anti-HB antibody in women (75.7%) than in men (68.4%). Other studies from China and Iran also reported higher rate of protective levels of anti-HBS in female children than in male children and infants.19,20 However certain other authors from Iran and Brazil have reported no difference in the distribution of sex and protective antibody levels.24,27
There is no clarity on the period of protection conferred by childhood hepatitis B vaccination.23,28 This issue is especially important in countries with high or intermediate prevalence as risk of HBV is greatest during childhood through horizontal transmission.29 Lao28 has convincingly argued the case to reexamine the claims of persistence of immune protection provided by childhood vaccination programmes and the possible need for booster dose in children. Without knowledge about this important aspect, control of HBV infection through childhood vaccination programmes may not be completely effective.
Our study has the advantage of including only children who had documented evidence of hepatitis B immunization; this is also one of the few studies carried out in India reporting the protective antibody levels in children after childhood vaccination. However, our study was carried out in a tertiary care hospital, and some of the children in our study were suffering from serious comorbid conditions which could have affected the immune response to the vaccination.
Conclusion
A high percentage of children in our study was detected to have protective antibody levels after childhood vaccination with Hep B vaccine. These figures are comparable and also higher than those reported by authors from other developed countries such as China and the USA. The protective titres, however, continued to decrease with age, and further studies may be required to assess the clinical and larger public health implications of the same.
Disclosure of competing interest
All authors have none to declare.
References
- 1.World Health Organization, Hepatitis. SEARO. Available at: http://www.searo.who.int/india/topics/hepatitis/en/[accessed April 20, 2020].
- 2.Puri P. Tackling the hepatitis B disease burden in India. J Clin Exp Hepatol. 2014;4:312–319. doi: 10.1016/j.jceh.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batham A., Narula D., Toteja T., Sreenivas V., Puliyel J.M. Sytematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatr. 2007;44:663–674. [PubMed] [Google Scholar]
- 4.Ray G. Current scenario of hepatitis B and its treatment in India. J Clin Transl Hepatol. 2017;5:277–296. doi: 10.14218/JCTH.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan J., Shil A., Mohanty S.K. Hepatitis B vaccination coverage across India: exploring the spatial heterogeneity and contextual determinants. BMC Publ Health. 2019;19:1263. doi: 10.1186/s12889-019-7534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma R., Khanna P., Prinja S., Rajput M., Chawla S., Bairwa M. Hepatitis B Vaccine in national immunization schedule: a preventive step in India. Hum Vaccine. 2011;7:1387–1388. doi: 10.4161/hv.7.12.17878. [DOI] [PubMed] [Google Scholar]
- 7.Puliyel J., Naik P., Puliyel A. Evaluation of the protection provided by hepatitis B vaccination in India. Indian J Pediatr. 2018;85:510–516. doi: 10.1007/s12098-017-2601-0. [DOI] [PubMed] [Google Scholar]
- 8.Lahariya C., Subramanya B.P., Sosler S. An assessment of hepatitis B vaccine introduction in India: lessons for roll out and scale up of new vaccines in immunization programs. Indian J Publ Health. 2013;57:8. doi: 10.4103/0019-557X.111357. [DOI] [PubMed] [Google Scholar]
- 9.Immunization_Handbook_for_Health_Workers-English.pdf. n.D.
- 10.Immunogenicity of a Recombinant Hepatitis B Vaccine in Adults | JAMA Internal Medicine | JAMA Network. Available at: https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/622555 [accessed April 30, 2020].
- 11.Bender T.J., Sharapov U., Utah O. Hepatitis B vaccine immunogenicity among adults vaccinated during an outbreak response in an assisted living facility—Virginia, 2010. Vaccine. 2014;32:852–856. doi: 10.1016/j.vaccine.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal R., Babu J.J., Hemalatha R., Reddy A.V., Sharma D., Kumar T. Effect of inclusion of hepatitis B vaccine in childhood immunization program in India: a retrospective cohort study. Indian Pediatr. 2014;51:875–879. doi: 10.1007/s13312-014-0520-y. [DOI] [PubMed] [Google Scholar]
- 13.Qawasmi M., Samuh M., Glebe D., Gerlich W.H., Azzeh M. Age-dependent decrease of anti-HBs titers and effect of booster doses using 2 different vaccines in Palestinian children vaccinated in early childhood. Hum Vaccines Immunother. 2015;11:1717–1724. doi: 10.1080/21645515.2015.1041687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K.H., Shim K.S., Lim I.S. Changes in hepatitis B virus antibody titers over time among children: a single center study from 2012 to 2015 in an urban of South Korea. BMC Pediatr. 2017;17:164. doi: 10.1186/s12887-017-0924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO | Hepatitis B. WHO. Available at: https://www.who.int/ith/vaccines/hepatitisB/en/[accessed April 30, 2020].
- 16.Mahmood S., Shah K.U., Khan T.M. Immune persistence after infant hepatitis-B vaccination: a systematic review and meta-analysis. Sci Rep. 2018;8:12550. doi: 10.1038/s41598-018-30512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuridan E., Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 18.Yue X., Ge C., Zhuge S. Changes and analysis of anti-HBs titres after primary immunization in 1- to 16-year-old Chinese children: a hospital-based study. J Viral Hepat. 2018;25:373–380. doi: 10.1111/jvh.12818. [DOI] [PubMed] [Google Scholar]
- 19.Rezaei M., Nooripoor S., Ghorbani R., Ramezanshams F., Mamishi S., Mahmoudi S. Seroprotection after hepatitis B vaccination in children aged 1 to 15 years in central province of Iran, Semnan. J Prev Med Hyg. 2014;55:1–3. [PMC free article] [PubMed] [Google Scholar]
- 20.He F., Ma Y., Zhou T. The serum anti-HBs level among children who received routine hepatitis B vaccination during infancy in mianyang city, China: a cross-sectional study. Viral Immunol. 2016;29:40–48. doi: 10.1089/vim.2015.0073. [DOI] [PubMed] [Google Scholar]
- 21.Qawasmi M., Samuh M., Glebe D., Gerlich W.H., Azzeh M. Age-dependent decrease of anti-HBs titers and effect of booster doses using 2 different vaccines in Palestinian children vaccinated in early childhood. Hum Vaccines Immunother. 2015;11:1717–1724. doi: 10.1080/21645515.2015.1041687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanetti A.R., Mariano A., Romanò L. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–1384. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 23.Petersen K.M., Bulkow L.R., McMahon B.J. Duration of hepatitis B immunity in low risk children receiving hepatitis B vaccinations from birth. Pediatr Infect Dis J. 2004;23:650–655. doi: 10.1097/01.inf.0000130952.96259.fd. [DOI] [PubMed] [Google Scholar]
- 24.Aghakhani A., Banifazl M., Izadi N. Persistence of antibody to hepatitis B surface antigen among vaccinated children in a low hepatitis B virus endemic area. World J Pediatr. 2011;7:358. doi: 10.1007/s12519-011-0286-4. [DOI] [PubMed] [Google Scholar]
- 25.Gilca V., De Serres G., Boulianne N. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine. 2013;31:448–451. doi: 10.1016/j.vaccine.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Alssamei F.A.A., Al-Sonboli N.A., Alkumaim F.A. Assessment of immunization to hepatitis B vaccine among children under five years in rural areas of taiz. Yemen Hepat Res Treat. 2017;2017 doi: 10.1155/2017/2131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandre K.V.F., Martins R.M.B., Souza MM de, Rodrigues I.M.X., Teles S.A. Brazilian hepatitis B vaccine: a six-year follow-up in adolescents. Mem Inst Oswaldo Cruz. 2012;107:1060–1063. doi: 10.1590/S0074-02762012000800016. [DOI] [PubMed] [Google Scholar]
- 28.Lao T.T. Immune persistence after hepatitis B vaccination in infancy - fact or fancy? Hum Vaccines Immunother. 2016;12(5):1172–1176. doi: 10.1080/21645515.2015.1130195. PMID:26810256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lao T.T. Long-term persistence of immunity after hepatitis B vaccination: is this substantiated by the literature? Hum Vaccines Immunother. 2017;13(4):918–920. doi: 10.1080/21645515.2016.1267084. [DOI] [PMC free article] [PubMed] [Google Scholar]