Abstract
Objectives
The study aimed at identifying salivary microbiota in caries-free Chinese preschool children using high-throughput sequencing.
Methods
Saliva samples were obtained from 35 caries-free preschool children (18 boys and 17 girls) with primary dentition, and 16S ribosomal DNA (rDNA) V3–V4 hypervariable regions of the microorganisms were analyzed using Illumina MiSeq.
Results
At 97% similarity level, all of these reads were clustered into 334 operational taxonomic units (OTUs). Among these, five phyla (Firmicutes, Proteobacteria, Actinobacteria, Bacteroidetes, and Candidate division TM7) and 13 genera (Streptococcus, Rothia, Granulicatella, Prevotella, Enterobacter, Veillonella, Neisseria, Staphylococcus, Janthinobacterium, Pseudomonas, Brevundimonas, Devosia, and Gemella) were the most dominant, constituting 99.4% and 89.9% of the salivary microbiota, respectively. The core salivary microbiome comprised nine genera (Actinomyces, Capnocytophaga, Gemella, Granulicatella, Lachnoanaerobaculum, Neisseria, Porphyromonas, Rothia,and Streptococcus). Analysis of microbial diversity and community structure revealed a similar pattern between male and female subjects. The difference in microbial community composition between them was mainly attributed to Neisseria (P=0.023). Furthermore, functional prediction revealed that the most abundant genes were related to amino acid transport and metabolism.
Conclusions
Our results revealed the diversity and composition of salivary microbiota in caries-free preschool children, with little difference between male and female subjects. Identity of the core microbiome, coupled with prediction of gene function, deepens our understanding of oral microbiota in caries-free populations and provides basic information for associating salivary microecology and oral health.
Keywords: Salivary microbiota, Caries-free, Preschool children, Primary dentition, Illumina MiSeq, 16S rDNA V3-V4 hypervariable regions
1 Introduction
The human body is considered a superorganism, with a combination of microbial and human attributes (Gillet al., 2006). Various microorganisms have been found residing in different parts of the human body, at an estimated ratio of 1:1 to the number of human cells (Senderet al., 2016). Previous studies have successfully characterized human microbiota, and reported that the microorganisms play an important role in host homeostasis (Ismailet al., 2009; Kauet al., 2011) as well as health (Tappenden and Deutsch, 2007; Cogenet al., 2008). Advances in metagenomics, coupled with the rise of personalized and precise medicine concepts, have generated vital data on human microbiota, which is expected to improve medical therapies. The oral cavity, which harbors about 1000 microbial species, is one of the most diverse and important habitats for human microorganisms (Wade, 2013). The mouth of a healthy individual harbors relatively stable microbial communities, with disturbances in these communities implicated in a series of local and systemic infectious diseases, including caries, periodontal, liver, and inflammatory bowel diseases (Liuet al., 2012; Saidet al., 2014; Struzycka, 2014; Linget al., 2015).
Studies on oral microbiota in children have revealed the changes in microbial communities that are associated with oral diseases, including dental caries, periodontitis, and pigmentation (Jianget al., 2013; Campiscianoet al., 2017; Zhanget al., 2017). However, only a handful of studies have reported on the oral microbiota in caries-free children (Xinet al., 2013; Leeet al., 2016). Furthermore, the identity and function of microorganisms in the saliva of caries-free preschool children in China remain unclear. Approximately half of all detected oral microorganisms are unculturable (Dewhirstet al., 2010), posing a big challenge for researchers seeking to analyze oral microbiota comprehensively. However, this challenge can be overcome by using culture-independent sequencing techniques which identify microorganisms based on hypervariable regions in the 16S ribosomal DNA (rDNA) gene (Yeet al., 2012). Recent advances in high-throughput sequencing have enhanced studies into oral microbiota. These techniques collect more information, with a higher efficiency as well as a greater width of coverage, by allowing the detection not only of dominant microbial taxa, but also of rare and unknown microorganisms when compared to traditional sequencing (Bennet al., 2018). However, to the best of our knowledge, a very limited number of studies have used high-throughput sequencing to identify the oral microbiota of primary dentition in caries-free preschool children.
Therefore, this study aimed at identifying the composition and gene functions of salivary microbiota of primary dentition in caries-free Chinese preschool children using Illumina MiSeq high-throughput sequencing, targeting the 16S rDNA V3–V4 hypervariable regions. Furthermore, we compared the results from male and female subjects to reveal the effects of gender on the diversity, composition, and structure of salivary microbiota. Our findings are expected to provide new insights into the composition and structure of the oral microbiome and reveal its relationship with health in preschool-age children. In future, the resulting microbial data are expected to be applied in the assessment of oral health and in disease diagnosis and prognosis.
2 Results
2.1. Demographic characteristics
The demographic characteristics of the 35 subjects (18 boys and 17 girls) recruited are summarized in Table 1. Their average age was (48.4±3.1) months, with no statistical differences (P=0.640) observed between males and females with regard to age.
Table 1.
Demographic characteristic of involved subjects
| Group | Number of subjects | Age (month) |
|---|---|---|
| Male | 18 | 48.6±3.0a |
| Female | 17 | 48.2±3.3a |
| Total | 35 | 48.4±3.1 |
a No statistical differences (P=0.640) observed between males and females according to Student’s t-test. Data are expressed as number or mean±standard deviation (SD).
2.2. Sequencing output and microbial composition
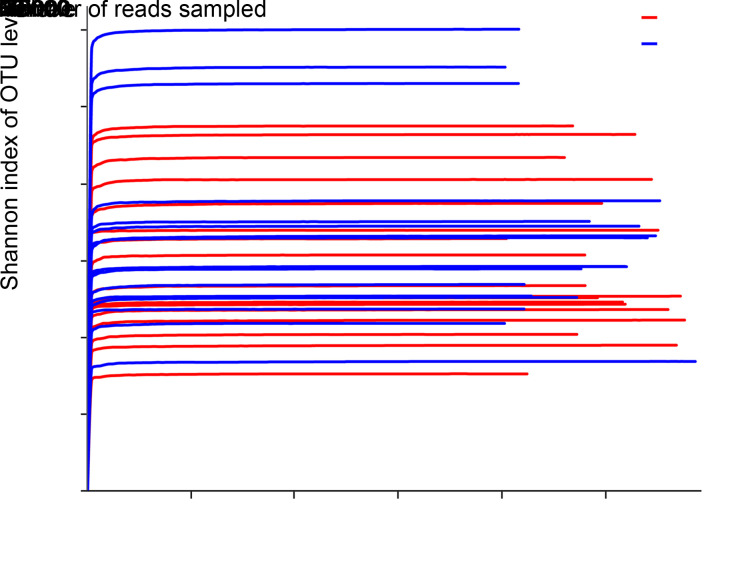
A Good's coverage index of more than 0.999 was obtained for each sample, indicating that the identified sequences represented a vast majority of the microorganisms in these saliva samples. Rarefaction curves indicated that the sequencing technique used effectively revealed complete bacterial richness (Fig. 1). These two indicators combined suggested that the sequencing depth was sufficient and that additional sampling was not necessary.
Fig. 1. Rarefaction curves for estimating richness among the 35 samples in the current study. The ordinate shows the Shannon index of the operational taxonomic unit (OTU) level expected to be calculated after sampling the number of sequences shown on the abscissa. The flat curve indicates that microbial richness will not significantly increase with increase in sequencing number and that the depth of sequencing is sufficient.
High-throughput Illumina MiSeq sequencing revealed a total of 1 824 344 reads, among which 1 712 531 (93.9%) high-quality reads passed quality control and were therefore selected for further analysis. An average of 48 929 reads per sample (standard deviation (SD)=5706; range: 39 624‒58 231) were obtained, and all reads were found to be over 420 bp, with an average length of (446.5±4.2) bp. At the 97% similarity level, all reads were clustered into 334 OTUs, which represented 13 phyla, 24 classes, 40 orders, 63 families, and 113 genera.
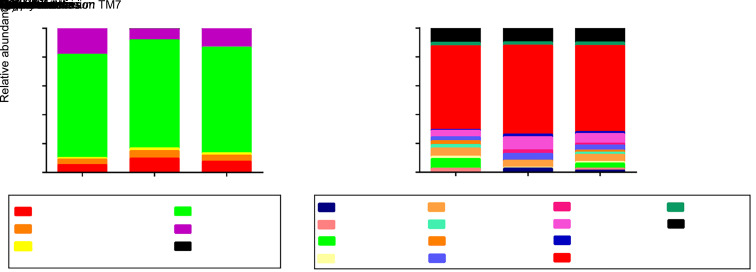
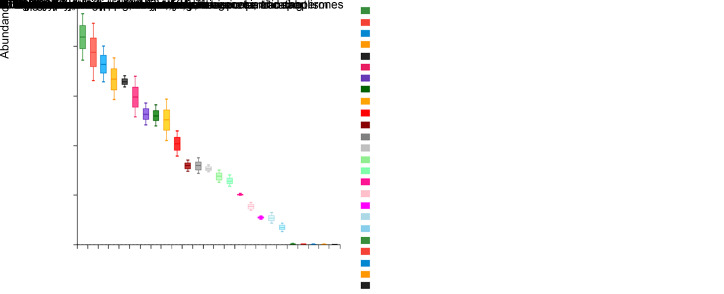
Five phyla, including Firmicutes(73.4%), Proteobacteria(12.8%), Actinobacteria(7.3%), Bacteroidetes (4.4%), and Candidate division TM7 (1.5%), were the most dominant across all studied subjects. These constituted 99.4% of the entire salivary microbiota, whereas other phyla were detected in relatively low proportions (Fig. 2a). At the genus level, 13 genera, including Streptococcus (59.8%), Rothia (6.7%), Granulicatella (5.1%), Prevotella (3.6%), Enterobacter (3.6%), Veillonella (2.2%), Neisseria (1.5%), Staphylococcus (1.3%), Janthinobacterium (1.3%), Pseudomonas (1.3%), Brevundimonas (1.2%), Devosia (1.2%), and Gemella (1.1%) were dominant. These represented 89.9% of all salivary microbiota (Fig. 2b).
Fig. 2. Distribution of dominant microorganisms at different taxonomic levels. The dominant taxa (>1% relative abundance) at two levels are shown. (a) Distribution at the phylum level; (b) Distribution at the genus level.
2.3. Microbial diversity and overall community structure between male and female subjects
There were no statistically significant differences between male and female subjects with regard to Ace, Chao, Shannon, and Simpson α diversity indices (Table 2; Fig. S1). This implied that gender had no effect on the richness or evenness of salivary microbiota populations in preschool children with caries-free primary dentition.
Table 2.
α diversity indices of male and female subjects
| Group | Ace | Chao | Shannon | Simpson |
|---|---|---|---|---|
| P-value* | 0.597 | 0.409 | 0.391 | 0.146 |
| Male (n=18) | 143.0±40.0 | 138.8±39.6 | 1.49±0.49 | 0.431±0.158 |
| Female (n=17) | 149.0±33.2 | 146.4±36.8 | 1.64±0.60 | 0.358±0.142 |
* Mann-Whitney U-test. Data are expressed as mean±standard deviation.
The high similarity of salivary microbial communities between male and female subjects was also reflected in the Venn diagram (Fig. S2). In summary, there was a high overlap in OTUs between the groups. Specifically, a total of 265 OTUs were shared by both groups of subjects, accounting for 90.8% and 86.3% of their total OTUs, respectively. In addition, 27 and 42 exclusive OTUs were found in male and female subjects, respectively, accounting for a respective 9.2% and 13.7% of their total OTUs.
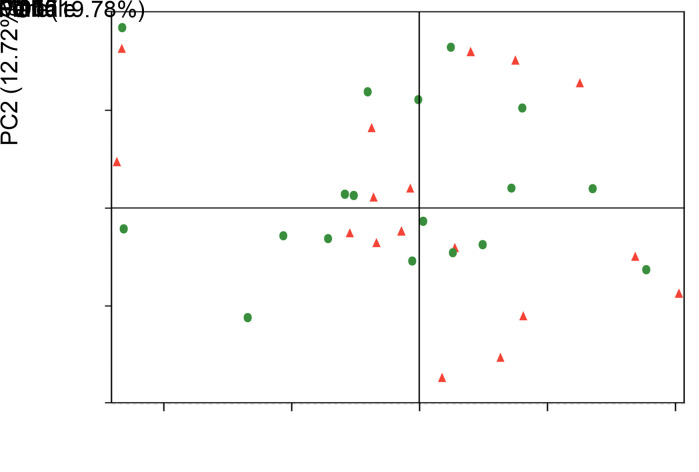
No clear separation of clusters was revealed by a principal coordinate analysis (PCoA)-based comparison of overall microbial community structure between male and female groups, using weighted UniFrac distance measurements (β diversity; Fig. 3). The dissimilarity test corroborated the PCoA results, revealing no marked difference between these two groups (analysis of similarities (ANOSIM) test, R=-0.0189, P=0.78). These results indicated that gender did not significantly affect the structure of salivary microbial communities.
Fig. 3. Principal coordinate analysis (PCoA) plot analysis based on weighted UniFrac distances at the operational taxonomic unit (OTU) level, at 97% identity. Each sample is represented by a dot. Balls represent samples from male subjects, whereas triangles denote samples from female subjects. The samples did not form well-separated clusters between these two groups, suggesting a similar structure in salivary microbial communities between male and female subjects. PC: principal component.
2.4. Taxonomic differences between male and female subjects
As mentioned earlier, a total of 13 phyla were identified, among which five (Firmicutes, Proteobacteria, Actinobacteria, Bacteroidetes, and Candidate division TM7) were dominant in both male and female subjects, albeit at no statistical difference (Fig. S3). In addition, Deinococcus-Thermus was only found in female subjects. No intergroup differences were observed among the rest of low-abundance phyla.
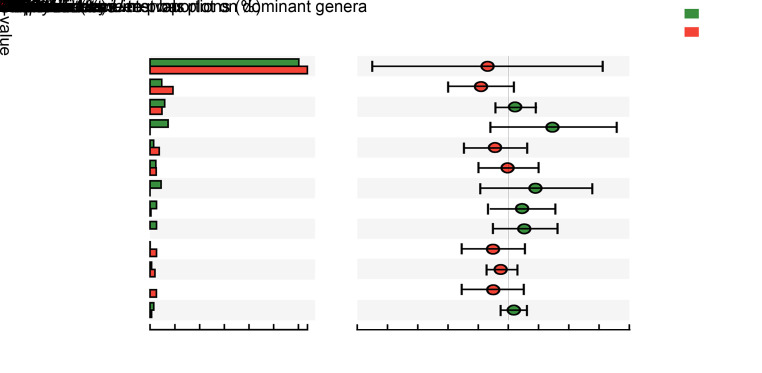
At the genus level, we detected 113 genera, 13 of which were dominant. Among these, only Neisseria showed a statistical difference between the groups: a significantly higher abundance (P=0.023) of this genus was observed in male than in female subjects (Fig. 4). No statistical differences were observed between the groups with regards to the other low-abundance genera.
Fig. 4. Comparison in relative abundance of dominant genera between male and female subjects. Green and red bars represent male and female subjects, respectively. Data are shown as mean±standard deviation (SD) (n=18 for males and n=17 for females), and the statistical difference is marked by red asterisks (* P<0.05) based on non-parametric Mann-Whitney U test. Only Neisseria showed statistical differences between male and female subjects, and had a higher relative abundance in male subjects.
2.5. Salivary core microbiome in caries-free preschool children
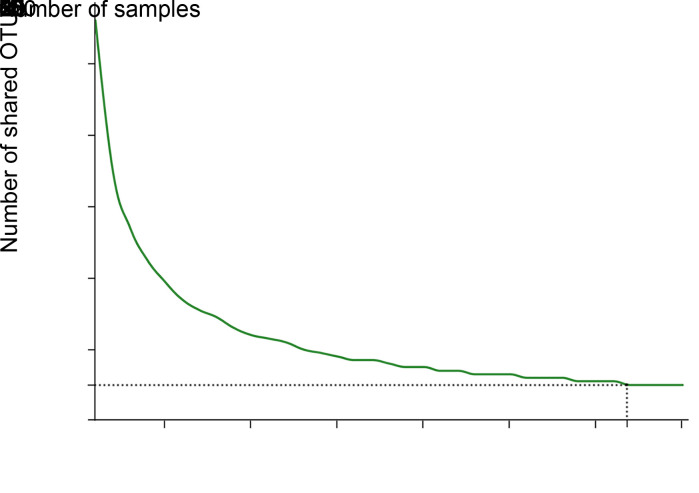
A core microbiome is defined as a set of microbial taxa shared by all subjects (Turnbaughet al., 2007). In the present study, analysis of the salivary core microbiome from caries-free preschool children with primary dentition revealed a decrease in the number of core OTUs with increase in sample size. Specifically, the number of shared OTUs first decreased to 10 when the sampling number reached 32, and then remained constant (Fig. 5). This result highlighted the importance of sample size (at least 32 in this study) for the reliable definition of a core microbiome.
Fig. 5. Core taxa analysis based on operational taxonomic unit (OTU) level at 97% identity. The ordinate shows that the number of core OTUs decreased when the sample size increased, as shown on the abscissa. The number of shared OTUs first decreased to 10 when the sample size reached 32, and then remained unchanged when the samples exceeded 32.
At the phylum level, we detected four phyla across all subjects, including Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. However, a total of nine genera, including Actinomyces, Capnocytophaga, Gemella, Granulicatella, Lachnoanaerobaculum, Neisseria, Porphyromonas, Rothia,and Streptococcus, were detected in all subjects(Table 3). These genera accounted for 75.2% of all identified sequences, indicating that the core microbiome represents a significantly high proportion of total salivary microbiota in caries-free preschool children with primary dentition.
Table 3.
Salivary core microbiome at phylum and genus levels
| Phylum | Genus |
|---|---|
| Actinobacteria | Actinomyces |
| Rothia | |
| Bacteroidetes | Capnocytophaga |
| Porphyromonas | |
| Firmicutes | Streptococcus |
| Gemella | |
| Granulicatella | |
| Lachnoanaerobaculum | |
| Proteobacteria | Neisseria |
2.6. Prediction of functions and pathways
OTU standardization using PICRUSt software revealed corresponding information on Clusters of Orthologous Genes (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology (KO). We then compared the COG and KO information to datasets in the Evolutionary Genealogy of Genes Non-supervised Orthologous Groups (EggNOG) and KEGG databases to predict the corresponding functions (EggNOG) and pathways (KEGG) of all salivary microbial genes in caries-free primary dentition. Based on these analyses, the following represented the top ten functions and pathways:function unknown; amino acid transport and metabolism; general function prediction only; carbohydrate transport and metabolism; translation, ribosomal structure and biogenesis; transcription; replication, recombination and repair; cell wall/membrane/envelope biogenesis; inorganic ion transport and metabolism; and energy production and conversion. Conversely, chromatin structure and dynamics, RNA processing and modification, cytoskeleton, extracellular structure, and nuclear structurerepresented the five lowest functions and pathways (Fig. 6).
Fig. 6. Functions and pathways presented by salivary microbial genes in caries-free primary dentition, predicted by comparing information with datasets from the Evolutionary Genealogy of Genes Non-supervised Orthologous Groups (EggNOG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. The ordinate shows abundance of microbial genes, whereas the abscissa shows the kind of functions and pathways which these genes presented.
3 Discussion
In 2007, the US National Institutes of Health (NIH) launched the Human Microbiome Project (HMP), a genetic engineering project aimed at characterizing human microbiota and ascertaining their effects on human physiology and pathology (Petersonet al., 2009). The oral cavity, which has a humid environment with a relatively constant temperature and contains a large variety of microorganisms (partly due to its various anatomical structures), is one of the most important microbial habitats. Previous studies have shown that the dominant microorganisms in saliva, which detach from oral tissues, are often commensal (Pereiraet al., 2012). For example, these microorganisms may originate from the biofilm matrix polymers degraded by bacterial enzymes, as well as from desquamation of epithelial surface cells (Kaplanet al., 2004). Since diversity and composition of salivary microbiota are affected by interactions between microorganisms and their hosts, we believe that saliva may be an ideal medium for exploring oral microbiota. Studies on oral microbiota are challenging on the whole, due to the fact that about half of all oral microorganisms are unculturable. However, culture-independent approaches, such as high-throughput or next-generation sequencing, can be used to overcome this limitation (Yeet al., 2012). In particular, the Illumina MiSeq platform is considered superior because it can provide a similar read length at a lower cost when compared to other related technologies such as Roche/454 pyrosequencing (Fadroshet al., 2014). In addition, previous studies have demonstrated the importance of different hypervariable regions (V1-V9) in the microbial 16S rRNA gene for species identification. The V3-V4 and V4-V5 regions have provided the most accurate results irrespective of the sequencing technology used (Claessonet al., 2010). Based on the aforementioned facts, we sought to identify and characterize salivary microbiota using Illumina MiSeq sequencing and targeting the V3-V4 regions.
Microbial richness and diversity in the environment can be determined by single-sample diversity analysis (α diversity), including a series of statistical indices to estimate species abundance and diversity. As shown in Table 2, richness estimators (Chao and Ace) and diversity indices (Shannon and Simpson) were introduced for α diversity analysis in this study (Ritz et al., 2020; Zhou et al., 2020). In addition, β diversity analysis can be used to determine similarity or difference in community structure among different groups. We carried out PCoA, a non-binding data dimension reduction analysis method based on the weighted UniFrac distance, to visualize the similarities in microbial structure among subjects. Specifically, we divided recruited subjects into two groups based on gender. α and β diversity indices revealed a high but not significantly different species richness, diversity, and community structure of salivary microbiota in the groups, indicating that gender does not affect these metrics. Furthermore, when it came to community composition of salivary microbiota, only genus Neisseria showed a higher relative abundance in the male group. The high similarities in these parameters suggest that the gender factor could be negligible for future investigations of salivary microbiota in primary dentition.
Our results further revealed the relationship between sample size and overlapping species. Briefly, calculating species overlap is a reliable way of analyzing the presence of microbial communities in the oral cavity, although the core microbiome requires that core taxa be shared by all subjects. Generally, this enables screening out of transient bacteria to enable detection of only those with the most stable composition (Belda-Ferreet al., 2012; Grice and Segre, 2012). We found a distinct and rapid decline in overlapping species as the number of tested samples increased, although the number of overlapping species remained unchanged when the sample size exceeded 32. This implies that a small sample size is not enough to eliminate the contribution of transient bacteria to overlapping species, and a sample size of >32 may be more reliable for defining a core microbiome.
The oral core microbiome was first defined in 2009 using next generation sequencing, based on analysis of microorganisms residing in five different oral niches (dental surfaces, buccal mucosa, hard palate, tongue, and saliva) in three healthy adults (Zauraet al., 2009). Since then, numerous studies have demonstrated the existence of a core microbiome in the oral cavity, and some have revealed the core microbiome in dental plaque of caries-free children. Xin et al. (2013) analyzed the oral microbiota of ten caries-free Chinese children aged between six and eight years old, and found that 13 genera were shared by all subjects. Moreover, Lee et al. (2016) found ten conserved genera in all ten five-year old caries-free Korean preschool children. We detected nine genera in all subjects, with three of them (Capnocytophaga, Neisseria,and Streptococcus) being consistent with those in two previous studies. This suggests that these three genera are conserved in caries-free children in spite of the differences in oral niches, geography, and age.
Previous studies have documented that some oral microorganisms are associated with certain oral diseases. For example, Streptococcus mutans is a well-known cariogenic species due to its high capacity for acid production and acid tolerance (Loesche, 1986). Surprisingly, we found a higher proportion of Streptococcus across all salivary microbiota detected in caries-free preschool children. We hypothesized that several species of Streptococcus were being detected, including S. mutans, Streptococcus sanguinis, Streptococcus oralis, Streptococcus sobrinus, and Streptococcus mitis, among others, which play distinct roles during the development of dental caries. Therefore, the high proportion of Streptococcus would not be considered an unhealthy status, because its existence cannot be simply equated to dental caries. Our findings are consistent with previous studies that found no statistical differences in Streptococcus between caries and caries-free populations (Linget al., 2010; Xuet al., 2014; Jianget al., 2016). As mentioned above, Capnocytophaga and Neisseria were conserved taxa in caries-free children. A previous study by Jiang et al. (2011) reported a significantly higher frequency of Capnocytophaga in caries-free when compared to caries-susceptible Chinese preschool children. In addition, Anderson et al. (2016) found that Neisseria was more prevalent in caries-free Swedish preschool children than in those with caries. Taken together, these findings indicate that Capnocytophaga and Neisseria may be important core microorganisms for children's dental health.
Biomineralization, including remineralization, is a dynamic process that occurs in the hard tissue of teeth, such as enamel, dentin, and cementum. Teeth are at risk of demineralization throughout life because they are constantly exposed to food, drink, oral microbiota, and other substances (Abou et al., 2016). Inorganic ions, including calcium, phosphate, and fluoride, play an important role in the battle between demineralization and remineralization processes and change the susceptibility of teeth to acid attack (Hara and Zero, 2010; Lussiet al., 2012; Meyeret al., 2018). Biological apatite, such as hydroxyapatite, is an important inorganic component of dental hard tissues. Carbonates and other ionic substitutions can significantly disrupt the crystal structure in hydroxyapatite and weaken it, thereby increasing its solubility and susceptibility to dental caries (Featherstone and Lussi, 2006; Qu and Zhou, 2020). The expansion of genes associated withcarbohydrate transport and metabolism is an essential property for caries pathogens in the oral cavity. Taking S. mutans as an example, the transport of saccharides is primarily conducted by the activity of adenosine triphosphate (ATP)-binding cassette (ABC) transporters (Tao et al., 1993; Webbet al., 2008), while the uptake and metabolism of saccharides are predominantly conducted by the activity of the phosphotransferase system (PTS) (Vadeboncoeur and Pelletier, 1997; Deutscheret al., 2006). They are encoded in the genome of S. mutans and give it the ability to adjust carbohydrate metabolism in response to the changeable oral environment. In this study, we found that salivary microbiota genes were enriched in inorganic ion transport and metabolism and carbohydrate transport and metabolism. This implies that they may play an important role in the processes of demineralization and remineralization as well as organic acid production and tolerance, which can modify teeth's susceptibility to caries.
Rudney et al. (2010) performed a metaproteomic analysis of human salivary microbiota using three-dimensional peptide fractionation and tandem mass spectrometry, and found that the most predominant pathway/protein assignments within major COGs groups are the following: translation, ribosomal structure, and biogenesis; carbohydrate transport and metabolism; and amino acid transport and metabolism (which is consistent with our findings). However, our study is limited in its metagenomics scope; we have no information about metatranscriptomics. Previous study of gut microbiota revealed that quite a few microbial transcripts were not differentially expressed according to their genomic abundance. For instance, ribosomal biogenesis is genomically stable but differentially transcribed among subjects; ribosomal protein-coding genes tended to be overexpressed and variable at the RNA level, relative to their metagenomic abundance (Franzosaet al., 2014). So, we believe that we need to fill this knowledge gap in our future research by incorporating the study of metatranscriptomics to elucidate oral microbial function.
This study was also limited by a small sample size, which may have influenced microbial diversity to a certain extent. Furthermore, we did not collect dental plaque data, indicating the need for future studies to unravel more microbial information.
In conclusion, our results reveal the identity, composition, and structural characteristics of microorganisms in the saliva of caries-free primary dentition of preschool children. Taken together, these results deepen our understanding of the oral microecology in healthy children, and lay a foundation for future research into the relationship between oral microecology and health or disease in children.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81801028) and the Zhejiang Provincial Natural Science Foundation of China (Nos. LQ19H140002 and LGF18H140004). We are grateful to and dearly miss our departed mentor Professor Hui CHEN.
Appendix
The raw sequence reads generated in this study deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (accession Nos. SUB7100449, PRJNA610499, and SRP252283).
Author contributions
Lei XU, Zhifang WU, and Shuli DENG contributed to the conception and design of this study. Lei XU, Zhifang WU, Yuan WANG, Sa WANG, Chang SHU, and Zhuhui DUAN performed the experiments. Lei XU, Zhifang WU, and Shuli DENG performed the data analysis. Lei XU wrote the manuscript. Lei XU, Zhifang WU, and Shuli DENG revised the manuscript. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Lei XU, Zhifang WU, Yuan WANG, Sa WANG, Chang SHU, Zhuhui DUAN, and Shuli DENG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the ethics committee of the Affiliated Hospital of Stomatology, Zhejiang University School of Medicine and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from parents or guardians of all participants for being included in this study.
References
- Abou NE, Aljabo A, Strange A, et al. , 2016. Demineralizationremineralization dynamics in teeth and bone. Int J Nanomed, 11: 4743-4763. 10.2147/IJN.S107624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Grindefjord M, Dahllöf G, et al. , 2016. Oral microflora in preschool children attending a fluoride varnish program: a cross-sectional study. BMC Oral Health, 16: 130. 10.1186/s12903-016-0325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, et al. , 2012. The oral metagenome in health and disease. ISME J, 6(1): 46-56. 10.1038/ismej.2011.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn A, Heng N, Broadbent JM, et al. , 2018. Studying the human oral microbiome: challenges and the evolution of solutions. Aust Dent J, 63(1): 14-24. 10.1111/adj.12565 [DOI] [PubMed] [Google Scholar]
- Campisciano G, Toschetti A, Comar M, et al. , 2017. Shifts of subgingival bacterial population after nonsurgical and pharmacological therapy of localized aggressive periodontitis, followed for 1 year by Ion Torrent PGM platform. Eur J Dent, 11(1): 126-129. 10.4103/ejd.ejd_309_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Wang Q, O'Sullivan O, et al. , 2010. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res, 38(22): e200. 10.1093/nar/gkq873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Nizet V, Gallo RL, 2008. Skin microbiota: a source of disease or defence? Br J Dermatol, 158(3): 442-455. 10.1111/j.1365-2133.2008.08437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW, 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev, 70(4): 939-1031. 10.1128/MMBR.00024-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, et al. , 2010. The human oral microbiome. J Bacteriol, 192(19): 5002-5017. 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrosh DW, Ma B, Gajer P, et al. , 2014. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome, 2: 6. 10.1186/2049-2618-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone JDB, Lussi A, 2006. Understanding the chemistry of dental erosion. Monogr Oral Sci, 20: 66-76. 10.1159/000093351 [DOI] [PubMed] [Google Scholar]
- Franzosa EA, Morgan XC, Segata N, et al. , 2014. Relating the metatranscriptome and metagenome of the human gut. Proc Natl Acad Sci USA, 111(22): E2329-E2338. 10.1073/pnas.1319284111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, et al. , 2006. Metagenomic analysis of the human distal gut microbiome. Science, 312(5778): 1355-1359. 10.1126/science.1124234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA, 2012. The human microbiome: our second genome. Annu Rev Genomics Hum Genet, 13: 151-170. 10.1146/annurev-genom-090711-163814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara AT, Zero DT, 2010. The caries environment: saliva, pellicle, diet, and hard tissue ultrastructure. Dent Clin North Am, 54(3): 455-467. 10.1016/j.cden.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Ismail AS, Behrendt CL, Hooper LV, 2009. Reciprocal interactions between commensal bacteria and γδ intraepithelial lymphocytes during mucosal injury. J Immunol, 182(5): 3047-3054. 10.4049/jimmunol.0802705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Gao XL, Jin LJ, et al. , 2016. Salivary microbiome diversity in caries-free and caries-affected children. Int J Mol Sci, 17(12): 1978. 10.3390/ijms17121978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jiang YT, Li CL, et al. , 2011. Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresis. Microb Ecol, 61(2): 342-352. 10.1007/s00248-010-9753-z [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang J, Chen H, 2013. Pyrosequencing analysis of oral microbiota in children with severe early childhood dental caries. Curr Microbiol, 67(5): 537-542. 10.1007/s00284-013-0393-7 [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Ragunath C, Velliyagounder K, et al. , 2004. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother, 48(7): 2633-2636. 10.1128/aac.48.7.2633-2636.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, et al. , 2011. Human nutrition, the gut microbiome and the immune system. Nature, 474(7351): 327-336. 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Nam OH, Lee HS, et al. , 2016. Diversity and homogeneity of oral microbiota in healthy Korean pre-school children using pyrosequencing. Acta Odontol Scand, 74(5): 335-336. 10.3109/00016357.2015.1132006 [DOI] [PubMed] [Google Scholar]
- Ling ZX, Kong JM, Jia P, et al. , 2010. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol, 60(3): 677-690. 10.1007/s00248-010-9712-8 [DOI] [PubMed] [Google Scholar]
- Ling ZX, Liu X, Cheng YW, et al. , 2015. Decreased diversity of the oral microbiota of patients with hepatitis B virus-induced chronic liver disease: a pilot project. Sci Rep, 5: 17098. 10.1038/srep17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Faller LL, Klitgord N, et al. , 2012. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS ONE, 7(6): e37919. 10.1371/journal.pone.0037919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ, 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev, 50(4): 353-380. 10.1128/MMBR.50.4.353-380.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussi A, Hellwig E, Klimek J, 2012. Fluorides—mode of action and recommendations for use. Schweiz Monatsschr Zahnmed, 122(11): 1030-1042. [PubMed] [Google Scholar]
- Meyer F, Amaechi BT, Fabritius HO, et al. , 2018. Overview of calcium phosphates used in biomimetic oral care. Open Dent J, 12: 406-423. 10.2174/1874210601812010406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JV, Leomil L, Rodrigues-Albuquerque F, et al. , 2012. Bacterial diversity in the saliva of patients with different oral hygiene indexes. Braz Dent J, 23(4): 409-416. 10.1590/S0103-64402012000400017 [DOI] [PubMed] [Google Scholar]
- Peterson J, Garges S, Giovanni M, et al. , 2009. The NIH Human Microbiome Project. Genome Res, 19(12): 2317-2323. 10.1101/gr.096651.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zhou XD, 2020. Novel insights on the etiology, diagnosis and prevention of dental erosion. Chin J Stomatol, 55(5): 289-295 (in Chinese). 10.3760/cma.j.cn112144-20200322-00168 [DOI] [PubMed] [Google Scholar]
- Ritz S, Hahn D, Wami HT, et al. , 2020. Gut microbiome as a response marker for pancreatic enzyme replacement therapy in a porcine model of exocrine pancreas insufficiency. Microb Cell Fact, 19: 221. 10.1186/s12934-020-01482-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudney JD, Xie H, Rhodus NL, et al. , 2010. A metaproteomic analysis of the human salivary microbiota by three-dimensional peptide fractionation and tandem mass spectrometry. Mol Oral Microbiol, 25(1): 38-49. 10.1111/j.2041-1014.2009.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said HS, Suda W, Nakagome S, et al. , 2014. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res, 21(1): 15-25. 10.1093/dnares/dst037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R, 2016. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell, 164(3): 337-340. 10.1016/j.cell.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Struzycka I, 2014. The oral microbiome in dental caries. Pol J Microbiol, 63(2): 127-135. 10.33073/pjm-2014-018 [DOI] [PubMed] [Google Scholar]
- Tao L, Sutcliffe IC, Russell RRB, et al. , 1993. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans . J Dent Res, 72(10): 1386-1390. 10.1177/00220345930720100701 [DOI] [PubMed] [Google Scholar]
- Tappenden KA, Deutsch AS, 2007. The physiological relevance of the intestinal microbiota-contributions to human health. J Am Coll Nutr, 26(6): 679S-683S. 10.1080/07315724.2007.10719647 [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, et al. , 2007. The Human Microbiome Project. Nature, 449(7164): 804-810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C, Pelletier M, 1997. The phosphoenolpyruvate: sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev, 19(3): 187-207. 10.1111/j.1574-6976.1997.tb00297.x [DOI] [PubMed] [Google Scholar]
- Wade WG, 2013. The oral microbiome in health and disease. Pharmacol Res, 69(1): 137-143. 10.1016/j.phrs.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Webb AJ, Homer KA, Hosie AH, 2008. Two closely related ABC transporters in Streptococcus mutans are involved in disaccharide and/or oligosaccharide uptake. J Bacteriol, 190(1): 168-178. 10.1128/JB.01509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin BC, Luo AH, Qin J, et al. , 2013. Microbial diversity in the oral cavity of healthy Chinese Han children. Oral Dis, 19(4): 401-405. 10.1111/odi.12018 [DOI] [PubMed] [Google Scholar]
- Xu H, Hao WJ, Zhou Q, et al. , 2014. Plaque bacterial microbiome diversity in children younger than 30 months with or without caries prior to eruption of second primary molars. PLoS ONE, 9(2): e89269. 10.1371/journal.pone.0089269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye DD, Fan MM, Guan Q, et al. , 2012. A review on the bioinformatics pipelines for metagenomic research. Zool Res, 33(6): 574-585 (in Chinese). 10.3724/SP.J.1141.2012.06574 [DOI] [PubMed] [Google Scholar]
- Zaura E, Keijser BJF, Huse SM, et al. , 2009. Defining the healthy "core microbiome" of oral microbial communities. BMC Microbiol, 9: 259. 10.1186/1471-2180-9-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Li Y, Xun Z, et al. , 2017. A preliminary study on the relationship between iron and black extrinsic tooth stain in children. Lett Appl Microbiol, 64(6): 424-429. 10.1111/lam.12728 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Cui P, et al. , 2020. Gut microbiome changes associated with HIV infection and sexual orientation. Front Cell Infect Microbiol, 10: 434. 10.3389/fcimb.2020.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The raw sequence reads generated in this study deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (accession Nos. SUB7100449, PRJNA610499, and SRP252283).