Key Points
Question
Is there an intensive care unit (ICU) communication and care-planning approach that might be used to reduce nonbeneficial treatments?
Findings
In this quality improvement study of 209 patients, the use of protocoled time-limited trials (TLTs) as the default communication and care-planning approach for critically ill patients with advanced medical illnesses was associated with significant reductions in ICU length of stay and use of invasive procedures without changes in hospital mortality or family satisfaction.
Meaning
For patients with advanced illnesses who prefer aggressive care, TLTs may prioritize patients’ values and preferences and may reduce ICU treatments that prolong suffering without benefit.
This quality improvement study uses data from medical intensive care units to investigate the association between time-limited trials and nonbeneficial treatments.
Abstract
Importance
For critically ill patients with advanced medical illnesses and poor prognoses, overuse of invasive intensive care unit (ICU) treatments may prolong suffering without benefit.
Objective
To examine whether use of time-limited trials (TLTs) as the default care-planning approach for critically ill patients with advanced medical illnesses was associated with decreased duration and intensity of nonbeneficial ICU care.
Design, Setting, and Participants
This prospective quality improvement study was conducted from June 1, 2017, to December 31, 2019, at the medical ICUs of 3 academic public hospitals in California. Patients at risk for nonbeneficial ICU treatments due to advanced medical illnesses were identified using categories from the Society of Critical Care Medicine guidelines for admission and triage.
Interventions
Clinicians were trained to use TLTs as the default communication and care-planning approach in meetings with family and surrogate decision makers.
Main Outcomes and Measures
Quality of family meetings (process measure) and ICU length of stay (clinical outcome measure).
Results
A total of 209 patients were included (mean [SD] age, 63.6 [16.3] years; 127 men [60.8%]; 101 Hispanic patients [48.3%]), with 113 patients (54.1%) in the preintervention period and 96 patients (45.9%) in the postintervention period. Formal family meetings increased from 68 of 113 (60.2%) to 92 of 96 (95.8%) patients between the preintervention and postintervention periods (P < .01). Key components of family meetings, such as discussions of risks and benefits of ICU treatments (preintervention, 15 [34.9%] vs postintervention, 56 [94.9%]; P < .01), eliciting values and preferences of patients (20 [46.5%] vs 58 [98.3%]; P < .01), and identifying clinical markers of improvement (9 [20.9%] vs 52 [88.1%]; P < .01), were discussed more frequently after intervention. Median ICU length of stay was significantly reduced between preintervention and postintervention periods (8.7 [interquartile range (IQR), 5.7-18.3] days vs 7.4 [IQR, 5.2-11.5] days; P = .02). Hospital mortality was similar between the preintervention and postintervention periods (66 of 113 [58.4%] vs 56 of 96 [58.3%], respectively; P = .99). Invasive ICU procedures were used less frequently in the postintervention period (eg, mechanical ventilation preintervention, 97 [85.8%] vs postintervention, 70 [72.9%]; P = .02).
Conclusions and Relevance
In this study, a quality improvement intervention that trained physicians to communicate and plan ICU care with family members of critically ill patients in the ICU using TLTs was associated with improved quality of family meetings and a reduced intensity and duration of ICU treatments. This study highlights a patient-centered approach for treating critically ill patients that may reduce nonbeneficial ICU care.
Trial Registration
ClinicalTrials.gov Identifier: NCT04181294
Introduction
Overuse of invasive intensive care unit (ICU) treatments for patients with advanced medical illnesses and poor prognoses may lead to medical care that provides minimal benefit and prolongs suffering.1,2,3 Previous studies showed that over 20% of patients receiving invasive treatments in medical ICUs had severely reduced likelihoods of meaningful recovery.4 Although the appropriateness of ICU care in this population is subject to varying opinions, there is a general consensus that the intensity of treatments should align with the patients’ prognosis, preferences, and values.5 Previous studies suggest that many patients with advanced medical illnesses, when informed of their therapeutic options, would forgo invasive therapies and prefer palliative approaches.6,7,8,9,10,11 Unfortunately, structured care planning and communication between clinicians, critically ill patients, and families are inconsistent.12,13 As a result, critical care services are frequently delivered to patients who may not choose such care if they were fully informed of its risks, benefits, and anticipated outcomes.1,2,5 Furthermore, even when the prognoses, risks, and benefits of ICU care are discussed, patients and families frequently remain uncertain about the appropriateness of ICU care.14,15,16 In such situations, the default decision in most ICUs is to pursue aggressive ICU treatments often without reassessment of that decision.17,18
Time-limited trials (TLTs) of ICU treatments have been recommended as an approach to reduce nonbeneficial treatments among critically ill patients with advanced medical illnesses.5,17,19,20 Time-limited trials involve detailed discussions of patients’ preferences for care and prognosis followed by agreements between clinicians and patients or their surrogate decision makers to use certain medical therapies for defined periods of time. Follow-up meetings are held to see whether patients improve or worsen according to predetermined clinical parameters, and the next steps in care are negotiated based on these results.17 Time-limited trials promote regular structured dialogue between clinicians, patients, and families, and consensus in decision-making. They also set rational boundaries to treatments based on patients’ goals of care while reassuring families that all indicated interventions have been pursued. The objective of this study was to examine whether a multicomponent quality improvement intervention that uses protocoled TLTs as the default ICU care-planning approach for critically ill patients with advanced medical illnesses was associated with decreased the duration and intensity of nonbeneficial ICU care.
Methods
Study Design and Setting
This prospective quality improvement study was conducted in the medical ICUs of 3 academic public hospitals in the Los Angeles County Department of Health Services: Harbor-University of California, Los Angeles, Olive View, and Los Angeles County-University of Southern California Medical Centers. The study was conducted from June 1, 2017, to December 31, 2019. All ICUs were staffed by trainees (interns, residents, and fellows). Each ICU was managed by physician and nurse directors who championed and implemented quality improvement activities. The study population and protocol were preregistered on ClinicalTrials.gov.21 The institutional review boards and leadership of each hospital approved the project and waived the need for informed consent because the delivery of the intervention was considered a quality improvement project intended to increase clinical behaviors (timely performance of family meetings and shared decision-making) that are recommended in practice statements from professional societies.19,22 The study is reported in accordance with the Standards for Quality Improvement Reporting Excellence (SQUIRE) 2.0 reporting guideline.23
Study Population
Patients at risk for potentially nonbeneficial ICU treatments because of advanced medical illnesses were identified by assigning categories based on the Society of Critical Care Medicine (SCCM) guidelines for admission and triage.24 All ICU physicians were trained to perform daily assessments of ICU patients using these guidelines. Our experience with training ICU teams to classify patients using this system has previously been published.4 ICU physicians were asked to categorize patients based on their likelihood of benefit from ICU treatments each day. All new admissions deemed to be critically ill, though less likely to benefit from aggressive ICU treatments owing to underlying medical conditions or severity of acute illness (category 3 in SCCM guidelines), were eligible. Because patient populations at risk for nonbeneficial treatments varied at each hospital, the ICU directors created common clinical examples to help clinicians recognize patients considered at risk for nonbeneficial treatments. Although these assessments of benefit are subjective, this approach was chosen because it is pragmatic and guideline-recommended and mirrors clinical practice. Patients who were initially assessed by clinicians to have a high likelihood of benefit but experienced clinical deterioration during ICU hospitalization to potentially nonbeneficial states were excluded. Patients who could not communicate for themselves and did not have surrogate decision makers were also excluded.
Quality Improvement Interventions
The framework for meeting with families and initiating TLTs is shown in eFigure 1 in the Supplement. Barriers to ICU communication, conceptual frameworks for developing interventions, and implementation strategy were previously described.25 Quality improvement interventions targeted clinicians and medical ICUs, not individual patients or family members. Training of clinicians was divided into 3 components delivered over the course of 4 to 6 weeks: (1) focus groups of physicians to identify barriers to using TLTs, (2) didactic sessions to define TLTs and review protocols for using TLTs, and (3) simulations of family meetings with actors as family members using the TLT protocol. Simulation sessions were facilitated by palliative care faculty with formal training in teaching communication skills. A TLT conversation guide was created to assist clinicians during family meetings; it consisted of a checklist of key components to be discussed in family meetings and sample phrases to use while discussing each component (eTable 1 in the Supplement). These components included (1) introductions, (2) explaining medical conditions, (3) defining acute care needs and prognosis, (4) eliciting patients’ values and preferences, (5) planning a TLT, and (6) setting timelines for follow-up meetings. The format was adapted from conversation guides on http://www.vitaltalk.org. Clinicians were encouraged, but not mandated, to use the conversation guide during family meetings. Other quality improvement interventions included care managers to schedule family meetings as well as regular meetings between clinicians and institutional ICU directors to discuss challenging cases and receive feedback on the improvement strategy. The conceptual framework for these interventions was based on the Capability, Opportunity, Motivation Behavior framework by Michie et al26 and addressed barriers identified in our preliminary studies that inhibit capabilities, opportunities, and motivation for effective shared decision-making (eFigure 2 in the Supplement).25,27,28
Quality improvement interventions were implemented sequentially at each hospital. Data were collected for 4 months before and after the intervention. Study timelines are shown in eTable 2 in the Supplement.
Data Collection
Clinical data were collected prospectively using electronic health records. Clinical outcomes including ICU and hospital lengths of stay (LOS) and outcomes of hospitalization (death, discharge to hospice, skilled nursing facility, or home) were collected after discharge. The ICU clinicians were asked to notify study personnel when family meetings were performed. Trained study personnel attended family meetings occurring on weekdays during daytime work hours and collected information using a standardized data collection form. Formal family meetings were defined as those scheduled by ICU teams or at the request of the patients’ family member(s), occurring in designated meeting rooms outside of the ICU (as opposed to ad hoc meetings, which were informal updates and discussions at bedside). The Family Satisfaction in the Intensive Care Unit (FS-ICU) survey was used to evaluate satisfaction with care and decision-making. The FS-ICU survey is a validated tool that assesses satisfaction with ICU care (24 items) with subscale rankings for satisfaction with medical care (14 items) and satisfaction with decision-making (10 items). Owing to limitations in study personnel, surveys were distributed to family members in 2 of the 3 hospitals (Harbor-University of California, Los Angeles Medical Center and Los Angeles County-University of Southern California Medical Center). Surveys were distributed after at least 72 hours of ICU hospitalization to ensure that families had opportunities to communicate with ICU care clinicians. All surveys were anonymous, and no identifying information about patients or respondents were collected. The institutional review board at each institution approved the use of anonymous surveys.
Statistical Analysis
Preintervention and postintervention clinical outcomes and use of ICU treatments were compared using t tests or Wilcoxon rank sum tests for continuous variables and χ2 tests for dichotomous variables. The primary outcome was ICU LOS. Based on our previous studies examining prevalence of potentially nonbeneficial ICU treatments, we estimated studying 130 patients during each study period (mean [SD] ICU LOS, 6.5 [3.7] days) and having 80% power to detect a difference of 1.3 ICU days.4 Multivariable linear regression analysis was used to adjust for prespecified covariates of age, comorbidities (Charlson Comorbidity Index), severity of illness (Acute Physiology and Chronic Health Evaluation [APACHE] score), primary ICU diagnosis, and hospital in the before-after analysis. Interrupted time-series analysis using segmented linear regression was performed as a sensitivity analysis to examine trends in log-transformed ICU LOS before and after the intervention.29,30 Interrupted time-series models were adjusted for covariates (age, Charlson Comorbidity Index, APACHE score, primary ICU diagnosis, and hospital) and included a constant, a baseline slope term to control for secular trends and terms estimating changes in level, and a slope of ICU LOS after the intervention was introduced. The unit of analysis was individual hospitalizations. Interrupted time-series analyses were conducted using SAS Proc Autoreg, version 9.3 (SAS Institute Inc) for time-series models. Distributions of ICU LOS between study periods were also examined with cumulative distribution functions and compared using the Kolmogorov-Smirnov test.31
Secondary outcomes included hospital LOS, days receiving life-sustaining treatments (mechanical ventilation, vasopressor medications, and renal replacement therapy), number of attempts at cardiopulmonary resuscitation, number of invasive procedures (central venous catheterization, thoracentesis, paracentesis, lumbar puncture, and endoscopy), and hospital mortality. Prespecified exploratory subgroup analyses examined primary and secondary outcomes stratified by survivors and nonsurvivors. The main process measure was quality of family meetings. The proportion of patients who had formal family meetings, median ICU day of first meetings, and how frequently key content elements were discussed were compared before and after the intervention. The FS-ICU surveys were also compared between study periods. Total satisfaction and subscale scores were calculated by linearly transforming scores from 0 to 100, oriented so that higher scores indicate greater satisfaction, and averaging survey items as previously described.32,33 P < .05 was considered statistically significant, and all P values were 2-sided. Analyses were performed using R software, version 3.6.2 (R Foundation) and SAS, version 9.3 (SAS Institute Inc).
Results
Patient Characteristics
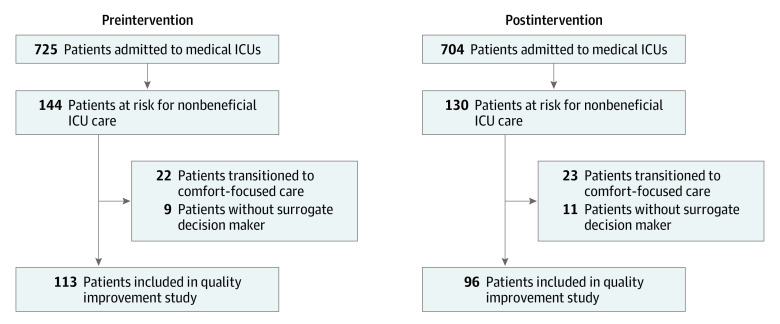
There were 725 patients admitted to the medical ICUs of participating hospitals during the preintervention period and 704 patients during the postintervention period (Figure 1). Of these, 144 patients in the preintervention and 130 patients in the postintervention periods were considered by ICU clinicians to be at risk for nonbeneficial treatments. After excluding patients who were transitioned to comfort-focused care after initial discussions with patients or surrogate decision makers (n = 45) and those without surrogate decision makers (n = 20), there were a total of 209 patients (mean [SD] age, 63.6 [16.3] years; 127 men [60.8%]; 101 Hispanic patients [48.3%]), including 113 (54.1%) in the preintervention period and 96 (45.9%) in the postintervention period (Figure 1 and Table 1). Distributions of sex, race/ethnicity, Charlson Comorbidity Index, and APACHE II score were similar between study periods (Table 1). Debilitating and progressive medical conditions, such as advanced dementia (preintervention, 21 of 113 [18.6%] vs postintervention 16 of 96 [16.7%]) and malignancy (32 [28.3%] vs 31 [32.3%]), were common in both periods (Table 1). The most common ICU diagnoses were acute respiratory failure (preintervention, 41 [36.3%] vs postintervention, 33 [34.4%]), cardiopulmonary arrest (31 [27.4%] vs 30 [32.3%]), and shock (33 [29.2%] vs 17 [17.7%]) (Table 1).
Figure 1. Patient Enrollment in Preintervention and Postintervention Study Periods.
ICU indicates intensive care unit.
Table 1. Baseline Characteristics of Study Population in Preintervention and Postintervention Periods.
| Variable | No. (%)a | |
|---|---|---|
| Preintervention (n = 113) | Postintervention (n = 96) | |
| Age, mean (SD), y | 62.2 (16.2) | 65.2 (16.3) |
| Women | 45 (39.8) | 37 (38.5) |
| Race/ethnicity | ||
| White | 19 (16.8) | 17 (17.7) |
| Black | 20 (17.7) | 16 (16.7) |
| Hispanic | 57 (50.4) | 44 (45.8) |
| Asian | 17 (15.0) | 19 (19.8) |
| Medical comorbidities | ||
| Myocardial infarction | 16 (14.2) | 16 (16.7) |
| Congestive heart failure | 22 (19.5) | 15 (15.6) |
| Dementia | 21 (18.6) | 16 (16.7) |
| Cerebrovascular disease | 9 (8.0) | 16 (16.7) |
| Chronic obstructive pulmonary disease | 22 (19.5) | 20 (20.8) |
| Diabetes | 44 (38.9) | 36 (37.5) |
| Chronic kidney disease | 19 (17.0) | 25 (26.0) |
| Malignancy | 32 (28.3) | 31 (32.3) |
| Cirrhosis | 16 (14.2) | 16 (16.7) |
| Charlson Comorbidity Index, mean (SD) | 6.3 (2.9) | 6.7 (2.1) |
| Primary ICU diagnosis | ||
| Acute respiratory failure | 41 (36.3) | 33 (34.4) |
| Cardiopulmonary arrest | 31 (27.4) | 30 (32.3) |
| Shock | 33 (29.2) | 17 (17.7) |
| Other | 8 (7.1) | 16 (16.7) |
| APACHE II score, mean (SD) | 23.0 (7.7) | 23.3 (8.3) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit.
Values are expressed as No. (%) unless otherwise specified.
Family Meetings
Formal family meetings occurred for 68 of 113 (60.2%) and 92 of 96 (95.8%) patients in the preintervention and postintervention periods, respectively (P < .001) (Table 2). In the preintervention period, median ICU days to first family meeting was 5.5 (interquartile range [IQR], 2.0-9.0) days. This was reduced to 1.0 (IQR, 1.0-2.0) days after the intervention (P < .001) (Table 2). In the preintervention period, many key components of family meetings were infrequently discussed (Table 2), including discussions of risks and benefits of ICU treatments (15 of 43 meetings [34.9%]), eliciting values and preferences of patients (20 [46.5%]), identifying clinical markers of improvement (9 [20.9%]), and making recommendations for next steps in management (18 [41.9%]). In the postintervention periods, most of these components were addressed in greater than 90% of family meetings (56 [94.9%], 58 [98.3%], 52 [88.1%], and 57 [96.6%] of 59, respectively; P < .01 for all) (Table 2).
Table 2. Study Outcomes.
| Variable | No. (%)a | P value | |
|---|---|---|---|
| Preintervention (n = 113) | Postintervention (n = 96) | ||
| ICU LOS, median (IQR), d | 8.7 (5.7-18.3) | 7.4 (5.2-11.5) | .02 |
| Multivariable analysis, reduction, % (95% CI)b | 24.9 (8.6-38.2) | .004 | |
| Hospital LOS, median (IQR), d | 14.2 (7.4-27.2) | 10.7 (6.7-18.1) | .01 |
| Multivariable analysis, reduction, % (95% CI)b | 28.8 (10.4-43.4) | .005 | |
| Family meetings | 68 (60.2) | 92 (95.8) | <.001 |
| Day of first meeting, median (IQR) | 5.5 (2.0-9.0) | 1.0 (1.0-2.0) | <.001 |
| Family meeting components | |||
| No. | 43 | 59 | |
| Description of medical issues | 42 (97.7) | 59 (100) | .24 |
| Discuss prognosis | 34 (79.1) | 59 (100) | <.001 |
| Discuss risk/benefit | 15 (34.9) | 56 (94.9) | <.001 |
| Elicit values and preferences | 20 (46.5) | 58 (98.3) | <.001 |
| Identify improvement markers | 9 (20.9) | 52 (88.1) | <.001 |
| Recommendations for next steps | 18 (41.9) | 57 (96.6) | <.001 |
| Questions | 32 (74.4) | 59 (100) | <.001 |
| Elicit understanding | 24 (55.8) | 55 (93.2) | <.001 |
| ICU procedures | |||
| Cardiopulmonary resuscitation in ICU | 14 (12.4) | 6 (6.3) | .32 |
| Vasopressor | 62 (54.9) | 50 (52.1) | .64 |
| Treatment time, median (IQR), d | 6.0 (3.0-11.3) | 4.0 (3.0-6.5) | NA |
| Noninvasive ventilation | 17 (15.0) | 13 (13.5) | .76 |
| Mechanical ventilation | 97 (85.8) | 70 (72.9) | .02 |
| Treatment time, median (IQR), d | 8.0 (5.0-17.5) | 7.0 (5.0-12.0) | NA |
| Renal replacement therapy | 34 (30.1) | 19 (19.8) | .09 |
| Thoracentesis | 5 (4.4) | 3 (3.1) | .39 |
| Paracentesis | 9 (8.0) | 4 (4.2) | .53 |
| Lumbar puncture | 5 (4.4) | 0 | .11 |
| Gastrointestinal endoscopy | 9 (8.0) | 7 (7.3) | .59 |
| Bronchoscopy | 28 (24.8) | 10 (10.4) | .03 |
| Central venous catheter | 81 (71.7) | 38 (40.0) | <.001 |
| Do-not-resuscitate order | |||
| Present on ICU admission | 63 (55.8) | 55 (57.3) | .82 |
| Present any time during hospitalization | 80 (70.8) | 86 (89.6) | <.001 |
| ICU mortality | 51 (45.1) | 39 (40.6) | .51 |
| Hospital mortality | 66 (58.4) | 56 (58.3) | .99 |
| Hospital disposition of survivors | |||
| Hospice | 10 (8.9) | 11 (11.5) | .13 |
| Skilled nursing facility | 30 (26.6) | 18 (18.8) | |
| Home | 7 (6.2) | 11 (11.5) | |
| Family satisfaction with ICU care (FS-ICU) | |||
| No. | 36 | 33 | NA |
| Total score, mean (SD) | 83.4 (13.3) | 84.8 (6.3) | .55 |
| Satisfaction with medical care, mean (SD) | 83.1 (13.3) | 83.6 (7.2) | .86 |
| Satisfaction with decision-making subscale, mean (SD) | 83.6 (14.9) | 86.2 (8.3) | .38 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; FS-ICU, Family Satisfaction in the Intensive Care Unit; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; NA, not applicable.
Values are expressed as No. (%) unless otherwise specified.
Shown are back-transformed (100 × [1-eβ]) β coefficients for the multivariable linear regression model of log LOS adjusted for age, Charlson Comorbidity Index, APACHE II score, and ICU admission diagnosis and hospital.
Clinical Outcomes
The primary and secondary outcomes are summarized in Table 2. The median ICU LOS was significantly reduced between the preintervention and postintervention periods (8.7 [IQR, 5.7-18.3] days vs 7.4 [IQR, 5.2-11.5] days; P = .02). Similarly, the median hospital LOS was also shorter in the postintervention period (14.2 [IQR, 7.4-27.2] days vs 10.7 [IQR, 6.7-18.1] days; P = .01). Many ICU procedures were used less frequently in the postintervention period (Table 2). For example, 97 of 113 patients (85.8%) received mechanical ventilation in the preintervention period compared with 70 of 96 patients (72.9%) after intervention (P = .02). Of patients receiving mechanical ventilation, median duration of treatment was reduced from 8.0 (IQR, 5.0-17.5) days to 7.0 (IQR, 5.0-12.0) days. Do-not-resuscitate orders were present in 63 (55.8%) and 55 (57.3%) patients on ICU admission in the preintervention and postintervention periods, respectively. More patients received do-not-resuscitate orders during hospitalization in the postintervention (86 patients [89.6%]) compared with preintervention (80 patients [70.8%]) periods (P < .01). Despite reductions in LOS and intensity of treatments, hospital mortality was similar between the preintervention and postintervention periods (66 [58.4%] vs 56 [58.3%], respectively; P = .99). Reductions in the median ICU LOS was greater in nonsurvivors (10.1 [IQR, 5.6-18.8] days vs 7.4 [IQR, 4.8-12.3] days in the preintervention and postintervention periods, respectively; P = .02) compared with survivors (7.8 days [5.7-16.4 days] vs 7.3 days [5.3-11.4 days]; P = .38) (Table 3). Similarly, reductions in the intensity of ICU treatments were greater in nonsurvivors compared with survivors (mechanical ventilation preintervention and postintervention, 60 [90.9%] and 42 [75.0%], P = .02 vs 37 [78.7%] and 28 [70.0%], P = .35) (Table 3).
Table 3. Study Outcomes Stratified by Survivors and Nonsurvivors of Hospitalization.
| Variable | Nonsurvivors, No. (%)a | P value | Survivors, No. (%) | P value | |||
|---|---|---|---|---|---|---|---|
| Preintervention (n = 66) | Postintervention (n = 56) | Preintervention (n = 47) | Postintervention (n = 40) | ||||
| ICU LOS, median (IQR), d | 10.1 (5.6 to 18.8) | 7.4 (4.8 to 12.3) | .02 | 7.8 (5.7 to 16.4) | 7.3 (5.3 to 11.4) | .38 | |
| Multivariable analysis, reduction, % (95% CI)b | 30.2 (9.0 to 46.5) | .01 | 18.9 (−11.1 to 40.8) | .19 | |||
| Hospital LOS, median (IQR), d | 14.3 (6.6 to 22.9) | 8.4 (5.3 to 14.4) | .01 | 13.9 (8.0 to 42.4) | 13.0 (8.8 to 21.6) | .44 | |
| Multivariable analysis, reduction, % (95% CI)b | 35.1 (12.3 to 51.8) | .01 | 19.7 (−14.6 to 43.8) | .23 | |||
| Family meetings | 43 (65.2) | 54 (96.4) | <.001 | 18 (38.3) | 38 (95.0) | <.001 | |
| Day of first meeting, median (IQR) | 6 (2 to 11) | 2 (1 to 2) | NA | 4 (1 to 6) | 1 (1 to 2) | NA | |
| ICU procedures | |||||||
| Cardiopulmonary resuscitation in ICU | 10 (15.2) | 6 (10.7) | .57 | 4 (8.5) | 0 | .31 | |
| Vasopressor | 41 (62.1) | 33 (58.9) | .72 | 21 (44.7) | 17 (42.5) | .73 | |
| Treatment time, median (IQR), d | 7.0 (4.0 to 13.0) | 5.0 (2.5 to 8.0) | NA | 5.0 (2.5 to 8.5) | 4.0 (3.3 to 5.8) | NA | |
| Noninvasive ventilation | 9 (13.6) | 6 (10.7) | .62 | 8 (17.0) | 7 (17.5) | .95 | |
| Mechanical ventilation | 60 (90.9) | 42 (75.0) | .02 | 37 (78.7) | 28 (70.0) | .35 | |
| Treatment time, median (IQR), d | 9.5 (5.3 to 17.8) | 7.0 (4.0 to 12.3) | NA | 7.0 (4.5 to 18.5) | 7.0 (5.0 to 11.5) | NA | |
| Renal replacement therapy | 20 (30.3) | 9 (16.1) | .06 | 14 (29.8) | 10 (25.0) | .62 | |
| Thoracentesis | 4 (6.1) | 1 (1.8) | .20 | 1 (2.3) | 2 (5.0) | .46 | |
| Paracentesis | 6 (9.1) | 3 (5.4) | .62 | 3 (6.4) | 1 (2.5) | .39 | |
| Lumbar puncture | 3 (4.5) | 0 | .27 | 2 (4.3) | 0 | .42 | |
| GI endoscopy | 5 (7.6) | 3 (5.4) | .40 | 4 (8.5) | 4 (10.0) | .97 | |
| Bronchoscopy | 18 (27.3) | 6 (10.7) | .09 | 10 (21.3) | 4 (10.0) | .42 | |
| Central venous catheter | 49 (74.2) | 27 (48.2) | .01 | 32 (68.1) | 11 (27.5) | <.001 | |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; GI, gastrointestinal; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; NA, not applicable.
Values are expressed as No. (%) unless otherwise specified.
Shown are back-transformed (100 × [1-eβ]) β coefficients for the multivariable linear regression model of log LOS adjusted for age, Charlson Comorbidity Index, APACHE II score, and ICU admission diagnosis and hospital.
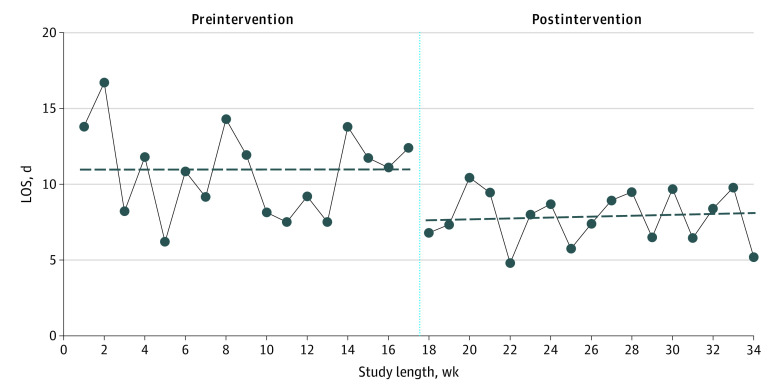
Multivariable linear regression analysis showed that ICU LOS was reduced by 24.9% (95% CI, 8.6%-38.2%) in the postintervention group after controlling for age, comorbid conditions, ICU diagnosis, severity of illness, and hospital (Table 2). Trends in study outcomes before and after the study interventions were consistent in all 3 hospitals (eTable 3 in the Supplement). Interrupted time-series analysis showed an abrupt decrease in ICU LOS of 3.3 days (unadjusted) at the start of the postintervention period (95% CI, –6.52 to –0.08 days; P = .045) (Figure 2 and eTable 4 in the Supplement). This decrease in ICU LOS remained similar (3.7 days; 95% CI, –7.28 to –0.16 days) after adjusting for covariates (eTable 4 in the Supplement). Control charts of ICU LOS by individual patients showed reductions in variability of ICU LOS and prolonged ICU hospitalizations in the postintervention period. Intensive care unit LOS for 18 hospitalizations were above the upper boundary (2 SD) in the preintervention period compared with 4 hospitalizations in the postintervention period (eFigure 3 in the Supplement). Cumulative distribution curves for ICU LOS before and after the study intervention showed that probabilities of prolonged ICU hospitalizations were lower in the postintervention period (65.5% vs 81.3% for hospitalizations with ICU LOS <14 days between the preintervention and postintervention periods; P = .03) (eFigure 4 in the Supplement).
Figure 2. Interrupted Time-Series Analysis of Intensive Care Unit (ICU) Length of Stay (LOS).
The solid line indicates trends in median ICU LOS by study weeks. The vertical dotted line separates the preintervention and postintervention periods. The dashed horizontal lines represent predicted values from segmented regression analysis (preintervention, β = −0.001; postintervention, β = 0.024). There was an abrupt decrease of 3.3 days at the start of the postintervention period (95% CI, –6.52 to –0.08 days; P = .045).
Satisfaction With Care
Of 165 patients with ICU admissions at Harbor-University of California, Los Angeles Medical Center and Los Angeles County-University of Southern California Medical Center, 69 (41.8%) completed the FS-ICU survey (Table 2). Family satisfaction with care, as assessed by the FS-ICU mean (SD) total score, was 83.4 (13.3) points and 84.8 (6.3) points in the preintervention and postintervention periods, respectively (P = .55). Satisfaction with the medical care subscale was 83.1 (13.3) points preintervention and 83.6 (7.2) points postintervention (P = .86). Satisfaction with the decision-making subscale was 83.6 (14.9) points and 86.2 (8.3) points in the preintervention and postintervention periods, respectively (P = .38).
Discussion
In this study, we implemented a quality improvement intervention that trained physicians to communicate and plan ICU care with family members of gravely ill patients using TLTs. After the intervention, family meetings occurred more frequently and earlier in the ICU hospitalization and were more likely to address topics that are important for effective shared decision-making. The intervention was associated with decreases in ICU and hospital LOS and use of invasive ICU treatments without a change in the hospital mortality. In addition, unwanted variation in ICU LOS and probability of prolonged hospitalizations were reduced. Prespecified subgroup analyses showed greater decreases in LOS and invasive treatments among those who died; these exploratory analyses suggest greater reductions in invasive treatments may occur among those who are unlikely to survive hospitalization despite aggressive ICU care.
Our findings are consistent with previous studies of communication interventions in ICU patients.32,34,35,36,37,38 Curtis et al38 used communication facilitators among ICU patients at high risk for death to reduce intensity of end-of-life ICU treatments. White and colleagues37 showed that family support interventions delivered by trained interprofessional teams improved quality of communication and reduced ICU LOS among seriously ill ICU patients. Previous studies such as these have generally examined patients at high risk for death, typically enrolling those on prolonged mechanical ventilation or for whom physicians estimated high risks of dying.32,34,37,38,39 In contrast, our study addressed situations in which clinicians believed that ICU treatments may be nonbeneficial, but patients or surrogate decision makers still favored pursuing intensive care. In such situations, it is especially important to mitigate risks for conflict by reassuring families that all indicated treatments have been pursued, developing rapport, and allowing time for emotional adjustment.40 Time-limited trials represent a way to achieve these goals and allow families and physicians to reach consensus. Another important distinction from previous studies was that our intervention was performed in a large public health care system serving racially diverse and primarily indigent patients. This patient population has been underrepresented in previous studies of ICU communication.32,34,35,36,37,38 Our findings support the feasibility of pragmatic communication interventions in this population despite disproportionate challenges in factors such as language concordance and health literacy.41
Distinctions between our study and previous work highlight the importance of understanding context and environment when evaluating complex ICU communication interventions. Guidelines from the SCCM on family-centered ICU care recommend routine family conferences using structured approaches for communication.42,43 Among the ICUs in our study, compliance with these recommendations was low at baseline with numerous opportunities for improvement in communication practices. Our study was also conducted in teaching hospitals. Clinicians in these teaching environments may have more malleable practice patterns compared to ICUs staffed by experienced clinicians with more established practice preferences. Interventions were also conducted in the home institutions of the investigators and project champions. Project champions included medical directors and administrative leaders of participating ICUs, increasing the likelihood of uptake of study interventions into practice.
Finally, it is important to clarify the goal of TLTs in our study. For critically ill patients with advanced medical illnesses, decisions to pursue aggressive ICU treatments are value laden and preference sensitive. Time-limited trials were not intended to limit care or pressure families into uncomfortable decisions. Instead, the goal was to create opportunities for clinicians to understand the values and preferences of patients and families, discuss risks and benefits of ICU treatments, and align ICU care with these preferences. Through this process of sharing information and examining the effects of ICU treatments together, it may have been easier to recognize when invasive treatments were not achieving their intended aims and place rational limits to minimize unnecessary suffering.44
Limitations
Our study has some important limitations. First, the before-and-after design makes the study susceptible to temporal trends that could bias patient selection and study outcomes. However, several findings support the interpretation that such biases were small. Baseline characteristics of the preintervention and postintervention study groups were similar. In addition, similar proportions of patients admitted to ICUs were enrolled between study periods, suggesting that clinicians’ propensities to consider patients at risk for nonbeneficial treatments did not change. Study outcomes also remained statistically significant after adjustment for differences in baseline characteristics and temporal trends using regression analyses. In order to minimize biases in patient selection, approaches to identify patients at risk for nonbeneficial ICU treatments remained consistent between study periods. Quality improvement training focused on improving communication and using TLTs and did not modify definitions of nonbeneficial treatments or prognostication. Second, it is not possible to know which elements of our multicomponent intervention facilitated changes in physician behaviors and clinical outcomes. For example, decreases in ICU LOS and ventilator days may also be related to conducting family meetings earlier in the ICU hospitalization. However, we chose a multifaceted approach because previous studies showed that interventions need to target multiple aspects of physician practice to be effective.45 Third, FS-ICU surveys may not be sensitive to feelings of discomfort or dissatisfaction with TLTs and family meetings. Qualitative assessments of family members’ experiences need to be performed to capture these perspectives. Finally, we were not able to evaluate the sustainability of our intervention. Important future directions include examining whether our intervention translates to other health care environments and what factors affect whether improvements are sustained.
Conclusions
In summary, a quality improvement intervention that trained physicians to communicate with family members of critically ill patients in the ICU using TLTs was associated with improved quality of family meetings and reduced intensity and duration of nonbeneficial ICU treatments without changing hospital mortality or worsening family satisfaction. Our study highlights an approach that prioritizes patients’ values and preferences and may reduce disproportionate use of nonbeneficial ICU treatments.
eTable 1. Time-Limited Trial Conversation Guide
eTable 2. Study Timeline
eTable 3. Study Outcomes by Hospital
eTable 4. Interrupted Time-Series Analysis of Intensive Care Unit Length of Stay
eFigure 1. Study Flowchart for Conducting Family Meetings and Implementing Time-Limited Trials
eFigure 2. Conceptual Framework for Study Interventions
eFigure 3. Shewhart Control Chart of Intensive Care Unit Length of Stay by Individual Patients in Preintervention and Postintervention Periods
eFigure 4. Cumulative Distribution Curves for Patients in the Preintervention and Postintervention Periods
References
- 1.Curtis JR, Rubenfeld GD. Improving palliative care for patients in the intensive care unit. J Palliat Med. 2005;8(4):840-854. doi: 10.1089/jpm.2005.8.840 [DOI] [PubMed] [Google Scholar]
- 2.Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. Chest. 2008;134(4):835-843. doi: 10.1378/chest.08-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh TN, Kleerup EC, Wiley JF, et al. The frequency and cost of treatment perceived to be futile in critical care. JAMA Intern Med. 2013;173(20):1887-1894. doi: 10.1001/jamainternmed.2013.10261 [DOI] [PubMed] [Google Scholar]
- 4.Chang DW, Dacosta D, Shapiro MF. Priority levels in medical intensive care at an academic public hospital. JAMA Intern Med. 2017;177(2):280-281.10.1001/jamainternmed.2016.8060 doi: 10.1001/jamainternmed.2016.8060 [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Engelberg RA, Bensink ME, Ramsey SD. End-of-life care in the intensive care unit: can we simultaneously increase quality and reduce costs? Am J Respir Crit Care Med. 2012;186(7):587-592. doi: 10.1164/rccm.201206-1020CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC, Barnato AE, Linde-Zwirble WT, et al. ; Robert Wood Johnson Foundation ICU End-Of-Life Peer Group . Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. doi: 10.1097/01.CCM.0000114816.62331.08 [DOI] [PubMed] [Google Scholar]
- 7.Fields MJ CC. Approach Death, Improving Care at the End of Life. National Academy Press; 1997. [PubMed] [Google Scholar]
- 8.Pritchard RS, Fisher ES, Teno JM, et al. ; SUPPORT Investigators . Study to understand prognoses and preferences for risks and outcomes of treatment: influence of patient preferences and local health system characteristics on the place of death. J Am Geriatr Soc. 1998;46(10):1242-1250. doi: 10.1111/j.1532-5415.1998.tb04540.x [DOI] [PubMed] [Google Scholar]
- 9.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24(6):695-701. doi: 10.1007/s11606-009-0952-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: a study of the US Medicare population. Med Care. 2007;45(5):386-393. doi: 10.1097/01.mlr.0000255248.79308.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankl D, Oye RK, Bellamy PE. Attitudes of hospitalized patients toward life support: a survey of 200 medical inpatients. Am J Med. 1989;86(6 pt 1):645-648. doi: 10.1016/0002-9343(89)90436-1 [DOI] [PubMed] [Google Scholar]
- 12.Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J. 2004;24(2):200-205. doi: 10.1183/09031936.04.00010104 [DOI] [PubMed] [Google Scholar]
- 13.McNeely PD, Hebert PC, Dales RE, et al. Deciding about mechanical ventilation in end-stage chronic obstructive pulmonary disease: how respirologists perceive their role. CMAJ . 1997;156(2):177-183. [PMC free article] [PubMed]
- 14.Elpern EH, Patterson PA, Gloskey D, Bone RC. Patients’ preferences for intensive care. Crit Care Med. 1992;20(1):43-47. doi: 10.1097/00003246-199201000-00014 [DOI] [PubMed] [Google Scholar]
- 15.Wu C, Melnikow J, Dinh T, et al. Patient admission preferences and perceptions. West J Emerg Med. 2015;16(5):707-714. doi: 10.5811/westjem.2015.7.27458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodlin SJ, Zhong Z, Lynn J, et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333-2339. doi: 10.1001/jama.282.24.2333 [DOI] [PubMed] [Google Scholar]
- 17.Quill TE, Holloway R. Time-limited trials near the end of life. JAMA. 2011;306(13):1483-1484. doi: 10.1001/jama.2011.1413 [DOI] [PubMed] [Google Scholar]
- 18.Bernacki RE, Block SD; American College of Physicians High Value Care Task Force . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003. doi: 10.1001/jamainternmed.2014.5271 [DOI] [PubMed] [Google Scholar]
- 19.Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared decision-making in intensive care units. executive summary of the American College of Critical Care Medicine and American Thoracic Society policy statement. Am J Respir Crit Care Med. 2016;193(12):1334-1336. doi: 10.1164/rccm.201602-0269ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kon AA, Shepard EK, Sederstrom NO, et al. Defining futile and potentially inappropriate interventions: a policy statement from the Society of Critical Care Medicine Ethics Committee. Crit Care Med. 2016;44(9):1769-1774. doi: 10.1097/CCM.0000000000001965 [DOI] [PubMed] [Google Scholar]
- 21.Time limited trials to reduce non-beneficial intensive care unit treatments. ClinicalTrials.gov identifier: NCT04181294. Updated February 20, 2020. Accessed November 29, 2019. https://clinicaltrials.gov/ct2/show/NCT04181294
- 22.Truog RD, Campbell ML, Curtis JR, et al. ; American Academy of Critical Care Medicine . Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College of Critical Care Medicine. Crit Care Med. 2008;36(3):953-963. doi: 10.1097/CCM.0B013E3181659096 [DOI] [PubMed] [Google Scholar]
- 23.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi: 10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633-638. doi: 10.1097/00003246-199903000-00048 [DOI] [PubMed] [Google Scholar]
- 25.Chang D, Parrish J, Kamangar N, Liebler J, Lee M, Neville T. Time-limited trials among critically ill patients with advanced medical illnesses to reduce nonbeneficial intensive care unit treatments: protocol for a multicenter quality improvement study. JMIR Res Protoc. 2019;8(11):e16301. doi: 10.2196/16301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53. doi: 10.1186/s13012-015-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss CH, Krishnan JA, Au DH, et al. ; ATS Ad Hoc Committee on Implementation Science . An official American Thoracic Society Research Statement: implementation science in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2016;194(8):1015-1025. doi: 10.1164/rccm.201608-1690ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. doi: 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 31.McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163-169. doi: 10.1586/erp.11.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis JR, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183(3):348-355. doi: 10.1164/rccm.201006-1004OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wall RJ, Engelberg RA, Downey L, Heyland DK, Curtis JR. Refinement, scoring, and validation of the Family Satisfaction in the Intensive Care Unit (FS-ICU) survey. Crit Care Med. 2007;35(1):271-279. doi: 10.1097/01.CCM.0000251122.15053.50 [DOI] [PubMed] [Google Scholar]
- 34.Mosenthal AC, Murphy PA, Barker LK, Lavery R, Retano A, Livingston DH. Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma. 2008;64(6):1587-1593. doi: 10.1097/TA.0b013e318174f112 [DOI] [PubMed] [Google Scholar]
- 35.Daly BJ, Douglas SL, O’Toole E, et al. Effectiveness trial of an intensive communication structure for families of long-stay ICU patients. Chest. 2010;138(6):1340-1348. doi: 10.1378/chest.10-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilly CM, De Meo DL, Sonna LA, et al. An intensive communication intervention for the critically ill. Am J Med. 2000;109(6):469-475. doi: 10.1016/S0002-9343(00)00524-6 [DOI] [PubMed] [Google Scholar]
- 37.White DB, Angus DC, Shields AM, et al. ; PARTNER Investigators . A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378(25):2365-2375. doi: 10.1056/NEJMoa1802637 [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Treece PD, Nielsen EL, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med. 2016;193(2):154-162. doi: 10.1164/rccm.201505-0900OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316(1):51-62. doi: 10.1001/jama.2016.8474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vink EE, Azoulay E, Caplan A, Kompanje EJO, Bakker J. Time-limited trial of intensive care treatment: an overview of current literature. Intensive Care Med. 2018;44(9):1369-1377. doi: 10.1007/s00134-018-5339-x [DOI] [PubMed] [Google Scholar]
- 41.Turnbull AE, Davis WE, Needham DM, White DB, Eakin MN. Intensivist-reported facilitators and barriers to discussing post-discharge outcomes with intensive care unit surrogates: a qualitative study. Ann Am Thorac Soc. 2016;13(9):1546-1552. doi: 10.1513/AnnalsATS.201603-212OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson JE, Aslakson RA, Long AC, et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45(1):103-128. doi: 10.1097/CCM.0000000000002169 [DOI] [PubMed] [Google Scholar]
- 43.Davidson JE, Powers K, Hedayat KM, et al. ; American College of Critical Care Medicine Task Force 2004-2005, Society of Critical Care Medicine . Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004-2005. Crit Care Med. 2007;35(2):605-622. doi: 10.1097/01.CCM.0000254067.14607.EB [DOI] [PubMed] [Google Scholar]
- 44.Anesi GL, Admon AJ, Halpern SD, Kerlin MP. Understanding irresponsible use of intensive care unit resources in the USA. Lancet Respir Med. 2019;7(7):605-612. doi: 10.1016/S2213-2600(19)30088-8 [DOI] [PubMed] [Google Scholar]
- 45.Khandelwal N, Long AC, Lee RY, McDermott CL, Engelberg RA, Curtis JR. Pragmatic methods to avoid intensive care unit admission when it does not align with patient and family goals. Lancet Respir Med. 2019;7(7):613-625. doi: 10.1016/S2213-2600(19)30170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Time-Limited Trial Conversation Guide
eTable 2. Study Timeline
eTable 3. Study Outcomes by Hospital
eTable 4. Interrupted Time-Series Analysis of Intensive Care Unit Length of Stay
eFigure 1. Study Flowchart for Conducting Family Meetings and Implementing Time-Limited Trials
eFigure 2. Conceptual Framework for Study Interventions
eFigure 3. Shewhart Control Chart of Intensive Care Unit Length of Stay by Individual Patients in Preintervention and Postintervention Periods
eFigure 4. Cumulative Distribution Curves for Patients in the Preintervention and Postintervention Periods