Key Points
Question
How does adding a 24-week self-directed strengthening exercise regimen and physical activity guidance supported by automated behavior-change text messages to web-based osteoarthritis (OA) information affect pain and function in people with knee OA?
Findings
This randomized clinical trial of 206 adults with a clinical diagnosis of knee OA found that a web-based exercise intervention supported by text messaging improved knee pain and function at 24 weeks compared with web-based information alone.
Meaning
This freely available digital intervention is a useful and effective option for improving access to recommended OA exercise and/or supporting clinicians in providing exercise management to people with knee OA at scale across the population.
Abstract
Importance
Exercise therapies are advocated in osteoarthritis (OA) clinical guidelines. However, challenges to accessing exercise may be limiting widespread uptake.
Objective
To evaluate the effects of a self-directed web-based strengthening exercise and physical activity program supported by automated behavior-change text messages on knee pain and function for people with knee OA.
Design, Setting, and Participants
The participant-blinded and assessor-blinded randomized clinical trial enrolled 206 people who met clinical criteria for knee OA in communities across Australia from July 2018 to August 2019, with follow-up taking place at 24 weeks.
Interventions
The control group was given access to a custom-built website with information on OA and the importance of exercise and physical activity. The intervention group was given access to the same information plus a prescription for a 24-week self-directed strengthening regimen and guidance to increase physical activity, supported by automated behavior-change text messages encouraging exercise adherence.
Main Outcomes and Measures
Primary outcomes were change in overall knee pain (numeric rating scale, 0-10) and difficulty with physical function (Western Ontario and McMaster Universities Osteoarthritis Index, 0-68) over 24 weeks. Secondary outcomes were another knee pain measure, sport and recreation function, quality of life, physical activity, self-efficacy, overall improvement, and treatment satisfaction.
Results
Of 206 participants, 180 (87%; mean [SD] age, 60 [8.4] years; 109 [61%] women) completed both 24-week primary outcomes. The intervention group showed greater improvements in overall knee pain (mean difference, 1.6 units; 95% CI, 0.9-2.2 units; P < .001) and physical function (mean difference, 5.2 units; 95% CI, 1.9-8.5 units; P = .002) compared with the control. There was evidence of differences in the proportion of participants exceeding the minimal clinically important improvement in pain (intervention group, 72.1%, vs control, 42.0%; risk difference, 0.30 [95% CI, 0.16-0.44]; P <. 001) and function (intervention group, 68%, vs control, 40.8%; risk difference, 0.27 [95% CI, 0.13-0.41]; P < .001) favoring the intervention. Between-group differences for all secondary outcomes favored the intervention except for physical activity, self-efficacy for function, and self-efficacy for exercise, for which there was no evidence of differences.
Conclusions and Relevance
This randomized clinical trial found that a self-directed web-based strengthening exercise regimen and physical activity guidance supported by automated behavior-change text messages to encourage exercise adherence improved knee pain and function at 24 weeks. This unsupervised, free-to-access digital intervention is an effective option to improve patient access to recommended OA exercise and/or to support clinicians in providing exercise management for people with knee OA at scale across the population.
Trial Registration
Australian New Zealand Clinical Trials Registry Identifier: ACTRN12618001167257
This randomized clinical trial evaluates whether adding a self-directed web-based exercise program and physical activity guide supported by automated behavior-change text messages to an informational osteoarthritis website improves pain and function in people with knee osteoarthritis.
Introduction
Knee osteoarthritis (OA) is highly prevalent and has no known cure.1 Recommended first-line management includes education and strengthening exercise.2,3,4,5,6 However, exercise remains underutilized,7,8,9,10 partly because of limited access to appropriately trained health professionals to prescribe and support exercise.11,12,13,14 Access challenges will continue to worsen given the aging population and rising obesity, and the burden of knee OA is forecast to overwhelm health care systems by 2030.15 Therefore, there is an urgent need to innovate how exercise is prescribed.16
Digital technologies may be a feasible solution given that people with knee OA are increasingly seeking information about their condition from internet sources.17 This information is of variable quality, inconsistently evidence based, and potentially difficult for the general public to comprehend.18 Some high-quality online OA self-management platforms are freely available through consumer organizations (eg, MyJointPain.org.au). However, they provide general exercise information only. This is problematic as a lack of clear instructions prevents people with knee OA from exercising.13,19 Adherence to exercise is typically poor in people with knee OA,20,21 which may explain why the clinical benefits of exercise are not sustained.21,22,23 Barriers include participation costs and lack of support.13 Cell phone text messages positively influence chronic disease self-management and physical activity behavior.24,25 Thus, text messages may help support exercise participation without the need for health professional involvement.
We developed a 24-week self-directed intervention consisting of a website and automated text messages. The website contains educational information on OA and exercise, plus guidance to increase physical activity and a prescription for a structured 24-week self-directed strengthening regimen.26 The text messaging system was developed using behavior change theory27 to support home exercise and has been shown to increase physiotherapist-prescribed home exercise adherence.28 We hypothesized that use of the website and cell phone–based intervention would lead to greater improvements in knee pain and function in people with knee OA compared with a control website offering only educational information on OA and the importance of exercise and physical activity. The control website content was similar to content currently available through high-quality online platforms.
Methods
Trial Design
Study Oversight
We conducted a parallel, 2-arm, superiority randomized clinical trial that was prospectively registered in the Australian New Zealand Clinical Trials Registry (ACTRN12618001167257). Reporting aligned with the Consolidated Standards of Reporting Trials (CONSORT) guideline,29 relevant Consolidated Standards of Reporting Trials Extension (CONSORT Extension) guideline,30,31,32 and the template for intervention description and replication (TIDieR) guideline.33 The study protocol is available in Supplement 1 and has been published elsewhere.26 Approval was obtained from The University of Melbourne Human Research Ethics Committee (No. 1851085). Digital informed consent was obtained using an online form prior to baseline assessments.
Study Participants
Study participants were recruited nationwide in Australia from July 2018 to August 2019 via online advertisements and the Centre for Health Exercise and Sports Medicine’s volunteer database. Screening was via an electronic survey developed using REDCap software (Vanderbilt University), and eligibility was confirmed by telephone. Inclusion criteria were: (1) OA clinical criteria (age ≥45 years, activity-related knee pain, and morning knee stiffness ≤30 minutes)5; (2) knee pain on most days for 3 months or more; (3) average overall knee pain severity of 4 or greater on an 11-point numeric rating scale (NRS) during the previous week; (4) own a cell phone with text messaging; (5) home internet access; and (6) ability to consent, participate, and complete assessments. Exclusion criteria are presented in eTable 1 in Supplement 2.
Study Design and Procedures
Randomization, Allocation Concealment, and Blinding
Participants were randomized with a 1:1 ratio. Computer-generated randomization was prepared by the biostatistician (J.K.) in permuted blocks of sizes 6 to 12. To ensure concealment, the randomization schedule was accessed via a password-protected computer program by a researcher not involved in participant screening, recruitment scheduling, or assessment (S.S. or P.C.). Limited disclosure was used to blind participants who were also assessors, as all outcomes were participant reported. Participants were informed that the study was investigating a range of digital resources (eg, computer, cell phone) to promote knee pain self-management that might include exercise and email or text messaging but were not given specific details of either the intervention or the control groups nor the hypothesis under investigation. The biostatisticians (J.K. and S.J.C.C.) were blinded.
Intervention: My Knee Exercise Website Plus Text Messages
A detailed description of the intervention (My Knee Exercise website and automated text messages) and its development have been published.26,27 Intervention design features are summarized in eTable 2 in Supplement 2. The website is accessed via https://mykneeexercise.org.au/, and the text messaging system, My Exercise Messages, was adapted for release as an app and is downloadable via app stores. Access is free of charge for both.
The study coordinator (R.K.N.) sent an email to participants in the intervention group describing the intervention (access to the My Knee Exercise website containing a prescription for a 24-week knee strengthening regimen supported by periodic text messages), website access details (the web address and a unique login username and password), and a request to commence the exercise regimen within 1 week. The website was divided into 4 sections (eTable 3 in Supplement 2).
All participants in the intervention group received the same standardized website and were permitted to access it at will for 24 weeks. Participant details were added to the text messaging system, triggering the 24-week automated messages designed to encourage adherence to the prescribed strengthening exercise. Descriptions of all text message types and their frequencies during the 24 weeks are provided in eTable 4 in Supplement 2. Messages were personalized with first names. No changes were made to the website or text messaging system during the trial.
Control Website: My Knee Education Website
The study coordinator (R.K.N.) sent an email to the participants in the control group describing the intervention (access to the My Knee Education website containing information on knee pain, knee OA, and the importance of exercise and physical activity), website access details (the web address and a unique login username and password), and a request to access the website within 1 week. At enrollment, control participants received a text message prompting them to access the website.
The control website contained the same textual information as the My Knee Education section of the intervention website, but references to the specific strengthening exercise regimen and physical activity guidance were removed. Only general recommendations were retained, similar to those freely accessible from OA web-based consumer resources (eg, generic information on the importance of strengthening exercises and meeting physical activity guidelines).
Outcomes
Outcomes were participant reported and collected via electronic surveys at baseline and at 24 weeks. The 2 primary outcomes were reliable and valid (1) pain and (2) physical function measures recommended for knee OA clinical trials.34,35,36 Average overall knee pain in the past week was assessed using an 11-point NRS with terminal descriptors of no pain (score 0) and extreme pain (score 10).37 Limitations with physical functioning during the past week were measured by the physical function subscale of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC, Likert version 3.1),35 extracted from the Knee Injury and Osteoarthritis Outcome Score (KOOS),38 with a total score ranging from 0 (no dysfunction) to 68 (maximum dysfunction).
Secondary outcomes were (1) KOOS pain, function in sport and recreation, and knee-related quality-of-life subscales38; (2) Assessment of Quality of Life (version AQoL-6D)39; (3) Physical Activity Scale for the Elderly40 (PASE); (4) Arthritis Self-Efficacy Scale (ASES)41 pain and physical function subscales; (5) Self-Efficacy for Exercise scale (SEE)42; (6) participant-perceived change overall since baseline (24-week follow-up only)43; and (7) overall satisfaction (24-week follow-up only).
Adverse events were participant-reported at 24 weeks, defined as any problem believed to be caused by the study intervention requiring treatment or medication and/or interfering with function for 2 days or more. Custom-developed surveys collected cointervention use (pain medications and other knee OA treatments) at 24 weeks (retrospective recall over previous 6 months). Exercise adherence was assessed by the number of days that knee exercises were performed during the previous week and the Exercise Adherence Rating Scale section B44 at 24 weeks. A range of process measures was collected (eTable 5 in Supplement 2). Another article reports qualitative evaluation.45
Sample Size
We aimed to detect a conservative effect size of 0.40 (small to moderate between-group difference)46 for the primary outcomes. We believed self-directed unsupervised exercise may have smaller effects than the 0.49 for pain and 0.52 for physical function observed with land-based supervised exercise for knee OA.23 To obtain 80% power, a 2-sided significance level of .05, and a correlation between baseline and follow-up measurements of 0.35 with a 15% loss to follow-up,47,48 103 participants were required per arm. Assuming between-participant SDs of 2.3 for pain and 11.7 for WOMAC function48 and a pre-post correlation of 0.35, this sample allowed greater than 99% power to detect a minimal clinically important difference (MCID) in pain of 1.8 units49 and 95% power to detect a MCID in function of 6 units.50
Statistical Analysis
Analyses were performed by biostatisticians (S.J.C.C. and J.K.) using Stata, release 16 (StataCorp LLC), and intention-to-treat with all available data from all randomized participants using their randomized group allocation. Baseline characteristics of participants who provided both primary outcomes and those who did not were compared using t tests or χ2 tests. P values were 2-tailed and statistical significance was defined as P < .05. Missing outcomes were imputed using chained equations with predictive mean matching and 5 nearest neighbors for continuous outcomes, and logistic regression imputation models for binary improvement outcomes. Imputation models for continuous outcomes at 24 weeks included all primary and secondary outcomes at both baseline and 24 weeks, along with age, sex, body mass index (calculated as weight in kilograms divided by height in meters squared), education level, geographical location, employment status, duration of symptoms, and laterality. The imputation model for the binary variable of global improvement was similar except that all secondary outcome variables at 24 weeks were omitted owing to the tendency for perfect prediction. Data were imputed for each treatment group separately. Estimates from 15 imputed data sets were combined using Rubin rules.51
For continuous outcomes, the mean (95% CI) difference in change (baseline minus follow-up) between groups was estimated using linear regression models, adjusted for baseline scores. The proportion of participants with self-perceived improvement overall and the magnitude of improvements that met or exceeded MCIDs for NRS pain and WOMAC function were compared between groups using logistic regression models, with results presented as risk ratios and risk differences (both obtained using marginal standardization).52 Validity of model assumptions and imputed data sets was assessed using standard diagnostic plots. Analyses were repeated using complete-case data.
Results
Study Participants
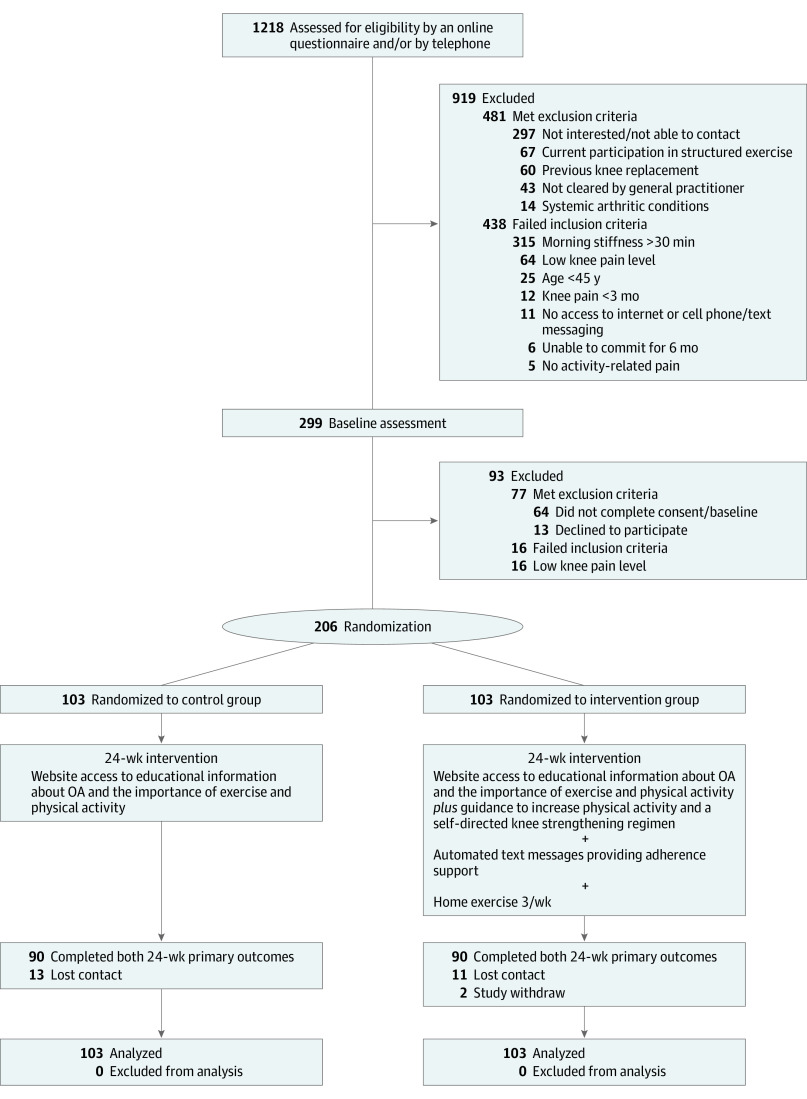
We randomized 206 participants, of which 180 (87%; mean [SD] age, 60 [8.4] years; 109 [61%] women) completed both primary outcome measures at 24 weeks (Figure). Groups were similar at baseline (Table 1). Participants who did not complete both primary outcomes at 24 weeks reported greater past use of injections, nonsteroidal anti-inflammatory drugs, and oral opioids to manage knee pain and higher rates of foot problems at baseline (eTable 6 in Supplement 2). Website and text message usage are outlined in eTable 7 in Supplement 2. Fourteen (8%) participants reported no website access in the first month (intervention: n = 3 [3%]; control: n = 11 [13%]) and 85 (49%) participants reported no website access in the past month (intervention: n = 35 [39%]; control: n = 50 [60%]) in the past month. The mean (SD) number of website logins per participant was 6.0 (4.9) in the intervention group and 3.2 (2.6) in the control group. In the intervention group, the mean (SD) number of text messages sent to each participant during the 24 weeks was 60.0 (7.5) and the average participant reply rate was 73% (34%).
Figure. Participant Flow Through the Randomized Clinical Trial.
OA denotes osteoarthritis.
Table 1. Baseline Characteristics of Participants by Group.
| Characteristic | Mean (SD) | Standardized difference, %a | |
|---|---|---|---|
| Intervention (n = 103) | Control (n = 103) | ||
| Age, y | 60.3 (8.2) | 59.0 (8.5) | 15.4 |
| Female, No. (%) | 60 (58) | 66 (64) | −12.0 |
| Height, m | 1.7 (0.1) | 1.7 (0.1) | 5.3 |
| Body mass, median (IQR), kg | 89.0 (76.0-105.0) | 90.0 (76.0-106.0) | 2.4 |
| BMI, median (IQR) | 31.1 (26.6-34.9) | 31.6 (26.9-36.4) | −0.2 |
| Geographic location,b No. (%) | |||
| Metropolitan | 64 (62) | 58 (56) | 11.9 |
| Regional | 39 (38) | 45 (44) | −11.9 |
| Education level | |||
| Secondary | 49 (48) | 48 (47) | 2.0 |
| Tertiary (university or equivalent) | 54 (52) | 55 (53) | −2.0 |
| Currently employed, No. (%) | 62 (60) | 70 (68) | −16.2 |
| Symptom duration, y | 8.5 (10.0) | 7.7 (8.0) | 8.1 |
| Laterality, No. (%) | |||
| Unilateral OA | 38 (37) | 42 (41) | −8.0 |
| Bilateral OA | 65 (63) | 61 (59) | 8.0 |
| Problems in other joints, No. (%) | |||
| Back | 35 (34) | 37 (36) | −4.1 |
| Hip | 27 (26) | 31 (30) | −8.6 |
| Shoulder | 26 (25) | 28 (27) | −4.4 |
| Hand | 26 (25) | 25 (24) | 2.3 |
| Neck | 23 (22) | 21 (20) | 4.7 |
| Foot | 21 (20) | 27 (26) | −13.8 |
| Treatments for knee OA in past 6 mo, No. (%) | |||
| ≥1 Treatment | 87 (84) | 96 (93) | −28.0 |
| Land-based exercises | 55 (53) | 49 (48) | 11.7 |
| Heat/cold treatment | 51 (50) | 66 (64) | −29.7 |
| Massage | 32 (31) | 42 (41) | −20.3 |
| Knee braces | 26 (25) | 39 (38) | −27.4 |
| Orthotics, arch supports | 23 (22) | 24 (23) | −2.3 |
| Manual therapy | 21 (20) | 22 (21) | −2.4 |
| Hydrotherapy | 20 (19) | 14 (14) | 15.7 |
| Walking stick | 16 (16) | 10 (10) | 17.6 |
| TENS | 13 (13) | 11 (11) | 6.1 |
| Injections (eg, cortisone, hylan G-F 20, platelet-rich plasma) | 9 (9) | 9 (9) | 0.0 |
| Therapeutic ultrasound | 8 (8) | 9 (9) | −3.5 |
| Acupuncture | 8 (8) | 8 (8) | 0.0 |
| Arthroscopic surgery | 4 (4) | 8 (8) | −16.6 |
| Low-level laser therapy | 0 | 2 (2) | −19.9 |
| High-tibial osteotomy surgery | 0 | 2 (2) | −19.9 |
| Ligament reconstruction | 0 | 3 (3) | −24.5 |
| Current pain medication use,c No. (%) | |||
| ≥1 Medication used | 86 (83) | 91 (88) | −14.0 |
| Analgesics (paracetamol combinations) | 66 (64) | 73 (71) | −14.6 |
| Topical anti-inflammatory drugs | 59 (57) | 69 (67) | −20.1 |
| Nonsteroidal anti-inflammatory drugs | 55 (53) | 63 (61) | −15.8 |
| Cyclooxygenase-2 inhibitors | 12 (12) | 16 (16) | −11.4 |
| Oral opioids | 5 (5) | 5 (5) | 0.0 |
| Oral corticosteroids | 1 (1.0) | 0 | 14.0 |
| Comorbid conditions, No. (%) | |||
| ≥1 Comorbid condition | 55 (53) | 57 (55) | −3.9 |
| High blood pressure | 36 (35) | 37 (36) | −2.0 |
| Depression | 14 (14) | 15 (15) | −2.8 |
| Diabetes | 10 (10) | 6 (6) | 14.6 |
| Spine condition, including arthritis | 9 (9) | 11 (11) | −6.6 |
| Lung disease | 7 (7) | 7 (7) | 0.0 |
| Other | 10 (10) | 9 (9) | 3.4 |
| Exercise importanced | 6.1 (1.2) | 6.1 (1.2) | 0.8 |
Abbreviations: BMI, body mass index calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range; OA, osteoarthritis; TENS, transcutaneous electrical nerve stimulation.
Calculated as intervention minus control group.
Based on residential postal code, in accordance with Australian Statistical Geography Standard.
Defined as ≥1 per week during the prior 6 mo.
Rated by level of agreement with the statement “How important is it to you to do regular exercise to manage your knee condition?” Scores range from 1-7, with higher scores indicating higher agreement.
At 24 weeks, the number of participant-reported knee exercise sessions in the previous week was similar in both groups (eTable 8 in Supplement 2). There was evidence of between-group differences in adherence to knee exercise measured by the Exercise Adherence Rating Scale (mean difference, 2.6 units; 95% CI, 0.8-4.4 units; P = .005), favoring intervention. Few adverse events were reported, and none were serious (eTable 9 in Supplement 2). More intervention participants experienced knee pain than control participants (n = 8 [9.6%] vs n = 1 [1.3%]; P = .019). Use of pain medications and other treatments for the knee during the 24 weeks was similar across groups, except more control participants used massage, heat or cold, and topical anti-inflammatories (eTable 9 in Supplement 2).
Primary Outcomes
There was evidence of greater improvements in overall pain (mean difference, 1.6 units; 95% CI, 0.9-2.2 units; P < .001) and in WOMAC function (mean difference, 5.2 units; 95% CI, 1.9-8.5 units; P = .002) favoring the intervention (Tables 2 and 3). More participants in the intervention group reached MCIDs in pain and in function than in the control group (Table 4). Analyses using complete case data produced similar results (eTables 10 and 11 in Supplement 2).
Table 2. Mean (SD) Scores on Continuous Outcome Measures Across Time, by Group.
| Outcome measurea | Baseline | Follow-up (24 wk) | ||
|---|---|---|---|---|
| Intervention (n = 103) | Control (n = 103) | Intervention (n = 91)b | Control (n = 92)c | |
| Primary outcomes | ||||
| Overall average knee pain (NRS) | 6.3 (1.5) | 6.2 (1.5) | 3.5 (2.2) | 5.0 (2.4) |
| Physical function (WOMAC) | 26.7 (11.8) | 25.0 (12.2) | 16.6 (13.0) | 20.7 (13.9) |
| Secondary outcomes | ||||
| Pain (KOOS) | 50.8 (16.0) | 53.1 (14.6) | 69.1 (17.0) | 60.5 (19.1) |
| Sport and recreation (KOOS) | 31.7 (19.2) | 30.0 (21.5) | 47.7 (23.0) | 39.6 (26.4) |
| Knee-related quality of life (KOOS) | 35.0 (18.0) | 34.3 (15.7) | 49.9 (18.5) | 43.3 (21.4) |
| Health-related quality of life (AQoL) | 0.68 (0.16) | 0.68 (0.17) | 0.75 (0.16) | 0.69 (0.20) |
| Physical activity levels (PASE) | 146.9 (71.7) | 136.0 (68.2) | 157.5 (75.6) | 144.5 (73.0) |
| Self-efficacy (ASES) | ||||
| Pain | 6.0 (1.7) | 6.0 (1.7) | 6.7 (2.3) | 6.0 (1.9) |
| Function | 7.9 (1.5) | 8.1 (1.3) | 8.2 (2.0) | 8.3 (1.3) |
| Self-efficacy exercise (SEE) | 60.6 (21.5) | 58.8 (18.6) | 55.4 (22.7) | 52.7 (20.0) |
| Treatment satisfaction | NA | NA | 5.6 (1.5) | 4.4 (1.7) |
Abbreviations: AQoL, Assessment of Quality of Life; ASES, Arthritis Self-Efficacy Scale; KOOS, Knee Injury and Osteoarthritis Outcome score; NA, not applicable; NRS, numerical rating scale; PASE, Physical Activity Scale for the Elderly; SEE, Self-Efficacy for Exercise; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Instruments and scoring ranges: AQoL, −0.04 to 1.0, with higher scores indicating better quality of life; ASES function subscale, 1-10, with higher scores indicating greater self-efficacy; ASES pain subscale, 1-10, with higher scores indicating greater self-efficacy; KOOS, 0-100, with lower scores indicating worse pain/symptoms/function/quality of life; NRS, 0-10 with higher scores indicating worse pain; PASE, 0-400+, with higher scores indicating more activity; SEE scale, 0-90, with high scores indicating greater self-efficacy; treatment satisfaction, 1-7, with higher scores indicating greater treatment satisfaction; and WOMAC physical function subscale, 0-68, with higher scores indicating worse function.
For overall knee pain, n = 91; for physical function (WOMAC) and pain (KOOS), n = 90; and for all other outcomes, n = 89.
For overall knee pain, n = 92; for physical function (WOMAC), n = 90; for pain (KOOS), n = 89; for sport and recreation (KOOS) and knee-related quality of life (KOOS), n = 88; for AQoL, n = 87; and for all other outcomes, n = 86.
Table 3. Change Within Groups and Difference in Change Between Groups for Continuous Outcomes, Using Multiply Imputed Data.
| Outcome measuresa | Change within groups, mean (SD) | Difference in change between groupsb | ||
|---|---|---|---|---|
| Baseline minus week 24 | Baseline to week 24 | |||
| Intervention (n = 103) | Control (n = 103) | Mean difference (95% CI) | P value | |
| Primary outcomes | ||||
| Overall average knee pain (NRS)c | 2.8 (2.1) | 1.2 (2.2) | 1.6 (0.9 to 2.2) | <.001 |
| Physical function (WOMAC)d | 10.1 (10.8) | 4.4 (11.9) | 5.2 (1.9 to 8.5) | .002 |
| Secondary outcomes | ||||
| Pain (KOOS)d | −18.5 (15.3) | −6.4 (15.9) | −11.3 (−15.7 to −7.0) | <.001 |
| Sport and recreation (KOOS)d | −16.9 (22.2) | −8.4 (22.8) | −9.1 (−15.3 to −2.9) | .004 |
| Knee-related quality of life (KOOS)d | −15.3 (19.6) | −8.2 (17.8) | −7.4 (−12.6 to −2.2) | .005 |
| Health-related quality of life (AQoL)d | −0.07 (0.16) | −0.00 (0.14) | −0.07 (−0.11 to −0.02) | .002 |
| Physical activity levels (PASE)d | −14.0 (67.1) | −9.6 (78.7) | −9.6 (−29.8 to 10.5) | .35 |
| Self-efficacy (ASES)d | ||||
| Pain | −0.7 (2.6) | −0.0 (1.9) | −0.6 (−1.3 to −0.0) | .046 |
| Function | −0.3 (2.2) | −0.2 (1.3) | 0.0 (−0.4 to 0.5) | .89 |
| Self-efficacy exercise (SEE) | 4.8 (23.5) | 6.2 (19.5) | −2.4 (−7.9 to 3.1) | .39 |
| Treatment satisfactionc | NA | NA | 1.2 (0.7 to 1.6) | <.001 |
Abbreviations: AQoL, Assessment of Quality of Life; ASES, Arthritis Self-Efficacy Scale; KOOS, Knee Injury and Osteoarthritis Outcome score; NA, not applicable; NRS, numeric rating scale; PASE, Physical Activity Scale for the Elderly; SEE, Self-Efficacy for Exercise; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Instruments and scoring ranges: AQoL, −0.04 to 1.0, with higher scores indicating better quality of life; ASES function subscale, 1-10, with higher scores indicating greater self-efficacy; ASES pain subscale, 1-10, with higher scores indicating greater self-efficacy; KOOS, 0-100, with lower scores indicating worse pain/symptoms/function/quality of life; NRS, 0-10 with higher scores indicating worse pain; PASE, 0-400+, with higher scores indicating more activity; SEE scale, 0-90, with high scores indicating greater self-efficacy; treatment satisfaction, 1-7, with higher scores indicating greater treatment satisfaction; and WOMAC physical function subscale, 0-68, with higher scores indicating worse function.
Adjusted for baseline value of outcome where possible.
For change within groups, positive changes indicate improvement. For difference in change between groups, positive differences favor the intervention.
For change within groups, negative changes indicate improvement. For difference in change between groups, negative differences favor the intervention.
Table 4. Percentage of Participants Achieving Minimal Clinically Important Improvements and Reporting Global Improvement, Using Multiply Imputed Data.
| Measurement | Intervention | Control | Relative risk | Risk difference | ||
|---|---|---|---|---|---|---|
| (95% CI)a | P value | (95% CI)b | P value | |||
| Improvement, units | ||||||
| NRS, ≥1.8c | 72.1 | 42.0 | 1.72 (1.25-2.18) | <.001 | 0.30 (0.16-0.44) | <.001 |
| WOMAC, ≥6c | 68.0 | 40.8 | 1.66 (1.19-2.13) | <.001 | 0.27 (0.13-0.41) | <.001 |
| Improved overalld | 57.3 | 27.4 | 2.09 (1.26-2.92) | <.001 | 0.30 (0.16-0.44) | <.001 |
Abbreviations: NRS, numeric rating scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Relative risk of >1 favors the intervention.
Risk difference of >0 favors the intervention.
Minimal clinically important improvements.
Rated using 7-point scales with terminal descriptors of much worse to much better, with those indicating moderately better or much better classified as improved.
Secondary Outcomes
There was evidence of between-group differences favoring the intervention in most secondary outcomes—all 3 KOOS subscales (pain, sports/recreation, and quality of life), AQoL, ASES pain, participant change since baseline, and overall satisfaction. Changes in PASE, ASES function, and SEE were similar in both groups (Table 3). More participants in the intervention group reported overall improvement than in the control group (Table 4). Analyses using complete case data produced similar results (eTable 10 and eTable 11 in Supplement 2).
Discussion
This randomized clinical trial provides robust evidence of the effectiveness of adding a 24-week self-directed strengthening exercise regimen and physical activity guidance supported by automated text messages to web-based information similar to what is currently available online through reputable OA consumer organizations. We found significant between-group differences in pain of 1.6 units (95% CI, 0.9-2.2 units) and function of 5.2 units (95% CI, 1.9-8.5 units) favoring the intervention. While these were just below the MCIDs for pain (1.8 units) and function (6 units), the 95% CIs include these differences within the plausible ranges. Additionally, substantially more participants reached the MCIDs in the intervention group than in the control group, while within-group changes in pain and function exceeded MCIDs in the intervention group but not in the control group. Furthermore, effect sizes (pain, 0.68 and function, 0.39) favoring the intervention group were similar to those observed with land-based therapist-supervised exercise.23
Taken together, the findings of the present study demonstrate that this easily scalable, unsupervised, free-to-access intervention is effective and may be clinically relevant on a population level. Further supporting its effectiveness are the findings of beneficial effects on secondary outcomes. An additional knee pain measure, function in sport and recreation, 2 quality-of-life measures, pain self-efficacy (3 KOOS subscales, AQoL, and ASES pain), perceived improvement overall, and treatment satisfaction all showed the intervention’s significant benefit, although other measures (PASE, ASES function, and SEE) showed similar changes in both groups.
To our knowledge, only 2 other randomized clinical trials53,54 have evaluated web-based interventions without health professional contact in OA. One trial evaluated a self-directed progressive lower-limb strength, flexibility, and walking program (Help My Knees)53 in people with knee OA compared with a wait-list control group. Unlike the present study findings, no between-group differences were found with the primary outcome of total WOMAC score (combined measure of pain, stiffness, and function) or secondary outcomes of physical function and pain at 4 months. The other trial evaluated a 9-module physical activity program (Join2Move)54 in adults with knee and/or hip OA compared with a wait-list control. Similar to the present study findings, there was evidence of between-group differences in physical function (KOOS; mean difference, 6.5 units; 95% CI, 1.8-11.2 units) and in proportion of participants reporting improvements (odds ratio, 10.7; 95% CI, 4.3-26.4), favoring intervention at 3 months. In contrast with the present study findings, there was no evidence of between-group differences in quality of life or function in sport and recreation at 3 months.
Conflicting findings across studies may partially be explained by low engagement levels with web-based programs. In the Help My Knees study,53 20% of participants did not access the program during the 4-month intervention. Similarly, only 55% of participants completed the second of 9 modules of Join2Move.54 The present intervention had much higher levels of user engagement—participant-reported website access was 97% in the first month and 61% in the final month. These differences may be due to variation in the type and level of support provided. In Help My Knees and Join2Move, automated support and exercise reminders seemed to occur just once per week. In contrast, the present intervention used automated text messages (average, 2.5 messages sent per participant per week), designed to address common exercise barriers and facilitators in knee OA.13 Such communication has been shown to enhance adherence to physiotherapist-prescribed exercise in people with knee OA.28 The text messages provided along with the web-based exercise program may have facilitated better engagement and enhanced its effectiveness. Furthermore, additional qualitative research45 highlights that participants valued the text messages as a prompt to exercise, keeping them accountable to the program.
In the present study, 72% of intervention participants experienced clinically important improvements in pain and 68% in function, demonstrating that most participants experienced meaningful improvements in knee OA symptoms without needing health professional contact. Conversely, 30% of participants did not benefit from the unsupervised approach, suggesting that more intensive, personalized management may be required. This gap could be addressed through a stepped-care approach where people move through a hierarchy of evidence-based interventions based on outcomes.55
Our free-to-access, unsupervised program could serve as an entry-level intervention, with participants who do not experience clinical benefits progressing to subsequent steps for more intensive, personalized management. Such an approach has the potential to better distribute limited health care resources and reduce demand for contact with health professionals, thus improving access for those requiring it. The present intervention may also be valuable in low-income to middle-income countries with large unmet needs for physiotherapy care.56 Further research is required to evaluate the effectiveness of this program when stepped care and/or face-to-face care is incorporated.
Strengths and Limitations
There are several study strengths. The robust randomized clinical trial design with reliable and valid outcomes, participant blinding and assessor blinding, and excellent participant retention enhances internal validity. Generalizability is maximized by broad inclusion criteria, recruitment of participants nationwide, and no restrictions placed on cointervention and medication use. Another strength is the rigorous intervention design, which was evidence informed, incorporated behavior change theory, and included input from clinicians and people with knee OA.
Several limitations are acknowledged. There was potential for bias with participants self-selecting to volunteer for a study investigating different digital resources to support knee OA management, which may have led to inclusion of people with more favorable views of technology. Participants had completed high school or higher education and reported moderate to high self-efficacy at baseline, meaning the study findings may not generalize to people with lower levels of education or self-efficacy. At baseline, participants not completing 24-week outcomes used more analgesics and injections, although symptom severity was similar between groups; therefore, the intervention may not meet the needs of those who prefer pharmacological treatments. We did not include a long-term follow-up; therefore, whether the intervention effects are maintained beyond 24 weeks is unknown. Finally, because the comparator was web-based education, it remains unknown how the intervention compares with clinician-delivered interventions (1-on-1 or group based).
Conclusions
A web-based intervention of self-directed strengthening exercise and physical activity guidance supported by automated behavior-change text messages improved knee pain and function at 24 weeks. The benefits may be clinically relevant and indicate that this unsupervised, free-to-access intervention could be an effective option for improving patient access to recommended OA exercise and supporting clinicians in providing exercise management to people with knee OA at scale across the population.
Trial Protocol
eTable 1. Trials exclusion criteria
eTable 2. Outline of the key design features of the My Knee Exercise website and the SMS system
eTable 3. Description of the content in the four sections of the intervention website, My Knee Exercise. The My Knee Education textual content presented here is common in both the intervention and control group
eTable 4. Description of all message types used within the SMS system
eTable 5. Process measures collected
eTable 6. Baseline characteristics and outcome scores of participants who did and did not complete both primary outcomes, reported as mean (standard deviation) unless otherwise stated. P-values based on t-tests for continuous characteristics and chi-squared tests for categorical characteristics
eTable 7. Measures of perceived website and SMS usefulness, website and SMS usage and exercise importance taken at 24-weeks, reported as mean (SD), unless otherwise stated
eTable 8. Mean (SD) of adherence measures within groups, and difference in adherence measures between groups, using complete case data
eTable 9. Number (percentage) of participants with adverse events, co-interventions, or pain medication use during the 24 weeks
eTable 10. Change within groups, and difference in change between groups (adjusted for baseline value of outcome where possible) for continuous outcomes, using complete case data
eTable 11. Proportion (percentage) of participants achieving minimal clinically important improvements (1.8 units for NRS overall pain, 6 units for WOMAC function) and proportion (percentage) of participants reporting global improvement, using complete case data
Data Sharing Statement
References
- 1.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160-167. doi: 10.1097/BOR.0000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220-233. doi: 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578-1589. doi: 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Fernandes L, Hagen KB, Bijlsma JW, et al. ; European League Against Rheumatism (EULAR) . EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125-1135. doi: 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence . United Kingdom. Clinical guideline: osteoarthritis care and management in adults. 2014. Accessed March 4, 2021. https://www.nice.org.uk/guidance/cg177
- 6.The Royal Australian College of General Practitioners . Guideline for the Management of Knee and Hip Osteoarthritis. Accessed March 4, 2020. https://www.racgp.org.au/download/Documents/Guidelines/Musculoskeletal/guideline-for-the-management-of-knee-and-hip-oa-2nd-edition.pdf
- 7.Ingelsrud LH, Roos EM, Gromov K, Jensen SS, Troelsen A. Patients report inferior quality of care for knee osteoarthritis prior to assessment for knee replacement surgery: a cross-sectional study of 517 patients in Denmark. Acta Orthop. 2020;91(1):82-87. doi: 10.1080/17453674.2019.1680180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagen KB, Smedslund G, Østerås N, Jamtvedt G. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2016;68(10):1443-1452. doi: 10.1002/acr.22891 [DOI] [PubMed] [Google Scholar]
- 9.Runciman WB, Hunt TD, Hannaford NA, et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med J Aust. 2012;197(2):100-105. doi: 10.5694/mja12.10510 [DOI] [PubMed] [Google Scholar]
- 10.Haskins R, Henderson JM, Bogduk N. Health professional consultation and use of conservative management strategies in patients with knee or hip osteoarthritis awaiting orthopaedic consultation. Aust J Prim Health. 2014;20(3):305-310. doi: 10.1071/PY13064 [DOI] [PubMed] [Google Scholar]
- 11.Briggs AM, Houlding E, Hinman RS, et al. Health professionals and students encounter multi-level barriers to implementing high-value osteoarthritis care: a multi-national study. Osteoarthritis Cartilage. 2019;27(5):788-804. doi: 10.1016/j.joca.2018.12.024 [DOI] [PubMed] [Google Scholar]
- 12.Egerton T, Diamond LE, Buchbinder R, Bennell KL, Slade SC. A systematic review and evidence synthesis of qualitative studies to identify primary care clinicians’ barriers and enablers to the management of osteoarthritis. Osteoarthritis Cartilage. 2017;25(5):625-638. doi: 10.1016/j.joca.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Dobson F, Bennell KL, French SD, et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am J Phys Med Rehabil. 2016;95(5):372-389. doi: 10.1097/PHM.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 14.Ackerman IN, Livingston JA, Osborne RH. Personal perspectives on enablers and barriers to accessing care for hip and knee osteoarthritis. Phys Ther. 2016;96(1):26-36. doi: 10.2522/ptj.20140357 [DOI] [PubMed] [Google Scholar]
- 15.Ackerman IN, Bohensky MA, Zomer E, et al. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet Disord. 2019;20(1):90. doi: 10.1186/s12891-019-2411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyles JP, Hunter DJ, Bennell KL, et al. ; Joint Effort Initiative Members . Priorities for the effective implementation of osteoarthritis management programs: an OARSI international consensus exercise. Osteoarthritis Cartilage. 2019;27(9):1270-1279. doi: 10.1016/j.joca.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 17.Jellison SS, Bibens M, Checketts J, Vassar M. Using Google Trends to assess global public interest in osteoarthritis. Rheumatol Int. 2018;38(11):2133-2136. doi: 10.1007/s00296-018-4158-2 [DOI] [PubMed] [Google Scholar]
- 18.Murray KE, Murray TE, O’Rourke AC, Low C, Veale DJ. Readability and quality of online information on osteoarthritis: an objective analysis with historic comparison. Interact J Med Res. 2019;8(3):e12855. doi: 10.2196/12855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley M, Dickson K, Hallett R, et al. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4:CD010842. doi: 10.1002/14651858.CD010842.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolson PJA, Hinman RS, Kasza J, Bennell KL. Trajectories of adherence to home-based exercise programs among people with knee osteoarthritis. Osteoarthritis Cartilage. 2018;26(4):513-521. doi: 10.1016/j.joca.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Pisters MF, Veenhof C, van Meeteren NL, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review. Arthritis Rheum. 2007;57(7):1245-1253. doi: 10.1002/art.23009 [DOI] [PubMed] [Google Scholar]
- 22.Pisters MF, Veenhof C, Schellevis FG, Twisk JW, Dekker J, De Bakker DH. Exercise adherence improving long-term patient outcome in patients with osteoarthritis of the hip and/or knee. Arthritis Care Res (Hoboken). 2010;62(8):1087-1094. doi: 10.1002/acr.20182 [DOI] [PubMed] [Google Scholar]
- 23.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554-1557. doi: 10.1136/bjsports-2015-095424 [DOI] [PubMed] [Google Scholar]
- 24.Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013;97:41-48. doi: 10.1016/j.socscimed.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 25.Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health. 2015;36:393-415. doi: 10.1146/annurev-publhealth-031914-122855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelligan RK, Hinman RS, Kasza J, Bennell KL. Effectiveness of internet-delivered education and home exercise supported by behaviour change SMS on pain and function for people with knee osteoarthritis: a randomised controlled trial protocol. BMC Musculoskelet Disord. 2019;20(1):342. doi: 10.1186/s12891-019-2714-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelligan RK, Hinman RS, Atkins L, Bennell KL. A short message service intervention to support adherence to home-based strengthening exercise for people with knee osteoarthritis: intervention design applying the behavior change wheel. JMIR mHealth uHealth. 2019;7(10):e14619. doi: 10.2196/14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennell K, Nelligan RK, Schwartz S, et al. Behavior change text messages for home exercise adherence in knee osteoarthritis: randomized trial. J Med Internet Res. 2020;22(9):e21749. doi: 10.2196/21749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Hopewell S, Schulz KF, et al. ; CONSORT . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28-55. doi: 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 30.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295-309. doi: 10.7326/0003-4819-148-4-200802190-00008 [DOI] [PubMed] [Google Scholar]
- 31.Eysenbach G; CONSORT-EHEALTH Group . CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res. 2011;13(4):e126. doi: 10.2196/jmir.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwarenstein M, Treweek S, Gagnier JJ, et al. ; CONSORT Group; Pragmatic Trials in Healthcare Group . Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 34.McAlindon TE, Driban JB, Henrotin Y, et al. OARSI clinical trials recommendations: design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):747-760. doi: 10.1016/j.joca.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 35.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840. [PubMed] [Google Scholar]
- 36.Rolfson O, Wissig S, van Maasakkers L, et al. Defining an international standard set of outcome measures for patients with hip or knee osteoarthritis: consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care Res (Hoboken). 2016;68(11):1631-1639. doi: 10.1002/acr.22868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S240-S252. doi: 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 38.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi: 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 39.Osborne RH, Hawthorne G, Lew EA, Gray LC. Quality of life assessment in the community-dwelling elderly: validation of the Assessment of Quality of Life (AQoL) Instrument and comparison with the SF-36. J Clin Epidemiol. 2003;56(2):138-147. doi: 10.1016/S0895-4356(02)00601-7 [DOI] [PubMed] [Google Scholar]
- 40.Martin KA, Rejeski WJ, Miller ME, James MK, Ettinger WH Jr, Messier SP. Validation of the PASE in older adults with knee pain and physical disability. Med Sci Sports Exerc. 1999;31(5):627-633. doi: 10.1097/00005768-199905000-00001 [DOI] [PubMed] [Google Scholar]
- 41.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37-44. doi: 10.1002/anr.1780320107 [DOI] [PubMed] [Google Scholar]
- 42.Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nurs Res. 2000;49(3):154-159. doi: 10.1097/00006199-200005000-00007 [DOI] [PubMed] [Google Scholar]
- 43.ten Klooster PM, Drossaers-Bakker KW, Taal E, van de Laar MA. Patient-perceived satisfactory improvement (PPSI): interpreting meaningful change in pain from the patient’s perspective. Pain. 2006;121(1-2):151-157. doi: 10.1016/j.pain.2005.12.021 [DOI] [PubMed] [Google Scholar]
- 44.Newman-Beinart NA, Norton S, Dowling D, et al. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: the Exercise Adherence Rating Scale (EARS). Physiotherapy. 2017;103(2):180-185. doi: 10.1016/j.physio.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 45.Nelligan RK, Hinman RS, Teo PL, Bennell KL. Exploring attitudes and experiences of people with knee osteoarthritis toward a self-directed ehealth intervention to support exercise: qualitative study. JMIR Rehabil Assist Technol. 2020;7(2):e18860. doi: 10.2196/18860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Taylor & Francis; 2013. doi: 10.4324/9780203771587 [DOI] [Google Scholar]
- 47.Bennell KL, Nelligan R, Dobson F, et al. Effectiveness of an internet-delivered exercise and pain-coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann Intern Med. 2017;166(7):453-462. doi: 10.7326/M16-1714 [DOI] [PubMed] [Google Scholar]
- 48.Bennell KL, Campbell PK, Egerton T, et al. Telephone coaching to enhance a home-based physical activity program for knee osteoarthritis: a randomized clinical trial. Arthritis Care Res (Hoboken). 2017;69(1):84-94. doi: 10.1002/acr.22915 [DOI] [PubMed] [Google Scholar]
- 49.Bellamy N, Carette S, Ford PM, et al. Osteoarthritis antirheumatic drug trials; iii, setting the delta for clinical trials: results of a consensus development (Delphi) exercise. J Rheumatol. 1992;19(3):451-457. [PubMed] [Google Scholar]
- 50.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi: [DOI] [PubMed] [Google Scholar]
- 51.Carpenter JG, Kenward MG. Multiple Imputation and Its Application. Wiley; 2013. doi: 10.1002/9781119942283 [DOI] [Google Scholar]
- 52.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962-970. doi: 10.1093/ije/dyu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen KD, Arbeeva L, Callahan LF, et al. Physical therapy vs internet-based exercise training for patients with knee osteoarthritis: results of a randomized controlled trial. Osteoarthritis Cartilage. 2018;26(3):383-396. doi: 10.1016/j.joca.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bossen D, Veenhof C, Van Beek KE, Spreeuwenberg PM, Dekker J, De Bakker DH. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: randomized controlled trial. J Med Internet Res. 2013;15(11):e257. doi: 10.2196/jmir.2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Von Korff M, Tiemens B. Individualized stepped care of chronic illness. West J Med. 2000;172(2):133-137. doi: 10.1136/ewjm.172.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jesus TS, Landry MD, Hoenig H. Global need for physical rehabilitation: systematic analysis from the Global Burden of Disease Study 2017. Int J Environ Res Public Health. 2019;16(6):E980. doi: 10.3390/ijerph16060980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Trials exclusion criteria
eTable 2. Outline of the key design features of the My Knee Exercise website and the SMS system
eTable 3. Description of the content in the four sections of the intervention website, My Knee Exercise. The My Knee Education textual content presented here is common in both the intervention and control group
eTable 4. Description of all message types used within the SMS system
eTable 5. Process measures collected
eTable 6. Baseline characteristics and outcome scores of participants who did and did not complete both primary outcomes, reported as mean (standard deviation) unless otherwise stated. P-values based on t-tests for continuous characteristics and chi-squared tests for categorical characteristics
eTable 7. Measures of perceived website and SMS usefulness, website and SMS usage and exercise importance taken at 24-weeks, reported as mean (SD), unless otherwise stated
eTable 8. Mean (SD) of adherence measures within groups, and difference in adherence measures between groups, using complete case data
eTable 9. Number (percentage) of participants with adverse events, co-interventions, or pain medication use during the 24 weeks
eTable 10. Change within groups, and difference in change between groups (adjusted for baseline value of outcome where possible) for continuous outcomes, using complete case data
eTable 11. Proportion (percentage) of participants achieving minimal clinically important improvements (1.8 units for NRS overall pain, 6 units for WOMAC function) and proportion (percentage) of participants reporting global improvement, using complete case data
Data Sharing Statement