Summary:
Mammalian cryptochromes regulate sleep and metabolism as components of the circadian clock. In this issue of Cell Chemical Biology, Miller et al. (2020) use phenotypic chemical screens to identify selective modulators of two cryptochrome isoforms. Binding specificity depends on conformational patterning of the ligand-binding pocket and a disordered C-terminal domain.
New drugs to better sleep, improve moods, and perhaps even reduce obesity – these are the long-term goals of a recent study aimed at perturbing circadian rhythms and modulating metabolism with small molecules. The targets? The human cryptochrome isoforms: Cry1 and Cry2. Cryptochromes (Crys) are fascinating proteins that assume many different functions throughout biology. Originally discovered for their light-sensing ability in plants, they are now known to mediate responses to varied stimuli in many different organisms (Chaves et al., 2011). Crys are evolutionarily related to the photolyase DNA-repair enzymes, and like photolyases, their activities often rely on the photochemistry of their bound flavin cofactors (Chaves et al., 2011). Although the mammalian Crys maintain the flavin-binding pocket, their functions are largely flavin independent. Cry1 and Cry2 form complexes with the Period proteins to act as repressors in the transcriptional-translational feedback loop (TTFL) that underpins the circadian clock (Takahashi, 2017). They bind to and directly oppose the transcriptional activator of clock-controlled genes: the heterodimer composed of Clock and BMAL1 (Fig. 1). Despite considerable sequence similarity, the two Cry isoforms are not redundant; they are expressed differently and their pattern of transcriptional repression throughout the circadian day is not the same (Takahashi, 2017). Although detailed molecular work is beginning to provide a structural and biochemical basis for these fascinating differences in mechanism and function (Fribourgh et al., 2020; Rosensweig et al., 2018), there are many unanswered questions regarding the physiological roles of Cry1 and Cry2. Yet, the stakes to advance our understanding may be high. For example, naturally occurring Cry variants impact sleep behavior in humans, largely by interfering with their affinity for the Clock-BMAL1 complex (Patke et al., 2017). Hence, small-molecule modulators of Cry function, particularly those that are isoform-specific, are highly sought.
Fig. 1.
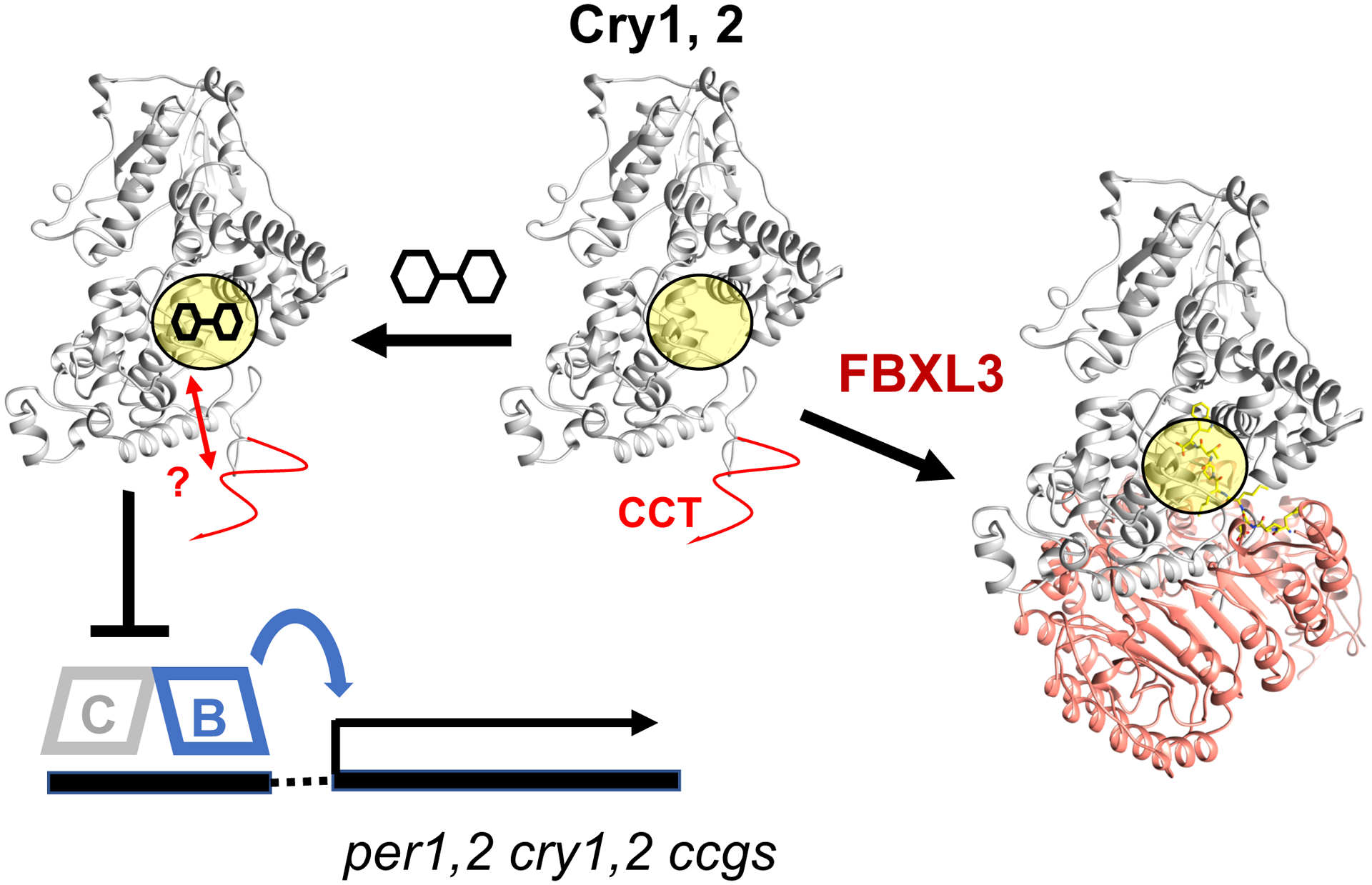
Competing for a binding pocket: Cry1,2 directly repress the heterodimeric transcription factor composed of Clock (C) and BMAL1 (B), which activates expression of clock-controlled genes (ccgs), including period 1 and 2 (per1,2). Small molecule compounds compete for the same primary pocket (yellow) as the E3 ubiquitin component FBXL3, and thereby enhance Cry repressor activity. An unstructured Cry C-terminal domain (CCT) confers compound specificity by possibly interacting with the occupied recognition pocket (red arrows).
About ten years ago, Hirota, Kay, and coworkers, implemented high-throughput cell-based assays to screen hundreds of thousands of compounds for their ability to perturb circadian oscillations of gene expression (Hirota et al., 2012). The screens were based on the rhythmic activation of a luciferase reporter gene under the control of a Bmal1 or Per2 promoter in human cell lines. Several different classes of compounds were found to affect the circadian period. The molecules were subsequently shown to target proteins that compose or regulate the core TTFL. One such carbazole derivative known as KL001 lengthens the circadian cycle by interacting directly with both Cry1 and Cry2 (Hirota et al., 2012). Remarkably, KL001 elicits its activity by binding into the cavity that normally harbors the flavin cofactor in light-sensing cryptochromes (Nangle et al., 2013). This pocket is also recognized by the E3 ubiquitin ligase subunit FBXL3 to direct the Cry proteins toward degradation (Xing et al., 2013). Thus, KL001 interferes with Cry ubiquitination and thereby enhances cellular stability (Fig. 1). However, KL001 has similar affinity and activity against both Cry isoforms.
This brings us to the current work of Miller, Kay, and Hirota, et al. (Miller et al., 2020a). The authors took a candidate testing approach to follow up on a hit from their extensive screens (Hirota et al., 2012). The compound KL201 was found to lengthen the circadian period but did not affect the activities of kinases known to regulate the clock. Rather, KL201 increased Cry protein stability in a cell-based degradation assay. The selectivity of KL201 for Cry1 over Cry2 was shown in Cry-knockout cell lines. KL201 only appreciably altered period2-driven reporter genes when Cry1 was present. KL201 also repressed mRNA levels of Clock-BMAL1 target genes in a Cry1-dependent manner. In vitro analysis confirms that KL201 preferentially binds Cry1.
Chemically, KL201 is based on a thienopyrimidine scaffold coupled to a third cyclohexyl ring, which distinguishes it from previously discovered circadian modulators. Cell assays combined with crystallographic analysis of the compound bound to the Cry1 photolyase homology region (PHR) define the interaction mode and rationalize the compound efficacy. KL201 contains a cyclohexylthienopyrimidine coupled to a bromophenyl moiety through an amide linkage. The bromophenyl moiety along with the size of the cyclohexyl ring are especially critical for activity.
As expected, KL201 binds deeply into the primary pocket of Cry1, mimicking the interaction with FAD seen in other cryptochromes (Fig. 2). The compound makes hydrophobic interactions between its two ring systems and the aromatic residues in the binding cleft, whereas hydrogen-bonding moieties are largely satisfied by ordered water molecules. The specificity of KL201 for Cry1 is remarkable because most pocket residues are conserved by Cry1 and Cry2, hence the lack of selectivity shown by KL001. However, comparing the crystal structures of KL201 bound to Cry1 and KL001 bound to Cry2 provides insight into the specificity of KL201. Conserved residues assume substantially different conformations in Cry1 bound to KL201 compared to Cry2 bound to KL001 (Fig. 2). It follows that second shell residues that differ between the isoforms may dictate the conformational response of the conserved pocket. Along these lines, a variable “lid” loop that interacts with the Per proteins juxtaposes the binding pocket and may influence residues that interact with the modulators (e.g., W399, Fig. 2). Thus, the structures provide an avenue to explore the underlying structural principles for selectively targeting the human Cry proteins.
Fig. 2.
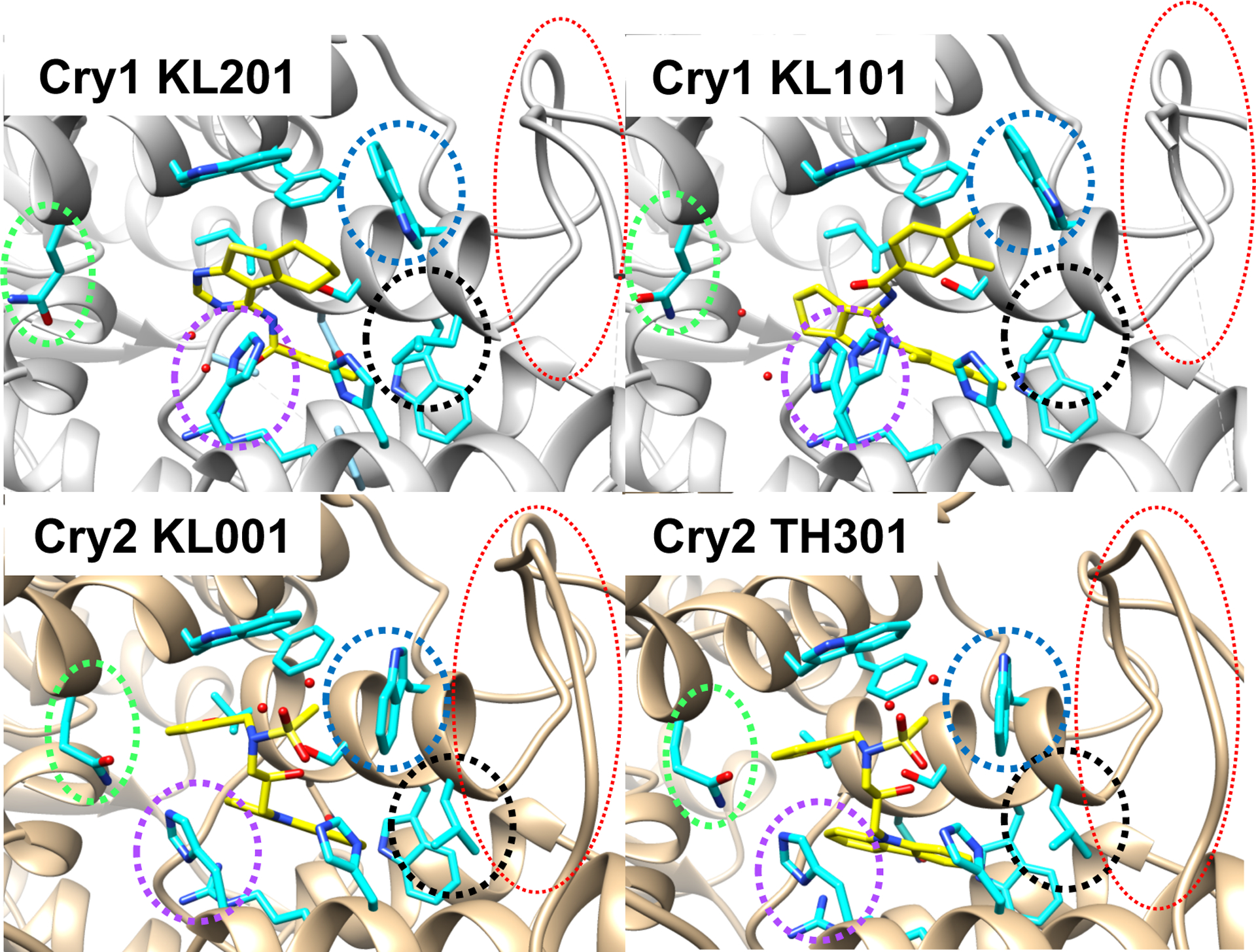
Conformational differences in conserved residues may contribute to compound specificity. The crystallographic structures of Cry1 bound to KL101, KL201, and Cry2 bound to KL001 and TH301. Pocket residues and loop regions whose confirmations differ in the Cry1 and Cry2 structures highlighted by dotted circles (Gln289 (green), His355 (purple), Trp399 (blue), Leu400 (black), lid-loop (red); Cry1 numbering).
Like KL001, KL201 blocks the site where the C-terminus of the E3-ligase component FBXL3 binds to Cry (Xing et al., 2013). Knockdown of FBXL3 substantially lengthens the circadian period on its own, but nonetheless, removes most effects of KL201. Hence, period lengthening by KL201 depends at least in part on its ability to compete for the primary recognition pocket also utilized by FBXL3 (Fig. 1).
KL201 is not the only isoform-specific Cry inhibitor recently found by this group. In a parallel report, they also describe the discovery of two other compounds: KL101, which, like KL201, is specific for Cry1, and TH301, which is specific for Cry2 (Miller et al., 2020b). In characterizing the actions of KL101 and TH301, the researchers revealed an intriguing twist to the recognition of the compounds – isoform selectivity depends on the “intrinsically disordered” Cry C-terminal domain (CCT) that varies in sequence and length across the protein family (Chaves et al., 2011). Nonetheless, the CCT is known to play key roles in function for many types of cryptochromes. For example, the regions coded by exon11 in Cry1 mediate affinity for Clock:Bmal1 and mutations that cause miss-splicing and removal of exon11 alter human sleep behavior (Patke et al., 2017). In type I insect Cry, the short CCT binds into the flavin pocket and is released upon light stimulation. In this case, regions coded for by Cry1 exon10 confer specificity for KL101, whereas regions coded for by Cry2 exon10 confer specificity for TH301. Thus, interactions among the compounds, the CCT, and the PHR pocket may collectively generate selective binding (Fig. 1). Alternatively, because exon10 is attached to the PHR C-terminus, the extension may act to alter the conformational properties of the binding pocket without contacting it directly. A fascinating issue pervading the two papers centers on this question of what drives selectivity when the residues comprising the binding pockets of the respective proteins are virtually identical. Clearly, the origins of selectivity must lie in regions of the proteins where they are different, but exactly how these differences impact recognition and manifest in terms of conformational templating, overall dynamics, and even entropic effects of the disordered extensions is an exciting direction for further investigation.
Importantly, the isoform-specific regulators have implications for Cry-dependent physiology. The researchers studied potential effects of Cry1/2 on energy metabolism and found that both proteins enhance differentiation of brown adipose tissue (BAT) under certain conditions. Remarkably, expression of specific genes associated with BAT enhancement were differentially affected by Cry1 and Cry2 and also by the isoform-selective compounds. How the Cry proteins drive BAT differentiation is not yet known in detail but could involve repression of bmal1 expression or interactions with completely different targets (Miller et al., 2020b). Cry1 and Cry2 influence transcription throughout the genome and have partners beyond CLOCK:BMAL1 (Takahashi, 2017). The Isoform-specific small-molecules described in these two studies promise to be invaluable tools for delineating a potentially wide range of non-redundant functions for these regulators, which could have high relevance for human health. For example, unlike white adipose tissue that stores calories, BAT consumes energy for thermogenesis. Thus, encouraging BAT differentiation with Cry-selective compounds may have substantial value for treating obesity and diabetes.
In all, this comprehensive work emphasizes the power of an non-biased phenotypic screening approach, especially when it is supported by well-defined cell-based assays, crystallographic analysis, and, importantly, a sufficient knowledge base that facilitates rational pursuit of candidate targets. In perhaps a pleasing parallel, it turns out that the discovered compounds, which share features of the flavin isoalloxazine ring, activate the mammalian cryptochromes by recognizing the analogous binding pocket where flavin conveys signals to their photosensory homologs.
Acknowledgement:
BRC acknowledges support of grant R35GM122535 from the National Institutes of Health.
Footnotes
Competing interests: The author declares that no competing interests exist.
References
- Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen LO, Van Der Horst GTJ, Batschauer A, and Ahmad M (2011). The cryptochromes: blue light photoreceptors in plants and animals. Annual review of plant biology 62, 335–364. [DOI] [PubMed] [Google Scholar]
- Fribourgh JL, Srivastava A, Sandate CR, Michael AK, Hsu PL, Rakers C, Nguyen LT, Torgrimson MR, Parico GCG, Tripathi S, et al. (2020). Dynamics at the serine loop underlie differential affinity of cryptochromes for CLOCK:BMAL1 to control circadian timing. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, et al. (2012). Identification of Small Molecule Activators of Cryptochrome. Science 337, 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Aikawa Y, Sugiyama A, Nagai Y, Hara A, Oshima T, Amaike K, Kay SA, Itami K, and Hirota T (2020a). An isoform-selective modulator of Cryptochrome 1 regulates circadian rhythms in mammals. Cell Chemical Biology. [DOI] [PubMed] [Google Scholar]
- Miller S, Son YL, Aikawa Y, Makino E, Nagai Y, Srivastava A, Oshima T, Sugiyama A, Hara A, Abe K, et al. (2020b). Isoform-selective regulation of mammalian cryptochromes. Nat Chem Biol 16, 676–+. [DOI] [PubMed] [Google Scholar]
- Nangle S, Xing WM, and Zheng N (2013). Crystal structure of mammalian cryptochrome in complex with a small molecule competitor of its ubiquitin ligase. Cell Res 23, 1417–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patke A, Murphy PJ, Onat OE, Krieger AC, Ozcelik T, Campbell SS, and Young MW (2017). Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 169, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosensweig C, Reynolds KA, Gao P, Laothamatas I, Shan YL, Ranganathan R, Takahashi JS, and Green CB (2018). An evolutionary hotspot defines functional differences between CRYPTOCHROMES. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS (2017). Transcriptional architecture of the mammalian circadian clock. Nature Reviews Genetics 18, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing WM, Busino L, Hinds TR, Marionni ST, Saifee NH, Bush MF, Pagano M, and Zheng N (2013). SCFFBXL3 ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 496, 64–+. [DOI] [PMC free article] [PubMed] [Google Scholar]


